Abstract
Background
Preeclampsia (PE): a hypertensive disorder of pregnancy characterized by de novo development of concurrent hypertension and proteinuria. The prevailing theory determined that PE starts from the placenta and ends in the maternal endothelium. Role of endothelial dysfunction in the onset of PE has been reported in different populations. Therefore, present study was designed to investigate the localization and expression of endothelial nitric oxide synthase (eNOS) and role of oxidative stress markers in preeclamptic Pakistani women.
Methods
A total of 400 blood samples (PE = 200, controls = 200) and 100 placental tissues (PE = 50, controls = 50) were recruited from pregnant women. Reactive oxygen species (ROS), thiobarbituric acid reactive substances (TBARS), superoxide dismutase (SOD), catalase (CAT), and guaiacol peroxidase (POD) levels were analyzed through spectrophotometer. Immunohistochemistry and quantitative real‐time polymerase chain reaction (qRT‐PCR) were carried out to estimate the localization and expression of eNOS in the placentas of PE patients and healthy pregnant women.
Results
Significantly increased levels of POD (0.01), TBARS (0.04), and ROS (p ≤ .001) were determined in preeclamptic women while, nonsignificant change in SOD and CAT was observed in both groups. Reduced eNOS immunoreactivity (p ≤ .001) and mRNA abundance (p ≤ .001) was observed in preeclamptic group as compared to control group.
Conclusion
Considering the results of current study, it is concluded that decreased eNOS expression and oxidative stress could play a role in the pathology of PE seen both in placenta and ultimately in maternal endothelium. However, large studies are necessary to validate these findings to prevent maternal and neonatal morbidity and mortality in Pakistani population.
Keywords: endothelial nitric oxide synthase, oxidative stress, placenta, preeclampsia
Oxidative stress and decreased placental eNOS expression might constitute a characteristic finding in the preeclamptic placenta and support the hypothesis that complex mechanisms involving eNOS pathways and oxidative stress may promote microvascular oxidative damage and favor abnormal placenta perfusion, probably by contributing to the reduced placental blood flow and increased resistance to flow in the feto‐maternal circulation.
1. INTRODUCTION
Preeclampsia (PE), a hypertensive disorder of pregnancy characterized by de novo development of concurrent hypertension and proteinuria, is a major contributor to maternal mortality, premature birth, intrauterine growth retardation, and perinatal mortality (Steegers et al., 2010). The only effective treatment available for PE is setting up premature delivery before 34 weeks of gestation to avoid severe maternal complications, taking into account that this attitude involves serious neonatal damage (Lisonkova & Joseph, 2013; Pennington et al., 2012).
The principal contributor to the pathogenesis of PE is placenta, as the syndrome is resolved when placenta is delivered (Raghupathy, 2013). Mechanistically, it was suggested that PE results from disturbed trophoblastic invasion of spiral arteries with remodeling, leading to reduced placental perfusion and endothelial dysfunction (George & Granger, 2010). Central to the effects of PE are the resulting presence of uteroplacental hypoxia, an imbalance in angiogenic and antiangiogenic proteins (Wang et al., 2009), oxidative stress (Gupta et al., 2005), maternal endothelial dysfunction (Gilbert et al., 2008), and elevated systemic inflammation (Manuel‐Apolinar et al., 2013). Due to impaired trophoblastic invasion, intermittency of arterial blood flow occurs resulting in periods of ischemia/reperfusion, creating a hypoxic environment which favors oxidative stress, consequent oxidative damage and inflammation (Myatt & Webster, 2009).
Oxidative stress is the imbalance between antioxidants defense mechanism and formation of ROS (Touyz, 2004). ROS are signaling molecules that regulate many functions in human physiology (Kalyanaraman, 2013). During normal pregnancy, ROS generations are known to be increased and are necessary for proper physiology, and utilize the antioxidant molecules at cellular levels (Dias, Cavalli, & Sandrim, 2018; Mutinati et al., 2013; Sinzato et al., 2018; Yang et al., 2012). The excess amount of ROS is the outcome of oxidative stress and endothelial dysfunction (Matsubara et al., 2015; McMaster et al., 2018). Increased antioxidant activity is necessary in placenta with the advancing gestation to prevent oxidative stress which leads to poor fetal outcome (Aouache, Biquard, Vaiman, & Miralles, 2018; Mahadik & Ali Sina, 2003; Shetty et al., 2018; Wang & Walsh, 1996).
Not all free radicals cause disturbances in the organism (Kalyanaraman, 2013), and NO is an example of such radicals (Moncada & Higgs, 1993; Moncada et al., 1991; Palmer et al., 1988). NO is a low molecular weight mediator, it mediates function of endothelium by regulating platelet aggregation, vascular tone, leukocyte adhesion, and smooth muscle cells development (Qian & Fulton, 2013). Endothelial dysfunction is characterized by decreased bioavailability of NO through reduced production or increased consumption by oxidative stress. Decreased concentrations of NO in plasma and the placenta have been found in women who have PE, and although its role in the pathophysiology of PE has not been well defined, it is postulated that low NO concentrations might contribute through a lack of paracrine vasodilatory effect on the uteroplacental blood flow (Brosens, Pijnenborg, & Brosens, 2002; Dikensoy et al., 2009). NO is synthesized by the endothelial nitric oxide synthase (eNOS) enzyme by the activity of eNOS (OMIM 163729, GenBank NM_000603.5) (Qian & Fulton, 2013). eNOS is constitutively expressed in endothelium and maintains vascular tone through the intrinsic synthesis of NO from the reduction of L‐arginine to L‐citruline (Moncada & Higgs, 1993; Moncada et al., 1991; Palmer et al., 1988). In placenta, eNOS expression is associated with cytotrophoblast to syncytiotrophoblast differentiation (Eis, Brockman, Pollock, & Myatt, 1995). The regulation of eNOS in PE is still controversial; in fact, the activity of eNOS has been shown to be decreased, unchanged, or increased in PE compared with normal pregnancy (Kim et al., 2006).
The pregnancy complicated by preeclampsia has been widely studied in biological basis and clinical outcomes, but it is unfortunate that the mechanism of this complication is not clear yet. Considering clinical symptoms of PE, different mega projects and meta‐analysis were conducted to better understand the underlying mechanisms but there are many gaps to be filled. Present study was directed to determine the role of oxidative stress markers in susceptibility to preeclampsia, in addition to elaborating the localization and expression of eNOS in placenta of preeclamptic Pakistani women.
