Abstract
Afterglow luminescent probes with high signal-to-background ratio show promise for in vivo imaging; however, such probes that can be selectively delivered into target sites and switch on afterglow luminescence remain limited. We optimize an organic electrochromic material and integrate it into near-infrared (NIR) photosensitizer (silicon 2,3-naphthalocyanine bis(trihexylsilyloxide) and (poly[2-methoxy-5-(2-ethylhexyloxy)-1,4-phenylenevinylene]) containing nanoparticles, developing an H2S-activatable NIR afterglow probe (F12+-ANP). F12+-ANP displays a fast reaction rate (1563 ± 141 M−1 s−1) and large afterglow turn-on ratio (~122-fold) toward H2S, enabling high-sensitivity and -specificity measurement of H2S concentration in bloods from healthy persons, hepatic or colorectal cancer patients. We further construct a hepatic-tumor-targeting and H2S-activatable afterglow probe (F12+-ANP-Gal) for noninvasive, real-time imaging of tiny subcutaneous HepG2 tumors (<3 mm in diameter) and orthotopic liver tumors in mice. Strikingly, F12+-ANP-Gal accurately delineates tumor margins in excised hepatic cancer specimens, which may facilitate intraoperative guidance of hepatic cancer surgery.
Subject terms: Bioanalytical chemistry, Nanoparticles, Nanoparticles
Afterglow imaging probes are of interest for in vivo imaging due to high signal-to-background ratios. Here, the authors report on an afterglow nanoparticle which is activated by hydrogen sulphide and demonstrate the measurement of hydrogen sulphide levels and the labelling of hepatic cancer specimens in vivo.
Introduction
Fluorescence imaging probes are indispensable in biomedical research and clinical diagnosis owing to their low cost, simple operation, and noninvasiveness; these characteristics enable real-time detection of physiological and pathological processes at the molecular and cellular levels1–4. However, because fluorescence imaging requires real-time light excitation, the inevitable light absorption, scattering, and autofluorescence of biological tissues can limit penetration depth and elicit a low signal-to-background ratio (SBR), compromising the sensitivity for in vivo imaging5–7. Afterglow luminescent probes (also called persistent luminescent probes), which can trap excitation energy in defects and slowly release photons after cessation of light excitation, have recently emerged as promising tools for overcoming the limitations of fluorescence probes in biosensing and molecular imaging8–11. Afterglow imaging with these luminescent probes does not require real-time excitation with an external light, causing no autofluorescence interference from biological tissues and offering greatly improved SBR and sensitivity to visualize disease sites in vivo12–15.
During the past few years, several prominent afterglow probes have been developed using rare-earth heavy metal ion (e.g., europium or praseodymium)-doping inorganic nanoparticles9,16–20 or organic and polymeric afterglow materials4,21–24. Richard and co-workers25 optimized chromium-doped zinc gallate (ZnGa1.995Cr0.005O4) to develop inorganic nanoparticles that can emit intense long-lasting near-infrared (NIR) afterglow luminescence for noninvasive tracking of labeled cells and imaging of vascularization and grafted tumor cells. Pu and co-workers26 proposed poly[2-methoxy-5-(2-ethylhexyloxy)-1,4-phenylenevinylene] (MEH-PPV)-based semiconducting polymer nanoparticles (SPNs) to develop biocompatible organic afterglow probes for sensitive detection of tumor metastasis and monitoring of hepatotoxicity in vivo26. Despite encouraging results, these afterglow probes encounter limitations when applied for in vivo diagnosis. Inorganic nanoparticle-based afterglow probes may be hampered by systemic toxicity concerns related to potential leakage of heavy metal ions27,28. Current MEH-PPV-based organic afterglow probes could be compromised by poor targeting ability to facilitate efficient delivery and accumulation in disease sites (e.g., tumors) after systemic injection29–31. In addition, most afterglow probes are designed as “always-on” luminescent probes; a continuous signal may produce a high background signal regardless of proximity or interaction with the molecular target of interest, thereby interfering with sensitivity and real-time imaging capacity13,25,27,29. By contrast, activatable afterglow luminescent probes with “off–on” signals in response to a biological target of interest can substantially reduce the background signal, improving SBR for real-time imaging. However, given a lack of effective activation approaches, activatable afterglow probes have been rarely developed for in vivo imaging26,32.
Hydrogen sulfide (H2S) is a highly reactive endogenous signaling molecule, playing a critical role in diverse physiological functions33–35. Aberrant concentration and tissue distribution of H2S are associated with many diseases (e.g., liver inflammation, hypertension, diabetes, and cancers)36–39. Therefore, precise spatiotemporal detection of H2S in living subjects is essential to the study of the biological functions and accurate diagnosis of H2S-related diseases. Although numerous H2S-activatable fluorescent probes have been developed, the insufficient reaction kinetics, poor in vivo targeting ability, and limited tissue penetration of fluorescence have restricted the capacity to detect H2S’ concentrations and locations in living animals40–46. Recently, we reported the engineering of an organic π-electron structure-based electrochromic material (EM 12+) as an H2S-responsive chromophore, which was amenable to build H2S-activatable NIR fluorescent probes for noninvasive imaging of H2S in living mice47.
In this paper, we optimize the structure of EM 12+ into a bis(pentafluorophenyl)-substituted EM F12+ that exhibits longer absorption wavelengths and significantly improved reaction kinetics toward H2S under physiological conditions. We showcase its feasibility to control afterglow luminescence from MEH-PPV (580 nm) and doping NIR photosensitizer (silicon 2,3-naphthalocyanine bis(trihexylsilyloxide), NIR775; 780 nm) when integrated into an MEH-PPV- and NIR775-containing afterglow nanoparticle (F12+-ANP). Significant activation of afterglow luminescence by a factor of ~122-fold can be achieved by incubating F12+-ANP with H2S within 1 min, followed by irradiation with an 808-nm laser, allowing for rapid quantification of the H2S concentration in blood samples of healthy persons and patients diagnosed with hepatic cancers (HCCs) or colorectal cancers (CRCs). Moreover, through incorporation of β-galactose (β-Gal) ligands on the surface, we build a hepatic tumor-targeting and H2S-activatable afterglow probe (F12+-ANP-Gal) that offers sensitive detection of subcutaneous and orthotopic liver tumors in living mice and delineation of liver tumor lesions in clinically excised HCC specimens. The demonstration of H2S-activatable afterglow luminescent probes feasible for sensitive and reliable detection of endogenous H2S underscores the potential of smart activatable afterglow luminescent probes for in vivo imaging.
Results
Design of H2S-activatable NIR afterglow probe
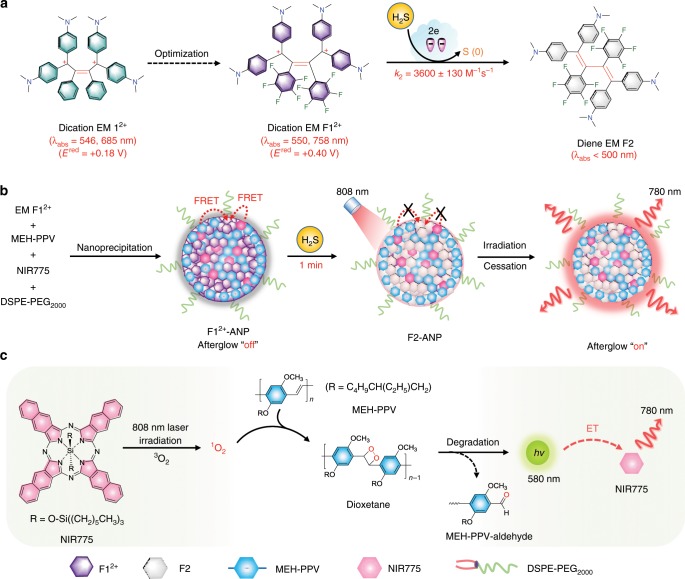
Figure 1 shows the design of an H2S-activatable NIR afterglow probe (F12+-ANP). NIR775 was chosen as an NIR-excitable photosensitizer due to its stronger ability to generate singlet oxygen (1O2) compared to MEH-PPV, amplifying the afterglow of MEH-PPV upon pre-irradiation by 808-nm light26,47. Furthermore, efficient intraparticle energy transfer (ET) from MEH-PPV to NIR775 can promote a red shift of afterglow wavelength into the NIR window, permitting improved penetration depth in biological tissues. We employed EM F12+ as a H2S-responsive chromophore because it is designed to have longer ultraviolet–visible–NIR (UV–visible–NIR) absorptions (550 and 758 nm) than EM 12+ (546 and 685 nm), ensuring extensive spectral overlaps with MEH-PPV and NIR775 emissions (Fig. 1a). Additionally, EM F12+ possesses a more positive reduction potential (Ered = + 0.40 V vs. SCE) than EM 12+ (+0.25 V vs. SCE), allowing for faster reaction to H2S via two-electron reduction. Within the compact nanoprobe F12+-ANP, the fluorescence and 1O2 generation ability of MEH-PPV and NIR775 are each quenched by EM F12+ due to efficient fluorescence resonance ET (FRET) from MEH-PPV and NIR775 to EM F12+; the afterglow of F12+-ANP is substantially quenched at the “off” state (Fig. 1b). In the presence of H2S, the EM F12+ can be rapidly reduced into diene EM F2 with distinct blue-shift absorption (λabs < 500 nm); the FRET process is hence eliminated, leading to recovery of 1O2 production in F2-ANP under 808-nm light irradiation. Therefore, NIR afterglow is activated at “on” state through 1O2-dependent oxidation of MEH-PPV, followed by an efficient intraparticle ET process. Ultralow background noise and deep-tissue penetration are achieved given advantages of long-lasting afterglow luminescence and rapid reaction kinetics. The probe thus presents a high SBR for rapid and sensitive detection of H2S levels in human blood samples, clinical liver tumor resections, and living mice.
Fig. 1. Schematic illustration of the design of F12+-ANP.
a Optimization of dication EM 12+ into F12+ by introducing two electron-withdrawing pentafluorophenyl groups, and proposed chemical conversion of F12+ into diene F2 upon reduction by H2S. b Preparation of F12+-ANP and proposed mechanism of H2S-mediated fast activation of NIR afterglow luminescence at 780 nm following pre-irradiation with an 808-nm laser. c The detailed photoreaction processes to produce NIR afterglow luminescence within activated F12+-ANP (i.e., F2-ANP). F12+-ANP was designed to contain hydrophobic EM F12+, MEH-PPV, and NIR775 via DSPE-PEG2000-assisted nanoprecipitation. F12+-ANP is initially afterglow “off” owing to the presence of F12+ that can quench both the fluorescence and 1O2 production of MEH-PPV and NIR775 via two efficient FRET processes. Upon reaction with H2S, F12+ was reduced into F2, and the FRET-based quenching processes were eliminated within F2-ANP, resulting in the recovery of fluorescence and 1O2 production capacity. Upon irradiation with the 808-nm laser, NIR775 can sensitize 3O2 to generate 1O2, which then oxidizes the vinylene bond of MEH-PPV to form unstable dioxetane. After cessation of irradiation, the subsequent slow degradation into MEH-PPV-aldehyde and releases photons at 580 nm, followed by efficient intraparticle ET to NIR775, ultimately switching “on” NIR afterglow luminescence at 780 nm.
