Abstract
Uranium is a key element in the nuclear industry, but its unintended leakage has caused health and environmental concerns. Here we report a sp2 carbon-conjugated fluorescent covalent organic framework (COF) named TFPT-BTAN-AO with excellent chemical, thermal and radiation stability is synthesized by integrating triazine-based building blocks with amidoxime-substituted linkers. TFPT-BTAN-AO shows an exceptional UO22+ adsorption capacity of 427 mg g−1 attributable to the abundant selective uranium-binding groups on the highly accessible pore walls of open 1D channels. In addition, it has an ultra-fast response time (2 s) and an ultra-low detection limit of 6.7 nM UO22+ suitable for on-site and real-time monitoring of UO22+, allowing not only extraction but also monitoring the quality of the extracted water. This study demonstrates great potential of fluorescent COFs for radionuclide detection and extraction. By rational designing target ligands, this strategy can be extended to the detection and extraction of other contaminants.
Subject terms: Polymers, Sensors and biosensors, Polymers
Porous materials for uranium capture have been developed in the past, but materials for simultaneous uranium capture and detection are scarce. Here the authors develop a stable covalent organic framework capable of adsorbing and detecting uranyl ions.
Introduction
With a low-carbon footprint, nuclear energy has a critical role in the global energy system1–3. Owing to the widespread use of nuclear power, large-scale uranium mining, nuclear accidents, and improper disposal of nuclear wastes, a large quantity of radioactive uranium has penetrated into the environment mainly in the form of UO22+ 4–6. Thus, regenerable materials for concurrent UO22+ detection and extraction are demanded for environmental monitoring and protection.
Some porous materials such as porous organic polymers (POPs)7, metal-organic frameworks (MOFs)8, and hydrogels4 have been developed for this purpose. However, the performance of amorphous POPs is affected by its irregular pores, burying a large fraction of porosity9, and hindering fast mass transfer needed for real-time response10. Although MOFs have regular pores and good crystallinity8, stability under extreme conditions (acid, base, temperature, and radiation) remains a challenge11–13. High stability is particularly important for the extraction of UO22+, as the sample matrix is likely to be strongly radioactive and acidic. Therefore, it remains a synthetic challenge for real-time detection and regenerable extraction of UO22+.
Covalent organic frameworks (COFs) are a class of porous crystalline polymers with significant advantages for application in catalysis14–18, gas storage19–22, and metal ion extraction23–27 owing to excellent chemical and thermal stability, flexible topological connectivity, and tunable functionality28–30. COFs with tunable porosity and large specific surface area might be ideal for extracting radionuclides such as UO22+. In addition, post-modification can rationally place various functional units within the periodic arrays to optimize the performance. At present, various COFs based on the Schiff base reaction (for example, COF-TpAb-AO1, and o-TDCOF3) have been developed for the extraction of UO22+. However, their major covalent bonds, such as the boron–oxygen and imine bonds, are susceptible to irradiation, acid, and base, which greatly limit their regeneration and practical application3,31,32.
Recently, considerable attention has been paid to the construction of olefin-based COFs synthesized by the Knoevenagel condensation reaction. Although the sp2-carbon bond are very stable, the reversibility of sp2-carbon bond formation is poor, making the synthesis of sp2-carbon-linked COFs extremely challenging33. Since 2016, several examples of sp2-carbon COFs have been reported, such as sp2c-COF34, TP-COF35, Por-sp2c-COF33, and g-C34N6-COF36. However, their application for the detection or extraction of UO22+ has not been explored. More importantly, the exploration of COFs for fluorescence detection of UO22+ is still in its infancy, and most UO22+-sensing platforms are often hampered by poor selectivity and a long response time37–40.
We herein report a sp2 carbon-conjugated COF for simultaneous detection and extraction of UO22+ by integrating triazine-based building blocks with amidoxime-substituted linkers. This sp2 carbon-conjugated COF not only has good luminescence yield, but also excellent chemical and thermal stability. Its selective binding of UO22+ is obtained by introducing amidoxime functional groups as ligands in the open 1D channels. Its real-time fluorescence response to UO22+ can be visually observed and recycling was also confirmed using reversible uranium binding.
Results
sp2 carbon-conjugated COF for reversible uranium binding
To achieve an acid, base, and radiation stable, strongly fluorescent and selective UO22+-binding COF, we had the following materials design considerations. Most current COFs relied on the boron–oxygen and imine bonds35. However, such reversible bonds lead to relatively poor stability. In addition, their weak π-electron delocalization over the framework hinders fluorescence yield36. Carbon–carbon double bonds (-C═C-) are more stable and can keep conjugated π-electrons, which can overcome the above challenges36,41. In addition, their intrinsic open 1D channels and regular porous structures facilitate exposure of binding sites, boosting rapid diffusion, and mass transfer. Thus, by introducing specific metal-binding sites on the pore walls, the sp2 carbon-conjugated COF may serve for high performance UO22+ extraction under harsh conditions.
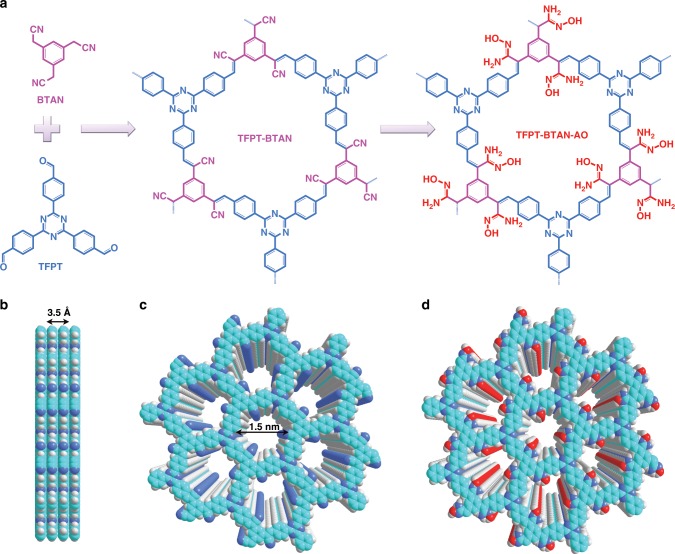
Our synthesis is depicted in Fig. 1. 2,4,6-tris(4-formylphenyl)-1,3,5-triazine (TFPT) and 2,2′,2″-(benzene-1,3,5-triyl)triacetonitrile (BTAN) were polymerized through the Knoevenagel reaction, yielding a cyano-based COF (TFPT-BTAN). To overcome the low reversibility of the Knoevenagel reaction, we optimized the solvent, catalyst, and temperature (Supplementary Table 1 and Supplementary Fig. 1 in Supplementary Information), and highly crystalline TFPT-BTAN was prepared by condensing TFPT and BTAN in a mixture of o-DCB and 4 M DBU (10:1 by vol.) at 90 °C. Subsequently, TFPT-BTAN was subjected to amidoximation by treating it with an excess of NH2OH·HCl at 85 °C for 24 h to give TFPT-BTAN-AO. Our synthetic strategy not only constructed a highly stable π-conjugated skeleton as the fluorophore, but also introduced dense amidoxime groups as the uranium receptors. These unique features are expected to facilitate real-time detection and efficient extraction of UO22+.
