Abstract
Background
The present research was designed to explore the association between single nucleotide polymorphisms (SNPs) at the 3′‐untranslated region (3′‐UTR) of methylenetetrahydrofolate reductase (MTHFR) and the risk of cervical cancer (CC).
Methods
From May 2015 to October 2016, a total of 197 patients (diagnosed with CC and precancerous lesions, and underwent surgical treatments) were enrolled in the study. Meanwhile, a total of 80 healthy cases were used as the controls. PCR‐DNA analysis was used to explore the genotype of the SNPs (rs4846048 and rs55763075) of the MTHFR 3′‐UTR as well as the association between allelic frequencies and the CC risk. Then, the role of rs4846048 SNPs in the association of microRNA‐522 (miR‐522) and MTHFR was evaluated through luciferase reporter assay. Meanwhile, the modulatory influence of miR‐522 on cell apoptosis and viability of Hela cells was also detected by flow cytometry and MTT assay.
Results
The rs4846048 AG and G allele frequencies were significantly higher in CC subgroup compared with the control group. Methylenetetrahydrofolate reductase rs4846048 A/G alleles contributed to miR‐522 binding, and miR‐522 negatively modulated the expressions of MTHFR. Furthermore, miR‐522 overexpression increased cell viability but decreased apoptotic cells in Hela cells.
Conclusion
The preliminary report revealed that the SNP rs4846048 of MTHFR enhanced the risk of CC through association with miR‐522, which further regulated cell viability and apoptosis in Hela cells.
Keywords: cervical cancer, miR‐522, MTHFR, SNPs
The present research was designed to explore the association between single nucleotide polymorphisms (SNPs) at the 3′‐untranslated region (3′‐UTR) of methylenetetrahydrofolate reductase (MTHFR) and the risk of cervical cancer (CC). This study revealed that the SNP rs4846048 of MTHFR enhanced the risk of CC through association with miR‐522, which further regulated cell viability and apoptosis in Hela cells.
![]()
1. INTRODUCTION
As one of the most frequent malignant genital tumors, an estimated 470,000 and 135,000 new cases with cervical cancer (CC) occur in the global and China, with about 200,000 and 50,000 deaths, respectively (Jemal et al., 2011). Nowadays, it has been identified that the infection of human papillomavirus (HPV) is the principal cause of CC (Muñoz et al., 2003). However, among women with a HPV infection rate of 15%–40%, the incidence of CC only accounts for 0.015%, implying that the individual differences exist in cancer susceptibility. Thus, the occurrence of CC may result from the intense crosstalk of environmental carcinogenic factors and genetic susceptibility factors (Duenas‐Gonzalez, Serrano‐Olvera, Cetina, & Coronel, 2014).
Methylenetetrahydrofolate reductase (MTHFR; OMIM accession number: 607093), which located on 1p36.3, is a core enzyme in the metabolism of folic acid (Goyette et al., 1994). Single nucleotide polymorphisms (SNPs) of MTHFR may reduce enzyme activity and increase thermal instability, thus resulting in dysfunction of MTHFR activity, folate and methionine levels in the circulation pool (Bagley & Selhub, 1998; Frosst et al., 1995). The accumulation of 5,10‐methylenetetrahydrofolate and the reduction of 5‐methyltetrahydrofolate might cause errors in DNA synthesis and methylation, eventually inducing dysfunction of a series of biosynthesis and metabolic pathways (Friso et al., 2002; Sah et al., 2018). More than 20 SNPs of MTHFR have been identified, among which the catalytic area of 677C→T and functional adjustment area 1298A→C have been reported to be closely linked with human disease (Nasr, Sami, & Ibrahim, 2012).
MicroRNAs (miRNAs), a family of small noncoding and endogenous RNAs with a length of 19–25 nucleotides, play a critical role in regulating the expression levels of target gene via binding to the 3′‐untranslated region (3′‐UTR) of mRNA sequence, thus resulting in translational repression or degradation (Doeppner et al., 2013). Several studies have revealed that SNPs located in binding site of miRNA may alter the miRNA target genes expression and accelerate the human susceptibility to cancers (Teo et al., 2012; Zhang et al., 2013), including CC (Guo, Cai, Yang, & Jiang, 2014). The association between 3′‐UTR SNPs of MTHFR and the risk of CC has not been fully elucidated yet, and especially the related regulatory mechanism. Therefore, this research aimed to assess the relation of MTHFR gene 3′‐UTR SNPs and CC, and further explore the potential mechanism of these association on the basis of regulation between MTHFR 3′‐UTR SNPs and miRNA. This study might provide new underlying mechanisms of CC pathogenesis and might provide new clues for the treatment of CC.
