Abstract
Evidence showed preventive impacts of the highly active antiretroviral therapy (HAART) on the Human Immunodeficiency Virus (HIV) transmission amomg heterosexual population, however, that is of deficit among men who have sex with men (MSM). The aim was to systematically examine the efficacy of HAART on HIV transmission and the association between the HAART initiation and unprotected anal intercourse (UAI) in MSM population. Three electronic databases were fully searched for articles published in peer-reviewed journals between 1996 and 2017. Of 1616 identified articles, fifteen articles were eligible for meta-analyses. The summary incidence rate (IR) of HIV was 6.63/100 person-year (95%CI 2.06–11.20/100 person-year)(p = 0.004). The pooled per-contact rate (PCR) of HIV was 0.42% (95% CI 0.21–0.63%)(p < 0.05). The HAART initiation (vs non-HAART) was not associated with engaging in UAI, with odds ratio (OR) 1.09 (95% CI 0.90–1.34)(p > 0.05). In the stratified analysis, participants with no less than 6 months recall period was slightly more likely to engage in UAI (OR 1.32; 95% CI 1.01–1.74)(p < 0.05). It indicated that HAART has potential efficacy on reducing infectivity of HIV positive individuals in anal intercourses. The relationship between the HAART initiation and UAI was not significant and may be influenced by some social-demographic factors. Consistent condom use and education on safe sex among MSM are crucial.
Subject terms: HIV infections, Medical research
Introduction
Globally, over 78 million people were infected with HIV. The prevalence of people living with HIV was 36.7 million1. Among men who have sex with men (MSM), the average percentage of HIV prevalence ranged between 6% (the Asia and Pacific region) and 15% (the western and central Africa region)2. In young (under 25) MSM, around 4.2% of the population was infected with HIV2. Although there is a trend of reduction of HIV incidence in the general population in most countries, it showed an upward trend among MSM2,3. Two Australian studies predicted that, for homosexual males, the mean number of homosexual partners was 11 in the past 1 year4, compared with heterosexual men, which was about 1.55. Theoretically, with high possibility of partner change rate, the MSM is regarded as the core group in the transmission of sexual transmitted diseases (STIs) including HIV6. Also, the MSM-population is supposed to be the risk group in the pattern of HIV transmission. A UNAIDS report estimated that the risk of HIV acquisition in MSM was 19 times higher than that in the heterosexual population2. Compared with different patterns of sexual intercourses, the risk of HIV transmission was the highest via the UAI7.
The multiple combination of antiretroviral drugs (ARVs), highly active antiretroviral therapy (HAART), was proved to successfully suppress the HIV virus in blood8. A landmark randomized clinic trail (RCT) HPTN 052 demonstrated that the HAART reduced individuals’ risk of HIV-1 transmission via sex with condoms by 96% between heterosexual sero-discordant partners9. Similar results were found in an observational cohort study in Africa10. However, both studies only detected the effect of HAART on the vaginal transmission, whether the HAART reducing the risk of HIV acquisition via penis-anal intercourses is unknown. Some researchers believed that the rectal tissue is more susceptible to HIV RNA virus than vaginal tissue, which would increase individuals’ risk of HIV acquisition11.
In 2012, WHO published a guideline regarding HAART as a secondary preventive strategy called the treatment as prevention (TasP) based on previous empirical evidences12. However, the preventive effect of HAART on MSM population is uncertain and may be influenced by many factors. For instance, some researchers pointed that the unstable and weak relationship between MSM may avert the preventive effect of the HAART7. In addition, the viral load remained at high level in rectal tissues, even though it has been suppressed at an undetectable level in plasma13,14. Hence, behavioural factors like condomless anal intercourses may induce negative impact on the TasP15. In contrast, a prospective cohort study illustrated none HIV negative participants were linked-infected by their HIV positive partners via unsafe sex16.
It is imperative to fill the gap with specific evidences of the efficacy of TasP on HIV transmission in MSM. Detecting the association between UAI and HAART and potential influential factors for UAI would help researchers and policy-makers tailored their public health projects for this sub-group population. In this meta-analysis, the term HIV is represented the HIV-1 type infection.
The aim of this systematic review and meta-analysis was twofold: 1) to examine the efficacy of HAART on the risk of HIV transmission among MSM, 2) to examine the likelihood of engaging in sexual risk behaviours which measured as UAI among MSM while HAART initiation.
Results
Characteristics of studies
Eighteen studies published between 2001 and 2006 were initially involved in the meta-analysis. Excluding 3 qualitative researches, 7 cross-sectional studies and 8 cohort studies were finally rolled in further analysis, contributing to an overall study population of 26040. Data were collected at clinical sites or gay communities via self-administrated questionnaires and medical records. Over half of the studies (8/15) adopted the 6-month recall period. Three articles17–19 from the same prospective national cohort were also included due to different study periods and samples (Table 1). The median of sample size (n = 14) was 714.5 (IQR 1410), ranging from 155 to 12573. Researches mainly conducted in developed countries (i.e. UK, Australia and USA, n = 12). The majority of the participants (91.83%) were HIV positive status. The median age of MSM (n = 12) ranged from 28 to 45, with mean age 35.7 ± 4.44 y. Ten studies reported ethnicities of participants, mainly consisting of white (80%, n = 16150), the others were Asians, African Americans and Latino/Hispanic. Nearly half of the participants held the degree of college or above (41%, n = 10932). However, few studies reported the income level (n = 4), alcohol intake (n = 4) and substance use (n = 5) and the number of sexual partners ((n = 4) (Table 2).
Table 1.
