Abstract
Background
A high prevalence of sleep disordered breathing (SDB) has been reported in persons with Marfan syndrome (MFS), a single gene disorder of connective tissue resulting in premature death from aortic rupture. The burden of SDB and accompanying hemodynamic stress could warrant broad screening in this population. Our goal was to assess the utility of traditional SDB screening tools in our sample of persons with MFS.
Methods
Participants were recruited during an annual Marfan Foundation meeting and Marfan status confirmed using the Ghent criteria. Screening questionnaires were administered and SDB assessed by home sleep testing. We assessed accuracy of screening tools using receiver‐operating characteristic curve analyses.
Results
The prevalence of moderate‐severe SDB was 32% in our sample of 31 MFS participants. The Stop‐Bang questionnaire had the highest positive predictive value (PPV) of 60% and the highest negative predictive value (NPV) of 100% using the high‐ and moderate‐risk cut‐offs, respectively, and the Berlin questionnaire had a PPV of 50% and an NPV of 92.3% at the high‐risk cut‐off. When those with mild SDB were included, the Stop‐Bang and the Sleep Apnea Clinical Score (SACS) questionnaires demonstrated useful screening accuracies with PPVs of 94.7% and 92.9%, and NPVs of 63.6% and 47.1%, respectively, at the moderate‐risk cut‐offs.
Conclusion
A survey of SDB in a sample of persons with MFS reveals not only a high burden of SDB but also that conventional screening instruments have utility if adapted appropriately. Future studies should validate the utility of these screening tools given concerns that SDB may contribute to progression of aortic pathology in MFS.
Keywords: connective tissue disorder, screening accuracy, SDB, surveys
A high prevalence of sleep‐disordered breathing (SDB) has been reported in persons with Marfan syndrome (MFS), a single gene disorder of connective tissue resulting in premature death from aortic rupture. The burden of SDB and accompanying hemodynamic stress warrants for its screening in this population. In persons with MFS, conventional SDB screening questionnaires can effectively identify persons at risk for SDB.

1. INTRODUCTION
Marfan syndrome (MFS) is a systemic disorder of connective tissue caused by mutations in the FBN1 gene. Aortic root aneurysms complicated by subsequent dissection and rupture are major causes of morbidity and mortality (Judge & Dietz, 2005). Prolonged and cyclic hemodynamic stress on a weakened aortic wall is thought to facilitate aortic deterioration. The standard approaches to minimize this stress and prevent dissection include the use of anti‐hypertensives such as beta‐blockers and angiotensin receptor blockers (Teixido‐Tura et al., 2018) and, when necessary, surgical intervention to reinforce the structural integrity of the proximal aortic wall (Robicsek & Thubrikar, 1994).
Sleep disordered breathing (SDB), a known source of cardiovascular stress, is prevalent in MFS Kohler et al., 2013; Kohler et al., 2009). It is characterized by repetitive upper airway obstruction that presents both a mechanical and hypoxic load on the heart and aorta (Schneider et al., 1997; Serizawa et al., 2008). The first study evaluating SDB in a group of MFS persons was conducted by Cistulli et al in 1993. They showed a 64% prevalence of SDB in a small cohort of persons with MFS compared to matched controls (Cistulli & Sullivan, 1993). Later, other studies (Kohler et al., 2009; Rybczynski et al., 2010) reported prevalence rates of 32% and 31%, with one demonstrating increased odds of aortic adverse events in the presence of SDB. A case report raises the possibility that SDB treatment with CPAP may attenuate aortic dilatation (Cistulli, Wilcox, Jeremy, & Sullivan, 1997). Given the high prevalence of SDB and accompanying hemodynamic stress, the development of valid screening methods for SDB in the MFS population is needed.
Current screening tools for adults include the Berlin questionnaire, STOP‐Bang, and the Sleep Apnea Clinical Score (SACS) (Nagappa et al., 2015; Netzer, Stoohs, Netzer, Clark, & Strohl, 1999; Prasad et al., 2017; Verbraecken, Hedner, & Penzel, 2017). The performance of these tools differ depending on the prevalence of SDB and characteristics of the studied population as demonstrated in prior studies (Mulherin & Miller, 2002). We reasoned that the screening performance of these instruments in the MFS population might be different due to the unique anatomical and genetic features of the MFS population.
The primary purpose of this study was to examine the performance of traditional screening surveys in a convenience sample of persons with MFS.
2. METHODS
2.1. Study overview and participant population
Participants were recruited as part of a cross‐sectional study conducted at the annual Marfan Foundation meeting conducted in Baltimore, Maryland. The hosting faculty, human subjects intuitional review board and the Marfan Foundation allowed for the participants of the meeting to be informed of the study through scheduled on‐site provider contact and attendance at the convention. The research team set up a booth at the conference to inform interested persons. Eligible participants were adults over the age of 18 with self‐reported MFS which was later confirmed using the revised Ghent nosology (Loeys et al., 2010; Radonic et al., 2011). Demographic and anthropometric information were collected and several SDB screening questionnaires were completed. A home sleep apnea test (HST) kit (AccuSom™; Novasom, Inc) was given to subjects to self‐apply overnight while sleeping in their hotel rooms after training by research staff. The study was approved by Committee 5 of the Johns Hopkins Institutional Review Board (IRB Number: NA_00073250) on human research and all participants provided written informed consent.
2.2. Specific procedures
2.2.1. Anthropometry
Participants had the following measurements taken using standard techniques: height; weight; and waist, hip and neck circumferences (Barrios, Martin‐Biggers, Quick, & Byrd‐Bredbenner, 2016).