2. MATERIALS AND METHODS
2.1. Ethical compliance
Approval was obtained from the Medical Ethics Committee of the People's Liberation Army Hospital. The study procedure was performed after the written informed patient consent.
2.2. Study population
In this study, a total of 197 cases of patients who diagnosed with CC and precancerous lesions, and underwent surgical treatments in Department of obstetrics and gynecology of the People's Liberation Army Hospital from May 2015 to October 2016 were recruited. All the included patients were divided into three groups based on histopathology, including low‐grade squamous intraepithelial lesion (LSIL; n = 45), high‐grade squamous intraepithelial lesion (HSIL; n = 60), and CC (n = 92). The control group was randomly selected the 80 healthy cases in our hospital during the same period. Each specimen was collected about 2 ml of the peripheral blood and immediately preserved at −80°C for subsequent experiments.
2.3. DNA extraction and genotyping
Genomic DNA from each sample was extracted and was subjected to DNA genotyping. Briefly, genomic DNA (10 ng of each sample) was augmented by PCR in a total system of DNA (1 μl), upstream and downstream primer (1 μl), 2 × Taq PCR Master Mix (10 μl), and DNase‐free water (10 μl). The primer sequences for rs4846048 and rs55763075 of MTHFR (NCBI Reference Sequence: NC_000001.11, https://www.ncbi.nlm.nih.gov/nuccore/NC_000001.11?report=fasta&from=11785723&to=11806103&strand=true) were as follows: rs4846048 (F‐TATCTTTGGGGCTGTGTCCT, R‐TCTCTACCCAAAGGCATCGG); rs55763075 (F‐CTGTGCTCTTTTGGTGGG, R‐CGGGCTCCAAGTGTAAGTTC). The PCR conditions were shown in sequence as follows: 95°C (5 min), 95°C (30 s, 30 cycles), 52°C (30 s), 72°C (45 s), and 25°C (2 min). Subsequently, the PCR products were digested overnight and the fragments were electrophoresed, stained, and photographed. Sequencing was performed using the ABI 3730xl DNA Analyzer (Applied Biosystems).
2.4. Cell culture and miR‐522 transfection
HEK‐293 cells and Hela cells were suspended in culture medium (Dulbecco's modified Eagle's medium [Gibco] with 10% fetal bovine serum [Hyclone]) and seeded on culture plates or dishes, and then cultured in a humidified atmosphere at 37°C with 5% CO2.
For miR‐522 transfection, Hela cells were transfected with miR‐522 mimic, and miR‐522 inhibitor, or transfected with the respective controls, referred as mimic control and inhibitor control on use of Lipofectamine 2000 (Invitrogen) in the light of the manufacturer's instruction.
2.5. Luciferase reporter assay
The 3′‐UTR fragments of MTHFR containing either SNP‐1 or SNP‐2 (which referred as the A or G alleles of rs4846048) were amplified using PCR and then cloned into pGLO‐promoterless luciferase‐based plasmids, which were shown as pGLO‐SNP‐1 and pGLO‐SNP‐2, respectively. Thereafter, reporter plasmids and miR‐522 mimic/mimic control were cotransfected into HEK‐293 cells for 48 hr and the luciferase activity was detected with Luciferase Assay Kit (Promega), following the manufacturer's protocol. Cotransfection with a Renilla luciferase vector was employed as the normalization, and the firefly luciferase activity was normalized to Renilla luciferase activity.
2.6. Quantitative Real‐time PCR
Total RNA of Hela cells was extracted after corresponding administrations employing TRIzol reagent in accordance with the product instructions. The ratio of optical densities at 260 nm/280 nm was used to verify the purity of RNA. The extracted RNA was reversed into cDNA by using TaqMan® miRNA Reverse Transcription Kit (BioTeke), and then, TaqMan Universal Master Mix II was used for the measurement of miR‐522 expression levels in cells. The classic method was performed to analyze the levels of miR‐522, and U6 snRNA was used for normalization in each sample.
2.7. Cell viability assay
Cell viability of Hela cells was monitored by MTT assay. Briefly, transfected or nontransfected cells at indicated time were added with MTT solution (5 mg/ml for final concentration) and placed in the incubator for 4 hr at 37°C. Then, the supernatants were cautiously taken out, followed by the dissolution of purple crystals in dimethyl sulfoxide (200 μl). Finally, the absorbance was read by a microplate reader (ELX‐800; BioTek) at a wavelength of 490 nm.
