Abstract
Objective
To investigate the expression of long‐chain noncoding growth stasis specific protein 6 antisense RNA1 (lncRNA DLX6‐AS1) in nasopharyngeal carcinoma (NPC) tissues and cells, and its regulatory effect on malignant phenotypes of NPC cells.
Methods
The expressions of DLX6‐AS1, miR‐199a‐5p, and HIF‐1α mRNA in NPC issues and cells were detected by qRT‐PCR. The proliferation, metastasis, and invasion of cells were monitored via MTT and transwell assay. The interactions between DLX6‐AS1 and miR‐199a‐5p, miR‐199a‐5p and HIF‐1α were verified by luciferase activity assay. Western blot was performed to determine the regulatory effect of DLX6‐AS1 and miR‐199a‐5p on HIF‐1α protein.
Results
The expression of lncRNA DLX6‐AS1 was up‐regulated in NPC tissues and cells. The proliferation, migration, and invasion of NPC were enhanced by overexpressed DLX6‐AS1 but inhibited by DLX6‐AS1 knockdown. In addition, DLX6‐AS1 can be used as a kind of ceRNA to regulate miR‐199a‐5p and, thereby modulating the expression of HIF‐1α.
Conclusion
We found that DLX6‐AS1 was a cancer‐promoting lncRNA to facilitate the progression of NPC, and its underlying mechanism was suppressing miR‐199a‐5p expression. This study can provide novel clues for the treatment of NPC.
Keywords: DLX6‐AS1, HIF‐1α, Long noncoding RNA, miR‐199a‐5p, Nasopharyngeal carcinoma
This study is to investigate the expression of long‐chain noncoding growth stasis specific protein 6 antisense RNA1 (lncRNA DLX6‐AS1) in nasopharyngeal carcinoma (NPC) tissues and cells, and its regulatory effect on malignant phenotypes of NPC cells. In conclusion, we found that DLX6‐AS1 was a cancer‐promoting lncRNA to facilitate the progression of NPC, and its underlying mechanism was suppressing miR‐199a‐5p expression. This study can provide novel clues for the treatment of NPC.
1. INTRODUCTION
Nasopharyngeal carcinoma (NPC) is a type of head and neck cancer, which is vastly more common in certain regions of southern China and southeast Asia than elsewhere. It is estimated that there are over 60,000 newly diagnosed cases with NPC every year in China, and such a public health issue has been brought into the center of people's concern (Tan, Tang, & Tang, 2015). A slew of patients with primary NPC remain gripped by the relapse and distant metastasis, despite of the fact that they are sensitive to radiotherapy and chemotherapy (Cheng et al., 2000). Notwithstanding the tremendous achievements in radiotherapy and chemotherapy, these approaches only provide very limited survival advantages due to the relapse and distant metastasis (Liu et al., 2017). Correspondingly, it's imperative to probe into the biological mechanism of NPC.
Long noncoding RNA (lncRNA) is a class of noncoding RNA (ncRNA) that is more than 200 nucleotides without protein‐coding ability. lncRNA, failing to translate proteins, wields enormous influence in biological processes, such as the proliferation, apoptasis, metastasis, tumorigenesis, and progression (Batista & Chang, 2013; Dhamija & Diederichs, 2016; Ponting, Oliver, & Reik, 2009). A growing body of evidences demonstrate that lncRNAs can modulate tumor progression (Adams, Parsons, Walker, Zhang, & Slack, 2017; Smolle, Bauernhofer, Pummer, Calin, & Pichler, 2017; Wapinski & Chang, 2011). For instance, lncRNA 00,858, as a competitive endogenous RNA of miR‐422a, potentiates the cell growth of nonsmall cell lung cancer (Zhu, Wang, Wang, & Zhao, 2017). The increased expression of lncRNA ZFAS1 is related to epithelial‐mesenchymal transformation of gastric cancer (Zhou et al., 2016). lncRNA LOC100129148 and EWSAT1 performs a carcinogenic role in NPC (Song & Yin, 2016; Sun, Peng, Chen, Song, & Zhou, 2017). It has been well documented that high expression of long‐chain non‐coding growth stasis specific protein 6 antisense RNA1 (lncRNA DLX6‐AS1) (HGNC#37151) is associated with tissue differentiation and TNM stage of lung cancer (Li et al., 2015). DLX6‐AS1 can also accelerate tumor proliferation and metastasis in osteosarcoma (Zhang, Meng, Mei, Zhao, & Chen, 2019). The expression of DLX6‐AS1 is up‐regulated and is correlated with poor prognosis in patients with hepatocellular carcinoma (Zhang, He, Jin, Gang, & Jin, 2017). Nevertheless, the role of DLX6‐AS1 in NPC remains far from being thoroughly elucidated.
miRNA is a class of highly conserved noncoding RNA molecules of 21 ~ 25nt length, which are involved in the regulation of gene transcription and expression by binding directly to the target mRNA (Voinnet, 2009). The suppressive influences that miR‐199a‐5p and miR‐199b‐5p impose on the progression of hepatocellular carcinoma by inhibiting ROCK1 have been revealed in previous articles (Zhan et al., 2017). miR‐199a‐5p restrains the proliferation but enhances the apoptosis of hemangioma cells (Wang et al., 2018). Additionally, miR‐199b‐5p facilitates the growth and metastasis of cervical cancer by down‐regulating KLK10 (Xu, Duan, Wang, & Yin, 2018).