2. MATERIALS AND METHODS
2.1. Ethical compliance
The present study was conducted with prior approval from ethical committees of Quaid‐i‐Azam University, Islamabad and collaborating hospitals including Pakistan Institute of Medical Sciences (PIMS), Islamabad and Quaid‐e‐Azam International Hospital, Islamabad. All participants were informed about the study objectives and signed an informed consent. The study protocol was done in accordance with the principles of the Declaration of Helsinki.
2.2. Patient identification and sample collection
Approximately 400 blood samples of pregnant women (200 = controls, 200 = PE) in the third trimester according to the diagnostic criteria for PE were recruited during the period of September 2015 to July 2017. A total 100 placental tissues (PE = 50, controls = 50) were collected after simple vaginal delivery (SVD) and cesarean section (C‐section).
2.3. Inclusion and exclusion criteria
Diagnostic criteria for preeclampsia were blood pressure ≥140/90 mmHg, and proteinuria ≥+1 on Dipstick test. The normotensive control group has women with uncomplicated gestation and blood pressure <125/85 mmHg and no proteinuria. Women with less than 35 years of age were included in the study. Exclusion criteria were diabetes, asthma, kidney disease, hematological disorder, autoimmune disease, urinary tract infection, current or past history of smoking, and eclampsia.
2.4. Sampling and storage
Venous blood samples were collected from all subjects during the antepartum period, before the onset of delivery in labeled tubes, serum was separated and stored at −80°C for analysis of oxidative stress markers and antioxidant enzymes. Fresh placental villous biopsies were taken from different sites of each placenta immediately after vaginal delivery or cesarean section in less than 10 minutes according to the protocol described by Burton et al., 2014. Tissues were washed in phosphate‐buffered saline (PBS) and half tissue was fixed in 10% formaldehyde for immunohistochemical processing and half tissue was immediately snap‐frozen in liquid nitrogen and kept at −80°C for subsequent mRNA studies using quantitative real‐time polymerase chain reaction (qRT‐PCR) and other analysis.
2.5. Oxidative stress markers activity
Oxidative stress markers were estimated in serum samples of patients with PE and normotensive pregnant women.
2.6. Estimation of ROS
Hayashi et al. (2007) protocol was followed for ROS detection. (Hayashi et al., 2007). 0.1 M sodium acetate buffer was prepared by dissolving 4.1 g of sodium acetate in 500 ml of distilled water. The pH was maintained at 4.8. Then 10 mg of N, N‐diethyl‐p‐phenylenediamine sulfate salt (DEPPD) in 100 ml of sodium acetate buffer was dissolved and a second solution was prepared by adding 50 mg of ferrous sulfate (FeSO4) in 10 ml of sodium acetate buffer. Both the solutions were mixed in a ratio of 1:25 and incubated in dark for 20 min at room temperature. Then 20 μl was taken from the solutions mixture, 1.2 ml of buffer and 20 μl of serum sample were taken in a cuvette and the absorbance was checked at 505 nm using Smart Spec TM plus Spectrophotometer. Three readings were taken for each sample after every 15 s.
2.7. TBARS
Method given by Wright et al., 1981 was used to estimate lipid peroxidation (Wright et al., 1981). Reaction solution consisted of 0.02 mM ferric chloride (FeCl3) of 100 mM, 0.2 ml ascorbic acid of 100 mM, 0.2 ml of serum sample, and 0.58 ml phosphate buffer (0.1 M) with pH value of 7.4 made the total volume 1 ml. In water bath at 37°C, final mixture was incubated for 1 hr and 1 ml of 10% trichloroacetic acid was used to end the reaction. Then 1 ml of 0.67% thiobarbituric acid was added and all the tubes were kept for 20 min in the water bath. After centrifuging for 15 min at 25,000 rpm and at 535 nm, readings were noted from spectrophotometer.
2.8. CAT activity
With small modification, method by Chance & Maehly, 1955 was used to find out the activities of CAT (Chance & Maehly, 1955). To measure CAT levels in serum samples, 0.1 ml serum sample, 2.5 ml of 50 mM phosphate buffer (pH 5.0), and 0.4 ml of 5.9 mM H2O2 were added in a cuvette. After 1 min the absorbance of solution at wavelength of 240 nm variations were noted.
2.9. SOD activity
By using the protocol of Kakkar et al. (1984), activity of SOD was determined (Kakkar et al., 1984). For this purpose, 0.3 ml of serum sample, 1.2 ml of sodium pyrophosphate buffer (0.052 mM; pH 7.0), and 0.1 ml of phenazine methosulfate (186 μM) were mixed and the reaction was started by the addition of 0.2 ml of NADH (780 μM). Finally, after 1 min, the reaction was ended by adding 1 ml of glacial acetic acid and readings were noted at 560 nm and results were explained as units/mg of protein.
2.10. POD activity
POD activity was determined by using method as recommended by Chance & Maehly, 1955 (Chance & Maehly, 1955). Reaction was carried out by adding 0.3 ml of 40 Mm H2O2, 2.5 ml of 50 mM phosphate buffer (pH = 5.0), and 0.1 ml of 20 mM guaiacol into 0.1 ml of serum sample. After 1 minute, changes in absorbance were noted at 470 nm.
2.11. Immunohistochemistry
For immunohistochemistry, one full‐thickness placental tissue section was placed in 10% buffered formalin for 12–24 hr before embedding in paraffin wax, according to standard procedures and cut into serial of 5 μm sections and put on super frosted glass slides (Micro slides, Santa Cruz Biotechnologies). Tissue sections were incubated overnight for dewaxing. Antigen retrieval was done by heating slides for 2 min in tris‐buffer saline (TBS). After drying, slides were washed with phosphate‐buffered saline (PBS) and incubated for 1 hour with incubation solution (0.05% bovine serum albumin, 0.01% Triton X and 10% goat serum). Again, slides were washed with PBS and then incubated with specific rabbit polyclonal primary antibody against eNOS (Catalogue no. ab‐5589; Abcam Biotechnology, Inc., Cambridge, United Kingdom) for 48 hr at 4°C. After incubation, slides were washed with PBS and incubated with Goat Anti‐Rabbit IgG (Alexa Fluor® 488) antibody (Catalogue no. ab‐150077; Abcam Biotechnology, Inc.) for 2 hr. Slides were then washed with PBS and kept for drying, mount with mounting medium and observed under fluorescent microscope (Bx51, Olympus, Tokyo, Japan).
