Abstract
Continuous intraocular pressure (IOP) monitoring for improving glaucoma diagnosis and treatment has remained a challenge for the past 60 years because glaucoma is the second leading cause of irreversible blindness worldwide. Several devices with different measurement principles and recently developed biosensors with semiconductor materials offer exciting properties. However, none of these devices for continuous IOP monitoring have been fully integrated into clinical practice, primarily due to technical problems. This review summarizes state-of-the-art biosensors developed for IOP monitoring by explaining their basic functions and applications, the main technology (pressure transductors, piezoresistive sensors, capacitive sensors, and resonant sensors), measurement approach (noninvasive, minimally invasive or invasive (surgically implantable)), and telemetry characteristics. To provide updated information for clinicians and researchers, we also describe the advantages and limitations of the application of these new sensors to eye care management. Despite significant improvements in IOP biosensor technology, the accuracy of their measurements must be improved to obtain a clear equivalence with actual IOP (measured in units of mmHg) to facilitate their clinical application. In addition, telemetry systems may be simplified to prevent adverse outcomes for patients and to guarantee the safety of stored data.
Keywords: Monitoring intraocular pressure, Glaucoma monitoring, Intraocular pressure sensor, Glaucoma sensors, Contact lens sensor
Resumen
El seguimiento continuo de la presión intraocular (PIO) para mejorar el diagnóstico y tratamiento del glaucoma ha sido un reto durante los últimos 60 años, ya que el glaucoma es la segunda causa principal de ceguera irreversible a nivel mundial. Diversos dispositivos con diferentes principios de medición, y los recientemente desarrollados biosensores con materiales semiconductores, ofrecen propiedades fascinantes. Sin embargo, ninguno de estos dispositivos para supervisar de manera continua la PIO se ha integrado plenamente en la práctica clínica, principalmente a causa de problemas técnicos. Esta revisión realiza un resumen de los biosensores de vanguardia desarrollados para supervisar la PIO, explicando sus funciones y aplicaciones básicas, su tecnología principal (transductores de presión, sensores piezoresistentes, sensores capacitivos, y sensores resonantes), enfoque de medición (no invasivo, mínimamente invasivo o invasivo (quirúrgicamente implantable)), y características telemétricas. Para proporcionar una información actualizada a los clínicos e investigadores, describimos también las ventajas y limitaciones de la aplicación de estos nuevos sensores a la terapéutica de la salud ocular. A pesar de las mejoras considerables en cuanto a la tecnología de los biosensores de PIO, la precisión de sus mediciones debe mejorarse para obtener una clara equivalencia con la PIO real (medida en unidades de mmHg) a fin de facilitar su aplicación clínica. Además, los sistemas telemétricos deben simplificarse para prevenir resultados adversos para los pacientes, y garantizar la seguridad de los datos almacenados.
Palabras clave: Seguimiento de la presión intraocular, Supervisión del glaucoma, Sensor de la presión intraocular, Sensores del glaucoma, Sensor de lentes de contacto
Introduction
Glaucoma is the second leading cause of irreversible blindness worldwide.1 The prevalence of glaucoma ranges from 1 to 4%, depending on the population studied and the diagnostic criteria used.1, 2 Moreover, glaucomatous optic neuropathy leads to certain characteristic changes in the optic nerve head and visual field loss. Glaucoma is also typically associated with increased intraocular pressure (IOP).3
One of the most important parameters in glaucoma diagnosis and treatment is IOP measurement because the primary treatment goal is to reduce IOP to prevent progressive optic nerve damage.4, 5, 6 Due to the circadian rhythm, IOP values fluctuate throughout the day, with maximum values at daybreak and minimum values at the end of the afternoon.7, 8 Determining the presence of increased IOP is difficult on the basis of clinical measurements performed in the consulting room because peak IOP occurs outside of office hours. Although daily IOP fluctuations of up to 5 mmHg have been reported in healthy subjects and may not be related to eye damage, glaucoma patients show higher IOP variations that are related to eye damage and disease progression.9 Additionally, IOP may be influenced by different factors, including ocular factors such as accommodation; the action of extraocular muscles (convergence) or blinking; corporal factors such as body position,10 Valsalva maneuvers, physical exercise and blood pressure11; and external factors such as tight neck ties or atmospheric pressure.12
Although many patients are controlled with treatment and the damage is stopped, other patients show glaucoma progression despite treatment or normal IOP values. In these cases, IOP monitoring would be useful to explain this complex disease with multiple etiologies.13, 14 Until a few years ago, the only method to measure IOP variations throughout the day was to obtain a 24-h tension curve in a hospital setting using standard tonometers (Goldman or Perkins).15 Almost 60 years ago, this method of IOP monitoring was investigated for its applicability to the diagnosis and treatment of glaucoma without success.16 Maurice16 developed an indentation tonometer with automated recording. The device was a metallic structure fixed to the head of the patient and was not pursued beyond the prototype. However, recently developed biosensors with new semiconductor materials offer exciting properties, opening the possibility to include these devices in clinical practice. Furthermore, different approaches using minimally or invasive biosensors, including contact lenses (CLS) that can indirectly measure IOP in patients,17, 18 implantable sensors that can monitor the actual IOP value,4, 19, 20, 21 and others,22, 23, 24 have been proposed for IOP monitoring. Some minimally invasive devices for use in IOP monitoring are based on the principle described by Lam et al.25; according to this principle, a 1 mmHg change in IOP causes a 3 μm change in the corneal curvature with a corneal radius of 7.80 mm. Similar outcomes were obtained by Hjortdal et al.26 in an in vitro study assessing ten enucleated human eyes with pressure ranging from 2 to 100 mmHg that was controlled by a column of isotonic saline. Implantable sensors are based on direct aqueous humor pressure with different measurement principles.
The rationale for obtaining IOP measurements over a 24-h cycle is that IOP exhibits time-dependent variation that can reach up to 5 mmHg over a 24-h period in healthy eyes; IOP is even more variable in eyes with glaucoma.15 For these reasons, the development of continuous IOP monitoring systems along with study designs that specifically address these issues will help clarify the nature of IOP variations and their role in glaucoma pathogenesis,12 including those in patients with normal tension glaucoma.12, 27, 28
The purpose of this paper is to review state-of-the-art sensors developed for IOP monitoring; explain their basic functions, possible applications, advantages and limitations; and provide an updated introduction to these new devices for eye care in glaucoma diagnosis and management for clinicians and researchers.
