Introduction
Historically, refractory/relapsed (r/r) acute B-lymphoblastic leukemia (ALL) in adults has a dismal prognosis, with less than 10% of patients being long-term survivors, which is only achievable through a bone marrow (BM) transplantation.1 The emergence of targeted therapies for ALL has shown that is possible to reach deep remissions even in these patients, achieving negative minimal residual disease (MRD) status, which is paramount for cure.2
Blinatumomab is a bispecific T-cell engager which binds human CD19 (B lymphoblasts) and CD3ɛ (T-cell), stimulating the formation of a cytolytic synapse and cell lysis.2 Inotuzumab Ozogamicin (IO) is an anti-CD22 monoclonal antibody which delivers a potent cytotoxic agent (calicheamicin) into the cell, inducing apoptosis.3
Little has been said about their role in extramedullary disease. Patients with central nervous system (CNS) infiltration and/or isolated extramedullary disease have been excluded from clinical trials, and it seems there is an over-representation of extramedullary relapses following blinatumomab exposure in these studies.4 Herein, we report on a patient with refractory B-cell ALL and extramedullary disease, treated from the beginning with Blinatumomab, and her differential behavior after this drug and subsequent exposure to IO.
Case presentation
A previously healthy 29-year-old female presented with left shoulder swelling, pain and fatigue for 30 days before medical care. Nuclear resonance showed a tumor measuring 14 cm × 11 cm × 5 cm in her left scapula, which was biopsied and initially misdiagnosed as a sarcoma. Subsequently, she underwent an exploratory laparotomy with a right colectomy due to an acute intestinal obstruction. The histologic study of the right colon revealed infiltration by B-lineage lymphoblasts. The BM aspiration revealed a massive infiltration by lymphoblasts (positive for CD19, CD10, CD22, CD34, TdT, cyIgM and CD13 and negative for CD20). Her scapular biopsy was reviewed and also supported a B-cell ALL. The BM karyotyping was diploid. The fluorescence in situ hybridization was negative for BCR/ABL rearrangement and demonstrated a partial deletion of the KMT2A gene on chromosome 11q23. The cerebral spinal fluid analysis was negative for blasts.
In short, she started chemotherapy with the Hyper-CVAD regimen, being refractory after two cycles. A salvage regimen with FLAG-IDA (idarubicin, fludarabine, cytarabine and filgrastim) was tentatively administered, but her disease persisted (98% of the lymphoblasts in the BM assessment).
Taking this chemorefractoriness into account, she was started on Blinatumomab in an inpatient setting (9 mcg/day on the first 7 days, followed by 28 mcg/day on days 8–28). On the whole, she succeeded in receiving all of the scheduled drug, in spite of a grade 2 cytokine release syndrome in the first week of infusion and acute pancreatitis which developed after the planned increment in the blinatumomab dose on day 8. These two adverse events were managed with methylprednisolone, temporary discontinuation of the drug and fasting during pancreatitis.
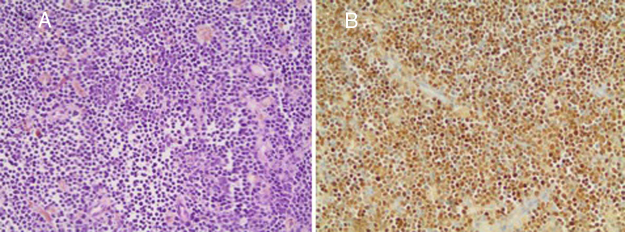
After the first cycle of blinatumomab, a new BM assessment was compatible with morphologic remission, and minimal residual disease by flow cytometry resulted negative. Re-evaluation of extranodal disease, using an 18FDG-PET-CT, demonstrated the persistence of the hypermetabolic lesion (Maximum Standard Uptake Value – SUVmax: 6.1) on the scapula. A new biopsy was performed, confirming the B-cell ALL persistence (Figure 1).
Figure 1.
Histological confirmation of acute lymphoblastic leukemia, despite monoclonal antibody therapy. (A) The core-biopsy shows numerous small- to medium-sized lymphocytes among scattered hemosiderin-containing macrophages (Hematoxylin–Eosin, 200×). (B) The TdT expression by immunohistochemistry (200×).