2.8. Apoptosis assay
The cell apoptosis was assessed by FITC‐tagged Annexin‐V Apoptosis Detection Kit (WLA001c; Wanleibio). Briefly, cells were collected and rinsed with sterile phosphate buffer saline and then carefully resuspended in binding buffer (100 μl). Subsequently, cells were stained with the mixture of 10 μl of FITC‐tagged Annexin‐V (20 μg/ml) and 5 μl of propidium iodide (50 μg/ml) for 30 min in the darkness. After incubation, the stained cells were resuspended again in the binding buffer (400 μl). Flow cytometry analysis was immediately performed by a FACS can.
2.9. Western blot
The protein was extracted and subsequently quantified using RIPA lysis buffer and BCA™ Protein Assay Kit (Thermo), respectively. Then, equal amount of protein samples were resolved over 12% SDS‐PAGE (Beyotime) and transferred to the PVDF membranes (Millipore). The membranes were blocked with 5% nonfat milk and then were orderly incubated with primary antibodies (4°C, overnight) and secondary antibody marked by horseradish peroxidase (room temperature, 2 hr). Signals of protein were detected by enhanced chemiluminescence method.
2.10. Statistical analysis
The obtained data are shown as the mean ± standard deviation (SD). GraphPad Prism 6 software was applied for statistical analyses. The p‐values in the experiments of luciferase assay, MTT, and RT‐PCR assays were analyzed using a one‐way ANOVA, and in comparison with clinical and general characteristics were calculated through chi‐square test. Odds ratio (OR) and 95% CI were measured by analysis of univariate and multivariate logistic regression. The comparison of p‐value of <0.05 was indicated to be a statistical significance.
3. RESULTS
3.1. Comparison of participants' characteristics
No significant difference was observed in the clinical characteristics (such as age, BMI, blood pressure, and the content of folic acid) of samples in the control group and experimental group (p > .05; Table 1). Meanwhile, the general characteristics of the populations in the two groups also showed no significant difference (p > .05; Table 1).
Table 1.
The clinical characteristics of subjects in the control group and experimental group
| Characteristics | Control group (n = 80) | Experimental group (n = 197) | t/χ 2 | p‐value | OR | 95% CI |
|---|---|---|---|---|---|---|
| Age (x ± s, year) | 46.54 ± 11.45 | 46.13 ± 11.22 | −0.271 | .820 | ||
| BMI (kg/m2) | 22.30 ± 0.47 | 22.33 ± 0.53 | 0.501 | .373 | ||
| SBP (mmHg) | 106.90 ± 10.78 | 109.00 ± 11.03 | 1.446 | .542 | ||
| DBP (mmHg) | 73.18 ± 5.32 | 73.29 ± 5.13 | 0.166 | .576 | ||
| Folic acid (nmol/L) | 16.36 ± 0.61 | 14.09 ± 0.93 | −20.247 | .000* | ||
| Education degreea | 0.123 | .94 | ||||
| 1 | 39 | 95 | ||||
| 2 | 18 | 48 | ||||
| 3 | 23 | 54 | ||||
| Smoking | 0.363 | .547 | 0.729 | 0.260–2.044 | ||
| Yes | 74 | 186 | ||||
| No | 6 | 11 | ||||
| Pregnant times | 0.376 | .54 | 0.846 | 0.496–1.444 | ||
| ≤2 | 47 | 125 | ||||
| >2 | 33 | 72 | ||||
| Age of first delivery | 0.391 | .532 | 1.199 | 0.679–2.118 | ||
| ≤25 years | 56 | 132 | ||||
| >25 years | 24 | 65 | ||||
| Family history of cancer | 0.911 | .340 | 0.479 | 0.103–2.239 | ||
| Yes | 2 | 10 | ||||
| No | 78 | 187 |
P‐values were calculated through chi‐square test.
Abbreviations: BMI, body mass index; CI, confidence intervals; DBP, diastolic blood pressure; OR, odds ratio; SBP, systolic blood pressure.
Education degree: 1 referred as elementary school and below; 2 referred as middle school; and 3 referred as college degree and above.
Means p < .05 (control group vs. experimental group). OR and 95% CI were measured by analysis of univariate and multivariate logistic regression.