Basic information of eighteen study designs.
| Author and Years | Locations | study design | study setting | data collection methods | Study period | Sample size for analysis | Recall period (within past months) | Response rate (%) | Length of follow-up |
|---|---|---|---|---|---|---|---|---|---|
| Brennan. DJ. 2010 | USA | cross sectional study | community | self-reported questionnaires | unknown | 346 | 12 | Not report | — |
| Cowan. SA. 2012* | Demark | cohort study | gay community | self-reported questionnaires | Jan. 1995–Jan. 2010 | — | — | — | |
| Cox.J. 2004 | Canada | cross sectional study | 5 ambulatory HIV clinics | self-reported questionnaires | Oct. 2002–Feb.2003 | 346 | 6 | 50 | — |
| Cunha. CB. 2014 | Brazil | cross sectional study | 1 clinic | self-reported questionnaires, medical records | Aug. 2010–Jun. 2012 | 155 | 3 | 93.2 | — |
| Dukers. NH. 2001 | Netherland | cohort study | clinics | self-reported questionnaires, medical records | Jan. 1992–Jan. 2000 | 365 | 6 | — | Unclear, probably 1 year |
| Dukers. NH. 2002 | Netherland | cross sectional study | STD clinics | self-reported questionnaires, medical records | 1999–2001 | 3090 | 6 | Not report | — |
| Fisher. M. 2010* | UK | cohort study | 1 clinic | self-reported questionnaires, medical records | 2000–2006 | — | — | — | |
| Gorbach. PM. 2011 | USA | cohort study | clinics | self-reported questionnaires, medical records | 2002–2006 | 187 | 3 | — | 12 months |
| Jansen. I. AV. 2011 | Netherland | cohort study | Public Health Service of Amsterdam | self-reported questionnaires, medical records | Oct. 1984–Dec. 2009 | 1642 | 6 | — | 11223 person-year |
| Jin. F. 2010 | Australia | cohort study | gay community | self-reported questionnaires, medical records | Jul. 2001–Jun. 2007 | 1136 | 6 | — | 5160 person-year |
| Magidson. JF. 2015 | Latin America (i.e. Argentina, Brazil, Chile, Columbia, Mexico, Peru, Venezuela) | cross sectional study | community | self-reported questionnaires |
Oct. 2012- Nov. 2012 |
2350 | 3 | 79.8 | — |
| Mori. SF. 2005 | USA | cross sectional study | community agent and clinics | self-reported questionnaires | unclear | 1870 | 3 | Not report | — |
| Porco. TC. 2004* | USA | cohort study | community | self-reported questionnaires | 1994–1999 | — | — | — | — |
| Rodger. AJ. 2016 | European countries (i.e. UK) | cohort study | 75 clinics | self-reported questionnaires, medical records | Sep. 2010–may. 2014 | 680 | 6 | — | 1238 couple-year |
| Safren. SA. 2016 | Thailand and Brazil | cohort study | clinics | self-reported questionnaires | Mar. 2011–May. 2013 | 749 | 2 | — | 15 months |
| Scott. HM. 2014 | UAS | cohort study | not known | self-reported questionnaires | 1992–1999 | 12573 | 6 | — | 18 months |
| Stephenson. JM. 2003 | UK | cross sectional study | an outpatient clinic | self-reported questionnaires | Jul. 1999–Aug. 2000 | 405 | 12 | 97.9 | — |
| Stolte. IG. 2004 | Netherland | cohort study | Multiple Health Service | self-reported questionnaires | Sep. 1999–May. 2002 | 146 | 6 | — | 21.6 months |
*Studies were not included in meta-analysis.
Table 2.
Characteristics of studies tested the efficacy of HAART on HIV transmission.
| Author and Years | ethnicity (%) | age (mean/median) Y(range/IQR) | sero-status of participants | education (college or above, n) | heavy alcohol user*(n) | income | substance use | number of sexual partners during recall period |
|---|---|---|---|---|---|---|---|---|
| Dukers. NH. 2002 | 77.1 Dutch | 34(28–40) | positive and negative | not report | not report | not report | not report | not report |
| Jin. F. 2010 | unclear | 35(18–75) | negative | not report | not report | not report | unknown | not report |
| Rodger. AJ. 2016 | 89.1 white, 0.9 Afrian American, 8.8 Asian and others | HIV positive 41.7(35.5–46.8); HIV negative 40.1(31.9–46.5) | sero-discordant couples | 339/680 | not report | not report | not report | not report |
| Safren. SA. 2016 | not report | 30–49 | positive | not report | Unclear (OR 1.04) | not report | not report | not report |
| Scott. HM. 2014 | 79.4 white, 5.3 African American, 10,9 Latino, 4.3 Asian and others | unclear | negative | 7884/12573 | 2219/12573 | not report | 2504 | mainly over 5 |
HAART and HIV IR
Four effect sizes contributing from three independent studies tested the IR of HIV in the era of HAART. The overall estimate of IR was 6.63/100 person-year (95% CI 2.06–11.20/100 person-year), p = 0.004 (Fig. 1). The heterogeneity test showed wide heterogeneous (p < 0.05; I2 = 99.88), thus the random-effects model was used. Egger’s regression intercept test showed no publication bias (p > 0.05). Sensitivity analysis showed no single effect size influenced the overall result.
Figure 1.
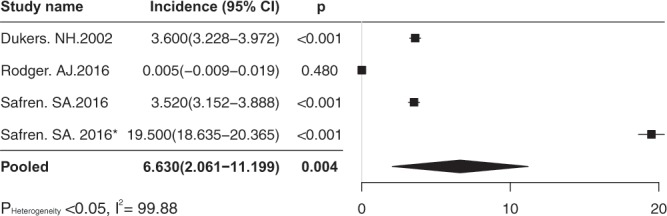
The forest plot of pooled estimate of IR, 100 person-year, in the era of HAART.
HAART and PCR
Two studies contributing six independent effect sizes examined the PCR of HIV transmission in the era of HAART. The pooled estimate of PCR was 0.42% (95% CI 0.21–0.63%), p < 0.001 (Fig. 2). The heterogeneity test showed substantial heterogeneous across effect sizes (p < 0.05; I2 = 76.55). The random-effects model was used. Egger’s regression intercept test showed no publication bias (p > 0.05). Sensitivity analysis showed the summary PCR did not influenced by removing each independent effect size.
Figure 2.