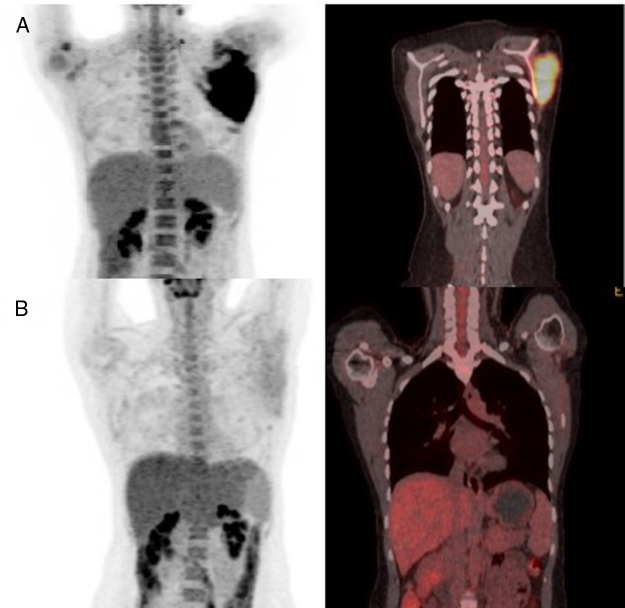
While receiving the second cycle of blinatumomab, she complained of a worsening of her left shoulder mass and noted a new left cervical lymphadenopathy, measuring 1.5 cm, denoting progression of the extramedullary disease. After this cycle, a new BM aspiration showed no blasts and the MRD evaluation remained negative. A new 18FDG-PET-CT demonstrated progressive extramedullary disease (Figure 2).
Figure 2.
The 18FDG PET-scan of the patient after blinatumomab therapy (A) and after inotuzumab plus radiotherapy (B).
Considering this, we decided to try IO. The drug was administered in our outpatient clinic as monotherapy, as originally described (0.8 mg/m2 on day 1 and 0.5 mg/m2 on days 8 and 15).5 After two cycles of IO, the patient and the medical team noticed a clinical reduction in the scapulary tumor. Local irradiation of 30 Gy was delivered as consolidation. After IO and radiotherapy, a new PET-CT was performed, demonstrating a complete remission status, along with MRD negativity found in the BM assessment (Figure 2). Thus, she was referred to haploidentical allogeneic stem-cell transplantation (SCT), but died prematurely from transplantation-related complications – thrombotic microangiopathy and sepsis.
Discussion
Extramedullary involvement is described in 20% of patients with ALL, and it is more common in the T-cell phenotype, as well as in the lymphoma presentation, without blood or BM disease.6 Despite being rarely seen, the B-cell ALL may involve different sites, including head and neck, gonads, retroperitoneum, mediastinum, pleura, breast, gastrointestinal tract, kidneys, brain and soft tissue.6 Not rarely, this tissue infiltration may be misdiagnosed as Ewing sarcoma, as in the presented case, and reinforced in previous pathologic reviews.7
A retrospective study of 65 patients with r/r ALL concluded that extramedullary disease at the time of the treatment with blinatumomab was the only predictor of failure in the multivariate analysis (OR = 13.16, p = 0.03).8 It remains unknown whether increasing the dose of blinatumomab for ALL will be able to overcome this resistance, since for non-Hodgkin lymphomas a higher dose has been studied and reasonable results have also been achieved for this disease.9 Interestingly, the current focus is on strategies to overcome the T-cell exhaustion and reactivate its function, since they are the responsible for inducing cell death with this drug. As previously demonstrated, some of these extramedullary relapses are driven by upregulation of the programmed death-ligand 1 (PD-L1) on tumor cells, and current trials combining blinatumomab with nivolumab or pembrolizumab are ongoing (ClinicalTrials.gov identifier: NCT03160079 and NCT02879695).10
The role of IO in extramedullary disease has also largely not been studied. Patients with extramedullary disease were excluded from the clinical trials, but its action mechanism, based on direct cytotoxicity of calicheamicin on tumor cells, may explain its performance in our case.3 Admittedly, although local radiotherapy cooperated in achieving tumor resolution in our case, IO clearly evoked an apparent clinical response seen even before radiation.