Fig. 1. Schematic of synthetic TFPT-BTAN-AO.
a Synthesis of TFPT-BTAN and TFPT-BTAN-AO. b Side and top c views of an eclipsed AA-stacking model of TFPT-BTAN (light green, C; blue, N; light gray, H). d Graphic view of TFPT-BTAN-AO (light green, C; red, O; blue, N; light gray, H).
The chemical structure and composition of TFPT-BTAN were determined by Fourier transform infrared (FT-IR) and solid-state 13C CP/MAS NMR spectroscopy. In the FT-IR spectra of TFPT-BTAN (Supplementary Fig. 2), the characteristic vibration peak of -CN (at ~2241 cm−1) was observed for both BTAN monomer and TFPT-BTAN. A stretching vibration peak of C═O (at ~1698 cm−1) was found in the TFPT monomer and it completely disappeared in TFPT-BTAN, indicating a high degree of condensation. The solid-state 13C CP/MAS NMR of TFPT-BTAN further confirmed highly efficient condensation, supported by the peaks at ~113 and ~171 ppm assigned to the carbon atoms in cyano and triazine moieties, respectively (Supplementary Fig. 3).
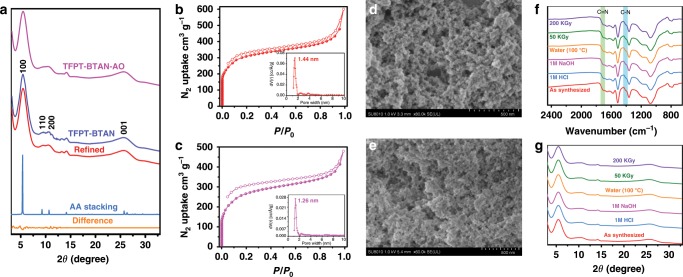
To determine the structure of TFPT-BTAN, powder X-ray diffraction (PXRD) experiments were performed (Fig. 2a). A strong peak at 5.8° (2θ) is assigned to the diffraction from the (100) plane, indicating a highly crystalline of TFPT-BTAN37. The other peaks at 9.8°, 11.2°, and 26.3° (2θ) are assigned to the diffractions of (110), (200), and (001) planes, respectively. Our PXRD pattern matches well with the AA stacking model of the simulated TFPT-BTAN structure, and the Pawley refined PXRD pattern fits well with experimental data (Rp = 2.85% and Rwp = 4.27%), as demonstrated by the negligible difference (Supplementary Fig. 4 and Supplementary Table 2). The above results indicate that TFPT-BTAN has open 1D channels (1.5 nm in diameter) and the interlayer distance of the framework is 3.5 Å. To evaluate the details of the pore features of TFPT-BTAN, N2 adsorption–desorption experiments were performed. The Brunauer-Emmett-Teller (BET) surface area of TFPT-BTAN was determined to be 1062 m2 g−1, and the pore-size distribution centered at 1.44 nm based on non-local density functional theory, which matches well with the model (Fig. 2b)30.
Fig. 2. Characterization of TFPT-BTAN and TFPT-BTAN-AO.
a PXRD profiles. Nitrogen adsorption–desorption isotherms of b TFPT-BTAN and c TFPT-BTAN-AO. Insets: the pore-size distributions calculated from non-local density functional theory. SEM images of d TFPT-BTAN and e TFPT-BTAN-AO. f FT-IR spectra and g PXRD profiles of TFPT-BTAN-AO before and after treatment with water (100 °C), HCl (1 M), NaOH (1 M), and γ-ray irradiation (50 kGy, 200 kGy).
We prepared TFPT-BTAN-AO by reacting crystalline and porous TFPT-BTAN with NH2OH·HCl. The PXRD pattern of TFPT-BTAN-AO shows a diffraction pattern comparable to the one of TFPT-BTAN with a strong diffraction peak at 5.8° (Fig. 2a), indicating that crystallinity was well retained after the amidoximation process. N2 adsorption–desorption isotherm were performed to verify pore accessibility after the post-modification process, affording isotherms comparable to those of TFPT-BTAN. The BET surface area was evaluated to be 803 m2 g−1, indicating that porosity was well retained after the amidoximation process (Fig. 2c). As shown in Supplementary Fig. 5, the vibration peak of -CN (at ~2241 cm−1) disappeared in TFPT-BTAN-AO, and the vibration peaks of the amidoxime groups can be observed at 1403 and 1707 cm−1, confirming the successful amidoximation1. Furthermore, solid-state 13C CP/MAS NMR spectra confirmed this successful conversion, as indicated by the disappearance of the peak at ~113 ppm that is assigned to carbon atoms in the cyano groups together with the concomitant appearance of a peak at ~156 ppm assigned to the carbon atoms in amidoxime groups (Supplementary Fig. 3)1,35. The obtained TFPT-BTAN and TFPT-BTAN-AO were pale-yellow powders. The scanning electron micrograph of TFPT-BTAN shows a porous network structure (Fig. 2d), which did not change after amidoxime functionalization (Fig. 2e), indicating that TFPT-BTAN-AO can be rapidly and thoroughly penetrated with UO22+.
Thermogravimetric analysis profiles revealed that both the TFPT-BTAN and TFPT-BTAN-AO were stable up to 320 °C, indicating that good thermal stability (Supplementary Fig. 6). In addition, we treated TFPT-BTAN-AO under different harsh conditions for 12 h to study chemical stability. The FT-IR spectra (Fig. 2f and Supplementary Fig. 7a) and PXRD (Fig. 2g and Supplementary Fig. 7b) both show that TFPT-BTAN-AO retained the same characteristic peaks after the treatments, confirming its remarkable chemical stability. In order to reflect the superior stability of TFPT-BTAN-AO compared with other COFs, two β-ketoenamine COFs (Tp-Bpy and Tp-BD) were synthesized by the reported method30. After treatment with high concentrations of nitric acid (3.0 M and 5.0 M), the crystallinity of Tp-Bpy and Tp-BD was completely destroyed (Supplementary Fig. 7c and d), whereas our TFPT-BTAN-AO maintained good crystallinity and stability. The results show that TFPT-BTAN-AO has superior stability in high concentration nitric acid compared with β-ketoenamine COFs. Moreover, TFPT-BTAN-AO retained a high residual mass (≥93.5%) after treatment with different concentrations of nitric acid, further indicating its excellent stability (Supplementary Table 3). All of these indicated successful synthesis of stable TFPT-BTAN-AO.