2.12. eNOS mRNA expression
qRT‐PCR was used to determine the expression of eNOS mRNA in placental tissues. Results were then analysed using Qiagen tool for mean expression by comparing with glyceraldehyde 3‐phosphate dehydrogenase (GAPDH) as housekeeping gene.
2.13. mRNA extraction
Placental tissues that were immediately snap‐frozen in liquid nitrogen and kept at −80°C were homogenized in lysis buffer containing β‐mercaptoethanol by using VWR® Deluxe Universal 200 Homogenizer. RNA was extracted with and Pure LinkTM RNA isolation kit (Invitrogen, Thermo Fisher Scientific) according to manufacturer details. RNA concentration was determined by nanodrop 1000TM UV/VIS spectrophotometer (Thermo Scientific, nanodrop technologies). Complementary DNA (cDNA) was synthesized from RNA by Protoscript® cDNA synthesis kit (New England Biolabs).
2.14. qRT‐PCR
The resulting cDNA was amplified by MyGo Pro real‐time PCR Thermocycler (IT‐IS International Ltd, UK). Primers efficiency was determined by cDNA amplification for eNOS and GAPDH with their respective primers and product was run on 2.5% agarose gel for product size confirmation (Table 1). Data were analyzed by Pfaffl method (Pfaffl, 2001). Fold change of gene expression was determined by using REST‐384 Tool version 2 (Qiagen).
Table 1.
Conditions of qRT‐PCR analysis of eNOS and GAPDH
| Variants | Primers | Temperature (°C) | Bands patterns (bp) |
|---|---|---|---|
| eNOS | F: 5ʹ‐CCTCGTCCCTGTGGAAAGAC−3ʹ, R: 5ʹ‐TGCTTCATGAAAGAGGCCGT−3 | 60 | 121 |
| GAPDH | F: 5ʹ‐GCTCTCTGCTCCTCCTGTTC−3, R: 5ʹ‐CCATGGTGTCTGAGCGATGT−3 | 58 | 80 |
2.15. Statistical analysis
All data were expressed as a median along with 95% confidence intervals (CI). Quantitative data will express as mean ± SEM. Significant differences between groups for characteristics of study subjects, antioxidant levels, and immunohistochemistry results were determined by performing Welch two sample t test, while, paired t test was used for comparison of fold expression of gene by using package of R 3.5.1 (R Development Core Team, 2018).
3. RESULTS
The characteristics of study subjects have been summarized in Table 2. There was significant (p < .001) decrease in gestational age and infant weight (p = .006) of preeclamptic patients as compared to control group. Systolic pressure and diastolic blood pressure were significantly higher (p < .001) in patients with PE than control group. No trace of protein was found in control group but in PE group (2.37 ± 0.006 g/24 hr).
Table 2.
Characteristics of study subjects
| Parameters | Controls | PE Patients | p value |
|---|---|---|---|
| Age (years) | 27.19 ± 0.59 | 27.31 ± 0.64 | .88 |
| BMI (kg/m2) | 26.15 ± 0.36 | 26.51 ± 0.40 | .52 |
| Gestational age (Weeks) | 38.44 ± 0.20 | 34.61 ± 0.65 | <.001 |
| Systolic blood pleasure (mmHg) | 113.8 ± 0.64 | 156.36 ± 3.34 | <.001 |
| Diastolic blood pleasure (mmHg) | 72.38 ± 0.57 | 101.13 ± 2.02 | <.001 |
| Proteins (g/24 hr) | 0.0 ± 0.0 | 2.37 ± 0.006 | <.001 |
| Infants’ weight (kg) | 3.01 ± 0.05 | 2.71 ± 0.06 | .006 |
3.1. Estimation of oxidative stress markers
Nonsignificant change was observed in SOD (p = .97), CAT (p = 81), and POD (p = .11) levels in both groups. While, significantly elevated levels of ROS (p < .001) and TBARS (p = .04) were observed in PE women as compared to normotensive group causing oxidative stress (Table 3).
Table 3.
Oxidative stress markers in control and preeclamptic groups
| Parameters | Controls | PE Patients | p value |
|---|---|---|---|
| SOD (U/ml) | 14.74 ± 0.07 | 14.75 ± 0.01 | .97 |
| CAT (U/ml) | 10.49 ± 1.10 | 10.14 ± 01.04 | .81 |
| POD (nmole) | 12.71 ± 0.56 | 13.72 ± 0.47 | .11 |
| TBARS (nM/ml) | 27.80 ± 1.78 | 33.41 ± 2.09 | .04 |
| ROS (U/ml) | 1.77 ± 0.02 | 2.1 ± 0.07 | <.001 |
3.2. Immunoreactivity
The immunohistochemical localization of eNOS was identified by green immunofluorescence seen only in the presence of primary antibody not in the absence of primary antibody (negative control). In the terminal villi of placenta, difference in eNOS immunostaining in both villous vascular endothelium and the syncytiotrophoblast was seen quantitatively between the two groups of placentas when compared to histological sections. Control group exhibit significantly (p < .001) greater number of immunoreactive cells as compared to preeclamptic group which revealed suppressed eNOS expression (Figure 1).
Figure 1.
