Abstract
Introduction
Adherence to non-vitamin-K oral anticoagulants (NOACs) may be lower than to vitamin K antagonists because NOACs do not require routine monitoring.
Objective
We assessed the impact of an educational program on adherence and persistence with apixaban in patients with non-valvular atrial fibrillation (NVAF).
Methods
Patients with NVAF eligible for NOACs with one or more stroke risk factor (prior stroke/transient ischemic attack, age ≥ 75 years, hypertension, diabetes, or symptomatic heart failure) were randomized (1:1) to standard of care (SOC) or SOC with additional educational (information booklet, reminder tools, virtual clinic access). The primary outcome was adherence to apixaban (2.5 or 5 mg twice daily) at 24 weeks. Patients receiving the educational program were re-randomized (1:1) to continue the program for 24 further weeks or to switch to secondary SOC. Implementation adherence and persistence were reassessed at 48 weeks.
Results
In total, 1162 patients were randomized (SOC, 583; educational program, 579). Mean implementation adherence ± standard deviation (SD) at 24 weeks was 91.6% ± 17.1 for SOC and 91.9% ± 16.1 for the educational program arm; results did not differ significantly between groups at any time-point. At 48 weeks, implementation adherence was 90.4% ± 18.0, 90.1% ± 18.6, and 89.3% ± 18.1 for continued educational program, SOC, and secondary SOC, respectively; and corresponding persistence was 86.1% (95% confidence interval [CI] 81.3–89.7), 85.2% (95% CI 81.5–88.2), and 87.8% (95% CI 83.4–91.1). Serious adverse events were similar across groups.
Conclusion
High implementation adherence and persistence with apixaban were observed in patients with NVAF receiving apixaban. The educational program did not show additional benefits.
Clinical trial registration
This study is registered at ClinicalTrials.gov [NCT01884350].
Electronic supplementary material
The online version of this article (10.1007/s40256-019-00356-2) contains supplementary material, which is available to authorized users.
Key Points
| Patients with non-valvular atrial fibrillation who do not receive adequate anticoagulation are at increased risk of embolic events compared with those continuously receiving the recommended treatment; poor adherence to anticoagulation can impact stroke protection. |
| This was the first prospective, randomized study investigating the impact of an education program on total days of adherence and persistence to a non-vitamin-K oral anticoagulant (apixaban). |
| Implementation adherence and persistence rates were high over the first 48 weeks of administration, with attrition over time, with or without additional patient education; lack of impact of the educational intervention may be related to the experience of the investigators (high level of standard care) and limited uptake of digital support strategies. |
Introduction
Vitamin K antagonists (VKAs) are highly effective for stroke prevention in non-valvular atrial fibrillation (NVAF), reducing the risk by about two-thirds [1]. However, VKAs have drawbacks limiting their use: extensive drug and food interactions, narrow therapeutic range, and need for frequent international normalized ratio (INR) monitoring and dose adjustments [1]. Accordingly, ongoing patient education is necessary with VKA treatment.
In patients with NVAF, non-VKA oral anticoagulants (NOACs) are at least as effective as warfarin for preventing stroke or systemic embolism (SE), while reducing the risk of intracranial hemorrhage by half [2–6]. In particular, apixaban has shown superiority over warfarin in preventing stroke or SE and is associated with a lower risk of major bleeding complications and mortality [2]. NOACs have fewer food and drug interactions than VKAs and do not require routine coagulation monitoring, overcoming many of the limitations of VKAs [7].
In the absence of routine anticoagulation monitoring, it is important that patients treated with NOACs take their medication as prescribed during all three phases of adherence: initiation (when the first dose is taken); implementation (the extent to which a patient’s actual dosing corresponds to the prescribed dosing); and persistence (absence of discontinuation, defined as the time when the next dose is omitted and no more doses are taken) [8]. Poor adherence may decrease protection from stroke [9].
Information on adherence to NOACs during the implementation phase is limited, with most studies instead describing persistence. Compared with warfarin, persistence (at 2.0, 1.9, 2.8, and 1.8 years) with NOACs in pivotal trials has been shown to be lower for dabigatran, similar for rivaroxaban or edoxaban, and higher for apixaban, respectively [2–6].
Dedicated educational interventions aim to improve adherence by improving patients’ understanding of NVAF, goals of treatment, and risks associated with non-adherence. Many observational studies, but only a few small randomized clinical studies, have specifically investigated the impact of educational programs on adherence to warfarin [10, 11]. While several observational studies have assessed adherence to NOACs [12–19], no studies have investigated the impact of education on NOAC adherence in patients with NVAF.
This study, the AEGEAN (Assessment of an Education and Guidance program for Eliquis Adherence in non-valvular Atrial fibrillatioN), investigated the impact of a proactive educational program on implementation adherence (from first administration of apixaban to discontinuation/completion of follow-up) and persistence, using an electronic monitoring device (EMD) that recorded detailed information on the timing of medication access [20].
Methods
Study Population
This phase IV randomized study was conducted at 171 sites in seven European countries (Belgium, France, Germany, Italy, Spain, Switzerland, and the UK). Patients with newly diagnosed or existing documented NVAF or atrial flutter, and a CHADS2 score ≥ 1 were eligible. Patients were either VKA experienced (≥ 3 months’ treatment; target was one-third of randomized population) or VKA naïve (≤ 30 days’ VKA treatment in the last 12 months). All patients were NOAC naïve. Patients with NVAF or atrial flutter who were receiving aspirin ≤ 165 mg once daily for an indication other than atrial fibrillation were eligible. The inclusion of patients receiving concomitant aspirin was in line with those of the pivotal ARISTOTLE trial [2].
Patients were excluded if they had other conditions requiring anticoagulation; had conditions associated with increased risk of hemorrhage; were at high risk of bleeding (e.g., due to other concomitant antiplatelet treatment or as a result of elective surgery or invasive procedures); were unable to self-administer study medication; were hospitalized; or were in long-term residential care (full details in the Electronic Supplementary Material [ESM]).