Pressure-sensing technology
Pressure sensors can be classified in terms of the technology used to complete IOP measurement, including pressure transductors, piezoresistive sensors, capacitive sensors, and resonant sensors. Pressure transducers can measure absolute or relative pressure but need a reference point measurement, which is usually obtained with an applanation tonometer (gold standard).10 Piezoresistive sensors are formed by strain gauges to detect strain due to applied pressure, and resistance increases as pressure deforms the material (organic polymers and semiconductors metal alloys).29 Capacitive sensors use a diaphragm and pressure cavity to create a variable capacitor to detect strain due to applied pressure, and capacitance decreases as pressure deforms the diaphragm.4 Resonant sensors use changes in resonant frequency in a sensing mechanism to measure stress or changes in gas density caused by applied pressure. Resonant sensors have been made with vibrating wire, vibrating cylinders, quartz, and silicon microelectromechanical system (MEMS).19 Generally, this technology provides very stable readings over time.
All these technologies offer enough sensitivity and biocompatibility for the purpose of IOP measurement, but the nanostructure disposition of new organic polymers (piezoresistive sensors) allow three-to-ten times greater sensitivity than that exhibited by conventional inorganic metals, such as semiconductor metal gauges.29 Another advantage of piezoresistive materials is that they permit the fabrication of strain-sensitive active elements that are flexible and transparent29; these characteristics have great potential in eye research because they can be included in a CLS.
The technology used for IOP monitoring could be classified by the following major methods: noninvasive (can be used in standard consulting room, ambulatory, etc.), minimally invasive (usually with CLS) or invasive including implantable or intraocular devices that require eye surgery. Despite these differences, the measuring principle in most of these new noninvasive or minimally invasive devices is similar because they attempt to detect small sensor deformations due to an IOP change that induces a cornea shape change and register these variations in physical units (such as volts or ohms). However, their clinical applications show substantial differences.
Noninvasive methods
Margalit et al.23 proposed a new method for IOP monitoring based on the illumination of the iris with a yttrium aluminum garnet (YAG) laser (532 nm wavelength) and subsequent analysis of the reflections with a specific camera, a complementary metal-oxide semiconductor (CMOS) camera. The camera must be slightly defocused to convert the tilting movement of the inspected surface into a transversal movement of speckle patterns. IOP fluctuations generate a constantly changing speckle pattern from the reflection of the illuminated object (iris or sclera) that the camera can record. This prototype must reduce these dimensions and simplify the measurement procedure for IOP self-monitoring by patients in a manner similar to some rebound tonometers (Icare Home®, Icare, Finland, Oy)13 but with the advantage of providing measurements independent of corneal thickness IOP measurements.
Ambulatory (consulting room) IOP measurements could be possible with repeated measurements using noninvasive tonometers (such as air-puff and rebounding). The automated recording indentation tonometer16 has evolved toward self-monitoring, and different tonometers, such as Icare® Home (Icare Finland Oy, Vantaa, Finland), Tono-Pen (Reichert Technologies, Depew, NY, USA) or Eye Pressure Monitor (Bausch and Lomb, Rochester, NY, USA), are utilized for their usability and portability. These devices do not require anesthetic and estimate IOP based on different rebound systems on the cornea.13
Minimally invasive methods
The first wireless IOP measurement was obtained in 1967 by Collins30 who studied a primitive version of a capacitive pressure sensor using a passive CLS transponder. This device used a gas bubble encapsulated in a rigidly suspended flexible film. Changes in IOP led to changes in the volume of the gas bubble, which were detected by the change in the resonance frequencies of two spiral coils with a resultant change in resonant frequency. In 1974, Greene et al.31 estimated the changes in the corneoscleral angle resulting from IOP variations, measuring IOP in a continuous manner using a soft silastic CLS embedded with strain gauge transducers.
In 1979, Cooper et al.27 designed a miniature guard ring using applanation sensors mounted in acrylic or Sauflon haptic elements individually designed for humans, rabbits or dogs. The holder of the human applanation sensor was modeled from a standard acrylic haptic ring without a central optic. The haptic ring was placed in the conjunctival fornix, and IOP was monitored with an automatic continual frequency monitor that induced electromagnetic oscillations in the applanation sensors. The resonant frequency of the applanation sensors should be directly proportional to IOP variations.
In 2009, the Sensimed company proposed a new CLS comprising two platinum-titanium resistive strain-sensing gauges.18 A microprocessor embedded in the CLS can record circumferential changes in the area of the limbus and wirelessly sends an output signal that is proportional to the changes in the strain gauges (in millivolts). Currently, this commercialized IOP monitoring sensor is the only class IIa device with European Conformity (CE-mark), and it is marketed as the Sensimed Triggerfish CLS. Later, Chen et al.32 developed a similar CLS using an inorganic metal sensor embedded in a CLS. Unfortunately, Chen's prototype is not currently commercialized.
The Sensimed Triggerfish device is being used in 36 registered clinical trials, but these reports provided their results with different approaches involving pressure measurement scales, millivolts or arbitrary units33 rather than mmHg units for IOP values. Some reports use zero as the first measured value, showing increases or decreases with time,18 indicating that some results show negative IOP values even with an IOP of 30 mmHg,18 which is biologically impossible. Because a constant equivalence between millivolts or ohms with IOP (mmHg) does not exist, the results obtained using the Sensimed device could be nonintuitive for daily use in clinical practices.