Selective sensing of UO22+
After demonstrating the synthesis, we then studied the sensing performance of our COF. The normalized fluorescence spectra of TFPT-BTAN and TFPT-BTAN-AO dispersed in water are shown in Supplementary Fig. 8. Compared with TFPT-BTAN, the aminoximation process introduced a large amount of -OH and -NH2, thereby reducing the conjugation effect in the TFPT-BTAN-AO. As a result, the emission spectrum of TFPT-BTAN-AO blue shifted compared to TFPT-BTAN, which further indicates successful amidoximation. By excitation at 277 nm, TFPT-BTAN-AO emitted bright blue fluorescence at 460 nm, showing a high absolute fluorescence quantum yield of 4.3%. With a high-quantum yield and specific affinity uranium-binding groups, we then studied its UO22+-sensing performance. A stock solution of TFPT-BTAN-AO was added to the solution containing UO22+, and then diluted with ultrapure water for fluorescence measurement (Supplementary Fig. 9). The fluorescence of TFPT-BTAN-AO was significantly quenched by UO22+, and the optimal pH for detection was at 6.0 (Supplementary Fig. 10). Importantly, TFPT-BTAN-AO responded very quickly to UO22+, and the system can reach equilibrium within 2 s (Supplementary Fig. 11), much faster than other detection systems (Supplementary Table 4). These results suggest that the 1D channels could promote the rapid diffusion and mass transfer of UO22+ and achieve real-time detection.
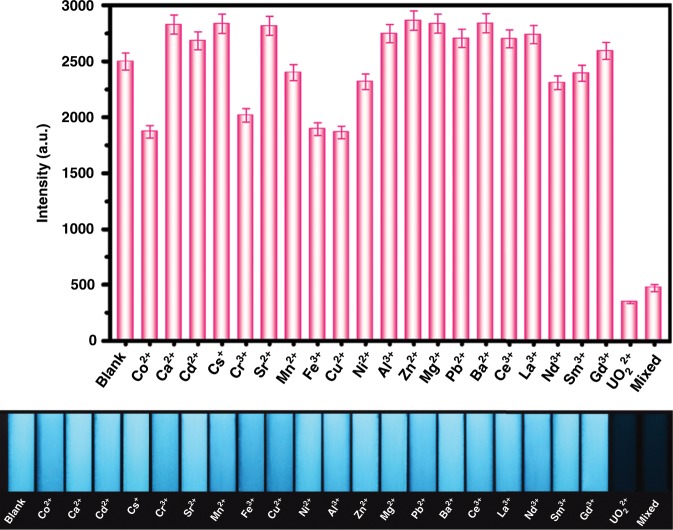
To test selectivity, UO22+ (20 μM) was added directly to the TFPT-BTAN-AO aqueous dispersion, whereas other metal ions were added at 50 μM (Fig. 3 and Supplementary Fig. 12). Only UO22+ caused significant fluorescence quenching, other metal ions have little effect on the UO22+ detection, and it can be visually observed under a portable 365 nm UV lamp, indicating that TFPT-BTAN-AO has good selectivity for UO22+ attributable to the specific affinity between UO22+ and amidoxime groups1, and the fluorescence quenching property of UO22+.
Fig. 3. Selectivity investigations.
Fluorescence intensity of TFPT-BTAN-AO at 460 nm in the presence of various metal ions and mixed ions. Concentrations of UO22+ and other metal ions were 20 μM and 50 μM, respectively. Photographs showing the fluorescence emission change (under a portable 365 nm UV lamp) of TFPT-BTAN-AO with various metal ions. Error bars represent S.D. n = 3 independent experiments.
Highly sensitive sensing of UO22+
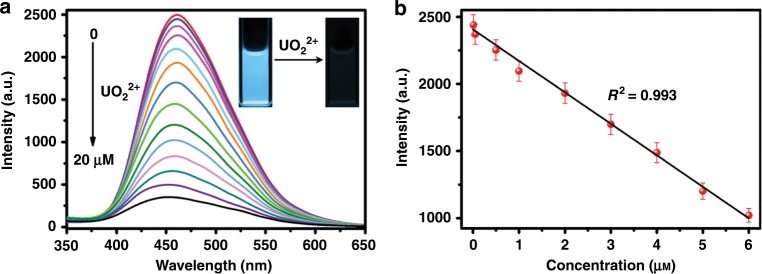
To explore the sensitivity, the fluorescence spectra of TFPT-BTAN-AO were measured at different UO22+ concentrations (Fig. 4a). The fluorescence intensity of TFPT-BTAN-AO decreased with the increasing concentration of UO22+ and 87% of the fluorescence was quenched with 20 μM UO22+. In addition, the fluorescence response to UO22+ was clearly observed under a 365 nm UV lamp. Figure 4b shows a good calibration curve for the fluorescence intensity of TFPT-BTAN-AO at 460 nm versus UO22+ concentration (0.02–6.0 μM) with a high correlation coefficient of 0.993. The limit of detection of the TFPF-BTAN-AO was determined as 6.7 nM UO22+ (Supplementary Fig. 13), well below the World Health Organization contamination limit for UO22+ in drinking water (63 nM)7. Therefore TFPF-BTAN-AO can be used for high sensitivity detection of UO22+.
Fig. 4. Sensitivity investigations.
a Fluorescence emission spectra of TFPT-BTAN-AO upon gradual addition of UO22+ (λex = 277 nm). Inset photos show the fluorescence emission change (under a 365 nm UV lamp) of TFPT-BTAN-AO after addition of UO22+. b Fluorescence intensity at 460 nm versus the concentration of UO22+. Inset: The linear calibration plot for UO22+ detection. Error bars represent S.D. n = 3 independent experiments.
Interaction between TFPT-BTAN-AO and UO22+
FT-IR and X-ray photoelectron spectroscopy (XPS) were applied to investigate the effective uranium-binding and fluorescence quenching. The FT-IR spectrum of TFPT-BTAN-AO after treatment with UO22+ shows a new peak at 916 cm−1, which can be attributed to the vibration of O═U═O (Supplementary Fig. 14)42. In addition, the appearance of N-H bending vibration at 1613 cm−1 after treatment with UO22+ indicates the presence of a chemisorption process8. After treating the TFPT-BTAN-AO with UO22+, distinctive U 4 f-binding energy peaks emerged, revealing that UO22+ was successfully loaded onto the TFPT-BTAN-AO (Supplementary Fig. 15)43,44.