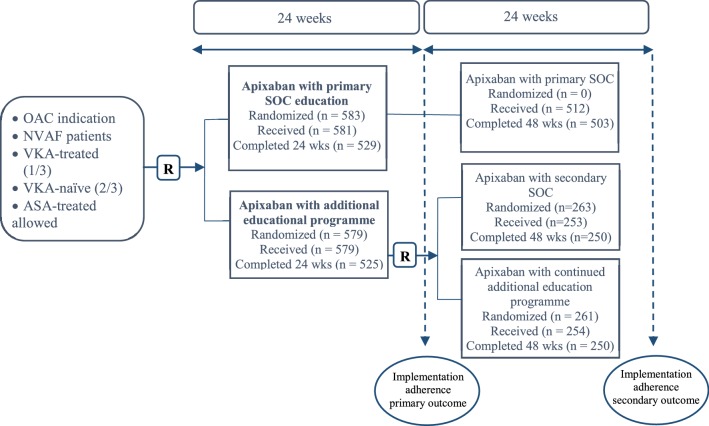
Patients consented to, and initiated therapy with, apixaban (Eliquis®, Bristol-Myers Squibb/Pfizer EEIG, Uxbridge, UK) and were subsequently asked to participate in the monitoring phase of the study. After patients provided consent to participate, they were centrally randomized (via electronic case report form) in a 1:1 ratio to receive standard-of-care (SOC) patient information or an additional educational program. After 24 weeks, patients receiving the educational program were re-randomized 1:1 to continue receiving the educational program or switch to SOC for an additional 24 weeks (Fig. 1). The trial design and the protocol were approved by the relevant national regulatory authorities and national and/or local ethics committees. All patients provided written informed consent. The trial is registered at http://www.clinicaltrials.gov (NCT01884350).
Fig. 1.
Study design. Patients consented to and initiated therapy with apixaban and were subsequently asked to participate in the implementation phase of the study. Following consent, patients were centrally randomized in a 1:1 ratio to receive standard-of-care patient information or an additional educational program. For subject discontinuations see Table 3 in the Electronic Supplementary Material. ASA acetylsalicylic acid, NVAF non-valvular atrial fibrillation, OAC oral anticoagulant, R randomization, SOC standard of care, VKA vitamin-K antagonist
Study Procedures
Most patients received standard apixaban 5 mg tablets twice daily for 48 weeks. Apixaban 2.5 mg tablets twice daily were allocated to patients with creatinine clearance of 15–29 ml/min or two or more of the following: age ≥ 80 years, body weight ≤ 60 kg, or serum creatinine ≥ 133 µmol/l. Commercial packages (over-labelled for clinical trial use only, according to country regulations) were provided. All sites were provided with 5 mg tablets and 2.5 mg tablets in separately labelled, numbered packs. During the telephone randomisation procedure, the dose-reduction criteria were entered, and the site staff were advised which subject pack they would dispense (5 or 2.5 mg packs), according to the dose-reduction criteria for apixaban in the approved summary of product characteristics. Sites were allocated a new patient pack with the appropriate dose for any subsequent dose reduction required (at a subsequent visit).
Patients were asked not to break the 5 mg tablets if they were allocated to the 2.5 mg dose twice daily and not to take two 2.5 mg tablets of apixaban if they were allocated to 5 mg twice daily. The EMD could accommodate blister packs of either dosage.
All patients received SOC information about apixaban treatment from the investigator and the standard patient information leaflet and information card, according to the usual practice at each study center. Patients assigned to the additional educational program also received (1) a patient education booklet explaining NVAF and anticoagulant treatment for stroke prevention; (2) one or more of the reminder tools (chosen at the patient’s discretion): key ring, short message service alert on mobile phone, or smartphone application; and (3) access to a virtual clinic organized at the country level, utilizing staff from an existing anticoagulant clinic (virtual clinic locations listed in ESM 3). Patients were called by the virtual clinic within 1 week of randomization to ensure they had correctly understood the disease, rationale for anticoagulant treatment, and importance of adherence. They were also given general information and advice regarding anticoagulant treatment, information on symptoms of bleeding or thromboembolic events, and a toll-free number for further questions. Follow-up calls to reinforce the importance of adherence and provide additional advice (if needed) occurred 10–20 days after the first call and monthly thereafter. Patients could call the virtual clinic as often as needed. All information was provided using the official local language(s). After randomization, five visits were scheduled during the study (weeks 4, 12, 24, 36, and 48; Table 1 in the ESM).
Outcome Measures
The primary outcome was implementation adherence of the dosing regimen (the proportion of days adhering to the prescribed regimen) at 24 weeks, assessed using an EMD, Helping Hand® (WestRock Switzerland Ltd., Sion, Switzerland), an established monitoring device previously utilized in adherence studies [21–24]. This study used the basic model (model number: 1110-01), which had no display or reminder function to enhance implementation (see Fig. 1 in the ESM). The EMD was operated by inserting commercial blister packs into the device, which then electronically recorded every time the blister was removed (date and time). It was assumed that a single dose of study medication was administered every time the blister pack was removed. Sites uploaded data from the device at every study visit, but investigators and patients remained blinded to the data. The secondary outcome of the study was implementation adherence to the dosing regimen at 48 weeks. Days of adherent and non-adherent implementation were defined as follows:
- Day of adherent implementation: 24-h window during which
- the treatment was taken twice daily as prescribed (one tablet two times daily)
- only one isolated tablet was missed in 24 h, and no tablet was missed the previous day or the day after) (see exception below).
- Day of non-adherent implementation: 24-h window during which
- two consecutive tablets within 24 h were missed
- one tablet was missed for several consecutive days, with the first day considered a day of adherent implementation
- one tablet was missed on alternate days, every second day with a missed tablet constituting a day of non-adherent implementation.
The details on how adherence units were calculated can be found in the submitted statistical analysis plan (ESM, Sect. 8.4.2). Thus, the primary outcome is a measure of the optimality of medication intake and reflects both implementation and persistence, with any non-persistent period corresponding to non-adherent days. Other study objectives included percentage of “persistent” patients (i.e., patients who temporarily, but not permanently, discontinued their treatment regimen at the discretion of the investigator) and evaluation of the effect of the educational program on clinical outcomes (death, myocardial infarction, stroke, transient ischemic attack or SE, venous thromboembolism, major bleeding [non-fatal] or clinically relevant non-major bleeding, and fatal bleeding). Bleeding events were defined according to the International Society on Thrombosis and Haemostasis criteria (ESM 4). All ischemic, hemorrhagic events and deaths were adjudicated by an independent committee (ESM 2) who were blinded to patients’ study group.
