Highlights
-
•
Age-associated CVDs impose a great burden on current health systems. Despite the fact that current strong evidence supports the links among aging, telomere attrition, and CVDs, there is no clear direction for the development of telomere therapeutics against CVDs.
-
•
This review focuses on immune modulation, CHIP, pharmaceutical interventions, and gene therapy for their therapeutic roles in age-associated CVDs.
-
•
The future goal of telomere cardiovascular therapy in young subjects is to prevent senescence and diseases, whereas in older adult subjects, the goal is restoration of cardiovascular functions. Further studies on the telomere-CHIP-atherosclerosis axis may shed insights on how to achieve these 2 different therapeutic targets.
Key Words: aging, atherosclerosis, cardiomyocytes, immune modulation, telomeres
Abbreviations and Acronyms: AAV, adeno-associated virus; CVD, cardiovascular diseases; CHIP, clonal hematopoiesis of indeterminate potential; LTL, leukocyte telomere length; TCA, telomere-CHIP-atherosclerosis; TERC, telomerase RNA component; TERT, telomerase reverse transcriptase; TRF2, telomere repeat-binding factor 2
Summary
Telomeres are double-stranded repeats of G-rich tandem DNA sequences that gradually shorten with each cell division. Aging, inflammation, and oxidative stress accelerate the process of telomere shortening. Telomerase counteracts this process by maintaining and elongating the telomere length. Patients with atherosclerotic diseases and cardiovascular risk factors (e.g., smoking, obesity, sedentary lifestyle, and hypertension) have shorter leukocyte telomere length. Following myocardial infarction, telomerase expression and activity in cardiomyocytes and endothelial cells increase significantly, implying that telomerase plays a role in regulating tissue repairs in heart diseases. Although previous studies have focused on the changes of telomeres in heart diseases and the telomere length as a marker for aging cardiovascular systems, recent studies have explored the potential of telomeres and telomerase in the treatment of cardiovascular diseases. This review discusses the significant advancements of telomere therapeutics in gene therapy, atherosclerosis, anti-inflammation, and immune modulation in patients with cardiovascular diseases.
Central Illustration
Telomeres are repetitive hexanucleotides (TTAGGG)n found at the end of linear chromosomes; they prevent chromosome ends from being recognized as DNA strand breaks and becoming inappropriately degraded by DNA damage responses 1, 2. In humans, telomeres are 10 to 15 kb of tandem DNA repeats. There are significant variations of telomere length among individuals, but telomeres invariably shorten with age and cell division (3).
Although there could be asynchrony of telomere length among different tissues (4), peripheral leukocyte DNA has been most commonly used in clinical studies to measure leukocyte telomere length (LTL) (5). Several methods have been used to measure LTL, including terminal restriction fragment analysis by hybridization with telomere sequence probes, single telomere amplification and blotting, flow cytometry of cells following hybridization with fluorescent peptide nucleic acid probes, quantitative fluorescence in situ hybridization with fluorescent telomere peptide nucleic acid probes, and quantitative polymerase chain reaction assays (6). Due to the different methods used in clinical trials and large interbatch coefficients of variations, there is no current gold standard of telomere length measurement, and therefore, comparison of telomere lengths between different clinical trials could be misleading (7).
Traditional risk factors for cardiovascular diseases (CVD), such as smoking, diabetes mellitus, dyslipidemia, hypertension, obesity, and shift work, have been associated with short LTL (8). In the prospective WOSCOPS (West of Scotland Primary Prevention Study) trial, subjects in the lowest tertile of LTL had a 44% increased risk of 5-year major cardiovascular events compared with subjects in the highest tertile of LTL (9). Another meta-analysis study with 43,725 participants and 8,400 patients revealed that short LTL had a pooled relative risk for coronary heart disease of 1.54 (95% confidence interval: 1.30 to 1.83). In addition, short LTL was associated with coronary artery disease risk independent of traditional vascular risk factors. The association of short LTL with cerebrovascular disease is less significant than that with coronary artery disease (10). Short LTL also affects the prognosis of coronary artery disease. In a prospective WHI (Women’s Health Initiative) study with 1,525 post-menopausal women, shorter LTL was associated with higher risks of mortality (11). Further analysis showed that patients with myocardial infarction had shorter LTL, which was equivalent to that observed in individuals without myocardial infarction but who were 8 to 12 years older in biological age (12). Shorter LTL was also associated with increased proinflammatory activity in high-risk unstable plaque on virtual histology intravascular ultrasound (13) and delayed re-endothelialization after drug-eluting stent implantation (14) in acute coronary syndrome. When patients developed chronic heart failure, they were also observed to have shorter LTL (15). Moreover, short LTL was also associated with congestive heart failure severity and clinical outcomes (16).
Despite the fact that current robust epidemiological and animal study evidence supports the telomere attrition links between age and CVDs, there is no clear route that leads to the development of telomere therapeutics against CVDs in the future due to the following limitations. First, in adult somatic cells, manipulation of the telomere system bears an oncogenic risk 17, 18. Thus, therapeutic techniques based on the overexpression of telomerase and other telomere-related signals should be applied after considering cell-type and tissue interactions. Second, there is still no clear mechanistic insight into the link between telomere and/or telomerase and atherosclerosis development (19). Third, the telomere system is complex and regulated by various feedback mechanisms, including circadian rhythm oscillations (20), and a direct interruption of 1 target in the telomere pathway can lead to various side effects.
Telomeres and Telomerase
Because DNA polymerase is unable to replicate the 3′ ends of chromosomes fully, the so-called “end-replication problem,” telomeres shorten during each cell replicated cycle (21). When telomeres reach a critically short length, genomic instability activates the DNA repair system and induces replicative arrest, senescence, and cell death (22).
In primary human cells, each time a cell divides, 50 to 100 bases are lost from the telomeres on each chromosome. This loss is much larger than the estimation from end-replication mechanisms, indicating that there are other contributing factors for telomere attrition in human cells (23). Oxidative stress and tissue inflammation have been observed to accelerate telomere shortening and reduced replicative lifespans (24). Telomere shortening is considered a biological molecular clock and is the underlying mechanism proposed to explain the limited lifespan of cells in culture, known as the Hayflick limit (25). During the progressive accumulation of senescent cells in aging, there is a marked increase in the secretion of proinflammatory cytokines, adhesion molecules, growth factors, and proteases from senescent cells 26, 27. This inflammatory signaling initiates a vicious cycle that enhances telomere dysfunction, triggers replicative senescence, and promotes aging and development of age-associated diseases (28).
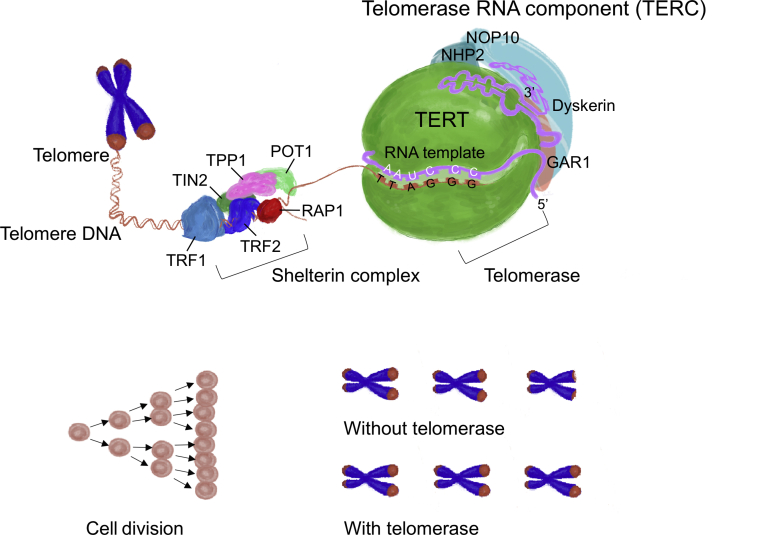
Telomerase is a crucial component in telomere maintenance and regulation. It consists of an RNA template known as telomerase RNA component (TERC) and a DNA reverse transcriptase polymerase known as telomerase reverse transcriptase (TERT) (Figure 1). Telomerase synthesizes new telomeric DNAs to compensate for the loss during cell divisions (29). The overexpression of telomerase extends the lifespan of cells in culture and transforms them into cancerous cells (30). Adult somatic cells typically have a low or undetectable level of telomerase activity with limited longevity; high telomerase activity is restricted to germ cells, pluripotent embryonic stem cells, and hematopoietic progenitor cells (31).