3.2. Analysis of MTHFR polymorphisms in 3'‐UTR
Then, the genotypes of 277 cases of blood samples were investigated. It seemed that there existed rs4846048 SNPs including AG and AA rather than GG in the control group and experimental group. However, this study could not observe polymorphic results in the corresponding site of rs55763075 in those samples. As a consequence, the genotype frequencies of AA and AG, as well as A and G alleles in 197 patients and 80 healthy controls were further investigated. The results showed that there was a statistically different distribution frequency of MTHFR SNP rs4846048 between experimental subgroups and control group (p < .05). In addition, in subgroup of CC, the frequencies of AG and G alleles were significant higher than in other three groups (control group, HSIL group, and LSIL group; p < .05). However, there was no statistical difference in distribution frequencies of two genotypes and alleles in HSIL and LSIL groups, compared with control group (p > .05; Table 2). Based on the genotype frequencies, the risk of CC was analyzed in control, HSIL, and LSIL groups, respectively. Results showed that the AG genotype was closely related with the risk of CC in controls (OR [95% CI] = 2.62 [1.36, 5.06]; p = .004), HSIL (OR [95% CI] = 2.27 [1.13, 4.55]; p = .021), and LSIL patients (OR [95% CI] = 5.41 [1.86, 15.70]; p = .002). Meanwhile, the G allele was closely associated with the risk of CC, CC versus control, OR (95% CI) = 2.62 (1.36, 5.06), p = .004; CC versus HSIL, OR (95% CI) = 2.27 (1.13, 4.55), p = .021; and CC versus LSIL, OR (95% CI) = 5.41 (1.86, 15.70), p = .002. Besides, Hardy–Weinberg equilibrium (HWE) testing suggested that the genotypes in control group (χ 2 = 0.736; p = .3911) conformed to HWE expectation. Thus, these results revealed that the MTHFR rs4846048 SNP was associated with CC risk.
Table 2.
Comparison of rs4846048 genotypes and allele frequencies in the control group and experimental group
| Group | Cases | rs4846048 (cases) | Allele (frequency) | ||
|---|---|---|---|---|---|
| AA | AG | A | G | ||
| Control | 80 | 66 | 14 | 146 | 14 |
| CC | 92 | 55 | 37 | 147 | 37 |
| HSIL | 60 | 48 | 12 | 108 | 12 |
| LSIL | 45 | 41 | 4 | 86 | 4 |
| Overall comparison: χ 2, p | 21.160, .000* | 18.249, .000* | |||
| Multiple comparison: χ 2, p | CC versus control | 10.587, .001 | 8.744, .003 | ||
| CC versus HSIL | 6.795, .009 | 5.489, .019 | |||
| CC versus LSIL | 14.143, .000* | 11.654, .001 | |||
| HSIL versus control | 0.142, .707 | 0.127, .721 | |||
| HSIL versus LSIL | 2.458, .117 | 2.255, .133 | |||
| LSIL versus control | 1.733, .188 | 1.598, .206 | |||
NCBI Reference Sequence: NC_000001.11 (https://www.ncbi.nlm.nih.gov/nuccore/NC_000001.11?report=fasta&from=11785723&to=11806103&strand=true).
Abbreviations: CC, cervical cancer; HSIL, high‐grade squamous intraepithelial lesion; LSIL, low‐grade squamous intraepithelial lesion.
Means p < .05 compared between groups.
3.3. miR‐522 could bind to MTHFR rs4846048 SNP and negatively regulate the expression of MTHFR
As shown in Figure 1a, miR‐522 mimic transfection significantly declined luciferase activity in both pGLO‐SNP‐1 and pGLO‐SNP‐2 plasmids transfected HEK‐293 cells, compared with respective mimic controls (p < .05). The binding efficiency of miR‐522 and MTHFR is not related with rs4846048 A→G SNPs. Furthermore, the influence of miR‐522 on the expression level of MTHFR on Hela cells was also studied. Hela cells were transfected with miR‐522‐overexpressing vector (miR‐522 mimic), and RT‐PCR assay was used to verify transfected efficiency. As expected, miR‐522 levels were significantly elevated after miR‐522 mimic transfection in Hela cells (Figure 1b, p < .01). Results of Western blot in Figure 1c revealed that miR‐522 mimic obviously downregulated the expression of MTHFR, while miR‐522 inhibitor upregulated MTHFR expression in Hela cells. These data suggested that miR‐522 might negatively regulate MTHFR levels via binding to its 3′‐UTR.
Figure 1.
