Dear Editor,
Since human lymphoma cells have a specific antigen expression pattern according to cell types and stages of differentiation, immunotherapy has become a promising field in lymphoma treatment. CD30 is a type 1 transmembrane receptor that is consistently overexpressed on all stages of cells in classical Hodgkin lymphoma (HL) and anaplastic large-cell lymphoma (ALCL); meanwhile, on normal cells, CD30 expression is limited, indicating that CD30 is an ideal immunotherapeutic target for these lymphoma subtypes1,2. CD30 antibody–drug conjugate brentuximab vedotin (BV) has been applied either as a single agent or combining it with frontline regimens. Encouraging results with a high response rate and good safety profile were reported in newly diagnosed patients; on the other hand, in refractory/relapse (r/r) patients, although the overall reaction rate of BV was still impressive, the CR rate seemed unsatisfactory3,4. The same situation was faced when anti-PD-1 antibody was used in r/r HL patients5,6. Therefore, there is still a challenge to treat r/r patients with these targeted therapy drugs. Recently, a new immunotherapy technique, named chimeric antigen receptor (CAR) T cell therapy, was developed7. CAR-T therapy targeting CD19 has been reported with exciting results in r/r B cell acute lymphoblastic leukemia (B-ALL), and also demonstrated efficacy in r/r B cell non-Hodgkin lymphomas (B-NHL)8,9.
Based on the effectiveness of the CAR-T system and the specificity of CD30 expression, we designed a new third-generation anti-CD30 CAR, and conducted a pilot study to test the efficacy and safety of anti-CD30 CAR-T cell therapy on r/r CD30+ lymphoma patients. Study design, data collection, and analysis are described in the Supplementary Methods. This study design was approved by the institutional review board of Tongji Hospital, Tongji Medical College, and Huazhong University of Science and Technology, and registered with the Chinese Clinical Trial Registry (ChiCTR, number ChiCTR-OPN-16009069). All patients provided written informed consent before enrollment in accordance with the Declaration of Helsinki.
From 1 September 2016 to 1 November 2018, nine r/r patients, including six HL and three ALCL patients, were enrolled in this study (Supplementary Fig. S1). All the nine patients were confirmed with CD30+ lymphoma by immunohistochemistry. The previous regimens included multiple lines of chemotherapy, radiotherapy, or even autologous transplantation. However, the disease status before CD30 CAR-T infusion was two patients with partial remission (PR), two patients with stable disease (SD), and five patients with progressive disease (PD) (Table 1). Three of the PD patients had extremely high tumor burden with multiple extranodal involvements before infusion. The detailed clinical characteristics are shown in Supplementary Table S1. The patients were given the FC (fludarabin 25 mg/m2 and cyclophosphamide 20 mg/kg for 3 days) regimen for lymphodepletion 4 days before CAR-T cell infusion. According to previous reports, the dose of CD30 CAR-T cell could be up to 2 × 107 per kg, which was much higher than that reported in CD19 CAR-T trials10,11. Besides, patients’ general conditions were also taken into consideration. As a result, a total dose of 0.7–3.2 × 107 per kg of CAR-T cells was infused, with a median dose of 1.4 × 107 cells per kg. No acute adverse events (AEs) were observed during CAR-T cell infusion. We observed manifestations of patients, and monitored the inflammatory cytokines continuously after CAR-T cell infusion.
Table 1.
Clinical characteristics of patients.