Figure 1.
The Human Telomerase Complex
The human telomerase complex consists of human telomerase reverse transcriptase (TERT), telomerase RNA component (TERC), dyskerin, and ribonucleoproteins (GAR1, NHP2, and NOP10). The telomerase complex protects chromosome ends by lengthening telomeres in DNA strands. TERT is responsible for catalyzing the addition of DNA nucleotides to the ends of telomeres. TERC serves as an RNA template for telomere replications by TERT. Shelterin complex protects telomeres and regulates telomerase activity. Shelterin has 6 subunits, including telomere repeat binding factor 1 (TRF1), telomere repeat binding factor 2 (TRF2), repressor/activator protein 1 (RAP1), protection of telomere 1 (POT1), TRF1- and TRF2-interacting nuclear protein 2 (TIN2), and ACD shelterin complex subunit and telomerase recruitment factor (TPP1). Without telomerase, telomeres lose approximately 120 bp of DNA in human cells. Shortened and dysfunctional telomeric ends will result in genomic instability, tumorigenesis, and cardiovascular aging.
Telomere length is determined by genetic and nongenetic factors. Based on genome-wide association studies, several genomic variants were identified to be associated with the average LTL (32). A large-scale meta-analysis also reported a consistent and high heritability for telomere length (10). Several nongenetic factors that contribute to inflammation and oxidative stress were reported to have significant impacts on LTL, such as psychosocial stress, alcohol consumption, physical inactivity, unhealthy diet, smoking, and obesity 33, 34. Thus, shortened LTL reflects inheritable genomic features, previous mitotic history, cumulative exposure to inflammation and oxidation stress, and the availability of telomerase activity for cells.
Although its causal relationships are still unclear, based on epidemiology studies, telomere attrition is considered to be associated with aging and aging-related diseases, such as CVDs, chronic lung disease, metabolic disorders, neurodegenerative diseases, cognitive disorders, and dysregulated immune function (35). The lifespan of older adults was reported to positively correlate with telomere length (36).
It was observed that the specific telomere syndromes or telomeropathies that affect humans, such as Hoyeraal-Hreidarsson syndrome, dyskeratosis congenita, and aplastic anemia, are caused by germline mutations in telomere maintenance genes. These telomere syndromes present a diverse manifestation but share the features of premature aging, loss of tissue regenerative capacity, increases in inflammation, and prominent organ failure (37).
Gene Therapy With Telomere and Telomerase for CVDs
Robust epidemiological and genetic evidence linking telomere length and CVD risk support the therapeutic hypothesis that genetic manipulations of the telomere system can be a potential treatment target for CVDs. Mice with genetic knockout of TERC or TERT had progressively shorter telomeres over generations and showed features such as severe developmental defects, aging pathologies, and premature death (38). Reconstitution of TERC or TERT expression in the TERC- or TERT-deficient mice with critically short telomeres resulted in elongation of telomeres, less DNA damage, decreases in aging biomarkers, and delay in age-related pathologies (39). In these mice models, short telomeres and associated pathologies were treated and halted by telomerase re-expression. These findings provided the concept for therapeutic strategies to delay age-associated pathologies by transiently increasing telomerase expression.
Telomerase gene therapy was first achieved by delivering mouse TERT with an adeno-associated virus (AAV) into young and old mice. This nonintegrative gene therapy resulted in elongated telomeres, extended lifespans, and delayed age-associated pathologies, such as insulin sensitivity, osteoporosis, and neuromuscular coordination, in both age groups (40). Importantly, telomerase-treated mice did not develop cancer at a higher rate than the corresponding control group (41). With the nonintegrative and replication incompetent properties of AAVs, this strategy restricted TERT expression to a few cell divisions and provided a relatively genome-safe TERT activation. Thus, these studies in mice supported the feasibility of telomerase activation treatment to overcome the adverse consequences of critically short telomeres. Applications of AAV-TERT gene therapy in specific telomere syndromes also showed expected therapeutic effects in preclinical mice models, such as aplastic anemia and pulmonary fibrosis 42, 43. A report for age-associated diseases, such as CVDs, demonstrated improved ventricular function and limited infarct scars after acute myocardial infarction with TERT gene therapy in a preclinical mouse model (44). TERT gene therapy is a promising candidate that deserves further research efforts for clinical implementation for the treatment of age-associated diseases.
Apart from direct TERT delivery by nonintegrative AAV vectors, new gene therapy methods using modified mRNA for in vitro encoding of TERT in human fibroblasts can transiently increase telomerase activity, rapidly extend telomeres, and increase proliferative capacity without the risks of insertional mutagenesis and off-target effects (45). In addition to proof-of-concept experimental data in mice, the development of safe strategies for transient and controllable telomerase activation in humans can be a subject of future studies.
Pharmaceutical Interventions for Telomeres and Telomerase Activity
Because of the pertinence of telomerase in antiaging gene therapy in mice models, several studies focused on the therapeutic interventions for telomerase modulations in humans. Several cardiovascular medications, which have been used for decades and have been shown to have significant survival benefits in patients, possess the effects of telomere length maintenance and senescence prevention.
Statins (3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors) exert various pleiotropic effects to prevent the development of atherosclerotic plaque (46). A cross-sectional analysis of 3,496 subjects from the U.S. National Health and Nutrition Examination Survey showed that telomere length appeared to be longer with a longer duration of statin usage (47). Statin therapy was associated with higher telomerase activity independently of multiple covariates, such as age, sex, smoking, lipid profile, and inflammation (48). Statins can enhance telomerase activity and protect telomeres through upregulation of the telomere repeat-binding factor (TRF)-2 in endothelial cells and endothelial progenitor cells (49). A more specific analysis of human T-lymphocytes showed that atorvastatin in pharmacologically relevant doses led to a transient increase in telomerase activity in T-cells. This effect, which could be blocked by inhibitors of Akt and phosphatidylinositol-4,5-bisphosphate 3 (PI3)-kinase, was more pronounced in the CD4+ than in the CD8+ T-cell subsets (50). In addition, it also prevented telomere shortening by accelerating DNA repair through Nijmegen breakage syndrome-1 protein stabilization and telomere maintenance in vascular smooth muscle cells (51).
The crosstalk between angiotensin II and telomere systems are noteworthy. Overexpression of TERT in vivo modified the angiotensin II−induced microvascular endothelial dysfunction (52). Angiotensin II induces oxidative stress and senescence in vascular smooth muscle cells with telomerase-independent oxidative stress-induced senescence and telomerase-dependent replicative senescence (53). Acute exposure of vascular smooth muscle cells to angiotensin II results in vascular smooth muscle senescence, which is not associated with telomerase activity changes and cannot be reversed by TERT overexpression. However, long-term exposure of vascular smooth muscle cells to angiotensin II induced reduction in proliferation and replicative senescence with telomere shortening (54). Angiotensin II receptor blockers (e.g., losartan) and angiotensin-converting enzyme inhibitors (e.g., captopril) were both shown to protect endothelial progenitor cells from senescence and dysfunction through telomerase cross-talk 55, 56. However, some studies showed that captopril and losartan had no effect on telomere attrition caused by cardiac hypertrophy after abdominal aortic constriction in rats (57). Therefore, the clinical use of angiotensin-converting enzyme inhibitors or angiotensin II inhibitors for the modification of telomere systems requires further clinical studies.