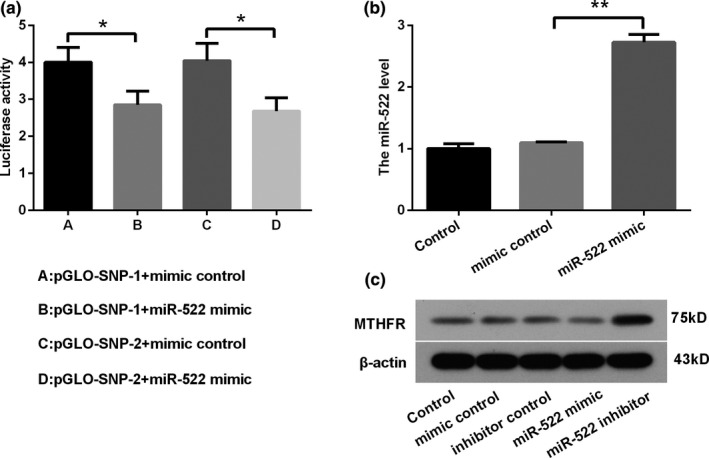
miR‐522 negatively regulated MTHFR expression by binding to its 3′‐UTR. (a) The binding of miR‐522 and MTHFR was verified by luciferase activity assay. (b) RT‐PCR assay was performed to assess the level of miR‐522 in Hela cells after transfection. (c) MTHFR expression was detected by Western blot after transfection in Hela cells. *p < .05 and **p < .01 were calculated using a one‐way ANOVA. *p < .05 was considered to have a significant difference. Abbreviations: 3′‐UTR, 3′‐untranslated region; miR‐522, microRNA‐522; MTHFR, methylenetetrahydrofolate reductase; qRT‐PCR, quantitative real‐time PCR; SNP, single nucleotide polymorphisms
3.4. miR‐522 promoted cell viability but reduced cell apoptosis in Hela cells
The study further investigated whether miR‐522 had an influence on cell viability and apoptosis in Hela cells. Results in Figure 2a indicated that miR‐522 mimic significantly increased viability of Hela cells compared with the mimic control (p < .01). However, a completely opposite effect was found in miR‐522 inhibitor‐transfected Hela cells, as miR‐522 inhibitor significantly decreased OD values (at 490 nm) compared with the inhibitor control (p < .05). Meanwhile, flow cytometry assay showed that miR‐522 mimic reduced the ratio of apoptotic cells, while miR‐522 inhibitor enhanced the apoptotic cell numbers (Figure 2b).
Figure 2.
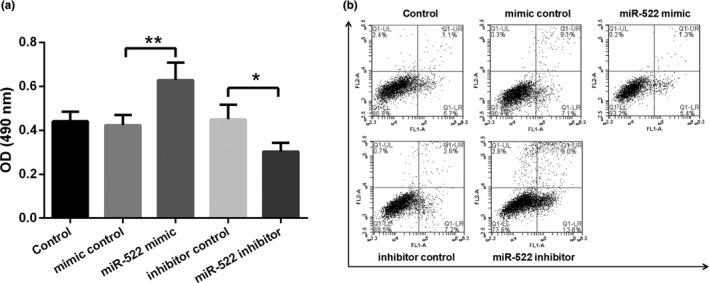
miR‐522 enhanced cell viability but inhibited cell apoptosis in Hela cells. (a) Cell viability of Hela cells was assessed by MTT assay after transfection. (b) Apoptotic cells were detected using flow cytometry analysis. *p < .05 and **p < .01 were calculated using a one‐way ANOVA. *p < .05 was considered to have a significant difference. Abbreviations: miR‐522, microRNA‐522; OD, optical density
4. DISCUSSION
An increasing number of unveilings indicated that the MTHFR polymorphisms were associated with susceptibility of CC (Delgado‐Enciso et al., 2006; Kohaar et al., 2010; Wu, Yang, Lin, Li, & Wen, 2013). The current study provided the evidence that the SNP rs4846048 of MTHFR was associated with the increased CC risk. The rs4846048 AG and G allele frequencies were significantly higher in CC patients compared with the healthy cases. The rs4846048 A/G alleles of MTHFR promoted its binding of miR‐522, and miR‐522 negatively regulated the MTHFR expressions. In addition, miR‐522 overexpression promoted cell viability but inhibited cell apoptosis in human CC cell line (Hela cells). Cheng et al. have presented that MTHFR C677T was an ethnicity‐dependent risk factor for the occurrence and development of CC through meta‐analysis (Wu, Gong, et al., 2013). Meanwhile, Zhuo et al. proved that MTHFR C667T was associated with declined susceptibility of CC among Caucasians by using meta‐analyses in 4,421 individuals (Zhuo, Zhang, Ling, Zhu, & Chen, 2012). Two polymorphic sites (rs4846048 and rs55763075) of MTHFR have been forecasted according to the combination of bioinformatics method and references researches. A previous study by Jeon et al. have reported that MTHFR rs4846048 might potentially associate with colorectal cancer (CRC) susceptibility in Koreans (Jeon et al., 2015). Wang et al. also demonstrated that the genotype frequency of MTHFR rs4846048 genotype was higher in gastric cardia adenocarcinoma (GCA) patients than in healthy controls, implying there was association between rs4846048 and GCA susceptibility among the Han population of China (Wang et al., 2015). However, evidences proved that MTHFR rs4846048 associated with a decreased risk of breast cancer and esophageal squamous cell carcinoma in a Chinese population (Lu et al., 2015; Tang et al., 2014). These previous reports suggest that rs4846048 of MTHFR might play an important role in cancer susceptibility. However, the association between rs4846048 SNPs and CC risk has not been reported yet. In this study, it demonstrated that the MTHFR rs4846048 polymorphism was existed in objected populations and the rs4846048 AG and G allele frequencies were remarkably higher in CC patients than the healthy cases. The G allele frequency in control group was 8.75% (14/160) in our study. The previous studies about GCA (Wang et al., 2015) and esophageal squamous cell carcinoma (Wang et al., 2015) suggested that the MTHFR rs4846048 G allele frequency was about 10% in Han Chinese population, which was similar with our findings. These results firstly revealed the association between rs4846048 SNPs with elevated CC risk and might provide new clues for potential biomarkers of CC prevention and treatment. In addition, the rs55763075 polymorphism was not found in CC patients, which might be attributing to the limitation of sample size, geographical distribution differences, genetic diversity, and so on. However, more investigations need to be done to identify it.