Statistical Analysis
The study had 85% power to detect a between-group difference of 4% in the percentage of days with a correct execution of the dosing regimen during 24 weeks, assuming an intra-group SD of 21.1%. Anticipating a dropout rate of 10%, 1112 patients, 556 per group, were needed to obtain a sample size of approximately 1000 evaluable patients (primary outcome analysis).
The primary outcome, calculated as the percentage of days of implementation adherence during the period for each study group, was tested by the two-sample independent t tests at the 5% significance level (two-sided). Only the time period for which patients were persistent was taken into consideration for the analysis. Homogeneity of variances was checked graphically and with the F test. The Cochran t test was used for unequal variances between the two groups.
Within each study group, the percentage of days with a correct execution of apixaban dosing during the first 12, 12- to 24, and 24- to 48-week periods was compared using a paired t test, at a two-sided α level of significance of 0.05.
The percentage of patients within each group who were “persistent” over time was described using Kaplan–Meier estimates. If treatment with apixaban was withheld temporarily by the investigator during the study period, the time window during which apixaban was withheld was not considered for adherence measurement purposes. Study treatment that was withheld for more than 30 consecutive days was considered a permanent discontinuation.
Descriptive statistics were used to summarize clinical outcomes per group to evaluate the effect of the educational program on efficacy and safety outcomes. The study was not powered to detect outcome differences between the groups.
Results
Patients and Procedures
Between 21 November 2013 and 19 December 2014, a total of 1228 patients consented and were enrolled in the AEGEAN study. Among them, 1162 were randomized to either SOC (n = 583) or the additional educational program (n = 579). Of 524 patients remaining in the additional educational program at week 24, a total of 263 were re-randomized to SOC (secondary SOC) and 261 continued the additional educational program to week 48 (patient flow shown in Fig. 2 in the ESM). Baseline patient characteristics were well-balanced between the two initial randomized groups (Table 1).
Table 1.
Baseline patient characteristics
| Characteristics | Additional educational program (Na = 579) | Primary SOC (Na = 583) |
|---|---|---|
| Age (years) | 73.1 ± 9.1 | 72.6 ± 8.9 |
| Age ≥ 75 years | 282 (48.7) | 276 (47.3) |
| Female | 234 (40.4) | 232 (39.8) |
| Weight (kg) | 81.7 ± 16.7 | 82.8 ± 17.7 |
| Heart rate (bpm) | 74.9 ± 16.7 | 74.3 ± 15.9 |
| SBP (mmHg) | 132.7 ± 16.4 | 135.0 ± 17.1 |
| DBP (mmHg) | 78.0 ± 10.7 | 79.5 ± 10.8 |
| MMSE | 28.2 ± 1.7 | 28.3 ± 1.6 |
| Type of diagnosis | ||
| AF | 557 (96.4) | 562 (96.4) |
| Atrial flutter | 19 (3.3) | 18 (3.1) |
| AF + atrial flutter | 2 (0.3) | 3 (0.5) |
| Type of NVAF | ||
| Paroxysmal | 301 (52.4) | 280 (48.4) |
| Persistent | 137 (23.9) | 148 (25.6) |
| Permanent | 136 (23.7) | 150 (26.0) |
| Stroke and bleeding risk factors | ||
| Congestive heart failure | 93 (16.1) | 83 (14.2) |
| Hypertension | 497 (85.8) | 516 (88.5) |
| Diabetes mellitus | 151 (26.1) | 154 (26.4) |
| History of stroke or TIA | 107 (18.5) | 94 (16.1) |
| History of coronary heart disease/stent or PVD | 142 (24.5) | 138 (23.7) |
| Renal failureb | 11 (1.9) | 7 (1.2) |
| CHADS2 | 2.1 ± 1.0 | 2.1 ± 1.1 |
| CHA2DS2-VASc | 3.6 ± 1.3 | 3.6 ± 1.4 |
| HAS-BLED | 1.7 ± 0.8 | 1.7 ± 0.8 |
| High risk of bleeding (HAS-BLED ≥ 3) | 87 (15.2) | 87 (15.1) |
| Baseline VKA status | ||
| VKA naïvec | 370 (63.9) | 371 (63.6) |
| VKA treatedd | 207 (35.8) | 210 (36.0) |
| Concomitant medications at baseline | 5.1 ± 2.9 | 5.0 ± 2.7 |
| Previous treatment with ASA | 114 (19.7) | 116 (19.9) |
| Concomitant treatment with ASA | 100 (17.9) | 89 (14.8) |
| Dose of study drug assigned at baseline (mg) | ||
| 5 | 499 (86.2) | 512 (88.1) |
| 2.5 | 80 (13.8) | 69 (11.9) |
ASA acetylsalicylic acid, AF atrial fibrillation, DBP diastolic blood pressure, MMSE Mini Mental State Examination, NVAF non-valvular atrial fibrillation, PVD peripheral vascular disease, SBP systolic blood pressure, SOC standard of care, TIA transient ischemic attack, VKA vitamin K antagonist
Values are mean ± standard deviation or n (%)
aN represents the number of patients contributing to summary statistics. All percentages are based on the number of patients with available data for each characteristic
bCreatinine clearance < 30 ml/min
cVKA-naïve patients must not have received VKA treatment for > 30 days within the last 12 months
dVKA-treated patients must have received the VKA treatment for > 3 months
The proportions of patients communicating with the virtual clinic and using reminders at 24 weeks are presented in Table 2 in the ESM. Overall, > 95% of patients in the additional education program group had communicated with the virtual clinic during the first 24 weeks. Most patients used the home phone (> 80%) as the preferred method of communication with the clinic and dose reminders (> 40%) as the preferred type of reminder. Few (< 1%) patients used email or a smartphone app. Nearly half (45.3%) of the patients in the additional education program group chose to receive one reminder; few patients (< 1%) chose to receive two reminders.