Furthermore, a CLS must be worn for an extended period of time to measure changes in IOP due to the circadian rhythm. This wear is associated with increased physiological overnight corneal swelling.34 A safety study on the extended wear of the Triggerfish device showed similar overnight corneal swelling, but corneal curvature irregularities increased slightly35 compared with those of standard hydrogel contact lens (CL) used for vision correction.36 This CLS is thicker than a standard CL, and the reason for this difference may be related to the material of this lens (silicone with high oxygen permeability). Due to their rigidity, the electronic circuit and other integrated elements of the Triggerfish lens could be a potential source of additional complications, and medium- and long-term follow-up trials may be necessary.10
Nevertheless, the corneal swelling induced during overnight CLS wear increases corneal thickness and could affect the biomechanical behavior of the corneal tissue. The effect of this change on the sensor measurement has not been clearly described. Since these sensors indirectly detect IOP variations using corneal shape changes,37 overnight corneal swelling could induce an erroneous clinical interpretation of IOP variation, and this issue must be resolved to clarify the impact of corneal swelling on corneal biomechanical properties and the clinical interpretation of IOP measurements taken by CLS.38
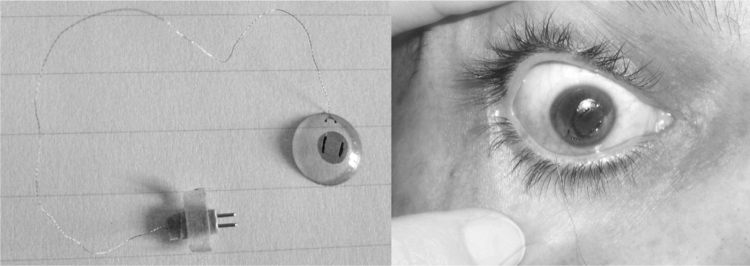
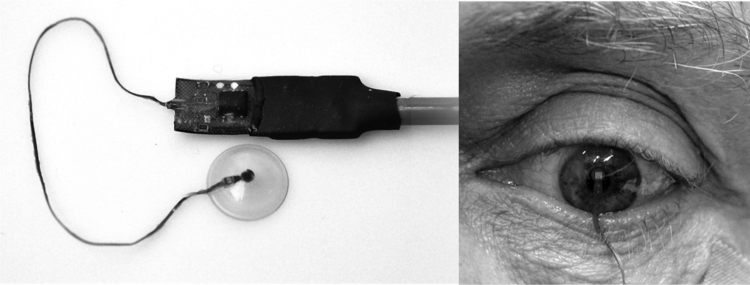
In 2011, Sanchez et al.37 conducted an in vivo proof-of-concept study of a gas-permeable doughnut-shaped CLS with a membrane based on a flexible, piezoresistive nanocomposite organic sensor (Fig. 1). This sensor has a bilayer film structure resulting from the softness of the nanocrystals of the conducting salt, which are embedded on one side of the polymeric matrix; the deformities of these nanocrystals under slight strain change their conducting properties.29 The piezoresistive sensor is glued over the CL hole to allow contact with the anterior corneal surface. This prototype registers changes in IOP using changes in electrical resistance (ohms). A similar prototype developed by Ziemer Ophthalmic Systems (Fig. 2) uses a dynamic contour tonometry (DCT) lens with a piezoresistive sensor to measure IOP and ocular pulse amplitude with a Pascal tonometer.13, 39 Although the existence of an association between ocular pulse amplitude and glaucoma remains controversial, the clinical relevance of ocular pulse amplitude has been consistently suggested.40
Figure 1.
Gas-permeable doughnut-shaped CL (left) with a piezoresistive nanocomposite developed by Sanchez et al. Proof-of-concept prototype of a CL in a volunteer's eye (right).
Courtesy of Dr. Sanchez.
Figure 2.
DCT lens (left) with piezoresistive nanocomposite manufactured by Ziemer Ophthalmic Systems Company. Proof-of-concept prototype of the CL in a volunteer's eye (right).
Courtesy of Dr. Kanngiesser.
Implantable or intraocular methods
Several implantable or intraocular methods for IOP measurement have been proposed in different studies with animal models (including cats, rabbits or macaques). Unfortunately, these devices may be difficult to use in human research because they require sophisticated surgeries or induce nonethical side effects. For example, in 1980, Wolbarsht et al.41 developed a sensor comprising a pressure transducer in an elastic band capable of measuring IOP. This band was implanted around the eyeball in cats between the upper straight muscle and sclera. In 2013, Chitnis et al.19 proposed a device that requires direct access to the vitreous space; a hypodermic needle (30 G) penetrates the sclera through the pars plana, providing direct access to the vitreous space in an animal model study (including four rabbits). The micromachined capacitive pressure sensor connects to the back end of the needle, and a flexible polyimide coil connects to the capacitor to form a circuit; the resonance frequency is a function of IOP. Because only one device shows a linear correlation between resonant frequency (MHz) and IOP and other sensors were likely damaged in surgery or while explanting the eye, this approach is not acceptable for humans. Finally, Downs et al.6, 10 used strain gauges on the back of the transducer membrane to register IOP variations thorough membrane deformations (with a function similar to that of the Sensimed sensor). The sensor is a 4 mm diameter titanium membrane hermetically laser-welded into a titanium housing and fixed with stainless steel bone screws at the orbit of three rhesus macaques that measured the IOP values of the anterior chamber for seven months. The combined data from the three animals revealed a weak trend of nocturnal elevation in IOP distributions, although one implant failed 5 weeks after implantation for unknown reasons. Although animal model studies could provide excellent support to clarify the relationship between glaucoma and the nycthemeral rhythm of IOP or to study new drug treatments to manage IOP, these results suggest limited use in humans.
Implantable devices that require surgery similar to cataract removal,21, 42, 43 could be acceptable to patients and surgeons. The first step toward the development of an implantable biosensor in anterior chamber was proposed by Svedbergh et al.44 who designed an intraocular lens (IOL) with a pressure sensor in the lens haptics. The sensor had a capacitive spiral circuit that needs no energy supply; the resonance frequency of this circuit is correlated with IOP. Walter et al., 45 later tested a completely encapsulated sensor with a telemetric signal and energy transfer integrated into a silicone disk in an animal (rabbit) model study.
In 2012, Ha et al.4 developed a capacitive pressure sensor incorporating a polymer-based MEMS for implantation in the anterior chamber of a mouse eye. This sensor has a high sensitivity to pressure changes and low temperature sensitivity, as is common in these types of sensors. This sensor shows an IOP sensing resolution of less than 1 mmHg when the sensor is combined with an application-specific integrated circuit (ASIC).