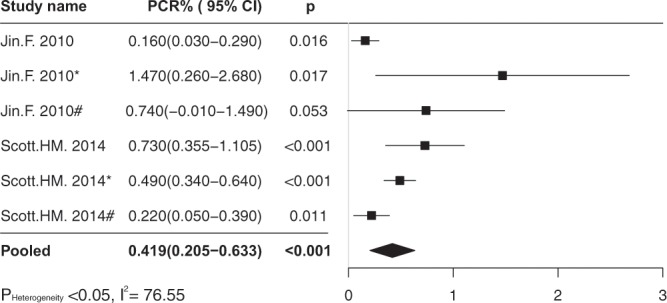
The forest plot of pooled estimate of per-contact rate (%) in the era of HAART.
HAART and UAI
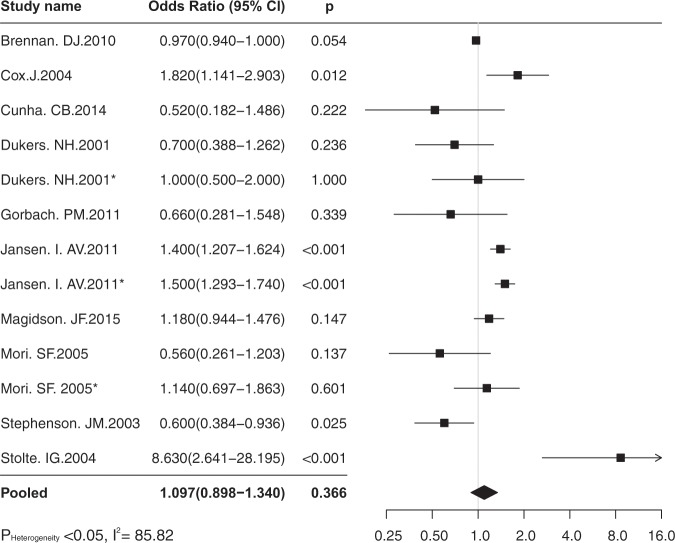
Ten studies contributing thirteen independent effect size tested the association between HAART (vs not HAART) and risk sexual behaviours. The percentage of HIV positive participants on HAART ranged from 23% to 100%. Only 2 studies17,20 reported the average length of HAART initiation (70 months and 10.8 months). Four studies presented the average duration of HIV infection, varying from 5.5 (IQR 0.2–16.4) to 12 (IQR 7–6) years20–23 (Table 3). The pooled estimate of OR was 1.09 (95% CI 0.90–1.34, P = 0.366), p > 0.05 (Fig. 3). The heterogeneity test showed wide heterogeneous across studies (p < 0.05, I2 = 85.82), thus, the random-effects model was used. The overall effect size was not influenced by removing single effect sizes when running the sensitivity analysis. There was no evidence of publication bias investigated by the Egger’s regression intercept (p > 0.05).
Table 3.
Findings of independent studies.
| Author and year | Measurement of effect size | Findings |
|---|---|---|
| Brennan. DJ. 2010 | OR |
Examination conducted between HAART belief of individuals on HAART and UAI. Scale contains 3 items, of which condom motivaion examined the association about perosonal attitudes towards condom use while on HAART with UAI. OR: 0.97 (95% CI 0.94–1.00) |
| Cowan. SA. 2012 | incidence (absolute number) | Yearly incidence of HIV diagnosed MSM in the Danish Cohort Study: median number 93/per year (range 71–137). |
| Cox.J. 2004 | Adjusted OR |
84% participants were on ART, 79% had at least one sexual partners, 194 anal sex, of which 93 with HIV positive, 72/93 UAI, 101 with HIV negative or unkown partners, 45/101 UAI. OR (UAI vs PI) 0.52 (95%CI 0.33–0.83), OR (UAI vs NRRIT) 1.95 (95%CI 1.00–3.80). AOR (UAI vs ART) 1.82 (95%CI 1,14–2.90) |
| Cunha. CB. 2014 | Adjusted OR |
63 UAI vs 92 non-UAI. 126 were on ART, of which 80 were non-UAI, 46 were on UAI. (HAART was remained as a confounder variable in multivariate model). AOR(ART vs UAI) 0.52 (95% CI 0.18–1.47) |
| Dukers. NH. 2001 | OR |
OR (ART vs UAI with Steady partners) 0.7 (0.4–1.3), OR (ART vs UAI with casual partners) 1.0 (0.5–1.9) |
| Dukers. NH. 2002 | Annual HIV incidence (new infection/HIV negative and recent infection) |
Overall HIV prevalence 14.7(454/3090). Incidence: 3.0 infections/100 person-year (95%CI 1.8–4.6) |
| Fisher. M. 2010 | RR | RR (HAART vs HIV transmission risk) 0.14 (95% CI 0.07–0.27) |
| Gorbach. PM. 2011 | OR | OR(HAART vs UAI): 0.66 (95% CI 0.28–1.54) |
| Jansen. I. AV. 2011 | OR, IRR(incidence risk ratio) |
217 of 1642 MSM seroconvetion, counted as yearly incidence/100 person-year, counted as yearly rate of UAI. OR (UAI vs HAART): 1.4 (95% CI 1.16–1.56) 1996–2003, 1.5 (95% CI 1.33–1.79) 2003–2009, compared with 1992–1996(pre-ART). Proportion UAI with steady patner was 60%, with casual partners 26% in 2009. In 180 sero-converted, 134 was allocated to a casual partners and 46 was allocated to the steady partners. |
| Jin. F. 2010 | PCR, OR |
46 sero-conversion. Total episode of UAI 228056. counted UAI with HIV positive, negative and unknown partners with three types of sex. PCR for insertive UAI with circumcised 0.11% (95% CI 0.02–0.24), and 0.62% (95% CI 0.07–1.68) without circumcised. PCR for receptive UAI with ejaculation inside OR 1.43 (95% CI 0.48–2.85), withdraw OR 0.65 (95% CI 0.15–1.53). Regardless of circumcision, PCR for insertive UAI was 0.16% (95% CI 0.05–0.31), receptive UAI with ejaculation inside was 1.47% (95% CI 0.51–2.93) and withdraw was 0.74% (95% CI 0.18–1.68) |
| Magidson. JF. 2015 | AOR(adjusted for sociodemographic characteristics |
Among 2350 HIV positive MSM, 684 not on ART, 1666 on ART. 949 not took UAI, 848 took UAI. AOR (ART vs UAI with sero-different partners) 1.18, 95% CI 0.94–1.47). OR (UAI vs 100% adherence of ART) 1.55 |
| Mori. SF. 2005 | OR | OR (ART vs steady partner UAI) 0.56 (95% CI 0.26–1.20), OR (ART vs casual partner UAI) 1.14 (95% CI 0.70–1.87) |
