Highlights
-
•
Increased FA of bilateral slMFB can be found in delusional SSD-patients.
-
•
Findings are supported by a psychopathological model of paranoia and grandiosity.
-
•
Findings are in line with a model of underlying network physiology (slMFB).
Keywords: Paranoia, Grandiosity, Psychosis, Reward system, White matter, DTI
Abstract
In many cases delusions in schizophrenia spectrum disorders (SSD) are driven by strong emotions such as feelings of paranoia or grandiosity. We refer to these extreme emotional experiences as psychotic affectivity. We hypothesized that increased structural connectivity of the supero-lateral medial forebrain bundle (slMFB), a major tract of the reward system, is associated with delusional psychotic affectivity. Forty-six patients with SSD and 44 healthy controls (HC) underwent diffusion weighted magnetic resonance imaging (DW-MRI)-scans. The slMFB and a comparison tract (corticospinal tract) were reconstructed using diffusion tensor imaging (DTI)-based tractography. Fractional anisotropy (FA) was sampled across the tracts. We used a mixed-model analyses of variance controlling for age and gender to compare FA of bilateral slMFB between SSD-patients and HC. Correlations of FA of bilateral slMFB and the PANSS-positive item delusions were calculated. In addition, FA was compared between three clinically homogeneous SSD-subgroups in terms of psychotic affectivity (severe, mild and no PA, sPA, mPA, nPA) and HC. FA of the slMFB did not differ between all SSD-patients and HC. In SSD-patients there was a positive correlation between delusions and FA in bilateral slMFB. Likewise, SSD-subgroups of psychotic affectivity and HC differed significantly in FA of the slMFB. Results were driven by higher FA in the right slMFB in sPA as compared to nPA and to HC. There was no significant effect for the comparison tract. In conclusion, increased structural connectivity of the slMFB may underlie delusional experiences of paranoia and grandiosity in SSD.
1. Introduction
In many cases delusions in schizophrenia spectrum disorders (SSD) are driven by strong emotions (Freeman et al., 2013; Garety et al., 2013; Heinz and Schlagenhauf, 2010; Lake, 2008; Strik et al., 2017). Mostly, the emotional valence is related to anxiety (e.g. an exceptional existential threat such as poisoning or persecution), whilst feelings of grandiosity (e.g. delusions of unrealistic power, or spiritual salvation) occur less frequently (Freeman et al., 2013; Garety et al., 2013; Paolini et al., 2016; Schoretsanitis et al., 2016; Strik et al., 2017). However, the emotional valence may switch rapidly between feelings of threat and power (Strik et al., 2017). Furthermore, in about 20% of SSD-patients paranoid and grandiose delusions co-occur even within the same observation period (Garety et al., 2013). There is also evidence that paranoia and grandiosity may share functional and structural alterations of the limbic system (Stegmayer et al., 2014b, 2017). These findings are in line with concepts viewing delusions of paranoia and grandiosity as a psychotic mood disorder (Lake, 2008). In this study, we refer to these extreme emotional experiences of either grandiosity or paranoid threat as psychotic affectivity (Lang et al., 2016, 2015a; Schoretsanitis et al., 2016; Stegmayer et al., 2014b, 2017; Strik et al., 2017).
Cognitive models of psychosis suggest that delusional patients base their decisions on limited evidence which is referred to as “jumping to conclusions” (Dudley et al., 2016; Garety and Freeman, 2013). In addition, delusion formation may stem from logical attribution errors assigning salience to neutral or irrelevant stimuli. This is referred to as the incentive salience hypothesis (Heinz and Schlagenhauf, 2010; Kapur, 2003; Speechley et al., 2010). On a neurobiological level the incentive salience hypothesis states that delusions in schizophrenia are associated with increased activation of the ventral tegmental area (VTA) and the ventral striatum (Heinz and Schlagenhauf, 2010; Jensen et al., 2008; Knolle et al., 2018; Murray et al., 2008; Romaniuk et al., 2010; Schlagenhauf et al., 2009). This overactivation is thought to be mediated by increased dopaminergic input from the VTA to the ventral striatum, which may lead to prediction errors of salience, attributing meaning to irrelevant stimuli (Heinz and Schlagenhauf, 2010; Kapur, 2003). The bed nucleus of the stria terminalis (BNST) is essential for the attribution of the emotional valence (Lebow and Chen, 2016). Overactivation of the BNST may thus underlie feelings of sustained threat or power in SSD-patients with delusions of paranoia or grandiosity (Avery et al., 2016; Davis et al., 2010; Walker et al., 2009, 2003).
The supero-lateral medial forebrain bundle (slMFB) is a polysynaptic pathway connecting cerebellar regions, brain stem, VTA, the BNST, the ventral striatum and the orbitofrontal cortex (Bracht et al., 2015, 2014b; Coenen et al., 2012, 2018b; Rivas-Grajales et al., 2018b; Schlaepfer et al., 2013; Zhang et al., 2016). Given that all the above mentioned brain regions have been linked to the formation of delusions, the slMFB may play a core role in delusions and in the attribution of their emotional valence (Avery et al., 2016; Coenen et al., 2011; Haber and Behrens, 2014; Heinz and Schlagenhauf, 2010; Lebow and Chen, 2016).
Diffusion weighted MRI (DW-MRI) enables an in vivo assessment of white matter microstructure (Jones et al., 2013). The most commonly used DW-MRI-based measure to probe white matter microstructure is the fractional anisotropy (FA). FA is influenced by barriers to diffusion of water molecules which may be related to anatomical alterations in white matter microstructure (Jones et al., 2013). Whole brain approaches such as Tract-Based Spatial Statistics (TBSS) (Smith et al., 2006)) compare white matter microstructure on a voxel-by-voxel level whilst tractography approaches average diffusion properties such as FA across tracts of interest for a specific research question (Jones et al., 2013). Given that schizophrenia is considered as a disorder of dysconnectivity (Friston, 1998), DW-MRI has been used extensively to study white matter microstructure alterations in schizophrenia (Ellison-Wright and Bullmore, 2009; Fitzsimmons et al., 2013). Whilst mainly decreases of FA have been reported (Fitzsimmons et al., 2013; Kelly et al., 2018), focal increases in FA have been linked to the presence of specific psychotic symptoms such as auditory verbal hallucinations or formal thought disorders (Hubl et al., 2004; Viher et al., 2018).
Two previous DW-MRI-studies reconstructed the striato-nigro-striatal (SNS)-tract (a segment of the slMFB) in schizophrenia. One study did not find group differences of structural connectivity based on a probabilistic fiber tracking approach (Bracht et al., 2014a). Another tractography study investigated chronic schizophrenia patients and found higher tract-dispersion (TD, an index of white matter morphology (Savadjiev et al., 2014)) but no differences of FA in the right SNS-tract (Rivas-Grajales et al., 2018a). However, to date no tractography study reconstructed the slMFB to investigate its role in the pathophysiology of delusional psychotic affectivity.
It was the first aim of this study to explore the neurobiological correlates of delusions in patients with schizophrenia spectrum disorders (SSD). Multiple training studies suggest that repeated neuronal activity induces structural plasticity that may lead to increases in FA (Piervincenzi et al., 2017; Salminen et al., 2016; Scholz et al., 2009; Steele et al., 2013). Given that the incentive salience hypothesis (Heinz and Schlagenhauf, 2010; Kapur, 2003) postulates an overactivation of the VTA and the ventral striatum (Heinz and Schlagenhauf, 2010; Jensen et al., 2008; Murray et al., 2008; Romaniuk et al., 2010) in delusion formation such increases in focal activity may lead to structural alterations of the slMFB in delusional psychotic patients. Thus, we hypothesized a positive correlation between the severity of delusions (as measured by the Positive and Negative Syndrome Scale (PANSS) (Kay et al., 1987)) and FA of the slMFB.
