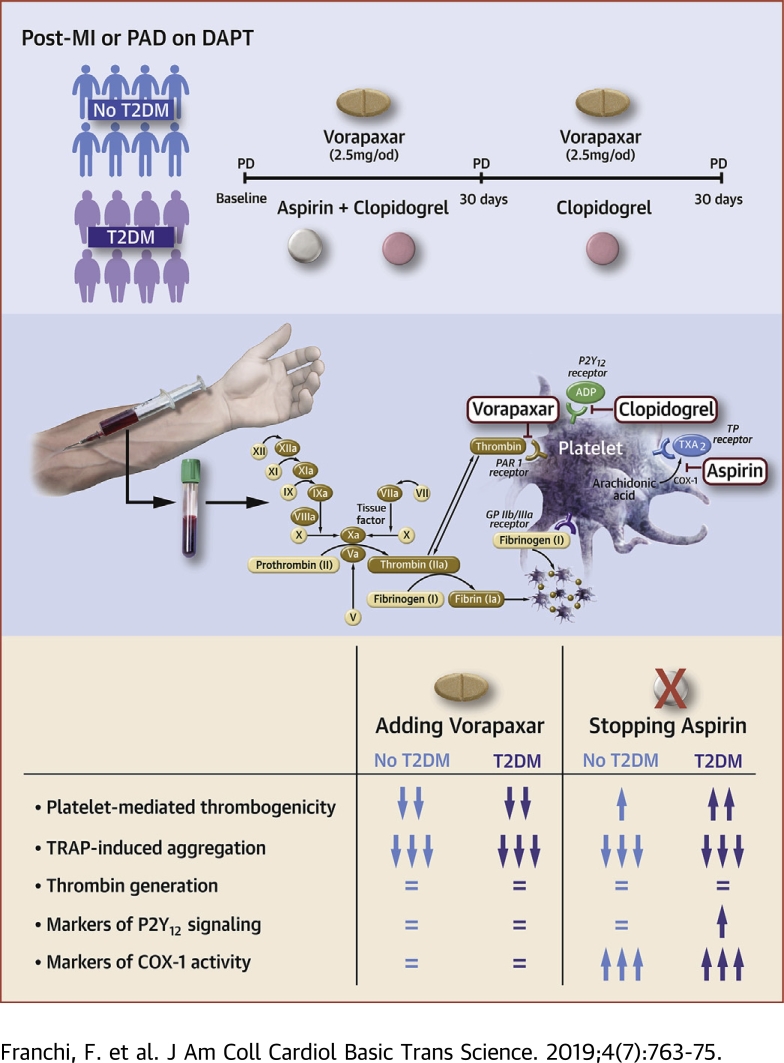
Visual Abstract
Key Words: dual antiplatelet therapy, pharmacodynamics, platelets, thrombin, vorapaxar
Abbreviations and Acronyms: ADP, adenosine diphosphate; CAT, collagen-related peptide + adenosine diphosphate + thrombin receptor activating peptide; CI, confidence interval; COX, cyclooxygenase; DAPT, dual antiplatelet therapy; DM, diabetes mellitus; LTA, light transmittance aggregometry; MI, myocardial infarction; MPA, maximum platelet aggregation; o.d., once daily; PAD, peripheral arterial disease; PAR, protease-activated receptor; PD, pharmacodynamic; TRAP, thrombin receptor activating peptide; TXB2, thromboxane B2; VASP, vasodilator-stimulated phosphoprotein
Highlights
-
•
Vorapaxar reduces thrombotic cardiovascular events in patients with atherosclerotic disease, with enhanced effects in those with DM.
-
•
Adjunctive vorapaxar therapy reduces platelet-mediated thrombogenicity without affecting clot kinetics in both patients with and those without DM having prior MI/PAD on dual antiplatelet therapy with aspirin and clopidogrel.
-
•
The pharmacodynamic effects of vorapaxar occur via selective blockade of the PAR-1 on the platelet membrane without apparent interplay with other platelet signaling pathways.
-
•
Aspirin withdrawal, which leaves patients on a background of clopidogrel and vorapaxar, increases markers specific to COX-1–mediated blockade, leading to an increase in platelet-mediated global thrombogenicity, particularly among patients with DM.
Summary
Vorapaxar reduces thrombotic cardiovascular events at the expense of increased bleeding. However, the differential pharmacodynamic (PD) effects of vorapaxar according to diabetes mellitus (DM) status are unknown. Moreover, although withdrawal of aspirin has emerged as a bleeding reduction strategy, the PD effects of stopping aspirin in patients treated with vorapaxar also are unknown. In this prospective PD investigation, vorapaxar was associated with reduced platelet-mediated thrombogenicity without affecting clot kinetics irrespective of DM status. However, platelet-mediated thrombogenicity increased after aspirin withdrawal, particularly among patients with DM. (Optimizing anti-Platelet Therapy In diabetes MellitUS-5 Study [OPTIMUS-5]; NCT02548650)
Patients with diabetes mellitus (DM) remain at increased risk for recurrent atherothrombotic events despite standard-of-care oral antiplatelet therapy 1, 2. This risk is in part due to their hyperreactive platelet phenotype, which can contribute to inadequate response to oral antiplatelet agents, including dual antiplatelet therapy (DAPT) with aspirin and clopidogrel, which is commonly used for secondary prevention of ischemic recurrences 2, 3, 4. Importantly, platelets of patients with DM are characterized by up-regulation of platelet signaling pathways that are not inhibited by DAPT, including thrombin-mediated signaling (2). Of note, thrombin is the most potent inducer of platelet activation and plays a key role in thrombus formation 5, 6. Hence, modulating the effects of thrombin represents an attractive option to reduce the risk of thrombotic complications, particularly in high-risk patients such as those with DM (7).
Vorapaxar is a novel, orally active, highly selective, competitive, slowly reversible protease-activated receptor (PAR)-1 inhibitor, which exerts potent inhibition of thrombin-mediated platelet aggregation 7, 8, 9. In a large-scale clinical trial, vorapaxar (as adjunct to standard-of-care antiplatelet therapy, mostly aspirin and clopidogrel) significantly reduced recurrent thrombotic events in patients with previous atherothrombosis, particularly those with prior myocardial infarction (MI) or peripheral arterial disease (PAD), albeit at the cost of increased bleeding 10, 11, 12. Notably, the absolute risk reduction of thrombotic complications associated with the adjunctive use of vorapaxar was greater among DM compared with patients without DM (13). These clinical observations in conjunction with the distinctive platelet phenotype of patients with DM make these patients particularly attractive for treatment with vorapaxar. However, the differential pharmacodynamic (PD) effects of vorapaxar in DM compared with patients without DM are unknown. Moreover, despite the proven efficacy of vorapaxar in reducing thrombotic complications, the increased risk of bleeding complications remains of concern. Withdrawal of aspirin when potent adjunctive antithrombotic therapies are used has been suggested as a strategy to reduce the risk of bleeding (14). Reduction of bleeding complications with aspirin withdrawal has been consistently shown among patients undergoing coronary stenting treated with a P2Y12 receptor inhibitor (mostly clopidogrel) and requiring oral anticoagulant therapy (i.e., blockade of circulating thrombin) 15, 16, 17, 18. However, the PD effects associated with a combination of vorapaxar and clopidogrel, without aspirin, is unknown.