| Porco. TC. 2004 | transmission probability per partnership | 534 uninfected participants at baseline. Transmission probability per-partnership was 0.0276 on pre-HAART, and 0.011 on post-HAART was 0.048. |
| Rodger. AJ. 2016 | Rate of sero-conversion, | 10 MSM couples, 1 heterosexual couples. But non-linked sero-conversion happened (0%) |
| Safren. SA. 2016 | probability of transmitting HIV | Estimated HIV transmission per 100 persons in Thailand was 3.52%, in Brazil was 1.95%. |
| Scott. HM. 2014 | per-contact risk | In the pre-HAART era, 52/1813 seroconversions. In the early HAART era, 584/42395 seroconversions. With HIV positive partners, receptive UAI 0.60 (95% CI 0.34–1.09). Estimated PCR of receptive UAI with seropositive partners in pre-HAART: 0.6% (95%CI 0.34–1.09%), early HAART 0.73% (95% CI 0.45–0.98%) |
| Stephenson. JM. 2003 | OR |
113 were not on HAART, 292 were on ART. UAI (on ART) 101/285, UAI(not on ART) 51/107; insertive UAI (on ART) 76/285, insertive UAI (not on ART) 39/107. OR (UAI vs HAART in the past 12 months), 0.60 (0,39–0.95). |
| Stolte. IG. 2004 | adjusted OR | (reference group: non-UAI with casual partners) Adjusted OR(ART-ralated belief vs UAI with casual partner) 8.63 (95% CI 2.64–28.18) |
Figure 3.
The forest plot of pooled estimates of association between HAART and UAI.
By previous literature review, we stratified data into sub-analyses (Table 4). On HAART group (vs non-HAART) with the recall period over 6 months had more slightly likelihood of engaging in UAI (OR 1.32; 95% CI 1.01–1.74, p < 0.047). While the other factors (i.e. study setting, Data collection method) remained none association between two variables (HAART and UAI).
Table 4.
Stratified analysis for UAI and HAART.
| Variables | Number of effect size (n = 13) | OR(95% CI) | p value | Heterogeneity | |
|---|---|---|---|---|---|
| p value | I-square | ||||
| Study setting | |||||
| Clinics | 11 | 1.09(0.84–1.42) | 0.5 | 0.00 | 76.68 |
| Non-clinics | 2 | 1.04(0.86–1.24) | 0.71 | 0.09 | 65.47 |
| Data collection method | |||||
| Self-reported | 7 | 1.11(0.84–1.47) | 0.47 | 0.00 | 79.85 |
| Self-reported & medical records | 6 | 1.16(0.91–1.48) | 0.22 | 0.02 | 62.596 |
| Recall period | |||||
| Less than 6 months | 6 | 0.82(0.58–1.15) | 0.244 | 0.03 | 58.19 |
| More than 6 months(include 6 months) | 7 | 1.32(1.01–1.74) | 0.047 | 0.00 | 91.729 |
| Sero-status of participants | |||||
| Positive only | 10 | 0.95(0.79–1.15) | 0.61 | 0.02 | 55.24 |
| Negative with(out) positive | 3 | 1.60(1.12–2.12) | 0.001 | 0.01 | 77.96 |
| Median age | |||||
| Less than 35 | 4 | 1.45(1.16–1.82) | 0.001 | 0.00 | 75.22 |
| More than 35 (include 35) | 9 | 0.90(0.71–1.13) | 0.35 | 0.03 | 53.39 |
| Sample size | |||||
| Less than 300 | 3 | 1.39(0.28–6.96) | 0.69 | 0.00 | 86.63 |
| More than 300 (include 300) | 10 | 1.09(0.90–1.33) | 0.37 | 0.00 | 87.05 |
Discussion
Effects of HAART on HIV infectiousness
Few international studies examined the IR among MSM before HAART therapy adopted. Therefore, comparable groups’ data (non-HAART) in most researches were absent.
In general, the pooled IR (6.63/100 person-year, p < 0.05)) in our meta-analysis was within the range of the incidence rate of HIV (1.2 to 14.4/100 person-year) in MSM population reported by WHO24. The overall weighted IR was highly influenced by the number contributed from Brazil25. This extreme data indicated that the risk of HIV transmission among MSM may be different within distinct regions. However, the assumption, whether the diversity of IR came from different samples or it was only an extreme case, needs further proof.
A prospective cohort study among four effect sizes demonstrated that the HAART had a preventive effect on HIV transmission via condomless anal intercourse at the individual-level16. It moderated the infectivity of HIV positive individuals17. However, at the population level, studies calculated the HIV IR in the era of pre-HAART and post-HAART seperately showed an increasing trend during 1991 and 2001 in Netherland26. This contradicted phenomenon revealed that there might be some environmental factors around MSM influence the preventive impact of HAART, even averted the preventive efficacy at this population.