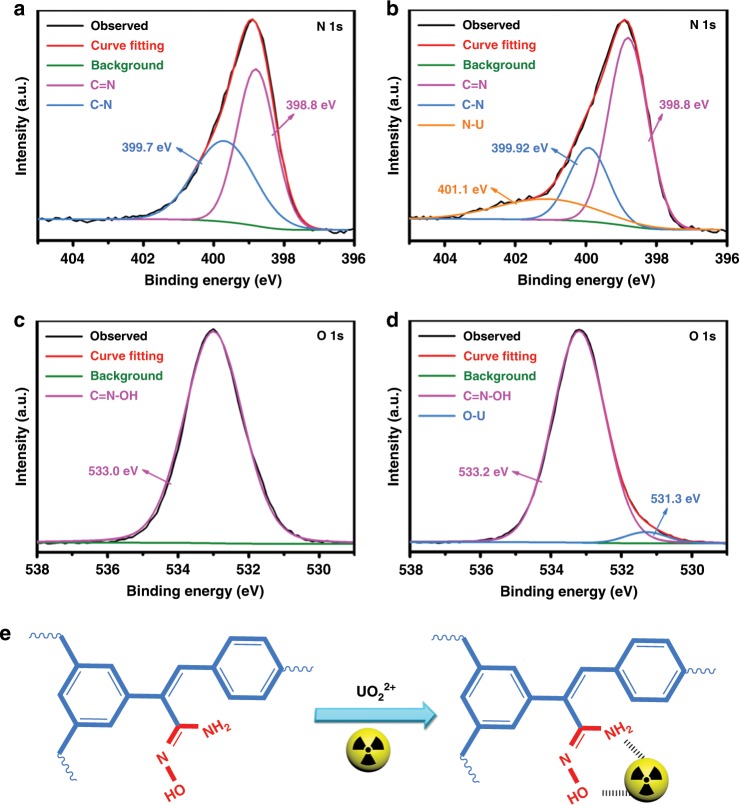
In the high-resolution XPS spectrum of N 1 s of the TFPT-BTAN-AO (Fig. 5a), the two binding energy peaks at 399.7 and 398.8 eV are assigned to C-N and C═N, respectively, which correspond to the nitrogen atoms in the TFPT-BTAN-AO framework42,45. After treatment with UO22+, the N 1 s XPS spectrum of the TFPT-BTAN-AO was observed under the same measurement conditions (Fig. 5b). Comparing the N 1 s binding energy peaks in the two samples, a new N-U peak (401.1 eV) formed, and the peak located at 399.7 eV of the TFPT-BTAN-AO moved 0.22 eV to a higher binding energy after treatment with UO22+. However, the N 1 s peaks at 398.8 eV in Fig. 5a, b show no shift after treatment with UO22+, revealing that the nitrogen atoms of the C═N did not bind to UO22+. Comparing the O 1 s peaks in the two samples (Fig. 5c, d), it is clearly observed that a new O-U peak (531.3 eV) formed, and the O 1 s core peak of the TFPT-BTAN-AO moved 0.2 eV to a higher binding energy after treatment with UO22+. Based on the above results, it is speculated that the adsorption of UO22+ onto the TFPT-BTAN-AO is a chemical process, and both the amino and hydroxyl groups in TFPT-BTAN-AO are coordinated with UO22+ (Fig. 5e), similar to the previously reported binding mode46.
Fig. 5. XPS data, fits, and interaction between TFPT-BTAN-AO and UO22+.
XPS spectra of the N 1 s region of TFPT-BTAN-AO a before and b after treatment with UO22+. XPS spectra of the O 1 s region of TFPT-BTAN-AO c before and d after treatment with UO22+. e Schematic diagram of the interaction between TFPT-BTAN-AO and UO22+.
The quenching effect of UO22+ on TFPT-BTAN-AO was further studied by time-resolved fluorescence spectroscopy. The fluorescence decay profile shows that TFPT-BTAN-AO has a lifetime of 3.1 ns (Supplementary Fig. 16, red curve). Upon addition of UO22+, the lifetime decreased to 1.8 ns (blue curve), which is consistent with a decrease in fluorescence intensity. UO22+ induced quenching of TFPT-BTAN-AO likely to proceed by a photoinduced electron transfer (PET) process from the framework to UO22+.
Efficient extraction of UO22+
Given the very stable (-C═C-) bonds and abundant selective UO22+-binding groups on the 1D channels, this material may serve for high performance UO22+ extraction under harsh conditions. To test the importance of the regular porous structure for adsorbing UO22+, an amorphous analog named POP-TB was synthesized and subjected to the same amidoximation to give POP-TB-AO (Supplementary Figs. 17–19). Both the COF-based materials and their amorphous POP analogs were demonstrated very similar FT-IR spectra and nitrogen element contents before and after the amidoximation process (Supplementary Figs. 20 and 21), indicating similar amidoxime functionalization processes.
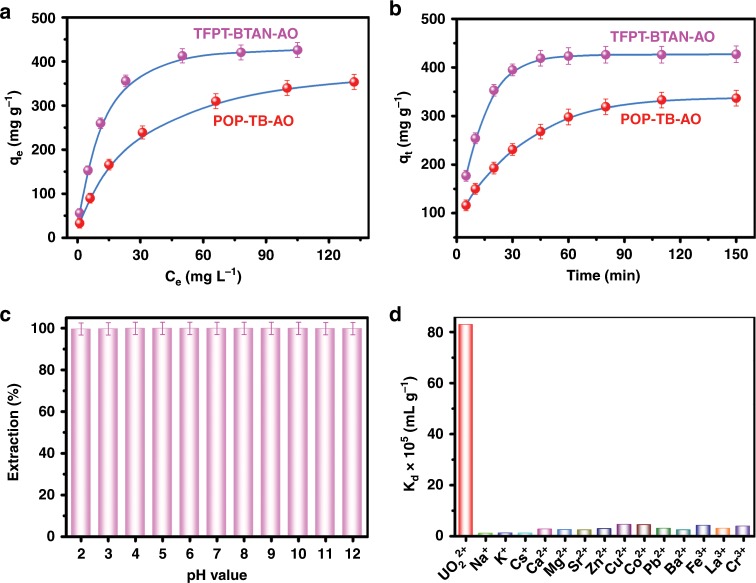
The adsorption isotherm of TFPT-BTAN-AO has a much steeper adsorption curve for UO22+ (Fig. 6a), indicating that its affinity for UO22+ is higher than that of corresponding POP-TB-AO24. All the adsorption experimental data are in good agreement with the Langmuir isotherm model, and the correlation coefficients are higher than 0.99 (Supplementary Fig. 22). Surprisingly, the maximum adsorption capacity for UO22+ on TFPT-BTAN-AO (427 mg g−1) is much higher than that of POP-TB-AO (353 mg g−1), and is located among the top of different types of adsorbents (Supplementary Table 5). Importantly, the superior performance of TFPT-BTAN-AO exceeded all previously reported COFs for UO22+ extraction, like COF-TpDb-AO (408 mg g−1)1, ACOF (169 mg g−1)43, and o-GS-COF (144.2 mg g−1)3. The uranium content loaded on the framework was calculated based on the ICP-MS results, this capacity means that 66.8% accessibility of the amidoxime groups in TFPT-BTAN-AO were used to extract UO22+. The TFPT-BTAN-AO showed exceptional performance in extraction of UO22+ in terms of saturated adsorption capacity as compared to POP-TB-AO, suggesting the important role of the adsorbent’s architecture. This surprisingly high saturation UO22+ extraction capacity can be attributed to synergistic effect of the rich and even distribution of amidoxime groups on the pore walls and the higher accessibility of UO22+ in the open 1D channel.
