Abstract
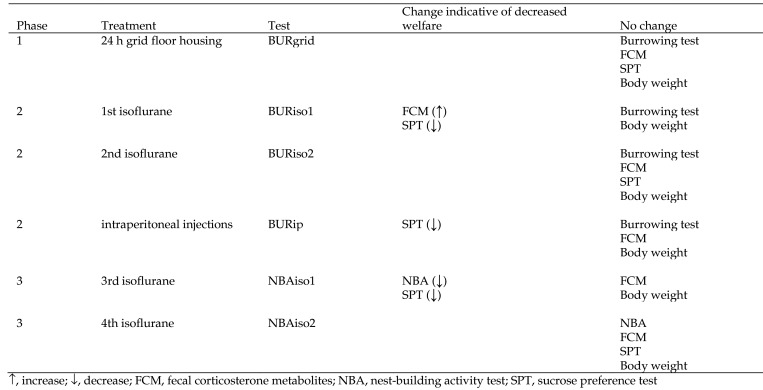
Nest building and burrowing are highly motivated natural behaviors in rodents, and changes in these behaviors can serve as welfare assessment tools. In this study, we investigated: 1) the limits of agreement between 2 observers for a refined scoring method for nest-building behavior; 2) the effect of repeated exposure to 15 min of isoflurane on nest-building behavior; 3) the effect of 24 h of grid-floor housing, repeated exposure to 15 min isoflurane, and daily intraperitoneal injection of 0.2 mL 0.9% isotonic saline for 3 d on burrowing behavior; and 4) the effect of exposure to grid-floor housing, isoflurane, and intraperitoneal injections on fecal corticosterone metabolites, body weight, fur status, and sucrose preference in mice. SPF C57BL/6NTac female mice (n = 27) were included in the study and were assessed first for burrowing behavior, followed by 2 wk of rest and then for nesting behavior. The refined scoring method for nest-building activity had good inter observer agreement. According to this method, a single exposure to anesthesia with isoflurane led to a decrease in nest-building activity and sucrose preference; a second exposure to anesthesia with isoflurane had no effect on nest building. Neither grid-floor housing nor repeated exposure to isoflurane anesthesia had any effect on burrowing behavior in mice. In contrast, intraperitoneal injections increased burrowing behavior. In conclusion, a refined scoring method for nest-building activity test that we developed for this study proved to be objective and sensitive to the effect of an initial exposure to anesthesia with isoflurane.
Abbreviations: FCM, fecal corticosterone metabolites; NBA, nest-building activity; SPT, sucrose preference test
Recent years have seen an increased focus on animal welfare in scientific research. Reduction of pain, distress, and suffering is a part of the regulations in most countries, and researchers are required to make efforts to assess the level of discomfort and harm inflicted by experiments.11 However, assessing pain and discomfort in mice is challenging. As prey animals, mice hide signs of pain and suffering to avoid becoming a target for predators and typically display only subtle (and retrospective) signs of suffering and pain, such as decreased body weight.22,28,32,35 Two promising and simple approaches to assess subtle changes in mice are the assessment of changes in nest-building activity (NBA) and burrowing behavior.8-10,19 These are spontaneous, highly motivated and species-specific behaviors that can be observed noninvasively.16 NBA and burrowing have been recommended as noninvasive tools in the assessment of welfare in mice and therefore potentially can be useful measures of welfare, when combined with other welfare parameters, such as body weight and fecal corticosterone metabolites. Burrowing is reduced after exposure to pain and restored after analgesia in both mice and rats.1,5-7,17,19,34 Similarly, NBA has been shown to be affected by pain, stress, and the administration of psychoactive drugs, as examples.2,18,29-31 In a previous study,12 we showed that burrowing decreased after exposure to 24 h of grid-floor housing, a stress-inducing element when introduced into the animals’ environment.13,21 Furthermore, in our previous study,12 we also found that NBA decreased after a single exposure to 15 min of isoflurane anesthesia, whereas NBA was not altered by repeated intraperitoneal injections of saline.12 Isoflurane has been reported as aversive and stressful,23,41 whereas intraperitoneal injections—particularly repeated intraperitoneal injections—are stressful for the animals involved, although experimental evidence supporting this statement is sparse.27 Therefore, in the current study, we aimed at investigating the reproducibility of our previous results while also assessing the effect of repeated exposure to isoflurane anesthesia and intraperitoneal injections of saline, which are commonly used in our unit. First, we aimed at establishing the limits of agreement of a refined scoring method of NBA. Second, we investigated the effect of exposure to isoflurane on NBA, fecal corticosterone metabolites (FCM), body weight, fur status, and sucrose preference. Third, we evaluated the effect of grid-floor housing and repeated exposure to isoflurane and intraperitoneal injections on burrowing, FCM, body weight, fur status, and sucrose preference.
We hypothesized that burrowing and NBA would decrease after exposure to any of the 3 chosen stressors: grid-floor housing, anesthesia with isoflurane, and intraperitoneal saline injections. We also hypothesized that these decreases in burrowing and NBA would be mirrored in FCM, body weight, fur status, and sucrose preference. Finally, we hypothesized that the refined scoring of NBA would prove to have good agreement between observers.