Methods
Study design and participants
The OPTIMUS (Optimizing anti-Platelet Therapy In diabetes MellitUS)-5 study (NCT02548650) was a prospective, parallel-design, open-label investigation aimed at assessing the PD effects of adjunctive use of vorapaxar in patients with a history of MI or PAD, with and without type 2 DM, on treatment with DAPT (aspirin and clopidogrel) and assessing the PD impact associated with discontinuation of aspirin therapy. The study was conducted in patients with a history of MI or PAD in line with the approved indication for use of vorapaxar 19, 20. In particular, vorapaxar is approved by the United States Food and Drug Administration and the European Medicines Agency for the reduction of thrombotic cardiovascular events in patients with a history of MI or PAD and is required to be used in addition to standard-of-care antiplatelet therapy with aspirin and/or clopidogrel 20, 21. The study was performed at the University of Florida Health–Jacksonville (Jacksonville, Florida). Patients with a history of MI or PAD older than 18 years on DAPT with aspirin and clopidogrel as part of their standard of care for at least 2 weeks were screened for study eligibility at the outpatient cardiology clinics of our institution (see Supplemental Material for details on study inclusion and exclusion criteria). The study complied with the Declaration of Helsinki and was approved by the Western Institutional Review Board. All patients gave written informed consent.
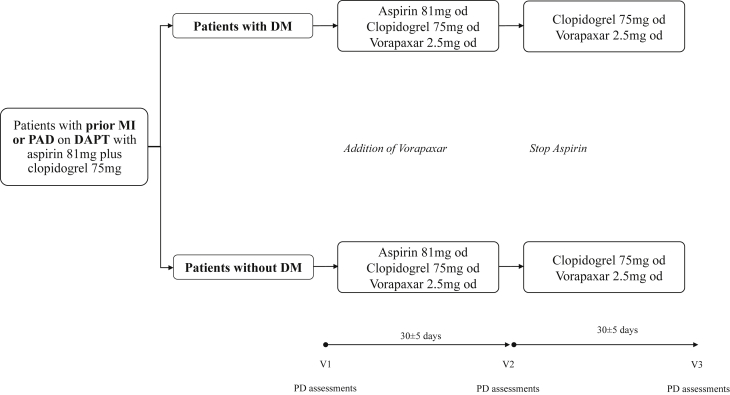
Patients on DAPT with aspirin and clopidogrel who met study entry criteria were divided into 2 cohorts according to the presence or absence of type 2 DM. Type 2 DM status was defined according to the World Heart Organization criteria, and patients needed to be on treatment with oral hypoglycemic agents and/or insulin for at least 2 months, without any changes in regimen (22). Vorapaxar (2.5 mg once daily [o.d.]) was added to the standard DAPT regimen of aspirin (81 mg o.d.) plus clopidogrel (75 mg o.d.), also known as triple therapy. Triple therapy was maintained for 30 ± 5 days. Patients then stopped taking aspirin and maintained dual therapy with vorapaxar (2.5 mg o.d.) plus clopidogrel (75 mg o.d.) for 30 ± 5 days. Blood sampling for PD testing was conducted at 3 time points: baseline (while patients were on standard DAPT); after 30 ± 5 days of triple therapy; and 30 ± 5 days after dual therapy. At each time point, blood was collected before the morning dose of clopidogrel and vorapaxar, in order to measure trough levels of platelet inhibition. Laboratory personnel were blinded to treatment assignments. Compliance to randomized treatment was assessed by pill count and patient interview. After completing the study, patients resumed their standard DAPT regimen. A flow diagram of the study design is illustrated in Figure 1.
Figure 1.
Study Design
DAPT = dual antiplatelet therapy; DM = diabetes mellitus; MI = myocardial infarction; od = once daily; PAD = peripheral arterial disease; PD = pharmacodynamics.
Blood sampling and laboratory assessments
Peripheral venous blood samples were drawn through a short venous catheter inserted into a forearm vein and collected in citrate, EDTA, and serum tubes as appropriate for assessments. The first 2 to 4 ml of blood was discarded to avoid spontaneous platelet activation. Blood sampling for PD assessments was performed at 3 time points as indicated in the study design section. Multiple assays were used, including light transmittance aggregometry (LTA); whole blood vasodilator-stimulated phosphoprotein (VASP); TEG 6s (Haemonetics Corp., Braintree, Massachusetts) thrombelastograph coagulation analyzer, which also included the platelet mapping assay using adenosine diphosphate (ADP); and enzyme-linked immunosorbent assay–based assessment of serum thromboxane B2 (TXB2) 23, 24, 25, 26. A detailed description of the assays is provided in the Supplemental Material. Assessments were performed and described with the following objectives: 1) to define the PD effect of vorapaxar on thrombin-mediated effects on platelets and systemically; to this extent, LTA following thrombin receptor activating peptide (TRAP) (15 μM) stimuli and markers of clot kinetics using the TEG 6s system were utilized, respectively; 2) to define the PD effect of vorapaxar on platelet-mediated thrombogenicity; to this extent, LTA following stimuli with combination of 2 μg/ml collagen-related peptide + 5 μM ADP + 15 μM TRAP (CAT) was used; and 3) to define the PD effect of vorapaxar on P2Y12 inhibition induced by clopidogrel and the impact of aspirin withdrawal; to this extent LTA following stimuli with ADP (20 μM) and VASP as well as markers sensitive to cyclooxygenase (COX)-1 blockade, including LTA following arachidonic acid (1 mM) and collagen (3 μg/ml) stimuli, and measurement of serum TXB2 levels, respectively, were assessed. LTA results are reported as maximum platelet aggregation (MPA%), VASP results as platelet reactivity index, and serum TXB2 levels in ng/ml.
Study endpoints and sample size calculation
The primary endpoint of our study was the comparison of CAT-induced MPA measured by LTA between triple (vorapaxar plus DAPT) and dual (vorapaxar plus clopidogrel) therapy. The rationale for choosing CAT-induced MPA for the primary endpoint was that this combination of agonist is more reflective of global thrombogenicity as it stimulates multiple platelet signaling pathways. We hypothesized that dual therapy would be noninferior to triple therapy after 30 ± 5 days of treatment in both patients with and without DM. Under the null hypothesis that the mean CAT-induced MPA between dual and triple therapy is not equal to 0 and a common standard deviation of 13%, a sample size of 28 patients per group with a valid primary endpoint time point allowed for the 95% confidence interval (CI) to stay within ±10% with 80% power and 2-sided α = 0.05. Considering the 2 groups (patients with and without DM), a total of 56 patients with valid primary endpoint data needed to be included. Assuming up to 40% rate of invalid results due to hemolysis or dropout, we estimated that up to 79 patients would need to be enrolled. Noninferiority was assessed using a 95% CI of the difference in mean MPA between the 2 arms. As there were no preliminary data in this setting, the 10% noninferiority margin was arbitrarily defined. Mean values of platelet aggregation and variability were estimated based on previous data of vorapaxar (26). Our approach for the statistical assumption is in agreement with recommendations for pilot investigations (27).
Other exploratory objectives included comparisons between patients with and without DM of all PD parameters measured by multiple assays, and comparisons between levels of platelet inhibition achieved by DAPT (baseline therapy) versus levels achieved by adding vorapaxar. The effects of additive inhibition of the thrombin-mediated platelet activation pathway, with or without aspirin therapy, on serum thromboxane levels also were evaluated.