2.2.2. Home sleep test
All participants underwent a home sleep test (HST) using the AccuSom™ device (Claman, Murr, & Trotter, 2001). Participants were provided formal instruction on applying the device before use. The device measures nasal and oral airflow (using sound), heart rate, oxygen saturation, respiratory effort, and snoring sound intensity (Hunsaker & Riffenburgh, 2006). The system also uses a voice alert to prompt the patient to readjust a dislodged sensor. Participants used the device for one night and returned it for data download, scoring, and study interpretation. The device does not differentiate sleep and wake, so the respiratory event index (REI) measurement was based on total night recording time (Reichert, Bloch, Cundiff, & Votteri, 2003). To maintain privacy, a pseudonym based on a study identification was assigned to each participant.
Scoring of the HST was performed based on the system's automated scoring algorithm that identifies hypopneas as a reduction in airflow ≥30% with an oxygen desaturation of 3% or more and an apnea as a complete absence of airflow with or without desaturation, both respiratory events lasting ≥10 s. The automated scoring was reviewed by two investigators (MS, SP) using the American Academy of Sleep Medicine (AASM) manual updated in 2016 (AASM Manual for the Scoring of Sleep & Associated Events, 2016), to improve overall accuracy. SDB was defined as an apnea‐hypopnea‐index (AHI) of ≥15 events per hr (moderate‐severe SDB).
2.2.3. Questionnaires
Three traditional screening questionnaires; the Berlin questionnaire, the STOP‐Bang, and the SACS were administered. Survey scoring criteria were used to stratify participants into groups with moderate‐risk and high‐risk for SDB (see Supporting Information). Snoring was determined by yes or no responses on the Berlin questionnaire.
2.3. Statistical analysis
The Mann‐Whitney's test was used to compare anthropometric, demographic, sleep study, and survey characteristics for those below and above an AHI of 15 events/hr. Data are presented as means ± SD or frequencies where appropriate. Screening accuracy was examined by calculating the area under the curve (AUC) for the receiver‐operating characteristic (ROC). The sensitivities, specificities, positive predictive values (PPV), and negative predictive values (NPV) were also calculated.
In post hoc analyses, we repeated the screening assessment with an SDB threshold of AHI ≥5 events/hr (mild‐severe SDB). All statistical analyses were performed using XLSTAT (XLSTAT, Addinsoft, 2017). Two‐tailed p values of less than .05 were considered to indicate statistical significance.
3. RESULTS
3.1. Participant characteristics
A total of 50 participants who completed the sleep surveys underwent the home sleep studies. At the time of data analyses, participants were contacted to validate MFS status using the revised Ghent criteria (Loeys et al., 2010; Penpattharakul & Pithukpakorn, 2016; Radonic et al., 2011) and 31 participants met the criteria. The prevalence of moderate‐severe SDB among the 31 participants was 32%.
Anthropometric, demographic, and sleep study characteristics are shown in Table 1 for those with and without moderate‐severe SDB. No significant differences were noted in anthropometry and demographics except for greater neck size in those with moderate‐severe SDB. As expected, AHI was significantly greater in persons with moderate‐severe SDB but the prevalence of snoring and sleepiness did not differ between groups. The proportion of persons classified with the Stop‐Bang as having a moderate‐risk for SDB was greater in those with moderate‐severe SDB. Similarly, the group with moderate‐severe SDB had a greater proportion of persons classified with the Berlin questionnaire as having a high‐risk for SDB.
Table 1.
Participant demographic, anthropometric, and SDB characteristics by presence of moderate‐severe SDB (AHI ≥15 events/hr)
| AHI <15/hr | AHI ≥15/hr | p value | |
|---|---|---|---|
| n | 21 | 10 | — |
| Women (%) | 15 (71) | 4 (40) | .190 |
| Age, years | 53.2 ± 14.6 | 56.2 ± 15.9 | .459 |
| Height, cm | 179.4 ± 12.2 | 188.5 ± 8.3 | .024 |
| Weight, kg | 88.8 ± 21.5 | 91.8 ± 29.2 | .553 |
| BMI, kg/m2 | 27.5 ± 5.7 | 25.6 ± 6.9 | .291 |
| Neck circumference, cm | 36.4 ± 3.4 | 39.6 ± 4.5 | .028 |
| Waist circumference, cm | 99.5 ± 15.2 | 104.9 ± 19.0 | .519 |
| Hip circumference, cm | 113.7 ± 18.1 | 111.8 ± 16.2 | .619 |
| AHI, (h−1) | 6.0 ± 4.1 | 32.4 ± 22.9 | <.0001 |
| ODI 3%, (h−1) | 2.3 ± 2.1 | 17.9 ± 18.2 | <.0001 |
| Snoring, n (%) | 12 (57) | 8 (80) | .352 |
| Sleepy, ESS ≥11 n (%) | 3 (14) | 3 (30) | .611 |
| STOP‐Bang, mod‐risk n (%) | 9 (45) | 10 (100) | <.0001 |
| SACS, mod‐risk n %) | 7 (33) | 7 (70) | .099 |
| STOP‐Bang, high‐risk n (%) | 4 (19) | 6 (60) | .058 |
| SACS, high‐risk n (%) | 0 (0) | 2 (10) | .318 |
| Berlin questionnaire, high‐risk n (%) | 9 (43) | 9 (90) | .006 |
Abbreviations: AHI, apnea hypopnea index; ESS, epworth sleepiness scale; ODI, oxygen desaturation index; SACS, sleep apnea clinical score; SDB, sleep disordered breathing.