Materials and Methods
The experimental procedures were all carried out in accordance with EU Directives11 and approved by the Danish Animal Experiments Inspectorate under the Ministry of Environment and Food in Denmark (license number, 2016-15-0201-00871). Reporting of the study follows the ARRIVE guidelines for reporting animal research.20
Animals and housing.
SPF C57BL/6NTac female mice (n = 27; age, 6 wk at arrival; Taconic, Silkebourg, Denmark) were housed in 9 cages in groups of 3. The C57BL/6 strain was chosen because of its vast use in research.38 All cages were transparent standard Makrolon type 4 cages (540 × 320 × 180 mm; Tecniplast, Buggiatate, Italy) with a 70-mm raised lid. Individually ventilated racks containing 16 cages each were used, and each cage contained Aspen bedding (LBS Biotechnology, Horley, United Kingdom), a handful of Enviro-Dri paper nesting material (LBS Biotechnology), 2 biting blocks (50 × 10 × 10 mm) in aspen wood (Tapvei, Paekcneak, Estonia), a dark colored acrylic shelter (Bach Vent, Denmark), a cardboard tube (LBS serving Biotechnology) and a hemp rope (length, 30 cm; diameter, 6mm) hanging from the lid in the center of the cage (Fyns Kran Udstyr, Odense, Denmark). The mice were kept at 20 to 22 °C, 45% to 65% humidity, under a 12:12-h dark:light cycle (lights on, 0600) and had unlimited access to both chow (type 1324, Maintenance Diet Rats/Mice, Altromin, Lage, Germany) and tap water in water bottles. The mice were monitored daily both by male and female animal caretakers, and the cages were changed once weekly during assessments. The cardboard tubes from each cage were used for handling mice during cage changes. All experimental assessments were made by female observers.
Timeline.
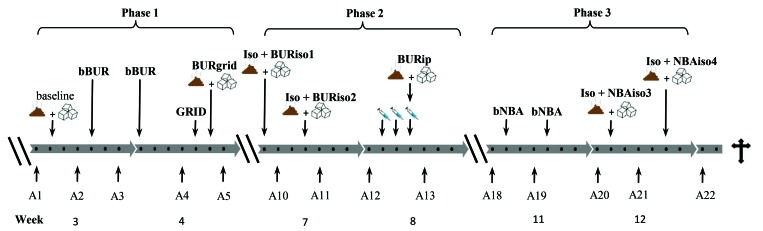
The study consisted of 3 phases: 1) burrowing after exposure to grid-floor housing; 2) burrowing after subjection to isoflurane and intraperitoneal injections; and 3) NBA after exposure to isoflurane (Figure 1).
Figure 1.
Schematic timeline of the experimental period. Phase 1: burrowing behavior test (BUR) used to assess the effect of grid floor housing (BURgrid); Phase 2: burrowing used to assess the effect of repeated exposure to isoflurane anesthesia (BURiso1 and BURiso2) and repeated intraperitoneal injections (BURip); Phase 3: nest-building activity (NBA) used to assess the effect of repeated exposure to isoflurane anesthesia (NBAiso3 and NBAiso4). A1–22, assessment of body weight and fur status; bBUR, baseline burrowing test; BURgrid, burrowing test after exposure to grid-floor housing; BURip, burrowing after repeated intraperitoneal injections; BURiso1, burrowing after the 1st exposure to isoflurane; BURiso2, burrowing after the 2nd exposure to isoflurane; bNBA, baseline nest building activity test; collection of feces for fecal corticosterone metabolites; GRID, grid-floor housing; intraperitoneal injections; Iso, isoflurane; NBAiso3, nest-building activity after the 3th exposure to isoflurane; NBAiso4, nest-building activity after the 4th exposure to isoflurane; sucrose preference test.
At arrival, the mice were randomly allocated into 9 cages with 3 mice in each cage. All 9 cages were tested in a randomized order throughout the study. After one week of acclimation (week 1), all 3 mice in each cage were ear marked for identification and left in the cage for another week (week 2). In the beginning of phase 1 (week 3), all mice were habituated to a sucrose preference test (SPT) and a burrowing tube before baseline values of burrowing and SPT were assessed (week 3 to 4). In addition, 24 h of fecal boli were collected for baseline values of FCM (bFCM). Burrowing baseline values (bBUR) consisted of an average of 2 baseline values assessed with 3 d between assessments. In week 4 (day 5), all mice were exposed to 24 h of grid-floor housing. Hereafter, the mice were transferred to a new clean cage for assessment of burrowing (BURgrid, day 6), SPT (SPT[BURgrid]) and collection of feces for FCM (FCM[BURgrid]).