Fig. 6. UO22+ adsorption isotherms and kinetics investigations.
a Adsorption isotherm of UO22+ on TFPT-BTAN-AO and POP-TB-AO (pH 4.0). b Adsorption kinetics of UO22+ on TFPT-BTAN-AO and POP-TB-AO (pH 4.0). c The removal efficiency of UO22+ under different pH conditions. d The selective adsorption of the test ions. Error bars represent S.D. n = 3 independent experiments.
In addition to the high adsorption capacity, TFPT-BTAN-AO also extracted UO22+ from aqueous solution more rapidly compared with POP-TB-AO (Fig. 6b). All the adsorption kinetics data are in good agreement with the pseudo-second-order model, and the correlation coefficients are higher than 0.995 (Supplementary Fig. 23). It is worth noting that TFPT-BTAN-AO can achieve a saturation capacity of about 98% within 45 min. This is in sharp contrast to the long contact times required for other uranium sorbent materials, which typically range from hours to days7,47–49. For comparison, POP-TB-AO took 85 min to reach 95% of its equilibrium adsorption capacity.
Apart from rapid equilibration, TFPT-BTAN-AO also has higher extraction capacity with an equilibrium capacity of 417 mg g−1, whereas POP-TB-AO only reaches 336 mg g−1 (Supplementary Fig. 24). Considering that TFPT-BTAN-AO and POP-TB-AO have similar chemical compositions, the high absorption capacity and rapid adsorption kinetics should be attributed to their different pore structures. The rapid kinetics of TFPT-BTAN-AO can be attributed to the hierarchical pores structure and the evenly and densely distributed chelating sites on the pore walls to facilitate rapid diffusion of UO22+ throughout the framework (Supplementary Fig. 25). In contrast, the pore structure in POP-TB-AO is irregular, making it more susceptible to clogging, thus greatly impairing their adsorption performance.
For practical applications, extraction of UO22+ under various harsh conditions is highly desirable. Considering that UO22+ is mainly present in the acidic environment and hydrolysis occurs at a higher pH (Supplementary Fig. 26), adsorption experiments were performed at pH < 5.0. Supplementary Fig. 27 shows the adsorption capacity of UO22+ on TFPT-BTAN-AO at different pH values. Obviously, the adsorption capacity increases as the system pH value rises from 1.0 to 4.0.
As nuclear fuel reprocessing and wastewater are usually treated under highly acidic conditions, it is necessary to evaluate the effect of solution acidity on UO22+ extraction by TFPT-BTAN-AO (Supplementary Fig. 27). Surprisingly, TFPT-BTAN-AO showed high adsorption capacity in highly acidic media, the saturated capacity in 3.0 M nitric acid was calculated to be 128 mg g−1, this capacity is much higher than COF-IHEP1 (70 mg g−1, 2 M nitric acid)50. To further confirm the chemical stability and excellent uranium extraction performance of TFPT-BTAN-AO, the adsorption capacities of TFPT-BTAN-AO after treatment with water (100 °C), HCl (1 M), HNO3 (0.1–5.0 M), NaOH (1 M), and γ-ray irradiation (50 kGy, 200 kGy) were also studied (Supplementary Fig. 28). The results verified that the uranium extraction performance of TFPT-BTAN-AO was almost unchanged after treatment under various extreme conditions, indicating that TFPT-BTAN-AO has excellent stability and practical application potential. This feature is a significant advantage over imine-based COFs sorbents that typically suffer from the decomposition of structures under extreme conditions1,3.
In addition, the uranium concentration reduced from 9.952 ppm to 8.45 ppb and 6.17 ppb at pH values of 2 and 12, respectively (Fig. 6c). It is well below the US Environmental Protection Agency (EPA) uranium-containing wastewater discharge standard (30 ppb)1. To evaluate the affinity of TFPT-BTAN-AO toward UO22+, additional selective extraction experiments with 9.952 ppm UO22+ (V/m = 5000 mL g−1) were carried out. The calculated distribution coefficient Kd (8.3 × 106 mL g−1) is much larger than the distinguishing standard (1.0 × 104 mL g−1), which is generally considered to be a good adsorbent (Fig. 6d), indicating the excellent affinity of TFPT-BTAN-AO for UO22+47. We attribute the enormous distribution coefficient to the specific affinity for UO22+ provided by the 1D open channel amidoxime groups in TFPT-BTAN-AO. The above results indicate the superiority of TFPT-BTAN-AO as a promising candidate for uranium extraction.
Reversible binding for regeneration
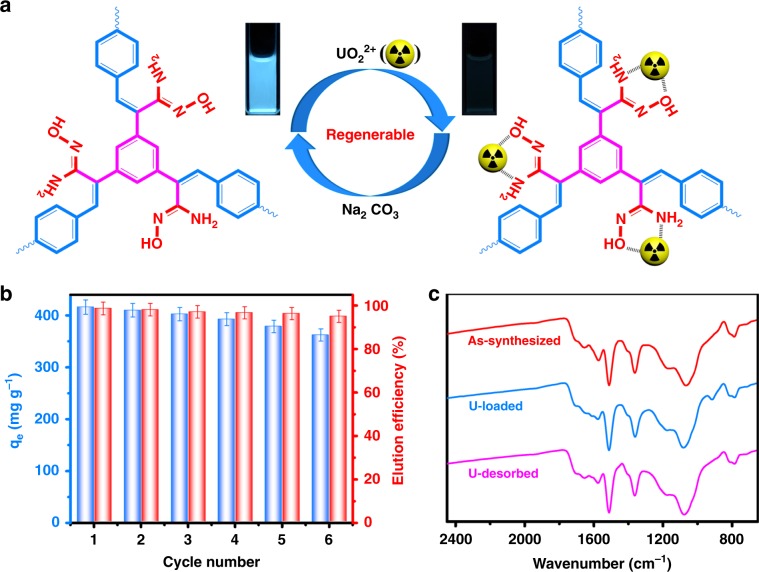
One of the unique advantages of TFPT-BTAN-AO is that the very stable framework provides the most critical foundation for the reversible binding of UO22+. We found that the fluorescence of TFPT-BTAN-AO can be easily recovered by Na2CO3 (1 M) aqueous solution (Fig. 7a). To confirm the practical reusability of TFPT-BTAN-AO as an adsorbent, we conducted multiple adsorption–desorption experiments and found that sodium carbonate has a high elution efficiency (>95%) even after six cycles (Fig. 7b). In addition, elution of UO22+ with sodium carbonate did not affect the adsorption performance of TFPT-BTAN-AO, and maintained a high adsorption capacity (>87%) even after six cycles. More importantly, owing to the excellent stability of the framework, TFPT-BTAN-AO crystal structure and functional groups were well preserved after recycling, as evidenced by PXRD and FT-IR results (Supplementary Fig. 29 and Fig. 7c). It is worth noting that TFPT-BTAN-AO can be cycled at least six times without noticeable influence of response to UO22+ or sensitivity, and this exceptional regeneration can be observed by the naked eye under a portable UV lamp (Supplementary Fig. 30). So far, this is the first demonstration of COF-based regenerable detection and extraction of UO22+. The above results indicate that TFPT-BTAN-AO has great potential for simultaneous detection and extraction of UO22+. Importantly, such regeneration is almost impossible for previously reported imine-based COFs adsorbents.