Statistical analysis
Categorical variables are expressed as frequencies and proportions. Continuous variables were analyzed for normal distribution with the Kolmogorov-Smirnov test and are expressed as mean ± SD. Comparisons between categorical variables were performed using the 2-tailed Fisher exact test or the Pearson chi-square test. The Student's t-test was used to compare continuous variables. A full-factorial repeated measure analysis of variance method for dependent variables with a general linear model was used to evaluate intragroup comparisons between time points (3). Least square mean differences in MPA between groups and the corresponding 2-sided 95% CI for the difference were obtained to assess noninferiority based on the linear model. The Student's t-test was used to compare continuous variables between groups. Platelet reactivity results are reported as mean ± SD for the detailed analyses. p Values are used to report superiority testing, and CIs are used to determine noninferiority. A 2-tailed p < 0.05 is considered to indicate a statistically significant difference for all the analyses performed. Given the exploratory nature of superiority intragroup comparisons, adjustment for multiple comparisons was not performed. Statistical analysis was performed using SPSS version 25.0 software (SPSS Inc., Chicago, Illinois).
The safety population was composed of all patients exposed to at least 1 dose of study medication (any time from enrollment until completion of the study). Any adverse event during the study period was recorded. The PD population included all patients with PD data and without a major protocol deviation thought to affect the PD effects of vorapaxar, aspirin, and clopidogrel. The PD population was used for analysis of all primary and secondary PD variables.
Results
Patient population
Between March 25, 2016 and October 22, 2018, 132 patients were screened. A total of 71 patients on maintenance therapy agreed to participate in the study; 5 patients were not eligible for randomization due to the presence of an exclusion criteria. A total of 66 patients (30 with DM, 36 without DM) were exposed to at least 1 dose of study medication, representing the safety population. Two patients were not compliant with medications and therefore were excluded from the PD analysis. Thus, a total of 64 patients (30 with DM, 34 without DM) represented the PD population of the study. Of these patients, 56 (28 with DM, 28 without DM) had valid primary endpoint data. Prior MI and PAD were the enrollment criteria for 41 (64%) and 23 (36%) patients, respectively. Baseline characteristics of the study population are summarized in Table 1. Patients with DM were older and had higher rates of PAD, hypertension, and hyperlipidemia. No ischemic or Bleeding Academic Research Consortium type 2 to 5 bleeding events were observed. One DM patient had a Bleeding Academic Research Consortium type 1 bleeding (hematuria) while on triple therapy that led to study drug discontinuation, and 11 patients (7 with DM, 4 without DM) had nonbleeding adverse events (see Supplemental Material for details).
Table 1.
Baseline Characteristics of the PD Population
| DM (n = 30) | Non-DM (n = 34) | p Value | |
|---|---|---|---|
| Age, yrs | 61 ± 8 | 56 ± 9 | 0.015 |
| Male | 23 (77) | 23 (67) | 0.579 |
| BMI, kg/m2 | 31 ± 5 | 32 ± 8 | 0.757 |
| Race | 0.487 | ||
| White | 15 (50) | 22 (65) | |
| Black | 14 (47) | 11 (32) | |
| Other | 1 (3) | 1 (3) | |
| Enrollment criteria∗ | 0.002 | ||
| Prior MI | 13 (43) | 28 (82) | |
| PAD | 17 (57) | 6 (18) | |
| CKD | 2 (7) | 2 (6) | 1 |
| Hypertension | 29 (97) | 25 (74) | 0.015 |
| Dyslipidemia | 27 (90) | 22 (65) | 0.020 |
| Active smoking | 10 (33) | 14 (41) | 0.408 |
| CAD | 22 (73) | 30 (88) | 0.199 |
| Prior PCI | 20 (67) | 25 (73) | 0.593 |
| Prior CABG | 7 (23) | 4 (12) | 0.322 |
| Creatinine, mg/dl | 1.1 ± 0.3 | 0.9 ± 0.2 | 0.073 |
| CrCl, ml/min | 102 ± 40 | 117 ± 58 | 0.232 |
| Platelet count, 103/μl | 249 ± 62 | 227 ± 59 | 0.160 |
| Hematocrit, % | 39 ± 4 | 41 ± 4 | 0.182 |
| Hemoglobin, g/dl | 12.9 ± 1.3 | 13.5 ± 1.6 | 0.145 |
| Medications | |||
| Insulin therapy | 16 (53) | 0 (0) | <0.001 |
| OAD | 23 (77) | 0 (0) | <0.001 |
| Beta-blockers | 27 (90) | 30 (88) | 1 |
| ACE inhibitor/ARB | 25 (83) | 21 (62) | 0.093 |
| Statins | 30 (100) | 32 (94) | 0.494 |
Values are mean ± SD or n (%).
ACE = angiotensin-converting enzyme; ARB = angiotensin receptor blocker; BMI = body mass index; CABG = coronary artery bypass graft; CAD = coronary artery disease; CKD = chronic kidney disease; CrCl = creatinine clearance; DM = diabetes mellitus; MI = myocardial infarction; OAD = oral antidiabetic drug; PAD = peripheral arterial disease; PCI = percutaneous coronary intervention; PD = pharmacodynamic.
2 patients categorized as MI also had PAD (see Supplemental Material for definition of study entry criteria).
PD Findings
Effects of vorapaxar on thrombin-mediated effects
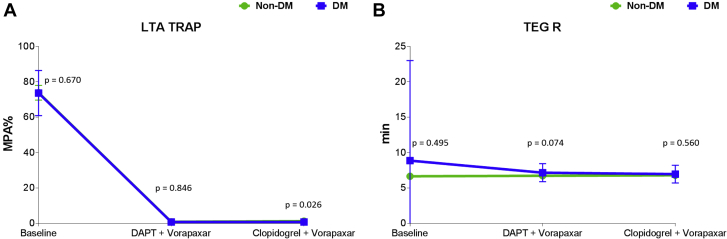
Adjunctive treatment with vorapaxar was associated with complete blockade of TRAP-induced platelet aggregation (p < 0.001). Complete suppression of TRAP-induced platelet aggregation persisted after discontinuation of aspirin therapy (p < 0.001). Overall, such effects were consistent irrespective of DM status (Figure 2A). On the contrary, vorapaxar did not affect markers of clot kinetics, including speed of thrombin generation, which remained unvaried even after discontinuation of aspirin therapy (Figure 2B). Findings were consistent irrespective of DM status (Supplemental Figures 1 to 4). These observations are supportive of the platelet-specific, and not systemic, effects of vorapaxar on modulating thrombin-mediated effects.
Figure 2.