Peroxisome proliferator-activated receptor agonists (e.g., pioglitazone) can increase the activity of telomerase and expression of TRF-2 in mice aorta and in mononuclear cells. Pioglitazone-treated mice were shown to possess the reduced senescence markers, p16, cell-cycle checkpoint kinase 2, and p53 (58). Angiotensin II−induced endothelial progenitor cells senescence was significantly reversed by pioglitazone through telomerase activity enhancement (59). Moreover, pioglitazone was able to increase the TERT and TRF-2 expression in the hearts of diabetic rats (60).
The low potency telomerase activator TA-65, a bioactive molecule extracted from Astragalus membranaceus, has been historically used in Chinese traditional medicine as an antiaging drug and has been shown to have effects on telomere lengthening in mice. TA-65 treatment induces telomerase-dependent elongation of short telomeres and reverses DNA damages in fibroblasts (61) and human T cells (62). Randomized, double-blind, and placebo-controlled clinical trials showed that TA-65 treatment increased high-density lipoprotein cholesterol and reduced C-reactive protein in patients with metabolic syndrome (63) and was also found to elongate telomeres (64).
In addition, sex hormones were also reported to activate TERT transcription. For decades, androgen therapy was considered as the first treatment choice for aplastic anemia, without a clear understanding of the underlying mechanism. A recent study showed that the upregulated telomerase activity is responsible for the effectiveness of the androgen treatment effect in aplastic anemia. In mice with aplastic anemia induced by short telomeres, testosterone therapy halted telomere attrition and prevented subsequent death, by enhancing telomerase expression and lengthening telomeres (65). Moreover, a synthetic androgen, danazol, which was used in the treatment of human telomeropathies, was shown to elongate telomeres in circulating leukocytes and improve hematological parameters (66).
Although specific telomere-lengthening effects of telomerase activation affect cardiovascular health and aging, noncanonical, extracellular, and non−telomere-lengthening functions of telomerase were recently described (67). The off-target effects of these telomerase activating or telomere-lengthening compounds, including those in mitogen signaling and oncogenesis, should be considered before clinical usage.
Inflammation, Atherosclerosis, and Clonal Hematopoiesis of Indeterminate Potential
Atherosclerosis is the dominant pathology of CVD, including myocardial infarction, heart failure, stroke, and peripheral artery diseases (68). The prevalence of these diseases progressively increases with age. In addition, the risk factors of atherosclerotic diseases, such as aging, smoking, obesity, sedentary lifestyle, and unhealthy diet, have been reported to be associated with telomere shortening based on observational epidemiological studies (69). As observed in 1 of these studies, each kilobase pair shortening of telomeres in peripheral blood cells was estimated to result in 2.8- to 3.2-fold higher risk of myocardial infarction and stroke (70).
Telomeres in coronary endothelial cells are shorter in patients with atherosclerosis than in healthy individuals (71). Telomere shortening of endothelial cells might play a role in atherogenesis by increasing proinflammatory reactions and promoting high-risk unstable atherosclerotic plaques (72). In human abdominal aorta analysis, shorter telomere and higher attrition were observed in aged vessels with increased shear wall stress (73). Significant telomere attrition, shorter telomeres, and DNA damages were demonstrated in biopsied tissue from a failing heart; these effects were only specific to cardiomyocytes, regardless of the age of the patients (74). Moreover, asynchronous shortening of the telomere length between cardiac atrial tissue and leukocytes served as a better biomarker than leukocyte length alone for post-cardiovascular surgery events (75).
Atherosclerosis is an inflammatory disease that involves vascular endothelium, smooth muscle, and blood cells (76). With aging, telomeres shorten, and blood cells start to accumulate somatic genetic mutations. As these blood cells gain a competitive expansion advantage, they give rise to some expanded clones of leukocytes that circulate in the peripheral blood, which is termed clonal hematopoiesis of indeterminate potential (CHIP) (77). More than 10% of septuagenarians exhibit CHIP, and its prevalence increases with age (78). Individuals with CHIP have increased cardiovascular mortalities independent of traditional risk factors (e.g., diabetes, hypertension, and dyslipidemia) (79). Current evidence shows that de novo mutations in DNMT3A, TET2, and ASXL1 facilitate the clonal expansion of leukocytes 80, 81. Macrophages from TET2-knockout mice resulted in the abnormal activation of the NLRP3 (Nucleotide-Binding Domain, Leucine-Rich–Containing Family, Pyrin Domain–Containing-3)-mediated inflammasome and contribute to enhanced atherosclerosis (82). Mice with CHIP mutations in hematopoietic cells also exhibited aggravated the development of heart failure (83).
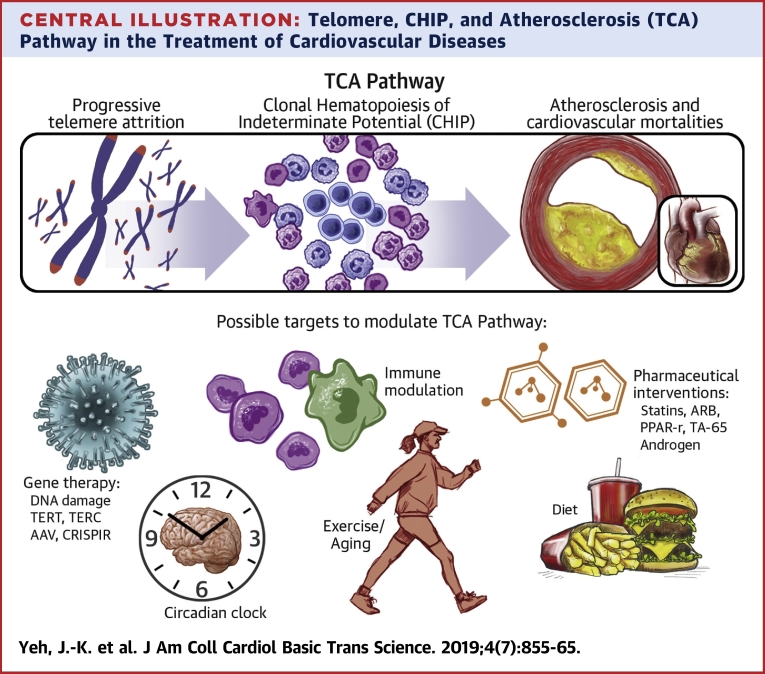
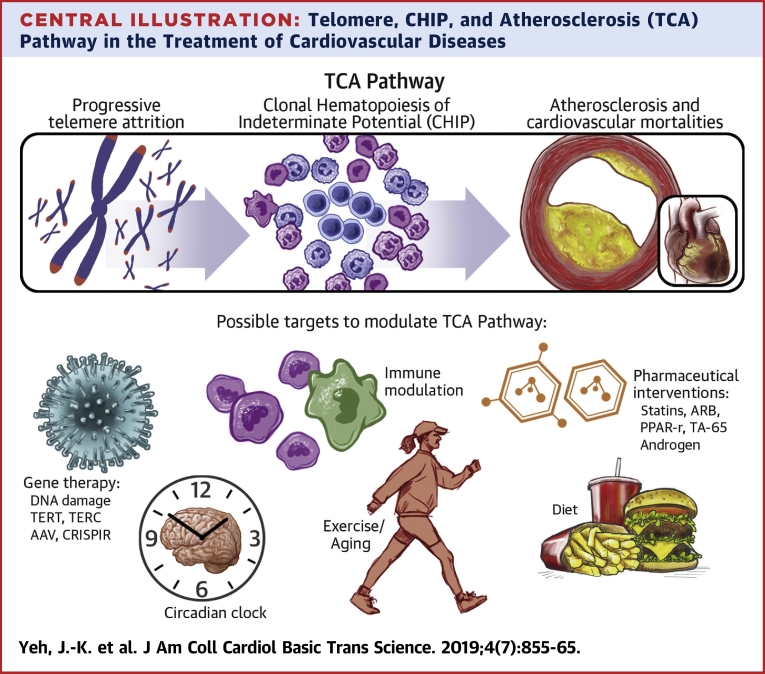
Both telomere attrition and CHIP increase with age. Accordingly, progressive leukocyte telomere attrition can lead to genomic instability, which later results in CHIP (84). Therefore, it is assumed that the manipulation of the telomere system would be a possible treatment target of CHIP-related CVDs. Although current clinical evidence supports this concept, further research needs to be conducted. In a whole-genome sequencing study, the strongest association of CHIP was found to be an 8-bp deletion in intron 3 of the TERT gene (85). In the same study, telomere lengths were observed to be significantly shortened in individuals with CHIP. Dyskeratosis congenita is a rare progressive congenital disease with skin pigmentation, nail dystrophy, and leukoplakia of the oral mucosa. Dyskeratosis congenita is characterized by short telomeres with poor telomere maintenance, mainly caused by some abnormal mutations in ribosome and telomerase RNA components (86). Clonal expansion of hematopoietic cells bearing nonsynonymous coding somatic mutations is a common feature that occurs in one-half of patients with dyskeratosis congenita (87). The telomerase complex controls hematopoietic cell differentiation and senescence in the induced pluripotent stem cell model (88). The telomere-CHIP-atherosclerosis (TCA) axis may provide several possible therapeutic targets, including modulating telomerase activity, rescuing senescent or mutated clonal cells, and inhibiting the inflammation from CHIP (Central Illustration). However, future investigations are required to understand the TCA axis before comprehensive clinical trials can be undertaken in the future.