The PCR refers to the probability of one person be infected by their sexual partners while exposed at a certain sexual pattern, measured as infectivity (β) of HIV7. Porco and colleagues (2004) calculated 60% decrease of the PCR of per-partnership in the post-HAART era compared with that in the pre-HAART era27. An across-country prospective cohort study (Opposites Attract study) investigated that the HAART had a positive impact on diminishing the infectivity of HIV viral hosts by suppressing the viral load in the plasma under 200 copies/ml (0 linked-infection, unpublished data), which is similar with that found by Rodger. AJ et al.16. Those optimistic findings were only shown between steady sexual relationships. However, evidences displayed that homosexual males were more prone to be involved in polygamous and vulnerable relationships4, which may contribute part of reasons for the uncertain efficacy of HAART on MSM. In addition, this research showed that the overall β of UAI is not zero even the HAART initiated. Thus, keep encouraging condom use among MSM is indispensable to prevent the HIV transmission. Researches have been proved that the preventive impact of condom use on HIV/STI transmission was effective if a person practices sexual activities with condoms all the time28. Consistently expanding HAART on MSM could be one approach to control the epidemic of HIV, which potentially decreases the average infectivity of the total population. Monitoring and guaranteeing the adherence of HAART intake should be a supplementary approach to maximise the impact of HAART.
Association between HAART and UAI
The pooled effect size of the third meta-analysis showed the HAART would not influence the people’s choice on condom use during sexual activities. However, in the stratified analysis, participants had sexual experience no less than previous 6 months were more likely to engage in UAI (p = 0.047) compared with that within past 3 months, which implies that the frequency of sexual intercourses may influence the association between UAI and HAART. On the other hand, there may be a recall bias, since participants with longer recall period had higher chance to be vague on the memory of past sexual experiences. The reduction of self-reported reliability of sexual behaviours was also reported in the study of Napper (2009), which mentioned this reduction could be detected if the recall period beyond 6 months29. However, Napper pointed out that this finding needs further proof on anal intercourses29.
Confounders in the third meta-analysis were probably multiple and probably involve both socio-demographic and individual sides. The age, income and educational level, alcohol and drug abuse have been found to be significantly associated with engaging in UAI, indicating that people are at both young and old age, with lower education, successive alcohol drinking and substance (i.e. “popper”) use before or during sex were more likely to engage in UAI18,20–22,30,31. The high income was found to be the risk factor for engaging UAI21. However, the lower income group held optimistic beliefs on the preventive impact of HAART20. Some researchers believed that people held positive beliefs on the HAART was more likely to engaging in UAI17,20. This psychological construction also found in the research of Huebner. DM and Gerend. MA. (2001), pointing out that the HIV infected MSM who believed HAART has the preventive capability were more prone to take UAI, especially with casual sexual partners32. Therefore, consistent providing knowledge about safe sex, such as the limitation of TasP strategies and the importance of condom use in anal sex, is essential in the post-HAART era. However, the “condom fatigue”, referring to people get tired of health education on condom use and lag to change sexual risk behaviours, was mentioned in both Cox. J. (2004) and Brennan. DJ. (2010) researches, which may decrease the efficacy of safe sex education in the long-term run20,21.
Conclusion
Even though the information of HAART on MSM is limited and HAART preventive efficacy on HIV transmission among MSM was hardly draw a robust conclusion at this stage, this meta-analysis was the first aggregated quantitative research focused on the HIV transmission among MSM and provided specific information of this public health issue. Individual epidemiological findings have illustrated an optimistic opportunity for TasP to control the epidemic of HIV in community. However, since related information was scarce, further researches could emphases on its efficacy on population-level and detect potential influential factors. Also, data were mainly contributed from open societies like the USA and Europe, researchers and public health policy-makers from other regions should interpret those findings carefully in local contexts. In our meta-analysis, we reviewed social and individual factors that may confound the relationship between HAART and UAI. However, the impact of characteristics of HAART was not mentioned, including the adverse effect and drug resistance. Therefore, we encourage future studies could be designed more comprehensively to explore the relationship between HAART and UAI in MSM.
Limitations
There are some limitations in this meta-analysis. First, this meta-analysis did not include unpublished articles due to limited accessible literature resources. Also, we excluded non-full articles because we did not access original data. Second, there are wide heterogeneities. We did not perform a stratified analysis for the meta-analysis of the HAART efficacy since the total number of effect sizes was too few to conduct the sub-group analysis. However, we conducted a stratified analysis for the third meta-analysis to examine the influential factors. Third, participants included in the meta-analysis were skewed on western countries and open societies. In eastern countries, cultures and social structures would be different. For instance, in some countries in Africa and Asia, admitting self-identities as gay are still illegal2. Hence, findings of this meta-analysis need carefully and cautiously to generalize into different contexts. In addition, due to few HIV data of MSM collected in Asian and African countries, we encourage further researches to turn eyes on investigating reasons behind this phenomenon, help policy-makers in those countries formulate public health policies on HIV interventions and devote to changing this social issue. Fourth, we reviewed the social and individual factors that may confound the relationship between HAART and UAI, however, the impact of characteristics of HAART was not mentioned, including the adverse effect and drug resistance. Fifth, there are a few biases in the meta-analysis. Data were collected from self-reports, which would introduce a recall bias. Participants recruited from clinics may introduce the selection bias, since people who attended to clinics may be more care about their health and this self-awareness may overestimate the efficacy of HAART on the HIV incidence and sexual behaviours in the overall MSM population.
Materials and Methods
Screening and inclusion criteria
The following criteria were carried out for literature research:
Participants were over 18 years old (including 18) or defined as adults according to National Laws.
Participants were homosexual males, self-identified as gay or engaging in male-to-male sexual behaviours regardless of original sexual orientation.
HAART were exposed on HIV positive rather than negative participants.
Studies performed to examine 1) the risk of HIV transmission, and/or 2) any types of UAI (i.e. receptive or insertive).
Studies published as journal articles in English with peer reviewed.
Full text articles are available online.
Articles published between January 1996 to February 2017.