3.2. Screening accuracy of questionnaires
3.2.1. Moderate‐severe SDB (AHI ≥15 events/hr)
At the high‐risk cut‐offs, the sensitivities for the STOP‐Bang, SACS and Berlin questionnaires were 60.0%, 20.0% and 90.0%, respectively, and the specificities were 80.0%, 100.0% and 57.1%, respectively (Table 2, upper panel). The AUCs were 70.0% (p = .033), 60.0% (p = .143), and 73.0% (p = .003) for the STOP‐Bang, SACS, and Berlin questionnaires, respectively (Figure 1a). At the moderate‐risk cut‐offs, the sensitivities for the STOP‐Bang and SACS questionnaires were 100.0% and 70.0%, respectively, and the specificities were 57.1% and 66.7%, respectively (Table 2, lower panel). The AUCs were 77.5% (p < .0001) and 67.5% (p = .063) for the Stop‐Bang and SACS questionnaires, respectively (Figure 1b).
Table 2.
Measures of survey screening accuracy are shown at an SDB threshold of AHI ≥15 events/hr
| Stop‐Bang | SACS | Berlin | |
|---|---|---|---|
| High‐risk cut‐off | Score ≥5 | Score ≥15 | a |
| Sensitivity, % | 60.0 (31.2–83.1) | 20.0 (4.9–52.2) | 90.0 (57.1–100.0) |
| Specificity, % | 80.0 (57.7–92.3) | 100.0 (81.4–100.0) | 57.1 (36.5–75.5) |
| PPV, % | 60.0 (29.6–90.4) | 100.0 (100.0–100.0) | 50.0 (26.9–73.1) |
| NPV, % | 80.0 (62.5–97.5) | 72.4 (56.1–88.7) | 92.3 (77.8–100.0) |
| AUC, % | 70.0 (51.6–88.4) | 60.0 (46.9–73.1) | 72.5 (57.6–87.4) |
| p value | .033 | .134 | .003 |
| Moderate‐risk cut‐off | Score ≥3 | Score ≥8 | a |
| Sensitivity, % | 100.0 (67.4–100.0) | 70.0 (39.2–89.4) | |
| Specificity, % | 57.1 (36.5–75.5) | 66.7 (45.2–82.8) | |
| PPV, % | 52.6 (30.2–75.1) | 50.0 (23.8–76.2) | |
| NPV, % | 100.0 (100.0–100.0) | 82.4 (64.2–100.0) | |
| AUC, % | 77.5 (66.3–88.7) | 67.5 (49.1–85.9) | |
| p value | <.0001 | .063 |
Data are presented as means and 95% confidence intervals. A p value <.05 was considered to indicate statistical significance.
Abbreviations: AHI, apnea hypopnea index; AUC, area under the receiver operating characteristic curve; NPV, negative predictive value; PPV, positive predictive value; SACS, sleep apnea clinical score; SDB, sleep disordered breathing.
The Berlin questionnaire only has a high‐ and low‐risk category because of its score structure unlike the Stop‐Bang and SACS that have high‐, intermediate‐, and low‐risk categories (see Supporting Information).
Figure 1.
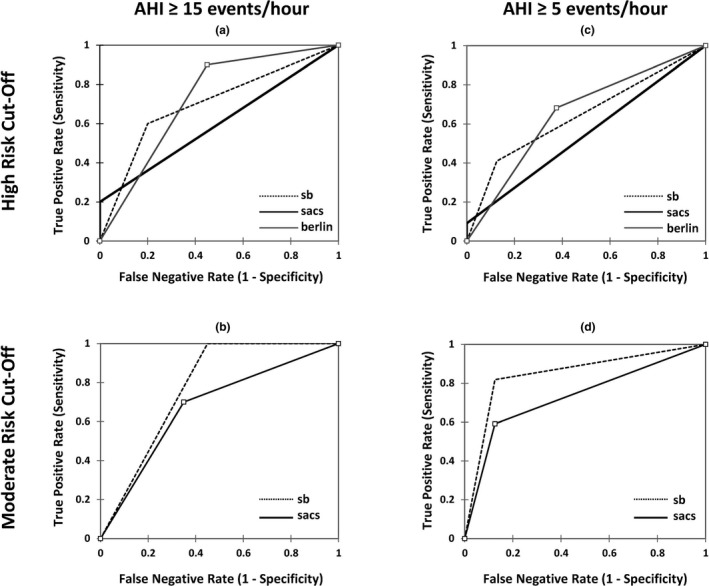
Receiver‐operating characteristic (ROC) curves for the screening questionnaires. Performance of screening surveys at an apnea hypopnea index (AHI) ≥15 events/hr (a and b) and AHI ≥5 events/hr (c and d) using the high‐ and moderate‐risk screening cut‐offs. (b and d) Only show ROC curves for the Stop‐Bang (sb) and the Sleep Apnea Clinical Score (sacs) because the Berlin questionnaire (berlin) does not have a moderate‐risk criteria (see Supporting Information)
3.2.2. Mild‐severe SDB (AHI ≥5 events/hr)
At the high‐risk cut‐offs, the sensitivities for the STOP‐Bang, SACS and Berlin questionnaires were 40.9%, 9.1%, and 68.2%, respectively, and the specificities were 87.5%, 100.0%, and 66.7%, respectively (Table 3, upper panel). The AUCs were 64.2% (p = .085), 54.5% (p = .147), and 65.3% (p = .143) for the Stop‐Bang, SACS, and Berlin questionnaires, respectively (Figure 1c). At the moderate‐risk cut‐offs, the sensitivities for the STOP‐Bang and SACS were 81.8% and 59.1%, respectively, and the specificities were 87.5% and 88.9%, respectively (Table 3, lower panel). The AUCs were 84.7% (p < .0001) and 73.3% (p = .005) for the STOP‐Bang and SACS questionnaires, respectively (Figure 1d).