After 2 wk of rest, phase 2 began in week 7, with mice being exposed, one cage at a time, to 15 min of anesthesia with isoflurane (IsoFlovet 100%, Zoetis Finland, Oy, Finland) on days 1 and 4. On both days, burrowing (BURiso1 and BURiso2), SPT (SPT[BURiso1] and SPT[BURiso2]), and FCM (FCM[BURiso1] and FCM[BURiso2]) were assessed. In week 8, the mice were subjected to a daily injection of 0.2 mL 0.9% isotonic saline with a 27 gauge needle for 3 d on day 2, 3 and 4 (by 3 different technicians) followed by assessment of burrowing (BURip), SPT (SPT[BURip]), and FCM (FCM[BURip]). Phase 3 began after another 2 wk of rest, and baseline values of NBA were assessed on day 1 and 4 (week 11) by 2 independent observers—the primary observer and an animal technician—for a limit-of-agreement calculation. NBA baseline values (bNBA) consisted of an average of the 2 baseline values made by the primary observer. Blinding of the observers was not possible given that all mice were exposed to the same stressors simultaneously. In week 12, the mice were exposed to 15 min of isoflurane anesthesia on days 2 and 6. Subsequently, the mice were transferred to a new clean cage, and NBA was assessed (NBAiso3 and NBAiso4) by the primary observer. On both days, this event was followed by assessments of SPT (SPT[NBAiso3] and SPT[NBAiso4]), and collection of feces for FCM (FCM[NBAiso3]) and FCM[NBAiso4]). Testing took place in the home cage at all times. Assessments of body weight and fur status were performed before and after exposure to a stressor and twice weekly in resting periods. The mice (age, 18 wk) were euthanized by using carbon dioxide in week 13.
Stressors.
Grid-flood housing, anesthesia with isoflurane, and intraperitoneal injections were used as stressors. The grid floor consisted of round grids (diameter, 1.1 mm) placed 8 mm apart vertically, intersecting with similar grids placed 3 cm apart horizontally. The isoflurane (IsoFlovet 100%, Zoetis Finland) was mixed with oxygen (3 L/min) and nitrous oxide (0.7 L/min) at a concentration of 4% to 5% during induction and 2% during maintenance. All 3 mice from each cage were anesthetized at the same time in a chamber designed for that purpose. Intraperitoneal injections were performed by using 27-gauge needles.
Burrowing.
Because of the learning element in the behavior,6 the mice were habituated to the burrowing tube overnight at 48 h before baseline assessment. Baseline values were assessed twice with 3 d between assessments, and the mean of the baseline values was used as a reference for experimental values. Immediately after exposure to the stressor (grid-floor housing, isoflurane, and intraperitoneal injections), the test was initiated (at 1100 each time).
For each burrowing assessment, nesting material and the acrylic shelter were replaced with a nontransparent burrowing tube (length, 20 cm; diameter, 6.8 cm; closed at one end and raised 3 cm at the other end) filled with 90 g aspen bedding material (Tapvei). The tube was placed in the back left corner of the cage. After 2 h (at 1300), the content remaining in the tube was weighed and the amount burrowed calculated. Cages with mice not displaying burrowing behavior at any time were excluded from all analyses.
NBA.
Baseline values for NBA were assessed with 3 d between assessments, and a mean therefore was used as the reference. All 3 mice from the same cage were exposed to isoflurane at the same time, and NBA evaluation was initiated immediately afterward. Specifically, at 1400, the nesting material in the cage was replaced with 45 g of new nesting material loosely spread in the back half of the cage. The shelter and cardboard tube were removed, whereas the other enrichment items (gnawing sticks and hemp rope) remained in the cage. Scoring by the primary investigator occurred 24 h later. All nests were scored by combining a previously described the scoring system14 with a score of the nest's height, which involved using a knitting needle that was converted into a ruler with 0.5-cm intervals. The nests were given a score of 0 for undisturbed nesting material (nesting material had not been moved nor manipulated), a score of 1 for disturbed nesting material (evident interaction with nesting material) that had not been assembled to form a nest site, and a score of 2 when the nesting material has been assembled to form a nest site.14 For each nest site (score, 2), the ruler was used as a supplementary scoring tool. Each side of the nest (when considering the nest site as a square with sides parallel to the cage) was measured by using the ruler and an average of the 4 sides was calculated and used as the overall score for height. The ruler was used on the highest spot of the nest, and the height of the nests measured from the base of the bedding upward (Figure 2) The ruler score was added to the total nest score: for example, a score of 2 was given for formation of a nest, and if the height measured by the ruler was an average of 2 cm, the total nest score would be 4. Cages with mice not displaying NBA behavior at any time were excluded from all analyses.
Figure 2.
Scoring of nests. Scoring sites for the nest building activity test are marked with a red cross, and the outer and inner walls of the nest are marked with black circles (A). The nest is considered a square with sides parallel to the walls of the cage. The scoring sites represent the highest point between the inner and outer wall of the nest at each of the 4 sides. (B) The height of these points are measured by using the ruler.
At baseline, 2 independent observers (the primary investigator and an observer not otherwise involved in the study) scored all nests by using the refined method, and the limits of agreement were calculated. Due to high interobserver reliability, it was considered sufficient that the primary investigator performed nest scorings at all other times.
FCM.
FCM were measured to avoid invasive handling procedures related to corticosterone measured in serum or urine.39 During the study, 24 h of feces were collected 7 times per cage (Figure 1). For each collection, the mice were transferred to clean cages twice, with 24 h between, allowing for a 24-h feces sample. Each sample was sorted for fecal boli and stored at –20 °C for later analysis. FCM concentration was quantified (catalog no. EIA-4164, Corticosterone [competitive] ELISA, DRG Instruments, Marburg, Germany) as described previously,37 (with the exception of samples being evaporated and dissolved in buffer after extraction instead of being diluted in ethanol) and according to the manufacturer's instructions.
SPT.