Dimensional assessments of psychopathology aid disentangling the heterogeneity of clinical presentations in psychosis, adding specificity to studies on the pathobiology of psychosis. One concept, the Systems Neuroscience of Psychosis (SyNoPsis) views psychosis to result from a fundamental communication breakdown of the language, the affectivity or the motor domain (Strik et al., 2017). SyNoPsis postulates that the neurobiology of specific psychosis symptoms is ideally studied contrasting patients with these symptoms with patients without these symptoms; instead of comparing all patients to healthy controls (Strik et al., 2017). The SyNoPsis instrument is the Bern Psychopathology Scale (BPS) that rates the global severity of impairments in the language, affectivity and the motor domain (Strik et al., 2010).
It is the second aim of this study to explore the role of the slMFB for psychotic affectivity in patients with SSD. In line with previous studies (Lang et al., 2016, 2015a; Schoretsanitis et al., 2016; Stegmayer et al., 2014b, 2017) we used the BPS to stratify patients into subgroups with severe, mild and no psychotic affectivity (sPA, mPA, nPA). We hypothesized increased FA in the slMFB in SSD-patients with sPA as compared to subgroups with mPA, nPA and HC.
Specificity of putative findings was tested twofold. First, a comparison tract (corticospinal tract, CST) was reconstructed. We did not expect any significant differences of FA in the CST between sPA, mPA, nPA and HC. Second, we stratified SSD-patients according to the severity in other psychopathological domains (motor and language) (Bracht et al., 2012; Steinau et al., 2017; Strik et al., 2017, 2010). We did not assume slMFB-microstructure to differ between subgroups of those comparison domains.
2. Methods
2.1. Participants
Forty-six patients with SSD (schizophrenia = 37, schizoaffective disorder = 2, and schizophreniform disorder = 7) according to the Diagnostic and Statistical Manual of Mental Health (DSM-5) and 44 healthy controls matched for age, gender and years of education have been included in the study. Patients were recruited at the inpatient and outpatient departments of the University Hospital of Psychiatry and Psychotherapy, Bern. Inclusion criterion for all participants was right-handedness as assessed with the Edinburgh Handedness Inventory (Oldfield, 1971). Exclusion criteria were contraindications to perform an MRI, neurological disorders or a history of head injuries with subsequent loss of consciousness or a history of substance use disorder other than nicotine. Exclusion criterion for controls was a history of a psychiatric disorder (as assessed with the Mini International Neuropsychiatric Interview (MINI)) (Sheehan et al., 1998). All but four patients were taking antipsychotic medication. Chlorpromazine equivalents were calculated to assess the current antipsychotic dosage. (Woods, 2003) The study was approved by the local ethics committee (KEK-BE 025/13) and adhered to the Declaration of Helsinki. All participants provided written informed consent. Demographic details are given in Table 1.
Table 1.
Clinical and demographic characteristics.
| Schizophrenia spectrum disorder | Healthy controls | Analyses | |
|---|---|---|---|
| Age (y) | 38.2 ± 11.5 | 38.8 ± 13.6 | T(88) = −0.23, P = .815 |
| Gender (women) | 37% | 41% | X2(1) = 0.15, P = .701 |
| Right handedness | 100% | 100% | |
| Education (years) | 13.4 ± 3.1 | 14.1 ± 2.7 | T(88) = −1.24, P = .220 |
| Duration of illness (years) | 12.3 ± 12.4 | ||
| Number of episodes | 6.8 ± 7.2 | ||
| PANSS positive | 18.4 ± 6.3 | ||
| PANSS negative | 18.5 ± 5.0 | ||
| PANSS total | 36.4 ± 9.1 | ||
| PANSS_P1 (delusions) | 3.5 ± 1.7 | ||
| PANSS_P5 (grandiosity) | 2.3 ± 1.3 | ||
| PANSS_P6 (suspiciousness) | 2.4 ± 1.3 | ||
| CAINS (sum score) | 18.8 ± 11.0 | ||
| SANS (global score) | 9.4 ± 5.0 | ||
| CPZ (mg) | 403.4 ± 347.3 |
2.2. Psychopathological assessment
Psychopathology was assessed at the day of the MRI-scan using the PANSS (Kay et al., 1987), the Clinical Assessment Interview for Negative Symptoms (CAINS) (Kring et al., 2013), the Scale for the Assessment of Negative Symptoms (SANS) (Andreasen, 1989), the Comprehensive Assessment of Symptoms and History (CASH) (Andreasen et al., 1992) and the BPS (Strik et al., 2010). In the present study, the severity of psychotic affectivity was assessed using the BPS global score of the affectivity domain. SSD-patients were rated on a seven-point Likert scale ranging from −3 (most severe psychotic anxiety, i.e. paranoid threat) to +3 (most severe psychotic grandiosity, i.e. delusions of power), while 0 represents normal affectivity (Lang et al., 2016; Schoretsanitis et al., 2016; Stegmayer et al., 2014b, 2017). Importantly, the severity of psychotic affectivity is rated based on the global clinical impression and does not result from the sum of single items. For example, the presence of few but severe affective signs (e.g. an isolated but severe paranoid threat of poisoning) would still result in a high global severity score of the affectivity domain. Internal consistency and external validity of the BPS has been demonstrated repeatedly (Bracht et al., 2012; Lang et al., 2016, 2015a, 2015b; Schoretsanitis et al., 2016; Stegmayer et al., 2014b, 2017; Steinau et al., 2017; Strik et al., 2010).
SSD-patients were grouped into clinically homogeneous subgroups according to the severity of psychotic affectivity based on the BPS affectivity domain global score. We defined three patient subgroups: The first subgroup incorporates SSD-patients with severe (sPA, global scores of the affectivity domain = −3, −2, +2, +3), the second group includes SSD-patients with mild psychotic affectivity (mPA, global scores of the affectivity domain = −1, +1), and the third group consists of SSD-patients with normal affectivity (no psychotic affectivity, nPA, global scores of the affectivity = 0). This approach is in line with previous studies of our group (Lang et al., 2015b; Stegmayer et al., 2014b, 2017).
The global scores of the language and the motor domain of the BPS were used to collate SSD-patients into subgroups of two comparison domains. Three patient subgroups were defined for both the motor and the language domain according to their global rating scores of the BPS (first group: −3, −2, +2, +3; second group: −1, +1; third group 0). For instance, stupor is rated as motor inhibition (-) and psychomotor agitation is rated as motor disinhibition (+) whilst mutism is rated as language inhibition (-) and increased speech production is rated as language disinhibition (+). In both domains we did not expect differences of FA in the slMFB. For a detailed description of the language and the motor domains we refer to the original publication of the BPS (Strik et al., 2010) and to its applications (Bracht et al., 2012; Stegmayer et al., 2014a; Viher et al., 2018).
2.3. MRI-acquisition
MRI-scans were acquired on a 3 Tesla MRI-scanner (Siemens Magnetom Trio; Siemens Medical Solutions, Erlangen, Germany) with a 12-channel radio frequency headcoil for signal reception.
3D-T1-weighted (Modified Driven Equilibrium Fourier Transform Pulse Sequence; MDEFT) images for each subject have been obtained, resulting in 176 sagittal slices with 256 × 256 matrix points with a non-cubic field of view (FOV) of 256 mm, yielding a nominal isotopic resolution of 1 mm3. Further scan parameters were as follows: Repetition time (TR) = 7.92 ms, echo time (TE) = 2.48 ms and flip angle = 16°
For DTI measurements, a spin echo planar imaging (EPI) sequence (59 slices, FOV = 256 × 256 mm2, sampled on a 128 × 128 matrix, slice thickness = 2 mm, gap between slices = 0 mm, resulting in 2 mm3 isotopic voxel resolution, TR/TE = 8000/92 ms) covering the whole brain (40 mT/m gradient, 6/8 partial Fourier, GRAPPA factor 2, bandwidth 1346 Hz/Px) was used. Diffusion-weighted images (DWI) were positioned in the axial plane parallel to the AC-PC line. Diffusion encoding gradients (b-value = 1300s/mm2) were applied along 42 directions. In addition, four non-diffusion weighted scans (b-value = 0 s/mm2) were collected. We used a balanced and rotationally invariant diffusion-encoding scheme over the unit sphere to generate the DTI data. Acquisition time was 6 min.