Thrombin-Mediated Effects on Platelets and Systemically
(A) Thrombin receptor activating peptide (TRAP)-induced maximal platelet aggregation (MPA%) measured by light transmittance aggregometry (LTA). The p values represent comparisons between the 2 groups at each time point. Error bars indicate SD. Intragroup comparisons for non-DM: Baseline versus DAPT + vorapaxar: mean difference = 72; 95% confidence interval (CI): 67 to 77; p < 0.001. Baseline versus clopidogrel + vorapaxar: mean difference = 72; 95% CI: 67 to 77; p < 0.001. DAPT + vorapaxar versus clopidogrel + vorapaxar: mean difference = 0.3; 95% CI: −1.6 to 0.72; p = 0.20. Intragroup comparisons for DM: Baseline versus DAPT + vorapaxar: mean difference = 73; 95% CI: 68 to 78; p < 0.001. Baseline versus clopidogrel + vorapaxar: mean difference = 73; 95% CI: 68 to 78; p < 0.001. DAPT + vorapaxar versus clopidogrel + vorapaxar: mean difference = 0; 95% CI: −0.35 to 0.35; p = 1.00. (B) Reaction time (R) measured by thromboelastography (TEG) using kaolin as agonist. The p values represent comparisons between the 2 groups at each time point. Error bars indicate SD. Intragroup comparisons for non-DM: Baseline versus DAPT + vorapaxar: p = 0.736. Baseline versus clopidogrel + vorapaxar: p = 0.829. DAPT + vorapaxar versus clopidogrel + vorapaxar: p = 0.886. Intragroup comparisons for DM: Baseline versus DAPT + vorapaxar: p = 0.436. Baseline versus clopidogrel + vorapaxar: p = 0.416. DAPT + vorapaxar versus clopidogrel + vorapaxar: p = 0.412. Abbreviations as in Figure 1.
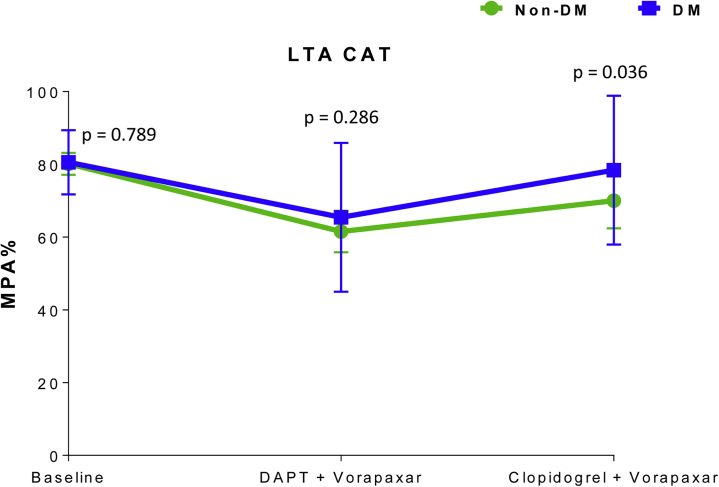
Effects of vorapaxar on global thrombogenicity
Adding vorapaxar to DAPT significantly reduced CAT-induced aggregation in both patients with DM (mean difference = 15; 95% CI: 7 to 23; p < 0.001) and patients without DM (mean difference = 20; 95% CI: 15 to 25; p < 0.001). However, stopping aspirin was associated with an increase in CAT-induced aggregation in both patients with DM (mean difference = 12; 95% CI: 3 to 21; p = 0.010) and patients without DM (mean difference =10; 95% CI: 4 to 16; p = 0.003), thus not meeting the primary endpoint of noninferiority (Figure 3). After aspirin withdrawal, CAT-induced aggregation was significantly lower compared with baseline in patients without DM (mean difference = 10; 95% CI: 3 to 16; p < 0.001) but not in DM (mean difference = 3; 95% CI; -6 to 11; p = 0.542) patients. Overall, the magnitude of increase in CAT-induced aggregation after aspirin withdrawal in the presence of vorapaxar and clopidogrel therapy was higher in DM patients compared with patients without DM (p = 0.036) (Figure 3).
Figure 3.
Markers of Platelet-Mediated Thrombogenicity
CAT-induced maximal platelet aggregation (MPA%) measured by light transmittance aggregometry (LTA). The p values represent comparisons between the 2 groups at each time point. Error bars indicate SD. The agonist CAT is a combination of collagen-related peptide, adenosine diphosphate, and thrombin receptor activating peptide. Intragroup comparisons for non-DM: Baseline versus DAPT + vorapaxar: mean difference = 20; 95% CI: 15 to 25; p < 0.001. Baseline versus clopidogrel + vorapaxar: mean difference = 10; 95% CI: 3 to 16; p < 0.001. DAPT + vorapaxar versus clopidogrel + vorapaxar: mean difference = 10; 95% CI: 4 to 16; p = 0.003. Intragroup comparisons for DM: Baseline versus DAPT + vorapaxar: mean difference = 15; 95% CI: 7 to 23; p < 0.001. Baseline versus clopidogrel + vorapaxar: mean difference = 3; 95% CI: −6 to 11; p = 0.542. DAPT + vorapaxar versus clopidogrel + vorapaxar: mean difference = 12; 95% CI: 3 to 21; p = 0.010. Abbreviations as in Figure 1.
Effects of vorapaxar on modulating P2Y12 inhibition induced by clopidogrel in the presence and absence of aspirin
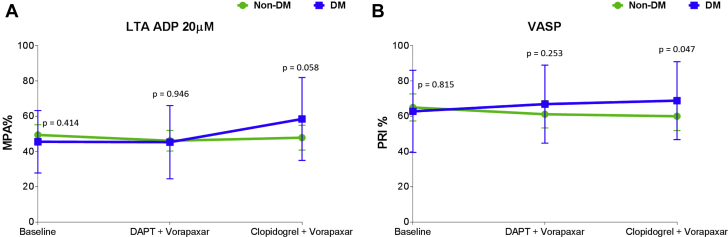
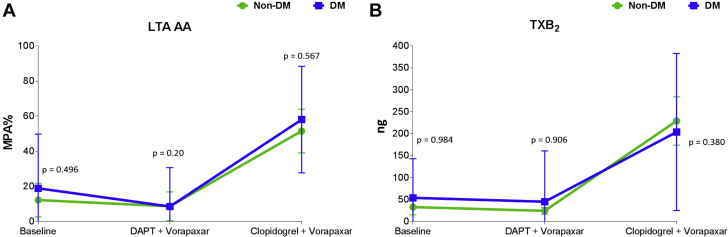
Adding vorapaxar to DAPT did not affect markers assessing P2Y12 inhibition using all assays, including LTA and VASP. After aspirin withdrawal, there were no significant differences in levels of these markers among patients without DM, but these levels modestly increased in patients with DM (Figure 4, Supplemental Figure 5). Aspirin withdrawal was associated with a marked increase in makers sensitive to COX-1 blockade, including arachidonic acid– and collagen-induced aggregation as well as serum TXB2 levels, in both patients with and without DM (Figure 5, Supplemental Figure 6).
Figure 4.
Markers of P2Y12 Signaling
(A) Adenosine diphosphate (ADP)-induced maximal platelet aggregation (MPA%) measured by light transmittance aggregometry (LTA). The p values represent comparisons between the 2 groups at each time point. Error bars indicate SD. Intragroup comparisons for non-DM: Baseline versus DAPT + vorapaxar: p = 0.166. Baseline versus clopidogrel + vorapaxar: p = 0.811. DAPT + vorapaxar versus clopidogrel + vorapaxar: p = 0.269. Intragroup comparisons for DM: Baseline versus DAPT + vorapaxar: p = 0.837. Baseline versus clopidogrel + vorapaxar: mean difference = 12; 95% CI: 5 to 20; p = 0.002. DAPT + vorapaxar versus clopidogrel + vorapaxar: mean difference = 13; 95% CI: 5 to 20; p = 0.002. (B) Platelet reactivity index (PRI) measured by vasodilator-stimulated phosphoprotein (VASP). The p values represent comparisons between the 2 groups at each time point. Error bars indicate SD. Intragroup comparisons for non-DM: Baseline versus DAPT + vorapaxar: p = 0.063. Baseline versus clopidogrel + vorapaxar: p = 0.067. DAPT + vorapaxar versus clopidogrel + vorapaxar: p = 0.959. Intragroup comparisons for DM: Baseline versus DAPT + vorapaxar: p = 0.296. Baseline versus clopidogrel + vorapaxar: p = 0.142. DAPT + vorapaxar versus clopidogrel + vorapaxar: p = 0.398. Abbreviations as in Figure 1.