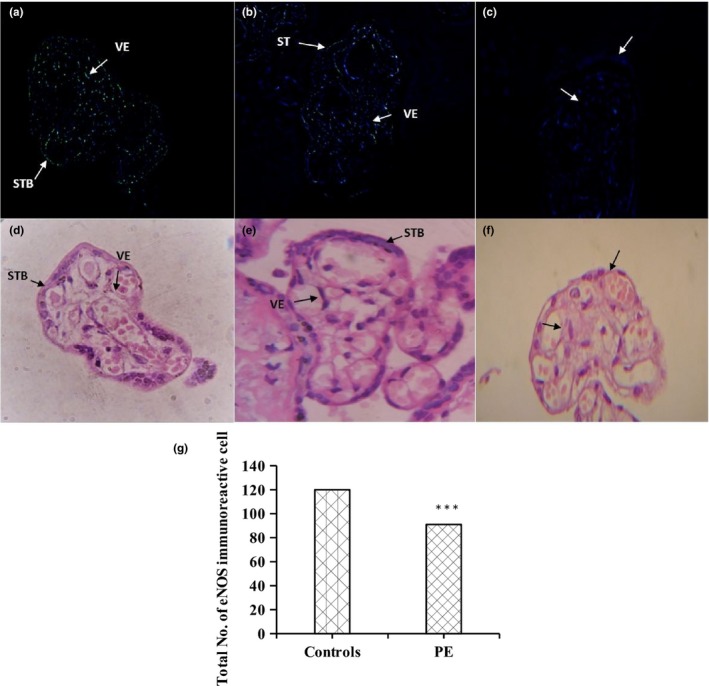
Fluorescent microscopic images of eNOS immunoreactivity in green (a, b, and c) in placental villi when compared to H&E stained microscopic images of placental villi (d, e, and f). A. Control group showed significant (<.001) increase in eNOS immunoreactive cells in villi of placenta as compared to preeclamptic group (b). (c) Represents negative control images. (g) Number of eNOS immunoreactive cells based on random sections observed in placental trophoblastic villi. eNOS immunoreactive cells were significantly (<0.001) reduced in PE group as compared to control group in paired t test analysis. Photographs were taken at 100× magnification. Syncytiotrophoblast (STB), Vascular Epithelium (VE). Significance level ***<.001
3.3. RT‐PCR
Relative RNA abundance of eNOS in placentas of preeclamptic and normotensive Pakistani women was determined by qRT‐PCR. The qRT‐PCR reaction produced a product of 122 bp for eNOS and 87 bp for GAPDH (Figure 2). The expression of eNOS was compared in both groups and results were described as fold change. There was significant (p < .001) downregulation of eNOS in preeclamptic group as compared to control group. Expression of eNOS was lowered in preeclamptic group by approximately 0.514 folds, as shown in Figure 3.
Figure 2.
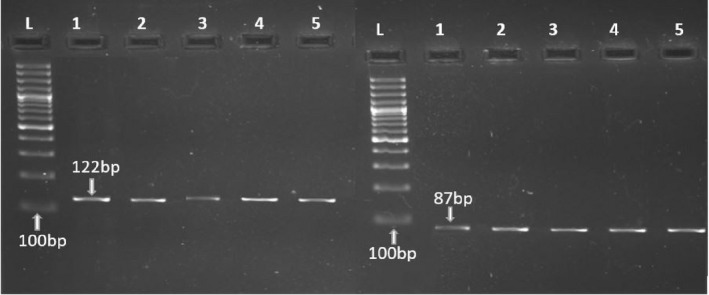
Gel images predicting the PCR product of eNOS primers (a) with product size of 121 bp and GAPDH primers (b) with product size of 87 bp compared with 100 bp ladder in cDNA samples of preeclamptic women (lane 1–3) and normotensive pregnant women (lane 4, 5)
Figure 3.
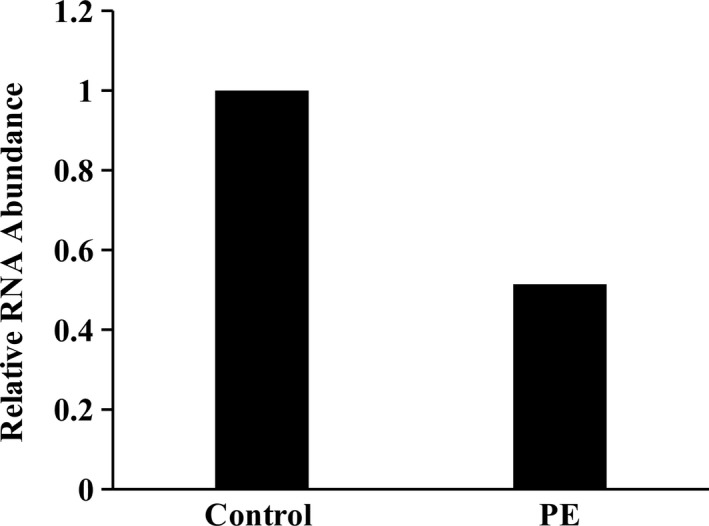
Data obtained after qRT‐PCR on placental samples of control and preeclamptic group were analyzed for relative RNA abundance of eNOS as mean ± SEM. There was significant (<.001) decrease in the abundance of eNOS mRNA in preeclamptic group as compared to normotensive group. Significance level ***<.001
4. DISCUSSION
Preeclampsia is an important maternal health problem, especially in developing countries like Pakistan. Identification of various factors could help in understanding this complication and provide clues for its management and treatment. Endothelial dysfunction and oxidative stress markers were linked with susceptibility to PE (Aggarwal, Jain, & Jha, 2010; Gannoun et al., 2015; Rahimi et al., 2013). This study was the first to assess the potential role of antioxidants levels, eNOS localization, and expression in preeclamptic women as compared to healthy normotensive pregnant women in Pakistan.
It has been suggested that oxidative stress on endothelium may itself induced by hypertension. In the present study, elevated ROS and TBARS levels were observed in PE women as compared to normotensive controls. Similar studies showed that increased ROS bioactivity exceeding antioxidant activity leads to oxidative stress (Matsubara et al., 2015). It is one of the risk factors for the development of PE through endothelial dysfunction and increased contractility resulting in placental hypoxia and ischemia (Matsubara et al., 2015; Touyz, 2004).
Results of the current study revealed that levels SOD, POD, and CAT have nonsignificant decrease in preeclamptic groups as compared to controls in accordance with previous findings (Aouache et al., 2018; Bernardi, Constantino, Machado, Petronilho, & Dal‐Pizzol, 2008; Bernardi et al., 2015; Singh et al., 2010; Var et al., 2003). Many studies determined contradictory results of elevated ROS and TBARS but reduced antioxidants in PE (Cohen et al., 2015; Mutlu‐Türko et al., 1998). Increased TBARS levels showed the increase lipid peroxidation in cells and may suggest decreased enzymatic free radical scavenging capacity in preeclamptic patients (Biri et al., 2007; Gupta et al., 2009; Saxena et al., 2015).