Optimization of EM 12+ into EM F12+
We first optimized EM 12+ into EM F12+ by replacing the two phenyl rings with electron-withdrawing pentafluorophenyl rings (Fig. 1a). Density functional theory (DFT) calculation predicted that the introduction of two pentafluorophenyl groups would lead to an energetically lower-lying lowest unoccupied molecular orbital of F12+ (−8.1 eV) than 12+ (−7.8 eV), which was validated by cyclic voltammetry (CV) analysis (Supplementary Fig. 1c). The Ered of F12+ (+0.40 V vs. SCE) was higher than that of 12+ (+0.25 V vs. SCE), suggesting that F12+ could act as a stronger oxidant toward H2S. High-performance liquid chromatography (HPLC) analysis showed that purple F12+ could be efficiently reduced into light yellow diene F2 by NaHS (H2S donor) in phosphate-buffered saline (PBS) buffer (pH 7.4), resulting in a noticeable blue shift of UV–visible–NIR absorption from 550 and 758 nm to 430 nm (Supplementary Fig. 2b, c). The second-order reaction rate (k2) between F12+ and NaHS in PBS buffer (pH 7.4) was found to be 3600 ± 130 M−1 s−1, ~11.8-fold faster than that of 12+ (304 ± 8 M−1 s−1; k2 is defined as mean ± standard deviation from three independent measurements) (Supplementary Fig. 2d–i). Moreover, the introduction of electron-withdrawing groups contributed to a red shift of the NIR absorption band from 685 nm in 12+ to 758 nm in F12+, causing extensive spectral overlap between F12+ absorption and NIR775 emission (Supplementary Fig. 3a). Therefore, F12+ quenched NIR775 fluorescence more efficiently (~1428-fold) than 12+ (~740-fold) (Supplementary Fig. 3). F12+ was also highly stable following incubation in the PBS buffer (pH 7.4) for 7 days, and little photobleaching occurred upon irradiation with an 808-nm laser (1 W cm−2) for 3 min (Supplementary Fig. 4). These results indicate that F12+ was a more efficient H2S-responsive chromophore than 12+.
Preparation of F12+-ANP
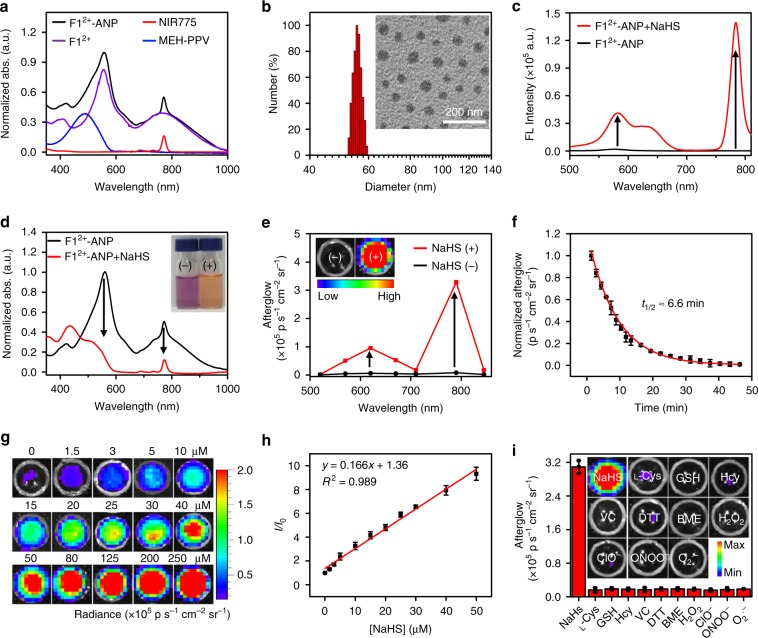
We then employed F12+ to prepare an H2S-activatable NIR afterglow luminescent probe (F12+-ANP) by doping it into NIR775 and MEH-PPV-based afterglow SPNs via DSPE-PEG-assisted encapsulation (Fig. 1b). The encapsulating ratio of MEH-PPV, NIR775, and F12+(BF4−)2 within F12+-ANP was optimized at ~28/2.2/58 (by mass) (Supplementary Fig. 5), unveiling characteristic absorption bands of MEH-PPV, NIR775, and F12+ (Fig. 2a). Dynamic light scattering (DLS) and transmission electron microscopy analysis revealed that F12+-ANP appeared as mono-disperse nanoparticles, with a mean hydrodynamic size of ~55 nm (Fig. 2b). As expected, the fluorescence of MEH-PPV (580 nm) and NIR775 (780 nm) within F12+-ANP was efficiently quenched by F12+ owing to extensive spectral overlaps between their fluorescence emissions and F12+ absorption (Fig. 2c). Fluorescence lifetime measurement revealed that the lifetimes of MEH-PPV and NIR775 within F12+-ANP were dramatically shortened as compared with that in the absence of F12+ (Supplementary Fig. 6), suggesting that efficient FRET processes occurred between MEH-PPV and F12+, and NIR775 and F12+, with ET efficiency estimated to be 97.1% and 99.9%, respectively (Supplementary Fig. 7 and Supplementary Note. 1). Upon incubation with NaHS (90 μM) in PBS buffer (pH 7.4) for 1 min, F12+ absorption above 500 nm within F12+-ANP nearly disappeared, and the solution color changed from violet to light orange (Fig. 2d). Accordingly, the initially quenched fluorescence at 580 and 780 nm were activated by H2S (Fig. 2c). The k2 between F12+-ANP and H2S in PBS buffer (pH 7.4) was 1563 ± 141 M−1 s−1 (Supplementary Fig. 8), much faster than other reported optical probes amenable for in vivo imaging of H2S (Supplementary Dataset 1).
Fig. 2. Characterization of F12+-ANP in vitro.
a Comparison of the UV–visible-NIR absorption spectra of F12+-ANP, F12+, NIR775, and MEH-PPV. b DLS and transmission electron microscopy (TEM) image (inset) of F12+-ANP. c Fluorescence and d absorption spectra of F12+-ANP (58/28/2.2 μg mL−1 F12+(BF4−)2/MEH-PPV/NIR775) in the absence or presence of NaHS (200 μM, 1 min). Inset: photographs of F12+-ANP before (−) and after (+) incubation with NaHS in PBS buffer. Fluorescence spectra was acquired by synchronous fluorescence scanning (λex = 400‒800 nm, offset = 100 nm). e Afterglow luminescence spectra and images (inset) of F12+-ANP (58/28/2.2 μg mL−1 F12+(BF4−)2/MEH-PPV/NIR775) with and without incubation with NaHS (200 μM) in PBS buffer (pH 7.4) at 37 °C for 1 min, followed by irradiation with 808-nm laser (1 W cm−2, 1 min). After cessation of laser, the afterglow images were acquired under an open filter, with an acquisition time of 60 s. f Decay of afterglow luminescence of H2S-activated F12+-ANP in PBS buffer at 37 °C. g Afterglow luminescence images of F12+-ANP (58/28/2.2 μg mL−1 F12+(BF4−)2/MEH-PPV/NIR775) upon incubation with varying concentrations of NaHS (0, 1.5, 3, 5, 10, 15, 20, 25, 30, 40, 50, 80, 125, 200, and 250 μM) at 37 °C for 1 min. h Plot of the afterglow luminescence intensity of F12+-ANP and the concentration of NaHS from 0 to 50 μM. i Afterglow luminescence intensities and images (inset) of F12+-ANP upon incubation with different reductants or ROS (200 µM NaHS, 1.25 mM l-cysteine (l-Cys), 10 mM glutathione (GSH), 1 mM homocysteine (Hcy), 1.25 mM ascorbic acid (VC), 1.25 mM dithiothreitol (DTT), 100 μM β-mercaptoethanol (BME), 1 mM H2O2, 1 mM ClO−, ONOO− (1 mM NaNO2 + 1 mM H2O2), O2.− (100 μM xanthine + 22 mU xanthine oxidase)) for 10 min. The solutions were then irradiated with the 808-nm laser (1 W cm−2, 1 min), and the NIR afterglow images were collected for 60 s with a 790 nm filter after the end of irradiation. Data denote mean ± standard deviation (s.d.) (n = 3). Source data are provided as a Source Data file.
As 1O2 is indispensable in oxidizing MEH-PPV into PPV dioxetanes, which subsequently decompose into MEH-PPV-aldehyde and emit afterglow luminescence (Fig. 1c), the 1O2 generation ability of F12+-ANP before and after reaction with NaHS was measured. Irradiation of F12+-ANP with the 808-nm laser (1 W cm−2, 1 min) induced little enhancement in singlet oxygen sensor green (SOSG) fluorescence, indicating low ability to produce 1O2 (Supplementary Fig. 9). However, irradiation of F12+-ANP after incubation with NaHS for 1 min produced remarkably enhanced SOSG fluorescence. Generated 1O2 could evoke MEH-PPV oxidation as evidenced by the gradually declining MEH-PPV absorption within activated F12+-ANP (i.e., F2-ANP) upon irradiation with the 808-nm laser (1 W cm−2) for 0–60 min (Supplementary Fig. 10).
We next investigated the afterglow luminescence of F12+-ANP in the absence or presence of NaHS in PBS buffer (pH 7.4) at 37 °C. After optimizing irradiation conditions (Supplementary Fig. 11), the solutions were pre-irradiated by the 808-nm laser at a power density of 1 W cm−2 for 1 min. F12+-ANP exhibited little afterglow in the absence of NaHS, whereas NaHS-treated F12+-ANP displayed obvious afterglow signals in the visible and NIR regions. Signal quantification from the images (Fig. 2e, inset) showed that afterglow intensities increased by ~122-fold. Notably, the afterglow signals in the NIR region were much higher than those in the visible region, presumably attributable to the ET process from MEH-PPV to NIR775 within activated F12+-ANP as demonstrated by the spectral overlaps between them (Supplementary Fig. 12a, b). The ET process between MEH-PPV and NIR775 was further confirmed by the shorter fluorescence lifetime of MEH-PPV in activated F12+-ANP (~49 ps) than that in the absence of NIR775 (~106 ps), with the ET efficiency of ~53.8% (Supplementary Fig. 12c and Supplementary Note. 2). These results suggest that H2S can effectively turn on the NIR afterglow luminescence of F12+-ANP. Through continuous acquisition of afterglow images, the afterglow could persist for over 40 min after laser cessation, with a half-life of ~6.6 min (Fig. 2f and Supplementary Fig. 13). Moreover, the afterglow signal could be recharged by multiple irradiation with the 808-nm laser; the afterglow signal of activated F12+-ANP was still ~50-fold higher than that of unactivated F12+-ANP after irradiation for 15 cycles over 3 days, while their size and morphology were negligibly changed after irradiation, reflecting the potential for long-term imaging (Supplementary Figs. 14 and 15).
We subsequently examined the sensitivity and specificity of F12+-ANP toward H2S (Fig. 2g, h). Afterglow images of F12+-ANP became brighter with NaHS concentration, showing a linear correlation between afterglow intensities and NaHS concentrations from 0 to 50 μM (limit of detection: ~0.1 μM (3σ/k). Only H2S could turn on the afterglow of F12+-ANP (Fig. 2i), consistent with those measured by fluorescence (Supplementary Fig. 16), demonstrating that F12+-ANP was a specific probe for H2S. In addition, F12+-ANP was highly stable under different physiological conditions (Supplementary Figs. 17 and 18); the fluorescence and afterglow of F12+-ANP were similarly activated by NaHS in different cell culture mediums (Supplementary Fig. 19) or in PBS buffers with pH ranging from 5.0 to 8.0 (Supplementary Fig. 20). Taking advantage of high-sensitivity, specificity, and ultrafast reaction kinetics toward H2S, F12+-ANP was applied to quantify H2S concentration in mouse blood (Supplementary Fig. 21). The H2S concentration in mouse whole blood was measured to be 24.8 ± 2.2 μM (the concentration is expressed as mean ± standard deviation of three samples) using NaHS as an internal standard, which was within reported values35,48,49.