Figure 5.
Markers Sensitive to Cyclooxygenase-1 Blockade
(A) Arachidonic acid (AA)-induced maximal platelet aggregation (MPA%) measured by light transmittance aggregometry (LTA). The p values represent comparisons between the 2 groups at each time point. Error bars indicate SD. Intragroup comparisons for non-DM: Baseline versus DAPT + vorapaxar: mean difference = 2; 95% CI: −10 to 15; p = 0.734. Baseline versus clopidogrel + vorapaxar: mean difference = 42; 95% CI: 26 to 58; p < 0.001. DAPT + vorapaxar versus clopidogrel + vorapaxar: mean difference = 44; 95% CI: 28 to 60; p < 0.001. Intragroup comparisons for DM: Baseline versus DAPT + vorapaxar: mean difference = 9; 95% CI: −2 to 21; p = 0.108. Baseline versus clopidogrel + vorapaxar: mean difference = 40; 95% CI: 25 to 54; p < 0.001. DAPT + vorapaxar versus clopidogrel + vorapaxar: mean difference = 49; 95% CI: 36 to 63; p < 0.001. (B) Serum thromboxane B2 (TXB2). The p values represent comparisons between the 2 groups at each time point. Error bars indicate SD. Intragroup comparisons for non-DM: Baseline versus DAPT + vorapaxar: p = 0.545. Baseline versus clopidogrel + vorapaxar: p < 0.001. DAPT + vorapaxar versus clopidogrel + vorapaxar: p < 0.001. Intragroup comparisons for DM: Baseline versus DAPT + vorapaxar: p = 0.671. Baseline versus clopidogrel + vorapaxar: p < 0.001. DAPT + vorapaxar versus clopidogrel + vorapaxar: p < 0.001. Abbreviations as in Figure 1.
Discussion
Vorapaxar is a PAR-1 inhibitor clinically approved for reduction of thrombotic cardiovascular events in patients with a history of MI or PAD treated with standard-of-care antiplatelet therapy with aspirin and/or clopidogrel 19, 20, 21. The OPTIMUS-5 study was conducted to provide insights on the PD effects of vorapaxar in this setting, particularly exploring profiles among patients with and without DM treated with DAPT (aspirin and clopidogrel), which to date have not been explored. Moreover, in light of the emerging interest in an aspirin-free approach as a strategy to reduce the risk of bleeding complications in patients treated with more potent antithrombotic therapies, this investigation also explored the PD effects associated with aspirin withdrawal in the presence of vorapaxar and clopidogrel therapy. The results of OPTIMUS-5 can be summarized as follows: 1) adjunctive treatment with vorapaxar reduces platelet-mediated thrombogenicity without affecting clot kinetics in both patients with and without DM treated with DAPT (aspirin and clopidogrel); 2) reduction of platelet-mediated thrombogenicity induced by vorapaxar occurs selectively via PAR-1 blockade without apparent interplay with other platelet signaling pathways (e.g., P2Y12 or thromboxane); and 3) platelet-mediated thrombogenicity is increased after aspirin withdrawal, particularly among patients with DM.
The TRA 2°P–TIMI 50 (Thrombin Receptor Antagonist in Secondary Prevention of Atherothrombotic Ischemic Events–Thrombolysis In Myocardial Infarction 50) trial, conducted in patients with previous atherothrombosis, demonstrated that, when added to standard-of-care treatment, including antiplatelet therapy with aspirin and clopidogrel, vorapaxar significantly reduces recurrent thrombotic events (10). This benefit was limited to patients with a history of prior MI or PAD but not to patients with a prior cerebrovascular event, in whom vorapaxar was associated with increased harm (i.e., increased rates of intracranial hemorrhage) 10, 11, 12. Notably, in the cohort of patients with DM and prior MI included in the TRA 2°P trial, vorapaxar reduced the primary composite endpoint at 3 years by 27% (p = 0.002) and led to a greater absolute risk reduction (absolute risk difference: −3.50%) compared with those without DM (absolute risk difference: −1.36%), with a number needed to treat of 29 (13). The benefit of vorapaxar also was consistent in patients with PAD, although no specific subgroup data according to DM status were available 12, 13. These observations make patients with DM an attractive population for treatment with vorapaxar. The reason for the enhanced benefit of vorapaxar among patients with DM has been hypothesized to be attributed to an up-regulated status of thrombin-mediated platelet activation, which makes these patients more susceptible to the antithrombotic effects of the drug (13). However, in our investigation we found that vorapaxar completely abolished TRAP-induced aggregation irrespective of DM status. In addition, the effects on global thrombogenicity were similar in patients with and without DM. Thus, it is more likely that the clinical observations from TRA 2°P–TIMI 50 can be attributed to the greater baseline risk of patients with DM, which allows for a greater magnitude of treatment effects with vorapaxar. This observation is consistent with other secondary prevention studies in patients with DM using potent antiplatelet therapies (28).
The increased risk of bleeding complications with vorapaxar remains a clinical concern. PD investigations have suggested that in the presence of potent P2Y12 blockade, aspirin provides limited adjunctive antithrombotic effects 14, 29, 30. Although the GLOBAL LEADERS trial, which tested a strategy of ticagrelor monotherapy after only 1 month of DAPT versus standard DAPT in a large all-comers population undergoing percutaneous coronary intervention, failed to meet its primary endpoint for superior efficacy of aspirin withdrawal, such an experimental strategy was not associated with any safety concerns (31). Several ongoing investigations are evaluating the safety and efficacy of dropping aspirin in the presence of potent P2Y12 receptor blockade in patients undergoing percutaneous coronary intervention 14, 32. However, a number of other studies, mostly conducted in patients with atrial fibrillation undergoing coronary stenting and requiring treatment with both oral anticoagulant (OAC) and antiplatelet therapy, have shown that stopping aspirin and maintaining treatment with OAC and a P2Y12 inhibitor (mostly clopidogrel) significantly reduced bleeding complications without any apparent trade-off in ischemic events 15, 16, 17, 18, 33. Accordingly, current recommendations are to minimize the duration of DAPT in patients also taking OAC 34, 35. OPTIMUS-5 also explored the effects of dropping aspirin in the setting of a platelet-specific, rather than systemic, modulation of thrombin-mediated effects. We showed that stopping aspirin was associated with an increase in markers specific to COX-1–mediated blockade leading to an increase in platelet-mediated global thrombogenicity. Importantly, the magnitude of increase was higher in patients with DM compared with patients without DM, and patients with DM achieved levels of global thrombogenicity while on vorapaxar and clopidogrel that were similar to those while on aspirin and clopidogrel.