Modulation of vascular function occurs through interference of ROS and NO. eNOS function and expression seem to be suppressed by the increased production of ROS. Further, peroxynitrite (ONOO−) is formed as result of NO scavenging by ROS which not only oxidizes lipids, proteins, and DNA but also disturb the vascular signaling pathways (Farrow et al., 2008). Thus, ROS increase and subsequent increased ONOO− formation are known to reduce the bioavailability of NO and cause endothelial dysfunction. These effects could be key elements in the pathogenesis of PE (Matsubara et al., 2015).
In the present study, reduced eNOS activity has been observed in preeclamptic patients, these results are in consistent with the results of Kim et al., 2006 and Schönfelder et al., 2004. These results support the notion that, there will be adverse effect on placental hemodynamic functioning if the activity of eNOS becomes reduced in vivo (Kim et al., 2006). Reduced eNOS will decrease the bioavailability of NO leading to endothelial dysfunction which is the feature of this disorder (Motta‐Mejia et al., 2017). Fetal placental hemodynamics is likely to be influenced by NO production from endothelium of placental villi. However, platelets and leukocytes aggregation in intervillous spaces might be prevented by NO produced by syncytiotrophoblast (Brennecke, Gude, Iulio, & King, 1997). There have been some conflicting results with regard to eNOS activity in preeclamptic patient's placenta showing no significant difference in eNOS activity in preeclamptic women (Matsubara et al., 2015, 2001; Orange et al., 2003). In addition to eNOS reduced expression and activity, lower substrate levels for eNOS and elevated inhibitors of eNOS can also contribute toward reduced NO bioavailability (Motta‐Mejia et al., 2017; Myatt & Roberts, 2015).
In accordance with these findings, eNOS mRNA expression has also been shown to be decreased in preeclamptic pregnant women as compared to normotensive pregnant women, which is in accordance with other investigative groups (Kim et al., 2006; Schönfelder et al., 2004). However, some conflicting results showed that eNOS mRNA expression was greater in PE women than in normotensive pregnant women placental tissues, probably due to an adaptive or compensatory mechanism (Napolitano et al., 2000).
In comparison with the placental expression, controversial studies have been reported on systemic eNOS expression in PE patients. Significantly lower eNOS expression was observed in the preeclamptic women when compared with control group in accordance with the findings of Seligman et al., 1994 and Var et al., 2003. However, higher eNOS expression in the peripheral circulation of pregnancies complicated by PE than in normal pregnancies was observed, as a compensatory response to maintain blood flow in fetoplacental unit (Norris et al., 1999; Smárason et al., 1997) and unchanged expression of eNOS has also been reported in preeclamptic patients as compared to healthy pregnant women (Curtis et al., 1995; Davidge, Stranko, & Roberts, 1996).
5. CONCLUSION
In conclusion, our data suggested that oxidative stress and suppressed placental eNOS expression might constitute a characteristic finding in the preeclamptic placenta and support the hypothesis that complex mechanisms involving eNOS pathways and oxidative stress may promote microvascular oxidative damage and favor abnormal placenta perfusion, probably by contributing to the reduced placental blood flow and increased resistance to flow in the feto‐maternal circulation.
6. FUTURE PERSPECTIVE
Therapeutic options are currently limited, but understanding the factors involved in endothelial dysfunction could help design new approaches for prediction and management of preeclampsia. Umbilical‐placental vascular resistance has been improved and reduced in vivo by NO donating drugs (Schiessl et al., 2005). Therefore, for NO donating drugs and antioxidants therapy can be given for better management of PE which will reduce the maternal and neonatal morbidity and mortality in Pakistan.
DECLARATION OF INTEREST
The authors report no declarations of interest.
AUTHOR CONTRIBUTION
SJ lead the idea, conceived study and helped in preparing the draft. Samples were collected by GS. Antioxidant assays were done by QA. GS and AU performed immunohistochemistry and qRT‐PCR. GS, SR, and IA helped in writing the results. GS, IA, and SR wrote the paper with input from all other authors SJ, SR, and AA made substantial contribution in interpretation of data and revising the manuscript for intellectual content. All authors read and approved the final manuscript.
ACKNOWLEDGMENTS
We are grateful to Department of animal sciences, Quaid‐i‐Azam University, Islamabad, Pakistan and Higher Education Commission, Pakistan for funding and assisting us in designing the study and interpretation of results. Furthermore, we are grateful to the Deanship of Scientific Research at King Saud University for providing funding for this research through Research Group Project number 193.
Shaheen G, Jahan S, Ain QU, et al. Placental endothelial nitric oxide synthase expression and role of oxidative stress in susceptibility to preeclampsia in Pakistani women. Mol Genet Genomic Med. 2020;8:e1019 10.1002/mgg3.1019
REFERENCES
- Aggarwal, P. K. , Jain, V. , & Jha, V. (2010). Endothelial nitric oxide synthase, angiotensin‐converting enzyme and angiotensinogen gene polymorphisms in hypertensive disorders of pregnancy. Hypertension Research, 33, 473–477. 10.1038/hr.2010.23 [DOI] [PubMed] [Google Scholar]