Implementation Adherence and Persistence
In the additional educational program group, the mean ± SD (median [minimum, maximum]) percentage of days of implementation adherence (i.e., the primary outcome) was 91.9 ± 16.1% (98.2% [3%, 100%]) (Fig. 2). In the SOC group, the mean implementation adherence was 91.6 ± 17.1% (98.2% [1%, 100%]) (Fig. 2). By 48 weeks, implementation adherence had declined to 90.4 ± 18.0% (97.3% [2%, 100%]) in the continued additional educational program group; 90.1 ± 18.6% (97.9% [1%, 100%]) in the SOC group; and 89.3 ± 18.1% (97.9% [2%, 100%]) in the secondary SOC group. There were no significant differences between groups at 24 or 48 weeks (Fig. 3; p > 0.7 for all between-group comparisons). Up to 48 weeks, the average percentage of missed doses was low in each of the additional educational program, SOC, and secondary SOC groups (24 weeks: 4.4 ± 8.2%, 4.9 ± 8.5%, and 4.7 ± 7.2%, respectively; 48 weeks: 4.9 ± 8.6%, 5.9 ± 9.3%, and 5.7 ± 7.8%). At 24 weeks, the percentage of fully adherent subjects was 28.1% in the primary SOC group and 26.5% in the additional educational program group, with no significant difference between groups. The median duration of full adherence was 58.0 days in the primary SOC group and 59.0 days in the additional educational program group.
Fig. 2.
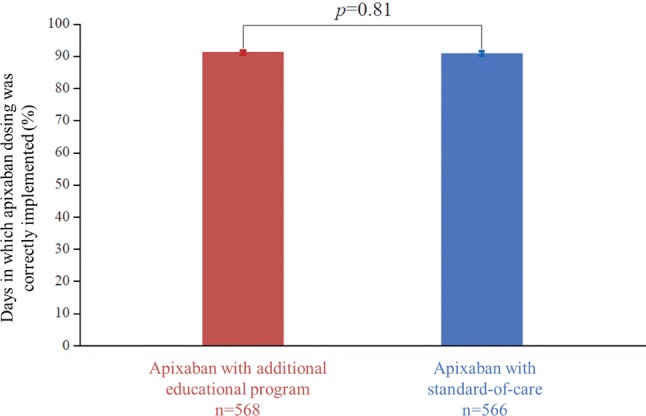
Implementation of the correct apixaban dose by study group at week 24 (mean; primary outcome). Implementation adherence (treatment taken as prescribed with one or less dose missed within 24 h and no tablet missed on the previous 2 consecutive days) was monitored by an electronic monitoring device. Not all patients handed in the device at week 24
Fig. 3.
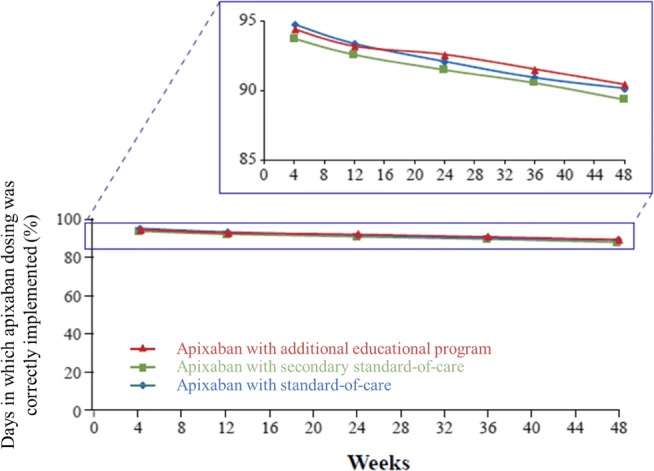
Overall implementation of the correct apixaban dose (mean) by study group. P > 0.7 for all between-group comparisons at 24 and 48 weeks. Apixaban with standard of care = patients randomized to receive only standard-of-care information following apixaban initiation; Apixaban with additional educational program = patients randomized to receive an additional educational program following apixaban initiation; Apixaban with secondary standard of care = patients with educational program switched to standard-of-care information only following 24 weeks of apixaban treatment. Additional data from all patients handing in the device at the end of the trial were included for calculating implementation adherence by week, up to week 48
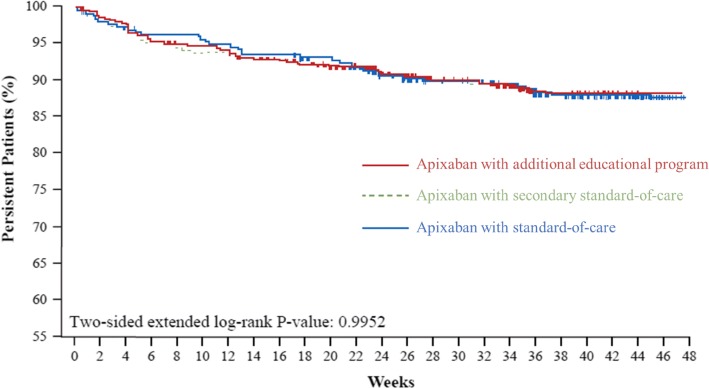
Persistence declined gradually across 48 weeks in all groups (Fig. 4). However, the percentage of patients persistent at 48 weeks was high: 86.1% (95% confidence interval [CI] 81.3–89.7), 85.2% (95% CI 81.5–88.2), and 87.8% (95% CI 83.4–91.1) in the continued additional educational program, SOC, and secondary SOC groups, respectively (p > 0.5 for all between-group comparisons). At week 48, only 9.2% (n = 100) of patients had missed three consecutive doses at any time during the study period. At this timepoint, the mean ± standard error percentage of days of implementation adherence was 90.8 ± 18.0% (median 98.1% [1%, 100%]) in previously VKA-treated patients and 89.6 ± 18.5% (97.6% [2%, 100%]) in VKA-naïve patients (p = 0.32). In addition, the likelihood of having < 80% implementation adherence in previously VKA-treated and VKA-naïve patients was similar in all groups (p > 0.05 for all between-group treatment comparisons). Patients were still considered persistent if they temporarily, but not permanently, discontinued their treatment regimen. The mean total interruption of study drug up to 24 weeks was 1.2 (± 3.6) days in the continued additional educational program group, 1.8 (± 8.3) days in the primary SOC group and 1.9 (± 5.5) days in the secondary SOC group. Mean total interruption of study drug up to 48 weeks was 2.2 (± 5.9) days in the continued additional educational program group, 3.8 (± 19.6) days in the primary SOC group and 4.8 (± 18.0) days in the secondary SOC group.
Fig. 4.