Central Illustration.
Telomere, CHIP, and Atherosclerosis (TCA) Pathway in the Treatment of Cardiovascular Diseases
Progressive telomere attrition can lead to genomic instability, which later results in clonal hematopoiesis of indeterminate potential (CHIP). Individuals with CHIP have increased atherosclerosis and cardiovascular mortalities independent of traditional risk factors, such as diabetes, hypertension, and dyslipidemia. Gene therapy, pharmaceutical interventions, immune modulation, circadian clock, exercise, and diet are possible targets to modulate the telomere/telomerase system, CHIP, and atherosclerosis pathway. AAV = adeno-associated virus; ARB = angiotensin II receptor blocker; CRISPR = clustered regularly interspaced short palindromic repeats; IL = interleukin; NF-κB = nuclear factor-kappa B; PPAR = peroxisome proliferator-activated receptor; TCA = telomere-CHIP-atherosclerosis; TERC = telomerase RNA component; TERT = telomerase reverse transcriptase; TNF = tumor necrosis factor.
Furthermore, several aspects of the TCA axis remain to be understood. First, telomere attrition and CHIP are a progressive, long-term processes, like atherosclerosis, and thus require better cellular or animal models to simulate these 2 chronic processes (e.g., low-density lipoprotein receptor knockout mice for atherosclerosis). Second, the evidence for the connection between telomere attrition and CHIP is based on a clinical association study. Therefore, a future study is required to understand their underlying mechanisms. Third, the telomere length variations between individuals and different attrition rates between tissues are not directly linked to CHIP occurrence and atherosclerosis. The connections could be affected by other factors, such as different responses to critical short telomeres or inflammation. Even among patients with dyskeratosis congenita, >10% of them do not develop CHIP (87). Lastly, there is still no evidence showing that CHIP can be prevented or reversed via telomere modulation.
Immune Modulation
Inflammation is a protective response to injury of a process that delivers leukocytes to sites of infection or tissue damage. Acute inflammation usually lasts for hours and has many positive (e.g., interleukin-6/tumor necrosis factor-α) and negative (e.g., interleukin-10) regulators. If infection and tissue damage persists, if the healing process is somehow disturbed, or if 1 of the negative control mechanisms fails, inflammation may progress to a chronic state that can last for weeks, months, or maybe years (89). In many common chronic diseases, such as atherosclerosis, the chronic inflammatory process does not follow a manifestation of an acute reaction but begins as a low grade and smoldering response (90).
Aging, DNA damage, and stem cell failure are closely associated with low levels of chronic inflammation (91). Chronic low-grade inflammation increases oxidative stress and enhances telomere dysfunction (92). The links between telomere dysfunction and chronic inflammation are bi-directional and can result in complex vicious cycles. Telomerase is active in the human coronary artery and its activity is increased during atherosclerosis formation (93). Different proinflammatory mediators increased TERT mRNA and telomerase activity in macrophages in atherosclerosis through nuclear factor-κB signaling (93). Premature telomere erosion in peripheral blood mononuclear cells is a common phenomenon in obesity, myocardial infarction, and atherosclerosis (8). In telomerase-deficient mice, marked increases in proinflammatory cytokines interleukin-6, CXCL16 (Chemokine (C-X-C motif) ligand 16), and tumor necrosis factor-α were observed in pulmonary tissues (94). Chronic inflammation aggravates telomere dysfunction and cellular senescence through oxidative stress activation and cyclooxygenase-2−dependent reactive oxygen species production (95). From this evidence, the telomere system and chronic inflammation are suspected to be linked closely.
The aging process in humans is associated with changes in circadian rhythm patterns (96). Circadian rhythm controls telomeres and telomerase activity through circadian locomotor output cycles kaput gene−aryl hydrocarbon receptor nuclear translocator-like protein 1 heterodimers (97). Mice and humans with circadian rhythm abnormalities not only have increased vascular senescence (98), impaired endothelial progenitor cell function, enhanced atherosclerosis (99), and obesity (100) but are also prone to chronic inflammation and sepsis (101). The intersection of circadian mechanics into the linkage between telomere and chronic inflammation provides more opportunities in combating atherosclerosis. For example, experimental evidence in mice indicate that melatonin regulates the transactivation of telomerase and the expression of core clock and clock-related genes (102). Melatonin inhibits smooth muscle cell inflammation and atherosclerosis in mice 103, 104. For example, physicians in emergency departments are known to lose their telomerase oscillation, and have low telomerase activities and circadian misalignments that increases CVD risk factors (105). Further research on the connections between telomerase and circadian rhythm will shed more light upon this area.
However, the connections between telomere and atherosclerosis are not definitive. Although mice with double deficiency in ApoE and TERC have extensive telomere attritions, a substantial reduction of atherosclerosis was observed in them compared to mice with normal telomerases (106). Short telomeres result in immunosenescence and lead to protection from atherosclerosis (107). Moreover, not all clinical studies have found significant associations between telomere lengths in white blood cells and morbidity or mortalities (108).
Aging Cardiovascular Patients and Telomere Therpay
According to World Health Organization data, by 2020, all countries across the world will face significant challenges to their health and social systems due to the aging demographic shift (109). The number of people aged 60 years and older will outnumber children younger than 5 years. The pace of the aging population will be faster in future decades. There are 3 significant differences in telomere therapy between the young and older adult population. First, in older adult individuals, the evidence supporting the telomere length and remaining lifespan is controversial (110). The contradictory results of these studies suggest that in septuagenarians and octogenarians, the role of telomere in survival becomes less important (111). A recent study indicated that healthy lifestyle habits such as not smoking and not being obese at the age of 71 were the most significant associated factors with survival at the age of 85 years or older in men (112). The efficacy of manipulation of the telomere system with younger subjects versus older subjects will need exploratory studies in the future. Second, the goals of telomere cardiovascular therapy in young subjects and older adults subjects are different. In young subjects, the goal of telomere therapy is to prevent cardiovascular senescence and diseases, whereas in older adult subjects, the goal of telomere therapy is to restoration of cardiovascular functions. Third, the prevention of CHIP and atherosclerosis with telomere targets in young subjects requires long-term treatments. Moreover, the efficacy and effectiveness of the treatment involving CHIP detection, prevention, and correction in octogenarians are questionable. Further clinical studies are required to overcome these limitations.