There is accumulating evidence that lncRNA can interact directly with miRNA and regulate their expression and activity (Bayoumi et al., 2016; Cao, Jiang, Tang, & Liang, 2017). This study indicated that, in NPC tissues, the expression of DLX6‐AS1 was up‐regulated compared with paired adjacent normal nasopharyngeal epithelial tissues, and contributed to inhibiting the expression of miR‐199a‐5p. Moreover, both knockdown of DLX6‐AS1 and overexpressed miR‐199a‐5p repressed the proliferation, migration and invasion of NPC cells in vitro. Luciferase reporter gene assay uncovered that HIF‐1α was a direct target of miR‐199a‐5p in NPC cells. These results and findings implied a underlying DLX6‐AS1/miR‐199a/b‐5p/HIF‐1α pathway and provided new insights into the tumorigenesis and progression of NPC.
2. MATERIALS AND METHODS
2.1. General materials
72 patients with NPC who underwent biopsy in our hospital from 2012 to 2017 were enrolled in this study. Of the patients, were 44 male and 28 female, the age of which ranged from 21 ~ 68 years old. The NPC tissue samples and non‐NPC tissues were taken and confirmed by pathological examination after operation. Neither the radiotherapy and chemotherapy nor targeted drugs were given to any patients. Prior patient consents were obtained. The collected cancer tissues and normal tissues were frozen at −80°C.
2.2. Cell culture
Nasopharyngeal epithelial cells NP69, NPC cell lines S18, S26, CNE‐1, CNE‐2, HONE‐1, and 5‐8F were purchased from Shanghai Enzyme Research Biotechnology Co, Ltd. The cells were cultured in RPMI‐1640 medium (Gibco, Carlsbad, CA, USA) containing 10% fetal bovine serum (FBS; Gibco) and 100 U/ml penicillin/streptomycin (Life Technologies, USA) at 37°C and 5% CO2.
2.3. RT‐qPCR
Total RNA was extracted from cancer cells by using RNAiso Plus reagent (Takara Biotechnology Co., Ltd, Dalian). In order to detect the expression of miR‐199a/b‐5p, the total RNA was reversely transcripted by MMLV. cDNA taken as a template, real‐time PCR was conducted using PCR kit (Qiagen). The transcripts were quantified by real‐time PCR and standardized as GAPDH expression. With regard to the expression analysis of DLX6‐AS1 and HIF‐1α mRNA, the first chain cDNA was synthesized by using cDNA synthesis kit (Takara) according to the manufacturer's instructions. cDNA expression at mRNA level was detected by Syber Green PCR Master Mix (Applied Biosystems). The comparative cycle threshold (Ct) was employed to calculate the relative expression of genes (2‐△△Ct). The specific primer sequences of GAPDH were shown in Table 1.
Table 1.
qRT‐PCR primer sequences
| Name | Primer sequences |
|---|---|
| lncRNA DLX6‐AS1 | Forward: 5’‐AGTTTCTCTCTAGATTGCCTT−3 |
| Reverse: 5’‐ATTGACATGTTAGTGCCCTT−3' | |
| GAPDH | Forward: 5’‐AGCCACATCGCTCAGACAC−3' |
| Reverse: 5’‐GCCCAATACGACCAAATCC−3' |
2.4. Genotyping
The GenBank reference for DLX6‐AS1 is NC_000007.14, and HIF‐1α is NC_000014.9.
2.5. Cell construction
In accordance with the database of National Biotechnology Information Center, DLX6‐AS1 sequence lacking poly‐A tail was synthesized and subcloned into pcDNA3.1 (Jikai, Shanghai, China). Using siRNA‐DLX6‐AS1, the expression of DLX6‐AS1 was knocked down. According to the manufacturer's instructions, no‐load plasmid, pcDNA‐DLX6‐AS1 or si‐DLX6‐AS1 was transfected into C666‐1 and HNE1 cells cultured in the 6‐well plate using Lipofectamine™ 2000 (Invitrogen). After 24 hr or 48 hr of transfection, the cells were harvested and processed for further analysis.
2.6. MTT assay
The exponentially grown NPC cells were cultured in 96‐well plate for 12 hr. Within 24 hr, siRNA was added to interfere with DLX6‐AS1 expression, and siRNA transfection was carried out with Lipofectamine RNAimax reagent (Invitrogen). The medium was replaced at a specified time with a complete medium encompassing 10 μl MTT reagent, and then the cells were incubated at 37°C for 1 hr. Vmax microspectrophotometer (Molecular Devices) was placed to measure absorbance at 570 nm.
2.7. Transwell assay
The cells were inoculated in a 6‐well plate equipped with chambers, and two wells were set in each group. NPC cells were suspended in the upper chamber (approximately 105 cells/well) (well diameter of 8 μm; BD Biosciences, San Jose, CA, USA) of the serum‐free medium. The lower chamber was 500 μl high glucose DMEM medium with 10% fetal bovine serum (FBS). After 24 hr, the cells on the surface of the upper chamber were gently wiped off with cotton swabs, while the cells invading the lower chamber were fixed with methanol and then stained with crystal violet. Five visual fields (including the center and periphery of the membrane) were randomly selected under an inverted microscope. Furthermore, the number of cells invading the lower chamber were counted. Matrix gel was coated on the bottom of the transwell chamber during the invasion assay, and the other steps were the same as the migration assay.
2.8. Dual luciferase reporter gene assay
DNA oligonucleotides and pMiR‐Reporter were adopted to construct luciferase reporter vectors (pMiR‐DLX6‐AS1‐WT/ pMiR‐DLX6‐AS1‐Mut and pMiR‐HIF‐1 α‐WT/pMiR‐HIF‐1 α‐Mut). pMiR‐DLX6‐AS1‐WT or pMiR‐DLX6‐AS1‐Mut and miR‐26a mimics or negative control (NC) were co‐transfected into HEK293 cells. Luciferase activity was measured by Dual Luciferase Reporter Assay Kit (Promega) at 48 h post‐transfection.