To avoid variation in sucrose consumption on the very first exposure to the sucrose solution, the mice were habituated to drinking a 2.0% sucrose solution from a water bottle for 2 h (1800 to 2000) on the day before assessment of baseline values of SPT (bSPT).36 The following day, the mice underwent a 24-h choice test, initiated at 1800, with continuous access to food and 2 water bottles (one containing tap water and one containing 2% sucrose). No food or water deprivation was applied before the test. After 12 h, the bottles were carefully switched, while avoiding potential leakage from the bottles, to avoid side preference in drinking behavior. After 24 h, the test was stopped. All bottles were weighed before and after the test, and sucrose preference calculated as a percentage of the consumed sucrose solution from the total amount of liquid consumed. This procedure was performed for every assessment of SPT.
Body weight and fur condition assessments.
Assessments of body weight and fur condition were conducted the day before collection of feces for bFCM, before assessing values for baseline burrowing, before exposure to each of the stressors, and on the day after assessments of burrowing and NBA (Figure 1). The mice were weighed, and the fur condition of the mice was assessed according to a 4-point scale: 1, generally well-groomed fur and body; 2, slightly fluffy fur with some spiky patches; 3, fluffy fur on most of the body; and 4, fluffy, stained, dirty fur, possibly with some bald patches.26 The assessments were performed as quickly as possible to minimize disturbance of the cage.
Statistical analyses.
The number of mice was based on our previous study,13 where 8 cages with 3 mice in each were used. In the current study, we added one more cage to compensate for a possible lack of burrowing or NBA behavior. Therefore, 9 cages with 3 mice in each cage were used. Statistical analyses were performed by using SAS 9.3 (SAS Institute, Cary, NC) with a 1% significance level to compensate for multiple comparisons. A repeated-measures analysis using the SAS procedure PROC MIXED was used to compare baseline values with experimental values in burrowing, NBA, FCM, SPT, and body weight, with cage set as a random effect. Goodness of fit (linearity, variance homogeneity, and normal distribution of residuals) was investigated by visual inspection of plots and normality was met in every comparison. Cage was the unit of treatment, except for assessment of body weight (by mouse). Two different observers each scored all baseline values of NBA, and the agreement between observers was established by calculating Bland–Altman limits of agreement.
Results
An overview of all results is given in Figure 3. One cage of mice did not display burrowing behavior at any time point during the study and was excluded from all analyses regarding burrowing. Therefore, 8 cages were included in the analysis of burrowing, whereas 9 cages were included in the analysis of NBA, FCM, SPT, body weight, and fur status. Baseline assessments of burrowing and NBA were performed twice, and the results are based on an average thereof.
Figure 3.
Overview of results.
Burrowing behavior (phases 1 and 2).
At baseline (bBUR), the mice burrowed 48.64 ± 5.25 g (mean ± SEM). The mice burrowed 47.34 ± 6.88 g after 24 h on grid floor housing (BURgrid), 50.48 ± 8.50 g after the 1st exposure to anesthesia with isoflurane (BURiso1), 49.46 ± 7.82 g after the 2nd exposure to anesthesia with isoflurane (BURiso2), and 65.03 ± 8.15 g after 1 daily intraperitoneal injection of saline for 3 d (BURip), respectively. bBUR did not differ from BURgrid, BURiso1, or BURiso2. A significant difference was found between bBUR and BURip (mean difference, 16.38 g; 95% CI, 5.68 to 27.08; SE = 5.22; P = 0.004).
NBA (phase 3).
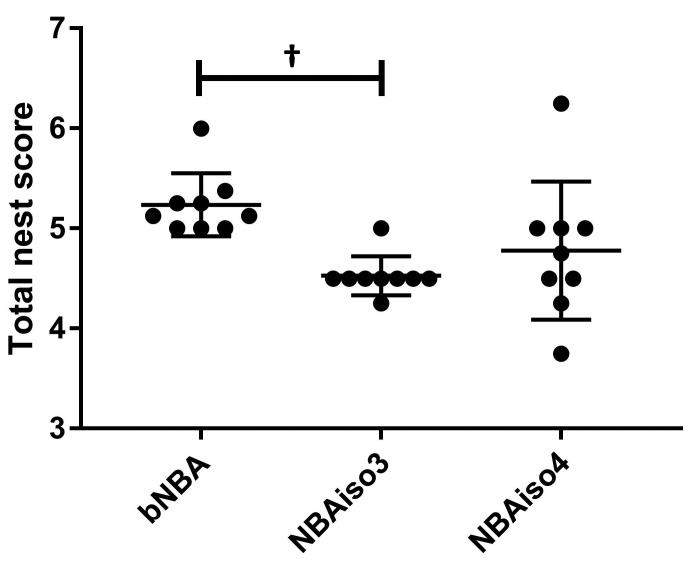
At baseline, the nests were scored by 2 different observers, and the limits of agreement between these 2 scorings were calculated to a difference of ±0.25 cm. At baseline (bNBA), the mice made nests with a score of 5.24 ± 0.11 (mean ± SEM). After the 3rd exposure to anesthesia with isoflurane (NBAiso3), the mice made nests with an average nest score of 4.53 ± 0.07, and after the 4th exposure to isoflurane (NBAiso4), the mice made nests with an average score of 4.78 ± 0.23. After the 3rd exposure to isoflurane (NBAiso3), the total nest score was significantly different from baseline (mean difference, –0.71; 95% CI, –1.09 to –0.33; SE, 0.18; P = 0.001). However, this was not the case for NBAiso4 (mean difference, –0.46, 95% CI, –0.84 to –0.08; SE, 0.18; P = 0.02; Figure 4).