Telomere Therapy From the Bench to the Bedside
Telomere biology could be potentially involved in the development of age-associated CVDs including atherosclerosis, hypertension, myocardial infarction, and heart failure. Critically shortened telomeres activate a series of downstream changes that induce cardiomyocyte cell cycle arrest and cellular senescence (38). The reduced proliferative potential of cardiovascular systems limits the regenerative capacity of aged and injured myocardium and vasculature (113). Thus, therapeutic strategies to restore the proliferative potential of adult cardiovascular systems are considered as a promising alternative treatment for CVDs. In mouse models, telomerase gene transfer therapy provides an attractive way for cardiovascular restoration and deserves future investigations. Although we are still far from applying the current knowledge in daily therapeutic protocols, many studies seem to agree with the fact that a combination of exercise, healthy diet, low everyday stress, and anti-inflammatory agents intake may prove to be beneficial in promoting human longevity by modulating the telomere system and in slowing down the effects of many chronic disorders. The present knowledge in this regard still requires input from different studies, and further investigations are needed to uncover the true molecular relationships involved in the previously described phenomena.
Footnotes
Dr. Wang was supported by the National Health Research Institute (NHRI-EX106-10617SI), National Science Council (105-2628-B-182-009-MY4), and Chang Gung Memorial Hospital (CMRPG3H0132, CMRPG3I0321, and CMRPG3H0842). All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Basic to Translational Scienceauthor instructions page.
References
- 1.Blackburn E.H. Telomere states and cell fates. Nature. 2000;408:53–56. doi: 10.1038/35040500. [DOI] [PubMed] [Google Scholar]
- 2.d’Adda di Fagagna F. Living on a break: cellular senescence as a DNA-damage response. Nat Rev Cancer. 2008;8:512–522. doi: 10.1038/nrc2440. [DOI] [PubMed] [Google Scholar]
- 3.Rehkopf D.H., Needham B.L., Lin J. Leukocyte telomere length in relation to 17 biomarkers of cardiovascular disease risk: a cross-sectional study of US adults. PLoS Med. 2016;13 doi: 10.1371/journal.pmed.1002188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang C.-Y. Asynchronous shortening of telomere length and cardiovascular outcomes. J Am Coll Cardiol Basic Trans Sci. 2018;3:601–603. doi: 10.1016/j.jacbts.2018.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fitzpatrick A.L., Kronmal R.A., Gardner J.P. Leukocyte telomere length and cardiovascular disease in the cardiovascular health study. Am J Epidemiol. 2007;165:14–21. doi: 10.1093/aje/kwj346. [DOI] [PubMed] [Google Scholar]
- 6.Fasching C.L. Telomere length measurement as a clinical biomarker of aging and disease. Crit Rev Clin Lab Sci. 2018;55:443–465. doi: 10.1080/10408363.2018.1504274. [DOI] [PubMed] [Google Scholar]
- 7.Martin-Ruiz C.M., Baird D., Roger L. Reproducibility of telomere length assessment: an international collaborative study. Int J Epidemiol. 2015;44:1673–1683. doi: 10.1093/ije/dyu191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Valdes A.M., Andrew T., Gardner J.P., Kimura M. Obesity, cigarette smoking, and telomere length in women. Lancet. 2005 doi: 10.1016/S0140-6736(05)66630-5. [DOI] [PubMed] [Google Scholar]
- 9.Brouilette S.W., Moore J.S., McMahon A.D. Telomere length, risk of coronary heart disease, and statin treatment in the West of Scotland Primary Prevention Study: a nested case-control study. Lancet. 2007;369:107–114. doi: 10.1016/S0140-6736(07)60071-3. [DOI] [PubMed] [Google Scholar]
- 10.Haycock P.C., Heydon E.E., Kaptoge S., Butterworth A.S., Thompson A., Willeit P. Leukocyte telomere length and risk of cardiovascular disease: systematic review and meta-analysis. BMJ. 2014;349:g4227. doi: 10.1136/bmj.g4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carty C.L., Kooperberg C., Liu J. Leukocyte telomere length and risks of incident coronary heart disease and mortality in a racially diverse population of postmenopausal women. Arterioscler Thromb Vasc Biol. 2015;35:2225–2231. doi: 10.1161/ATVBAHA.115.305838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Samani N.J., Boultby R., Butler R., Thompson J.R., Goodall A.H. Telomere shortening in atherosclerosis. Lancet. 2001;358:472–473. doi: 10.1016/S0140-6736(01)05633-1. [DOI] [PubMed] [Google Scholar]
- 13.Calvert P.A., Liew T.-V., Gorenne I. Leukocyte telomere length is associated with high-risk plaques on virtual histology intravascular ultrasound and increased proinflammatory activity. Arterioscler Thromb Vasc Biol. 2011;31:2157–2164. doi: 10.1161/ATVBAHA.111.229237. [DOI] [PubMed] [Google Scholar]
- 14.Armstrong E.J., Xing L., Zhang J. Association between leukocyte telomere length and drug-eluting stent strut coverage by optical coherence tomography. J Am Coll Cardiol. 2012;59:2218–2219. doi: 10.1016/j.jacc.2012.03.028. [DOI] [PubMed] [Google Scholar]
- 15.van der Harst P., van der Steege G., de Boer R.A. Telomere length of circulating leukocytes is decreased in patients with chronic heart failure. J Am Coll Cardiol. 2007;49:1459–1464. doi: 10.1016/j.jacc.2007.01.027. [DOI] [PubMed] [Google Scholar]
- 16.Haver V.G., Mateo Leach I., Kjekshus J. Telomere length and outcomes in ischaemic heart failure: data from the COntrolled ROsuvastatin multiNAtional Trial in Heart Failure (CORONA) Eur J Heart Fail. 2015;17:313–319. doi: 10.1002/ejhf.237. [DOI] [PubMed] [Google Scholar]
- 17.Smith L., Luchini C., Demurtas J. Telomere length and health outcomes: an umbrella review of systematic reviews and meta-analyses of observational studies. Ageing Res Rev. 2019;51:1–10. doi: 10.1016/j.arr.2019.02.003. [DOI] [PubMed] [Google Scholar]
- 18.Hannen R., Bartsch J.W. Essential roles of telomerase reverse transcriptase hTERT in cancer stemness and metastasis. FEBS Lett. 2018;592:2023–2031. doi: 10.1002/1873-3468.13084. [DOI] [PubMed] [Google Scholar]
- 19.De Meyer T., Nawrot T., Bekaert S., De Buyzere M.L., Rietzschel E.R., Andrés V. Telomere length as cardiovascular aging biomarker: JACC review topic of the week. J Am Coll Cardiol. 2018;72:805–813. doi: 10.1016/j.jacc.2018.06.014. [DOI] [PubMed] [Google Scholar]
- 20.Qu Y., Mao M., Li X. Telomerase reconstitution contributes to resetting of circadian rhythm in fibroblasts. Mol Cell Biochem. 2008;313:11–18. doi: 10.1007/s11010-008-9736-2. [DOI] [PubMed] [Google Scholar]
- 21.Levy M.Z., Allsopp R.C., Futcher A.B., Greider C.W., Harley C.B. Telomere end-replication problem and cell aging. J Mol Biol. 1992;225:951–960. doi: 10.1016/0022-2836(92)90096-3. [DOI] [PubMed] [Google Scholar]
- 22.Hackett J.A., Feldser D.M., Greider C.W. Telomere dysfunction increases mutation rate and genomic instability. Cell. 2001;106:275–286. doi: 10.1016/s0092-8674(01)00457-3. [DOI] [PubMed] [Google Scholar]
- 23.O’Sullivan R.J., Karlseder J. Telomeres: protecting chromosomes against genome instability. Nat Rev Mol Cell Biol. 2010;11:171–181. doi: 10.1038/nrm2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kawanishi S., Oikawa S. Mechanism of telomere shortening by oxidative stress. Ann N Y Acad Sci. 2004;1019:278–284. doi: 10.1196/annals.1297.047. [DOI] [PubMed] [Google Scholar]