Detection of H2S in HCC cells
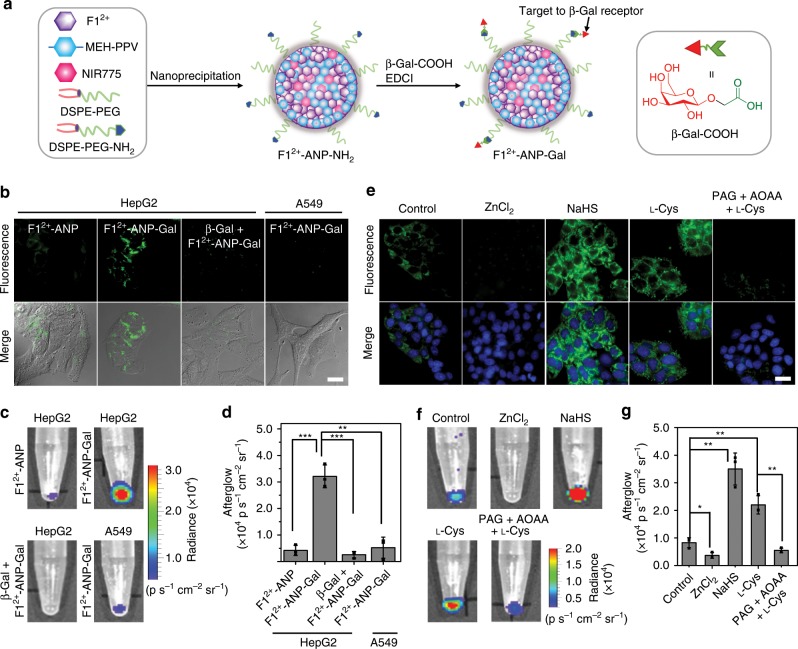
Since increased H2S level is found in malignant liver tumors50,51, we investigated the potential to detect H2S in human HCC HepG2 cells through afterglow imaging. The overexpression of cystathionine β-synthase (CBS) and cystathionine γ-lyase (CSE) for the biosynthesis of endogenous H2S in HepG2 cells was validated by Western Blot (WB) analysis (Supplementary Fig. 22). To enable efficient entry into HepG2 cells, β-Gal capable of targeting to β-Gal receptors overexpressed on HepG2 cells was introduced to the surface of F12+-ANP to prepare F12+-ANP-Gal (Fig. 3a). Akin to F12+-ANP, F12+-ANP-Gal manifested as mono-disperse nanoparticles in PBS buffer (pH 7.4) and exhibited significantly activated afterglow in response to H2S (Supplementary Fig. 23). F12+-ANP-Gal demonstrated low toxicity to HepG2 cells by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay, with little cell death observed before and after irradiation with the 808-nm laser (1 W cm−2, 1 min) (Supplementary Fig. 24). After optimizing incubation conditions (Supplementary Figs. 25 and 26), F12+-ANP-Gal could be taken up by HepG2 cells via β-Gal receptor-mediated endocytosis (Fig. 3b), resulting in intracellular fluorescence distributed mainly in lysosomes and mitochondria (Supplementary Fig. 27). We then collected cell pellets (~1 × 105 cells) and irradiated with the 808-nm laser (1 W cm−2, 1 min) to produce afterglow. As presented in Fig. 3c, HepG2 cells incubated with F12+-ANP-Gal showed a much brighter afterglow image compared to that of F12+-ANP, β-Gal-pre-treated HepG2 cells, or β-Gal receptor-deficient human lung cancer A549 cells incubated with F12+-ANP-Gal. The afterglow intensity in HepG2 cells incubated with F12+-ANP-Gal was more than 7-fold higher than that with F12+-ANP (Fig. 3d). We subsequently applied F12+-ANP-Gal to monitor H2S fluctuation in HepG2 cells (Fig. 3f, g). Afterglow signals in F12+-ANP-Gal-treated HepG2 cell pellets (~3 × 104 cells) increased noticeably when pre-treated with extraneous NaHS; pre-treatment with ZnCl2 to scavenge H2S reduced afterglow substantially. HepG2 cells stimulated with l-Cys (a precursor for biosynthesis of H2S) to upregulate intracellular H2S levels could augment afterglow, which was greatly inhibited when adding dl-propargylglycine (PAG; 50 μg mL−1) and aminooxyacetic acid (AOAA; 20 μM) to inhibit endogenous CSE and CBS activities. These results were confirmed by fluorescence imaging of H2S in individual HepG2 cells (Fig. 3e). We also found that afterglow signals in F12+-ANP-Gal-treated HepG2 cell pellets increased with the cell number, which could allow to detect 1000 and 5000 HepG2 cells in vitro and in vivo, respectively, much lower than fluorescence (Supplementary Figs. 28 and 29). Moreover, the afterglow luminescence of F12+-ANP-Gal could still be clearly observed in HepG2 cells even after irradiation for 12 cycles over 24 h, suggesting the potential for long-term afterglow imaging in live HepG2 cells (Supplementary Fig. 30). Thus, F12+-ANP-Gal was an efficient probe capable of sensitively detecting HepG2 cells through afterglow imaging of endogenous H2S.
Fig. 3. Preparation of F12+-ANP-Gal for imaging of H2S in cells.
a Schematic for the preparation of F12+-ANP-Gal. b Fluorescence imaging of HepG2 cells after incubation with F12+-ANP, F12+-ANP-Gal, F12+-ANP-Gal plus 20 mM free β-Gal, or A549 cells incubated with F12+-ANP-Gal for 3 h. Scale bar: 20 μm. c Afterglow luminescence images and intensities (d) of cells (~1 × 105 cells) treated with indicated conditions in b. e Fluorescence imaging of HepG2 cells incubated with F12+-ANP-Gal, F12+-ANP-Gal together with 300 µM ZnCl2, 1 mM NaHS, 200 μM l-Cys, or 200 μM l-Cys plus 50 mg L−1 PAG and 20 μM AOAA. Scale bar: 20 μm. f Afterglow luminescence images and intensities g of HepG2 cells (~3 × 104 cells) treated with indicated conditions in e. All the cells were incubated with F12+-ANP or F12+-ANP-Gal at a concentration of 58/28/2.2 μg mL−1 F12+(BF4−)2/MEH-PPV/NIR775 for 3 h. For afterglow luminescence imaging, the cell pellets were irradiated with the 808-nm laser (1 W cm−2) for 1 min. After cessation of laser, the afterglow images were acquired for 60 s with an open filter. Data denote mean ± s.d. (*P < 0.05, **P < 0.01, ***P < 0.001, n = 3). Statistical differences were analyzed by Student’s t test. Source data are provided as a Source Data file.
Afterglow imaging of H2S in subcutaneous liver tumors
Prior to afterglow imaging of H2S in vivo, the penetration depth of afterglow luminescence emitted from the H2S-activated F12+-ANP-Gal was examined. The afterglow and fluorescence signals each declined with the thickness of chicken tissues (Supplementary Fig. 31). However, because tissue autofluorescence was minimized without real-time excitation, the afterglow exerted significantly higher SBR than NIR fluorescence. At a thickness of ~4 cm, the SBR of afterglow remained as high as 18.7 ± 4.6 (SBR is expressed as mean ± standard deviation, n = 3), whereas the SBR of NIR fluorescence was close to 1. These results indicate that the penetration of afterglow luminescence was much deeper than that of NIR fluorescence, which was also confirmed by the larger SBR of afterglow (~201) versus fluorescence (~2.5) when detecting signals through a mouse body (~1.9 cm). We next determined the blood half-life (t1/2) of F12+-ANP-Gal following intravenous (i.v.) injection into mice. The t1/2 was measured to be ~7.5 h, permitting efficient circulation to facilitate delivery into tumor tissues. The biodistribution study showed that F12+-ANP-Gal accumulated mainly in the liver of healthy mice at 12 h post injection (%ID/g ≈ 23.6%, Supplementary Fig. 32).
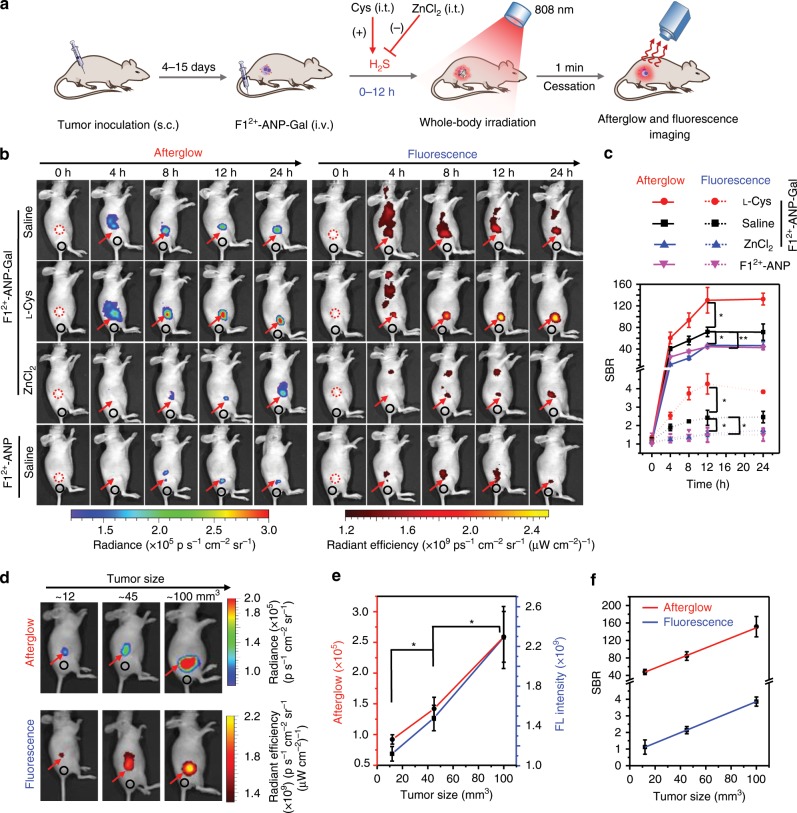
We next applied F12+-ANP-Gal for afterglow imaging of endogenous H2S in subcutaneous (s.c.) HepG2 tumors (Fig. 4a). After i.v. injection into mice, afterglow and fluorescence signals both increased gradually and peaked after 12 h (Fig. 4b and Supplementary Fig. 33). However, owing to the low background signal of afterglow, the SBR of afterglow was much higher than that of fluorescence (Fig. 4c). At 12 h, the average SBR of afterglow was 71.9 ± 8.8, ~30-fold higher than that of fluorescence (2.4 ± 0.4). When l-Cys was injected into tumors to upregulate H2S levels, afterglow and fluorescence signals were both significantly enhanced, whereas intratumoral (i.t.) injection of ZnCl2 to scavenge tumor H2S reduced tumor afterglow and fluorescence. The SBR of afterglow 12 h post injection of F12+-ANP-Gal and l-Cys increased to 133.8 ± 24.9, ~1.9- and ~2.9-fold higher than that of F12+-ANP-Gal alone and F12+-ANP-Gal plus ZnCl2 (46.7 ± 1.6), respectively. In addition, mice injected with untargeted F12+-ANP showed much lower afterglow signals and SBR (44.2 ± 3.1) in HepG2 tumors compared to F12+-ANP-Gal. These results aligned with ex vivo fluorescence imaging of resected tumor tissue slices (Supplementary Fig. 34), affirming that F12+-ANP-Gal could deliver into HepG2 tumors and efficiently detect tumor H2S levels.