Figure 4.
Total nest scores for mice at baseline, bNBA, and after the 3th and 4th exposure to anesthesia with isoflurane (NBAiso3 and NBAiso4, respectively); n = 9 cages. Values are presented as mean ± 1 SD; †, P ≤ 0.001.
FCM (phases 1, 2, and 3).
At baseline (bFCM), the level of FCM was found to be 0.24 nmol corticosterone excreted over 24 h. The only significant effect of treatment was an increase in FCM after the 1st exposure to isoflurane during burrowing assessment (FCM[BURiso1]: mean difference, 0.06 nmol; 95% CI, 0.02 to 0.10; SE, 0.02; P = 0.006).
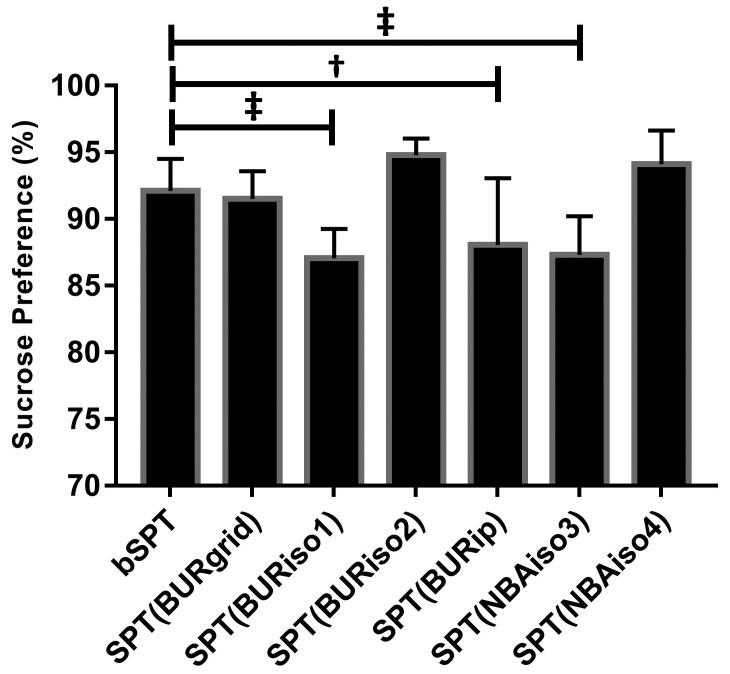
SPT (phases 1, 2, and 3).
A significant decrease in sucrose preference was found between baseline (bSPT) and after the 1st exposure to isoflurane during burrowing assessment (SPT[BURiso1]: mean difference, –5.51 g; 95% CI, –8.21 to –2.80; SE, 1.34; P = 0.0002), after repeated intraperitoneal injections (SPT[BURip]: mean difference, –4.59 g, 95% CI, –7.24 to –1.83; SE, 1.34: P = 0.002), and after the 2nd exposure to isoflurane during NBA assessment (SPT[NBAiso3]: mean difference, –5.39 g; 95% CI, –8.10 to –2.69; SE, 1.34; P = 0.0002; Figure 5).
Figure 5.
Sucrose preference (%) in mice (n = 9 cages) at baseline, bSPT, and after exposure to various stressors: 24 h grid-floor housing, SPT(BURgrid); 1st and 2nd exposure to anesthesia with isoflurane during burrowing assessment, SPT(BURiso1) and SPT(BURiso2); intraperitoneal injections, SPT(BURip); and 3th and 4th exposures to anesthesia with isoflurane during nest-building activity assessment, SPT(NBAiso3) and SPT(NBAiso4). Values are presented as mean ± 1 SD; †, P ≤ 0.001; ‡, P ≤ 0.0001.
Body condition assessments (phases 1, 2, and 3).
Throughout the study, all mice were weighed and the condition of the fur assessed. None of the stressors applied caused a decrease in body weight nor did the fur condition of the mice change during the study. All mice were well-groomed and had nonsoiled fur at all assessments.
Discussion
This study used a refined scoring method for the complexity of nests as a measure of nest building activity; the test had good inter observer agreement. This refined method revealed a decrease in NBA and sucrose preference after a single exposure to isoflurane anesthesia. However, nest building activity was not altered by a second exposure to anesthesia with isoflurane. In addition, we were unable to reproduce our previous results of reduced burrowing activity after exposure to grid-floor housing,12 nor did we see reduced burrowing activity after repeated exposure to isoflurane anesthesia or intraperitoneal injections. However, intraperitoneal injections were followed by a decrease in sucrose preference.