Fig. 7. UO22+ adsorption and regeneration investigations.
a Schematic diagram of TFPT-BTAN-AO regeneration detection and extraction of UO22+. b The UO22+ adsorption capacity (left axis) and elution efficiency (right axis) of TFPT-BTAN-AO in six successive adsorption–desorption cycles. c FT-IR spectra of TFPT-BTAN-AO, TFPT-BTAN-AO after extraction of UO22+, and TFPT-BTAN-AO after desorption of UO22+ by the Na2CO3 (1.0 mol L−1) aqueous solution. Error bars represent S.D. n = 3 independent experiments.
Discussion
In summary, we have successfully developed a strongly fluorescent COF for real-time detection and efficient extraction of UO22+. Different from previous COF sorbents relying on imine bond (-C═N-), our COF used very stable carbon–carbon double bonds (-C═C-). Our COF combines strong fluorescence, excellent stability, dense, and evenly distributed amidoxime groups, and highly accessible binding sites through the open 1D channel. With these advantages, TFPT-BTAN-AO achieved real-time sensitive detection, efficient extraction, and efficient regeneration by simply adding carbonate. Given the wealth of knowledge in designing contaminant-specific ligands, this strategy may extend to the detection and extraction of other environmental contaminants.
Methods
Materials
2,4,6-Tris(4-bromophenyl)-1,3,5-triazine, n-BuLi, hydroxylamine hydrochloride (NH2OH·HCl), DBU, and triethylamine were purchased from Saan Chemical Technology (Shanghai) Co., Ltd. Benzaldehyde and 1,3,5-tris(bromomethyl)benzene were purchased from Jilin Chinese Academy of Sciences-Yanshen Technology Co., Ltd. Mesitylene, 1,4-dioxane, acetone, chloroform (CHCl3), tetrahydrofuran (THF), N,N-dimethylformamide, sodium bicarbonate, sodium cyanide, n-hexane, n-BuOH, NaOH, EtONa, Cs2CO3, piperidine, pyridine, 1,2-dichlorobenzene, and MgSO4 were purchased from Sinopharm Chemical Reagent Co., Ltd. Ultrapure water was prepared from the Millipore system (18.25 MΩ-cm). All the purchased reagents were of analytical grade and used without further purification.
Synthesis of model compound
To a 25 mL Pyrex tube, benzaldehyde (63.67 mg, 0.60 mmol), 2,2′,2″-(benzene-1,3,5-triyl)triacetonitrile (39.04 mg, 0.20 mmol), o-DCB (5 mL) and DBU aqueous solution (0.5 mL, 4 M) were added. The mixture was sonicated for 10 minutes, degassed by three freeze–pump–thaw cycles, sealed under vacuum and heated at 90 °C for 3 days. The reaction mixture was cooled to room temperature, the precipitate was filtered and washed several times with ethanol. The product was obtained as a pale-yellow solid. Yield: 72%. 1H NMR (CDCl3, δ (ppm)): 7.99 (m, 6 H, ArH), 7.96 (s, 3 H, Ar-H), 7.71 (s, 3 H, vinyl), 7.52 (m, 9 H, Ar-H). 13C NMR (DMSO-d6, δ (ppm)): 150.44, 140.99, 138.61, 136.29, 134.54, 134.24, 129.01, 122.75, 114.31. Elemental analysis: calculated C (86.25%), H (4.61%), N (9.14%) and observed C (86.09%), H (4.45%), N (9.03%).
Synthesis of TFPT-BTAN COF
To a 25 mL Pyrex tube, 2,4,6-tris(4-formylphenyl)-1,3,5-triazine (78.68 mg, 0.20 mmol), 2,2′,2″-(benzene-1,3,5-triyl)triacetonitrile (39.04 mg, 0.20 mmol), o-DCB (5 mL) and DBU aqueous solution (0.5 mL, 4 M) were added. The mixture was sonicated for 10 minutes, degassed by three freeze–pump–thaw cycles, sealed under vacuum and heated at 90 °C for 5 days. The reaction mixture was cooled to room temperature, and a pale-yellow precipitate was collected by centrifugation, washed several times with methanol, CH2Cl2, and THF, respectively. It was then Soxhlet extracted in CH2Cl2 and THF for 24 h and dried under vacuum at 80 °C for 12 h to afford pale-yellow powder, 67% yield. Elemental analysis: calculated C (79.10%), H (5.53%), N (15.37%), and observed C (74.31%), H (5.72%), N (15.65%). For other conditions, follow the same experimental procedure to obtain TFPT-BTAN COF, as shown in the Supplementary Table 1.
Synthesis of TFPT-BTAN-AO
The TFPT-BTAN (0.4 g) was swollen in absolute ethanol (40 mL) for 20 min, followed by the addition of NH2OH·HCl (1.0 g) and N(CH2CH3)3 (1.5 g). After stirring at 85 °C for 24 h, the mixture was filtered, washed with excess water and finally dried at 60 °C under vacuum. The pale-yellow solid obtained was expressed as TFPT-BTAN-AO. Elemental analysis: calculated C (66.96%), H (6.09%), N (19.52%), and observed C (65.31%), H (6.22%), N (19.75%).
Synthesis of POP-TB
To a 25 mL Pyrex tube, 2,4,6-Tris(4-formylphenyl)-1,3,5-triazine (78.68 mg, 0.20 mmol), 2,2′,2″-(benzene-1,3,5-triyl)triacetonitrile (39.04 mg, 0.20 mmol), 1,4-dioxane (5 mL), and NaOH aqueous solution (0.5 mL, 4 M) were added. The mixture was sonicated for 10 minutes, degassed by three freeze–pump–thaw cycles, sealed under vacuum and heated at 90 °C for 5 days. The reaction mixture was cooled to room temperature, and a pale-yellow precipitate was collected by centrifugation, washed several times with methanol, CH2Cl2, and THF, respectively. It was then Soxhlet extracted in CH2Cl2 and THF for 24 h and dried under vacuum at 80 °C for 12 h to afford pale-yellow powder, 70% yield. Elemental analysis: observed C (74.16%), H (5.64%), N (15.53%).
Synthesis of POP-TB-AO
The POP-TB (0.4 g) was swollen in absolute ethanol (40 mL) for 20 min, followed by the addition of NH2OH·HCl (1.0 g) and N(CH2CH3)3 (1.5 g). After stirring at 85 °C for 24 h, the mixture was filtered, washed with excess water and finally dried at 60 °C under vacuum. The pale-yellow solid obtained was expressed as POP-TB-AO. Elemental analysis: observed C (65.15%), H (6.13%), N (19.58%).
Supplementary information
Acknowledgements
We gratefully acknowledge the supports from the National Natural Science Foundation of China (21675078, 21775065, and 21976077), and the Natural Science Foundation of Jiangxi Province (20165BCB18022).