Table 3.
Measures of survey screening accuracy are shown at an SDB threshold of AHI ≥5 events/hr
| Stop‐Bang | SACS | Berlin | |
|---|---|---|---|
| High‐risk cut‐off | Score ≥5 | Score ≥15 | a |
| Sensitivity, % | 40.9 (23.3–61.3) | 9.1 (1.5–29.3) | 68.2 (47.1–83.7) |
| Specificity, % | 87.5 (50.5–99.5) | 100.0 (65.0–100.0) | 66.7 (35.1–88.0) |
| PPV, % | 90.0 (71.4–100.0) | 100.0 (100.0–100.0) | 83.3 (66.1–100.0) |
| NPV, % | 35.0 (14.1–55.9) | 31.0 (14.2–47.9) | 46.2 (19.1–73.3) |
| AUC, % | 64.2 (48.1–80.3) | 54.5 (48.4–60.7) | 65.3 (44.8–85.9) |
| p value | .085 | .147 | .143 |
| Moderate‐risk cut‐off | Score ≥3 | Score ≥8 | a |
| Sensitivity, % | 81.8 (60.7–93.1) | 59.1 (38.7–76.7) | |
| Specificity, % | 87.5 (50.5–99.5) | 88.9 (54.0–99.8) | |
| PPV, % | 94.7(84.7–100.0) | 92.9 (79.4–100.0) | |
| NPV, % | 63.6 (35.2–92.1) | 47.1 (23.3–70.8) | |
| AUC, % | 84.7 (69.9–99.4) | 73.3 (57.2–89.4) | |
| p value | <.0001 | .005 |
Data are presented as means and 95% confidence intervals. A p value < .05 was considered to indicate statistical significance.
Abbreviations: AHI, apnea hypopnea index; AUC, area under the receiver operating characteristic curve; NPV, negative predictive value; PPV, positive predictive value; SACS, sleep apnea clinical score; SDB, sleep disordered breathing.
The Berlin questionnaire only has a high‐ and low‐risk category because of its score structure unlike the Stop‐Bang and SACS that have high‐, intermediate‐, and low‐risk categories (see Supporting Information).
4. DISCUSSION
The primary purpose of the study was to evaluate the utility of traditional screening tools in a sample of persons with MFS, a patient group with heightened susceptibility to SDB and deleterious cardiovascular outcomes. Our convenience sample of MFS volunteers demonstrated a high prevalence of SDB, confirming the findings from previous studies. We report three findings. First, the Stop‐Bang was the most effective screening tool for moderate‐severe (AHI ≥15 events/hr) and mild‐severe (AHI ≥5 events/hr) SDB. Second, the Berlin questionnaire showed a high sensitivity of 90% and modest specificity of 57.1% for moderate‐severe SDB, at the high‐risk cut‐off. Third, the SACS questionnaire showed a high specificity of 88.9% and modest sensitivity of 59.1% for mild‐severe SDB, at the moderate‐risk cut‐off. In the MFS population therefore, case‐by‐case identification of those at risk for SDB is achievable with the tailored use of traditional screening questionnaires.
4.1. Conceptual approach
The purpose of any SDB screening questionnaire is to recognize persons with a high likelihood of having SDB in a subsequent HST or PSG. An additional rationale is to identify persons with SDB in the context of their underlying disease. For single gene disorders like MFS, we do not know the clinically relevant cut‐off for SDB nor do we know if the standard risk cut‐offs on the screening questionnaires apply similarly. If undetected SDB presents an extra‐ordinary high risk for morbidity and mortality then the screening approach would need to be adapted to improve overall accuracy. In MFS therefore, unique susceptibility to SDB and vulnerability to cardiovascular adverse events warrants the consideration of this approach.
4.2. Recruitment strategy
Recruitment for specialized diagnostic studies requiring real‐time surveillance in rare genetic disorders like MFS presents major challenges due to cost associated with travel to study location, validation of disease phenotypes, and verification of genotype outside of centers of excellence. A unique strength of this study design was conducting the study during the annual national meeting of the Marfan Foundation where there is a strong sense of commitment by physicians and persons with MFS to new concepts and treatments for the disorder. Since the overnight sleep study measurements were performed in a hotel, rather than at home, the 3 days conference period allowed study coordinators to confirm data collection and assess quality. Anthropometric, questionnaire, and other clinical evaluation were also done over the same 3 days period.
4.3. SDB prevalence in MFS
In our convenience sample, the prevalence of moderate‐severe SDB was 32%. Cistulli et al, showed a prevalence of 64% for mild‐severe SDB (Cistulli & Sullivan, 1993), whereas studies by Kohler et al and Rybczynski et al reported SDB prevalences of 18% and 10%, respectively, for moderate‐severe SDB (Kohler et al., 2009; Rybczynski et al., 2010). The prevalence seen in this study may be related to selection bias and not generalizable to the MFS population. Nonetheless, this finding supports the evolving consensus of increased susceptibility to SDB in MFS. Factors such as craniofacial dysmorphism and abnormalities in the pharyngeal connective tissue are thought to be the main contributors (Cistulli & Sullivan, 1995; Isono, 2012) to SDB susceptibility. Fat distribution may also play a role, as visceral adiposity is a known indicator of SDB severity (Schwartz et al., 2010). In our sample, neck size was associated with the presence of SDB (Table 1) but BMI was not. Neck size might thus be a better early indicator for visceral adiposity in MFS as suggested in some prior studies of the general population (Tang & Friedman, 2018).