The combined purpose of our current and previous studies12 was to establish reproducible welfare assessment tests—based on innate, natural, and highly motivated behaviors—that could be carried out inside the home cage and that were simple to perform, thus enabling testing during working hours throughout the year. The tests should be able to detect very subtle negative effects on animal welfare, given that most animals in our facilities are primarily exposed to stressors that are considered to be mild. Our previous study12 showed promising results for using the NBA test as a tool in the welfare assessment of mice. However, we experienced difficulties in keeping the scoring objective and consistent, even though all nests were scored by the same observer. Nest scoring was performed as described previously,14 where the height of the nest domes is evaluated and scored accordingly. Although this method is indeed useful, it requires a skilled observer. Therefore, in the current study, we investigated the limits of agreement of a refined scoring method for NBA and incorporated a ruler to measure the heights of the nest walls. This scoring method proved to be objective, because 2 independent observers scored the same nest within ± 0.25 cm. Therefore, the combination of the previous scoring method14 and the ruler designed in the current study makes the NBA test more objective than our previous method,12 with good agreement between 2 independent observers.
In the present study, the NBA test was performed inside the home cage to minimize stress and unwanted effects caused by moving the animals to an external experimental setup.16 In accordance with our first study, a single exposure to isoflurane led to a decrease in nest building activity alongside a decrease in sucrose preference.12 However, a second exposure to isoflurane was not followed by a decrease in NBA, thus indicating a limitation to the ability of the NBA test to detect subtle changes in welfare. This result might also indicate that mice adapted to isoflurane exposure, even though isoflurane has been shown to be aversive to mice and rats.4,24,41 In contrast to our current study, another recent study showed that neither single nor repeated exposure to isoflurane influenced NBA.15 One possible explanation for this contrast could be that the scoring method used by the other authors is a modified version of another previously described method,8 which perhaps is not sufficiently sensitive to subtle changes. However, comparison between this previous study15 and our current study should be made with caution, as many differences exist. For example, the previous authors15 measured nest building on individual level, exposed the mice to 45 min of anesthesia, and used cotton squares as nesting material, whereas we tested on the group level, exposed the mice to 15 min of anesthesia, and used Enviro-Dri nesting material. The previous study15 also showed a decrease in burrowing after repeated exposure to isoflurane; this was not the case in our current study. One explanation could be differences in methodology during burrowing assessment. In contrast to our study, the previous study15 used a water bottle lying on the bottom of the cage as a burrowing tube, with food pellets as burrowing substrate. Comparison between studies lacking consistency in methodology should be made with caution.
Like the NBA test, the burrowing test was performed in the home cage. In addition, regular bedding from our animal facilities was used to simplify the test setup. Our previous study12 showed that the burrowing test could detect an effect caused by exposure to grid-floor housing and that no difference existed between performing the test just prior to the dark phase (after working hours) and performing it from 1100 to 1300 (during normal working hours). In the current and our previous study, the mice burrowed the same amount of bedding (approximately 48 g during 2 h) during baseline assessment in both the dark phase and during working hours. These results demonstrate that performing the test during working hours (light phase) provides similar results as when performing the test during the dark phase, as originally intended6—at least during baseline assessments. Unfortunately, in the current study, we were unable to reproduce the effect of exposure to grid-floor housing on burrowing when performed during working hours, even though we used mice of the same strain, sex, and age and kept external influences—such as climate, daily routines, and feeding regimen—the same. However, although differences in external influences between the 2 studies cannot be ruled out completely, we do not believe that any potential differences would have profound effects and thereby explain the lack of reproducibility of burrowing.
Furthermore, the burrowing test did not detect an effect of either single or repeated exposure to isoflurane anesthesia. These results are in contrast to one previous study,17 showing that burrowing decreases after subjection to anesthesia with sevoflurane in an induction chamber, as well as another,15 showing that burrowing decreases after repeated isoflurane exposure. Furthermore, one previous study15 showed that corticosterone levels were not influenced by anesthesia, in contrast to our results showing that the 1st exposure to isoflurane caused a rise in FCM as well as a drop in sucrose preference. These findings indicate that the 1st exposure to isoflurane is stressful and induces anhedonic-like behavior,25,36 but the effect of exposure is not measureable by using the burrowing test. The conditions in the 3 studies are slightly different, for example the length of exposure to anesthesia, the type of burrowing tube used, and the time during the light dark cycle, and these differences should be taken into consideration when comparing the studies. However, whether these variations are the reason for the differences between our current study and the previous ones15,17 as well as the difference between our own 2 studies is unknown. One explanation could be the greater variance seen within the group after subjection to a stressor than at baseline. Nevertheless, these differences raise the question of reproducibility and of whether the burrowing test should be developed for use as a standard welfare measure.
Burrowing has been shown to decrease after postsurgical pain and nerve injuries5,17—effects that are considered not more severe than intraperitoneal injections and exposure to grid-floor housing.11 Therefore, the burrowing test seems to be useful for detecting more stressful stimuli than those we used in the present study, but there is a limit to how sensitive the test is. Exposure to grid-floor housing for 24 h, 2 rounds of isoflurane anesthesia, and 3 daily intraperitoneal injections of saline are likely too mild as stressors to evoke a change in the burrowing test.
Throughout the study, the mice were used as their own controls, by comparing experimental values with baseline values. However, this method can be considered a limitation to the study. Having a control group that was not exposed to stressors over time would have been advantageous. Furthermore, a crossover design would have been advantageous by making it possible for mice to be naïve to isoflurane exposure during NBA assessment. These caveats should be taken into consideration when interpreting the results from this study. In addition, only female mice were used in the study, due to the aggression that is commonly observed in group-housed males.40 No empirical evidence exists to indicate that female mice show more variation than males.3,33 However, the exclusion of male mice is a major limitation to our study. Therefore, an important study would be needed to investigate whether the refined nest building scoring we used in this study can be used in male mice as well.