Author contributions
J.-D.Q. conceived and designed the research. W.-R.C. performed the synthesis and conducted the experiments, and C.-R.Z., W.J. and F.-F.L. performed the characterizations. W.-R.C., R.-P.L., J.L. and J.-D.Q. participated in drafting the paper and gave approval to the final version of the manuscript.
Data availability
All data are either provided in the Article and its Supplementary Information or are available from the corresponding author upon request.
Competing interests
The authors declare no competing interests.
Footnotes
Peer review information Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information is available for this paper at 10.1038/s41467-020-14289-x.
References
- 1.Qi S, et al. Covalent organic frameworks as a decorating platform for utilization and affinity enhancement of chelating sites for radionuclide sequestration. Adv. Mater. 2018;30:1705479. doi: 10.1002/adma.201705479. [DOI] [PubMed] [Google Scholar]
- 2.Abney CW, et al. Materials for the recovery of uranium from seawater. Chem. Rev. 2017;117:13935–14013. doi: 10.1021/acs.chemrev.7b00355. [DOI] [PubMed] [Google Scholar]
- 3.Wen R, et al. Graphene-synergized 2D covalent organic framework for adsorption: a mutual promotion strategy to achieve stabilization and functionalization simultaneously. J. Hazard. Mater. 2018;358:273–285. doi: 10.1016/j.jhazmat.2018.06.059. [DOI] [PubMed] [Google Scholar]
- 4.Xiao F, et al. Smart photonic crystal hydrogel material for uranyl ion monitoring and removal in water. Adv. Funct. Mater. 2017;27:1702147. doi: 10.1002/adfm.201702147. [DOI] [Google Scholar]
- 5.Sun Q, et al. Bio-inspired nano-traps for uranium extraction from seawater and recovery from nuclear waste. Nat. Commun. 2018;9:1644. doi: 10.1038/s41467-018-04032-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ma S, et al. Efficient uranium capture by polysulfide/layered double hydroxide composites. J. Am. Chem. Soc. 2015;137:3670–3677. doi: 10.1021/jacs.5b00762. [DOI] [PubMed] [Google Scholar]
- 7.Xu M, et al. Highly fluorescent conjugated microporous polymers for concurrent adsorption and detection of uranium. J. Mater. Chem. A. 2019;7:11214–11222. doi: 10.1039/C8TA11764K. [DOI] [Google Scholar]
- 8.Liu W, et al. Highly sensitive and selective uranium detection in natural water systems using a luminescent mesoporous metal–organic framework equipped with abundant lewis basic sites: a combined batch, X-ray absorption spectroscopy, and first principles simulation investigation. Environ. Sci. Technol. 2017;51:3911–3921. doi: 10.1021/acs.est.6b06305. [DOI] [PubMed] [Google Scholar]
- 9.Aguila B, et al. Efficient mercury capture using functionalized porous organic polymer. Adv. Mater. 2017;29:1700665. doi: 10.1002/adma.201700665. [DOI] [PubMed] [Google Scholar]
- 10.Fu Y, et al. Sulfur-rich covalent triazine polymer nanospheres for environmental mercury removal and detection. Polym. Chem. 2018;9:4125–4131. doi: 10.1039/C8PY00419F. [DOI] [Google Scholar]
- 11.Xu L, et al. Nano-MOF+ technique for efficient uranyl remediation. ACS Appl. Mater. Interfaces. 2019;11:21619–21626. doi: 10.1021/acsami.9b06068. [DOI] [PubMed] [Google Scholar]
- 12.Ly HGT, et al. Superactivity of MOF-808 toward peptide bond hydrolysis. J. Am. Chem. Soc. 2018;140:6325–6335. doi: 10.1021/jacs.8b01902. [DOI] [PubMed] [Google Scholar]
- 13.Paille G, et al. A fully noble metal-free photosystem based on cobalt-polyoxometalates immobilized in a porphyrinic metal–organic framework for water oxidation. J. Am. Chem. Soc. 2018;140:3613–3618. doi: 10.1021/jacs.7b11788. [DOI] [PubMed] [Google Scholar]
- 14.Lu S, et al. Synthesis of ultrafine and highly dispersed metal nanoparticles confined in a thioether-containing covalent organic framework and their catalytic applications. J. Am. Chem. Soc. 2017;139:17082–17088. doi: 10.1021/jacs.7b07918. [DOI] [PubMed] [Google Scholar]
- 15.Aiyappa HB, et al. Cobalt-modified covalent organic framework as a robust water oxidation electrocatalyst. Chem. Mater. 2016;28:4375–4379. doi: 10.1021/acs.chemmater.6b01370. [DOI] [Google Scholar]
- 16.Pachfule P, et al. Diacetylene functionalized covalent organic framework (COF) for photocatalytic hydrogen generation. J. Am. Chem. Soc. 2018;140:1423–1427. doi: 10.1021/jacs.7b11255. [DOI] [PubMed] [Google Scholar]
- 17.Bhadra M, et al. Predesigned metal-anchored building block for in situ generation of Pd nanoparticles in porous covalent organic framework: application in heterogeneous tandem catalysis. ACS Appl. Mater. Interfaces. 2017;9:13785–13792. doi: 10.1021/acsami.7b02355. [DOI] [PubMed] [Google Scholar]
- 18.Bhadra M, et al. Triazine functionalized porous covalent organic framework for photo-organocatalytic E–Z isomerization of olefins. J. Am. Chem. Soc. 2019;141:6152–6156. doi: 10.1021/jacs.9b01891. [DOI] [PubMed] [Google Scholar]
- 19.Ning H, et al. Two-dimensional covalent organic frameworks for carbon dioxide capture through channel-wall functionalization. Angew. Chem. 2015;127:3029–3033. doi: 10.1002/ange.201411262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rabbani MG, et al. A 2D mesoporous imine-linked covalent organic framework for high pressure gas storage applications. Chem. Eur. J. 2013;19:3324–3328. doi: 10.1002/chem.201203753. [DOI] [PubMed] [Google Scholar]
- 21.Li Z, et al. An azine-linked covalent organic framework: synthesis, characterization and efficient gas storage. Chem. Eur. J. 2015;21:12079–12084. doi: 10.1002/chem.201501206. [DOI] [PubMed] [Google Scholar]
- 22.El-Mahdy AFM, et al. Hollow microspherical and microtubular [3+3] carbazole-based covalent organic frameworks and their gas and energy storage applications. ACS Appl. Mater. Interfaces. 2019;11:9343–9354. doi: 10.1021/acsami.8b21867. [DOI] [PubMed] [Google Scholar]
- 23.Sun Q, et al. Postsynthetically modified covalent organic frameworks for efficient and effective mercury removal. J. Am. Chem. Soc. 2017;139:2786–2793. doi: 10.1021/jacs.6b12885. [DOI] [PubMed] [Google Scholar]
- 24.Huang N, et al. Stable covalent organic frameworks for exceptional mercury removal from aqueous solutions. J. Am. Chem. Soc. 2017;139:2428–2434. doi: 10.1021/jacs.6b12328. [DOI] [PubMed] [Google Scholar]