4.4. Performance of screening questionnaires
In our sample of MFS persons, all three questionnaires demonstrated varying sensitivities and specificities depending on the screening cut‐offs and the definition of SDB. Five of the ten screening assessments demonstrated overall accuracies that were significantly better than chance (see p values, Tables 2 and 3). For moderate‐severe SDB (AHI ≥15 events/hr), the Stop‐Bang had the highest PPV of 60% and the highest NPV of 100% using the moderate‐ and high‐risk cut‐offs, respectively. It is notable that the Berlin questionnaire had a PPV of 50% and an NPV of 92.3% for moderate‐severe SDB at the high‐risk cut‐off. When those with mild SDB (AHI ≥5 events/hr) were included, the Stop‐Bang had the highest PPV of 94.7% and the highest NPV of 63.6% at the moderate‐risk cut‐offs. The SACS questionnaire also demonstrated useful screening accuracy with a PPV of 92.9% and an NPV of 47.1% for mild‐severe SDB (AHI ≥5 events/hr) at the moderate‐risk cut‐offs. Of note, using the mild‐severe SDB threshold at high‐risk cut‐offs, none of the questionnaires effectively identified SDB (see Table 3, upper panel), which implies that the high‐risk cut‐offs may be un‐suitable to capture those with mild SDB in this population.
In the general population, the above studied questionnaires have been shown to improve the diagnostic yield (Gamaldo et al., 2018; Ramachandran & Josephs, 2009) in those who exceed the standard thresholds. Our results indicate that the high‐risk cut‐off on the Stop‐Bang is best to rule‐in or improve the diagnostic yield for moderate‐severe SDB in MFS persons. On the other hand, the moderate‐risk cut‐off on the Stop‐Bang is ideal to rule‐out or exclude mild‐severe SDB. Altogether, these findings suggest that traditional questionnaires are useful for SDB screening in persons with MFS if applied correctly. Furthermore, the implications of misclassification should be considered when using these screening tools, as potential consequences of untreated SDB may outweigh concerns about over testing in the MFS population.
4.5. SDB threshold in MFS
We chose an AHI of ≥15 events/hr as the definition for SDB because moderate‐severe SDB presents a potential, significant marker for adverse cardiovascular events (Nieto et al., 2002; Seif et al., 2013). In contrast, mild SDB (AHI ≥5 events/hr and <15 events/hr) is not considered to manifest a significant hypoxic load. Nonetheless, mild SDB may yet pose health risks to MFS persons from surges in sympathetic activity due to recurrent arousals and mechanical stress from widening pleural pressure swings (Bauters et al., 2019; Guilleminault, Stoohs, Shiomi, Kushida, & Schnittger, 1996; Lugaresi, Cirignotta, Coccagna, & Piana, 1980; Stoohs & Guilleminault, 1991). At this point however, the nocturnal hemodynamic stresses just mentioned are yet to be demonstrated in the MFS population. Future studies are thus needed to determine if mild SDB increases the risk of adverse health events in this population. In addition, a critical look at the contribution of each of the survey components is required to identify (a) the factors most predictive of SDB and (b) if comorbid conditions such as fatigue suggested to be prevalent in MFS persons (Bathen, Velvin, Rand‐Hendriksen, & Robinson, 2014) affect the composite scores on these surveys. Ultimately, in‐laboratory or home sleep testing of all persons with MFS may be the most prudent screening strategy given the potential risk SDB poses to their cardiovascular health.
4.6. Limitations
This study has several limitations. First, underlying concerns for sleep disorders may have driven participation in the study and resulted in the increased prevalence of SDB reported. Regardless, the contemporaneous testing of SDB and completion of questionnaires provided the critical data to test the screening tools. Second, the study sample was small and did not allow for robust multivariable analyses. Future studies with a larger cohort of unselected participants are needed to suitably characterize the prevalence of SDB in persons with MFS and assess interactions between known risk factors. Third, SDB was assessed by HST, which was not reliable for distinguishing obstructive from central events. Because cardiovascular complications are common in MFS, the correct classification of the SDB phenotype is essential to establishing the potential connection with adverse cardiovascular events. Furthermore, the inability of our HST devices to assess for hypoventilation may have underestimated SDB.
4.7. Implications
Conventional screening questionnaires for SDB may require customization for populations that present unique risk factors for this impairment. Although other heritable disorders such as Ehlers‐Danlos Syndrome and Turner Syndrome show high prevalence of sleep apnea reflecting known structural predispositions, no assessment of the utility of current questionnaires in these disorders has been pursued (Gaisl et al., 2017; Guilleminault et al., 2013; Orliaguet et al., 2001). We submit that our approach to modifying the questionnaire thresholds for disease ascertainment in MFS be explored in other such genetically defined groups. The ideal solution may be the development of altogether new questionnaires weighted for specific disease features that confer risk for sleep apnea and related morbidity.