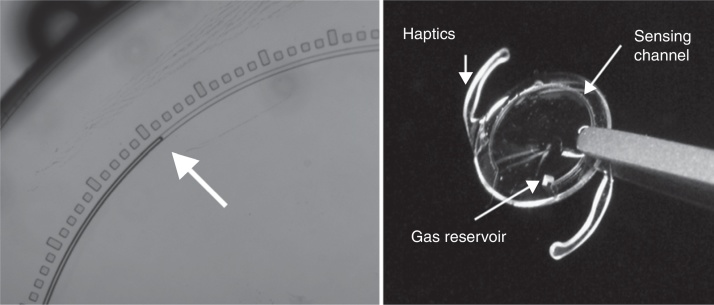
Melki et al.21 reported the first safety study of an implantable sensor (WIT®) that could monitor IOP in a single volunteer with 18 months of follow-up. The WIT® sensor has three major parts, an ASIC chip, a circular microcoil antenna and eight pressure sensitive capacitors (Fig. 3). These components are encapsulated in a silicone ring placed in the ciliary sulcus through a 5.5 mm corneal incision during programmed intracapsular cataract surgery with IOL implantation. IOP is monitored by an array of capacitive pressure sensors composed of two parallel plates, including a thin flexible membrane that is indented by IOP and a thicker, rigid base. When the membrane is mechanically deflected by IOP changes, it causes a change in the distance between the plates.46 Recently, in the ARGOS studies (clinical trial numbers NCT02945176 and NCT02434692), Koutsonas et al.42 presented one-year follow-up data from six patients implanted with the WIT® sensor and suggested that this sensor is a potential method for continuous IOP monitoring in glaucoma patients. Nevertheless, in the case of failure of the implantable sensor,6, 19 the replacement could be problematic because it involves a new eye surgery. For example, in the ARGOS study,42 one of six devices failed.
Figure 3.
A WIT device (left) compared to a standard soft contact lens (right).
Courtesy of Implandata Ophthalmic Products GmbH.
Using a similar approach, Donida et al.3, 43 developed a rollable, implantable IOP sensor with an appearance similar to that of the WIT® device. The system operates at 13.56 MHz and is powered by inductive coupling through a magnetic field generated by an external reader. The device comprises a MEMS-based piezoresistive pressure sensor, an ASIC that reads out the sensor data every 15 min and an external reader installed on customized glasses that can register the cardiac ocular pulse wave (the third harmonic of the cardiac rate). These data seem to provide information about the health of the retinal vasculature, which is related to glaucoma because decreased ocular pulse amplitude has been reported in patients with normal tension glaucoma.47
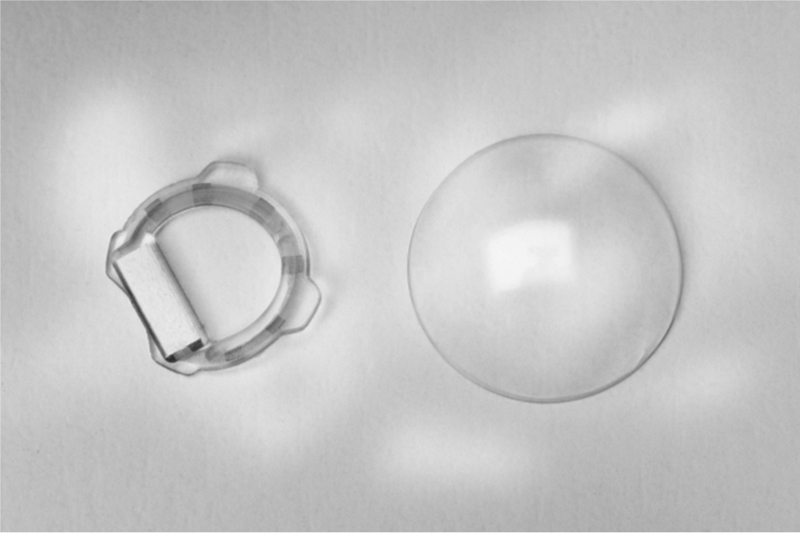
In 2014, Araci et al.22 published a method to obtain actual IOP measurements. However, this option does not permit IOP monitoring. The system uses a passive pressure sensor in an IOL based on the principles of microfluidic physics. The IOP sensor is composed of an airtight microfluidic channel that is open to the aqueous intraocular liquid at one end and connected to a gas reservoir at the end of the channel. Capillary forces and IOP drive the liquid into the microchannel, compressing the gas inside the reservoir until the gas pressure reaches equilibrium with the liquid pressure. Fig. 4 shows that the pressure readout is performed by a camera equipped with one optical adaptor and image analysis software for the detection of the aqueous-air interface position in micrometers.
Figure 4.
IOL with a microfluidic channel that is open to the aqueous intraocular liquid at one end and connected to a gas reservoir at the end of the channel. This channel has different marks to conduct the measurement.
Courtesy of Dr. Araci.
The main issue with all implantable sensors is related to the invasive procedure that is required to place the sensor into the eye. A large incision is necessary because these devices cannot be bent like current IOLs.21, 42 Thus, recommending this type of surgery with the single objective of measuring IOP is difficult, and the procedure must be scheduled to coincide with cataract surgery. Thus, a large number of patients with glaucoma cannot be monitored because the disease is usually diagnosed at 40 or 50 years old. Moreover, a large incision could undermine the advantages of small incisions in terms of visual outcome after cataract surgery when bent IOLs are implanted (especially by the amount of induced astigmatism). The risk of infection, fibrosis and other complications of IOP sensor surgery have been previously described19 and are different from those of the standard cataract surgery procedure.
However, implantable devices have some advantages. These devices obtain actual IOP measurements, and the measurement is taken inside the eye, independent of corneal thickness. Unfortunately, these devices require applanation tonometry to calibrate the sensor. Nevertheless, not all implantable devices allow IOP monitoring. The sensor developed by Araci (Fig. 4) is a passive pressure sensor included in an IOL,22 which allows practitioners to identify IOP changes. In addition, this device does not need an ASIC and telemetry system; thus, it could be a useful method for management of patients with normal tension glaucoma after cataract surgery because it provides an IOP measurement that is independent of cornea deformation.