Fig. 4. Afterglow imaging of H2S in s.c. HepG2 tumors.
a Schematic for noninvasive fluorescence and afterglow imaging of H2S in HepG2 tumor-bearing mice. b Afterglow (left) and fluorescence (right) imaging of HepG2 tumors in mice at 0, 4, 8, 12, and 24 h following i.v. injection of F12+-ANP-Gal or F12+-ANP in saline (saline), F12+-ANP-Gal with i.t. injection of l-Cys or ZnCl2. l-Cys (1 mM, 25 µL) or ZnCl2 (1 mM, 25 µL) was injected into tumors at 3.5 h post i.v. injection of F12+-ANP-Gal (211/100/8 μg F12+(BF4−)2/MEH-PPV/NIR775, 200 μL). c Quantification of SBRs for afterglow (solid) and fluorescence (dash) imaging of HepG2 tumors in mice treated with F12+-ANP-Gal or F12+-ANP alone, F12+-ANP-Gal plus l-Cys, or ZnCl2 at indicated time point. d Afterglow (up) and fluorescence (down) imaging of HepG2 tumors at size of ~12, ~45, and ~100 mm3 in mice at 12 h post i.v. injection of F12+-ANP-Gal. e Quantification of afterglow and fluorescence intensities of HepG2 tumors at different size. f Plots of the SBRs for afterglow and fluorescence imaging versus the tumor size revealed a strong correlation for F12+-ANP-Gal (Person’s r = 0.99). For afterglow imaging, the mouse body was irradiated with the 808-nm laser (1 W cm−2) for 1 min. After cessation of laser, the afterglow images were acquired for 60 s with an open filter. The fluorescence images were acquired with λex/em = 740/790 nm. Red arrows indicate the locations of HepG2 tumors in mice, and black circles indicate the background locations. Data denote mean ± s.d. (*P < 0.05, **P < 0.01, n = 3). Statistical differences were analyzed by Student’s t test. Source data are provided as a Source Data file.
We applied F12+-ANP-Gal to noninvasively monitor HepG2 tumor growth in living mice. After s.c. injection of HepG2 cells (2 × 106) at ~4, ~8, and ~15 days, average tumor sizes grew to ~12 (~2.8 × 2.9 mm2), ~45 (~4.4 × 4.5 mm2), and ~100 mm3 (~5.7 × 6.1 mm2), respectively. The afterglow and fluorescence signals of HepG2 tumors 12 h after i.v. injection of F12+-ANP-Gal increased with tumor size, and their SBRs paralleled those of tumor size (Fig. 4d, e). For a tumor measuring only ~12 mm3, the SBR of afterglow was 47.8 ± 5.9; conversely, the SBR of fluorescence was only 1.1 ± 0.4 (Fig. 4f). These results indicate that the afterglow emitted from F12+-ANP-Gal after activation by endogenous H2S was more appropriate than fluorescence when applied to detect tiny s.c. HepG2 tumors in vivo. Notably, we identified a strong correlation between the SBR of afterglow or fluorescence produced from F12+-ANP-Gal and apparent tumor size growth (Pearson’s r = 0.99). Therefore, F12+-ANP-Gal can offer valuable information about tumor growth in living mice.
Imaging of orthotopic liver tumors in mice
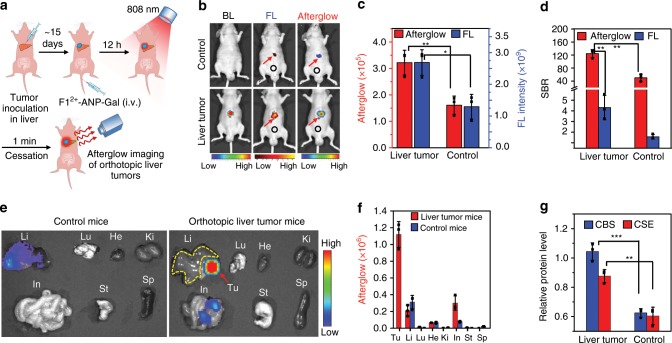
By virtue of the deep-tissue penetration of afterglow imaging, F12+-ANP-Gal was applied to detect orthotopic HepG2 liver tumors through afterglow imaging of H2S. Orthotopic liver tumors were established by inoculation of luciferase-transfected HepG2 cells (HepG2/Luc) into the lube of livers of mice (Fig. 5a). Strong bioluminescence (BL) in liver indicated the successful establishment of orthotopic liver tumors after 15 days (Fig. 5b). We then injected (i.v.) F12+-ANP-Gal into mice followed by irradiation of each mice body (face up) with the 808-nm laser (1 W cm−2, 1 min) after 12 h (Fig. 5a). NIR fluorescence and afterglow images both showed that mice with orthotopic liver tumors had brighter signals relative to control mice (Fig. 5b, c), with locations closely matching those of BL images. The SBR of afterglow in orthotopic liver tumors was 124.5 ± 12.5, 2.5-fold higher than that in livers of control mice (50.0 ± 10.9) (Fig. 5d). The SBR of afterglow was also much higher compared to that of fluorescence in liver tumors (4.3 ± 1.2). We subsequently conducted ex vivo imaging of main organs (e.g., liver, lung, heart, kidneys, intestines, stomach, and spleen) resected from control mice and orthotopic liver tumor mice. Figure 5e shows that tumor tissues in the resected liver exhibited the brightest afterglow image among all resected organs. The afterglow intensity in tumor tissues was ~5.3-fold higher than that in surrounding liver tissues and ~3.6-fold higher than normal liver tissues of control mice (Fig. 5f). NIR fluorescence imaging of the same tissues verified these findings (Supplementary Fig. 35), which were further confirmed via fluorescence imaging of tumor and normal tissue slices (Supplementary Fig. 36). Subsequent WB analysis revealed that enhanced afterglow and fluorescence in orthotopic HepG2/Luc tumors concurred with the higher expression of CBS and CSE enzymes compared to normal liver tissues (Fig. 5g and Supplementary Fig. 37). These findings demonstrate that F12+-ANP-Gal could offer a large SBR to accurately and noninvasively position orthotopic liver tumor foci via afterglow imaging of H2S.
Fig. 5. Noninvasive imaging of orthotopic liver tumors in mice.
a Schematic for afterglow imaging of orthotopic HepG2 tumors in living mice. b Bioluminescence (BL), fluorescence (FL), and afterglow imaging of control mice (Control) and orthotopic HepG2 tumor (liver tumor) bearing mice at 12 h post i.v. injection of F12+-ANP-Gal (211/100/8 μg F12+(BF4−)2/MEH-PPV/NIR775, 200 μL). c Afterglow (red) and FL (blue) intensities in the livers of control mice and orthotopic liver tumor-bearing mice. d Comparison of the SBRs for afterglow and fluorescence imaging of livers in control mice and orthotopic liver tumor-bearing mice. Red arrows indicate the locations of livers, and black circles indicate the background locations. e Representative ex vivo afterglow images of main organs (e.g., liver (Li), lung (Lu), heart (He), kidneys (Ki), intestines (In), stomach (St), spleen (Sp), and tumor (Tu)) resected from control mice (left) and orthotopic HepG2 tumor-bearing mice (right) at 12 h post i.v. injection of F12+-ANP-Gal. Red arrow and yellow dash box indicate the locations of HepG2 tumor in the liver and normal liver tissues chosen for region of interest (ROI), respectively. f Comparison of the average afterglow intensities of tumors and main organs resected from control (blue) and orthotopic liver tumor (red) mice. g WB analysis shows the relative CBS and CSE protein levels in the liver tissues resected from control mice and HepG2 tumor tissues resected from orthotopic liver tumor mice. All afterglow luminescence images were acquired for 60 s with an open filter, after pre-irradiation of mouse body or organs with the 808-nm laser (1 W cm−2, 1 min). All fluorescence images were acquired with λex/em = 740/790 nm. Data denote mean ± s.d. (*P < 0.05, **P < 0.01, n = 3). Statistical differences were analyzed by Student’s t test. Source data are provided as a Source Data file.
Detection of H2S in clinical blood samples
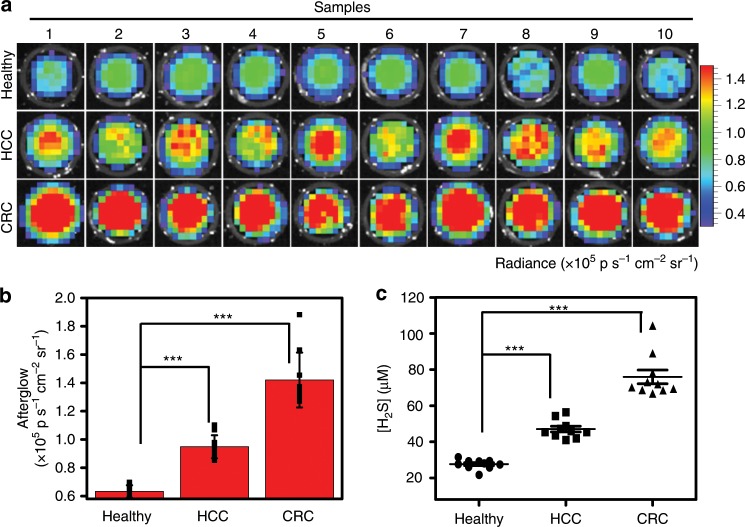
We employed the activatable afterglow to detect H2S levels in human blood samples collected from 10 healthy persons, 10 HCC patients, and 10 CRC patients in clinics. Following incubation of 2-fold diluted blood with F12+-ANP for 1 min, afterglow signals in HCC and CRC patients’ bloods were much higher than that in healthy persons (Fig. 6a, b). We took a standard curve established by the addition of NaHS into healthy persons’ blood as the internal standard, and the average H2S level in the whole blood of healthy persons was 27.6 ± 2.7 μM (the H2S level is expressed as mean ± standard deviation, n = 10); the level increased to 47.0 ± 5.0 and 75.9 ± 11.9 μM in the whole blood of HCC and CRC patients, respectively (Fig. 6c and Supplementary Fig. 38). These results revealed that blood H2S levels in HCC and CRC patients were upregulated compared to healthy persons, suggesting that H2S could serve as a potential blood marker for certain malignant cancers (e.g., HCC and CRC). Thus, F12+-ANP presents high potential to quantify blood H2S levels for cancer diagnosis.
Fig. 6. Detection of H2S in blood samples.
a Afterglow images of F12+-ANP in blood samples freshly collected from 10 healthy persons, 10 HCC patients, and 10 CRC patients. Freshly collected bloods were 2-fold diluted with PBS buffer (1×, pH 7.4), and then incubated with F12+-ANP (58/28/2.2 μg mL−1 F12+(BF4−)2/MEH-PPV/NIR775) at 37 °C for 1 min, followed by irradiation with the 808-nm laser (1 W cm−2) for 1 min. After removal of the laser, the afterglow luminescence images were immediately acquired for 60 s with an 790 nm filter. b Afterglow luminescence intensities of F12+-ANP in 2-fold diluted blood samples. c Quantification of H2S concentrations in whole blood of healthy persons and HCC or CRC patients. Data denote mean ± s.d. (***P < 0.001, n = 10). Statistical differences were analyzed by Student’s t test. Source data are provided as a Source Data file.