- Aouache, R. , Biquard, L. , Vaiman, D. , & Miralles, F. (2018). Oxidative stress in preeclampsia and placental diseases. International Journal of Molecular Sciences, 19, 1496 10.3390/ijms19051496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardi, F. , Constantino, L. , Machado, R. , Petronilho, F. , & Dal‐Pizzol, F. (2008). Plasma nitric oxide, endothelin‐1, arginase and superoxide dismutase in pre‐eclamptic women. Journal of Obstetrics and Gynaecology Research, 34, 957–963. 10.1111/j.1447-0756.2008.00860.x [DOI] [PubMed] [Google Scholar]
- Bernardi, F. C. , Vuolo, F. , Petronilho, F. , Michels, M. , Ritter, C. , & Dal‐Pizzol, F. (2015). Plasma nitric oxide, endothelin‐1, arginase and superoxide dismutase in the plasma and placentae from preeclamptic patients. Anais Da Academia Brasileira De Ciências, 87, 713–719. 10.1590/0001-3765201520140069 [DOI] [PubMed] [Google Scholar]
- Biri, A. , Bozkurt, N. , Gunaydin, G. , Korucuoglu, U. , Durak, I. , & Kavutcu, M. (2007). Antioxidant enzyme activities and lipid peroxidation in preeclampsia. International Journal of Gynecology & Obstetrics, 96, 196–197. 10.1016/j.ijgo.2006.10.011 [DOI] [PubMed] [Google Scholar]
- Brennecke, S. , Gude, N. , Di Iulio, J. , & King, R. (1997). Reduction of placental nitric oxide synthase activity in pre‐eclampsia. Clinical Science, 93, 51–55. 10.1042/cs0930051 [DOI] [PubMed] [Google Scholar]
- Brosens, J. J. , Pijnenborg, R. , & Brosens, I. A. (2002). The myometrial junctional zone spiral arteries in normal and abnormal pregnancies: A review of the literature. American Journal of Obstetrics and Gynecology, 187, 1416–1423. 10.1067/mob.2002.127305 [DOI] [PubMed] [Google Scholar]
- Burton, G. J. , Sebire, N. J. , Myatt, L. , Tannetta, D. , Wang, Y. L. , Sadovsky, Y. , … Redman, C. W. (2014). Optimising sample collection for placental research. Placenta, 35(1), 9–22. [DOI] [PubMed] [Google Scholar]
- Chance, B. , & Maehly, A. (1955). [136] Assay of catalases and peroxidases. Methods in Enzymology, 2, 764–775. [DOI] [PubMed] [Google Scholar]
- Cohen, J. M. , Beddaoui, M. , Kramer, M. S. , Platt, R. W. , Basso, O. , & Kahn, S. R. (2015). Maternal antioxidant levels in pregnancy and risk of preeclampsia and small for gestational age birth: A systematic review and meta‐analysis. PLoS ONE, 10, e0135192 10.1371/journal.pone.0135192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis, N. E. , Gude, N. M. , King, R. G. , Marriott, P. J. , Rook, T. J. , & Brennecke, S. P. (1995). Nitric oxide metabolites in normal human pregnancy and preeclampsia. Hypertension in Pregnancy, 14, 339–349. 10.3109/10641959509015680 [DOI] [Google Scholar]
- Davidge, S. T. , Stranko, C. P. , & Roberts, J. M. (1996). Urine but not plasma nitric oxide metabolites are decreased in women with preeclampsia. American Journal of Obstetrics and Gynecology, 174, 1008–1013. 10.1016/S0002-9378(96)70341-1 [DOI] [PubMed] [Google Scholar]
- Dias, M. C. , Cavalli, R. , & Sandrim, V. (2018). 80. Trans‐resveratrol increases nitric oxide and heme‐oxigenase‐1 production and decreases ROS levels in endothelial cells incubated with plasma from preeclamptic patients. Pregnancy Hypertension, 13, S69 10.1016/j.preghy.2018.08.206 [DOI] [Google Scholar]
- Dikensoy, E. , Balat, O. , Pence, S. , Balat, A. , Cekmen, M. , & Yurekli, M. (2009). The changes of plasma malondialdehyde, nitric oxide, and adrenomedullin levels in patients with preeclampsia. Hypertension in Pregnancy, 28, 383–389. 10.3109/10641950802629634 [DOI] [PubMed] [Google Scholar]
- Eis, A. , Brockman, D. , Pollock, J. , & Myatt, L. (1995). Immunohistochemical localization of endothelial nitric oxide synthase in human villous and extravillous trophoblast populations and expression during syncytiotrophoblast formation in vitro. Placenta, 16, 113–126. 10.1016/0143-4004(95)90000-4 [DOI] [PubMed] [Google Scholar]
- Epstein, F. H. , Moncada, S. , & Higgs, A. (1993). The L‐arginine‐nitric oxide pathway. New England Journal of Medicine, 329, 2002–2012. 10.1056/NEJM199312303292706 [DOI] [PubMed] [Google Scholar]
- Farrow, K. N. , Lakshminrusimha, S. , Reda, W. J. , Wedgwood, S. , Czech, L. , Gugino, S. F. , … Steinhorn, R. H. (2008). Superoxide dismutase restores eNOS expression and function in resistance pulmonary arteries from neonatal lambs with persistent pulmonary hypertension. American Journal of Physiology‐Lung Cellular and Molecular Physiology, 295(6), L979–L987. 10.1152/ajplung.90238.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannoun, M. B. A. , Zitouni, H. , Raguema, N. , Maleh, W. , Gris, J.‐C. , Almawi, W. , & Mahjoub, T. (2015). Association of common eNOS/NOS3 polymorphisms with preeclampsia in Tunisian Arabs. Gene, 569, 303–307. 10.1016/j.gene.2015.05.072 [DOI] [PubMed] [Google Scholar]
- George, E. M. , & Granger, J. P. (2010). Recent insights into the pathophysiology of preeclampsia. Expert Review of Obstetrics & Gynecology, 5, 557–566. 10.1586/eog.10.45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert, J. S. , Ryan, M. J. , LaMarca, B. B. , Sedeek, M. , Murphy, S. R. , & Granger, J. P. (2008). Pathophysiology of hypertension during preeclampsia: Linking placental ischemia with endothelial dysfunction. American Journal of Physiology‐Heart and Circulatory Physiology, 294, H541–H550. 10.1152/ajpheart.01113.2007 [DOI] [PubMed] [Google Scholar]
- Gupta, S. , Agarwal, A. , & Sharma, R. K. (2005). The role of placental oxidative stress and lipid peroxidation in preeclampsia. Obstetrical & Gynecological Survey, 60, 807–816. 10.1097/01.ogx.0000193879.79268.59 [DOI] [PubMed] [Google Scholar]