Technology limitations
The main limitations of the technology used to develop biosensors to measure or monitor IOP could be summarized in three major points. The first point is the limitations of the sensors and ASIC, which are related to the impact of other variables in sensor measurement. The second point is the limitations of the telemetry system, which must be able to register sensor measurements. The final point is related to the clinical use of these new sensors, especially side effects induced by the surgery required to place the sensor into the eye (invasive devices) and the indirect measurement of IOP conducted by noninvasive and minimally invasive devices expressed in different units that are not easily converted into IOP pressure units (mmHg).
Sensor limitations and ASIC
Most new sensors are commonly affected by thermal variations that can alter the measured value.3, 10, 37, 46 Thus, in vitro drift studies,6, 24 such as the study by Todanis et al.,46 were performed to address these shortcomings. WIT® devices showed an average drift of 3.47 mmHg after one year of testing by immersion in saline at a temperature of 36 °C and an absolute test pressure of 1000 hPa. This result indicated a drift rate of 2.46 mmHg per year with constant temperature and pressure. In Downs et al.,10 the signal noise of the pressure transducer was less than 0.4 mmHg, and the signal drift was less than 3 mmHg monotonic. If the sensor characteristics and feasibility can be established, it may be possible to successfully develop an ASIC with the correct signal registration as in these cases.6, 37
Ocular movements, the Valsalva maneuver, blinking and eye rubbing cause IOP variations. These variations cause sudden and significant IOP changes unrelated to the circadian rhythm but can be subtracted with an ASIC.
Telemetry limitations
Some systems3, 20, 46 permit the external transmission of measured values by radiofrequency such that the data can be stored for later analysis. The implant and external reader device are inductively coupled via a low-power electromagnetic link with a high-frequency field at 13.56 MHz.20 Whether this frequency is advisable has been questioned, although a report by Hirtl and Schmid48 concluded that this high frequency (13.56 MHz) does not cause any thermally induced adverse health effects. However, nonself-powered devices have problems related to the lack of a replaceable battery or an inductive powering/charging system.10
Clinical limitations
The measurement and ambulatory monitoring of IOP are challenges in the diagnosis and management of glaucoma. Thus, the use of different technologies have been proposed in the development of new methods that allow IOP monitoring through two main approaches. In one approach, devices are used in the ambulatory clinic (noninvasive or minimally invasive); in the second approach, implantable (intraocular) devices provide IOP values independent of corneal thickness. The nanotechnology required to develop highly sensitive IOP sensors was unavailable until a few years ago. These sensors have made the measurement of IOP variations possible through different designs, approaches, materials and telemetry systems. However, more information is needed regarding clinical studies, biocompatibility reports and clinical trials of these devices.
The major problem with minimally invasive sensors in terms of measurement and IOP monitoring is related to the measurement technique because all these sensors measure physical magnitude variations in different units (ohms, volts, and others),33, 37 and no standard pressure units (mmHg) are provided. In most noninvasive or minimally invasive devices, the measurement is obtained by the change in the corneal radius induced by IOP fluctuations (around 1 mmHg change 3 μm corneal curvature)25 but this change could be affected by different anatomical (corneal thickness, corneal curvature, corneal diameter, corneal biomechanical properties, scleral resistance, etc.) and biological (corneal swelling related with sensor wear time, etc.) factors that must be studied in detail with future research. In implantable devices, the measurement is collected by detecting a change in IOP. Therefore, biosensors provide an indirect method to record IOP changes, and transforming sensor outcomes to the standard pressure unit of IOP (mmHg) is necessary. Unfortunately, no clear, accepted equivalence in mmHg has been proposed in any current sensors. The influence of some factors such as temperature variation, saline solution contact, and signal processing must be clarified to find the equivalence between sensor outcomes and mmHg.6, 10, 19 Moreover, this lack of consistency in the units provided by each sensor and their transformation to mmHg makes systematic comparisons between studies difficult (or impossible) because each report presents data with different outcomes.
Attempts to continuously monitor IOP have been made, but none of the devices for built for this purpose have been fully integrated into clinical practice, primarily due to technical problems, lack of long-term stability, signal drift, telemetry frequency and other issues.6, 37, 43 Table 1 summarizes the main details and limitations of the current sensors proposed for measuring IOP.
Table 1.
Comparison of general characteristics of devices used to attempt IOP monitoring.
| Device | Direct/indirect IOP measurement | Corneal thickness dependent | Ambulatory IOP monitoring | Tested in humans/animals | Continuous measurements | Wireless data registration | Principal limitation |
|---|---|---|---|---|---|---|---|
| Noninvasive sensors | |||||||
| Margalit23 | Direct | No | No | Humans | Yes | Yes | A large system mounted in an optical bench. |
| Minimally invasive sensors | |||||||
| Maurice16 | Indirect | Yes | No | Humans | Yes | No | The tonometer needs to be fixed to the head. |
| Greene31 | Indirect | Yes | Yes | Rabbits | No | No | Rudimentary sensor at present. Could not be used during normal activities of animals. |
| Cooper27 | Indirect | No | Yes | Humans | Yes | No | Could not be used during normal life, difficult to close the eye. |
| Triggerfish33 | Indirect | Yes | Yes | Humans | Yes | Yes | Does not have clear equivalence between volts and mmHg pressure units. |
| Chen32 | Indirect | Yes | Yes | Synthetic eye model | Yes | Yes | Not tested in humans. |
| Sanchez37 | Indirect | Yes | Yes | Humans | Yes | Yes | Does not have clear equivalence between volts and mmHg pressure units. Mounted in a rigid gas-permeable contact lens. |
| DCT lens39 | Indirect | No | Yes | Humans | Yes | Yes | Mounted in a rigid gas-permeable contact lens. |
| Implantable or intraocular methods | |||||||
| Wolbarsht41 | Direct | No | No | Cats | Yes | Yes | Very invasive surgery. |
| Downs6 | Direct | No | No | Nonhuman primates | Yes | Yes | Anterior chamber permanently cannulated. |
| Chitnis19 | Direct | No | No | Rabbits | Yes | Yes | Wide antenna system permanently connected to the vitreous chamber. |
| Collins30 | Direct | No | No | Theoretical | Yes | Yes | Theoretical concept of wireless system with a coupled magnetic field. |
| Svedbergh44 | Direct | No | No | Animal model | Yes | Yes | Rudimentary sensor at present. |
| Walter45 | Direct | No | No | Rabbits/pigs | Yes | Yes | Lack of long-term stability and signal drift. |
| Ha4 | Direct | No | No | Mouse | Yes | Yes | Lack of long-term stability and signal drift. |
| Araci22 | Direct | No | No | Humans | No | No | Not able to monitor IOP. Pupil dilatation is required to achieve the measurement. |
| WIT21 | Direct | No | No | Humans | Yes | Yes | Not a rollable device. A large incision is necessary. |
| Donida43 | Direct | No | No | In vitro model | Yes | Yes | In vitro model with high precision manometer of sensor, wireless and ASIC system. |
IOP: intraocular pressure.