- 25.Ding S-Y, et al. Thioether-based fluorescent covalent organic framework for selective detection and facile removal of mercury(II) J. Am. Chem. Soc. 2016;138:3031–3037. doi: 10.1021/jacs.5b10754. [DOI] [PubMed] [Google Scholar]
- 26.Li Y, et al. Fabrication of hydrazone-linked covalent organic frameworks using alkyl amine as building block for high adsorption capacity of metal ions. ACS Appl. Mater. Interfaces. 2019;11:11706–11714. doi: 10.1021/acsami.8b18502. [DOI] [PubMed] [Google Scholar]
- 27.Mon M, et al. Fine-tuning of the confined space in microporous metal–organic frameworks for efficient mercury removal. J. Mater. Chem. A. 2017;5:20120–20125. doi: 10.1039/C7TA06199D. [DOI] [Google Scholar]
- 28.Cui W-R, et al. Covalent organic framework nanosheet-based ultrasensitive and selective colorimetric sensor for trace Hg2+ detection. ACS Sustain. Chem. Eng. 2019;7:9408–9415. doi: 10.1021/acssuschemeng.9b00613. [DOI] [Google Scholar]
- 29.Diercks CS, et al. Reticular electronic tuning of porphyrin active sites in covalent organic frameworks for electrocatalytic carbon dioxide reduction. J. Am. Chem. Soc. 2018;140:1116–1122. doi: 10.1021/jacs.7b11940. [DOI] [PubMed] [Google Scholar]
- 30.Cui W-R, et al. Covalent organic framework nanosheets for fluorescence sensing via metal coordination. ACS Appl. Nano Mater. 2019;2:5342–5349. doi: 10.1021/acsanm.9b01366. [DOI] [Google Scholar]
- 31.Ding S-Y, et al. Construction of covalent organic framework for catalysis: Pd/COF-LZU1 in suzuki-miyaura coupling reaction. J. Am. Chem. Soc. 2011;133:19816–19822. doi: 10.1021/ja206846p. [DOI] [PubMed] [Google Scholar]
- 32.Zhang G, et al. Water-dispersible PEG-curcumin/amine-functionalized covalent organic framework nanocomposites as smart carriers for in vivo drug delivery. Nat. Commun. 2018;9:2785. doi: 10.1038/s41467-018-04910-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen R, et al. Designed synthesis of a 2D porphyrin-based sp2 carbon-conjugated covalent organic framework for heterogeneous photocatalysis. Angew. Chem. Int. Ed. 2019;58:6430–6434. doi: 10.1002/anie.201902543. [DOI] [PubMed] [Google Scholar]
- 34.Jin E, et al. Two-dimensional sp2 carbon–conjugated covalent organic frameworks. Science. 2017;357:673. doi: 10.1126/science.aan0202. [DOI] [PubMed] [Google Scholar]
- 35.Zhao Y, et al. Fully conjugated two-dimensional sp2-carbon covalent organic frameworks as artificial photosystem I with high efficiency. Angew. Chem. Int. Ed. 2019;58:5376–5381. doi: 10.1002/anie.201901194. [DOI] [PubMed] [Google Scholar]
- 36.Xu J, et al. An olefin-linked covalent organic framework as a flexible thin-film electrode for a high-performance micro-supercapacitor. Angew. Chem. Int. Ed. 2019;58:12065–12069. doi: 10.1002/anie.201905713. [DOI] [PubMed] [Google Scholar]
- 37.Zhang Z, et al. 3,4-Hydroxypyridinone-modified carbon quantum dot as a highly sensitive and selective fluorescent probe for the rapid detection of uranyl ions. Environ. Sci. 2019;6:1457–1465. [Google Scholar]
- 38.Li Y, et al. Growth of high-quality covalent organic framework nanosheets at the interface of two miscible organic solvents. Nanoscale Horiz. 2018;3:205–212. doi: 10.1039/C7NH00172J. [DOI] [PubMed] [Google Scholar]
- 39.Zhang D, et al. Colorimetric peroxidase mimetic assay for uranyl detection in sea water. ACS Appl. Mater. Interfaces. 2015;7:4589–4594. doi: 10.1021/am507361x. [DOI] [PubMed] [Google Scholar]
- 40.Wen J, et al. Aggregation-induced emission active tetraphenylethene-based sensor for uranyl ion detection. J. Hazard. Mater. 2016;318:363–370. doi: 10.1016/j.jhazmat.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 41.Bi S, et al. Two-dimensional semiconducting covalent organic frameworks via condensation at arylmethyl carbon atoms. Nat. Commun. 2019;10:2467. doi: 10.1038/s41467-019-10504-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu X, et al. Amidoxime-functionalized hollow carbon spheres for efficient removal of uranium from wastewater. ACS Sustain. Chem. Eng. 2019;7:10800–10807. doi: 10.1021/acssuschemeng.9b01616. [DOI] [Google Scholar]
- 43.Li X, et al. Solvent- and catalyst-free synthesis of an azine-linked covalent organic framework and the induced tautomerization in the adsorption of U(vi) and Hg(ii) Green. Chem. 2019;21:649–657. doi: 10.1039/C8GC03295E. [DOI] [Google Scholar]
- 44.Aguila B, et al. Design strategies to enhance amidoxime chelators for uranium recovery. ACS Appl. Mater. Interfaces. 2019;11:30919–30926. doi: 10.1021/acsami.9b09532. [DOI] [PubMed] [Google Scholar]
- 45.Ma C, et al. Sunlight polymerization of poly(amidoxime) hydrogel membrane for enhanced uranium extraction from seawater. Adv. Sci. 2019;6:1900085. doi: 10.1002/advs.201900085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhao S, et al. A Dual-surface amidoximated halloysite nanotube for high-efficiency economical uranium extraction from seawater. Angew. Chem. Int. Ed. 2019;131:15121–15127. doi: 10.1002/ange.201908762. [DOI] [PubMed] [Google Scholar]
- 47.Xiong XH, et al. Ammoniating covalent organic framework (COF) for high-performance and selective extraction of toxic and radioactive uranium ions. Adv. Sci. 2019;6:1900547. doi: 10.1002/advs.201900547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barber PS, et al. Surface modification of ionic liquid-spun chitin fibers for the extraction of uranium from seawater: seeking the strength of chitin and the chemical functionality of chitosan. Green. Chem. 2014;16:1828–1836. doi: 10.1039/C4GC00092G. [DOI] [Google Scholar]
- 49.Zheng T, et al. Overcoming the crystallization and designability issues in the ultrastable zirconium phosphonate framework system. Nat. Commun. 2017;8:15369. doi: 10.1038/ncomms15369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yu J, et al. Phosphonate-decorated covalent organic frameworks for actinide extraction: a breakthrough under highly acidic conditions. CCS Chem. 2019;1:286–295. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are either provided in the Article and its Supplementary Information or are available from the corresponding author upon request.