- Gupta, S. , Aziz, N. , Sekhon, L. , Agarwal, R. , Mansour, G. , Li, J. , & Agarwal, A. (2009). Lipid peroxidation and antioxidant status in preeclampsia: A systematic review. Obstetrical & Gynecological Survey, 64, 750–759. 10.1097/OGX.0b013e3181bea0ac [DOI] [PubMed] [Google Scholar]
- Hayashi, I. , Morishita, Y. , Imai, K. , Nakamura, M. , Nakachi, K. , & Hayashi, T. (2007). High‐throughput spectrophotometric assay of reactive oxygen species in serum. Mutation Research/Genetic Toxicology and Environmental Mutagenesis, 631, 55–61. 10.1016/j.mrgentox.2007.04.006 [DOI] [PubMed] [Google Scholar]
- Kakkar, P. , Das, B. , & Viswanathan, P. (1984). A modified spectrophotometric assay of superoxide dismutase. Indian Journal of Biochemistry & Biophysics, 21, 130–132. [PubMed] [Google Scholar]
- Kalyanaraman, B. (2013). Teaching the basics of redox biology to medical and graduate students: Oxidants, antioxidants and disease mechanisms. Redox Biology, 1, 244–257. 10.1016/j.redox.2013.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, Y. , Park, H. , Lee, H. , Ha, E. , Suh, S. , Oh, S. , & Yoo, H.‐S. (2006). Reduced L‐arginine level and decreased placental eNOS activity in preeclampsia. Placenta, 27, 438–444. 10.1016/j.placenta.2005.04.011 [DOI] [PubMed] [Google Scholar]
- Lisonkova, S. , & Joseph, K. (2013). Incidence of preeclampsia: risk factors and outcomes associated with early‐ versus late‐onset disease. American Journal of Obstetrics and Gynecology, 209(6), 544.e1–544.e12. 10.1016/j.ajog.2013.08.019 [DOI] [PubMed] [Google Scholar]
- Mahadik, K. V. , & Ali Sina, S. (2003). Study of serum levels of superoxide dismutase in preeclampsia and eclampsia: Role of the test as a predictive tool. Journal of Obstetrics and Gynaecology Research, 29, 262–267. 10.1046/j.1341-8076.2003.00105.x [DOI] [PubMed] [Google Scholar]
- Manuel‐Apolinar, L. , López‐Romero, R. , Zarate, A. , Damasio, L. , Ruiz, M. , Castillo‐Hernández, C. , … Mera‐Jiménez, E. (2013). Leptin mediated ObRb receptor increases expression of adhesion intercellular molecules and cyclooxygenase 2 on murine aorta tissue inducing endothelial dysfunction. Int J Clin Exp Med, 6, 192–196. [PMC free article] [PubMed] [Google Scholar]
- Matsubara, K. , Higaki, T. , Matsubara, Y. , & Nawa, A. (2015). Nitric oxide and reactive oxygen species in the pathogenesis of preeclampsia. International Journal of Molecular Sciences, 16, 4600–4614. 10.3390/ijms16034600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara, S. , Takizawa, T. , Takayama, T. , Izumi, A. , Watanabe, T. , & Sato, I. (2001). Immuno‐electron microscopic localization of endothelial nitric oxide synthase in human placental terminal villous trophoblasts—normal and pre‐eclamptic pregnancy. Placenta, 22, 782–786. 10.1053/plac.2001.0718 [DOI] [PubMed] [Google Scholar]
- McMaster, K. , Vaka, V. , & LaMarca, B. (2018). 337: Mitochondrial dysfunction in preeclampsia demonstrated by increased reactive oxygen species production. American Journal of Obstetrics & Gynecology, 218, S209–S210. 10.1016/j.ajog.2017.10.273 [DOI] [Google Scholar]
- Moncada, S. , Palmer, R. , & Higgs, E. (1991). Nitric oxide: Physiology, pathophysiology, and pharmacology. Pharmacological Reviews, 43, 109–142. 10.1016/j.niox.2007.09.007 [DOI] [PubMed] [Google Scholar]
- Motta‐Mejia, C. , Kandzija, N. , Zhang, W. , Mhlomi, V. , Cerdeira, A. S. , Burdujan, A. , … Vatish, M. (2017). Placental vesicles carry active endothelial nitric oxide synthase and their activity is reduced in preeclampsia. Hypertension, 70(2), 372–381. 10.1161/HYPERTENSIONAHA.117.09321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutinati, M. , Piccinno, M. , Roncetti, M. , Campanile, D. , Rizzo, A. , & Sciorsci, R. (2013). Oxidative stress during pregnancy in the sheep. Reproduction in Domestic Animals, 48, 353–357. 10.1111/rda.12141 [DOI] [PubMed] [Google Scholar]
- Mutlu‐Türko, Ü. , Ademo, E. , Ibrahimo, L. , Aykac‐Toker, G. , & Uysal, M. (1998). Imbalance between lipid peroxidation and antioxidant status in preeclampsia. Gynecologic and Obstetric Investigation, 46, 37 10.1159/000009994 [DOI] [PubMed] [Google Scholar]
- Myatt, L. , & Roberts, J. M. (2015). Preeclampsia: syndrome or disease? Current Hypertension Reports, 17, 83 10.1007/s11906-015-0595-4 [DOI] [PubMed] [Google Scholar]
- Myatt, L. , & Webster, R. P. (2009). Vascular biology of preeclampsia. Journal of Thrombosis and Haemostasis, 7, 375–384. 10.1111/j.1538-7836.2008.03259.x [DOI] [PubMed] [Google Scholar]
- Napolitano, M. , Miceli, F. , Calce, A. , Vacca, A. , Gulino, A. , Apa, R. , & Lanzone, A. (2000). Expression and relationship between endothelin‐1 messenger ribonucleic acid (mRNA) and inducible/endothelial nitric oxide synthase mRNA isoforms from normal and preeclamptic placentas. The Journal of Clinical Endocrinology & Metabolism, 85, 2318–2323. 10.1210/jcem.85.6.6623 [DOI] [PubMed] [Google Scholar]
- Norris, L. A. , Higgins, J. R. , Darling, M. R. , Walshe, J. J. , & Bonnar, J. (1999). Nitric oxide in the uteroplacental, fetoplacental, and peripheral circulations in preeclampsia. Obstetrics & Gynecology, 93, 958–963. [DOI] [PubMed] [Google Scholar]
- Orange, S. , Painter, D. , Horvath, J. , Yu, B. , Trent, R. , & Hennessy, A. (2003). Placental endothelial nitric oxide synthase localization and expression in normal human pregnancy and pre‐eclampsia. Clinical and Experimental Pharmacology and Physiology, 30, 376–381. 10.1046/j.1440-1681.2003.03844.x [DOI] [PubMed] [Google Scholar]