Our finding that markers specifically assessing COX-1 blockade increase with aspirin withdrawal provides support that alternative antithrombotic treatment regimens cannot replace the selective effects of aspirin on platelet COX-1 blockade. The increase in platelet-mediated global thrombogenicity could also be attributed to the loss of synergism that is known to occur when aspirin and clopidogrel are concomitantly used (36). Such synergism may be less relevant in the presence of more potent P2Y12 blockade 29, 30. It may be hypothesized that the enhanced increase in platelet-mediated thrombogenicity among patients with DM may be attributed to the fact that these patients are more susceptible to such loss of synergism as reflected by the increase in markers of P2Y12 signaling. Overall, these findings should caution against strategies of aspirin withdrawal in the absence of effective alternative antithrombotic treatment (14). Indeed, our PD observations question any potential clinical advantage of dual therapy with vorapaxar and clopidogrel compared with standard-of-care DAPT with aspirin and clopidogrel, especially in patients with DM. The use of OAC, which provides a block of systemic levels of thrombin, may be more effective in this regard, as modulation of circulating thrombin can also indirectly affect platelet reactivity (37). Thus, this approach would result in more wide-ranging antithrombotic effects compared with vorapaxar, which only selectively inhibits the effects of thrombin on platelets via selective PAR-1 blockade (7). The benefits of the antithrombotic efficacy of an OAC in addition to a single antiplatelet agent (e.g., aspirin) was recently supported by a large-scale secondary prevention study conducted in patients with CAD and PAD, which showed a significant reduction in ischemic recurrences and reduced cardiovascular mortality associated with a very low dosing regimen of rivaroxaban in adjunct to aspirin (38).
Despite the known interplay between thrombin- and P2Y12-mediated signaling, adjunctive treatment with vorapaxar does not interfere with markers of P2Y12 receptor blockade (39). This is consistent with other studies modulating systemic levels of thrombin with oral anticoagulant therapies 40, 41, 42. These findings may be attributed to the fact that these patients already are on P2Y12-inhibiting therapy, which may not allow unraveling such interplay as previously observed in in vitro investigations and in platelets from nonmedicated patients 43, 44, 45.
Study limitations
Our study was not designed to assess clinical outcomes. Moreover, our study was conducted in patients treated with aspirin and clopidogrel, and it may be argued that many patients with a history of MI are currently treated with more potent P2Y12 inhibitors (prasugrel or ticagrelor). However, in the TRA 2°P study, clopidogrel was the P2Y12 inhibitor utilized in 99.3% of patients, and the approved indication for vorapaxar was based on this background antiplatelet therapy. Accordingly, our PD study was conducted to mimic how vorapaxar was approved for clinical use based on the TRA 2°P trial 10, 20, 21. Moreover, clopidogrel is still the most frequently used P2Y12 inhibitor and is the only agent of this class approved for treatment of PAD (46). If similar findings would have been observed in patients on DAPT with aspirin and prasugrel or ticagrelor is unknown. The PD effects associated with the addition of vorapaxar to patients with a history of MI, treated with potent P2Y12 inhibitors (prasugrel or ticagrelor) and the effects associated with aspirin withdrawal in these patients are currently under investigation (NCT02545933).
Conclusions
Adjunctive treatment with vorapaxar reduces platelet-mediated thrombogenicity without affecting clot kinetics in both patients with DM and patients without DM, while on DAPT. However, platelet-mediated thrombogenicity is increased after aspirin withdrawal, particularly among patients with DM. These PD observations do not support any potential clinical advantage of dual therapy with vorapaxar and clopidogrel compared with standard-of-care DAPT with aspirin and clopidogrel, especially in patients with DM. The PD findings of this study suggest that the enhanced clinical benefit among patients with DM associated with adjunctive treatment with vorapaxar in addition to standard-of-care oral antiplatelet therapy, including aspirin and clopidogrel, is more likely due to the greater baseline risk profile of these patients, which allows for a greater magnitude of treatment effects, rather than a differential effect on platelets from patients with DM compared to patients without DM.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: Vorapaxar is a PAR-1 inhibitor clinically approved for the reduction of thrombotic cardiovascular events in patients with a history of MI or PAD treated with standard-of-care antiplatelet therapy with aspirin and/or clopidogrel. This study provides evidence that adjunctive treatment with vorapaxar in addition to DAPT reduces platelet-mediated thrombogenicity without affecting clot kinetics in both patients and patients without DM. Notably, this reduction occurs selectively via PAR-1 blockade without apparent interplay with other platelet signaling pathways, and platelet-mediated thrombogenicity is increased after aspirin withdrawal, particularly among patients with DM. This has clinical implications as it demonstrates that the enhanced benefit of vorapaxar shown in clinical trials among patients with DM is likely just attributed to the greater baseline risk of patients with DM, thus underscoring the need for more aggressive treatment of these patients. In addition, the study shows that alternative antithrombotic treatment regimens cannot replace the selective effects of aspirin on platelet COX-1 blockade and cautions against strategies of aspirin withdrawal in the absence of effective alternative antithrombotic treatment.
TRANSLATIONAL OUTLOOK: Our study was conducted in patients treated with aspirin and clopidogrel, and it may be argued that many patients with a history of MI are currently treated with the more potent P2Y12 inhibitors prasugrel and ticagrelor. The PD effects associated with the addition of vorapaxar to treatment with prasugrel or ticagrelor and the effects associated with aspirin withdrawal in these patients need to be investigated in specifically designed studies. Use of direct oral anticoagulants, which inhibit systemic thrombin, may be more effective in reducing platelet-mediated global thrombogenicity, as modulation of circulating thrombin (rather than just its selective effect on the platelet PAR-1 receptor) can also indirectly affect platelet reactivity and result in more wide-ranging antithrombotic effects. The association between PD findings and clinical outcomes in patients with vascular disease manifestations treated with strategies that modulate the effects of thrombin warrants further investigation.
Footnotes
The present study was investigator-initiated. The study was originally funded by a grant from Merck until transfer of marketing rights of vorapaxar, including grant responsibilities, to Aralez. At the time of transfer, Merck had funded 60% of overall grant costs. Following such transfer, Aralez filed for bankruptcy and did not cover any of the residual grant costs, which was completed using research funds from the Division of Cardiology, University of Florida College of Medicine–Jacksonville. Upon completion of the study, Deerfield acquired marketing rights of vorapaxar and provided a nominal fee for the final phases of investigator-initiated research studies conducted with vorapaxar at our institution (NCT02548650 and NCT02545933). Dr. Franchi has received payment as an individual for consulting fees or honoraria from AstraZeneca and Sanofi. Dr. Rollini has received payment as an individual for consulting fees or honoraria from Chiesi. Dr. Angiolillo has received payment as an individual for consulting fees or honoraria from Amgen, Aralez, AstraZeneca, Bayer, Biosensors, Boehringer Ingelheim, Bristol-Myers Squibb, Chiesi, Daiichi-Sankyo, Eli Lilly, Haemonetics, Janssen, Merck, PhaseBio, PLx Pharma, Pfizer, Sanofi, and The Medicines Company; participation in review activities from CeloNova and St. Jude Medical; and institutional payments for grants from Amgen, AstraZeneca, Bayer, Biosensors, CeloNova, CSL Behring, Daiichi-Sankyo, Eisai, Eli Lilly, Gilead, Idorsia, Janssen, Matsutani Chemical Industry Co., Merck, Novartis, Osprey Medical, and Renal Guard Solutions. Dr. Jennings has received payments as an individual for consulting fees or honoraria from Bayer, Janssen, PhaseBio, and Portola; and institutional payments for grants from Janssen and In Motion Musculoskeletal Institute. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Basic to Translational Scienceauthor instructions page.