- 25.Shay J.W., Wright W.E. Hayflick, his limit, and cellular ageing. Nat Rev Mol Cell Biol. 2000;1:72–76. doi: 10.1038/35036093. [DOI] [PubMed] [Google Scholar]
- 26.Jiang H., Schiffer E., Song Z. Proteins induced by telomere dysfunction and DNA damage represent biomarkers of human aging and disease. Proc Natl Acad Sci U S A. 2008;105:11299–11304. doi: 10.1073/pnas.0801457105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.López-Otín C., Blasco M.A., Partridge L., Serrano M., Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gilley D., Tanaka H., Herbert B.-S. Telomere dysfunction in aging and cancer. Int J Biochem Cell Biol. 2005;37:1000–1013. doi: 10.1016/j.biocel.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 29.Shay J.W., Wright W.E. Telomeres and telomerase: three decades of progress. Nat Rev Genet. 2019;20:299–309. doi: 10.1038/s41576-019-0099-1. [DOI] [PubMed] [Google Scholar]
- 30.Mitchell J.R., Collins K. Human telomerase activation requires two independent interactions between telomerase RNA and telomerase reverse transcriptase. Mol Cell. 2000;6:361–371. doi: 10.1016/s1097-2765(00)00036-8. [DOI] [PubMed] [Google Scholar]
- 31.Wright W.E., Piatyszek M.A., Rainey W.E., Byrd W., Shay J.W. Telomerase activity in human germline and embryonic tissues and cells. Dev Genet. 1996;18:173–179. doi: 10.1002/(SICI)1520-6408(1996)18:2<173::AID-DVG10>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 32.Codd V., Nelson C.P., Albrecht E. Identification of seven loci affecting mean telomere length and their association with disease. Nat Genet. 2013;45:422–427. doi: 10.1038/ng.2528. 427e1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bekaert S., De Meyer T., Rietzschel E.R. Telomere length and cardiovascular risk factors in a middle-aged population free of overt cardiovascular disease. Aging Cell. 2007;6:639–647. doi: 10.1111/j.1474-9726.2007.00321.x. [DOI] [PubMed] [Google Scholar]
- 34.Yeh J.-K., Wang C.-Y. Telomeres and telomerase in cardiovascular diseases. Genes. 2016;7(9) doi: 10.3390/genes7090058. pii:E58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mangaonkar A.A., Patnaik M.M. Short telomere syndromes in clinical practice: bridging bench and bedside. Mayo Clin Proc. 2018;93:904–916. doi: 10.1016/j.mayocp.2018.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heidinger B.J., Blount J.D., Boner W., Griffiths K., Metcalfe N.B., Monaghan P. Telomere length in early life predicts lifespan. Proc Natl Acad Sci U S A. 2012;109:1743–1748. doi: 10.1073/pnas.1113306109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Calado R.T., Young N.S. Telomere diseases. N Engl J Med. 2009;361:2353–2365. doi: 10.1056/NEJMra0903373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leri A., Franco S., Zacheo A. Ablation of telomerase and telomere loss leads to cardiac dilatation and heart failure associated with p53 upregulation. EMBO J. 2003;22:131–139. doi: 10.1093/emboj/cdg013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Samper E., Flores J.M., Blasco M.A. Restoration of telomerase activity rescues chromosomal instability and premature aging in Terc-/- mice with short telomeres. EMBO Rep. 2001;2:800–807. doi: 10.1093/embo-reports/kve174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.de Jesus B.B., Vera E., Schneeberger K. Telomerase gene therapy in adult and old mice delays aging and increases longevity without increasing cancer. EMBO Mol Med. 2012;4:691–704. doi: 10.1002/emmm.201200245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bernardes de Jesus B., Blasco M.A. Telomerase at the intersection of cancer and aging. Trends Genet. 2013;29:513–520. doi: 10.1016/j.tig.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bär C., Povedano J.M., Serrano R. Telomerase gene therapy rescues telomere length, bone marrow aplasia, and survival in mice with aplastic anemia. Blood. 2016;127:1770–1779. doi: 10.1182/blood-2015-08-667485. [DOI] [PubMed] [Google Scholar]
- 43.Povedano J.M., Martinez P., Serrano R. Therapeutic effects of telomerase in mice with pulmonary fibrosis induced by damage to the lungs and short telomeres. Elife. 2018;7 doi: 10.7554/eLife.31299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bär C., Bernardes de Jesus B., Serrano R. Telomerase expression confers cardioprotection in the adult mouse heart after acute myocardial infarction. Nat Commun. 2014;5:5863. doi: 10.1038/ncomms6863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ramunas J., Yakubov E., Brady J.J. Transient delivery of modified mRNA encoding TERT rapidly extends telomeres in human cells. FASEB J. 2015;29:1930–1939. doi: 10.1096/fj.14-259531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang C.-Y., Liu P.-Y., Liao J.K. Pleiotropic effects of statin therapy: molecular mechanisms and clinical results. Trends Mol Med. 2008;14:37–44. doi: 10.1016/j.molmed.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tran P.T., Meeker A.K., Platz E.A. Association between statin drug use and peripheral blood leukocyte telomere length in the National Health and Nutrition Examination Survey 1999–2002: a cross-sectional study. Ann Epidemiol. 2018;28:529–534. doi: 10.1016/j.annepidem.2018.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Boccardi V., Barbieri M., Rizzo M.R. A new pleiotropic effect of statins in elderly: modulation of telomerase activity. FASEB J. 2013;27:3879–3885. doi: 10.1096/fj.13-232066. [DOI] [PubMed] [Google Scholar]
- 49.Spyridopoulos I., Haendeler J., Urbich C. Statins enhance migratory capacity by upregulation of the telomere repeat-binding factor TRF2 in endothelial progenitor cells. Circulation. 2004;110:3136–3142. doi: 10.1161/01.CIR.0000142866.50300.EB. [DOI] [PubMed] [Google Scholar]
- 50.Bennaceur K., Atwill M., Al Zhrany N. Atorvastatin induces T cell proliferation by a telomerase reverse transcriptase (TERT) mediated mechanism. Atherosclerosis. 2014;236:312–320. doi: 10.1016/j.atherosclerosis.2014.07.020. [DOI] [PubMed] [Google Scholar]
- 51.Mahmoudi M., Gorenne I., Mercer J., Figg N., Littlewood T., Bennett M. Statins use a novel Nijmegen breakage syndrome-1–dependent pathway to accelerate DNA repair in vascular smooth muscle cells. Circ Res. 2008;103:717–725. doi: 10.1161/CIRCRESAHA.108.182899. [DOI] [PubMed] [Google Scholar]
- 52.Ait-Aissa K., Kadlec A.O., Hockenberry J., Gutterman D.D., Beyer A.M. Telomerase reverse transcriptase protects against angiotensin II-induced microvascular endothelial dysfunction. Am J Physiol Heart Circ Physiol. 2018;314:H1053–H1060. doi: 10.1152/ajpheart.00472.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Matthews C., Gorenne I., Scott S. Vascular smooth muscle cells undergo telomere-based senescence in human atherosclerosis: effects of telomerase and oxidative stress. Circ Res. 2006;99:156–164. doi: 10.1161/01.RES.0000233315.38086.bc. [DOI] [PubMed] [Google Scholar]