Detection of liver tumor tissues in clinical specimens
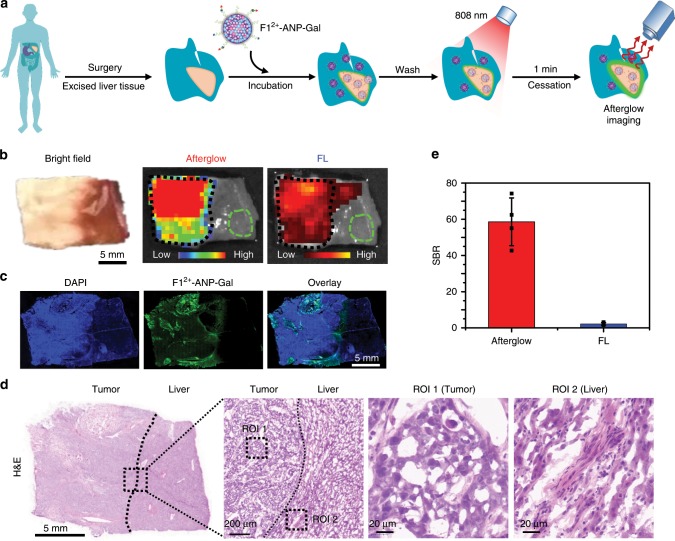
Having demonstrated the higher blood H2S levels in HCC patients’ bloods, we employed afterglow to delineate tumor margins in clinically excised HCC specimens. Excised liver tissues from four HCC patients were incubated with F12+-ANP-Gal in PBS buffer (pH 7.4) for 3 h to allow efficient internalization and activation by H2S. After being washed with PBS buffer, whole specimens were irradiated with the 808-nm laser (1 W cm−2, 1 min); the resulting afterglow and fluorescence images were acquired immediately (Fig. 7a). A strong afterglow image appeared in the left section of the specimen, corroborating the NIR fluorescence image of the tissue slice (Fig. 7b, c). Subsequent hematoxylin and eosin (H&E) staining revealed that the left section of the specimen was characterized by tumor tissue (Fig. 7d). Lesions with clear afterglow and fluorescence signals resulting from F12+-ANP-Gal staining accorded with the tumor location was indicated by H&E staining, as was observed in the other three specimens from different HCC patients (Supplementary Figs. 39–41). These results suggest that F12+-ANP-Gal was effective in delineating liver tumor lesions in clinical specimens. Upon dividing the signal intensity in tumor lesions by that in adjacent normal liver tissue, the average SBR in afterglow imaging of the four clinical specimens was 58.6 ± 13.2, significantly higher than that in fluorescence (2.2 ± 0.7) (Fig. 7e). Such a high afterglow SBR could promote detection of tumor lesions in the specimen covered with 1.5-cm-thick chicken tissue (Supplementary Fig. 42). These findings imply that F12+-ANP-Gal may have promise for detecting deeply located tumor cells, which could be applied to identify tumor margins and accurately guide liver tumor surgery in the future.
Fig. 7. Afterglow imaging of liver tumor tissues in HCC specimens.
a Schematic for afterglow imaging of tumor tissues in clinically excised liver specimens using F12+-ANP-Gal. b Representative photograph (bright field), afterglow, and FL images of the liver specimen resected from an HCC patient. The specimen was incubated with F12+-ANP-Gal (58/28/2.2 μg mL−1 F12+(BF4−)2/MEH-PPV/NIR775) in PBS buffer (1×, pH 7.4) at 37 °C for 3 h, and then rinsed with PBS buffer for three times. The whole specimen was irradiated with the 808-nm laser (1 W cm−2, 1 min). After cessation of the laser, the afterglow image was acquired under an open filter, with an acquisition time of 60 s. The fluorescence image was collected with λex/em = 740/790 nm. c Fluorescence images of liver tissue slices were dissected from the HCC specimen after incubation with F12+-ANP-Gal (green) for 3 h and stained with DAPI (blue). d H&E staining of the liver tissue slice dissected from the HCC specimen. Black dash boxes indicate the enlarged areas, in which box ROI 1 shows the tumor tissue and box ROI 2 indicates the normal liver tissue, respectively. e Quantitative analysis of the average SBRs for afterglow and fluorescence imaging of liver specimens resected from HCC patients. Data denote mean ± s.d. (n = 4). Black dash boxes in b indicate the location of tumors, and green dash boxes indicate the location of normal liver tissues selected as the background. Source data are provided as a Source Data file.
In vivo clearance and biosafety evaluations
To evaluate the in vivo clearance and biosafety, we injected (i.v.) F12+-ANP-Gal at the same dose as the imaging dose (211/100/8 μg F12+(BF4−)2/MEH-PPV/NIR775, 200 μL). The NIR fluorescence images showed that the NIR fluorescence was gradually observed in the liver, with intensity maximized at 12 h (Supplementary Fig. 43). After that, the NIR fluorescence in mice decreased overtime, and nearly returned to the background after 28 days, indicating that F12+-ANP-Gal could be cleared out after systemic administration. We then monitored the body weights, showing little difference between F12+-ANP-Gal-treated and saline-treated mice for 28 days (Supplementary Fig. 44a). Moreover, the subsequent biochemistry and hematology test of mice 1, 7, 14, and 28 days post injection of F12+-ANP-Gal showed that all the markers related to liver or kidney functions were within the reference range of healthy mice (Supplementary Fig. 44b, c); F12+-ANP-Gal did not cause obvious toxic effect on mice, which was further confirmed by the histologic analysis of resected main organs (e.g., liver, lung, kidney, heart, and spleen) (Supplementary Fig. 45).
Discussion
Afterglow luminescence holds great promise for sensitive and noninvasive imaging of biomolecules in living subjects26,31; however, real-time afterglow imaging remains rare due to lack of afterglow probes that can be rapidly activated by a molecular target to produce intense afterglow luminescence. Herein, we optimized the structure of an organic π-electron system-based EM 12+ into EM F12+ and engineered it as an effective H2S-responsive chromophore capable of integrating with NIR775 and MEH-PPV to develop H2S-activatable NIR afterglow probe (F12+-ANP) for in vivo imaging. F12+ with two electron-withdrawing pentafluorophenyl groups showed strong absorptions at 550 and 758 nm, overlapping more extensively with MEH-PPV (580 nm) and NIR775 emissions (780 nm) than 12+. F12+ also possessed a more positive value of Ered (+0.40 V vs. SCE) than 12+ (+0.25 V vs. SCE), permitting much faster reaction kinetics toward H2S (k2 = 3600 ± 130 M−1 s−1). These features offer F12+ noteworthy advantages over 12+ in designing activatable afterglow probes. The longer absorption of F12+ could (a) allow it to prevent NIR775 from generating 1O2, which is key to eliciting afterglow luminescence (Supplementary Fig. 9) and (b) directly quench visible and NIR afterglow luminescence of F12+-ANP. Accordingly, afterglow signals could be substantially quenched in an “off” state. Upon reaction with H2S, rapid reaction kinetics between F12+ and H2S could allow H2S to efficiently reduce F12+ into F2 within 1 min, thereby recovering afterglow luminescence by a factor of ~122-fold (Fig. 2e). Such activatable afterglow probes with large turn-on ratio and ultrafast reaction kinetics could facilitate sensitive and real-time detection of H2S in vitro and in vivo.
H2S is recognized as the third signaling gasotransmitter, participating in many essential physiological and pathological processes36–39. Throughout the past few years, many colorimetric and fluorescent probes have been developed for H2S measurement52–57. However, due to the low concentration and transient nature of H2S in biology58, the detection of endogenous H2S with these probes has been compromised by insufficient sensitivity, selectivity, or reaction kinetics. F12+-ANP possesses high sensitivity, selectivity, and an ultrafast response to H2S, rendering it suitable for quantification of H2S concentration in mouse blood samples (Supplementary Fig. 21). Parallel measurements of H2S levels in human blood freshly collected from healthy persons, HCC patients, and CRC patients were taken following incubation with F12+-ANP for 1 min. Findings showed that blood H2S levels in HCC and CRC patients were substantially higher than in healthy persons (Fig. 6), suggesting that H2S could be a potential blood marker for cancer diagnosis.
In addition to detecting blood H2S in vitro, F12+-ANP was engineered into a hepatic tumor-targeting and H2S-activatable afterglow luminescent probe, F12+-ANP-Gal, for afterglow imaging of hepatic tumor H2S in vivo. With a long blood circulation half-life and β-Gal receptor-mediated active delivery, F12+-ANP-Gal could efficiently enter s.c. HepG2 tumors after i.v. injection into mice, turning on afterglow luminescence for real-time and noninvasive monitoring of H2S fluctuation in tumors. Benefiting from low tissue autofluorescence in the absence of real-time light excitation, F12+-ANP-Gal with activated NIR afterglow presented a much higher SBR (47.8 ± 5.9) than NIR fluorescence (1.1 ± 0.4), permitting greater sensitivity to detect tiny s.c. HepG2 tumors (~12 mm3, Fig. 4d, f). The SBR resulting from afterglow images also increased linearly with tumor size, suggesting the potential of F12+-ANP-Gal for (a) accurate detection of early-stage liver tumors and (b) noninvasive monitoring of HepG2 tumor growth in living mice through afterglow imaging of H2S. Moreover, the enhanced penetration depth of NIR afterglow luminescence could allow F12+-ANP-Gal to sensitively detect orthotopic liver tumors, affording a large SBR (124.5 ± 12.5) to precisely position liver tumor foci in mice (Fig. 5). Of note, the H2S-induced rapid and substantial afterglow enhancement in F12+-ANP-Gal could also offer a large SBR (58.6 ± 13.2) to identify liver tumor lesions in excised HCC specimens, with tumor margins aligning with H&E staining (Fig. 7). These results may offer valuable information to guide surgical resection of liver tumor tissues in intraoperative HCC patients.
In conclusion, we report the design and development of H2S-activatable NIR afterglow luminescent probes by doping EM F12+ and NIR775 into MEH-PPV-based organic afterglow nanoparticles. Our findings demonstrate the probe’s utility in noninvasive and real-time imaging of H2S in living mice. F12+-ANP can be selectively and rapidly activated by H2S, leading to a large afterglow luminescence turn-on ratio (~122-fold) after pre-irradiation with an 808-nm laser, which is useful for quantifying the H2S concentration in the blood of healthy persons (27.6 ± 2.7 μM), HCC patients (47.0 ± 5.0 μM), and CRC patients (75.9 ± 11.9 μM). A hepatic tumor-targeting and H2S-activatable afterglow probe (F12+-ANP-Gal) is also built, offering a high SBR and deep-tissue penetration for high-sensitivity imaging of s.c. and orthotopic liver tumors via afterglow imaging of H2S in mice. Moreover, F12+-ANP-Gal can accurately delineate liver tumor margins in excised HCC specimens, which may be especially useful for image-guided HCC surgery. This study presents the potential of using H2S-activatable afterglow luminescent probes for in vivo imaging, which could detect H2S levels in other diseases (e.g., liver inflammation and hypertension) and promote early disease diagnosis in the future.
Methods
Chemicals and characterization
1H and 13C nuclear magnetic resonance (NMR) spectra were recorded on a BRUKER AscendTM 400 (1H/400 MHz and 13C/100 MHz) spectrometer. Mass spectra were recorded on a JEOL JMS-T100GCV spectrometer in FD mode (GC-MS&NMR Laboratory, Research Faculty of Agriculture, Hokkaido University). Redox potentials (Eox and Ered) were measured by CV in dry CH2Cl2 containing 0.1 M Bu4NBF4 as a supporting electrolyte at a scan rate of 100 mV s–1. A Pt disk and wire electrodes were used as the working and counter electrodes, respectively. The working electrode was polished using a water suspension Al2O3 (0.05 µmol) before use. Column chromatography was performed on a silica gel I-6-40 (YMC, 40–63 µm), a silica gel 60 N (Kanto Chemical Co., Inc., spherical neutral, 40–50 µm), or aluminum oxide 90 standardized (Merck, 63–200 µm). The DFT calculations were performed with the Gaussian 09 W program package. The geometries of the compounds were optimized by using the B3LYP method in combination with the 6-31G* basis set, unless otherwise indicated. Melting points were measured on a Yamato MP-21 melting point apparatus and are recorded uncorrected. Elemental analyses were performed on an Exeter Analytical CE440 (the Open Facility, Global Facility Center, Creative Research Institution, Hokkaido University). HPLC analysis was carried out on Thermo Fisher U3000 HPLC systems. Absorption spectra were performed an Ocean Optics UV–visible spectrometer. Fluorescence spectra were obtained on a HORIBA Jobin Yvon Fluoromax-4 fluorescence spectrometer. An Olympus IX73 fluorescent inverted microscope was used for fluorescence imaging for cells and tissue slices. DLS and zeta potential analysis were measured by a 90 Plus/BI-MAS equipment (Brookhaven, USA). Transmission electron microscopy images were conducted on a JEM-1011 transmission electron microscope (JEOL Ltd., Japan) with an accelerating voltage of 100 kV. MTT assay was performed on a microplate reader (Tcan). The afterglow luminescence spectra, afterglow, and fluorescence images in vitro or vivo were acquired with the IVIS Lumina XR III imaging system. An 808-nm high-power NIR laser (FC-808-10W-MM, Xilong Company, China) was used to irradiate the specified irradiation sites to generate the afterglow luminescence, unless otherwise noted.