| Patient no. | Diagnosis stage at entry | Previous treatment | Disease status at entry | CAR+ T cells infused per kg | CRS grade | CRES | Best response | Current outcome |
|---|---|---|---|---|---|---|---|---|
| 1 | NS HL, IIB | ABVD, BEACOPP, BV, DHAP, PD-1 antibody, RT, and ASCT | SD | 1 × 107 | 0 | None | CR × 29 months | Relapsed, dead from infection |
| 2 | NS HL, IIA | ABVD, PD-1 antibody, and RT | PD | 1.15 × 107 | 1 | None | CR × 38+ months | Alive, NED |
| 3 | ALCL, IIB | CHOP, CHOPE | PR | 7 × 106 | 0 | None | CR × 28+ months | Alive, NED |
| 4 | NS HL, IVEB | ABVD, ICE, GDP, RT, and ASCT | PD | 1.2 × 107 | 5 | None | NA | Dead from pleural hemorrhage |
| 5 | NS HL, IVEB | ABVD, GDP, DHAP, PD-1 antibody, and RT | PD | 2 × 107 | 2 | None | CR × 10 weeks | Relapsed, alive |
| 6 | ALCL, IVB | CHOP, CHOPE, hyper-CVAD A + B, and RT | PD | 3.2 × 107 | 3 | None | SD × 6 weeks | Dead from infection |
| 7 | ALCL, IIA | CHOP, CHOPE, and DHAP | PR | 2.45 × 107 | 0 | None | CR × 16 + months | Alive, NED |
| 8 | NS HL, IIIEA | ABVD, ICE, PD-1 antibody, and RT | SD | 1.4 × 107 | 1 | None | CR × 11 months | Relapsed, alive |
| 9 | NS HL, IIB | ABVD, R-DHAP, PD-1 antibody, and RT | PD | 3.1 × 107 | 1 | None | CR × 13 months | Relapsed, alive |
ABVD doxorubicin, bleomycin, vinblastine, dacarbazine, ALCL anaplastic large-cell lymphoma, ASCT autologous stem cell transplant, BEACOPP bleomycin, etoposide, doxorubicin, clophosphamide, vincristine, procarbazine, prednisone, BV brentuximab vedotin, CHOP(E) cyclophosphamide, doxorubicin, vincristine, prednisone (etoposide), CR complete remission, CRES CAR-T-related encephalopathy syndrome, CRS cytokine release syndrome, DHAP dexamethasone, high-dose cytarabine, cisplatin, GDP gemcitabine, dexamethasone, cisplatin, hyper-CVAD A cyclophosphamide, vincristine, doxorubicin, dexamethasone, hyper-CVAD ethotrexate, cytarabine, ICE ifosfamide, carboplatin, etoposide, NED no evidence of disease, NS HL nodular sclerosis Hodgkin lymphoma, PR partial remission, PD progressive disease, RT radiation therapy, SD stable disease.
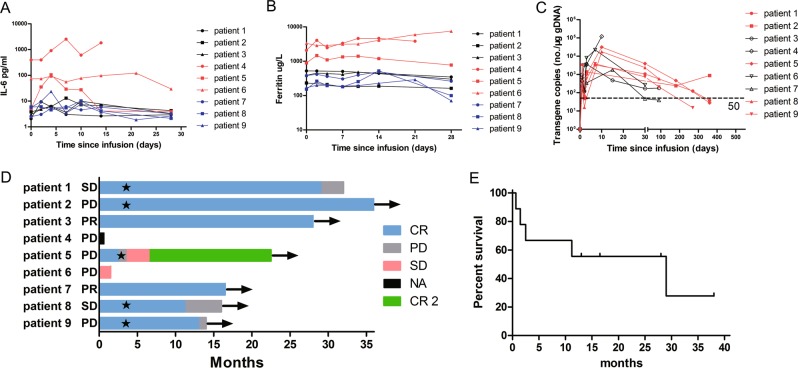
Six (66.7%) patients experienced CRS, four were of low-grade (grade 1–2), and no CAR-T-related encephalopathy syndrome was observed in all the nine patients (Table 1). All AEs are evaluated in Supplementary Table S2. The three patients, who had higher tumor burden as mentioned, experienced more severe AEs. They experienced remittent high fever, hypotension, hypoxia, poor physical status, and elongated time to recovery from grade 3/4 cytopenia (Supplementary Table S3). They had obviously higher IL-6 and ferritin levels, from baseline to peak, throughout the treatment course (Fig. 1a, b). One (#4) of the three patients had progressive peritoneal and pleural effusion, in which a large amount of CD30 CAR-T cells was detected, implicating a continuous recruitment and expansion of CAR-T cells in tumor tissue. Unfortunately, he died at day 20, 3 h after an uncontrollable sudden pleural hemorrhage, which caused continuous hypotension and hypoxia. Given the fact that before hemorrhage, the pleural effusion was clear and CRS grading was grade 2 (Supplementary Table S4), we inferred that he died from hypovolemic shock and severe pulmonary atelectasis caused by the hemothorax. The source of hemorrhage might be an intratumoral vessel, since the patient had multiple pulmonary and pleural infiltration loci. On the other hand, the six patients who had lower tumor burden experienced only mild fever or nausea, and cytopenia was recovered within 2 weeks. Skin rash similar with inflammatory purpura was observed in two patients (Supplementary Table S2). No other AEs were recorded spanning the period of hospitalization. There was also no significant elevation of inflammatory cytokines for these six patients, with the maximum fold change from baseline less than five (Fig. 1a, b). Although the grade of AEs and CRS seemed parallel with tumor burden (Supplementary Fig. S2), most of the AEs were mild and controllable in general. The discrepancies of CRS inferred that tumor burden might be a risk factor of more severe CRS. In addition, the tumor infiltration of vital organs might result in lethal complications.