- 54.Herbert K.E., Mistry Y., Hastings R., Poolman T., Niklason L., Williams B. Angiotensin II-mediated oxidative DNA damage accelerates cellular senescence in cultured human vascular smooth muscle cells via telomere-dependent and independent pathways. Circ Res. 2008;102:201–208. doi: 10.1161/CIRCRESAHA.107.158626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Imanishi T., Tsujioka H., Akasaka T. Endothelial progenitor cells dysfunction and senescence: contribution to oxidative stress. Curr Cardiol Rev. 2008;4:275–286. doi: 10.2174/157340308786349435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Donnini S., Terzuoli E., Ziche M., Morbidelli L. Sulfhydryl angiotensin-converting enzyme inhibitor promotes endothelial cell survival through nitric-oxide synthase, fibroblast growth factor-2, and telomerase cross. J Pharmacol Exp Ther. 2010;332:776–784. doi: 10.1124/jpet.109.159178. [DOI] [PubMed] [Google Scholar]
- 57.Sheng R., Gu Z.-L., Xie M.-L. Epigallocatechin gallate, the major component of polyphenols in green tea, inhibits telomere attrition mediated cardiomyocyte apoptosis in cardiac hypertrophy. Int J Cardiol. 2013;162:199–209. doi: 10.1016/j.ijcard.2011.07.083. [DOI] [PubMed] [Google Scholar]
- 58.Werner C., Gensch C., Pöss J., Haendeler J., Böhm M., Laufs U. Pioglitazone activates aortic telomerase and prevents stress-induced endothelial apoptosis. Atherosclerosis. 2011;216:23–34. doi: 10.1016/j.atherosclerosis.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 59.Imanishi T., Kobayashi K., Kuroi A., Ikejima H., Akasaka T. Pioglitazone inhibits angiotensin II-induced senescence of endothelial progenitor cell. Hypertens Res. 2008;31:757–765. doi: 10.1291/hypres.31.757. [DOI] [PubMed] [Google Scholar]
- 60.Makino N., Maeda T., Oyama J.-I., Higuchi Y., Mimori K. Improving insulin sensitivity via activation of PPAR-gamma increases telomerase activity in the heart of OLETF rats. Am J Physiol Heart Circ Physiol. 2009;297:H2188–H2195. doi: 10.1152/ajpheart.00421.2009. [DOI] [PubMed] [Google Scholar]
- 61.Bernardes de Jesus B., Schneeberger K., Vera E., Tejera A., Harley C.B., Blasco M.A. The telomerase activator TA-65 elongates short telomeres and increases health span of adult/old mice without increasing cancer incidence. Aging Cell. 2011;10:604–621. doi: 10.1111/j.1474-9726.2011.00700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Molgora B., Bateman R., Sweeney G. Functional assessment of pharmacological telomerase activators in human T cells. Cells. 2013;2:57–66. doi: 10.3390/cells2010057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fernandez M.L., Thomas M.S., Lemos B.S. TA-65, a telomerase activator improves cardiovascular markers in patients with metabolic syndrome. Curr Pharm Des. 2018;24:1905–1911. doi: 10.2174/1381612824666180316114832. [DOI] [PubMed] [Google Scholar]
- 64.Salvador L., Singaravelu G., Harley C.B., Flom P., Suram A., Raffaele J.M. A natural product telomerase activator lengthens telomeres in humans: a randomized, double blind, and placebo controlled study. Rejuvenation Res. 2016;19:478–484. doi: 10.1089/rej.2015.1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ziegler P., Schrezenmeier H., Akkad J. Telomere elongation and clinical response to androgen treatment in a patient with aplastic anemia and a heterozygous hTERT gene mutation. Ann Hematol. 2012;91:1115–1120. doi: 10.1007/s00277-012-1454-x. [DOI] [PubMed] [Google Scholar]
- 66.Townsley D.M., Dumitriu B., Young N.S. Danazol treatment for telomere diseases. N Engl J Med. 2016;375:1095–1096. doi: 10.1056/NEJMc1607752. [DOI] [PubMed] [Google Scholar]
- 67.Ait-Aissa K., Ebben J.D., Kadlec A.O., Beyer A.M. Friend or foe? Telomerase as a pharmacological target in cancer and cardiovascular disease. Pharmacol Res. 2016;111:422–433. doi: 10.1016/j.phrs.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Libby P., Ridker P.M., Hansson G.K. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473:317–325. doi: 10.1038/nature10146. [DOI] [PubMed] [Google Scholar]
- 69.Fernández-Alvira J.M., Fuster V., Dorado B. Short telomere load, telomere length, and subclinical atherosclerosis: the PESA study. J Am Coll Cardiol. 2016;67:2467–2476. doi: 10.1016/j.jacc.2016.03.530. [DOI] [PubMed] [Google Scholar]
- 70.Zee R.Y.L., Michaud S.E., Germer S., Ridker P.M. Association of shorter mean telomere length with risk of incident myocardial infarction: a prospective, nested case-control approach. Clin Chim Acta. 2009;403:139–141. doi: 10.1016/j.cca.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Minamino T., Miyauchi H., Yoshida T., Ishida Y., Yoshida H., Komuro I. Endothelial cell senescence in human atherosclerosis: role of telomere in endothelial dysfunction. Circulation. 2002;105:1541–1544. doi: 10.1161/01.cir.0000013836.85741.17. [DOI] [PubMed] [Google Scholar]
- 72.Kurz D.J., Decary S., Hong Y., Trivier E., Akhmedov A., Erusalimsky J.D. Chronic oxidative stress compromises telomere integrity and accelerates the onset of senescence in human endothelial cells. J Cell Sci. 2004;117:2417–2426. doi: 10.1242/jcs.01097. [DOI] [PubMed] [Google Scholar]
- 73.Okuda K., Khan M.Y., Skurnick J., Kimura M., Aviv H., Aviv A. Telomere attrition of the human abdominal aorta: relationships with age and atherosclerosis. Atherosclerosis. 2000;152:391–398. doi: 10.1016/s0021-9150(99)00482-7. [DOI] [PubMed] [Google Scholar]
- 74.Sharifi-Sanjani M., Oyster N.M., Tichy E.D. Cardiomyocyte-specific telomere shortening is a distinct signature of heart failure in humans. J Am Heart Assoc. 2017;6(9) doi: 10.1161/JAHA.116.005086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yin H., Akawi O., Fox S.A. Cardiac-referenced leukocyte telomere length and outcomes after cardiovascular surgery. J Am Coll Cardiol Basic Trans Sci. 2018;3:591–600. doi: 10.1016/j.jacbts.2018.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hansson G.K., Libby P. The immune response in atherosclerosis: a double-edged sword. Nat Rev Immunol. 2006;6:508–519. doi: 10.1038/nri1882. [DOI] [PubMed] [Google Scholar]
- 77.Libby P., Ebert B.L. CHIP (clonal hematopoiesis of indeterminate potential) Circulation. 2018;138:666–668. doi: 10.1161/CIRCULATIONAHA.118.034392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ebert B.L., Libby P. Clonal hematopoiesis confers predisposition to both cardiovascular disease and cancer: a newly recognized link between two major killers. Ann Intern Med. 2018;169:116–117. doi: 10.7326/M18-0737. [DOI] [PubMed] [Google Scholar]
- 79.Jaiswal S., Natarajan P., Silver A.J. Clonal hematopoiesis and risk of atherosclerotic cardiovascular disease. N Engl J Med. 2017;377:111–121. doi: 10.1056/NEJMoa1701719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jaiswal S., Fontanillas P., Flannick J. Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med. 2014;371:2488–2498. doi: 10.1056/NEJMoa1408617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fuster J.J., MacLauchlan S., Zuriaga M.A. Clonal hematopoiesis associated with TET2 deficiency accelerates atherosclerosis development in mice. Science. 2017;355:842–847. doi: 10.1126/science.aag1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Basiorka A.A., McGraw K.L., Eksioglu E.A. The NLRP3 inflammasome functions as a driver of the myelodysplastic syndrome phenotype. Blood. 2016;128:2960–2975. doi: 10.1182/blood-2016-07-730556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sano S., Oshima K., Wang Y. Tet2-mediated clonal hematopoiesis accelerates heart failure through a mechanism involving the IL-1β/NLRP3 inflammasome. J Am Coll Cardiol. 2018;71:875–886. doi: 10.1016/j.jacc.2017.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Aviv A., Levy D. Hemothelium, clonal hematopoiesis of indeterminate potential, and atherosclerosis. Circulation. 2019;139:7–9. doi: 10.1161/CIRCULATIONAHA.118.038434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zink F., Stacey S.N., Norddahl G.L. Clonal hematopoiesis, with and without candidate driver mutations, is common in the elderly. Blood. 2017;130:742–752. doi: 10.1182/blood-2017-02-769869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mitchell J.R., Wood E., Collins K. A telomerase component is defective in the human disease dyskeratosis congenita. Nature. 1999;402:551–555. doi: 10.1038/990141. [DOI] [PubMed] [Google Scholar]