Future perspectives
In recent years, significant technological advances have permitted the development of new sensors, telemetry systems, etc., with very interesting and promising results for measuring and monitoring IOP. Some of these sensors allow noninvasive indirect IOP measurement that should be improved to generalize their clinical use. However, new materials with biomedical applications, such as liquid crystal polymers, will be proposed in the future due to their biocompatibility, chemical inertness, flexibility, electromechanical properties and ease of micromachining,4 which should improve currently available sensors.
These new materials could be applied to developing new, minimally invasive sensors that are capable of measuring and monitoring IOP, opening up the possibility of developing new drug delivery systems that could improve glaucoma management and prevent patient noncompliance, which is often seen with standard pharmacological treatments.49, 50
IOP monitoring devices could be of great interest in glaucoma management, but other diseases or procedures could also benefit from these devices. For example, eye hypotony is possible after eye surgery (e.g., glaucoma filtration or retinal detachment surgery or vitrectomy). In these cases, early detection and management is of paramount importance to preserving eye function, and future sensors could help improve postsurgery patient management. Specifically, progress in glaucoma filtration surgery has led to the development of antiscarring agents50 and active microvalves51 for the prevention of ocular hypotony. These agents50 help avoid complications, such as blebbing, fibrosis, leaks and endophthalmitis, which may still occur during the healing process following glaucoma filtration surgery.
In summary, although the perfect device does not yet exist, the ideal sensor would be noninvasive and biocompatible and would provide a direct and stable IOP measurement. Moreover, this sensor would be self-powered, able to eliminate sudden fluctuations in IOP and drift in the signal registration, and capable of transferring IOP data at a safe frequency to a storage device or computer.
Conclusion
For diagnosis and management, patients with glaucoma require precise and accurate IOP measurements and monitoring, which could be improved by using new sensors that permit noninvasive measurements of actual IOP variations.
The accuracy of IOP measurements made by noninvasive sensors must be improved to develop a clear equivalence between indirect measures of actual IOP values and variations in mmHg to facilitate the clinical application of these new sensors. Telemetry systems may be simplified to avoid patient problems and to guarantee data storage that facilitates later clinical assessments, especially in the case of implantable sensors.
The combination of noninvasive sensors and a drug delivery system could be of great interest for improving treatment compliance in glaucoma patients, reducing glaucoma-related vision impairment, and managing posteye surgery patients to reduce complications from hypotony.
In summary, more research involving clinical trials is necessary to present evidence-based results that permit the use of these new technologies in the management of patients with glaucoma.
Literature search method
We performed an extensive electronic search of the Medline and PubMed databases using individual and combined key words (monitoring intraocular pressure, glaucoma monitoring, and intraocular pressure sensor) in March 2018 to identify the relevant publications in this field in the English language. We also included additional references from different sources that were cited in these articles. In total, we identified and retrieved 1350 publications. We refined the search results until 90 papers were selected. Publications involving tonometry-based IOP monitoring and self-tonometry reports about standard tonometers (Goldman or Perkins) or noncontact tonometers (air-puff, palpebral tonometers, etc.) and studies on the impact of corneal thickness on IOP measurements were excluded because these reports are not about new technologies for measuring IOP. We included references focused on prototypes and proof-of-concept IOP sensors that described their development, application or assessment. In vitro and in vivo studies as well as newly proposed human trials were included.
Among the 49 publications identified, those that described the design of methods for IOP monitoring or their applications were selected. A search was conducted in the literature cited by these articles, and additional papers were identified.
Conflict of interest
The authors have no conflicts of interest to declare.
Acknowledgments
The authors thank Implandata Ophthalmic Products GmbH (Kokenstrasse 5 30159 Hannover/Germany), Dr. Kanngiesser (Ziemer Ophthalmic Systems AG) and Dr. Araci for the illustrations included in this study.