Appendix
For an expanded Methods section as well as supplemental figures and references, please see the online version of this paper.
Appendix
References
- 1.Donahoe S.M., Stewart G.C., McCabe C.H. Diabetes and mortality following acute coronary syndromes. JAMA. 2007;298:765–775. doi: 10.1001/jama.298.7.765. [DOI] [PubMed] [Google Scholar]
- 2.Ferreiro J.L., Angiolillo D.J. Diabetes and antiplatelet therapy in acute coronary syndrome. Circulation. 2011;123:798–813. doi: 10.1161/CIRCULATIONAHA.109.913376. [DOI] [PubMed] [Google Scholar]
- 3.Angiolillo D.J., Jakubowski J.A., Ferreiro J.L. Impaired responsiveness to the platelet P2Y12 receptor antagonist clopidogrel in patients with type 2 diabetes and coronary artery disease. J Am Coll Cardiol. 2014;64:1005–1014. doi: 10.1016/j.jacc.2014.06.1170. [DOI] [PubMed] [Google Scholar]
- 4.Angiolillo D.J., Fernandez-Ortiz A., Bernardo E. Platelet function profiles in patients with type 2 diabetes and coronary artery disease on combined aspirin and clopidogrel treatment. Diabetes. 2005;54:2430–2435. doi: 10.2337/diabetes.54.8.2430. [DOI] [PubMed] [Google Scholar]
- 5.Davì G., Patrono C. Platelet activation and atherothrombosis. N Engl J Med. 2007;357:2482–2494. doi: 10.1056/NEJMra071014. [DOI] [PubMed] [Google Scholar]
- 6.Angiolillo D.J., Ueno M., Goto S. Basic principles of platelet biology and clinical implications. Circ J. 2010;74:597–607. doi: 10.1253/circj.cj-09-0982. [DOI] [PubMed] [Google Scholar]
- 7.Angiolillo D.J., Capodanno D., Goto S. Platelet thrombin receptor antagonism and atherothrombosis. Eur Heart J. 2010;31:17–28. doi: 10.1093/eurheartj/ehp504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Franchi F., Angiolillo D.J. Novel antiplatelet agents in acute coronary syndrome. Nat Rev Cardiol. 2015;12:30–47. doi: 10.1038/nrcardio.2014.156. [DOI] [PubMed] [Google Scholar]
- 9.Franchi F., Rollini F., Park Y., Angiolillo D.J. Platelet thrombin receptor antagonism with vorapaxar: pharmacology and clinical trial development. Future Cardiol. 2015;11:547–564. doi: 10.2217/fca.15.50. [DOI] [PubMed] [Google Scholar]
- 10.Morrow D.A., Braunwald E., Bonaca M.P. Vorapaxar in the secondary prevention of atherothrombotic events. N Engl J Med. 2012;366:1404–1413. doi: 10.1056/NEJMoa1200933. [DOI] [PubMed] [Google Scholar]
- 11.Scirica B.M., Bonaca M.P., Braunwald E. Vorapaxar for secondary prevention of thrombotic events for patients with previous myocardial infarction: a prespecified subgroup analysis of the TRA 2°P-TIMI 50 trial. Lancet. 2012;380:1317–1324. doi: 10.1016/S0140-6736(12)61269-0. [DOI] [PubMed] [Google Scholar]
- 12.Bonaca M.P., Scirica B.M., Creager M.A. Vorapaxar in patients with peripheral artery disease: results from TRA2°P-TIMI 50. Circulation. 2013;127:1522–1529. doi: 10.1161/CIRCULATIONAHA.112.000679. [DOI] [PubMed] [Google Scholar]
- 13.Cavender M.A., Scirica B.M., Bonaca M.P. Vorapaxar in patients with diabetes mellitus and previous myocardial infarction: findings from the thrombin receptor antagonist in secondary prevention of atherothrombotic ischemic events-TIMI 50 trial. Circulation. 2015;131:1047–1053. doi: 10.1161/CIRCULATIONAHA.114.013774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Capodanno D., Mehran R., Valgimigli M. Aspirin-free strategies in cardiovascular disease and cardioembolic stroke prevention. Nat Rev Cardiol. 2018;15:480–496. doi: 10.1038/s41569-018-0049-1. [DOI] [PubMed] [Google Scholar]
- 15.Dewilde W.J., Oirbans T., Verheugt F.W. Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: an open-label, randomised, controlled trial. Lancet. 2013;381:1107–1115. doi: 10.1016/S0140-6736(12)62177-1. [DOI] [PubMed] [Google Scholar]
- 16.Gibson C.M., Mehran R., Bode C. Prevention of bleeding in patients with atrial fibrillation undergoing PCI. N Engl J Med. 2016;375:2423–2434. doi: 10.1056/NEJMoa1611594. [DOI] [PubMed] [Google Scholar]
- 17.Cannon C.P., Bhatt D.L., Oldgren J. Dual antithrombotic therapy with dabigatran after PCI in atrial fibrillation. N Engl J Med. 2017;377:1513–1524. doi: 10.1056/NEJMoa1708454. [DOI] [PubMed] [Google Scholar]
- 18.Lopes R.D., Heizer G., Aronson R. Antithrombotic therapy after acute coronary syndrome or PCI in atrial fibrillation. N Engl J Med. 2019;380:1509–1524. doi: 10.1056/NEJMoa1817083. [DOI] [PubMed] [Google Scholar]
- 19.Magnani G., Bonaca M.P., Braunwald E. Efficacy and safety of vorapaxar as approved for clinical use in the United States. J Am Heart Assoc. 2015;4 doi: 10.1161/JAHA.114.001505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.U.S. Food and Drug Administration (FDA) Vorapaxar prescribing information. http://www.fda.gov/drugs/informationondrugs/ucm423935.htm Available at: Accessed June 1, 2019.
- 21.European Medicines Agency (EMA) Vorapaxar prescribing information. http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/002814/human_med_001839.jsp&mid=WC0b01ac058001d124 Available at: Accessed June 1, 2019.
- 22.World Health Organization (WHO) Definition and diagnosis of diabetes mellitus and intermediate hyperglycaemia: report of a WHO/IDF consultation. http://www.who.int/diabetes/publications/diagnosis_diabetes2006/en/ Available at: Accessed June 1, 2019.