2.9. Western blot
RIPA lysis buffer (Beyotime, China) embodying protease inhibitor cocktail (Roche) was used to prepare NPC cell lysate. The protein sample was added for SDS‐PAGE and transferred to the nitrocellulose membrane. After blocked with 5% fat‐free milk, the membrane was probed with the primary antibody HIF‐1α (1:1,000 dilution, Cell Signaling Technology) and the secondary antibody GAPDH (1:2,000 dilution, Santa Cruz Biotechnology). Subsequently, the membrane was incubated with horseradish peroxidase conjugated (HRP) secondary antibody (1:2,000, Santa Cruz Biotechnology) for 1 hr. Finally, an automatic imaging system (ChemiDocXRS imaging system) was employed for development and the gray value was calculated.
2.10. Statistical analysis
All assays were performed in triplicate. The statistics data among different groups were compared by t test. Differences with p < .05 were considered to be statistically significant. All statistical analysis was carried out using SPSS17.0 software.
3. RESULTS
3.1. LncRNA DLX6‐AS1 was highly expressed in NPC tissues and cells
First of all, we measured the expression of DLX6‐AS1 in 72 cases of NPC and non‐NPC tissues. As shown (Figure 1a), the expression of DLX6‐AS1 was significantly up‐regulated in NPC tissues compared with normal tissues. Besides, the expressions of DLX6‐AS1 in all of the six selected NPC cell lines, were higher than that in nasopharyngeal epithelium cell NP69 (Figure 1b).
Figure 1.

lncRNA DLX6‐AS1 was highly expressed in NPC tissues and cells. (a) The expressions of DLX6‐AS1 in 72 pairs of NPC tissues and normal tissues were detected by qRT‐PCR. (b) The expressions of DLX6‐AS1 in nasopharyngeal normal epithelial cells and NPC cells were determined by qRT‐PCR. *, **, *** represent P < 0.05, P < 0.01, P < 0.001 respectively
3.2. LncRNA DLX6‐AS1 could promote the proliferation, migration, and invasion of NPC cells
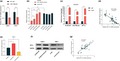
In order to probe into DLX6‐AS1 function in NPC, we successfully established DLX6‐AS1 overexpressed and knockdown cells with NPC cell lines C666‐1 and HNE1 cells respectively (Figure 2a). Then we carried out MTT assay to monitor the proliferation of NPC cells at 0, 12, 24, 48, 72, and 96 hr respectively. The results implied that the proliferation of NPC cells was facilitated by the overexpression of DLX6‐AS1 but restrained by the knockdown of DLX6‐AS1 (Figure 2b, c). By transwell assay, we figured out that overexpressed DLX6‐AS1 could expedite the migration and invasion of NPC cells (Figure 2d). By contrast, knockdown of DLX6‐AS1 would hinder the migration and invasion of NPC cells (Figure 2e).
Figure 2.
LncRNA DLX6‐AS1 can promote the proliferation, migration, and invasion of NPC cells. (a) The expression of DLX6‐AS1 in the constructed cells was monitored by qRT‐PCR. (b) The proliferation of C666‐1 cells with DLX6‐AS1 overexpressed was significantly promoted, which was examined by MTT assay. (c) The proliferation of HNE‐1 cells with DLX‐AS1 knockdown was significantly inhibited, which was examined by MTT assay. (d) Overexpression of DLX6‐AS1 promoted the migration of C666‐1 cells, while DLX‐AS1 knockdown inhibited the migration of HNE1 cells. (e) Overexpression of DLX6‐AS1 promoted the invasion of C666‐1 cells, while DLX‐AS1 knockdown inhibited the invasion of HNE1 cells. *, **, *** represent P < 0.05, P < 0.01, P < 0.001 respectively
3.3. miR‐199a‐5p was down‐regulated in NPC tissues and cell lines
Multiple microRNAs were found to be dysregulated in NPC, and miR‐199a‐5p and miR‐199b‐5p were reported to be down‐regulated in NPC tissues (Li, Hang, Ma, & Wu, 2016). In this study, we detected the expression levels of miR‐199a‐5p in NPC tissues and normal tissues by qRT‐PCR. Consistent with previous reports, the expression level of miR‐199a‐5p in normal nasopharyngeal epithelium was lower than that in NPC tissues (Figure 3a). In addition, the expression of miR‐199a‐5p in normal cells was higher than that in NPC cell lines (Figure 3b).
Figure 3.

miR‐199a‐5p was lowly expressed in NPC tissues and cells. (a) The expression of miR‐199a‐5p in 72 pairs of NPC tissues and normal tissues were detected by qRT‐PCR. (b) The expression of miR‐199a‐5p in nasopharyngeal normal epithelial cells and NPC cells was detected by qRT‐PCR. **, *** represent P < 0.01, P < 0.001 respectively
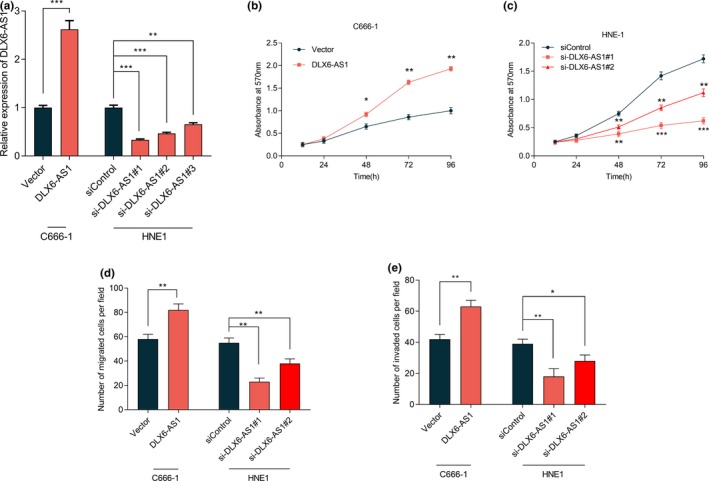
3.4. miR‐199a‐5p inhibited the proliferation, migration and invasion of NPC cells
In order to further validate the inhibitory effect of miR‐199a‐5p on NPC cells, we successfully transfected miR‐199a‐5p mimics and miR‐199a‐5p inhibitors into C666‐1 cells and HNE‐1 cells respectively (Figure 4a). It was confirmed by MTT assays that miR‐199a‐5p significantly stifled the proliferation of C666‐1 cells, while its inhibitor promoted the proliferation of HNE‐1 cells (Figure 4b, c). The results of transwell assays proved that miR‐199a‐5p could significantly inhibit the migration and invasion of NPC cells, while its inhibitor had opposite effects (Figure 4d, e).