- Palmer, R. , Ashton, D. , & Moncada, S. (1988). Vascular endothelial cells synthesize nitric oxide from L‐arginine. Nature, 333, 664–666. 10.1038/333664a0 [DOI] [PubMed] [Google Scholar]
- Pennington, K. A. , Schlitt, J. M. , Jackson, D. L. , Schulz, L. C. , & Schust, D. J. (2012). Preeclampsia: Multiple approaches for a multifactorial disease. Disease Models and Mechanisms, 5, 9–18. 10.1242/dmm.008516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl, M. W. (2001). A new mathematical model for relative quantification in real‐time RT–PCR. Nucleic Acids Research, 29, e45 10.1093/nar/29.9.e45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian, J. , & Fulton, D. (2013). Post‐translational regulation of endothelial nitric oxide synthase in vascular endothelium. Frontiers in Physiology, 4, 347 10.3389/fphys.2013.00347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghupathy, R. (2013). Cytokines as key players in the pathophysiology of preeclampsia. Medical Principles and Practice, 22, 8–19. 10.1159/000354200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Development Core Team . (2018). R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- Rahimi, Z. , Aghaei, A. , Rahimi, Z. , & Vaisi‐Raygani, A. (2013). Endothelial nitric oxide synthase (eNOS) 4a/b and G894T polymorphisms and susceptibility to preeclampsia. Journal of Reproduction & Infertility, 14, 184–189. [PMC free article] [PubMed] [Google Scholar]
- Saxena, S. , Thimmaraju, K. , Srivastava, P. C. , Mallick, A. K. , Das, B. , Sinha, N. , & Dalmia, K. (2015). Role of dyslipidaemia and lipid peroxidation in pregnancy induced hypertension. J Clin Sci Res, 4, 205–212. 10.15380/2277-5706.JCSR.14.059 [DOI] [Google Scholar]
- Schiessl, B. , Mylonas, I. , Hantschmann, P. , Kuhn, C. , Schulze, S. , Kunze, S. , … Jeschke, U. (2005). Expression of endothelial NO synthase, inducible NO synthase, and estrogen receptors alpha and beta in placental tissue of normal, preeclamptic, and intrauterine growth‐restricted pregnancies. Journal of Histochemistry & Cytochemistry, 53, 1441–1449. 10.1369/jhc.4A6480.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schönfelder, G. , Fuhr, N. , Hadzidiakos, D. , John, M. , Hopp, H. , & Paul, M. (2004). Preeclampsia is associated with loss of neuronal nitric oxide synthase expression in vascular smooth muscle cells of the human umbilical cord. Histopathology, 44, 116–128. 10.1111/j.1365-2559.2004.01806.x [DOI] [PubMed] [Google Scholar]
- Seligman, S. P. , Buyon, J. P. , Clancy, R. M. , Young, B. K. , & Abramson, S. B. (1994). The role of nitric oxide in the pathogenesis of preeclampsia. American Journal of Obstetrics and Gynecology, 171, 944–948. 10.1016/S0002-9378(94)70064-8 [DOI] [PubMed] [Google Scholar]
- Shetty, M. S. , Ramesh, A. , Shetty, P. K. , & Agumbe, P. (2018). Salivary and serum antioxidants in women with preeclampsia with or without periodontal disease. The Journal of Obstetrics and Gynecology of India, 68, 33–38. 10.1007/s13224-017-0993-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh, A. , Sharma, D. , Raghunandan, C. , & Bhattacharjee, J. (2010). Role of inflammatory cytokines and eNOS gene polymorphism in pathophysiology of pre‐eclampsia. American Journal of Reproductive Immunology, 63, 244–251. [DOI] [PubMed] [Google Scholar]
- Sinzato, Y. K. , Bevilacqua, E. M. , Volpato, G. T. , Hernandez‐Pando, R. E. , Rudge, M. V. , & Damasceno, D. C. (2018). Maternal oxidative stress, placental morphometry, and fetal growth in diabetic rats exposed to cigarette smoke. Reproductive Sciences, 26(9):1287‐1293. 10.1177/1933719118815589 [DOI] [PubMed] [Google Scholar]
- Smárason, A. Jr , Allman, K. G. , Young, D. , & Redman, C. W. (1997). Elevated levels of serum nitrate, a stable end product of nitric oxide, in women with pre‐eclampsia. BJOG: An International Journal of Obstetrics & Gynaecology, 104, 538–543. 10.1111/j.1471-0528.1997.tb11528.x [DOI] [PubMed] [Google Scholar]
- Steegers, E. A. , von Dadelszen, P. , Duvekot, J. J. , & Pijnenborg, R. (2010). Pre‐eclampsia. The Lancet, 376, 631–644. 10.1016/S0140-6736(10)60279-6 [DOI] [PubMed] [Google Scholar]
- Touyz, R. M. (2004). Reactive oxygen species, vascular oxidative stress, and redox signaling in hypertension: What is the clinical significance? Hypertension, 44, 248–252. 10.1161/01.HYP.0000138070.47616.9d [DOI] [PubMed] [Google Scholar]
- Var, A. , Yildirim, Y. , Onur, E. , Kuscu, N. K. , Uyanik, B. S. , Goktalay, K. , & Guvenc, Y. (2003). Endothelial dysfunction in preeclampsia. Gynecologic and Obstetric Investigation, 56, 221–224. 10.1159/000074824 [DOI] [PubMed] [Google Scholar]
- Wang, A. , Rana, S. , & Karumanchi, S. A. (2009). Preeclampsia: The role of angiogenic factors in its pathogenesis. Physiology, 24, 147–158. 10.1152/physiol.00043.2008 [DOI] [PubMed] [Google Scholar]
- Wang, Y. , & Walsh, S. W. (1996). Antioxidant activities and mRNA expression of superoxide dismutase, catalase, and glutathione peroxidase in normal and preeclamptic placentas. Journal of the Society for Gynecologic Investigation, 3, 179–184. 10.1177/107155769600300404 [DOI] [PubMed] [Google Scholar]
- Wright, J. , Colby, H. , & Miles, P. (1981). Cytosolic factors which affect microsomal lipid peroxidation in lung and liver. Archives of Biochemistry and Biophysics, 206, 296–304. 10.1016/0003-9861(81)90095-3 [DOI] [PubMed] [Google Scholar]
- Yang, X. , Guo, L. , Li, H. , Chen, X. , & Tong, X. (2012). Analysis of the original causes of placental oxidative stress in normal pregnancy and pre‐eclampsia: A hypothesis. The Journal of Maternal‐Fetal & Neonatal Medicine, 25, 884–888. 10.3109/14767058.2011.601367 [DOI] [PubMed] [Google Scholar]