Persons with MFS are at risk for aortic complications and SDB of even mild severity may be a risk factor for aortic disease (Kohler et al., 2009, 2013). These screening instruments help us identify the persons at risk for hypoxic and mechanical stress on the cardiovascular system from SDB. Further studies will determine whether identifying and relieving both the mechanical and hypoxic load will translate into a reduction in cardiovascular morbidity (Scharf, Brown, Saunders, & Green, 1979; Stoohs & Guilleminault, 1992). Finally, our recruitment strategy and method for data collection could serve as a means to decrease prohibitive time, costs, or retention issues in human subjects' research.
5. CONCLUSION
In persons with MFS, conventional screening questionnaires can effectively identify persons at risk for SDB. We provisionally recommend the use of the Stop‐Bang questionnaire for SDB screening at the risk cut‐offs discussed above. Validation in larger MFS cohorts including in‐laboratory sleep studies for detailed phenotyping is still needed. Ideally, the validation studies should be coupled with contemporaneous assessment for aortic disease progression. Finally, we offer that for rare single gene disorders, large foundation‐based conferences which include a clinical component can support pilot data acquisition that may not only avail new management approaches but also serve as a critical foundation for larger studies.
CONFLICT OF INTEREST
The authors certify that they have NO affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers' bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent‐licensing arrangements), or nonfinancial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
AUTHOR CONTRIBUTION
Mudiaga O. Sowho: Data collection, data analyses, and manuscript writing. Susheel Patil: Conceived and designed project, data collection, data analyses, and manuscript writing. Hartmut Schneider: Data analyses and manuscript writing. Gretchen MacCarrick: Project design and data collection. Jason P. Kirkness: Conceived and designed project, data collection, and manuscript writing. Lisa F. Wolfe: Project design and manuscript writing. Laura Sterni: Data collection and manuscript writing. Peter A. Cistulli: Manuscript writing. Enid R. Neptune: Conceived and designed project, data collection, data analyses, and manuscript writing. Enid Neptune takes responsibility for the content of the manuscript. We also thank Dr. Harry Dietz for his contributions to the manuscript.
Supporting information
ACKNOWLEDGMENT
We acknowledge the staff of the Johns Hopkins Marfan Clinic for their assistance in this study and Novasom Inc. for providing the home sleep testing devices used for this project.
Sowho MO, Patil S, Schneider H, et al. Sleep disordered breathing in Marfan syndrome: Value of standard screening questionnaires. Mol Genet Genomic Med. 2020;8:e1039 10.1002/mgg3.1039
Prior presentation: Submitted as an abstract to the ATS conference 2018.
Funding information
The study was supported by the Marfan Foundation, the Johns Hopkins Marfan Sleep Health Initiative, and the National Institute of Health (NIH) T32 Sleep and Genomics program (HL 110952‐6).
REFERENCES
- AASM Manual for the Scoring of Sleep and Associated Events ; Version 2.3. (2016). https://j2vjt3dnbra3ps7ll1clb4q2-wpengine.netdna-ssl.com/wp-content/uploads/2017/11/Summary-of-Updates-in-v2.3-FINAL.pdf.
- Barrios, P. , Martin‐Biggers, J. , Quick, V. , & Byrd‐Bredbenner, C. (2016). Reliability and criterion validity of self‐ measured waist, hip, and neck circumferences. BMC Medical Research Methodology, 16, 49 10.1186/s12874-016-0150-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bathen, T. , Velvin, G. , Rand‐Hendriksen, S. , & Robinson, H. S. (2014). Fatigue in adults with Marfan syndrome, occurrence and associations to pain and other factors. American Journal of Medical Genetics Part A, 164(8), 1931–1939. 10.1002/ajmg.a.36574 [DOI] [PubMed] [Google Scholar]
- Bauters, F. A. , Hertegonne, K. B. , De Buyzere, M. L. , Joos, G. F. , Chirinos, J. A. , & Rietzschel, E. R. (2019). Phenotype and risk burden of sleep apnea. Hypertension, 74(4), 1052–1062. 10.1161/HYPERTENSIONAHA [DOI] [PubMed] [Google Scholar]
- Cistulli, P. A. , & Sullivan, C. E. (1993). Sleep‐disordered breathing in Marfan's syndrome. American Review of Respiratory Disease, 147(3), 645–648. 10.1164/ajrccm/147.3.645 [DOI] [PubMed] [Google Scholar]
- Cistulli, P. A. , & Sullivan, C. E. (1995). Sleep apnea in Marfan's syndrome. Increased upper airway collapsibility during sleep. Chest, 108(3), 631–635. [DOI] [PubMed] [Google Scholar]