Figure 4.
miR‐199a‐5p suppressed the proliferation, migration, and invasion of NPC cells. (a) miR‐199a‐5p mimics and inhibitors were successfully transfected into in C666‐1 cells and HNE1 cells, respectively, which was measured by qRT‐PCR. (b) The proliferation of C666‐1 cells transfected with miR‐199a‐5p inhibitors was significantly inhibited, which was examined by MTT assay. (c) The proliferation of HNE‐1 cells transfected with miR‐199a‐5p inhibitors was significantly promoted, which was examined by MTT assay. (d) miR‐199a‐5p mimics inhibited the migration of C666‐1 cells, while miR‐199a‐5p inhibitors promoted the migration of HNE1 cells. (e) miR‐199a‐5p mimics inhibited the invasion of C666‐1 cells, while miR‐199a‐5p inhibitors promoted the invasion of HNE1 cells. *, **, *** represent P < 0.05, P < 0.01, P < 0.001 respectively
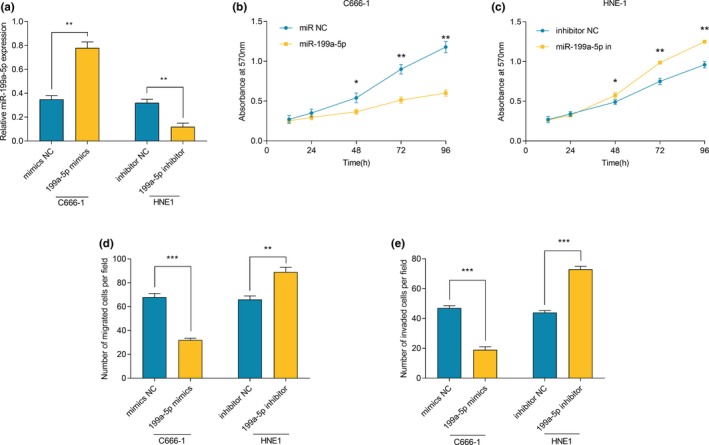
3.5. DLX6‐AS1 targeted miR‐199a‐5p
To clarify the underlying relationship between DLX6‐AS1 and miR‐199a‐5p, we referred to bioinformatics through StarBase (http://www.starbase.com). It showed that DLX6‐AS1 contained a potential binding site for miR‐199a‐5p (Figure S1). Then we detected the expression of miR‐199a‐5p in NPC cell lines after DLX6‐AS1 overexpression or knockdown. We noticed a decrease in miR‐199a‐5p expression when DLX‐AS1 is overexpressed and an increase in when DLX6‐AS1 is inhibited (Figure 5a). We also demonstrated that both miR‐199a‐5p diminished the luciferase activity of wild‐type pGL3‐DLX6‐AS1, but imposed no notable influence on mutant‐type pGL3‐DLX6‐AS1 (Figure 5b). Our data also exhibited that the expression level of DLX6‐AS1 in NPC samples was negatively correlated with the expression level of miR‐199a‐5p (Figure 5c). We further explored the effect of miR‐199a‐5p on the carcinogenic effect of DLX6‐AS1. MTT assay verified that, compared with overexpressed DLX6‐AS1 group, the proliferative and metastastic abilities of NPC cells were significantly inhibited after transfection of miR‐199a‐5p mimics (Figure 5d, e). Taken all results together, we ensured that miR‐199a‐5p could bind directly to DLX6‐AS1 at miRNA recognition site, and can be negatively regulated by DLX6‐AS1.
Figure 5.