2.4. Diffusion MRI data pre-processing
DWI-data were pre-processed using ExploreDTI v4.8.3 (Leemans et al., 2009). We used a B-matrix rotation to correct for motion artefacts and eddy current distortions (Leemans and Jones, 2009). Field inhomogeneities were corrected as described in Wu et al. (Wu et al., 2008). DWIs were non-linearly warped to the MDEFT-image using the fractional anisotropy map from the DWIs as a reference. Warps were calculated using Elastix (Klein et al., 2010). This results in corrected DWIs being localized in the same (undistorted) space as the MDEFT-image. A single diffusion tensor model was fitted to the diffusion data for calculation of quantitative parameters such as FA (Basser et al., 1994). Diffusion properties (FA, mean diffusivity (MD), radial diffusivity (RD), and axial diffusivity (AD)) were sampled along the tracts.
2.5. Tractography
Whole brain tractography was performed using ExploreDTI v4.8.3 (Leemans et al., 2009) and applying an algorithm similar to that of Basser et al. (Basser et al., 1994). Termination criteria were an angle threshold >45° and FA<0.2. Tracts were reconstructed based on anatomical landmarks as described in Bracht et al. (Bracht et al., 2015). For reconstruction of the slMFB one horizontal region of interest (ROI) was placed on the DWI surrounding the ventral tegmental area (VTA). Anatomical borders were laterally the substantia nigra, anteriorly the mammillary bodies and posteriorly the red nucleus. A second ROI was drawn on structural images surrounding caudate and putamen on a coronal section at the height of the nucleus accumbens (NAcc) (Bracht et al., 2015) (see Fig. 1 and supplementary material, S1). For reconstruction of the comparison tract (corticospinal tract) the precentral gyrus was encircled. A second ROI was drawn at the height of the pons on color coded DWI-images, where fibers of the corticospinal tract descend (Bracht et al., 2018) (see supplementary material, S2).
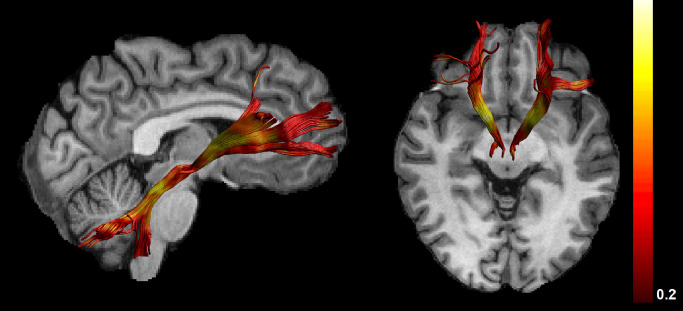
Fig. 1.
demonstrates reconstructions of the slMFB for an individual participant with view from the right side (left image) and view from above (right image). FA metrics are superimposed on the reconstructed tracts.
2.6. Group comparison between SSD-patients and healthy controls
Two-sample t-tests were used to compare demographic differences between SSD-patients and healthy controls (see Table 1). A mixed-model ANCOVA was used to investigate group differences in FA of the left and the right slMFB between HC and SSD. Group (SSD and HC) was entered as independent variable, mean-FA as dependent variable, hemisphere (left-right) as “within-subject” variable and age and gender as covariate.
2.7. Correlations between FA of the slMFB and delusions
Across all patients, we calculated Spearman correlations between FA of left and right slMFB and the PANSS-positive item delusions (PANSS_P1). Spearman correlations instead of Pearson correlations were used since PANSS-items are rather ordinal than continuous variables. Alterations in FA and AD may rather stem from axonal properties such as fiber coherence (Mezer et al., 2013), whilst MD and RD may be more sensitive to myelin changes (Song et al., 2002). Thus, where a main effect of FA (our primary outcome measure) was seen, results were followed-up with corresponding analyses of MD, RD and AD.
Exploratory correlations between FA of left and right slMFB and chlorpromazine equivalents (Woods, 2003) and duration of illness as well as psychopathology measures related to depression (Bracht et al., 2015, 2014b; Coenen et al., 2011; Schlaepfer et al., 2013) and negative and positive symptoms (Bopp et al., 2017; Bracht et al., 2014a) were calculated to improve specificity and to complement our analyses (results are presented in the supplementary material, S5).
2.8. Group comparisons between sPA, mPA, nPA-subgroups and HC
ANOVAS were used to explore clinical and demographic differences between sPA, mPA, nPA, and HC. In order to investigate group differences in FA of the left and the right slMFB between those clinically homogeneous subgroups in terms of psychotic affectivity a mixed-model ANCOVA was used. Group (sPA, mPA, nPA and HC) was entered as independent variable, mean-FA as dependent variable, hemisphere (left-right) as “within-subject” variable and age and gender as covariates. Significant main effects were followed up with post-hoc ANCOVAs including age and gender as covariates. Post-hoc tests were corrected for multiple comparisons using a False Discovery Rate correction (Benjamini and Hochberg, 1995). Where a main effect of FA (our primary outcome measure) was seen, results were followed-up with corresponding analyses of MD, RD and AD.
To enhance specificity of putative findings all analyses have also been performed for the comparison tract (corticospinal tract). Further, we stratified SSD-patients into severely, moderately and unaffected patients of psychopathological comparison domains (the motor domain and the language (associative) domain) (Bracht et al., 2012; Strik et al., 2017; Viher et al., 2018). We did not assume homogeneous subgroups of those comparison domains to differ in slMFB-microstructure.
Tract-specific group comparisons were complemented using whole brain TBSS analyses (see supplementary material, S10). Statistically we complement our analyses providing bootstrap confidence intervals for correlations and for effect sizes of subgroup comparisons (see supplementary material, S12 and S13).
3. Results
3.1. Group comparison between SSD-patients and healthy controls
Groups (SSD, HC) did not differ regarding age, gender, handedness and years of education (see table 1). The mixed-model ANCOVA for FA of the slMFB controlling for age and gender did not show a significant main effect of group for FA comparing all SSD-patients with HC (F (1, 86) = 1.10, P = .297, η2 = 0.012). Neither was there a main effect for FA of hemisphere (F (1, 86) = 0.25, P = .618, η2 = 0.003) nor a group x hemisphere interaction effect (F (1, 86) = 0.30, P = .586, η2 = 0.003). There were no significant effects for the comparison tract (see supplementary material, S6).
3.2. Correlations with delusions
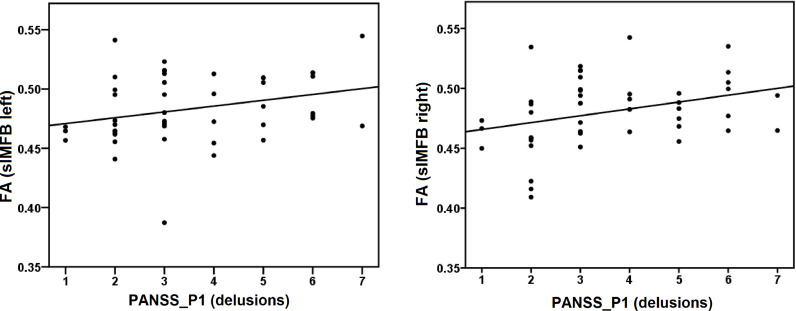
Across the whole group there was a positive correlation between delusions (PANSS_P1) and FA of the left (Rho = 0.337, P = .022) and of the right (Rho = 0.399, P = .006) slMFB (see Fig. 2).
Fig. 2.
demonstrates correlations between delusions PANSS_P1 (delusions) and FA of the left (Rho = 0.337, P = .022) and of the right (Rho = 0.339, P = .006) slMFB across all SSD-patients.
Follow-up analyses demonstrated negative correlations between delusions (PANSS_P1) and MD of left (Rho = −0.537, P < .001) and right (Rho = −0.467, P = .001) slMFB, as well as between delusions and AD of the left (Rho =- 0.488, P = .001) and the right (Rho = −0.326, P = .029) slMFB, and delusions and RD of the left (Rho = −0.455, P = .002) but not of the right (Rho = −0.288, P = .055) slMFB. There were no significant correlations between delusions (PANSS_P1) and FA of the comparison tract (see supplementary material, S3).