By document research and data comparison from miRNA SNP 2.0 (http://www.bioguo.org/miRNASNP/), it suggested that MTHFR rs4846048 located in complementary region of hsa‐miR‐522 seed sequence. Thus, it was worthy to verify whether miR‐522 could directly bind to 3′‐UTR of MTHFR and to explore the effect of SNPs of MTHFR rs4846048 on the binding efficiency. Mechanistically, it was firstly proved that MTHFR rs4846048 polymorphism affected the binding with miR‐522 and further negatively regulated the expression of MTHFR. Liu et al. reported that the SNP in MTHFR could enhance the binding of miR‐661 and MTHFR, leading to decreased expression of MTHFR in 293T cells, eventually associated with the increased risk of atherosclerosis (Liu et al., 2018). Another study also suggested that rs4846049 of MTHFR could modify miR‐149 binding and was associated with increased occurrence of coronary heart disease (Wu, Gong, et al., 2013). The results in the present study were partially consistent with previous study and might provide demystify for the underlying mechanism of CC development.
MicroRNA‐522 has been frequently identified as an oncogene in various cancers, such as non‐small‐cell lung cancer (Zhang et al., 2016), hepatocellular carcinoma (Shi, Qi, Liu, & Ding, 2016), CRC (Shuai, Wang, & Dong, 2018), and so on. The upregulation of miR‐522 could contribute the tumorigenesis and progression. However, the role of miR‐522 in CC has been rarely elucidated yet. These observations were consistent with previous reports about the role of miR‐522 in other cancers, which revealed that miR‐522 mimic transfection enhanced cell viability but inhibited apoptotic cell rates of Hela cells, along with the opposite effect of miR‐522 inhibitor transfection. However, the exact role of miR‐522 in CC and the potentially detailed mechanism need to be explored in future.
However, this study has several limitations. Firstly, the clinical sample size in this study was relatively small, especially the number of patients in control group. Secondly, the rs55763075 polymorphism of MTHFR was not detected in our study and a limited number of 3′‐UTR polymorphism were investigated. Finally, the exact role of miR‐522 in CC was not clarified fully. Thus, further studies with a large sample size are warranted.
5. CONCLUSION
Overall, the preliminary report demonstrated that MTHFR rs4846048 SNPs are related with enhanced risk of CC and the mechanism might be through binding to miR‐522 and further regulatory effect of miR‐522. These results might provide new insight for the understanding of pathological mechanisms for CC.
CONFLICT OF INTEREST
None declared.
AUTHORS' CONTRIBUTIONS
XZ carried out conception and design of the research. LS carried out acquisition of data. LS and JN carried out analysis and interpretation of data. LS and JN carried out drafting the manuscript. YL and JW carried out revision of manuscript for important intellectual content. All authors read and approved the final manuscript.
ETHICAL APPROVAL
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
INFORMED CONSENT
Informed consent was obtained from all individual participants included in the study.
Zhou X, Shan L, Na J, Li Y, Wang J. The SNP rs4846048 of MTHFR enhances the cervical cancer risk through association with miR‐522: A preliminary report. Mol Genet Genomic Med. 2020;8:e1055 10.1002/mgg3.1055
Funding information
This work was supported by Natural Science Project of Liaoning Province (grant number 20180551098).