miR‐199a‐5p interacted with DLX6‐AS1. (a) The changes of miR‐199a‐5p expression after overexpression or knockdown of DLX6‐AS1 were detected by qRT‐PCR. (b) Dual luciferase reporter activity assay was conducted to detect the luciferase activity of wild‐type and mutant DLX6‐AS1 plasmids co‐transfected with miR‐199a‐5p in HEK‐293 cells. (c) The correlation between DLX6‐AS1 expression and miR‐199a‐5p expression in clinical NPC samples was detected by qRT‐PCR (P<0.001). (d) miR‐199a‐5p reversed the effect of DLX6‐AS1 on proliferation in C666‐1 cells. (e) miR‐199a‐5p reversed the effect of DLX6‐AS1 on migration in C666‐1 cells. (f, g) miR‐199a‐5p reversed the effect of DLX6‐AS1 on migration and invasion in C666‐1 cells. *, ** represent P < 0.05, P < 0.01 respectively
3.6. miR‐199a‐5p could inhibit the expression of HIF‐1α, whereas lncRNA DLX6‐AS1 can induce the expression of HIF‐1α
Bioinformatics analysis also predicted that 3'‐UTR of hypoxia inducible factor 1 alpa (HIF‐1α) mRNA had 2 potential binding sites matching miR‐199a‐5p (Figure S1). We performed qRT‐PCR assay to detect the expression of HIF‐1α mRNA after transfection of miR‐199a‐5p mimics. Which was consistent with the expected, compared with control groups, the expression of HIF‐1α mRNA in NPC cells was significantly down‐regulated (Figure 6a). To further verify the interaction between miR‐199a‐5p and HIF‐1α, miR‐199a‐5p mimics was transfected into HEK293 cells, and luciferase reporter gene assay indicated that miR‐199a‐5p directly targeted HIF‐1α mRNA 3'‐UTR at the second binding sites (Figure 6b). Our data also exhibited that the expression level of miR‐199a‐5p in NPC samples was negatively correlated with the expression level of HIF‐1α (Figure 6c). Next, we detected the effects of DLX‐AS1 on the expression of HIF‐1α. In C666‐1 cells, after DLX‐AS1 was overexpressed, the expression of HIF‐1α mRNA was up‐regulated. In contrast, after knockdown of DLX6‐AS1, HIF‐1α mRNA was down‐regulated (Figure 6d). By western blot, we also discovered that the expression levels of HIF‐1α protein was modulated by DLX‐AS1 (Figure 6e). Importantly, in clinical samples, the expression of HIF‐1α was negatively associated with the expression of DLX‐AS1 (Figure 6f). Collectively, these data indicated that HIF‐1α was a target gene of miR‐199a‐5p and could be indirectly regulated by DLX‐AS1.
Figure 6.

DLX6‐AS1 modulated the expression of HIF‐1α by sponging miR‐199a‐5p. (a) The changes of HIF‐1α mRNA expression after transfection of miR‐199a‐5p mimics in C666‐1 cells and HNE‐1 cells were detected by qRT‐PCR. (b) Dual luciferase reporter activity assay was conducted to detect the luciferase activity of wild type and mutant HIF‐1α 3’UTR plasmids co‐transfected with miR‐199a‐5p in HEK‐293 cells. (c) The correlation between miR‐199a‐5p expression and HIF‐1α expression in clinical NPC samples was detected by qRT‐PCR (P<0.001). (d) The expression of HIF‐1α mRNA in C666‐1 cells was modulated by DLX6‐AS1, which was examined by qRT‐PCR. (e) The expression of HIF‐1α protein in C666‐1 cells was modulated by DLX6‐AS1, which was examined by western blot. (f) The correlation between DLX6‐AS1 expression and HIF‐1α expression in clinical NPC samples was detected by qRT‐PCR (P<0.001). (g) The correlation between DLX6‐AS1 and HIF1A mRNA was analyzed by correlation analysis. **, *** represent P < 0.01, P < 0.001 respectively
4. DISCUSSION
Recent studies revealed that lncRNAs play a vital role in the pathogenesis of cancer and provides new biological insights into tumor progression (Cheetham, Gruhl, Mattick, & Dinger, 2013; Chen, Wei, Wang, & Sun, 2017). lncRNA had been regarded as "garbage" produced in the process of transcription, which was a manifestation of the low fidelity of RNA polymeras (Ricciuti et al., 2016). The anti‐tumor function and tumor‐promoting function of lncRNA have been paid much attention. The dysfunction of lncRNA is closely related to the tumorigenesis, including NPC (Chen, Wang, Zhang, & Chen, 2014). The up‐regulated expression of UCA1 in pancreatic cancer promotes cell migration and invasion through Hippo signaling pathway (Zhang et al., 2018). CASC2 enhances the proliferation and metastasis of bladder cancer cells by activating wnt/β‐catenin signaling pathway (Pei et al., 2017). By mediating miR‐124/NF‐κB, NEAT1 facilitates NPC progression (Cheng & Guo, 2017). However, the function of lncRNAs on NPC remains largely unknown. To understand the precise molecular mechanisms of lncRNA function would fuel the development of lncRNA targeted therapy. LncRNA DLX6‐AS1 is an antisense transcription product of DLX6 gene, and the biological function of DLX6 gene has not been elucidated (Li et al., 2015). In this study, we determined that DLX6‐AS1 was up‐regulated in NPC. Addtionally, DLX6‐AS1 knockdown significantly inhibited the proliferation, migration, and invasion of NPC cells.
miRNAs exerts great impact on the tumor progression, whose function in tumor cells has been much heralded in recent years. It has been claimed that miR‐628‐5p reduced the carcinogenicity of ovarian cancer cells by targeting FGFR2 (Li et al., 2018). miR‐125a produces the suppressive impact on the proliferation, migration, and invasion of cervical cancer cells via targeting STAT3 (Fan et al., 2015). Through directly targeting SOX9, miR‐124 represses the proliferation, migration, and invasion of lung adenocarcinoma (Wang et al., 2016). Nevertheless, the function and abnormal expression mechanism of miR‐199a‐5p in NPC is far from being thoroughly clarified. Mounting articles have reported that lncRNAs, as an endogenous competitive RNA, can modulate the expression of miRNA, so lncRNA conducts a crucial role in the tumorigenesis. For instance, in gallbladder carcinoma, H19 binds to miR‐342‐3p to mediate FOXXM1 expression (Wang et al., 2016). In nonsmall cell lung cancer, lncRNA UCA1 conducts a carcinogenic role via targeting miR‐193a‐3p (Nie et al., 2016). lncRNA DLX6‐AS1 enhances the proliferation and invasion of pancreatic cancer cells by weakening the endogenous function of miR‐181b in pancreatic cancer (An et al., 2018). In this study, we discovered that DLX6‐AS1 facilitated the expression of HIF‐1α via targeting miR‐199a‐5p, thereby speeding up the proliferation and metastasis of NPC cells.