Synthesis of F12+(BF4−)2
The synthesis of F12+(BF4−)2 was depicted in Supplementary Fig. 46 and Supplementary Methods. The NMR spectra and single crystal analysis of F12+(BF4−)2 were summarized in Supplementary Figs. 47–53.
Preparation of F12+-ANP and F12+-ANP-Gal
All the nanoparticles were prepared using amphiphilic polymers-assisted nanoprecipitation method. Typically, to prepare F12+-ANP, F12+(BF4−)2 (0.52 mg), MEH-PPV (0.25 mg), NIR775 (0.02 mg), and DSPE-PEG2000 (10 mg) were dissolved in tetrahydrofuran (THF, 0.5 mL) to form a homogeneous solution. These solutions were rapidly injected into deionized (D.I.) water (9 mL) under continuous sonication. After addition, the mixture was kept sonicated for 10 min in an ice-water bath. Then, THF was removed with a rotary evaporator. The resulting deep color aqueous solution was washed with D.I. water three times, using a 10 K centrifugal filter (Millipore) under centrifugation at 2040 × g for 15 min. The amount of F12+(BF4−)2 and MEH-PPV (NIR775) were determined by indirectly measuring the UV–visible absorption spectra of filtrate, showing that nearly all F12+(BF4−)2, MEH-PPV, and NIR775 were encapsulated into nanoparticles.
To prepare F12+-ANP-NH2, F12+(BF4−)2 (0.52 mg), MEH-PPV (0.25 mg), NIR775 (0.02 mg), DSPE-PEG2000 (8 mg), and DSPE-PEG2000-NH2 (2 mg) were dissolved in THF (0.5 mL) to form a homogeneous solution. These solutions were rapidly injected into D.I. water (9 mL) under continuous sonication. After addition, the mixture was kept sonicated for 10 min in an ice-water bath. Then, THF was removed with a rotary evaporator. The resulting deep color aqueous solution was washed with D.I. water three times, using a 10 K centrifugal filter (Millipore) under centrifugation at 2040 × g for 15 min. The F12+-ANP-NH2 solution was finally concentrated to 252 μg mL−1 (based on the mass of MEH-PPV) by ultrafiltration, and used for the preparation of F12+-ANP-Gal. Typically, β-Gal-COOH (1.5 mg), 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (3.0 mg), and N-hydroxy succinimide (2 mg) were dissolved in 1.0 mL PBS (1×, pH 7.4) and the solution was stirred at room temperature (r.t.) for 1 h. Then, F12+-ANP-NH2 (522/252/19.8 μg mL−1 F12+(BF4−)2/MEH-PPV/NIR775) was added, and the mixture was kept stirring at r.t. for another 6 h. The reaction solution was then washed with PBS for three times via using centrifugal filter units with molecular weight cutoff of 10 kDa under centrifugation at 2040 × g to remove excess reactant. The stock solution was stored at 4 °C under dark. The amount of F12+(BF4−)2 and MEH-PPV (NIR775) were determined by indirectly measuring the UV–visible–NIR absorption of filtrate, showing that nearly all F12+(BF4−)2, MEH-PPV and NIR775 were encapsulated into nanoparticles.
Measurement of the fluorescence and afterglow luminescence
To measure the fluorescence of F12+-ANP or F12+-ANP-Gal in response to H2S, F12+-ANP, or F12+-ANP-Gal (2.2 μg NIR775, 28 μg MEH-PPV, 58 μg F12+(BF4−)2) in 1.0 mL PBS buffer (pH 7.4) were incubated with NaHS (200 µM) at 37 °C for 1 min, and the fluorescence spectra were recorded on a HORIBA Jobin Yvon Fluoromax-4 fluorometer with fluorescence synchronous scanning mode (λex = 400–800 nm, offset = 100 nm).
To measure the afterglow luminescence of F12+-ANP or F12+-ANP-Gal in response to H2S, F12+-ANP, or F12+-ANP-Gal (2.2 μg NIR775, 28 μg MEH-PPV, 58 μg F12+(BF4−)2) in 1.0 mL PBS buffer (pH 7.4) were incubated with 200 μM NaHS at 37 °C for 1 min. Then, the incubation solutions were illuminated by an 808-nm laser (1 W cm−2) for 1 min. The laser was removed, and the afterglow luminescence images were acquired on an IVIS Spectrum imaging system equipped with an open filter or a 790 nm emission filter, with an acquisition time of 60 s. The afterglow luminescence intensity in each image was quantified by applying region of interest (ROI) over the image, using the Living Image Software (4.5.2, PerkinElmer, MA, USA).
Cell culture
Human HCC HepG2 cells and human lung cancer A549 cancer cells were purchased from Stem Cell Bank, Chinese Academy of Sciences (Shanghai, China). All cell lines were routinely tested to exclude infection with mycoplasma. They were authenticated by the supplier using short tandem repeat test. Human HCC HepG2 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM). Human lung cancer A549 cancer cells were grown in F-12 medium. All the mediums were supplemented with 10% fetal bovine serum (FBS), 1% penicillin, and streptomycin. All cells were maintained at 37 °C in a humidified atmosphere with 5% CO2.
Imaging of H2S in HepG2 cells
HepG2 Cells were plated at a density of 5 × 104 cells per well onto a glass-bottom dish (In Vitro Scientific, D35-20-1-N), and grown for 12 h. Cells were then exposed to various concentrations of F12+-ANP-Gal (2, 7, 14, 28, 38, and 48 μg mL−1 based on mass concentration of MEH-PPV) at 37 °C for different times (0.5, 1, 2, 3, 4, and 6 h). To image the elevated H2S levels in living cells, HepG2 cells were incubated with F12+-ANP-Gal (2.2/28/58 μg mL−1 NIR775/MEH-PPV/F12+(BF4−)2) for 3 h, and then the medium was discarded and washed with cold PBS (1×, pH 7.4) once, followed by incubation at 37 °C for 1 h in the DMEM medium containing 1 mM NaHS. To elevate the endogenous H2S production, HepG2 cells were incubated with 0.2 mM l-Cys for 1 h; to image the down-regulated H2S levels in living cells, HepG2 cells were pre-treated with ZnCl2 (0.3 mM) for 10 min to scavenge the endogenous H2S; alternately, PAG (50 mg L−1) and AOAA (20 μM) were added into the cells, and then incubated for 0.5 h to inhibit the endogenous CSE and CBS activities, followed by adding l-Cys (0.2 mM) and incubating for another 1 h. Then, cells were incubated with F12+-ANP-Gal (2.2/28/58 μg mL−1 NIR775/MEH-PPV/F12+(BF4−)2) for 3 h. Cells were then stained with 2.0 µM Hoechst 33342 for another 20 min. Prior to fluorescence imaging, cells were washed with cold PBS (1×, pH 7.4) three times. After adding fresh medium, the fluorescence images were acquired on an Olympus IX73 fluorescent inverted microscope equipped with DAPI (4′,6-diamidino-2-phenylindole) and TRITC (tetramethylrhodamine) filter.
For afterglow imaging of cells, HepG2 cells were plated at a density of 3 × 104 (or 1 × 105) cells into culture dish. Cells were treated according to the above-mentioned procedures. Cells were harvested from the culture dish by digestion with trypsin. The cell pellets were then collected after centrifugation at 161 × g for 4 min. The cell pellets were irradiated with the 808-nm laser (1 W cm−2) for 1 min. After removal of the laser, the afterglow luminescence images were immediately acquired on an IVIS Spectrum imaging system equipped with an open filter, with an acquisition time of 60 s. The afterglow luminescence intensities in the cell pellets were quantified by applying ROI over the images, using the Living Image Software (4.5.2, PerkinElmer, MA, USA).
Investigation of tissue-penetration ability
To investigate the tissue-penetration ability, F12+-ANP-Gal (0.026/0.33/0.684 mg mL−1 NIR775/MEH-PPV/F12+(BF4−)2, 200 µL) in PBS buffer (pH 7.4) was incubated with NaHS (2.0 mM) at 37 °C for 10 min. The incubation solution was then irradiated with the 808-nm laser (1 W cm−2) for 1 min. After removal of laser, the solution was covered with chicken tissues at varying thickness (0, 1.5, 2.0, 3.0 and 4.0 cm). The afterglow images were acquired on an IVIS Spectrum imaging system equipped with an open filter, with an acquisition time of 60 s. To investigate the tissue-penetration ability through a mouse body, a solution of H2S-activated F12+-ANP-Gal (0.026/0.33/0.684 mg mL−1 NIR775/MEH-PPV/F12+(BF4−)2, 50 µL) was irradiated with the 808-nm laser (1 W cm−2, 1 min), and then placed under a living mouse. The afterglow luminescence images were acquired on an IVIS Spectrum imaging system equipped with an open filter, with an acquisition time of 60 s. The fluorescence images were acquired on an IVIS Lumina XR III imaging system with λex/λem = 740/790 nm. Each experiment was conducted for three times.
Animals and tumor models
BALB/c mice (~5–6 weeks old) were purchased from the Model Animal Research Center (MARC) of Nanjing University in China. All animal experiments were performed in compliance with the Guidelines established by the Institutional Animal Care and Use Committee (IACUC) of Nanjing University.
To establish tumors, HepG2 cells (2.0 × 106 cells, 50 μL) suspended in 50 v/v% mixture of Matrigel in supplemented DMEM (10% FBS, 1% pen/strep (100 U mL−1 penicillin and 100 µg mL−1 streptomycin)) were injected subcutaneously into the selected positions of nude mice. The tumor volume (V) was determined assuming ellipsoid shape with the formula of V = (L × W2)/2, where the length (L) and width (W) of each tumor was measured using a caliper. When the tumor volume reached about 12–100 mm3, fluorescence and afterglow imaging were conducted. To establish an orthotopic HCC model, a midline incision of the anterior abdominal wall was made. HepG2 cells transfected with luciferase (HepG2/Luc) (2 × 106, 100 μL) in serum-free culture medium were carefully injected into the lube of liver of mice under anesthesia by pentobarbital sodium. Tumor growths were monitored by BL imaging. After 2 weeks, mice with HCC model were successfully established for afterglow and fluorescence imaging.
Imaging of tumor H2S in vivo
To image H2S in HepG2 tumors, mice bearing s.c. HepG2 tumors were i.v. injected with F12+-ANP-Gal or F12+-ANP (211/100/8 μg F12+(BF4−)2/MEH-PPV/NIR775, in 200 μL saline). After 3.5 h, l-Cys (1 mM, 25 µL) or ZnCl2 (1 mM, 25 µL) was directly injected into tumors to regulate tumor H2S levels. The fluorescence and afterglow images were acquired at 0, 4, 8, 12, and 24 h using an IVIS Lumina XR III imaging system. To image H2S in different sizes of HepG2 tumors, HepG2 tumors at an average size of ~12, ~45, and ~100 mm3 were i.v. injected with F12+-ANP-Gal (211/100/8 μg F12+(BF4−)2/MEH-PPV/NIR775, in 200 μL saline). The fluorescence and afterglow images were acquired at 12 h post injection. To image H2S in orthotopic HepG2/Luc tumors, mice with orthotopic HepG2/Luc tumors and control mice were i.v. injected with F12+-ANP-Gal (211/100/8 μg F12+(BF4−)2/MEH-PPV/NIR775, in 200 μL saline). The fluorescence and afterglow images were acquired at 12 h post injection.