- Cistulli, P. A. , Wilcox, I. , Jeremy, R. , & Sullivan, C. E. (1997). Aortic dilatation in Marfan's syndrome: A contribution from obstructive sleep apnea? Chest, 111, 1763–1766. 10.1378/chest.111.6.1763 [DOI] [PubMed] [Google Scholar]
- Claman, D. , Murr, A. , & Trotter, K. (2001). Clinical validation of the Bedbugg in detection of obstructive sleep apnea. Otolaryngology ‐ Head and Neck Surgery, 125(3), 227–230. 10.1067/mhn.2001.118126 [DOI] [PubMed] [Google Scholar]
- Gaisl, T. , Giunta, C. , Bratton, D. J. , Sutherland, K. , Schlatzer, C. , Sievi, N. , … Kohler, M. (2017). Obstructive sleep apnea and quality of life in Ehlers‐Danlos syndrome: A parallel cohort study. Thorax, 72(8), 729–735. 10.1136/thoraxjnl-2016-209560 [DOI] [PubMed] [Google Scholar]
- Gamaldo, C. , Buenaver, L. , Chernyshev, O. , Derose, S. , Mehra, R. , Vana, K. , … Gurubhagavatula, I. (2018). Evaluation of clinical tools to screen and assess for obstructive sleep apnea. OSA assessment tools task force of the American Academy of sleep medicine. Journal of Clinical Sleep Medicine, 14(7), 1239–1244. 10.5664/jcsm.7232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilleminault, C. , Primeau, M. , Chiu, H. Y. , Yuen, K. M. , Leger, D. , & Metlaine, A. (2013). Sleep‐disordered breathing in Ehlers‐Danlos syndrome: A genetic model of OSA. Chest, 144(5), 1503–1511. 10.1378/chest.13-0174 [DOI] [PubMed] [Google Scholar]
- Guilleminault, C. , Stoohs, R. , Shiomi, T. , Kushida, C. , & Schnittger, I. (1996). Upper airway resistance syndrome, nocturnal blood pressure monitoring, and borderline hypertension. Chest, 109(4), 901–908. 10.1378/chest.109.4.901 [DOI] [PubMed] [Google Scholar]
- Hunsaker, D. H. , & Riffenburgh, R. H. (2006). Snoring significance in patients undergoing home sleep studies. Otolaryngology–head and Neck Surgery, 134(5), 756–760. 10.1016/j.otohns.2006.01.017 [DOI] [PubMed] [Google Scholar]
- Isono, S. (2012). Obesity and obstructive sleep apnoea: Mechanisms for increased collapsibility of the passive pharyngeal airway. Respirology, 17(1), 32–42. 10.1111/j.1440-1843.2011 [DOI] [PubMed] [Google Scholar]
- Judge, D. P. , & Dietz, H. C. (2005). Marfan's syndrome. Lancet, 366(9501), 1965–1976. 10.1016/S0140-6736(05)67789-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler, M. , Blair, E. , Risby, P. , Nickol, A. H. , Wordsworth, P. , Forfar, C. , & Stradling, J. R. (2009). The prevalence of obstructive sleep apnea and its association with aortic dilatation in Marfan's syndrome. Thorax, 64(2), 162–166. 10.1136/thx.2008.102756 [DOI] [PubMed] [Google Scholar]
- Kohler, M. , Pitcher, A. , Blair, E. , Risby, P. , Senn, O. , Forfar, C. , … Stradling, J. R. (2013). The impact of obstructive sleep apnea on aortic disease in Marfan's syndrome. Respiration, 86(1), 39–44. 10.1159/000340008 [DOI] [PubMed] [Google Scholar]
- Loeys, B. L. , Dietz, H. C. , Braverman, A. C. , Callewaert, B. L. , De Backer, J. , Devereux, R. B. , … De Paepe, A. M. (2010). The revised Ghent nosology for the Marfan syndrome. Journal of Medical Genetics, 47(7), 476–485. 10.1136/jmg.2009.072785 [DOI] [PubMed] [Google Scholar]
- Lugaresi, E. , Cirignotta, F. , Coccagna, G. , & Piana, C. (1980). Some epidemiological data on snoring and cardiocirculatory disturbances. Sleep, 3(3–4), 221–224. 10.1093/sleep/3.3-4.221 [DOI] [PubMed] [Google Scholar]
- Mulherin, S. A. , & Miller, W. C. (2002). Spectrum bias or spectrum effect? Subgroup variation in diagnostic test evaluation. Annals of Internal Medicine, 137, 598–602. 10.7326/0003-4819-137-7-200210010-00011 [DOI] [PubMed] [Google Scholar]
- Nagappa, M. , Liao, P. , Wong, J. , Auckley, D. , Ramachandran, S. K. , Memtsoudis, S. , … Chung, F. (2015). Validation of the STOP‐bang questionnaire as a screening tool for obstructive sleep apnea among different populations: A systematic review and meta‐analysis. PLoS ONE, 10(12), e0143697 10.1371/journal.pone.0143697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netzer, N. C. , Stoohs, R. A. , Netzer, C. M. , Clark, K. , & Strohl, K. P. (1999). Using the Berlin questionnaire to identify patients at risk for the sleep apnea syndrome. Annals of Internal Medicine, 131, 485–491. 10.7326/0003-4819-131-7-199910050-00041 [DOI] [PubMed] [Google Scholar]
- Nieto, F. J. , Young, T. B. , Lind, B. K. , Shahar, E. , Samet, J. M. , Redline, S. , … Pickering, T. G. (2002). Association of sleep‐disordered breathing, sleep apnea, and hypertension in a large community‐based study. Sleep Heart Health Study. JAMA, 283(14), 1829–1836. 10.1001/jama.283.14.1829 [DOI] [PubMed] [Google Scholar]
- Orliaguet, O. , Pépin, J. L. , Bettega, G. , Ferretti, G. , Mignotte, H. N. , & Lévy, P. (2001). Sleep apnoea and Turner's syndrome. European Respiratory Journal, 17(1), 153–155. [DOI] [PubMed] [Google Scholar]