Hypoxia is one of typical microenvironments of solid tumors. Previous studies have demonstrated that hypoxia was involved in biological processes of tumor cells, such as the proliferation, apoptosis, chemotherapy resistance, angiogenesis, and metastasis (Tong, Tong, & Liu, 2018). Compared with the low expression in normal tissues, HIF‐1α expression is notably increased in hypoxic microenvironment, thus emerging as a key mediator affecting the behaviors of tumor cells (Zhang & Zhang, 2018). When HIF‐1α accumulated in cells, the enhanced expression would be observed in downstream target genes such as vascular endothelial growth factor (VEGF) and glucose transporter 1 (GLUT1). Concurrently, the angiogenesis and energy metabolism were accelerated, thereby facilitating the proliferation and metastasis (Al‐Anazi et al., 2018; Hamann et al., 2018). Furthermore, the abnormality of upstream regulatory molecules will also lead to the increase in HIF‐1α expression. Our results gave the evidence that the aberrant expression of HIF‐1α in tumor tissues may be related to the disorder of DLX6‐AS1 and miR‐199a/b‐5p, which reveals the relationship between ncRNA and micro‐environment.
In this study, we found that lncRNA DLX6‐AS1 can inhibit the expression of miR‐199a‐5p. Some previous studies focused on that the abnormal expression of miR‐199a‐5p in a variety of tumor tissues can participate in the cell proliferation and motility through a variety of ways. For instance, the decreased expression of miR‐199a‐5p can inhibit the proliferation and invasion of ovarian cancer cells by blocking NF‐κB signaling pathway (Liu, Yao, & Wu, 2018). miR‐199a‐5p can impede the proliferation and metastasis of colonic cancer cells via inhibiting ROCK1 (Zhu et al., 2018). miR‐199a‐5p can also restrain the progression of thyroid carcinoma by inhibiting SNAI1 (Ma, Jia, & Ni, 2018). Consistent with the above reports, our results proved that miR‐199a‐5p emerged as tumor suppressor. Its inhibitory effect can reverse the increase in cell proliferation, migration and invasion induced by DLX6‐AS1 over‐expression.
In conclusion, our findings provide new evidence that lncRNA DLX6‐AS1 increases the expression of HIF‐1α and promotes the malignant phenotypes of NPC cells via targeting miR‐199a‐5p.
CONFLICT OF INTEREST
The authors declare that they have no competing interest.
AUTHOR CONTRIBUTION
Conceived and designed the experiments: TX; Performed the experiments: BY, LJ, CBJ, HR; Statastic analysis: QRR, DSH, HZ; Wrote the paper: LH, HYZ.
All authors read and approved the final manuscript.
Supporting information
ACKNOWLEDGMENTS
None.
Yang B, Jia L, Ren H, et al. LncRNA DLX6‐AS1 increases the expression of HIF‐1α and promotes the malignant phenotypes of nasopharyngeal carcinoma cells via targeting MiR‐199a‐5p. Mol Genet Genomic Med. 2020;8:e1017 10.1002/mgg3.1017
Bin Yang and Lin Jia are first authors.
Funding information
This study was funded by the CSCO‐Merck Serono Oncology Research Fund (No. Y‐MT2015‐022), and CSCO‐Stone Medicine Oncology Research Fund (No. Y‐sy2018‐142).
DATA AVAILABILITY STATEMENT
The data used to support the findings of this study are available from the corresponding author upon request.
REFERENCES
- Adams, B. D. , Parsons, C. , Walker, L. , Zhang, W. C. , & Slack, F. J. (2017). Targeting noncoding RNAs in disease. Journal of Clinical Investigation, 127(3), 761–771. 10.1172/JCI84424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al‐Anazi, A. , Parhar, R. , Saleh, S. , Al‐Hijailan, R. , Inglis, A. , Al‐Jufan, M., … Al‐Mohanna, F. (2018). Data on hypoxia‐induced VEGF, leptin and NF‐kB p65 expression. Data Brief, 21, 2395–2397. 10.1016/j.dib.2018.10.147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- An, Y. , Chen, X. M. , Yang, Y. , Mo, F. , Jiang, Y. , Sun, D. L. , & Cai, H. H. (2018). LncRNA DLX6‐AS1 promoted cancer cell proliferation and invasion by attenuating the endogenous function of miR‐181b in pancreatic cancer. Cancer Cell International, 18 (1), 143 10.1186/s12935-018-0643-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batista, P. J. , & Chang, H. Y. (2013). Long noncoding RNAs: Cellular address codes in development and disease. Cell, 152(6), 1298–1307. 10.1016/j.cell.2013.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayoumi, A. S. , Sayed, A. , Broskova, Z. , Teoh, J. P. , Wilson, J. , Su, H. , … Kim, I. M. (2016). Crosstalk between Long Noncoding RNAs and MicroRNAs in Health and Disease. International Journal of Molecular Sciences, 17(3), 356 10.3390/ijms17030356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao, M. X. , Jiang, Y. P. , Tang, Y. L. , & Liang, X. H. (2017). The crosstalk between lncRNA and microRNA in cancer metastasis: Orchestrating the epithelial‐mesenchymal plasticity. Oncotarget, 8(7), 12472–12483. 10.18632/oncotarget.13957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheetham, S. W. , Gruhl, F. , Mattick, J. S. , & Dinger, M. E. (2013). Long noncoding RNAs and the genetics of cancer. British Journal of Cancer, 108(12), 2419–2425. 