3.3. Group comparisons between sPA, mPA, nPA and healthy controls
SSD-patients were grouped into sPA (n == 16), mPA (n = =20) and nPA (n == 10). Subgroups (sPA, mPA, nPA and HC) did not differ regarding age (F (3, 89) = 0.26, P = .855), gender (X2(3) = 0.48, P = .922) or years of education (F (3, 89) = 0.98, P = .408). SSD-subgroups (sPA, nPA, mPA) differed significantly regarding PANSS-positive total scores, which were driven by higher PANSS-items delusions, excitement and suspiciousness/persecution and hostility in the sPA group (for demographic details and group comparisons see supplementary material, S7).
The mixed-model ANCOVA controlling for age and gender comparing FA of the slMFB showed a significant main effect of group (sPA, mPA, nPA, HC) (F (3, 84) = 3.06, P=.033, η2=0.098), but no significant main effect of hemisphere (F (1, 84) = 0.24, P= .629, η2 = 0.003), nor an interaction effect of group x hemisphere (F (3, 84) = 1.74, P= .166, η2 = 0.058).
The observed main effect was not present in the comparison tract (corticospinal tract) (see supplementary material, S9). Anatomical specificity of the main effect of group for FA of the slMFB was shown by a significant group x tract (levels tract (slMFB) and control tract (CST) interaction (F (3, 84) = 3.99, P=.010*). Furthermore, the FA in the slMFB did not differ between groups stratified according to the psychopathological comparison domains, i.e. motor and language (see supplementary material, table S11).
Post-hoc ANCOVAs controlling for age and gender comparing FA of left and right slMFB between sPA, mPA, nPA and HC demonstrated increases in FA for the right slMFB in sPA as compared to HC and nPA (see Table 2). All P-values are corrected for multiple comparisons using a False Discovery Rate correction (Benjamini and Hochberg, 1995).
Table 2.
Results of post-hoc ANCOVAs comparing FA between sPA, mPA, nPA and HC controlling for age and gender are displayed. Displayed p-values are corrected for multiple comparisons using a False Discovery Rate (Benjamini and Hochberg, 1995).
| Left slMFB | ||
|---|---|---|
| Contrast (mean FA ± SD) | Statistics | η2 |
| sPA (0.49±0.04) vs HC (0.48±0.03) | F(1,59) = 1.87, P = .266 | .032 |
| sPA (0.49±0.04) vs mPA (0.49±0.03) | F(1,35) = 0.01, P = .992 | <0.001 |
| sPA (0.49±0.04) vs nPA (0.47±0.01) | F(1,25) = 3.26, P = .204 | .129 |
| mPA (0.49±0.03) vs HC (0.48±0.03) | F(1,63) = 2.25, P = .247 | .036 |
| mPA (0.49±0.03) vs nPA (0.47±0.01) | F(1,29) = 6.10, P = .08 | .190 |
| nPA (0.47±0.01) vs HC (0.48±0.03) | F(1,53) = 1.02, P = .38 | .020 |
| Right slMFB | ||
|---|---|---|
| Contrast (mean FA ± SD) | Statistics | |
| sPA (0.50±0.02) vs HC (0.48±0.02) | F (1,59) = 7.86, P = .042* | .123 |
| sPA (0.50±0.02) vs mPA(0.48±0.03) | F (1,35) = 5.05, P = .096 | .136 |
| sPA (0.50±0.02) vs nPA (0.46±0.02) | F (1,25) = 11.49, P = =0.036* | .343 |
| mPA (0.48±0.03) vs HC (0.48±0.02) | F (1,63) < 0.001, P = .992 | <0.001 |
| mPA (0.48±0.03) vs nPA (0.46±0.02) | F (1,29) = 1.21, P = .376 | .044 |
| nPA (0.46±0.02) vs HC (0.48±0.02) | F (1,53) = 2.20, P = .257 | .042 |
Secondary analyses for significant post-hoc ANCOVAs demonstrated decreased MD for the right slMFB comparing sPA with nPA, and sPA with HC. There were no significant group differences for AD and RD (see supplementary material, S8).
Distribution of FA across BPS-global scores of affectivity are best explained by a quadratic regression model. Plotting FA-values across subgroups a U-shaped distribution with increases of FA in SSD-patients with both paranoia and grandiosity can be seen (see Fig. 3, and supplementary material, S4).
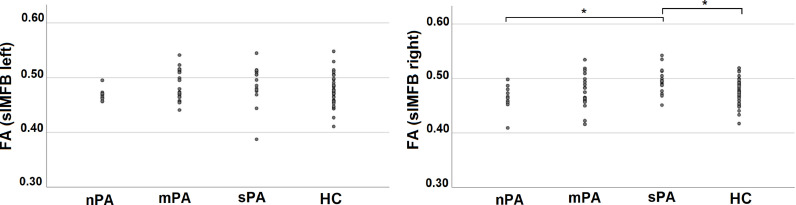
Fig. 3.
Mean-FA of the left slMFB (left side) and the right slMFB (right side) for homogeneous groups of psychotic affectivity. SSD-patients with sPA show increased FA in the right slMFB compared to nPA and HC.
4. Discussion
Delusions are thought to result from dopamine-driven aberrant salience (Heinz and Schlagenhauf, 2010; Kapur, 2003). Here, we explored for the first time whether the slMFB as a major white matter pathway of the reward system was linked to delusions of psychotic affectivity. Our study has two main findings. First, we demonstrated an association between the severity of delusions and white matter microstructure of both left (FA, MD, RD, AD) and right (FA, MD, AD) slMFB. Second, we found increased structural connectivity (as measured by increased FA and reduced MD) in the right slMFB in sPA compared to nPA and to HC. All findings were specific for the slMFB (and not present in a comparison tract). Further, group differences of FA in the slMFB were specific for the sPA group and disappeared when stratifying SSD-patients across psychopathological comparison dimensions (motor and language domain).
The identified positive correlation between FA in bilateral slMFB in SSD-patients and the severity of delusions corroborates the theoretical concept of the incentive salience hypothesis suggesting that increased (dopaminergic) input from the VTA to the ventral striatum attributes salience to otherwise neutral stimuli (Heinz and Schlagenhauf, 2010; Kapur, 2003). An overactivation of the VTA and the ventral striatum (Heinz and Schlagenhauf, 2010; Jensen et al., 2008; Murray et al., 2008; Romaniuk et al., 2010) may induce structural neuroplasticity (Salminen et al., 2016; Scholz et al., 2009; Steele et al., 2013; Stuber et al., 2008) leading to structural alterations as suggested by our findings of increased FA (and decreased MD, RD and AD) in the slMFB in delusional psychotic patients. However, our study design does not allow for conclusions on the direction of causality between functional and structural alterations in SSD-patients with delusions.
We complemented our analyses approach using a dimensional approach to stratify SSD-patients into homogeneous subgroups in terms of the severity of psychotic affectivity (Lang et al., 2016; Schoretsanitis et al., 2016; Stegmayer et al., 2014b, 2017; Strik et al., 2017). We found increased FA and reduced MD of the right slMFB in SSD-patients with sPA as compared to nPA and HC. There was a U-shaped FA-distribution of bilateral slMFB demonstrating increases of structural alterations with increases of either grandiosity or paranoia (see supplementary material, S4). Similar distributions of structural and functional alterations in SSD-patients with sPA have been reported for the volume of the ventral striatum and for the perfusion of the amygdala (Stegmayer et al., 2014b, 2017). Thus, grandiosity and paranoia may share structural and functional alterations of the limbic and the reward system.
Given that the sPA-subgroup had higher FA-values and more pronounced delusions than other PA-subgroups, the reported correlation between delusions (PANSS_P1) and FA across all SSD-patients is also captured by the identified group differences in FA between subgroups of PA. Rather than assessing the presence or severity of delusions, the BPS focusses on the emotional valence of delusions and therefore provides clinically relevant additional information. Our sPA-subgroup consisted mainly of SSD-patients with paranoid threat. This is corroborated by higher PANSS_P6 (persecution) and PANSS_P7 (hostility) values in the sPA-subgroup and points to a predominant paranoid syndrome in the sPA subgroup of this sample. Nevertheless, increases of FA are also seen in sPA with grandiosity (see supplementary material, S4).