Persistence by study group over 48 weeks (secondary outcome). Apixaban with standard of care = patients randomized to receive only standard-of-care information following apixaban initiation; Apixaban with additional educational program = patients randomized to receive an additional educational program following apixaban initiation; Apixaban with secondary standard of care = patients with educational program switched to standard-of-care information only following 24 weeks of apixaban treatment
Comparisons of implementation adherence between the SOC and the additional educational program for different subgroups of patients demonstrated no significant differences in the likelihood of having < 80% implementation adherence (Fig. 3 in the ESM; p > 0.1 for all subgroup comparisons).
Clinical Outcomes
Adjudicated clinical events are summarized in Table 2. Overall, 4.8% of patients had at least one adverse event leading to permanent discontinuation (4.5% in the additional educational group; 5.1% in the SOC group). Between 24 and 48 weeks, 2.2% of patients had an adverse event leading to permanent discontinuation (1.1, 2.2, and 3.0% in the additional educational, SOC, and secondary SOC groups, respectively). Bleeding events were low and evenly distributed across groups (Table 2).
Table 2.
Adjudicated clinical endpoints at 24 and 48 weeks of follow-up
| Endpoint | 24 weeks | 24–48 weeks | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Apixaban, AEP (N = 583) | Apixaban, pSOC (N = 579) | Apixaban, AEP (N = 261) | Apixaban, pSOC (N = 583) | Apixaban, sSOC (N = 263) | ||||||
| Patients | Events | Patients | Events | Patients | Events | Patients | Events | Patients | Events | |
| Death | 4 (0.7) | 4 | 4 (0.7) | 4 | 1 (0.4) | 1 | 1 (0.2) | 1 | 3 (1.1) | 3 |
| Cardiovascular death | 2 (0.3) | 2 | 4 (0.7) | 4 | 0 | 0 | 1 (0.2) | 1 | 2 (0.8) | 2 |
| Stroke, TIA, SE | 5 (0.9) | 6 | 1 (0.2) | 1 | 1 (0.4) | 1 | 1 (0.2) | 1 | 3 (1.1) | 3 |
| Ischemic stroke | 0 | 0 | 1 (0.2) | 1 | 1 (0.4) | 1 | 0 | 0 | 0 | 0 |
| Hemorrhagic stroke | 2 (0.3) | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (0.4) | 1 |
| TIA | 0 | 0 | 0 | 0 | 0 | 0 | 1 (0.2) | 1 | 1 (0.4) | 1 |
| SE | 3 (0.5) | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (0.4) | 1 |
| Myocardial infarction | 4 (0.7) | 4 | 2 (0.3) | 2 | 0 | 0 | 1 (0.2) | 1 | 0 | 0 |
| Venous thromboembolism | 1 (0.2)a | 1 | 0 | 0 | 1 (0.4) | 1 | 0 | 0 | 0 | 0 |
| Pulmonary embolism | 1 (0.2) | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Deep vein thrombosis | 1 (0.2) | 1 | 0 | 0 | 1 (0.4) | 1 | 0 | 0 | 0 | 0 |
| MB (non-fatal) or CRNMB | 9 (1.6)b | 10 | 7 (1.2) | 7 | 0 | 0 | 2 (0.3) | 2 | 2 (0.8) | 2 |
| MB (non-fatal) | 4 (0.7) | 4 | 2 (0.3) | 2 | 0 | 0 | 2 (0.3) | 2 | 1 (0.4) | 1 |
| CRNMB | 5 (0.9) | 5 | 5 (0.9) | 5 | 0 | 0 | 0 | 0 | 0 | 0 |
| Fatal bleeding | 1 (0.2) | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (0.4) | 1 |
AEP additional educational program, CRNMB clinically relevant non-major bleeding, MB major bleeding, pSOC primary standard of care, SE systemic embolism, sSOC secondary standard of care, TIA transient ischemic attack
Data are presented as N or N (%)
aOne patient, who had one event of deep vein thrombosis + pulmonary embolism
bOne patient had a major and fatal bleeding
Discussion
This was the first prospective, randomized study investigating the impact of an education program on adherence to an NOAC. In daily practice, many patients do not adhere to lifesaving treatments or to treatments that can prevent serious complications and/or improve quality of life [8, 25–27]. However, strict implementation of NOAC intake is crucial because its anticoagulant effect wanes within 12–24 h after the last intake [7]. The AEGEAN study showed that implementation adherence and persistence rates with apixaban therapy for NVAF are high over the first 48 weeks of administration (~ 90% for the implementation phase), with attrition of implementation adherence and persistence over time. While most studies present rates of adherent patients, rather than the total days of adherence as per the primary outcome in this study, rates of persistence compare favorably with previously reported rates in other NOAC studies (persistence of 56–74% over a median follow-up duration of up to 36 weeks) [17, 28, 29]. However, these studies were retrospective with significant measurement bias [28, 29]. Although the deployment of a 24- or 48-week proactive educational program in the AEGEAN study did not substantially increase the implementation adherence and persistence rates, the very high SOC rates are encouraging, and these data are consistent with the reported data for apixaban [28, 29]. The adherence and persistence findings may reflect the impact of continued follow-up of patients in the SOC group, with five follow-up visits in the first year, the importance of providing appropriate NOAC-specific information at initiation (as recommended [30]), as well as the use of the EMD. While the EMD used in this study did not incorporate a reminder function, the monitoring of medication use could itself act as a continuous reminder (the Hawthorne effect) [31] and act as a motivator for implementation adherence. In the AEGEAN study, the double-blind aspect of the study meant that adherence data feedback was not possible. However, a recently published telemonitoring study using daily patient feedback and employing an EMD showed very high adherence (89.6% over a 3-month observation period) to NOAC therapy despite the small sample size, probably largely attributable to being monitored [18].
Implementation adherence and persistence rates reported with VKAs are generally lower than those with NOACs, with historical cohort studies and clinical trials reporting rates of 42–78% (duration of follow-up of 3–84 months) [9]. This may be attributed to burdensome therapeutic monitoring and dose-adjustment requirements associated with these agents [32], as well as drug–drug and drug–food interactions that need to be explained in detail at treatment initiation and require ongoing education. One-third of the patients in AEGEAN were VKA experienced and switched to apixaban at randomization. In these patients, implementation adherence in the SOC group was similar to that observed in anticoagulant-naïve patients.