- 23.Capodanno D., Patel A., Dharmashankar K. Pharmacodynamic effects of different aspirin dosing regimens in type 2 diabetes mellitus patients with coronary artery disease. Circ Cardiovasc Interv. 2011;4:180–187. doi: 10.1161/CIRCINTERVENTIONS.110.960187. [DOI] [PubMed] [Google Scholar]
- 24.Franchi F., Rollini F., Aggarwal N. Pharmacodynamic comparison of prasugrel versus ticagrelor in patients with type 2 diabetes mellitus and coronary artery disease: the OPTIMUS (Optimizing Antiplatelet Therapy in Diabetes Mellitus)-4 Study. Circulation. 2016;134:780–792. doi: 10.1161/CIRCULATIONAHA.116.023402. [DOI] [PubMed] [Google Scholar]
- 25.Gurbel P.A., Bliden K.P., Tantry U.S. First report of the point-of-care TEG: a technical validation study of the TEG-6S system. Platelets. 2016;27:642–649. doi: 10.3109/09537104.2016.1153617. [DOI] [PubMed] [Google Scholar]
- 26.Storey R.F., Kotha J., Smyth S.S. Effects of vorapaxar on platelet reactivity and biomarker expression in non-ST-elevation acute coronary syndromes. The TRACER Pharmacodynamic Substudy. Thromb Haemost. 2014;111:883–891. doi: 10.1160/TH13-07-0624. [DOI] [PubMed] [Google Scholar]
- 27.Lancaster G.A., Dodd S., Williamson P.R. Design and analysis of pilot studies: recommendations for good practice. J Eval Clin Pract. 2004;10:307–312. doi: 10.1111/j..2002.384.doc.x. [DOI] [PubMed] [Google Scholar]
- 28.Bhatt D.L., Bonaca M.P., Bansilal S. Reduction in ischemic events with ticagrelor in diabetic patients with prior myocardial infarction in PEGASUS-TIMI 54. J Am Coll Cardiol. 2016;67:2732–2740. doi: 10.1016/j.jacc.2016.03.529. [DOI] [PubMed] [Google Scholar]
- 29.Armstrong P.C., Leadbeater P.D., Chan M.V. In the presence of strong P2Y12 receptor blockade, aspirin provides little additional inhibition of platelet aggregation. J Thromb Haemost. 2011;9:552–561. doi: 10.1111/j.1538-7836.2010.04160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Traby L., Kollars M., Kaider A., Eichinger S., Wolzt M., Kyrle P.A. Effects of P2Y12 receptor inhibition with or without aspirin on hemostatic system activation: a randomized trial in healthy subjects. J Thromb Haemost. 2016;14:273–281. doi: 10.1111/jth.13216. [DOI] [PubMed] [Google Scholar]
- 31.Vranckx P., Valgimigli M., Jüni P. Ticagrelor plus aspirin for 1 month, followed by ticagrelor monotherapy for 23 months vs aspirin plus clopidogrel or ticagrelor for 12 months, followed by aspirin monotherapy for 12 months after implantation of a drug-eluting stent: a multicentre, open-label, randomised superiority trial. Lancet. 2018;392:940–949. doi: 10.1016/S0140-6736(18)31858-0. [DOI] [PubMed] [Google Scholar]
- 32.Baber U., Dangas G., Cohen D.J. Ticagrelor with aspirin or alone in high-risk patients after coronary intervention: rationale and design of the TWILIGHT study. Am Heart J. 2016;182:125–134. doi: 10.1016/j.ahj.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 33.Lopes R.D., Hong H., Harskamp R.E. Safety and efficacy of antithrombotic strategies in patients with atrial fibrillation undergoing percutaneous coronary intervention: a network meta-analysis of randomized controlled trials. JAMA Cardiol. 2019 June 19 doi: 10.1001/jamacardio.2019.1880. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Angiolillo D.J., Goodman S.G., Bhatt D.L. Antithrombotic therapy in patients with atrial fibrillation treated with oral anticoagulation undergoing percutaneous coronary intervention. Circulation. 2018;138:527–536. doi: 10.1161/CIRCULATIONAHA.118.034722. [DOI] [PubMed] [Google Scholar]
- 35.Capodanno D., Huber K., Mehran R. Management of antithrombotic therapy in atrial fibrillation patients undergoing PCI: current status and future directions. J Am Coll Cardiol. 2019;74:83–99. doi: 10.1016/j.jacc.2019.05.016. [DOI] [PubMed] [Google Scholar]
- 36.Cadroy Y., Bossavy J.P., Thalamas C., Sagnard L., Sakariassen K., Boneu B. Early potent antithrombotic effect with combined aspirin and a loading dose of clopidogrel on experimental arterial thrombogenesis in humans. Circulation. 2000;101:2823–2828. doi: 10.1161/01.cir.101.24.2823. [DOI] [PubMed] [Google Scholar]
- 37.Borissoff J.I., Spronk H.M., ten Cate H. The hemostatic system as a modulator of atherosclerosis. N Engl J Med. 2011;364:1746–1760. doi: 10.1056/NEJMra1011670. [DOI] [PubMed] [Google Scholar]
- 38.Eikelboom J.W., Connolly S.J., Bosch J. Rivaroxaban with or without aspirin in stable cardiovascular disease. N Engl J Med. 2017;377:1319–1330. doi: 10.1056/NEJMoa1709118. [DOI] [PubMed] [Google Scholar]
- 39.Nylander S., Mattsson C., Ramström S., Lindahl T.L. Synergistic action between inhibition of P2Y12/P2Y1 and P2Y12/thrombin in ADP- and thrombin-induced human platelet activation. Br J Pharmacol. 2004;142:1325–1331. doi: 10.1038/sj.bjp.0705885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Franchi F., Rollini F., Cho J.R. Effects of dabigatran on the cellular and protein phase of coagulation in patients with coronary artery disease on dual antiplatelet therapy with aspirin and clopidogrel. Results from a prospective, randomised, double-blind, placebo-controlled study. Thromb Haemost. 2016;115:622–631. doi: 10.1160/TH15-06-0467. [DOI] [PubMed] [Google Scholar]
- 41.Borst O., Münzer P., Alnaggar N. Inhibitory mechanisms of very low-dose rivaroxaban in non-ST-elevation myocardial infarction. Blood Adv. 2018;2:715–730. doi: 10.1182/bloodadvances.2017013573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Franchi F., Rollini F., Garcia E. Effects of edoxaban on the cellular and protein phase of coagulation in patients with coronary artery disease on dual antiplatelet therapy with aspirin and clopidogrel: results of the EDOX-APT study. Thromb Haemost. 2019 doi: 10.1055/s-0039-1695772. Aug 30 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 43.Furugohri T., Isobe K., Honda Y. DU-176b, a potent and orally active factor Xa inhibitor: in vitro and in vivo pharmacological profiles. J Thromb Haemost. 2008;6:1542–1549. doi: 10.1111/j.1538-7836.2008.03064.x. [DOI] [PubMed] [Google Scholar]
- 44.Honda Y., Kamisato C., Morishima Y. Edoxaban, a direct factor Xa inhibitor, suppresses tissue-factor induced human platelet aggregation and clot-bound factor Xa in vitro: comparison with an antithrombin-dependent factor Xa inhibitor, fondaparinux. Thromb Res. 2016;141:17–21. doi: 10.1016/j.thromres.2016.02.028. [DOI] [PubMed] [Google Scholar]
- 45.Wong P.C., Jiang X. Apixaban, a direct factor Xa inhibitor, inhibits tissue-factor induced human platelet aggregation in vitro: comparison with direct inhibitors of factor VIIa, XIa and thrombin. Thromb Haemost. 2010;104:302–310. doi: 10.1160/TH10-02-0097. [DOI] [PubMed] [Google Scholar]
- 46.Jones W.S., Patel M.R. Antithrombotic therapy in peripheral artery disease: generating and translating evidence into practice. J Am Coll Cardiol. 2018;71:352–362. doi: 10.1016/j.jacc.2017.11.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.