REFERENCES
- Bagley, P. J. , & Selhub, J. (1998). A common mutation in the methylenetetrahydrofolate reductase gene is associated with an accumulation of formylated tetrahydrofolates in red blood cells. Proceedings of the National Academy of Sciences of the United States of America, 95(22), 13217–13220. 10.1073/pnas.95.22.13217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado‐Enciso, I. , Martinez‐Garza, S. G. , Rojas‐Martinez, A. , Espinoza‐Gomez, F. , Canseco‐Avila, L. M. , Vidal‐Gutierrez, O. , … Ortiz‐Lopez, R. (2006). The effect of MTHFR polymorphisms, pregnancy and first intercourse on cervical cancer in a population from the Northeastern Mexico. Revista de Investigacion Clinica, 58(5), 462–469. [PubMed] [Google Scholar]
- Doeppner, T. R. , Doehring, M. , Bretschneider, E. , Zechariah, A. , Kaltwasser, B. , Müller, B. , … Michel, U. (2013). MicroRNA‐124 protects against focal cerebral ischemia via mechanisms involving Usp14‐dependent REST degradation. Acta Neuropathologica, 126(2), 251–265. 10.1007/s00401-013-1142-5 [DOI] [PubMed] [Google Scholar]
- Duenas‐Gonzalez, A. , Serrano‐Olvera, A. , Cetina, L. , & Coronel, J. (2014). New molecular targets against cervical cancer. International Journal of Women's Health, 6, 1023–1031. 10.2147/IJWH.S49471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friso, S. , Choi, S.‐W. , Girelli, D. , Mason, J. B. , Dolnikowski, G. G. , Bagley, P. J. , … Selhub, J. (2002). A common mutation in the 5,10‐methylenetetrahydrofolate reductase gene affects genomic DNA methylation through an interaction with folate status. Proceedings of the National Academy of Sciences of the United States of America, 99(8), 5606–5611. 10.1073/pnas.062066299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frosst, P. , Blom, H. J. , Milos, R. , Goyette, P. , Sheppard, C. A. , Matthews, R. G. , … Rozen, R. (1995). A candidate genetic risk factor for vascular disease: A common mutation in methylenetetrahydrofolate reductase. Nature Genetics, 10(1), 111–113. 10.1038/ng0595-111 [DOI] [PubMed] [Google Scholar]
- Goyette, P. , Sumner, J. S. , Milos, R. , Duncan, A. M. , Rosenblatt, D. S. , Matthews, R. G. , & Rozen, R. (1994). Human methylenetetrahydrofolate reductase: Isolation of cDNA, mapping and mutation identification. Nature Genetics, 7(2), 195–200. 10.1038/ng0694-195 [DOI] [PubMed] [Google Scholar]
- Guo, H. , Cai, Y. , Yang, S. , & Jiang, P. (2014). Association of microRNA‐502‐binding site single nucleotide polymorphism of lysine ethyltransferase8 gene with cervical cancer risk in Chinese women. Journal of Bionanoscience, 8(1), 51–55. 10.1166/jbns.2014.1191 [DOI] [Google Scholar]
- Jemal, A. , Bray, F. , Center, M. M. , Ferlay, J. , Ward, E. , & Forman, D. (2011). Global cancer statistics. CA: A Cancer Journal for Clinicians, 65(2), 87–108. 10.3322/caac.20107 [DOI] [PubMed] [Google Scholar]
- Jeon, Y. J. , Kim, J. W. , Park, H. M. , Kim, J. O. , Jang, H. G. , Oh, J. , … Kim, N. K. (2015). Genetic variants in 3'‐UTRs of methylenetetrahydrofolate reductase (MTHFR) predict colorectal cancer susceptibility in Koreans. Scientific Reports, 5, 11006 10.1038/srep11006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohaar, I. , Kumar, J. , Thakur, N. , Hussain, S. , Niyaz, M. K. , Das, B. C. , … Bharadwaj, M. (2010). Homocysteine levels are associated with cervical cancer independent of methylene tetrahydrofolate reductase gene (MTHFR) polymorphisms in Indian population. Biomarkers, 15(1), 61–68. 10.3109/13547500903295881 [DOI] [PubMed] [Google Scholar]
- Liu, X. , Wang, L. , Chi, H. , Wang, J. , Zheng, Q. , Li, J. , … Yang, D. (2018). The SNP Rs915014 in MTHFR regulated by MiRNA associates with atherosclerosis. Cellular Physiology and Biochemistry, 45(3), 1149–1155. 10.1159/000487355 [DOI] [PubMed] [Google Scholar]
- Lu, Q. , Jiang, K. , Li, Q. , Ji, Y. J. , Chen, W. L. , & Xue, X. H. (2015). Polymorphisms in the MTHFR gene are associated with breast cancer risk and prognosis in a Chinese population. Tumour Biology, 36(5), 3757–3762. 10.1007/s13277-014-3016-4 [DOI] [PubMed] [Google Scholar]