This study tested the use of nest-building activity and burrowing tests to detect changes in welfare induced by mild stressors in female B6 mice. By using a refined scoring method developed for this study, the nest-building activity test proved objective and sensitive to the effect of a single exposure to anesthesia with isoflurane. However, the burrowing test was unable to detect the effect of repeated exposure to isoflurane anesthesia, raising the question of the applicability of the test in welfare assessment of mice.
References
- 1.Andrews N, Legg E, Lisak D, Issop Y, Richardson D, Harper S, Pheby T, Huang W, Burgess G, Machin I, Rice AS. 2012. Spontaneous burrowing behaviour in the rat is reduced by peripheral nerve injury or inflammation associated pain. Eur J Pain 16:485–495. 10.1016/j.ejpain.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 2.Arras M, Rettich A, Cinelli P, Kasermann HP, Burki K. 2007. Assessment of post-laparotomy pain in laboratory mice by telemetric recording of heart rate and heart rate variability. BMC Vet Res 3:1–10. 10.1186/1746-6148-3-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beery AK. 2018. Inclusion of females does not increase variability in rodent research studies. Curr Opin Behav Sci 23:143–149. 10.1016/j.cobeha.2018.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boulanger Bertolus J, Nemeth G, Makowska IJ, Weary DM. 2015. Rat aversion to sevoflurane and isoflurane. Appl Anim Behav Sci 164:73–80. 10.1016/j.applanim.2014.12.013. [DOI] [Google Scholar]
- 5.Bryden LA, Nicholson JR, Doods H, Pekcec A. 2015. Deficits in spontaneous burrowing behavior in the rat bilateral monosodium iodoacetate model of osteoarthritis: an objective measure of pain-related behavior and analgesic efficacy. Osteoarthritis Cartilage 23:1605–1612. 10.1016/j.joca.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 6.Deacon R. 2012. Assessing burrowing, nest construction, and hoarding in mice. J Vis Exp 59:e2607 10.3791/2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deacon RM, Raley JM, Perry VH, Rawlins JN. 2001. Burrowing into prion disease. Neuroreport 12:2053–2057. 10.1097/00001756-200107030-00052. [DOI] [PubMed] [Google Scholar]
- 8.Deacon RMJ. 2006. Assessing nest building in mice. Nat Protoc 1:1117–1119. 10.1038/nprot.2006.170. [DOI] [PubMed] [Google Scholar]
- 9.Deacon RMJ. 2006. Burrowing in rodents: a sensitive method for detecting behavioral dysfunction. Nat Protoc 1:118–121. 10.1038/nprot.2006.19. [DOI] [PubMed] [Google Scholar]
- 10.Deacon RMJ. 2009. Burrowing: a sensitive behavioural assay, tested in five species of laboratory rodents. Behav Brain Res 200:128–133. 10.1016/j.bbr.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 11.European Parliament and the Council of the European Union. 2010. Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the Protection of Animals Used for Scientific Purposes. Off J Eur Communities L276:33–79. [Google Scholar]
- 12.Gjendal K, Ottesen JL, Olsson IAS, Sørensen DB. 2018. Burrowing and nest building activity in mice after exposure to grid floor, isoflurane or ip injections. Physiol Behav 206:59–66. [DOI] [PubMed] [Google Scholar]
- 13.Heidbreder CA, Weiss IC, Domeney AM, Pryce C, Homberg J, Hedou G, Feldon J, Moran MC, Nelson P. 2000. Behavioral, neurochemical and endocrinological characterization of the early social isolation syndrome. Neuroscience 100:749–768. 10.1016/S0306-4522(00)00336-5. [DOI] [PubMed] [Google Scholar]
- 14.Hess SE, Rohr S, Dufour BD, Gaskill BN, Pajor EA, Garner JP. 2008. Home improvement: C57BL/6J mice given more naturalistic nesting materials build better nests. J Am Assoc Lab Anim Sci 47:25–31. [PMC free article] [PubMed] [Google Scholar]
- 15.Hohlbaum K, Bert B, Dietze S, Palme R, Fink H, Thöne-Reineke C. 2017. Severity classification of repeated isoflurane anesthesia in C57BL/6JRj mice-Assessing the degree of distress. PLoS One 12:1–21. 10.1371/journal.pone.0179588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jirkof P. 2014. Burrowing and nest building behavior as indicators of well-being in mice. J Neurosci Methods 234:139–146. 10.1016/j.jneumeth.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 17.Jirkof P, Cesarovic N, Rettich A, Nicholls F, Seifert B, Arras M. 2010. Burrowing behavior as an indicator of post-laparotomy pain in mice. Front Behav Neurosci 4:1–9. 10.3389/fnbeh.2010.00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jirkof P, Fleischmann T, Cesarovic N, Rettich A, Vogel J, Arras M. 2013. Assessment of postsurgical distress and pain in laboratory mice by nest complexity scoring. Lab Anim 47:153–161. 10.1177/0023677213475603. [DOI] [PubMed] [Google Scholar]