Search strategies and literature research
Electronic database PubMed, ScienceDirect and Google Scholar were fully searched. Terms‘HIV’, ‘antiretroviral’, ‘transmission’, ‘men who have sex with men’, ‘homosexual’, ‘behaviours’, ‘treatment as prevention’, ‘test and treat’, ‘anal sex’ were crossly combined and searched as either keywords in titles and abstracts or Medical Subject Heading (MeSH) terms (i.e. ‘HIV’ and ‘behaviour’). The literature research was conducted mainly in the form of two search strings: (1) ‘HIV’, ‘antiretroviral’, ‘transmission’, (‘men who have sex with men’ OR ‘homosexual’); (2) ‘HIV’, ‘antiretroviral’, (‘men who have sex with men’ OR ‘homosexual’), ‘behaviours’. Keywords ‘test and treat’, ‘treatment as prevention’ ‘anal sex’ were performed as supplementary search strategies with the other terms. Keywords ‘pre-exposure’, ‘hepatitis’, ‘HPV’, ‘herpes’, ‘drug resistance’ and ‘prevalence’ were excluded from title and abstract while searching at the database of Google Scholar. The search period was adopted from January 1996 to the present (February 2017).
Screening
2327 articles were found and 1616 articles were remained for further examination after removing duplications. Titles or abstracts were excluded if relating to:
irrelative study purposes: psychological and mental health,virology, health economics and policy, other STIs and AIDS-related diseases, drug resistance, belief or attitudes towards safe sex rather than behavioural changes, characters of participant, HAART scaling-up strategies.
irrelative exposures (HAART) or study groups: PrEP or PEP, alternative interventions (i.e. behaviours, partner notification, testing and counselling),heterosexual or women participants only.
irrelative study design: molecular epidemiology, qualitative researches, grey articles, news and reviews.
As a result, 75 articles were left for eligibility assessment via full text reviewed. The following principles were used to guide the full text article screening of this meta-analysis:
Studies performed to examine the efficacy of HAART on HIV transmission and/or the relationship of HAART and the UAI irrespective types of sexual patterns and partners.
Studies were focused on the change of actual sexual behaviours rather than the change of attitudes towards sexual behaviours.
If participants were made up of mixed subgroups (i.e. homosexual, heterosexual and bisexual), the subgroup of homosexual males or male couples would be included in this meta-analysis.
If the database overlapped between articles, with similar research purposes, the one contained the widest range of data would be included.
Outcomes were measured for HIV incidence or the likelihood of engaging UAI.
The ecological studies would not be included, because exposures were multiple and aggregated, potential confounders and the ecological fallacy may contribute great heterogeneity to the pooled analysis33.
57 articles were excluded via full text screening with following reasons listed in the Fig. 4. Finally, eighteen articles were included in this quantitative analysis. However, data presented in three articles were not eligible for meta-analyses27,34,35. Two studies measured by the incidence per year, which was not comparable with other studies34. The other study measured the association between HAART and HIV transmission risk by rate ratio (RR) was hardly fitted into the third meta-analysis35. Thus, there were fifteen full text articles enrolled in the final data analysis. This systematic review was carried out according to guidelines by PRISMA checklist36. The PRISMA flaw chat for the data selection procedure has been followed and presented in Fig. 4.
Figure 4.
PLASMA flow chat of literature search.
Data extraction
Each study was numbered by the first author and published year. A unified form was designed for the data extraction and imported into a Microsoft Excel database, including following categories:
Study design characters: authors and years, location, study design, study setting, data collection methods, study period, sample size for analysis, recall period (within past months), response rate, length of follow-up (person-year);
Participants’ characters: ethnicity, age, sero-status of participants, education, income, heavy alcohol users, substance use, number of sexual partners during recall period, since when diagnosed, type of data analysis;
Findings: measurement of effect size and findings.
If both adjusted and unadjusted effect sizes were reported by independent studies, the unadjusted one would be included for the meta-analysis. Additionally, if the effect size was stratified by regions or types of sexual patterns, the sub-level number would be added into the meta-analysis. The extracted items listed in standard forms (Tables 1, 2, 3 and 5) were tested by three different pilot studies21,22,37.
Table 5.
Characteristics of studies tested the association between HAART and UAI.
| Author and Years | ethnicity (%) | age (mean/median) Y(range/IQR) | sero-status of participants | education (college or above, n) | income | alcohol (n) | substance use | median number of sexual partners during recall period | since when HIV diagnosed (median/mean (IQR/range), y) | type of data analysis |
|---|---|---|---|---|---|---|---|---|---|---|
| Brennan. DJ. 2010 | 23 white, 4.6 African American, 47.5 Hispanic, 24.6 others | 43(38–48) | positive | 217 | not report | 109 (never), 129 (less 3 times/months), 104 (1–2 times a week or per day) | 143 never, 203 at leat once (in the past 3 months) | not report | 12(7–16) | multi-variate analysis |
| Cox.J. 2004 | Unclear | 45(24–73) | positive | 111 | 35% (<$20,000), 33%($20,000–39.,999), 32% (>=$40 000) | not report | not report | 36% had 6 or more partners | 10(0–19) | multi-variate analysis |
| Cunha. CB. 2014 | 52.9 White | 38(32–45) | positive | 128 | not report | 36.8% high use in the past 3 months | 57 use before sex in the past 3 months | commercial sex | 6(2.8–12.4) | multi-variate analysis |
| Dukers. NH. 2001 | 88.4 central and European white | 39.9(31.4–42.8) | positive | 145 | not report | not report | not report | not report | not report | multi-variate analysis |
| Gorbach. PM. 2011 | 71 white,21 Hispanic | 35(19–64) | positive | 165 | not report | not report | 21.1 use at last sex | 8.8 in the past 3 months | nuclear | multi-variate analysis |
| Jansen. I. AV. 2011 | 81 Dutch | 28.8(24.8–35.9) | negative | 901 | not report | not report | not report | unclear | not report | multi-variate analysis |
| Magidson. JF. 2015 | Unknown | 28(23–25) | positive | 78.2% | 74.4% middle income class | not report | not report | not report | not report | multi-variate analysis |
| Mori. SF. 2005 | 38.4 white, 35.3 African American, 17.8 Latino, 8.34 Asian and others | 40 | positive | 1153 | 1212 unemployment, 658 employment | 620 none, 1145 some, 100 daily | 331 use, 1538 non-use | not report | not report | multi-variate analysis |
| Stephenson. JM. 2003 | 90.3 white | 38(21–64) | positive | 228 | 199 full-time, 77 unemployment, 43 medically retired, 43 other | not report | not report | 12 in the past 12 months | 5.5(0.2–16.4) | multi-variate analysis |
| Stolte. IG. 2004 | 93.1 Dutch | 30.8(26.3–33.5) | negative | unclear | not report | not report | not report | not report | not report | multi-variate analysis |
Data analysis
Three independent meta-analysis were performed. The pooled risk of HIV transmission was measured separately by the IR (the number of new infections divided by at risk population over specific time) and the PCR (estimation based on Bernoulli model or boost rapping algorithm likelihood function) with 95% CI. The pooled OR with 95% CI was used to estimate the association between HAART and UAI, representing the probability of engaging UAI between the on-HAART group (index group) and the non-HAART group (reference group). The baseline data in cohort studies were included in meta-analysis. If OR is over 1, it represented that the index group is more likely to engage in UAI than the reference group. Otherwise, the index group is less likely to engage in UAI (OR < 1). If OR = 1, it means there is no association between both variables.