Previous fMRI and arterial spin labelling (ASL)-studies suggest that paranoia in psychosis may underlie increased amygdala activation and decreased functional connectivity between the prefrontal cortex and the amygdala (Hall et al., 2008; Hoptman et al., 2014, 2010; Pinkham et al., 2015; Stegmayer et al., 2017). These changes in functional connectivity could rely on structural alterations of pathways connecting the prefrontal cortex with the amygdala such as the uncinate fasciculus (Bracht et al., 2009; Von Der Heide et al., 2013) or the anterior thalamic radiation (Coenen et al., 2012). Even though the anterior thalamic radiation runs medially of the slMFB there is a partial overlap in the anterior limb of the internal capsule. Thus, given the current resolution of DTI it cannot be excluded that lateral segments of the ATR contribute to our results as well. The slMFB does not only connect to core regions of the reward system but also to relay stations of the motor system such as the cerebellum (Coenen et al., 2018a). Thus, the slMFB may play an essential role for the preparation of motor related responses to experiences of psychotic affectivity such as fight or flight reactions in SSD-patients with paranoid experiences.
Deep brain stimulation (DBS) is used to stimulate the slMFB in treatment resistant depression (Coenen et al., 2018a; Fenoy et al., 2018; Schlaepfer et al., 2013). Interestingly, in rare cases psychotic symptoms of grandiosity have been attributed to DBS-stimulation of the slMFB (Coenen et al., 2009). It is possible that reduced structural connectivity of the slMFB in depression (Bracht et al., 2014b) is being successfully reversed by slMFB-DBS. Therefore, structural connectivity of the slMFB may reflect a continuum from severe anhedonia to experiences of intense emotions such as psychotic affectivity as suggested by our findings of increased FA in sPA-SSD patients.
We identified increases in FA (and decreases in MD) in the slMFB in sPA. Alterations in FA and AD may rather stem from axonal properties such as fiber coherence (Mezer et al., 2013), whilst MD and RD may be more sensitive to myelin changes (Song et al., 2002). Thus, identified changes in sPA may be due to myelin alterations. However, changes in diffusion properties are unspecific and do not allow conclusions on changes in “fiber integrity” (Jones et al., 2013). Novel sub-compartment specific white matter mapping techniques may lead to more informative neurobiological conclusions (Bracht et al., 2016; Vanes et al., 2018).
Using TBSS we identified widespread distributions of FA reductions predominantly localized in the corpus callosum comparing all SSD-patients with HC (see supplementary material S10). This is in line with a broad body of literature (Fitzsimmons et al., 2013; Kelly et al., 2018). On the other hand, increases of FA (as identified in the slMFB in sPA) have been linked to positive psychotic symptoms (Hubl et al., 2004; Viher et al., 2018). Thus, our results corroborate both concepts pointing to global white matter alterations in schizophrenia and concepts linking overactivation of functionally connected brain regions to increases of structural connectivity.
4.1. Limitations
This study has some limitations. First, the sample size of SSD-PA subgroups is small. Second, there are non-significant trends for demographic and clinical differences between SSD-PA subgroups which may have contributed to group differences. Third, even though smoking may have an impact on FA in schizophrenia we did not collect these data (Gons et al., 2011; Zhang et al., 2010). Fourth, cross-sectional designs do not enable statements on causality and neuroplasticity. Thus, we cannot conclude on slMFB-microstructure as a state or a trait marker. Fifth, a non-significant finding in the comparison tract does not proof the absence of an actual effect.
5. Conclusions
In conclusion, this is the first tractography study to investigate the role of the slMFB for experiences of delusional psychotic affectivity in SSD. Our results suggest an association between experiences of paranoid threat and grandiosity in SSD with white matter microstructure of the slMFB. Our results thus corroborate the important role of the reward system in the pathophysiology of delusions (Heinz and Schlagenhauf, 2010; Kapur, 2003). Further, this study argues for investigating patients with distinct homogeneous behavioral phenotypes in order to study the neurobiology of psychotic symptom dimensions (Insel et al., 2010; Strik et al., 2017).
Declaration of Competing Interest
Dr. Walther received honoraria from Lundbeck, Lilly, Janssen, and Otsuka. All further authors report no competing interests.
Acknowledgments
This study received funding from the Swiss National Science Foundation (SNF grant #152619 to SW and AF) and the Bangerter-Rhyner Foundation (to SW).
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.nicl.2019.102044.
Appendix. Supplementary materials
References
- Andreasen N.C. The scale for the assessment of negative symptoms (SANS): conceptual and theoretical foundations. Br J Psychiatry Suppl. 1989:49–58. [PubMed] [Google Scholar]
- Andreasen N.C., Flaum M., Arndt S. The comprehensive assessment of symptoms and history (CASH). an instrument for assessing diagnosis and psychopathology. Arch Gen Psychiatry. 1992;49:615–623. doi: 10.1001/archpsyc.1992.01820080023004. [DOI] [PubMed] [Google Scholar]
- Avery S.N., Clauss J.A., Blackford J.U. The human BNST: functional role in anxiety and addiction. Neuropsychopharmacology. 2016;41:126–141. doi: 10.1038/npp.2015.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basser P.J., Mattiello J., LeBihan D. Estimation of the effective self-diffusion tensor from the NMR spin echo. J Magn Reson B. 1994;103:247–254. doi: 10.1006/jmrb.1994.1037. [DOI] [PubMed] [Google Scholar]
- Benjamini J., Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society. Series B (Methodological) 1995;57(1):289–300. [Google Scholar]
- Bopp M.H.A., Zollner R., Jansen A., Dietsche B., Krug A., Kircher T.T.J. White matter integrity and symptom dimensions of schizophrenia: a diffusion tensor imaging study. Schizophr Res. 2017;184:59–68. doi: 10.1016/j.schres.2016.11.045. [DOI] [PubMed] [Google Scholar]
- Bracht T., Doidge A.N., Keedwell P.A., Jones D.K. Hedonic tone is associated with left supero-lateral medial forebrain bundle microstructure. Psychol Med. 2015;45:865–874. doi: 10.1017/S0033291714001949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracht T., Heidemeyer K., Koschorke P., Horn H., Razavi N., Wopfner A., Strik W., Walther S. Comparison of objectively measured motor behavior with ratings of the motor behavior domain of the bern psychopathology scale (BPS) in schizophrenia. Psychiatry Res. 2012;198:224–229. doi: 10.1016/j.psychres.2011.12.038. [DOI] [PubMed] [Google Scholar]
- Bracht T., Horn H., Strik W., Federspiel A., Razavi N., Stegmayer K., Wiest R., Dierks T., Muller T.J., Walther S. White matter pathway organization of the reward system is related to positive and negative symptoms in schizophrenia. Schizophr Res. 2014;153:136–142. doi: 10.1016/j.schres.2014.01.015. [DOI] [PubMed] [Google Scholar]
- Bracht T., Horn H., Strik W., Federspiel A., Schnell S., Hofle O., Stegmayer K., Wiest R., Dierks T., Muller T.J., Walther S. White matter microstructure alterations of the medial forebrain bundle in melancholic depression. J Affect Disord. 2014;155:186–193. doi: 10.1016/j.jad.2013.10.048. [DOI] [PubMed] [Google Scholar]