The lack of a significant benefit of our proactive educational program on apixaban implementation adherence and persistence was unexpected. In addition to the high implementation adherence rate with SOC (~ 90%), several factors may have offset the effect of the educational program: (1) investigators in the AEGEAN trial are likely to be more experienced and well-trained in prescribing NOACs and providing more NOAC-specific information than the general population of physicians, narrowing the gap between SOC patient information and the additional educational program; (2) limited patient uptake of dose reminder strategies, especially those associated with email and cell phones, possibly because of the advanced age of the patients; and (3) the nature of the virtual clinic, as recent findings suggest that face-to-face engagement can maximize effectiveness [17]. Nonetheless, the virtual clinic contacted patients in the intervention arm regularly and proactively, albeit remotely, which would act as an intermittent reminder for medication adherence, and most virtual clinics reported positive patient interactions. It is also possible that a major differential effect would be expected on the initiation phase of adherence (patients who start/do not start treatment), which was not measured in this study as most patients had initiated treatment in hospital. Finally, a lack of observed effects from similar educational programs has been reported in clinical situations other than NVAF [33, 34], reinforcing the difficulties of such initiatives. In contrast, the recent FACILITA study showed that a mixed intervention, consisting of patient education and a simple calendar reminder of drug intake, was an effective strategy to improve dabigatran adherence (to 91% and 89% at 6 and 12 months, respectively, compared with 65% and 63% for the control group) [19]. Also, in the IMPACT-AF study, the use of oral anticoagulation in patients with atrial fibrillation was improved by a multifaceted and multilevel educational program, where the provision of physician education targeted the initiation phase of adherence as well as the implementation and persistence phases [35]. However, these studies had some notable differences from the present study. Most patients in IMPACT-AF (> 80%) were anticoagulated with VKA, with poor implementation of treatment at baseline (66%), leaving room for a significant improvement after intervention. Moreover, education was provided to not only patients but also their families and healthcare providers, an important aspect of VKA treatment. There was also regular monitoring with a feedback mechanism providing relevant information to coordinating centers, such as notifications when patients were not taking their drugs and allowing physician intervention to prevent discontinuation of anticoagulation and improve persistence [35]. Despite these efforts, the persistence rate observed in IMPACT-AF remained lower than seen in the current study.
Our study also has limitations. The selection of participating patients may not fully represent patients in real life, as patients willing to participate in an adherence study are more likely to be better adherers. Furthermore, patient consent to participate in the implementation phase was only obtained once patients had consented to initiating apixaban therapy. Thus, patients who did not consent to the study, possibly due to lack of motivation (and who might have exhibited poorer implementation adherence or persistence), were not included in the trial. While the follow-up schedule was selected to be close to the SOC for most countries, in some centers this would constitute additional visits that may have acted as a reminder and improved implementation adherence. Our results apply to the duration of the study, and adherence may further decrease over time beyond 48 weeks—we do not know the impact of education beyond this period. Measuring implementation adherence improves it per se, as the patient is aware they are being monitored, resulting in overestimation. However, this effect was minimized because neither the patient nor the investigator was aware of the implementation adherence data during the trial. AEGEAN used a low-bias, rich sampling method (EMD Helping Hand®) that precisely recorded the date and time of medication access, providing detailed information on implementation adherence (although pill disposal after extraction from the packet cannot be ruled out) [20]. The type of EMD used may also have influenced results across the study, as some models with displays provide instant feedback. Instant positive feedback following intake of medication may encourage future implementation adherence and persistence; thus, it is possible that overall adherence across all cohorts would increase if EMDs with displays were used. We believe our definition of a unit of non-adherence in implementation (missing two consecutive pills) is conservative. It has been argued that missing three consecutive doses of an oral anticoagulant with a 12-h half-life would have the same impact on its pharmacokinetic profile as missing one (double) dose in a once-daily regimen [36]. Thus, it is likely that the lower limit of apixaban’s therapeutic window, where the risk of thrombosis begins to increase, is reached only after missing three or more doses. While the cost of medication may also impact implementation adherence and persistence, medications were fully reimbursed in all countries that participated in this study and therefore medication acquisition costs would not have impacted the outcome. Finally, the study enrolled patients with AF who were relatively stable compared with an unselected AF population, as hospitalized patients and those with an increased risk of bleeding were excluded. This may have reduced the occurrence of clinical events such as bleeding, which could have impacted adherence and reduced the effect of the educational program on this outcome.
In conclusion, our study demonstrated high implementation adherence and persistence rates during the first 48 weeks in patients started on apixaban for NVAF and highlighted the importance of regular patient follow-up and education in maintaining favorable adherence to NOAC therapy in both SOC and intervention arms of the study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors acknowledge editorial support from Lucid Group (Stuart Wakelin, Claire Price, Angela Pozo Ramajo, Susan Cheer, Lisa Tatler, Jessica Stevens, Vatsala Malik, Eman Zaman, and Emer O’Connor), funded by Bristol-Myers Squibb and Pfizer. The Bristol-Myers Squibb and Pfizer Alliance thank the patients who volunteered to participate and their caregivers, physicians, and clinical study teams for their contribution to the advancement of patient care. The image of the EMD was provided courtesy of Aardex group.
Compliance with Ethical Standards
Funding
This work was supported by Bristol-Myers Squibb (Paris, France) and Pfizer Limited (Tadworth, UK). The sponsor was involved with the design and conduct of the study and the collection, management, analysis, and interpretation of the data.
Author contributions
Trial conduct and data analysis were overseen by the executive steering committee (ESM 3) in collaboration with the study sponsors (Bristol-Myers Squibb and Pfizer Ltd). The coordinating center was the ACTION Study Group at Pitié-Salpêtrière hospital. Data were collected, managed, and analyzed by two Clinical Research Organizations: Lincoln (data management, Boulogne-Billancourt, France) and Everest (data analysis, Ontario, Canada). Statistical analyses were validated by an independent statistician from ACTION Academic Research Organization (http://www.action-coeur.org). The study chairman had unrestricted access to data after database lock. The first author (GM) prepared the first draft of the manuscript, which was revised by all authors, who made the decision to submit the manuscript for publication; they take responsibility for data accuracy.