A Cochran’s Q test based on Chi-square test and an I2 test were used to examine the heterogeneity across independent studies. The fixed-effects model would be used if the data showed low or moderate heterogeneity (p > 0.05, I2 < 50%), otherwise, the random-effects model (p < 0.05, I2 > = 50%) would be carried out38. Publication bias was tested by the Egger’s regression intercept, if p > 0.05, it means no evidence on publication bias, otherwise, there would be a publication bias existing39. Statistical significance was 0.05 (P < 0.05) with 95% CI. Data were pooled and analysed by the software Comprehensive Meta-Analysis (version 2.0, Biostat, Englewood, New Jersey).
Acknowledgements
We would like to thank Mingyu Luo from the Centre for Disease Control, Zhejiang, and Dewei An from Shanghai Institute of Hypertension, Ruijin Hospital, Shanghai Jiaotong University, both who provided with supports on application of the analytical software.
Author contributions
Y.J., Y.B. formulated the research idea, study design and analysis and carried out the main writing of this meta-analysis. Y.J. completed the full data research and together with Y.B. discussing the plausibility of data. S.S. and Y.B. helped developed the idea scientifically and provide technical supports of data analysis while needed. All authors helped edit and revise the manuscript and approved the final manuscript.
Data availability
All data generated or analysed during this study are included in this published article.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.UNAIDS. Fact sheet November 2016: global HIV statistics. (2016).
- 2.UNAIDS. The gap report. (2014).
- 3.Hogg RS, Moore DM, Michelow WD, Montaner JS. Reduction of HIV incidence in men who have sex with men. The Lancet. Infectious diseases. 2010;10:655–656. doi: 10.1016/s1473-3099(10)70200-0. [DOI] [PubMed] [Google Scholar]
- 4.Grulich AE, de Visser RO, Smith AM, Rissel CE, Richters J. Sex in Australia: homosexual experience and recent homosexual encounters. Australian and New Zealand journal of public health. 2003;27:155–163. doi: 10.1111/j.1467-842X.2003.tb00803.x. [DOI] [PubMed] [Google Scholar]
- 5.de Visser RO, Smith AM, Rissel CE, Richters J, Grulich AE. Sex in Australia: heterosexual experience and recent heterosexual encounters among a representative sample of adults. Australian and New Zealand journal of public health. 2003;27:146–154. doi: 10.1111/j.1467-842X.2003.tb00802.x. [DOI] [PubMed] [Google Scholar]
- 6.Cowan, F. A. B. G. In ABC of Sexually Transmitted infection. (ed. Rogstad, K. E.) (BMJ, 2011).
- 7.Vittinghoff E, et al. Per-contact risk of human immunodeficiency virus transmission between male sexual partners. American journal of epidemiology. 1999;150:306–311. doi: 10.1093/oxfordjournals.aje.a010003. [DOI] [PubMed] [Google Scholar]
- 8.Collier AC, et al. Treatment of human immunodeficiency virus infection with saquinavir, zidovudine, and zalcitabine. AIDS Clinical Trials Group. The New England journal of medicine. 1996;334:1011–1017. doi: 10.1056/nejm199604183341602. [DOI] [PubMed] [Google Scholar]
- 9.Cohen MS, et al. Prevention of HIV-1 infection with early antiretroviral therapy. The New England journal of medicine. 2011;365:493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Donnell D, et al. Heterosexual HIV-1 transmission after initiation of antiretroviral therapy: a prospective cohort analysis. Lancet (London, England) 2010;375:2092–2098. doi: 10.1016/s0140-6736(10)60705-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fallon SJ, Forrest DW. Unexamined challenges to applying the treatment as prevention model among men who have sex with men in the United States: a community public health perspective. AIDS and behavior. 2012;16:1739–1742. doi: 10.1007/s10461-012-0258-2. [DOI] [PubMed] [Google Scholar]
- 12.World Health Organisation. Programmatic update: antiretroviral treatment as prevention, (TasP) of HIV and TB, http://apps.who.int/iris/bitstream/10665/70904/1/WHO_HIV_2012.12_eng.pdf (accessed 10618 Mar 12017) (2012).