References
- 1.Quigley H.A., Broman A.T. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90:262–267. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sung V.C., Koppens J.M., Vernon S.A. Longitudinal glaucoma screening for siblings of patients with primary open angle glaucoma: the Nottingham Family Glaucoma Screening Study. Br J Ophthalmol. 2006;90:59–63. doi: 10.1136/bjo.2005.072751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Piffaretti F., Barrettino D., Orsatti P. Rollable and implantable intraocular pressure sensor for the continuous adaptive management of glaucoma. Conf Proc IEEE Eng Med Biol Soc. 2013;2013:3198–3201. doi: 10.1109/EMBC.2013.6610221. [DOI] [PubMed] [Google Scholar]
- 4.Ha D., de Vries W.N., John S.W. Polymer-based miniature flexible capacitive pressure sensor for intraocular pressure (IOP) monitoring inside a mouse eye. Biomed Microdevices. 2012;14:207–215. doi: 10.1007/s10544-011-9598-3. [DOI] [PubMed] [Google Scholar]
- 5.Durairaj C. Optimal sampling scheme for estimation of intraocular pressure diurnal curves in glaucoma trials. Clin Pharmacokinet. 2015;54:95–105. doi: 10.1007/s40262-014-0183-9. [DOI] [PubMed] [Google Scholar]
- 6.Downs J.C., Burgoyne C.F., Seigfreid W.P. 24-Hour IOP telemetry in the nonhuman primate: implant system performance and initial characterization of IOP at multiple timescales. Invest Ophthalmol Vis Sci. 2011;52:7365–7375. doi: 10.1167/iovs.11-7955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Konstas A.G., Mylopoulos N., Karabatsas C.H. Diurnal intraocular pressure reduction with latanoprost 0.005% compared to timolol maleate 0.5% as monotherapy in subjects with exfoliation glaucoma. Eye (Lond) 2004;18:893–899. doi: 10.1038/sj.eye.6701345. [DOI] [PubMed] [Google Scholar]
- 8.Liu J.H., Zhang X., Kripke D.F. Twenty-four-hour intraocular pressure pattern associated with early glaucomatous changes. Invest Ophthalmol Vis Sci. 2003;44:1586–1590. doi: 10.1167/iovs.02-0666. [DOI] [PubMed] [Google Scholar]
- 9.De Smedt S. Noninvasive intraocular pressure monitoring: current insights. Clin Ophthalmol. 2015;9:1385–1392. doi: 10.2147/OPTH.S53772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Downs J.C. IOP telemetry in the nonhuman primate. Exp Eye Res. 2015;141:91–98. doi: 10.1016/j.exer.2015.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McMonnies C.W. Intraocular pressure and glaucoma: is physical exercise beneficial or a risk. J Optom. 2016;9:139–147. doi: 10.1016/j.optom.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sit A.J. Intraocular pressure variations: causes and clinical significance. Can J Ophthalmol. 2014;49:484–488. doi: 10.1016/j.jcjo.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 13.Ittoop S.M., SooHoo J.R., Seibold L.K. Systematic review of current devices for 24-h intraocular pressure monitoring. Adv Ther. 2016;33:1679–1690. doi: 10.1007/s12325-016-0388-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McMonnies C.W. The importance of and potential for continuous monitoring of intraocular pressure. Clin Exp Optom. 2017;100:203–207. doi: 10.1111/cxo.12497. [DOI] [PubMed] [Google Scholar]
- 15.Hughes E., Spry P., Diamond J. 24-Hour monitoring of intraocular pressure in glaucoma management: a retrospective review. J Glaucoma. 2003;12:232–236. doi: 10.1097/00061198-200306000-00009. [DOI] [PubMed] [Google Scholar]
- 16.Maurice D.M. A recording tonometer. Br J Ophthalmol. 1958;42:321–335. doi: 10.1136/bjo.42.6.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Agnifili L., Mastropasqua R., Frezzotti P. Circadian intraocular pressure patterns in healthy subjects, primary open angle and normal tension glaucoma patients with a contact lens sensor. Acta Ophthalmol. 2015;93:e14–e21. doi: 10.1111/aos.12408. [DOI] [PubMed] [Google Scholar]
- 18.Mansouri K., Medeiros F.A., Weinreb R.N. Intraocular pressure changes during sexual activity. Acta Ophthalmol. 2013;91:e324–e325. doi: 10.1111/aos.12023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chitnis G., Maleki T., Samuels B. A minimally invasive implantable wireless pressure sensor for continuous IOP monitoring. IEEE Trans Biomed Eng. 2013;60:250–256. doi: 10.1109/TBME.2012.2205248. [DOI] [PubMed] [Google Scholar]
- 20.Dresher R.P., Irazoqui P.P. A compact nanopower low output impedance CMOS operational amplifier for wireless intraocular pressure recordings. Conf Proc IEEE Eng Med Biol Soc. 2007;2007:6056–6059. doi: 10.1109/IEMBS.2007.4353729. [DOI] [PubMed] [Google Scholar]
- 21.Melki S., Todani A., Cherfan G. An implantable intraocular pressure transducer: initial safety outcomes. JAMA Ophthalmol. 2014;132:1221–1225. doi: 10.1001/jamaophthalmol.2014.1739. [DOI] [PubMed] [Google Scholar]
- 22.Araci I.E., Su B., Quake S.R. An implantable microfluidic device for self-monitoring of intraocular pressure. Nat Med. 2014;20:1074–1078. doi: 10.1038/nm.3621. [DOI] [PubMed] [Google Scholar]
- 23.Margalit I., Beiderman Y., Skaat A. New method for remote and repeatable monitoring of intraocular pressure variations. J Biomed Opt. 2014;19:027002. doi: 10.1117/1.JBO.19.2.027002. [DOI] [PubMed] [Google Scholar]
- 24.Xue N., Chang S.P., Lee J.B. A SU-8-based compact implantable wireless pressure sensor for intraocular pressure sensing application. Conf Proc IEEE Eng Med Biol Soc. 2011;2011:2854–2857. doi: 10.1109/IEMBS.2011.6090788. [DOI] [PubMed] [Google Scholar]
- 25.Lam A.K., Douthwaite W.A. The effect of an artificially elevated intraocular pressure on the central corneal curvature. Ophthalmic Physiol Opt. 1997;17:18–24. [PubMed] [Google Scholar]
- 26.Hjortdal J.O., Jensen P.K. In vitro measurement of corneal strain, thickness, and curvature using digital image processing. Acta Ophthalmol Scand. 1995;73:5–11. doi: 10.1111/j.1600-0420.1995.tb00004.x. [DOI] [PubMed] [Google Scholar]