Conflicts of interest
GM reports research grants to his institution from ICAN, Fédération Française de Cardiologie, Medtronic, MSD, Pfizer, Sanofi-Aventis, Servier, and INSERM; consultancy fees from Beth Israel Deaconess Medical, Brigham Women’s Hospital, Menarini, TIMI Study Group, and ACTELION; and educational support from Cardiovascular Research Foundation, CME Resources, Europa, Elsevier, Lead-Up, WebMD CCC. CB, AD’A, and HD have received consultancy fees from Bristol-Myers Squibb/Pfizer. BC reports personal fees from St Jude Medical and research grants from Daiichi Sankyo. HJC and LD report research grants to their institutions from Bristol-Myers Squibb/Pfizer. FE has received speaker honoraria from Bristol-Myers Squibb. DAL reports research grants to her institution from Bristol-Myers Squibb and Boehringer Ingelheim, and speaker and consultancy fees from Boehringer Ingelheim, Bayer, and Bristol Myers Squibb/Pfizer. BB is a former employee of Bristol-Myers Squibb. AC is an employee of Pfizer Inc. EV reports grants to his institution from Boehringer and Sanofi; has received consultancy fees from Abbott, Bristol-Myers Squibb, Celgene, Daiichi Sankyo, European Cardiovascular Research Center, Fresenius, LFB, Lilly, Medtronic, Pfizer, and Sorin Group, and has received lecture fees from Novartis.
Footnotes
Bruno Besse: Position at the time of manuscript preparation. No longer an employee of Bristol-Myers Squibb.
The full list of AEGEAN Investigators is given in the Electronic Supplementary Material.
References
- 1.Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146:857–867. doi: 10.7326/0003-4819-146-12-200706190-00007. [DOI] [PubMed] [Google Scholar]
- 2.Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, ARISTOTLE Committees and Investigators et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–992. doi: 10.1056/NEJMoa1107039. [DOI] [PubMed] [Google Scholar]
- 3.Connolly SJ, Ezekowitz M, Yusuf S, Eikelboom J, Oldgren J, Parekh A, RE-LY Steering Committee and Investigators et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–1151. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 4.Connolly SJ, Ezekowitz MD, Yusuf S, Reilly PA, Wallentin L, Randomized Evaluation of Long-Term Anticoagulation Therapy Investigators Newly identified events in the RE-LY trial. N Engl J Med. 2010;363:1875–1876. doi: 10.1056/NEJMc1007378. [DOI] [PubMed] [Google Scholar]
- 5.Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, Breithardt G, ROCKET AF Investigators et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–891. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 6.Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL, ENGAGE AF-TIMI 48 Investigators et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369:2093–2104. doi: 10.1056/NEJMoa1310907. [DOI] [PubMed] [Google Scholar]
- 7.Steffel J, Verhamme P, Potpara TS, Albaladejo P, Antz M, Desteghe L, ESC Scientific Document Group et al. The 2018 European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Eur Heart J. 2018;39:1330–1393. doi: 10.1093/eurheartj/ehy136. [DOI] [PubMed] [Google Scholar]
- 8.Vrijens B, De Geest S, Hughes DA, Przemyslaw K, Demonceau J, Ruppar T, ABC Project Team et al. A new taxonomy for describing and defining adherence to medications. Br J Clin Pharmacol. 2012;73:691–705. doi: 10.1111/j.1365-2125.2012.04167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rodriguez RA, Carrier M, Wells PS. Non-adherence to new oral anticoagulants: a reason for concern during long-term anticoagulation? J Thromb Haemost. 2013;11:390–394. doi: 10.1111/jth.12086. [DOI] [PubMed] [Google Scholar]
- 10.Khan TI, Kamali F, Kesteven P, Avery P, Wynne H. The value of education and self-monitoring in the management of warfarin therapy in older patients with unstable control of anticoagulation. Br J Haematol. 2004;126:557–564. doi: 10.1111/j.1365-2141.2004.05074.x. [DOI] [PubMed] [Google Scholar]
- 11.Clarkesmith DE, Pattison HM, Lane DA. Educational and behavioural interventions for anticoagulant therapy in patients with atrial fibrillation. Cochrane Database Syst Rev. 2013;2013:CD008600. doi: 10.1002/14651858.CD008600.pub2. [DOI] [PubMed] [Google Scholar]
- 12.Shore S, Ho PM, Lambert-Kerzner A, Glorioso TJ, Carey EP, Cunningham F, et al. Site-level variation in and practices associated with dabigatran adherence. JAMA. 2015;313:1443–1450. doi: 10.1001/jama.2015.2761. [DOI] [PubMed] [Google Scholar]
- 13.Diener HC, Aisenberg J, Ansell J, Atar D, Breithardt G, Eikelboom J, et al. Choosing a particular oral anticoagulant and dose for stroke prevention in individual patients with non-valvular atrial fibrillation: part 2. Eur Heart J. 2017;38:860–868. doi: 10.1093/eurheartj/ehx320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raparelli V, Proietti M, Cangemi R, Lip GYH, Lane DA, Basili S. Adherence to oral anticoagulant therapy in patients with atrial fibrillation: focus on non-vitamin K antagonist oral anticoagulants. Thromb Haemost. 2016;117:209–218. doi: 10.1160/TH16-10-0757. [DOI] [PubMed] [Google Scholar]
- 15.McHorney CA, Crivera C, Laliberté F, Nelson WW, Germain G, Bookhart B, et al. Adherence to non-vitamin-K-antagonist oral anticoagulant medications based on the Pharmacy Quality Alliance measure. Curr Med Res Opin. 2015;31:2167–2173. doi: 10.1185/03007995.2015.1096242. [DOI] [PubMed] [Google Scholar]
- 16.Hanemaaijer S, Sodihardjo F, Horikx A, Wensing M, De Smet PA, Bouvy ML, Teichert M. Trends in antithrombotic drug use and adherence to non-vitamin K oral anticoagulants in The Netherlands. Int J Clin Pharm. 2015;37:1128–1135. doi: 10.1007/s11096-015-0174-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shore S, Carey EP, Turakhia MP, Jackevicius CA, Cunningham F, Pilote L, et al. Adherence to dabigatran therapy and longitudinal patient outcomes: insights from the veterans health administration. Am Heart J. 2014;167:810–817. doi: 10.1016/j.ahj.2014.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Desteghe L, Vijgen J, Koopman P, Dilling-Boer D, Schurmans J, Dendale P, Heidbuchel H. Telemonitoring-based feedback improves adherence to non-vitamin K antagonist oral anticoagulants intake in patients with atrial fibrillation. Eur Heart J. 2018;39:1394–1403. doi: 10.1093/eurheartj/ehx762. [DOI] [PubMed] [Google Scholar]