- Bracht T., Jones D.K., Bells S., Walther S., Drakesmith M., Linden D. Myelination of the right parahippocampal cingulum is associated with physical activity in young healthy adults. Brain Struct Funct. 2016;221:4537–4548. doi: 10.1007/s00429-016-1183-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracht T., Steinau S., Federspiel A., Schneider C., Wiest R., Walther S. Physical activity is associated with left corticospinal tract microstructure in bipolar depression. Neuroimage Clin. 2018;20:939–945. doi: 10.1016/j.nicl.2018.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracht T., Tuscher O., Schnell S., Kreher B., Rusch N., Glauche V., Lieb K., Ebert D., Il'yasov K.A., Hennig J., Weiller C., van Elst L.T., Saur D. Extraction of prefronto-amygdalar pathways by combining probability maps. Psychiatry Res. 2009;174:217–222. doi: 10.1016/j.pscychresns.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Coenen V.A., Honey C.R., Hurwitz T., Rahman A.A., McMaster J., Burgel U., Madler B. Medial forebrain bundle stimulation as a pathophysiological mechanism for hypomania in subthalamic nucleus deep brain stimulation for parkinson's disease. Neurosurgery. 2009;64:1106–1114. doi: 10.1227/01.NEU.0000345631.54446.06. discussion 1114-1105. [DOI] [PubMed] [Google Scholar]
- Coenen V.A., Panksepp J., Hurwitz T.A., Urbach H., Madler B. Human medial forebrain bundle (MFB) and anterior thalamic radiation (ATR): imaging of two major subcortical pathways and the dynamic balance of opposite affects in understanding depression. J Neuropsychiatry Clin Neurosci. 2012;24:223–236. doi: 10.1176/appi.neuropsych.11080180. [DOI] [PubMed] [Google Scholar]
- Coenen V.A., Sajonz B., Reisert M., Bostroem J., Bewernick B., Urbach H., Jenkner C., Reinacher P.C., Schlaepfer T.E., Madler B. Tractography-assisted deep brain stimulation of the superolateral branch of the medial forebrain bundle (slMFB DBS) in major depression. Neuroimage Clin. 2018;20:580–593. doi: 10.1016/j.nicl.2018.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coenen V.A., Schlaepfer T.E., Maedler B., Panksepp J. Cross-species affective functions of the medial forebrain bundle-implications for the treatment of affective pain and depression in humans. Neurosci Biobehav Rev. 2011;35:1971–1981. doi: 10.1016/j.neubiorev.2010.12.009. [DOI] [PubMed] [Google Scholar]
- Coenen V.A., Schumacher L.V., Kaller C., Schlaepfer T.E., Reinacher P.C., Egger K., Urbach H., Reisert M. The anatomy of the human medial forebrain bundle: ventral tegmental area connections to reward-associated subcortical and frontal lobe regions. Neuroimage Clin. 2018;18:770–783. doi: 10.1016/j.nicl.2018.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M., Walker D.L., Miles L., Grillon C. Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology. 2010;35:105–135. doi: 10.1038/npp.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley R., Taylor P., Wickham S., Hutton P. Psychosis, delusions and the "Jumping to conclusions" reasoning bias: a systematic review and meta-analysis. Schizophr Bull. 2016;42:652–665. doi: 10.1093/schbul/sbv150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellison-Wright I., Bullmore E. Meta-analysis of diffusion tensor imaging studies in schizophrenia. Schizophr Res. 2009;108:3–10. doi: 10.1016/j.schres.2008.11.021. [DOI] [PubMed] [Google Scholar]
- Fenoy A.J., Schulz P.E., Selvaraj S., Burrows C.L., Zunta-Soares G., Durkin K., Zanotti-Fregonara P., Quevedo J., Soares J.C. A longitudinal study on deep brain stimulation of the medial forebrain bundle for treatment-resistant depression. Transl Psychiatry. 2018;8:111. doi: 10.1038/s41398-018-0160-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzsimmons J., Kubicki M., Shenton M.E. Review of functional and anatomical brain connectivity findings in schizophrenia. Curr Opin Psychiatry. 2013;26:172–187. doi: 10.1097/YCO.0b013e32835d9e6a. [DOI] [PubMed] [Google Scholar]
- Freeman D., Dunn G., Fowler D., Bebbington P., Kuipers E., Emsley R., Jolley S., Garety P. Current paranoid thinking in patients with delusions: the presence of cognitive-affective biases. Schizophr Bull. 2013;39:1281–1287. doi: 10.1093/schbul/sbs145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K.J. The disconnection hypothesis. Schizophr Res. 1998;30:115–125. doi: 10.1016/s0920-9964(97)00140-0. [DOI] [PubMed] [Google Scholar]
- Garety P.A., Freeman D. The past and future of delusions research: from the inexplicable to the treatable. Br J Psychiatry. 2013;203:327–333. doi: 10.1192/bjp.bp.113.126953. [DOI] [PubMed] [Google Scholar]
- Garety P.A., Gittins M., Jolley S., Bebbington P., Dunn G., Kuipers E., Fowler D., Freeman D. Differences in cognitive and emotional processes between persecutory and grandiose delusions. Schizophr Bull. 2013;39:629–639. doi: 10.1093/schbul/sbs059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gons R.A., van Norden A.G., de Laat K.F., van Oudheusden L.J., van Uden I.W., Zwiers M.P., Norris D.G., de Leeuw F.E. Cigarette smoking is associated with reduced microstructural integrity of cerebral white matter. Brain. 2011;134:2116–2124. doi: 10.1093/brain/awr145. [DOI] [PubMed] [Google Scholar]
- Haber S.N., Behrens T.E. The neural network underlying incentive-based learning: implications for interpreting circuit disruptions in psychiatric disorders. Neuron. 2014;83:1019–1039. doi: 10.1016/j.neuron.2014.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J., Whalley H.C., McKirdy J.W., Romaniuk L., McGonigle D., McIntosh A.M., Baig B.J., Gountouna V.E., Job D.E., Donaldson D.I., Sprengelmeyer R., Young A.W., Johnstone E.C., Lawrie S.M. Overactivation of fear systems to neutral faces in schizophrenia. Biol Psychiatry. 2008;64:70–73. doi: 10.1016/j.biopsych.2007.12.014. [DOI] [PubMed] [Google Scholar]
- Heinz A., Schlagenhauf F. Dopaminergic dysfunction in schizophrenia: salience attribution revisited. Schizophr Bull. 2010;36:472–485. doi: 10.1093/schbul/sbq031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoptman M.J., Antonius D., Mauro C.J., Parker E.M., Javitt D.C. Cortical thinning, functional connectivity, and mood-related impulsivity in schizophrenia: relationship to aggressive attitudes and behavior. Am J Psychiatry. 2014;171:939–948. doi: 10.1176/appi.ajp.2014.13111553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoptman M.J., D'Angelo D., Catalano D., Mauro C.J., Shehzad Z.E., Kelly A.M., Castellanos F.X., Javitt D.C., Milham M.P. Amygdalofrontal functional disconnectivity and aggression in schizophrenia. Schizophr Bull. 2010;36:1020–1028. doi: 10.1093/schbul/sbp012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubl D., Koenig T., Strik W., Federspiel A., Kreis R., Boesch C., Maier S.E., Schroth G., Lovblad K., Dierks T. Pathways that make voices: white matter changes in auditory hallucinations. Arch Gen Psychiatry. 2004;61:658–668. doi: 10.1001/archpsyc.61.7.658. [DOI] [PubMed] [Google Scholar]
- Insel T., Cuthbert B., Garvey M., Heinssen R., Pine D.S., Quinn K., Sanislow C., Wang P. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010;167:748–751. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- Jensen J., Willeit M., Zipursky R.B., Savina I., Smith A.J., Menon M., Crawley A.P., Kapur S. The formation of abnormal associations in schizophrenia: neural and behavioral evidence. Neuropsychopharmacology. 2008;33:473–479. doi: 10.1038/sj.npp.1301437. [DOI] [PubMed] [Google Scholar]
- Jones D.K., Knosche T.R., Turner R. White matter integrity, fiber count, and other fallacies: the do's and don'ts of diffusion MRI. Neuroimage. 2013;73:239–254. doi: 10.1016/j.neuroimage.2012.06.081. [DOI] [PubMed] [Google Scholar]