The fluorescence images were acquired on an IVIS Lumina XR III imaging system, with λex/λem = 740/790 nm. To acquire afterglow images, the mouse body at 0, 4, 8, 12, and 24 h post i.v. injection of F12+-ANP-Gal was irradiated under the 808-nm laser (1 W cm−2) for 1 min. After cessation of laser, the afterglow luminescence images were immediately acquired on an IVIS Spectrum imaging system equipped with an open filter, with an acquisition time of 60 s. Every experiment was conducted in three mice. ROIs were drawn over the tumor and the thigh of the mouse to quantify the fluorescence and afterglow intensities using the Living Image Software. The SBRs were calculated by dividing the fluorescence and afterglow intensities in tumor to that in thigh.
WB analysis of CSE and CBS expression
To conduct the WB analysis of mouse liver and orthotopic HepG2 tumor tissue homogenates, three normal mice and mice with orthotopic liver tumors were euthanized, and the livers from normal mice and the HepG2 tumor tissues from the orthotopic liver tumor-bearing mice were resected. Liver and HepG2 tumor tissues were then homogenized with lysis buffer. The homogenates were incubated on ice for 30 min, and the lysates were obtained by centrifugation (13,000 × g, 15 min) at 4 °C. Aliquots of proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (10%) and subsequently transferred to nitrocellulose membranes by electroblotting. The membrane was blocked in 5% skim milk powder in 0.1% Tris-buffered saline/Tween-20 (TBST, 20 mM Tris-HCl, pH 7.4, 137 mM NaCl, and 0.1% Tween) at r.t. for 2 h, and then incubated with antibody raised against CBS (Abcam, ab135626, lot: GR3265732-10, dilution 1 : 1000) for 2 h at r.t. After three washes with TBST, the membrane was incubated with a secondary antibody horseradish peroxidase (HRP)-conjugated goat anti-rabbit immunoglobulin G (IgG) for 2 h at r.t. The films were developed with the ECL system. The CBS protein and markers were visualized using G:BOX chemiXR5. Afterwards, the membrane was rinsed in D.I. water at r.t. for 5 min, and antibody stripping buffer (weak alkaline) was added. The membrane was then rinsed with D.I. water at r.t. for 5 min, blocked in 5% skim milk powder in 0.1% Tris-buffered saline/Tween-20 at r.t. for 2 h, and incubated with antibody raised against CSE (Abcam, ab151769, lot: GR3257101-3, dilution 1 : 1000) for 2 h at r.t. After three washes with TBST, the membrane was incubated with a secondary antibody HRP-conjugated goat anti-rabbit IgG at r.t. for 2 h. The films were developed with the ECL system. The CSE protein and markers were visualized using G:BOX chemiXR5. The resulting band intensities were quantified using a Gel-Pro32. Each experiment was repeated for three times.
Measurement of the H2S concentration in human blood
Whole-blood samples were collected from healthy volunteers and the patients with HCC and CRC at Affiliated Drum Tower Hospital of Nanjing University (Nanjing, China). For the detection of H2S in blood samples of healthy persons and patients diagnosing with HCC or CRC, freshly collected bloods were 2-fold diluted with PBS buffer (1×, pH 7.4), and then incubated with F12+-ANP (2.2/28/58 μg mL−1 NIR775/MEH-PPV/F12+(BF4−)2) at 37 °C for 1 min, followed by irradiation with the 808-nm laser (1 W cm−2) for 1 min. After removal of laser, the afterglow luminescence images were immediately acquired for 60 s with a 790 nm filter. The afterglow luminescence intensities at 790 nm for F12+-ANP were quantified by applying ROI over the image using the Living Image Software (4.5.2, PerkinElmer, MA, USA). With constructing a standard curve in healthy blood by an internal standard method, the concentrations of H2S in whole bloods of healthy persons and HCC or CRC patients were obtained.
Imaging of human HCC specimens
HCC specimens were collected from the patients with HCC at Affiliated Drum Tower Hospital of Nanjing University (Nanjing, China). This study was approved by institutional review board of Affiliated Drum Tower Hospital of Nanjing University, and all subjects provided written informed consent under institutional review board prior to sample collection. HCC specimens were resected from four HCC patients. HCC specimens were infiltrated with F12+-ANP-Gal (58/28/2.2 μg mL−1 F12+(BF4−)2/MEH-PPV/NIR775) in PBS buffer for 3 h. The specimens were then rinsed with PBS buffer (1×, pH 7.4) three times to remove nonspecifically absorbed F12+-ANP-Gal on the tissues. The specimens were irradiated with the 808-nm laser (1 W cm−2, 1 min). After removal of laser, the afterglow images were acquired on an IVIS Spectrum imaging system equipped with an open filter, with an acquisition time of 60 s. The fluorescence images were acquired with λex/em = 740/790 nm. ROIs were drawn over the tumor and adjacent normal liver tissues to quantify the fluorescence and afterglow intensities using the Living Image Software. The SBRs were calculated by dividing the fluorescence and afterglow intensities in tumor to that in normal liver tissues.
Immunohistochemistry studies
HCC tissues were fixed in 4% formalin and then embedded in paraffin before 10-µm sectioning. Histology samples were stained by H&E using a standard protocol. White light images were acquired using an IX73 optical microscope equipped with a color camera.
Statistical analysis
Results are expressed as the mean ± standard deviation unless otherwise stated. Statistical differences among experimental groups were analyzed by Student’s t test. P < 0.05 was considered statistically significant. All statistical calculations were performed using GraphPad Prism 6 including assumptions of tests used (GraphPad Software Inc., CA, USA).
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Description of Additional Supplementary Files
Acknowledgements
Yuhua Zhu from Nanjing University for the help on fluorescence lifetime measurements is acknowledged. Financial supports from the National Key R&D Program of China (2017YFA0701301), National Natural Science Foundation of China (21922406, 21775071, and 21632008), Natural Science Foundation of Jiangsu Province (BK20190055), the Fundamental Research Funds for the Central Universities (020514380185), Excellent Research Program of Nanjing University (ZYJH004), CAS Key Laboratory of Receptor Research (SIMM1904YKF-03), and Grant-in-aid for Scientific Research on Innovation Areas “Middle molecular strategy” from MEXT (Japan) are acknowledged.
Source data
Author contributions
D.Y., T.S., and L.W. conceived the research plan. L.W., Y.I., Y.H., W.Z., and Y.S. performed the experiments. K.S. and T.H. synthesized electrochromic materials. L.W., Y.I., T.S., and D.Y. analyzed the data. L.W., J.H., X.J., H.C., T.S. and D.Y. wrote the manuscript. All authors discussed the results and commented on the manuscript.
Data availability
The source data underlying Figs. 2b, e, f, h, i, 3d, g, 4c, e, f, 5c, d, f, g, 6b, c and 7e and Supplementary Figs. 2d–i, 5c, f, 8, 9d, 11, 12b, 14c, d, 15, 16d, 17a–c, j–l, 18, 19b, d, 20b, d, 22, 21a, c, 23a, b, f, 24, 28b, d, 29b, c, 30c, 31b, e, 32, 33, 35b, 37, 38b, 39d, 40d, 41d, 42c, d, 43b and 44 are provided as a Source Data file. The authors declare that all other data related to this study are available in the article/and or its Supplementary Information files or from the authors upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Peer review information Nature Communications thanks Xiaogang Liu, Xiaoyuan Chen and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Luyan Wu, Yusuke Ishigaki
Contributor Information
Takanori Suzuki, Email: tak@sci.hokudai.ac.jp.
Deju Ye, Email: dejuye@nju.edu.cn.
Supplementary information
Supplementary information is available for this paper at 10.1038/s41467-020-14307-y.
References
- 1.Razgulin A, Ma N, Rao J. Strategies for in vivo imaging of enzyme activity: an overview and recent advances. Chem. Soc. Rev. 2011;40:4186–4216. doi: 10.1039/c1cs15035a. [DOI] [PubMed] [Google Scholar]
- 2.Zhang J, et al. Fluorogenic probes for disease-relevant enzymes. Chem. Soc. Rev. 2019;48:683–722. doi: 10.1039/C7CS00907K. [DOI] [PubMed] [Google Scholar]
- 3.Zhang Y, et al. Non-invasive multimodal functional imaging of the intestine with frozen micellar naphthalocyanines. Nat. Nanotechnol. 2014;9:631. doi: 10.1038/nnano.2014.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang J, Li J, Lyu Y, Miao Q, Pu K. Molecular optical imaging probes for early diagnosis of drug-induced acute kidney injury. Nat. Mater. 2019;18:1133–1143. doi: 10.1038/s41563-019-0378-4. [DOI] [PubMed] [Google Scholar]
- 5.Wang S, et al. Anti-quenching NIR-II molecular fluorophores for in vivo high-contrast imaging and pH sensing. Nat. Commun. 2019;10:1058. doi: 10.1038/s41467-019-09043-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moore, C., Chen, F., Wang, J. & Jokerst, J. V. Listening for the therapeutic window: advances in drug delivery utilizing photoacoustic imaging. Adv. Drug Delivery Rev. 144, 78–89 (2019). [DOI] [PMC free article] [PubMed]
- 7.Yan R, et al. Activatable NIR fluorescence/MRI bimodal probes for in vivo imaging by enzyme-mediated fluorogenic reaction and self-assembly. J. Am. Chem. Soc. 2019;141:10331–10341. doi: 10.1021/jacs.9b03649. [DOI] [PubMed] [Google Scholar]
- 8.Palner M, Pu K, Shao S, Rao J. Semiconducting polymer nanoparticles with persistent near-infrared luminescence for in vivo optical imaging. Angew. Chem. Int. Ed. 2015;54:11477–11480. doi: 10.1002/anie.201502736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abdukayum A, Chen J-T, Zhao Q, Yan X-P. Functional near infrared-emitting Cr3+/Pr3+ Co-doped zinc gallogermanate persistent luminescent nanoparticles with superlong afterglow for in vivo targeted bioimaging. J. Am. Chem. Soc. 2013;135:14125–14133. doi: 10.1021/ja404243v. [DOI] [PubMed] [Google Scholar]
- 10.Maldiney T, et al. Controlling electron trap depth to enhance optical properties of persistent luminescence nanoparticles for in vivo imaging. J. Am. Chem. Soc. 2011;133:11810–11815. doi: 10.1021/ja204504w. [DOI] [PubMed] [Google Scholar]
- 11.Jiang Y, et al. A generic approach towards afterglow luminescent nanoparticles for ultrasensitive in vivo imaging. Nat. Commun. 2019;10:2064. doi: 10.1038/s41467-019-10119-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chuang Y-J, et al. Photostimulable near-infrared persistent luminescent nanoprobes for ultrasensitive and longitudinal deep-tissue bio-imaging. Theranostics. 2014;4:1112–1122. doi: 10.7150/thno.9710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ni X, et al. Near-infrared afterglow luminescent aggregation-induced emission dots with ultrahigh tumor-to-liver signal ratio for promoted image-guided cancer surgery. Nano Lett. 2019;19:318–330. doi: 10.1021/acs.nanolett.8b03936. [DOI] [PubMed] [Google Scholar]