Fig. 1. Patients’ characteristics and survival after CD30 CAR-T cell infusion.
a The serum IL-6 level of each patient was assessed before and at serial time points after cell infusion; the red lines representing the three patients with higher tumor burden were much higher than the others. b The serum ferritin level of each patient was assessed before and at serial time points after cell infusion; the red lines representing the three patients with higher tumor burden were much higher than the others. c The copies of anti-CD30 CAR transgenes, the red lines representing the five patients who received anti-PD-1 antibody last longer; the horizontal line denotes the lower limit of quantitation (50 copies/μg). d Clinical responses for the nine patients. On the left of the Y axis is the disease status before infusion; arrows indicate alive; the star marker indicated the start time of anti-PD-1 antibody therapy. Patient #5 received autologous transplantation 4 months after PD, and achieved CR again (CR2). e Progression-free survival of all nine patients. NA not applicable, PD progression of disease, SD stable disease, PR partial response, CR complete response.
All patients were kept in the hospital after infusion, until there was neither evidence of infection nor leukocytopenia, and then returned monthly to have treatment response evaluated. The assessment of treatment response was carried out by CT/MRI/PET-CT scan at each visit. Excitingly, seven patients successfully achieved CR at the first visit. Relapse was observed in four patients (#5, #8, #9, and #1) after 10 weeks, 11, 13, and 28 months of CR, respectively (Fig. 1d). The median progression-free survival for all the nine patients was 13 months, with three long-term CRs over 2 years (Fig. 1d). The expansion of CAR-T cells was monitored by droplet digital polymerase chain reaction (ddPCR); most patients had persistent lentiviral copies for half a year (Fig. 1c). This result was hitherto one of the best responses to CD30 CAR therapy ever reported.
In comparison with the encouraging results achieved by CD19 CAR on B-ALL, there is little to report on anti-CD30 CAR-T cell therapy10,11. The results of existing phase I studies were unsatisfactory, with the best response being three complete remissions (CR) out of nine patients10. The reason for the poor response may be a lack of lymphodepletion, efficiency of CAR itself, or other unrevealed factors. In this study, we used FC regimen as lymphodepletion, which may, to some extent, exert slight tumor-reductive activities. However, we believed that the good response was due to our newly designed CD30 CAR, not the preceding chemotherapy, since all patients had refractory/relapse disease from previous cytotoxic chemotherapy (Supplementary Table S1). Our CD30 CAR contained two costimulatory domains from CD28 and 4-1BB (Supplementary Methods, Fig. S3). CAR-T cells by using CD28 as a costimulator have enhanced activation and faster proliferation, while 4-1BB endodomain results in a lower rate of T cell exhaustion, and can promote the persistence of CAR-T cells. The combination of the two costimulatory domains can both facilitate CAR-T cell proliferation, and elongate CAR-T cell existence in vivo12,13.
We also tested the possibility of combining anti-PD-1 antibody with anti-CD30 CAR-T cell therapy. Previous studies showed that anti-PD-1 antibody therapy had a high overall reaction rate, but a poor CR rate around 20% in phase II studies of r/r HL14,15. In our study, five of the HL patients had already received no less than three courses of anti-PD-1 antibody in previous treatment, but the response was two SD and three PD. After CD30 CAR infusion, anti-PD-1 antibody treatment was applied from day 90 or from the time when the patient was found to have progression of disease before day 90. Interestingly, progression of the relapsed patient (#5) was well controlled, and the other four patients remained in CR state for at least another 8 months after anti-PD-1 therapy started (Supplementary Table S5). The ddPCR also showed longer persistence of lentiviral copies in those five patients who received anti-PD-1 antibody therapy (Fig. 1c). These results indicated a synergetic effect of our CD30 CAR-T and the following anti-PD-1 antibody therapy.
Taken together, although the scale was limited, we showed the safety and efficacy of our CD30 CAR. We believe that our work will provide a new option for treatment of CD30+ lymphomas.
Supplementary information
Acknowledgements
We would like to thank all members of the Department of Hematology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology for their support. We also appreciate Wuhan Bio-Raid Biotechnology Co., Ltd for their service in cell manufacturing. This work is supported by the funding from the Key Program of the National Natural Science Foundation of China (81830008 and 81630006 to J.-F.Z.), the National Natural Science Foundation of China (81300410 to D.W.; 81873452 to C.-R.L.; 81600120 to N.W.; 81670152 to L.H.; 81873444 to Y.X.; 81570196 to J.-F.Z.), the Milstein Medical Asian American Partnership Foundation (2018 MMAAP Foundation Hematology Fellowship Award to L.H.), and the Applied Basic Research Project of Wuhan City (2017060201010156 to Y.X.).