- Kapur S. Psychosis as a state of aberrant salience: a framework linking biology, phenomenology, and pharmacology in schizophrenia. Am J Psychiatry. 2003;160:13–23. doi: 10.1176/appi.ajp.160.1.13. [DOI] [PubMed] [Google Scholar]
- Kay S.R., Fiszbein A., Opler L.A. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Kelly S., Jahanshad N., Zalesky A., Kochunov P., Agartz I., Alloza C., Andreassen O.A., Arango C., Banaj N., Bouix S., Bousman C.A., Brouwer R.M., Bruggemann J., Bustillo J., Cahn W., Calhoun V., Cannon D., Carr V., Catts S., Chen J., Chen J.X., Chen X., Chiapponi C., Cho K.K., Ciullo V., Corvin A.S., Crespo-Facorro B., Cropley V., De Rossi P., Diaz-Caneja C.M., Dickie E.W., Ehrlich S., Fan F.M., Faskowitz J., Fatouros-Bergman H., Flyckt L., Ford J.M., Fouche J.P., Fukunaga M., Gill M., Glahn D.C., Gollub R., Goudzwaard E.D., Guo H., Gur R.E., Gur R.C., Gurholt T.P., Hashimoto R., Hatton S.N., Henskens F.A., Hibar D.P., Hickie I.B., Hong L.E., Horacek J., Howells F.M., Hulshoff Pol H.E., Hyde C.L., Isaev D., Jablensky A., Jansen P.R., Janssen J., Jonsson E.G., Jung L.A., Kahn R.S., Kikinis Z., Liu K., Klauser P., Knochel C., Kubicki M., Lagopoulos J., Langen C., Lawrie S., Lenroot R.K., Lim K.O., Lopez-Jaramillo C., Lyall A., Magnotta V., Mandl R.C.W., Mathalon D.H., McCarley R.W., McCarthy-Jones S., McDonald C., McEwen S., McIntosh A., Melicher T., Mesholam-Gately R.I., Michie P.T., Mowry B., Mueller B.A., Newell D.T., O'Donnell P., Oertel-Knochel V., Oestreich L., Paciga S.A., Pantelis C., Pasternak O., Pearlson G., Pellicano G.R., Pereira A., Pineda Zapata J., Piras F., Potkin S.G., Preda A., Rasser P.E., Roalf D.R., Roiz R., Roos A., Rotenberg D., Satterthwaite T.D., Savadjiev P., Schall U., Scott R.J., Seal M.L., Seidman L.J., Shannon Weickert C., Whelan C.D., Shenton M.E., Kwon J.S., Spalletta G., Spaniel F., Sprooten E., Stablein M., Stein D.J., Sundram S., Tan Y., Tan S., Tang S., Temmingh H.S., Westlye L.T., Tonnesen S., Tordesillas-Gutierrez D., Doan N.T., Vaidya J., van Haren N.E.M., Vargas C.D., Vecchio D., Velakoulis D., Voineskos A., Voyvodic J.Q., Wang Z., Wan P., Wei D., Weickert T.W., Whalley H., White T., Whitford T.J., Wojcik J.D., Xiang H., Xie Z., Yamamori H., Yang F., Yao N., Zhang G., Zhao J., van Erp T.G.M., Turner J., Thompson P.M., Donohoe G. Widespread white matter microstructural differences in schizophrenia across 4322 individuals: results from the enigma schizophrenia DTI working group. Mol Psychiatry. 2018;23:1261–1269. doi: 10.1038/mp.2017.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein S., Staring M., Murphy K., Viergever M.A., Pluim J.P. elastix: a toolbox for intensity-based medical image registration. IEEE Trans Med Imaging. 2010;29:196–205. doi: 10.1109/TMI.2009.2035616. [DOI] [PubMed] [Google Scholar]
- Knolle F., Ermakova A.O., Justicia A., Fletcher P.C., Bunzeck N., Duzel E., Murray G.K. Brain responses to different types of salience in antipsychotic naive first episode psychosis: an fMRI study. Transl Psychiatry. 2018;8:196. doi: 10.1038/s41398-018-0250-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kring A.M., Gur R.E., Blanchard J.J., Horan W.P., Reise S.P. The clinical assessment interview for negative symptoms (CAINS): final development and validation. Am J Psychiatry. 2013;170:165–172. doi: 10.1176/appi.ajp.2012.12010109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lake C.R. Hypothesis: grandiosity and guilt cause paranoia; paranoid schizophrenia is a psychotic mood disorder; a review. Schizophr Bull. 2008;34:1151–1162. doi: 10.1093/schbul/sbm132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang F.U., Muller-Stierlin A.S., Walther S., Stegmayer K., Becker T., Jager M. Dimensional approaches to schizophrenia: a comparison of the bern psychopathology scale and the five-factor model of the positive and negative syndrome scale. Psychiatry Res. 2016;239:284–290. doi: 10.1016/j.psychres.2016.03.032. [DOI] [PubMed] [Google Scholar]
- Lang F.U., Stierlin A.S., Stegmayer K., Walther S., Becker T., Jager M. Factor structure of the bern psychopathology scale in a sample of patients with schizophrenia spectrum disorders. Eur Psychiatry. 2015;30:880–884. doi: 10.1016/j.eurpsy.2015.07.010. [DOI] [PubMed] [Google Scholar]
- Lang F.U., Walther S., Stegmayer K., Anderson-Schmidt H., Schulze T.G., Becker T., Jager M. Subtyping schizophrenia: a comparison of positive/negative and system-specific approaches. Compr Psychiatry. 2015;61:115–121. doi: 10.1016/j.comppsych.2015.05.014. [DOI] [PubMed] [Google Scholar]
- Lebow M.A., Chen A. Overshadowed by the amygdala: the bed nucleus of the stria terminalis emerges as key to psychiatric disorders. Mol Psychiatry. 2016;21:450–463. doi: 10.1038/mp.2016.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leemans A., Jeurissen B., Sijbers J., Jones D.K. Proceedings of the International Society for Magnetic Resonance in Medicine 17th Annual Meeting April 18-24. Vol. 3536. Honolulu Hawaii; 2009. ExporeDTI: a graphical toolbox for processing, analyzing and visualizing diffusion MR data. [Google Scholar]
- Leemans A., Jones D.K. The B-matrix must be rotated when correcting for subject motion in DTI data. Magn Reson Med. 2009;61:1336–1349. doi: 10.1002/mrm.21890. [DOI] [PubMed] [Google Scholar]
- Mezer A., Yeatman J.D., Stikov N., Kay K.N., Cho N.J., Dougherty R.F., Perry M.L., Parvizi J., Hua le H., Butts-Pauly K., Wandell B.A. Quantifying the local tissue volume and composition in individual brains with magnetic resonance imaging. Nat Med. 2013;19:1667–1672. doi: 10.1038/nm.3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray G.K., Corlett P.R., Clark L., Pessiglione M., Blackwell A.D., Honey G., Jones P.B., Bullmore E.T., Robbins T.W., Fletcher P.C. Substantia nigra/ventral tegmental reward prediction error disruption in psychosis. Mol Psychiatry. 2008;13(239):267–276. doi: 10.1038/sj.mp.4002058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield R.C. The assessment and analysis of handedness: the edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Paolini E., Moretti P., Compton M.T. Delusions in first-episode psychosis: principal component analysis of twelve types of delusions and demographic and clinical correlates of resulting domains. Psychiatry Res. 2016;243:5–13. doi: 10.1016/j.psychres.2016.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piervincenzi C., Ben-Soussan T.D., Mauro F., Mallio C.A., Errante Y., Quattrocchi C.C., Carducci F. White matter microstructural changes following quadrato motor training: a longitudinal study. Front Hum Neurosci. 2017;11:590. doi: 10.3389/fnhum.2017.00590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkham A.E., Liu P., Lu H., Kriegsman M., Simpson C., Tamminga C. Amygdala hyperactivity at rest in paranoid individuals with schizophrenia. Am J Psychiatry. 2015;172:784–792. doi: 10.1176/appi.ajp.2014.14081000. [DOI] [PubMed] [Google Scholar]
- Rivas-Grajales A.M., Savadjiev P., Kubicki M., Nestor P.G., Niznikiewicz M., McCarley R.W., Westin C.F., Shenton M.E., Levitt J.J. Striato-nigro-striatal tract dispersion abnormalities in patients with chronic schizophrenia. Brain Imaging Behav. 2018 doi: 10.1007/s11682-018-9934-9. [DOI] [PubMed] [Google Scholar]