- 27.Cooper R.L., Beale D.G., Constable I.J. Continual monitoring of intraocular pressure: effect of central venous pressure, respiration, and eye movements on continual recordings of intraocular pressure in the rabbit, dog, and man. Br J Ophthalmol. 1979;63:799–804. doi: 10.1136/bjo.63.12.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hasegawa K., Ishida K., Sawada A. Diurnal variation of intraocular pressure in suspected normal-tension glaucoma. Jpn J Ophthalmol. 2006;50:449–454. doi: 10.1007/s10384-006-0343-z. [DOI] [PubMed] [Google Scholar]
- 29.Vladimir L., Irene S., Ana M. Non-invasive intraocular pressure monitoring with a contact lens engineered with a nanostructured polymeric sensing film. Sens Actuators A. 2011;170:36–43. [Google Scholar]
- 30.Collins C.C. Miniature passive pressure transensor for implanting in the eye. IEEE Trans Biomed Eng. 1967;14:74–83. doi: 10.1109/tbme.1967.4502474. [DOI] [PubMed] [Google Scholar]
- 31.Greene M.E., Gilman B.G. Intraocular pressure measurement with instrumented contact lenses. Invest Ophthalmol. 1974;13:299–302. [PubMed] [Google Scholar]
- 32.Chen G.Z., Chan I.S., Leung L.K. Soft wearable contact lens sensor for continuous intraocular pressure monitoring. Med Eng Phys. 2014;36:1134–1139. doi: 10.1016/j.medengphy.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 33.Mansouri K., Liu J.H., Weinreb R.N. Analysis of continuous 24-hour intraocular pressure patterns in glaucoma. Invest Ophthalmol Vis Sci. 2012;53:8050–8056. doi: 10.1167/iovs.12-10569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martin R., de Juan V., Rodríguez G. Measurement of corneal swelling variations without removal of the contact lens during extended wear. Invest Ophthalmol Vis Sci. 2007;48:3043–3050. doi: 10.1167/iovs.06-1372. [DOI] [PubMed] [Google Scholar]
- 35.Hubanova R., Aptel F., Chiquet C. Effect of overnight wear of the Triggerfish® sensor on corneal thickness measured by Visante® anterior segment optical coherence tomography. Acta Ophthalmol. 2014;92:e119–e123. doi: 10.1111/aos.12241. [DOI] [PubMed] [Google Scholar]
- 36.Mansouri K., Medeiros F.A., Tafreshi A. Continuous 24-hour monitoring of intraocular pressure patterns with a contact lens sensor: safety, tolerability, and reproducibility in patients with glaucoma. Arch Ophthalmol. 2012;130:1534–1539. doi: 10.1001/jamaophthalmol.2013.1350. [DOI] [PubMed] [Google Scholar]
- 37.Sánchez I., Laukhin V., Moya A. Prototype of a nanostructured sensing contact lens for noninvasive intraocular pressure monitoring. Invest Ophthalmol Vis Sci. 2011;52:8310–8315. doi: 10.1167/iovs.10-7064. [DOI] [PubMed] [Google Scholar]
- 38.Lau W., Pye D.C. Associations between diurnal changes in Goldmann tonometry, corneal geometry, and ocular response analyzer parameters. Cornea. 2012;31:639–644. doi: 10.1097/ICO.0b013e31822481ac. [DOI] [PubMed] [Google Scholar]
- 39.Ziemer ophthalmology. Available at https://www.ziemertonometry.com/dct-lens.html; Accessed 20.10.17.
- 40.Willekens K., Rocha R., Van Keer K. Review on dynamic contour tonometry and ocular pulse amplitude. Ophthalmic Res. 2015;55:91–98. doi: 10.1159/000441796. [DOI] [PubMed] [Google Scholar]
- 41.Wolbarsht M.L., Wortman J., Schwartz B. A scleral buckle pressure gauge for continuous monitoring of intraocular pressure. Int Ophthalmol. 1980;3:11–17. doi: 10.1007/BF00136208. [DOI] [PubMed] [Google Scholar]
- 42.Koutsonas A., Walter P., Roessler G. Implantation of a novel telemetric intraocular pressure sensor in patients with glaucoma (ARGOS study): 1-year results. Invest Ophthalmol Vis Sci. 2015;56:1063–1069. doi: 10.1167/iovs.14-14925. [DOI] [PubMed] [Google Scholar]
- 43.Donida A., Di Dato G., Cunzolo P. A circadian and cardiac intraocular pressure sensor for smart implantable lens. IEEE Trans Biomed Circuits Syst. 2015;9:777–789. doi: 10.1109/TBCAS.2015.2501320. [DOI] [PubMed] [Google Scholar]
- 44.Svedbergh B., Bäcklund Y., Hök B. The IOP-IOL. A probe into the eye. Acta Ophthalmol (Copenh) 1992;70:266–268. doi: 10.1111/j.1755-3768.1992.tb04135.x. [DOI] [PubMed] [Google Scholar]
- 45.Walter P., Schnakenberg U., vom Bögel G. Development of a completely encapsulated intraocular pressure sensor. Ophthalmic Res. 2000;32:278–284. doi: 10.1159/000055626. [DOI] [PubMed] [Google Scholar]
- 46.Todani A., Behlau I., Fava M.A. Intraocular pressure measurement by radio wave telemetry. Invest Ophthalmol Vis Sci. 2011;52:9573–9580. doi: 10.1167/iovs.11-7878. [DOI] [PubMed] [Google Scholar]
- 47.Schwenn O., Troost R., Vogel A. Ocular pulse amplitude in patients with open angle glaucoma, normal tension glaucoma, and ocular hypertension. Br J Ophthalmol. 2002;86:981–984. doi: 10.1136/bjo.86.9.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hirtl R., Schmid G. Numerical analysis of specific absorption rate in the human head due to a 13.56 MHz RFID-based intra-ocular pressure measurement system. Phys Med Biol. 2013;58:N267–N277. doi: 10.1088/0031-9155/58/18/N267. [DOI] [PubMed] [Google Scholar]
- 49.Slota C., Sayner R., Vitko M. Glaucoma patient expression of medication problems and nonadherence. Optom Vis Sci. 2015;92:537–543. doi: 10.1097/OPX.0000000000000574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim N.J., Harris A., Gerber A. Nanotechnology and glaucoma: a review of the potential implications of glaucoma nanomedicine. Br J Ophthalmol. 2014;98:427–431. doi: 10.1136/bjophthalmol-2013-304028. [DOI] [PubMed] [Google Scholar]
- 51.Sassetti F., Guarnieri F.A., Garelli L. Characterisation and simulation of an active microvalve for glaucoma. Comput Methods Biomech Biomed Engin. 2012;15:1273–1280. doi: 10.1080/10255842.2011.585978. [DOI] [PubMed] [Google Scholar]