Author contributions
C.-R.L., Y.-C.Z., Y.X. and J.-F.Z. were responsible for conception and design of this study; D.W., B.X., J.-H.X., L.-J.J., J.W. and Q.-X.W. performed the clinical research; C.Z., N.W., and L.H. performed laboratory work for this study; D.W., C.Z., Q.-X.W., N.W., and L.H. were responsible for acquisition and analysis of clinical data and statistical analysis; Y.-C.Z., Y.X. and J.-F.Z. coordinated the study and revised it critically for important intellectual content; D.W. wrote the paper.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Y. Xiao, Email: yixiao@tjh.tjmu.edu.cn
J.-F. Zhou, Email: jfzhou@tjh.tjmu.edu.cn
Supplementary information
Supplementary Information accompanies this paper at (10.1038/s41408-020-0274-9).
References
- 1.Horie R, Watanabe T. CD30: expression and function in health and disease. Semin. Immunol. 1998;10:457–470. doi: 10.1006/smim.1998.0156. [DOI] [PubMed] [Google Scholar]
- 2.Chiarle R, et al. CD30 in normal and neoplastic cells. Clin. Immunol. 1999;90:157–164. doi: 10.1006/clim.1998.4636. [DOI] [PubMed] [Google Scholar]
- 3.Younes A, et al. Brentuximab vedotin (SGN-35) for relapsed CD30-positive lymphomas. N. Engl. J. Med. 2010;363:1812–1821. doi: 10.1056/NEJMoa1002965. [DOI] [PubMed] [Google Scholar]
- 4.Gopal AK, et al. Durable remissions in a pivotal phase 2 study of brentuximab vedotin in relapsed or refractory Hodgkin lymphoma. Blood. 2015;125:1236–1243. doi: 10.1182/blood-2014-08-595801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ansell SM, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N. Engl. J. Med. 2015;372:311–319. doi: 10.1056/NEJMoa1411087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Armand P, et al. Programmed death-1 blockade with pembrolizumab in patients with classical hodgkin lymphoma after brentuximab vedotin failure. J. Clin. Oncol. 2016;34:3733–3739. doi: 10.1200/JCO.2016.67.3467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dotti G, Gottschalk S, Savoldo B, Brenner MK. Design and development of therapies using chimeric antigen receptor-expressing T cells. Immunol. Rev. 2014;257:107–126. doi: 10.1111/imr.12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park JH, et al. Long-term follow-up of CD19 CAR therapy in acute lymphoblastic leukemia. N. Engl. J. Med. 2018;378:449–459. doi: 10.1056/NEJMoa1709919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schuster SJ, et al. Chimeric antigen receptor T cells in refractory B-cell lymphomas. N. Engl. J. Med. 2017;377:2545–2554. doi: 10.1056/NEJMoa1708566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ramos CA, et al. Clinical and immunological responses after CD30-specific chimeric antigen receptor-redirected lymphocytes. J. Clin. Invest. 2017;127:3462–3471. doi: 10.1172/JCI94306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang CM, et al. Autologous T cells expressing CD30 chimeric antigen receptors for relapsed or refractory hodgkin lymphoma: an open-label phase I trial. Clin. Cancer Res. 2017;23:1156–1166. doi: 10.1158/1078-0432.CCR-16-1365. [DOI] [PubMed] [Google Scholar]
- 12.Karlsson H, et al. Evaluation of intracellular signaling downstream chimeric antigen receptors. PLoS ONE. 2015;10:e0144787. doi: 10.1371/journal.pone.0144787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramos CA, et al. In vivo fate and activity of second- versus third-generation CD19-specific CAR-T cells in B cell non-Hodgkin’s lymphomas. Mol. Ther. 2018;26:2727–2737. doi: 10.1016/j.ymthe.2018.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen R, et al. Phase II study of the efficacy and safety of pembrolizumab for relapsed/refractory classic Hodgkin lymphoma. J. Clin. Oncol. 2017;35:2125–2132. doi: 10.1200/JCO.2016.72.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Armand P, et al. Nivolumab for relapsed/refractory classic Hodgkin lymphoma after failure of autologous hematopoietic cell transplantation: extended follow-up of the Multicohort Single-Arm Phase II CheckMate 205 Trial. J. Clin. Oncol. 2018;36:1428–1439. doi: 10.1200/JCO.2017.76.0793. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.