- Rivas-Grajales A.M., Sawyer K.S., Karmacharya S., Papadimitriou G., Camprodon J.A., Harris G.J., Kubicki M., Oscar-Berman M., Makris N. Sexually dimorphic structural abnormalities in major connections of the medial forebrain bundle in alcoholism. Neuroimage Clin. 2018;19:98–105. doi: 10.1016/j.nicl.2018.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romaniuk L., Honey G.D., King J.R., Whalley H.C., McIntosh A.M., Levita L., Hughes M., Johnstone E.C., Day M., Lawrie S.M., Hall J. Midbrain activation during pavlovian conditioning and delusional symptoms in schizophrenia. Arch Gen Psychiatry. 2010;67:1246–1254. doi: 10.1001/archgenpsychiatry.2010.169. [DOI] [PubMed] [Google Scholar]
- Salminen T., Martensson J., Schubert T., Kuhn S. Increased integrity of white matter pathways after dual n-back training. Neuroimage. 2016;133:244–250. doi: 10.1016/j.neuroimage.2016.03.028. [DOI] [PubMed] [Google Scholar]
- Savadjiev P., Rathi Y., Bouix S., Smith A.R., Schultz R.T., Verma R., Westin C.F. Fusion of white and gray matter geometry: a framework for investigating brain development. Med Image Anal. 2014;18:1349–1360. doi: 10.1016/j.media.2014.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaepfer T.E., Bewernick B.H., Kayser S., Madler B., Coenen V.A. Rapid effects of deep brain stimulation for treatment-resistant major depression. Biol Psychiatry. 2013;73:1204–1212. doi: 10.1016/j.biopsych.2013.01.034. [DOI] [PubMed] [Google Scholar]
- Schlagenhauf F., Sterzer P., Schmack K., Ballmaier M., Rapp M., Wrase J., Juckel G., Gallinat J., Heinz A. Reward feedback alterations in unmedicated schizophrenia patients: relevance for delusions. Biol Psychiatry. 2009;65:1032–1039. doi: 10.1016/j.biopsych.2008.12.016. [DOI] [PubMed] [Google Scholar]
- Scholz J., Klein M.C., Behrens T.E., Johansen-Berg H. Training induces changes in white-matter architecture. Nat Neurosci. 2009;12:1370–1371. doi: 10.1038/nn.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoretsanitis G., Kutynia A., Stegmayer K., Strik W., Walther S. Keep at bay!–Abnormal personal space regulation as marker of paranoia in schizophrenia. Eur Psychiatry. 2016;31:1–7. doi: 10.1016/j.eurpsy.2015.10.001. [DOI] [PubMed] [Google Scholar]
- Sheehan D.V., Lecrubier Y., Sheehan K.H., Amorim P., Janavs J., Weiller E., Hergueta T., Baker R., Dunbar G.C. The mini-international neuropsychiatric interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33. quiz 34-57. [PubMed] [Google Scholar]
- Smith S.M., Jenkinson M., Johansen-Berg H., Rueckert D., Nichols T.E., Mackay C.E., Watkins K.E., Ciccarelli O., Cader M.Z., Matthews P.M., Behrens T.E. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Song S.K., Sun S.W., Ramsbottom M.J., Chang C., Russell J., Cross A.H. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage. 2002;17:1429–1436. doi: 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- Speechley W.J., Whitman J.C., Woodward T.S. The contribution of hypersalience to the "jumping to conclusions" bias associated with delusions in schizophrenia. J Psychiatry Neurosci. 2010;35:7–17. doi: 10.1503/jpn.090025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele C.J., Bailey J.A., Zatorre R.J., Penhune V.B. Early musical training and white-matter plasticity in the corpus callosum: evidence for a sensitive period. J Neurosci. 2013;33:1282–1290. doi: 10.1523/JNEUROSCI.3578-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stegmayer K., Horn H., Federspiel A., Razavi N., Bracht T., Laimbock K., Strik W., Dierks T., Wiest R., Muller T.J., Walther S. Supplementary motor area (SMA) volume is associated with psychotic aberrant motor behaviour of patients with schizophrenia. Psychiatry Res. 2014;223:49–51. doi: 10.1016/j.pscychresns.2014.05.002. [DOI] [PubMed] [Google Scholar]
- Stegmayer K., Horn H., Federspiel A., Razavi N., Bracht T., Laimbock K., Strik W., Dierks T., Wiest R., Muller T.J., Walther S. Ventral striatum gray matter density reduction in patients with schizophrenia and psychotic emotional dysregulation. Neuroimage Clin. 2014;4:232–239. doi: 10.1016/j.nicl.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stegmayer K., Strik W., Federspiel A., Wiest R., Bohlhalter S., Walther S. Specific cerebral perfusion patterns in three schizophrenia symptom dimensions. Schizophr Res. 2017;190:96–101. doi: 10.1016/j.schres.2017.03.018. [DOI] [PubMed] [Google Scholar]
- Steinau S., Stegmayer K., Lang F.U., Jager M., Strik W., Walther S. Comparison of psychopathological dimensions between major depressive disorder and schizophrenia spectrum disorders focusing on language, affectivity and motor behavior. Psychiatry Res. 2017;250:169–176. doi: 10.1016/j.psychres.2017.01.084. [DOI] [PubMed] [Google Scholar]
- Strik W., Stegmayer K., Walther S., Dierks T. Systems neuroscience of psychosis: mapping schizophrenia symptoms onto brain systems. Neuropsychobiology. 2017;75:100–116. doi: 10.1159/000485221. [DOI] [PubMed] [Google Scholar]
- Strik W., Wopfner A., Horn H., Koschorke P., Razavi N., Walther S., Wirtz G. The bern psychopathology scale for the assessment of system-specific psychotic symptoms. Neuropsychobiology. 2010;61:197–209. doi: 10.1159/000297737. [DOI] [PubMed] [Google Scholar]
- Stuber G.D., Klanker M., de Ridder B., Bowers M.S., Joosten R.N., Feenstra M.G., Bonci A. Reward-predictive cues enhance excitatory synaptic strength onto midbrain dopamine neurons. Science. 2008;321:1690–1692. doi: 10.1126/science.1160873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanes L.D., Mouchlianitis E., Wood T.C., Shergill S.S. White matter changes in treatment refractory schizophrenia: does cognitive control and myelination matter? Neuroimage Clin. 2018;18:186–191. doi: 10.1016/j.nicl.2018.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viher P.V., Stegmayer K., Giezendanner S., Federspiel A., Bohlhalter S., Wiest R., Strik W., Walther S. White matter correlates of the disorganized speech dimension in schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2018;268:99–104. doi: 10.1007/s00406-016-0753-y. [DOI] [PubMed] [Google Scholar]
- Von Der Heide R.J., Skipper L.M., Klobusicky E., Olson I.R. Dissecting the uncinate fasciculus: disorders, controversies and a hypothesis. Brain. 2013;136:1692–1707. doi: 10.1093/brain/awt094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker D.L., Miles L.A., Davis M. Selective participation of the bed nucleus of the stria terminalis and crf in sustained anxiety-like versus phasic fear-like responses. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:1291–1308. doi: 10.1016/j.pnpbp.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker D.L., Toufexis D.J., Davis M. Role of the bed nucleus of the stria terminalis versus the amygdala in fear, stress, and anxiety. Eur J Pharmacol. 2003;463:199–216. doi: 10.1016/s0014-2999(03)01282-2. [DOI] [PubMed] [Google Scholar]
- Woods S.W. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry. 2003;64:663–667. doi: 10.4088/jcp.v64n0607. [DOI] [PubMed] [Google Scholar]
- Wu M., Chang L.C., Walker L., Lemaitre H., Barnett A.S., Marenco S., Pierpaoli C. Comparison of epi distortion correction methods in diffusion tensor MRI using a novel framework. Med Image Comput Comput Assist Interv. 2008;11:321–329. doi: 10.1007/978-3-540-85990-1_39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Stein E.A., Hong L.E. Smoking and schizophrenia independently and additively reduce white matter integrity between striatum and frontal cortex. Biol Psychiatry. 2010;68:674–677. doi: 10.1016/j.biopsych.2010.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X.Y., Wang J.J., Zhu J.N. Cerebellar fastigial nucleus: from anatomic construction to physiological functions. Cerebellum Ataxias. 2016;3:9. doi: 10.1186/s40673-016-0047-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.