- 14.Wang J, et al. Autofluorescence-free targeted tumor imaging based on luminous nanoparticles with composition-dependent size and persistent luminescence. ACS Nano. 2017;11:8010–8017. doi: 10.1021/acsnano.7b02643. [DOI] [PubMed] [Google Scholar]
- 15.Ai T, et al. Near infrared-emitting persistent luminescent nanoparticles for hepatocellular carcinoma imaging and luminescence-guided surgery. Biomaterials. 2018;167:216–225. doi: 10.1016/j.biomaterials.2018.01.031. [DOI] [PubMed] [Google Scholar]
- 16.Moore JD, Lord RL, Cisneros GA, Allen MJ. Concentration-independent pH detection with a luminescent dimetallic Eu(III)-based probe. J. Am. Chem. Soc. 2012;134:17372–17375. doi: 10.1021/ja307098z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yan L-L, et al. Stereocontrolled self-assembly and self-sorting of luminescent europium tetrahedral cages. J. Am. Chem. Soc. 2015;137:8550–8555. doi: 10.1021/jacs.5b03972. [DOI] [PubMed] [Google Scholar]
- 18.Ai K, Zhang B, Lu L. Europium-based fluorescence nanoparticle sensor for rapid and ultrasensitive detection of an anthrax biomarker. Angew. Chem. Int. Ed. 2009;48:304–308. doi: 10.1002/anie.200804231. [DOI] [PubMed] [Google Scholar]
- 19.Liu C-L, et al. Intraligand charge transfer sensitization on self-assembled europium tetrahedral cage leads to dual-selective luminescent sensing toward anion and cation. J. Am. Chem. Soc. 2017;139:12474–12479. doi: 10.1021/jacs.7b05157. [DOI] [PubMed] [Google Scholar]
- 20.Moore EG, et al. 3-Hydroxypyridin-2-one complexes of near-infrared (NIR) emitting lanthanides: sensitization of holmium(III) and praseodymium(III) in aqueous solution. Angew. Chem. Int. Ed. 2008;47:9500–9503. doi: 10.1002/anie.200802337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ma H, Peng Q, An Z, Huang W, Shuai Z. Efficient and long-lived room-temperature organic phosphorescence: theoretical descriptors for molecular designs. J. Am. Chem. Soc. 2019;141:1010–1015. doi: 10.1021/jacs.8b11224. [DOI] [PubMed] [Google Scholar]
- 22.Kabe R, Adachi C. Organic long persistent luminescence. Nature. 2017;550:384. doi: 10.1038/nature24010. [DOI] [PubMed] [Google Scholar]
- 23.Yang J, et al. The influence of the molecular packing on the room temperature phosphorescence of purely organic luminogens. Nat. Commun. 2018;9:840. doi: 10.1038/s41467-018-03236-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang Z, et al. Intermolecular electronic coupling of organic units for efficient persistent room-temperature phosphorescence. Angew. Chem. Int. Ed. 2016;55:2181–2185. doi: 10.1002/anie.201509224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maldiney T, et al. The in vivo activation of persistent nanophosphors for optical imaging of vascularization, tumours and grafted cells. Nat. Mater. 2014;13:418. doi: 10.1038/nmat3908. [DOI] [PubMed] [Google Scholar]
- 26.Miao Q, et al. Molecular afterglow imaging with bright, biodegradable polymer nanoparticles. Nat. Biotechnol. 2017;35:1102. doi: 10.1038/nbt.3987. [DOI] [PubMed] [Google Scholar]
- 27.Zhen X, Xie C, Pu K. Temperature-correlated afterglow of a semiconducting polymer nanococktail for imaging-guided photothermal therapy. Angew. Chem. Int. Ed. 2018;57:3938–3942. doi: 10.1002/anie.201712550. [DOI] [PubMed] [Google Scholar]
- 28.Tu D, Zheng W, Liu Y, Zhu H, Chen X. Luminescent biodetection based on lanthanide-doped inorganic nanoprobes. Coord. Chem. Rev. 2014;273-274:13–29. doi: 10.1016/j.ccr.2013.11.017. [DOI] [Google Scholar]
- 29.Xie C, Zhen X, Miao Q, Lyu Y, Pu K. Self-assembled semiconducting polymer nanoparticles for ultrasensitive near-infrared afterglow imaging of metastatic tumors. Adv. Mater. 2018;30:1801331. doi: 10.1002/adma.201801331. [DOI] [PubMed] [Google Scholar]
- 30.Lyu Y, et al. Near-infrared afterglow semiconducting nano-polycomplexes for the multiplex differentiation of cancer exosomes. Angew. Chem. Int. Ed. 2019;58:4983–4987. doi: 10.1002/anie.201900092. [DOI] [PubMed] [Google Scholar]
- 31.Xie C, Lyu Y, Zhen X, Miao Q, Pu K. Activatable semiconducting oligomer amphiphile for near-infrared luminescence imaging of biothiols. ACS Appl. Bio Mater. 2018;1:1147–1153. doi: 10.1021/acsabm.8b00353. [DOI] [PubMed] [Google Scholar]
- 32.Chen L-J, et al. Activatable multifunctional persistent luminescence nanoparticle/copper sulfide nanoprobe for in vivo luminescence imaging-guided photothermal therapy. ACS Appl. Mater. Interfaces. 2016;8:32667–32674. doi: 10.1021/acsami.6b10702. [DOI] [PubMed] [Google Scholar]
- 33.Lippert AR, New EJ, Chang CJ. Reaction-based fluorescent probes for selective imaging of hydrogen sulfide in living cells. J. Am. Chem. Soc. 2011;133:10078–10080. doi: 10.1021/ja203661j. [DOI] [PubMed] [Google Scholar]
- 34.Hammers MD, et al. A bright fluorescent probe for H2S enables analyte-responsive, 3D imaging in live zebrafish using light sheet fluorescence microscopy. J. Am. Chem. Soc. 2015;137:10216–10223. doi: 10.1021/jacs.5b04196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Szabó C. Hydrogen sulphide and its therapeutic potential. Nat. Rev. Drug Discov. 2007;6:917. doi: 10.1038/nrd2425. [DOI] [PubMed] [Google Scholar]
- 36.Xu G, et al. Imaging of colorectal cancers using activatable nanoprobes with second near-infrared window emission. Angew. Chem. Int. Ed. 2018;57:3626–3630. doi: 10.1002/anie.201712528. [DOI] [PubMed] [Google Scholar]
- 37.Kolluru GK, Shen X, Bir SC, Kevil CG. Hydrogen sulfide chemical biology: pathophysiological roles and detection. Nitric Oxide. 2013;35:5–20. doi: 10.1016/j.niox.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Das A, et al. Impairment of an endothelial NAD+-H2S signaling network is a reversible cause of vascular aging. Cell. 2018;173:74–89. doi: 10.1016/j.cell.2018.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ning N, et al. Dysregulation of hydrogen sulphide metabolism impairs oviductal transport of embryos. Nat. Commun. 2014;5:4107. doi: 10.1038/ncomms5107. [DOI] [PubMed] [Google Scholar]
- 40.Zhao C, et al. Förster resonance energy transfer switchable self-assembled micellar nanoprobe: ratiometric fluorescent trapping of endogenous H2S generation via fluvastatin-stimulated upregulation. J. Am. Chem. Soc. 2015;137:8490–8498. doi: 10.1021/jacs.5b03248. [DOI] [PubMed] [Google Scholar]
- 41.Sasakura K, et al. Development of a highly selective fluorescence probe for hydrogen sulfide. J. Am. Chem. Soc. 2011;133:18003–18005. doi: 10.1021/ja207851s. [DOI] [PubMed] [Google Scholar]
- 42.Bae SK, et al. A ratiometric two-photon fluorescent probe reveals reduction in mitochondrial H2S production in parkinson’s disease gene knockout astrocytes. J. Am. Chem. Soc. 2013;135:9915–9923. doi: 10.1021/ja404004v. [DOI] [PubMed] [Google Scholar]
- 43.Qian Y, et al. Selective fluorescent probes for live-cell monitoring of sulphide. Nat. Commun. 2011;2:495. doi: 10.1038/ncomms1506. [DOI] [PubMed] [Google Scholar]
- 44.Du Z, et al. Quantitative monitoring and visualization of hydrogen sulfide in vivo using a luminescent probe based on a ruthenium(II) complex. Angew. Chem. Int. Ed. 2018;57:3999–4004. doi: 10.1002/anie.201800540. [DOI] [PubMed] [Google Scholar]
- 45.Liu Y, et al. Rationally designed pure-inorganic upconversion nanoprobes for ultra-highly selective hydrogen sulfide imaging and elimination in vivo. Chem. Sci. 2019;10:1193–1200. doi: 10.1039/C8SC04464C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen Y, et al. A ratiometric fluorescent probe for rapid detection of hydrogen sulfide in mitochondria. Angew. Chem. Int. Ed. 2013;52:1688–1691. doi: 10.1002/anie.201207701. [DOI] [PubMed] [Google Scholar]
- 47.Wu L, et al. Engineering of electrochromic materials as activatable probes for molecular imaging and photodynamic therapy. J. Am. Chem. Soc. 2018;140:16340–16352. doi: 10.1021/jacs.8b10176. [DOI] [PubMed] [Google Scholar]
- 48.Bhatia M, Sidhapuriwala JN, Wei Ng S, Tamizhselvi R, Moochhala SM. Pro-inflammatory effects of hydrogen sulphide on substance P in caerulein-induced acute pancreatitis. J. Cell. Mol. Med. 2008;12:580–590. doi: 10.1111/j.1582-4934.2007.00131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhou Y, et al. Inorganic–organic hybrid nanoprobe for NIR-excited imaging of hydrogen sulfide in cell cultures and inflammation in a mouse model. Small. 2014;10:4874–4885. doi: 10.1002/smll.201401867. [DOI] [PubMed] [Google Scholar]
- 50.Wu D, et al. Hydrogen sulfide in cancer: friend or foe? Nitric Oxide. 2015;50:38–45. doi: 10.1016/j.niox.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 51.Shi D-T, et al. Selective fluorogenic imaging of hepatocellular H2S by a galactosyl azidonaphthalimide probe. Chem. Commun. 2015;51:3653–3655. doi: 10.1039/C4CC09771H. [DOI] [PubMed] [Google Scholar]
- 52.Wang X, et al. A near-infrared ratiometric fluorescent probe for rapid and highly sensitive imaging of endogenous hydrogen sulfide in living cells. Chem. Sci. 2013;4:2551–2556. doi: 10.1039/c3sc50369k. [DOI] [Google Scholar]
- 53.Lin VS, Chen W, Xian M, Chang CJ. Chemical probes for molecular imaging and detection of hydrogen sulfide and reactive sulfur species in biological systems. Chem. Soc. Rev. 2015;44:4596–4618. doi: 10.1039/C4CS00298A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang F, et al. Realizing highly chemoselective detection of H2S in vitro and in vivo with fluorescent probes inside core-shell silica nanoparticles. Biomaterials. 2018;159:82–90. doi: 10.1016/j.biomaterials.2018.01.009. [DOI] [PubMed] [Google Scholar]
- 55.Lin VS, Lippert AR, Chang CJ. Cell-trappable fluorescent probes for endogenous hydrogen sulfide signaling and imaging H2O2-dependent H2S production. Proc. Natl Acad. Sci. USA. 2013;110:7131–7135. doi: 10.1073/pnas.1302193110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Adarsh N, Krishnan MS, Ramaiah D. Sensitive naked eye detection of hydrogen sulfide and nitric oxide by Aza-BODIPY dyes in aqueous medium. Anal. Chem. 2014;86:9335–9342. doi: 10.1021/ac502849d. [DOI] [PubMed] [Google Scholar]
- 57.Qian Y, et al. A fluorescent probe for rapid detection of hydrogen sulfide in blood plasma and brain tissues in mice. Chem. Sci. 2012;3:2920–2923. doi: 10.1039/c2sc20537h. [DOI] [Google Scholar]
- 58.Whitfield NL, Kreimier EL, Verdial FC, Skovgaard N, Olson KR. Reappraisal of H2S/sulfide concentration in vertebrate blood and its potential significance in ischemic preconditioning and vascular signaling. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008;294:1930–1937. doi: 10.1152/ajpregu.00025.2008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
The source data underlying Figs. 2b, e, f, h, i, 3d, g, 4c, e, f, 5c, d, f, g, 6b, c and 7e and Supplementary Figs. 2d–i, 5c, f, 8, 9d, 11, 12b, 14c, d, 15, 16d, 17a–c, j–l, 18, 19b, d, 20b, d, 22, 21a, c, 23a, b, f, 24, 28b, d, 29b, c, 30c, 31b, e, 32, 33, 35b, 37, 38b, 39d, 40d, 41d, 42c, d, 43b and 44 are provided as a Source Data file. The authors declare that all other data related to this study are available in the article/and or its Supplementary Information files or from the authors upon reasonable request.