- 13.Muessig KE, et al. Does ART prevent HIV transmission among MSM? AIDS (London, England) 2012;26:2267–2273. doi: 10.1097/QAD.0b013e328355713d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Politch JA, et al. Highly active antiretroviral therapy does not completely suppress HIV in semen of sexually active HIV-infected men who have sex with men. AIDS (London, England) 2012;26:1535–1543. doi: 10.1097/QAD.0b013e328353b11b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baggaley RF, White RG, Boily MC. HIV transmission risk through anal intercourse: systematic review, meta-analysis and implications for HIV prevention. International journal of epidemiology. 2010;39:1048–1063. doi: 10.1093/ije/dyq057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodger AJ, et al. Sexual Activity Without Condoms and Risk of HIV Transmission in Serodifferent Couples When the HIV-Positive Partner Is Using Suppressive Antiretroviral Therapy. Jama. 2016;316:171–181. doi: 10.1001/jama.2016.5148. [DOI] [PubMed] [Google Scholar]
- 17.Dukers NH, et al. Sexual risk behaviour relates to the virological and immunological improvements during highly active antiretroviral therapy in HIV-1 infection. AIDS (London, England) 2001;15:369–378. doi: 10.1097/00002030-200102160-00010. [DOI] [PubMed] [Google Scholar]
- 18.Jansen IA, et al. Ongoing HIV-1 transmission among men who have sex with men in Amsterdam: a 25-year prospective cohort study. AIDS (London, England) 2011;25:493–501. doi: 10.1097/QAD.0b013e328342fbe9. [DOI] [PubMed] [Google Scholar]
- 19.Stolte IG, Dukers NH, Geskus RB, Coutinho RA, de Wit JB. Homosexual men change to risky sex when perceiving less threat of HIV/AIDS since availability of highly active antiretroviral therapy: a longitudinal study. AIDS (London, England) 2004;18:303–309. doi: 10.1097/00002030-200401230-00021. [DOI] [PubMed] [Google Scholar]
- 20.Brennan DJ, Welles SL, Miner MH, Ross MW, Rosser BR. HIV treatment optimism and unsafe anal intercourse among HIV-positive men who have sex with men: findings from the positive connections study. AIDS education and prevention: official publication of the International Society for AIDS Education. 2010;22:126–137. doi: 10.1521/aeap.2010.22.2.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cox J, Beauchemin J, Allard R. HIV status of sexual partners is more important than antiretroviral treatment related perceptions for risk taking by HIV positive MSM in Montreal, Canada. Sexually transmitted infections. 2004;80:518–523. doi: 10.1136/sti.2004.011288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cunha CB, et al. Unprotected sex among men who have sex with men living with HIV in Brazil: a cross-sectional study in Rio de Janeiro. BMC public health. 2014;14:379. doi: 10.1186/1471-2458-14-379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stephenson JM, et al. Is use of antiretroviral therapy among homosexual men associated with increased risk of transmission of HIV infection? Sexually transmitted infections. 2003;79:7–10. doi: 10.1136/sti.79.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.World Health Organisation. Prevention and treatment of HIV and other sexualtransmitted infections among men who have sex with men and transgender people: recommendation for a public health approach, http://apps.who.int/iris/bitstream/10665/44619/10661/9789241501750_eng.pdf (accessed 9789241501718 Mar 9789241502017) (2011). [PubMed]
- 25.Safren SA, et al. Frequency and predictors of estimated HIV transmissions and bacterial STI acquisition among HIV-positive patients in HIV care across three continents. Journal of the International AIDS Society. 2016;19:21096. doi: 10.7448/ias.19.1.21096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dukers NH, et al. HIV incidence on the increase among homosexual men attending an Amsterdam sexually transmitted disease clinic: using a novel approach for detecting recent infections. AIDS (London, England) 2002;16:F19–24. doi: 10.1097/00002030-200207050-00001. [DOI] [PubMed] [Google Scholar]
- 27.Porco TC, et al. Decline in HIV infectivity following the introduction of highly active antiretroviral therapy. AIDS (London, England) 2004;18:81–88. doi: 10.1097/00002030-200401020-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith DK, Herbst JH, Zhang X, Rose CE. Condom effectiveness for HIV prevention by consistency of use among men who have sex with men in the United States. Journal of acquired immune deficiency syndromes (1999) 2015;68:337–344. doi: 10.1097/qai.0000000000000461. [DOI] [PubMed] [Google Scholar]
- 29.Napper LE, Fisher DG, Reynolds GL, Johnson ME. HIV risk behavior self-report reliability at different recall periods. AIDS and behavior. 2010;14:152–161. doi: 10.1007/s10461-009-9575-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gorbach PM, et al. Behaviors of recently HIV-infected men who have sex with men in the year postdiagnosis: effects of drug use and partner types. Journal of acquired immune deficiency syndromes (1999) 2011;56:176–182. doi: 10.1097/QAI.0b013e3181ff9750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morin SF, et al. Predicting HIV transmission risk among HIV-infected men who have sex with men: findings from the healthy living project. Journal of acquired immune deficiency syndromes (1999) 2005;40:226–235. doi: 10.1097/01.qai.0000166375.16222.eb. [DOI] [PubMed] [Google Scholar]
- 32.Huebner DM, Gerend MA. The relation between beliefs about drug treatments for HIV and sexual risk behavior in gay and bisexual men. Annals of behavioral medicine: a publication of the Society of Behavioral Medicine. 2001;23:304–312. doi: 10.1207/s15324796abm2304_10. [DOI] [PubMed] [Google Scholar]
- 33.Oxford Handbook of Public Health Practice 2nd edn, (Oxford University Press, 2006).
- 34.Cowan SA, et al. Stable incidence of HIV diagnoses among Danish MSM despite increased engagement in unsafe sex. Journal of acquired immune deficiency syndromes (1999) 2012;61:106–111. doi: 10.1097/QAI.0b013e31825af890. [DOI] [PubMed] [Google Scholar]
- 35.Fisher M, et al. Determinants of HIV-1 transmission in men who have sex with men: a combined clinical, epidemiological and phylogenetic approach. AIDS (London, England) 2010;24:1739–1747. doi: 10.1097/QAD.0b013e32833ac9e6. [DOI] [PubMed] [Google Scholar]
- 36.Moher D, Liberati A, Tetzlaff J, Altman DG. The PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jin F, et al. Per-contact probability of HIV transmission in homosexual men in Sydney in the era of HAART. AIDS (London, England) 2010;24:907–913. doi: 10.1097/QAD.0b013e3283372d90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ (Clinical research ed.) 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ (Clinical research ed.) 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this published article.