10.1038/bjc.2013.233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, J. , Wang, R. , Zhang, K. , & Chen, L. B. (2014). Long non‐coding RNAs in non‐small cell lung cancer as biomarkers and therapeutic targets. Journal of Cellular and Molecular Medicine, 18(12), 2425–2436. 10.1111/jcmm.12431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Q. N. , Wei, C. C. , Wang, Z. X. , & Sun, M. (2017). Long non‐coding RNAs in anti‐cancer drug resistance. Oncotarget, 8(1), 1925–1936. 10.18632/oncotarget.12461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng, N. , & Guo, Y. (2017). Long noncoding RNA NEAT1 promotes nasopharyngeal carcinoma progression through regulation of miR‐124/NF‐κB pathway. Onco Targets and Therapy, 10, 5843–5853. 10.2147/OTT.S151800 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Cheng, S. H. , Jian, J. J. , Tsai, S. Y. , Yen, K. L. , Chu, N. M. , Chan, K. Y. , … Huang, A. T. (2000). Long‐term survival of nasopharyngeal carcinoma following concomitant radiotherapy and chemotherapy. International Journal of Radiation Oncology Biology Physics, 48(5), 1323–1330. [DOI] [PubMed] [Google Scholar]
- Dhamija, S. , & Diederichs, S. (2016). From junk to master regulators of invasion: lncRNA functions in migration. EMT and Metastasis. International Journal of Cancer, 139(2), 269–280. 10.1002/ijc.30039 [DOI] [PubMed] [Google Scholar]
- Fan, Z. , Cui, H. , Xu, X. , Lin, Z. , Zhang, X. , Kang, L. , … Jiao, S. (2015). MiR‐125a suppresses tumor growth, invasion and metastasis in cervical cancer by targeting STAT3. Oncotarget, 6(28), 25266–25280. 10.18632/oncotarget.4457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamann, I. , Krys, D. , Glubrecht, D. , Bouvet, V. , Marshall, A. , Vos, L. , … Wuest, F. (2018). Expression and function of hexose transporters GLUT1, GLUT2, and GLUT5 in breast cancer‐effects of hypoxia. The FASEB Journal, 32(9), 5104–5118. 10.1096/fj.201800360R [DOI] [PubMed] [Google Scholar]
- Li, J. , Li, P. , Zhao, W. , Yang, R. , Chen, S. , Bai, Y. , … Zhang, G. (2015). Expression of long non‐coding RNA DLX6‐AS1 in lung adenocarcinoma. Cancer Cell International, 15, 48 10.1186/s12935-015-0201-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, M. , Qian, Z. , Ma, X. , Lin, X. , You, Y. , Li, Y. , … Jiang, H. (2018). MiR‐628‐5p decreases the tumorigenicity of epithelial ovarian cancer cells by targeting at FGFR2. Biochemical and Biophysical Research Communications, 495(2), 2085–2091. 10.1016/j.bbrc.2017.12.049 [DOI] [PubMed] [Google Scholar]
- Li, S. , Hang, L. , Ma, Y. , & Wu, C. (2016). Distinctive microRNA expression in early stage nasopharyngeal carcinoma patients. Journal of Cellular and Molecular Medicine, 20(12), 2259–2268. 10.1111/jcmm.12906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, X. , Tang, L. L. , Du, X. J. , Li, W. F. , Chen, L. , Zhou, G. Q. , … Ma, J. (2017). Changes in Disease Failure Risk of Nasopharyngeal Carcinoma over Time: Analysis of 749 Patients with Long‐Term Follow‐Up. International Journal of Cancer, 8(3), 455–459. 10.7150/jca.17104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, X. , Yao, B. , & Wu, Z. (2018). miRNA‐199a‐5p suppresses proliferation and invasion by directly targeting NF‐κB1 in human ovarian cancer cells. Oncology Letter, 16(4), 4543–4550. 10.3892/ol.2018.9170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, S. , Jia, W. , & Ni, S. (2018). miR‐199a‐5p inhibits the progression of papillary thyroid carcinoma by targeting SNAI1. Biochemical and Biophysical Research Communications, 497(1), 181–186. 10.1016/j.bbrc.2018.02.051 [DOI] [PubMed] [Google Scholar]
- Nie, W. , Ge, H. J. , Yang, X. Q. , Sun, X. , Huang, H. , Tao, X. , … Li, B. (2016). LncRNA‐UCA1 exerts oncogenic functions in non‐small cell lung cancer by targeting miR‐193a‐3p. Cancer Letters, 371(1), 99–106. 10.1016/j.canlet.2015.11.024 [DOI] [PubMed] [Google Scholar]
- Pei, Z. , Du, X. , Song, Y. , Fan, L. , Li, F. , Gao, Y. , … Zeng, J. (2017). Down‐regulation of lncRNA CASC2 promotes cell proliferation and metastasis of bladder cancer by activation of the Wnt/‐catenin signaling pathway. Oncotarget, 8(11), 18145–18153. 10.18632/oncotarget.15210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponting, C. P. , Oliver, P. L. , & Reik, W. (2009). Evolution and functions of long noncoding RNAs. Cell, 136(4), 629–641. 10.1016/j.cell.2009.02.006 [DOI] [PubMed] [Google Scholar]
- Ricciuti, B. , Mencaroni, C. , Paglialunga, L. , Paciullo, F. , Crin, L. , Chiari, R. , & Metro, G. (2016). Long noncoding RNAs: New insights into non‐small cell lung cancer biology, diagnosis and therapy. Medical Oncology, 33(2), 18 10.1007/s12032-016-0731-2 [DOI] [PubMed] [Google Scholar]