- Muñoz, N. , Bosch, F. X. , de Sanjosé, S. , Herrero, R. , Castellsagué, X. , Shah, K. V. , … Meijer, C. J. L. M. (2003). Epidemiologic classification of human papillomavirus types associated with cervical cancer. New England Journal of Medicine, 348(6), 518–527. 10.1056/NEJMoa021641 [DOI] [PubMed] [Google Scholar]
- Nasr, A. S. , Sami, R. M. , & Ibrahim, N. Y. (2012). Methylenetetrahydrofolate reductase gene polymorphisms (677C > T and 1298A > C) in Egyptian patients with non‐Hodgkin lymphoma. Journal of Cancer Research & Therapy, 8(3), 355–360. 10.4103/0973-1482.103512 [DOI] [PubMed] [Google Scholar]
- Sah, A. K. , Shrestha, N. , Joshi, P. , Lakha, R. , Shrestha, S. , Sharma, L. , … Rijal, B. (2018). Association of parental methylenetetrahydrofolate reductase (MTHFR) C677T gene polymorphism in couples with unexplained recurrent pregnancy loss. BMC Research Notes, 11(1), 233 10.1186/s13104-018-3321-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi, Y. H. , Qi, B. B. , Liu, X. B. , & Ding, H. M. (2016). Upregulation of miR‐522 is associated with poor outcome of hepatocellular carcinoma. European Review for Medical and Pharmacological Sciences, 20(15), 3194–3198. [PubMed] [Google Scholar]
- Shuai, F. , Wang, B. , & Dong, S. (2018). miR‐522‐3p promotes tumorigenesis in human colorectal cancer via targeting bloom syndrome protein. Oncology Research Featuring Preclinical and Clinical Cancer Therapeutics, 26(7), 1113–1121. 10.3727/096504018X15166199939341 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Tang, W. , Zhang, S. , Qiu, H. , Wang, L. , Sun, B. , Yin, J. , & Gu, H. (2014). Genetic variations in MTHFR and esophageal squamous cell carcinoma susceptibility in Chinese Han population. Medical Oncology, 31(5), 915 10.1007/s12032-014-0915-6 [DOI] [PubMed] [Google Scholar]
- Teo, M. T. W. , Landi, D. , Taylor, C. F. , Elliott, F. , Vaslin, L. , Cox, D. G. , … Kiltie, A. E. (2012). The role of microRNA‐binding site polymorphisms in DNA repair genes as risk factors for bladder cancer and breast cancer and their impact on radiotherapy outcomes. Carcinogenesis, 33(3), 581–586. 10.1093/carcin/bgr300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y. , Chen, S. , Kang, M. , Tang, W. , Gu, H. , Yin, J. , & Huang, Z. (2015). Genetic variations in MTHFR and gastric cardia adenocarcinoma susceptibility in the Chinese Han population. International Journal of Clinical and Experimental Medicine, 8(10), 18936–18944. [PMC free article] [PubMed] [Google Scholar]
- Wu, C. , Gong, Y. , Sun, A. , Zhang, Y. , Zhang, C. , Zhang, W. , … Ge, J. (2013). The human MTHFR rs4846049 polymorphism increases coronary heart disease risk through modifying miRNA binding. Nutrition, Metabolism and Cardiovascular Diseases, 23(7), 693–698. 10.1016/j.numecd.2012.02.009 [DOI] [PubMed] [Google Scholar]
- Wu, C. Y. , Yang, M. , Lin, M. , Li, L. P. , & Wen, X. Z. (2013). MTHFR C677T polymorphism was an ethnicity‐dependent risk factor for cervical cancer development: Evidence based on a meta‐analysis. Archives of Gynecology and Obstetrics, 288(3), 595–605. 10.1007/s00404-013-2721-3 [DOI] [PubMed] [Google Scholar]
- Zhang, S. , Chen, H. , Zhao, X. , Cao, J. , Tong, J. , Lu, J. , … Lu, D. (2013). REV3L 3′UTR 460 T>C polymorphism in microRNA target sites contributes to lung cancer susceptibility. Oncogene, 32(2), 242–250. 10.1038/onc.2012.32 [DOI] [PubMed] [Google Scholar]
- Zhang, T. , Hu, Y. , Ju, J. , Hou, L. , Li, Z. , Xiao, D. , … Zhang, L. (2016). Downregulation of miR‐522 suppresses proliferation and metastasis of non‐small cell lung cancer cells by directly targeting DENN/MADD domain containing 2D. Scientific Reports, 6, 19346 10.1038/srep19346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuo, W. L. , Zhang, L. , Ling, J. J. , Zhu, Y. , & Chen, Z. T. (2012). MTHFR C677T and A1298C polymorphisms and cervical carcinoma susceptibility: Meta‐analyses based on 4,421 individuals. Molecular Biology Reports, 39(9), 8723–8732. 10.1007/s11033-012-1732-7 [DOI] [PubMed] [Google Scholar]