- 19.Márquez-Contreras E, Martell-Claros N, Márquez-Rivero S, Hermida-Campa E, Gracia-Diez C, Sanchez-López E, Gil-Guillén V, Compliance and Inertia Working Group, Spanish Society of Hypertension (SEH-LELHA) Strategies for improving dabigatran adherence for stroke prevention in patients with non-valvular atrial fibrillation: education and drug intake reminders (FACILITA study) Curr Med Res Opin. 2018;34:1301–1308. doi: 10.1080/03007995.2018.1435519. [DOI] [PubMed] [Google Scholar]
- 20.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353:487–497. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- 21.Christensen A, Christrup LL, Fabricius PE, Chrostowska M, Wronka M, Narkiewicz K, Hansen EH. The impact of an electronic monitoring and reminder device on patient compliance with antihypertensive therapy: a randomized controlled trial. J Hypertens. 2010;28:194–200. doi: 10.1097/HJH.0b013e328331b718. [DOI] [PubMed] [Google Scholar]
- 22.Kuypers DR, Peeters PC, Sennesael JJ, Kianda MN, Vrijens B, Kristanto P, ADMIRAD Study Team et al. Improved adherence to tacrolimus once-daily formulation in renal recipients: a randomized controlled trial using electronic monitoring. Transplantation. 2013;95:333–340. doi: 10.1097/TP.0b013e3182725532. [DOI] [PubMed] [Google Scholar]
- 23.Dobbels F, De Bleser L, Berben L, Kristanto P, Dupont L, Nevens F, et al. Efficacy of a medication adherence enhancing intervention in transplantation: the MAESTRO-Tx trial. J Heart Lung Transplant. 2017;36:499–508. doi: 10.1016/j.healun.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 24.De Bleser L, De Geest S, Vandenbroeck S, Vanhaecke J, Dobbels F. How accurate are electronic monitoring devices? A laboratory study testing two devices to measure medication adherence. Sensors (Basel) 2010;10:1652–1660. doi: 10.3390/s100301652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ruddy K, Mayer E, Partridge A. Patient adherence and persistence with oral anticancer treatment. CA Cancer J Clin. 2009;59:56–66. doi: 10.3322/caac.20004. [DOI] [PubMed] [Google Scholar]
- 26.Wu JR, Moser DK, Lennie TA, Burkhart PV. Medication adherence in patients who have heart failure: a review of the literature. Nurs Clin N Am. 2008;43:133–153. doi: 10.1016/j.cnur.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 27.van der Wal MH, Jaarsma T. Adherence in heart failure in the elderly: problem and possible solutions. Int J Cardiol. 2008;125:203–208. doi: 10.1016/j.ijcard.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 28.Pan X, Kachroo S, Liu X, Kawabata H, Phatak H. Real world discontinuation rates with apixaban versus warfarin, dabigatran, or rivaroxaban among atrial fibrillation patients newly initiated on anticoagulation therapy: early findings. J Am Coll Cardiol. 2014;63:Suppl:A415. Abstract. doi: 10.1016/S0735-1097(14)60415-0. [DOI] [Google Scholar]
- 29.Yao X, Abraham NS, Alexander GC, Crown W, Montori VM, Sangaralingham LR, et al. Effect of adherence to oral anticoagulants on risk of stroke and major bleeding among patients with atrial fibrillation. J Am Heart Assoc. 2016;5:e003074. doi: 10.1161/JAHA.115.003074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lane DA, Aguinaga L, Blomström-Lundqvist C, Boriani G, Dan GA, Hills MT, et al. Cardiac tachyarrhythmias and patient values and preferences for their management: the European Heart Rhythm Association (EHRA) consensus document endorsed by the Heart Rhythm Society (HRS), Asia Pacific Heart Rhythm Society (APHRS), and Sociedad Latinoamericana de Estimulación Cardíaca y Electrofisiología (SOLEACE) Europace. 2015;17:1747–1769. doi: 10.1093/europace/euv233. [DOI] [PubMed] [Google Scholar]
- 31.Braunholtz DA, Edwards SJL, Lilford RJ. Are randomized clinical trials good for us (in the short term)? Evidence for a “trial effect”. J Clin Epidemiol. 2001;54:217–224. doi: 10.1016/S0895-4356(00)00305-X. [DOI] [PubMed] [Google Scholar]
- 32.Kneeland PP, Fang MC. Current issues in patient adherence and persistence: focus on anticoagulants for the treatment and prevention of thromboembolism. Patient Prefer Adherence. 2010;4:51–60. doi: 10.2147/ppa.s6101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cohen A, Assyag P, Boyer-Chatenet L, Cohen-Solal A, Perdrix C, Dalichampt M, Réseau Insuffisance Cardiaque (RESICARD) PREVENTION Investigators et al. An education program for risk factor management after an acute coronary syndrome: a randomized clinical trial. JAMA Intern Med. 2014;174:40–48. doi: 10.1001/jamainternmed.2013.11342. [DOI] [PubMed] [Google Scholar]
- 34.van Dalem J, Krass I, Aslani P. Interventions promoting adherence to cardiovascular medicines. Int J Clin Pharm. 2012;34:295–311. doi: 10.1007/s11096-012-9607-5. [DOI] [PubMed] [Google Scholar]
- 35.Vinereanu D, Lopes RD, Bahit MC, Xavier D, Jiang J, Al-Khalidi HR, IMPACT-AF investigators et al. A multifaceted intervention to improve treatment with oral anticoagulants in atrial fibrillation (IMPACT-AF): an international, cluster-randomised trial. The Lancet. 2017;390:1737–1746. doi: 10.1016/S0140-6736(17)32165-7. [DOI] [PubMed] [Google Scholar]
- 36.Vrijens B, Heidbuchel H. Non-vitamin K antagonist oral anticoagulants: considerations on once- vs. twice-daily regimens and their potential impact on medication adherence. Europace. 2015;17:514–523. doi: 10.1093/europace/euu311. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.