- Smolle, M. A. , Bauernhofer, T. , Pummer, K. , Calin, G. A. , & Pichler, M. (2017). Current Insights into Long Non‐Coding RNAs (LncRNAs) in Prostate Cancer. International Journal of Molecular Sciences, 18 (2), pii, E473 10.3390/ijms18020473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, P. , & Yin, S. C. (2016). Long non‐coding RNA EWSAT1 promotes human nasopharyngeal carcinoma cell growth in vitro by targeting miR‐326/‐330‐5p. Aging (Albany NY), 8(11), 2948–2960. 10.18632/aging.101103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, K. Y. , Peng, T. , Chen, Z. , Song, P. , & Zhou, X. H. (2017). Long non‐coding RNA LOC100129148 functions as an oncogene in human nasopharyngeal carcinoma by targeting miR‐539‐5p. Aging (Albany NY), 9(3), 999–1011. 10.18632/aging.101205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan, G. , Tang, X. , & Tang, F. (2015). The role of microRNAs in nasopharyngeal carcinoma. Tumour Biology, 36(1), 69–79. 10.1007/s13277-014-2847-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong, W. W. , Tong, G. H. , & Liu, Y. (2018). Cancer stem cells and hypoxia‐inducible factors (Review). International Journal of Oncology, 53(2), 469–476. 10.3892/ijo.2018.4417 [DOI] [PubMed] [Google Scholar]
- Voinnet, O. (2009). Origin, biogenesis, and activity of plant microRNAs. Cell, 136(4), 669–687. 10.1016/j.cell.2009.01.046 [DOI] [PubMed] [Google Scholar]
- Wang, S. H. , Ma, F. , Tang, Z. H. , Wu, X. C. , Cai, Q. , Zhang, M. D. , … Quan, Z. W. (2016). Long non‐coding RNA H19 regulates FOXM1 expression by competitively binding endogenous miR‐342‐3p in gallbladder cancer. Journal of Experimental & Clinical Cancer Research, 35(1), 160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, X. , Liu, Y. , Liu, X. , Yang, J. , Teng, G. , Zhang, L. , & Zhou, C. (2016). MiR‐124 inhibits cell proliferation, migration and invasion by directly targeting SOX9 in lung adenocarcinoma. Oncology Reports, 35(5), 3115–3121. 10.3892/or.2016.4648 [DOI] [PubMed] [Google Scholar]
- Wang, Y. , Dai, Y. X. , Wang, S. Q. , Qiu, M. K. , Quan, Z. W. , … Ou, J. M. (2018). miR‐199a‐5p inhibits proliferation and induces apoptosis in hemangioma cells through targeting HIF1A. International Journal of Immunopathology and Pharmacology, 31, 394632017749357. 10.1177/0394632017749357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wapinski, O. , & Chang, H. Y. (2011). Long noncoding RNAs and human disease. Trends in Cell Biology, 21(6), 354–361. 10.1016/j.tcb.2011.04.001 [DOI] [PubMed] [Google Scholar]
- Xu, L. J. , Duan, Y. , Wang, P. , & Yin, H. Q. (2018). MiR‐199b‐5p promotes tumor growth and metastasis in cervical cancer by down‐regulating KLK10. Biochemical and Biophysical Research Communications, 503(2), 556–563. 10.1016/j.bbrc.2018.05.165 [DOI] [PubMed] [Google Scholar]
- Zhan, Y. , Zheng, N. , Teng, F. , Bao, L. , Liu, F. , Zhang, M. , … Wang, Q. (2017). MiR‐199a/b‐5p inhibits hepatocellular carcinoma progression by post‐transcriptionally suppressing ROCK1. Oncotarget, 8(40), 67169–67180. 10.18632/oncotarget.18052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, J. , & Zhang, Q. (2018). VHL and Hypoxia Signaling: Beyond HIF in Cancer. Biomedicines, 6 (1), pii, E35 10.3390/biomedicines6010035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, L. , He, X. , Jin, T. , Gang, L. , & Jin, Z. (2017). Long non‐coding RNA DLX6‐AS1 aggravates hepatocellular carcinoma carcinogenesis by modulating miR‐203a/MMP‐2 pathway. Biomedicine & Pharmacotherapy, 884–891, 10.1016/j.biopha.2017.10.056 [DOI] [PubMed] [Google Scholar]
- Zhang, M. , Zhao, Y. , Zhang, Y. , Wang, D. , Gu, S. , Feng, W. , … Xu, M. (2018). LncRNA UCA1 promotes migration and invasion in pancreatic cancer cells via the Hippo pathway. Biochimica Et Biophysica Acta (BBA)‐Molecular Basis of Disease, 1864 (5), 1770–1782. 10.1016/j.bbadis.2018.03.005 [DOI] [PubMed] [Google Scholar]
- Zhang, N. , Meng, X. , Mei, L. , Zhao, C. , & Chen, W. (2019). LncRNA DLX6‐AS1 promotes tumor proliferation and metastasis in osteosarcoma through modulating miR‐641/HOXA9 signaling pathway. Journal of Cellular Biochemistry, 120(7), 11478–11489. 10.1002/jcb.28426 [DOI] [PubMed] [Google Scholar]
- Zhou, H. , Wang, F. , Chen, H. , Tan, Q. , Qiu, S. , Chen, S. , … Tu, J. (2016). Increased expression of long‐noncoding RNA ZFAS1 is associated with epithelial‐mesenchymal transition of gastric cancer. Aging (Albany NY), 8(9), 2023–2038. 10.18632/aging.101048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, Q. D. , Zhou, Q. Q. , Dong, L. , Huang, Z. , Wu, F. , & Deng, X. (2018). MiR‐199a‐5p Inhibits the Growth and Metastasis of Colorectal Cancer Cells by Targeting ROCK1. Technology in Cancer Research & Treatment, 17 10.1177/1533034618775509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, S. P. , Wang, J. Y. , Wang, X. G. , & Zhao, J. P. (2017). Long intergenic non‐protein coding RNA 00858 functions as a competing endogenous RNA for miR‐422a to facilitate the cell growth in non‐small cell lung cancer. Aging (Albany NY), 9(2), 475–486. 10.18632/aging.101171 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.