- Penpattharakul, W. , & Pithukpakorn, M. (2016). Revised Ghent criteria is comparable to original diagnostic criteria for Marfan syndrome with increased ability to clinically diagnose related disorders. Journal of the Medical Association of Thailand, 99(1), 34–39. [PubMed] [Google Scholar]
- Prasad, K. T. , Sehgal, I. S. , Agarwal, R. , Aggarwal, A. N. , Behera, D. , & Dhooria, S. (2017). Assessing the likelihood of obstructive sleep apnea: A comparison of nine screening questionnaires. Sleep and Breathing, 21(4), 909–917. 10.1007/s11325-017-1495-4 [DOI] [PubMed] [Google Scholar]
- Radonic, T. , de Witte, P. , Groenink, M. , de Bruin‐Bon, R. A. , Timmermans, J. , Scholte, A. J. , … Mulder, B. J. (2011). Critical appraisal of the revised Ghent criteria for diagnosis of Marfan syndrome. Clinical Genetics, 80(4), 346–353. 10.1111/j.1399-0004.2011.01646 [DOI] [PubMed] [Google Scholar]
- Ramachandran, S. K. , & Josephs, L. A. (2009). A meta‐analysis of clinical screening tests for obstructive sleep apnea. Anesthesiology, 110(4), 928–939. 10.1097/ALN.0b013e31819c47b6 [DOI] [PubMed] [Google Scholar]
- Reichert, J. A. , Bloch, D. A. , Cundiff, E. , & Votteri, B. A. (2003). Comparison of the NovaSom QSG, a new sleep apnea home‐diagnostic system, and polysomnography. Sleep Medicine, 4(3), 213–218. 10.1016/S1389-9457(02)00234-4 [DOI] [PubMed] [Google Scholar]
- Robicsek, F. , & Thubrikar, M. J. (1994). Hemodynamic considerations regarding the mechanism and prevention of aortic dissection. The Annals of Thoracic Surgery, 58(4), 1247–1253. 10.1016/0003-4975(94)90523-1 [DOI] [PubMed] [Google Scholar]
- Rybczynski, M. , Koschyk, D. , Karmeier, A. , Gessler, N. , Sheikhzadeh, S. , Bernhardt, A. M. , … von Kodolitsch, Y. (2010). Frequency of sleep apnea in adults with the Marfan syndrome. The American Journal of Cardiology, 105(12), 1836–1841. 10.1016/j.amjcard.2010.01.369 [DOI] [PubMed] [Google Scholar]
- Scharf, S. M. , Brown, R. , Saunders, N. , & Green, H. L. (1979). Effects of normal and loaded spontaneous inspiration on cardio‐vascular function. Journal of Applied Physiology, 47, 582–590. 10.1152/jappl.1979.47.3.582 [DOI] [PubMed] [Google Scholar]
- Schneider, H. , Schaub, C. D. , Andreoni, K. A. , Schwartz, A. R. , Smith, P. L. , Robotham, J. L. , & O'Donnell, C. P. (1997). Systemic and pulmonary hemodynamic responses to normal and obstructed breathing during sleep. Journal of Applied Physiology, 83(5), 1671–1680. 10.1152/jappl.1997.83.5.1671 [DOI] [PubMed] [Google Scholar]
- Schwartz, A. R. , Patil, S. P. , Squier, S. , Schneider, H. , Kirkness, J. P. , & Smith, P. L. (2010). Obesity and upper airway control during sleep. Journal of Applied Physiology, 108(2), 430–435. 10.1152/japplphysiol.00919.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seif, F. , Patel, S. R. , Walia, H. , Rueschman, M. , Bhatt, D. L. , Gottlieb, D. J. , … Mehra, R. (2013) Association between obstructive sleep apnea severity and endothelial dysfunction in an increased background of cardiovascular burden. Journal of Sleep Research, 22(4), 443–451. 10.1111/jsr.12026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serizawa, N. , Yumino, D. , Takagi, A. , Gomita, K. , Kajimoto, K. , Tsurumi, Y. , & Hagiwara, N. (2008). Obstructive sleep apnea is associated with greater thoracic aortic size. Journal of the American College of Cardiology, 52(10), 885–886. 10.1016/j.jacc.2008.05.039 [DOI] [PubMed] [Google Scholar]
- Stoohs, R. , & Guilleminault, C. (1991). Snoring during NREM sleep: Respiratory timing, esophageal pressure and EEG arousal. Respiration Physiology, 85(2), 151–167. 10.1016/0034-5687(91)90058-q [DOI] [PubMed] [Google Scholar]
- Stoohs, R. , & Guilleminault, C. (1992). Cardiovascular changes associated with obstructive sleep apnea syndrome. Journal of Applied Physiology, 72, 583–589. 10.1152/jappl.1992.72.2.583 [DOI] [PubMed] [Google Scholar]
- Tang, J. A. , & Friedman, M. (2018). Incidence of lingual tonsil hypertrophy in adults with and without obstructive sleep apnea. Otolaryngology Head and Neck Surgery, 158(2), 391–394. 10.1177/0194599817740333 [DOI] [PubMed] [Google Scholar]
- Teixido‐Tura, G. , Forteza, A. , Rodríguez‐Palomares, J. , González‐Mirelis, J. , Gutiérrez, L. , Sánchez, V. , … Evangelista, A. (2018). Losartan versus atenolol for prevention of aortic dilation in patients with Marfan syndrome. Journal of the American College of Cardiology, 72(14), 1613–1618. 10.1016/j.jacc.2018.07.052 [DOI] [PubMed] [Google Scholar]
- Verbraecken, J. , Hedner, J. , & Penzel, T. (2017). Pre‐operative screening for obstructive sleep apnea. European Respiratory Review, 26(143), 160012 10.1183/16000617.0012-2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