- 87.Perdigones N., Perin J.C., Schiano I. Clonal hematopoiesis in patients with dyskeratosis congenita. Am J Hematol. 2016;91:1227–1233. doi: 10.1002/ajh.24552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jose S.S., Tidu F., Burilova P., Kepak T., Bendickova K., Fric J. The telomerase complex directly controls hematopoietic stem cell differentiation and senescence in an induced pluripotent stem cell model of telomeropathy. Front Genet. 2018;9:345. doi: 10.3389/fgene.2018.00345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rymer J.A., Newby L.K. Failure to launch: targeting inflammation in acute coronary syndromes. J Am Coll Cardiol Basic Trans Sci. 2017;2:484–497. doi: 10.1016/j.jacbts.2017.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Felisbino M.B., McKinsey T.A. Epigenetics in cardiac fibrosis: emphasis on inflammation and fibroblast activation. J Am Coll Cardiol Basic Trans Sci. 2018;3:704–715. doi: 10.1016/j.jacbts.2018.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Khatami M. Theories of aging and chronic diseases: chronic inflammation an interdependent “roadmap” to age-associated illnesses. In: Khatami M., editor. Inflammation, Aging and Cancer: Biological Injustices to Molecular Village of Immunity that Guard Health. Springer International Publishing; Cham, Switzerland: 2017. pp. 91–174. [Google Scholar]
- 92.Demissie S., Levy D., Benjamin E.J. Insulin resistance, oxidative stress, hypertension, and leukocyte telomere length in men from the Framingham Heart Study. Aging Cell. 2006;5:325–330. doi: 10.1111/j.1474-9726.2006.00224.x. [DOI] [PubMed] [Google Scholar]
- 93.Gizard F., Heywood E.B., Findeisen H.M. Telomerase activation in atherosclerosis and induction of telomerase reverse transcriptase expression by inflammatory stimuli in macrophages. Arterioscler Thromb Vasc Biol. 2011;31:245–252. doi: 10.1161/ATVBAHA.110.219808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Liu T., Yu H., Ding L. Conditional knockout of telomerase reverse transcriptase in mesenchymal cells impairs mouse pulmonary fibrosis. PLoS One. 2015;10 doi: 10.1371/journal.pone.0142547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jurk D., Wilson C., Passos J.F. Chronic inflammation induces telomere dysfunction and accelerates ageing in mice. Nat Commun. 2014;2:4172. doi: 10.1038/ncomms5172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Brown S.A., Schmitt K., Eckert A. Aging and circadian disruption: causes and effects. Aging. 2011;3:813–817. doi: 10.18632/aging.100366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chen W.-D., Wen M.-S., Shie S.-S. The circadian rhythm controls telomeres and telomerase activity. Biochem Biophys Res Commun. 2014;451:408–414. doi: 10.1016/j.bbrc.2014.07.138. [DOI] [PubMed] [Google Scholar]
- 98.Wang C.-Y., Wen M.-S., Wang H.-W. Increased vascular senescence and impaired endothelial progenitor cell function mediated by mutation of circadian gene Per2. Circulation. 2008;118:2166–2173. doi: 10.1161/CIRCULATIONAHA.108.790469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.McAlpine C.S., Swirski F.K. Circadian influence on metabolism and inflammation in atherosclerosis. Circ Res. 2016;119:131–141. doi: 10.1161/CIRCRESAHA.116.308034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bass J., Takahashi J.S. Circadian integration of metabolism and energetics. Science. 2010;330:1349–1354. doi: 10.1126/science.1195027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wang C.-Y., Hsieh M.-J., Hsieh I.-C. CLOCK modulates survival and acute lung injury in mice with polymicrobial sepsis. Biochem Biophys Res Commun. 2016;478:935–941. doi: 10.1016/j.bbrc.2016.08.054. [DOI] [PubMed] [Google Scholar]
- 102.Blask D.E., Hill S.M., Dauchy R.T. Circadian regulation of molecular, dietary, and metabolic signaling mechanisms of human breast cancer growth by the nocturnal melatonin signal and the consequences of its disruption by light at night. J Pineal Res. 2011;51:259–269. doi: 10.1111/j.1600-079X.2011.00888.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Li H.-Y., Leu Y.-L., Wu Y.-C., Wang S.-H. Melatonin inhibits in vitro smooth muscle cell inflammation and proliferation and atherosclerosis in apolipoprotein E-deficient mice. J Agric Food Chem. 2019;67:1889–1901. doi: 10.1021/acs.jafc.8b06217. [DOI] [PubMed] [Google Scholar]
- 104.Li H., Li J., Jiang X. Melatonin enhances atherosclerotic plaque stability by inducing prolyl-4-hydroxylase α1 expression. J Hypertens. 2019;37:964–971. doi: 10.1097/HJH.0000000000001979. [DOI] [PubMed] [Google Scholar]
- 105.Morris C.J., Purvis T.E., Hu K., Scheer F.A.J.L. Circadian misalignment increases cardiovascular disease risk factors in humans. Proc Natl Acad Sci U S A. 2016;113:E1402–E1411. doi: 10.1073/pnas.1516953113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rasmiena A.A., Stefanovic N., Huynh K. Attenuation of atherosclerosis in ApoE- and ApoE/GPX1-deficient mice by plasmalogen enrichment. Atherosclerosis. 2015;241:e10. [Google Scholar]
- 107.Armanios M., Alder J.K., Parry E.M., Karim B., Strong M.A., Greider C.W. Short telomeres are sufficient to cause the degenerative defects associated with aging. Am J Hum Genet. 2009;85:823–832. doi: 10.1016/j.ajhg.2009.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bischoff C., Petersen H.C., Graakjaer J. No association between telomere length and survival among the elderly and oldest old. Epidemiology. 2006;17:190–194. doi: 10.1097/01.ede.0000199436.55248.10. [DOI] [PubMed] [Google Scholar]
- 109.World Health Organization . World Health Organization; Geneva, Switzerland: 2015. World Report on Ageing and Health. [Google Scholar]
- 110.Zhan Y., Hägg S. Telomere length and cardiovascular disease risk. Curr Opin Cardiol. 2019;34:270–274. doi: 10.1097/HCO.0000000000000613. [DOI] [PubMed] [Google Scholar]
- 111.Martin-Ruiz C.M., Gussekloo J., van Heemst D., von Zglinicki T., Westendorp R.G.J. Telomere length in white blood cells is not associated with morbidity or mortality in the oldest old: a population-based study. Aging Cell. 2005;4:287–290. doi: 10.1111/j.1474-9726.2005.00171.x. [DOI] [PubMed] [Google Scholar]
- 112.Franzon K., Byberg L., Sjögren P., Zethelius B., Cederholm T., Kilander L. Predictors of independent aging and survival: a 16-year follow-up report in octogenarian men. J Am Geriatr Soc. 2017;65:1953–1960. doi: 10.1111/jgs.14971. [DOI] [PubMed] [Google Scholar]
- 113.Booth S.A., Charchar F.J. Cardiac telomere length in heart development, function, and disease. Physiol Genomics. 2017;49:368–384. doi: 10.1152/physiolgenomics.00024.2017. [DOI] [PubMed] [Google Scholar]