Conclusion
This case highlights the challenge of treating B-cell ALL with extranodal disease with new drugs and the differential response seen with blinatumomab. Whereas the BM disease reached MRD-negative status, there was a clinical progression of extramedullary disease (scapular tumor and nodal disease). This extramedullary disease eventually responded to IO, another targeted therapy for B-cell ALL, there being no previous report of response by extramedullary disease. It seems that the tumor microenvironment, as well as other mechanisms of tumor evasion, is essential to the response to these new drugs, and strategies of drug combinations are still a pending matter for these new targeted therapies, certainly deserving to be confirmed and validated by larger case series and clinical trials.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgments
We are grateful to the patient, who consented to have her data published in this case report before her demise.
References
- 1.Gokbuget N., Dombret H., Ribera J.-M., Fielding A.K., Advani A., Bassan R. International reference analysis of outcomes in adults with B-precursor Ph-negative relapsed/refractory acute lymphoblastic leukemia. Haematologica [Internet] 2016;101(12):1524–1533. doi: 10.3324/haematol.2016.144311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Le Jeune C., Thomas X. Antibody-based therapies in B-cell lineage acute lymphoblastic leukaemia. Eur J Haematol. 2015;94(2):99–108. doi: 10.1111/ejh.12408. PMID: 24981395. [DOI] [PubMed] [Google Scholar]
- 3.Thota S., Advani A. Inotuzumab ozogamicin in relapsed B-cell acute lymphoblastic leukemia. Eur J Haematol. 2017;98(5):425–434. doi: 10.1111/ejh.12862. [DOI] [PubMed] [Google Scholar]
- 4.Kantarjian H., Stein A., Gökbuget N., Fielding A.K., Schuh A.C., Ribera J.-M. Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. N Engl J Med [Internet] 2017;376(9):836–847. doi: 10.1056/NEJMoa1609783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kantarjian H.M., DeAngelo D.J., Stelljes M., Martinelli G., Liedtke M., Stock W. Inotuzumab ozogamicin versus standard therapy for acute lymphoblastic leukemia. N Engl J Med [Internet] 2016;375(8):740–753. doi: 10.1056/NEJMoa1509277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Geethakumari P.R., Hoffmann M.S., Pemmaraju N., Hu S., Jorgensen J.L., O’Brien S. Extramedullary B lymphoblastic leukemia/lymphoma (B-ALL/B-LBL): a diagnostic challenge. Clin Lymphoma Myeloma Leuk [Internet] 2014;14(4):e115–e118. doi: 10.1016/j.clml.2014.01.004. Available from: http://linkinghub.elsevier.com/retrieve/pii/S2152265014000202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lucas D.R., Bentley G., Dan M.E., Tabaczka P., Poulik J.M., Mott M.P. Ewing sarcoma vs lymphoblastic lymphoma. Am J Clin Pathol [Internet] 2001;115(1):11–17. doi: 10.1309/K1XJ-6CXR-BQQU-V255. [DOI] [PubMed] [Google Scholar]
- 8.Aldoss I., Song J., Stiller T., Nguyen T., Palmer J., O’Donnell M. Correlates of resistance and relapse during blinatumomab therapy for relapsed/refractory acute lymphoblastic leukemia. Am J Hematol [Internet] 2017;92(9):858–865. doi: 10.1002/ajh.24783. [DOI] [PubMed] [Google Scholar]
- 9.Sanders S., Stewart D.A. Targeting non-Hodgkin lymphoma with blinatumomab. Expert Opin Biol Ther [Internet] 2017;17(8):1013–1017. doi: 10.1080/14712598.2017.1334053. [DOI] [PubMed] [Google Scholar]
- 10.Köhnke T., Krupka C., Tischer J., Knösel T., Subklewe M. Increase of PD-L1 expressing B-precursor ALL cells in a patient resistant to the CD19/CD3-bispecific T cell engager antibody blinatumomab. J Hematol Oncol [Internet] 2015;8(1):111. doi: 10.1186/s13045-015-0213-6. Available from: http://www.jhoonline.org/content/8/1/111. [DOI] [PMC free article] [PubMed] [Google Scholar]