- 19.Jirkof P, Leucht K, Cesarovic N, Caj M, Nicholls F, Rogler G, Arras M, Hausmann M. 2013. Burrowing is a sensitive behavioural assay for monitoring general wellbeing during dextran sulfate sodium colitis in laboratory mice. Lab Anim 47:274–283. 10.1177/0023677213493409. [DOI] [PubMed] [Google Scholar]
- 20.Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. 2010. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol 8:1–5. 10.1371/journal.pbio.1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krohn TC, Hansen AK, Dragsted N. 2003. Telemetry as a method for measuring the impact of housing conditions on rats’ welfare. Anim Welf 12:53–62. [Google Scholar]
- 22.Langford DJ, Bailey AL, Chanda ML, Clarke SE, Drummond TE, Echols S, Glick S, Ingrao J, Klassen-Ross T, Lacroix-Fralish ML, Matsumiya L, Sorge RE, Sotocinal SG, Tabaka JM, Wong D, van den Maagdenberg AM, Ferrari MD, Craig KD, Mogil JS. 2010. Coding of facial expressions of pain in the laboratory mouse. Nat Methods 7:447–449. 10.1038/nmeth.1455. [DOI] [PubMed] [Google Scholar]
- 23.Leach MC, Bowell VA, Allan TF, Morton DB. 2002. Degrees of aversion shown by rats and mice to different concentrations of inhalational anaesthetics. Vet Rec 150:808–815. 10.1136/vr.150.26.808. [DOI] [PubMed] [Google Scholar]
- 24.Makowska IJ, Weary DM. 2009. Rat aversion to induction with inhalant anaesthetics. Appl Anim Behav Sci 119:229–235. 10.1016/j.applanim.2009.04.003. [DOI] [Google Scholar]
- 25.Malatynska E, Steinbusch HWM, Redkozubova O, Bolkunov A, Kubatiev A, Yeritsyan NB, Vignisse J, Bachurin S, Strekalova T. 2012. Anhedonic-like traits and lack of affective deficits in 18-month-old C57BL/6 mice: Implications for modeling elderly depression. Exp Gerontol 47:552–564. 10.1016/j.exger.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 26.Mineur YS, Prasol DJ, Belzung C, Crusio WE. 2003. Agonistic behavior and unpredictable chronic mild stress in mice. Behav Genet 33:513–519. 10.1023/A:1025770616068. [DOI] [PubMed] [Google Scholar]
- 27.Morton DB, Jennings M, Buckwell A, Ewbank R, Godfrey C, Holgate B, Inglis I, James R, Page C, Sharman I, Verschoyle R, Westall L, Wilson AB; Joint Working Group on Refinement. 2001. Refining procedures for the administration of substanced. Lab Anim 35:1–41. 10.1258/0023677011911345. [DOI] [PubMed] [Google Scholar]
- 28.National Research Council (US) Committee on Recognition and Alleviation of Pain in Laboratory Animals. 2009. Recognition and alleviation of pain in laboratory animals. Washington (DC): National Academies Press. [PubMed] [Google Scholar]
- 29.Otabi H, Goto T, Okayama T, Kohari D, Toyoda A. 2016. Subchronic and mild social defeat stress alter mouse nest building behavior. Behav Processes 122:21–25. 10.1016/j.beproc.2015.10.018. [DOI] [PubMed] [Google Scholar]
- 30.Otabi H, Goto T, Okayama T, Kohari D, Toyoda A. 2017. The acute social defeat stress and nest-building test paradigm: A potential new method to screen drugs for depressive-like symptoms. Behav Processes 135:71–75. 10.1016/j.beproc.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 31.Pedersen CS, Sørensen DB, Parachikova AI, Plath N. 2014. PCP-induced deficits in murine nest building activity: employment of an ethological rodent behavior to mimic negative-like symptoms of schizophrenia. Behav Brain Res 273:63–72. 10.1016/j.bbr.2014.07.023. [DOI] [PubMed] [Google Scholar]
- 32.Peterson NC. 2004. Assessment of pain scoring. Contemp Top Lab Anim Sci 43:74. [PubMed] [Google Scholar]
- 33.Prendergast BJ, Onishi KG, Zucker I. 2014. Female mice liberated for inclusion in neuroscience and biomedical research. Neurosci Biobehav Rev 40:1–5. 10.1016/j.neubiorev.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 34.Rutten K, Schiene K, Robens A, Leipelt A, Pasqualon T, Read SJ, Christoph T. 2013. Burrowing as a nonreflex behavioural readout for analgesic action in a rat model of sub-chronic knee joint inflammation. Eur J Pain 18:204–212. 10.1002/j.1532-2149.2013.00358.x. [DOI] [PubMed] [Google Scholar]
- 35.Stasiak KL, Maul D, French E, Hellyer PW, VandeWoude S. 2003. Species-specific assessment of pain in laboratory animals. Contemp Top Lab Anim Sci 42:13–20. [PubMed] [Google Scholar]
- 36.Strekalova T, Steinbusch HWM. 2010. Measuring behavior in mice with chronic stress depression paradigm. Prog Neuropsychopharmacol Biol Psychiatry 34:348–361. 10.1016/j.pnpbp.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 37.Sundbom R, Jacobsen KR, Kalliokoski O, Hau J, Abelson KSP. 2011. Post-operative corticosterone levels in plasma and feces of mice subjected to permanent catheterization and automated blood sampling. In Vivo 25:335–342. [PubMed] [Google Scholar]
- 38.Taconic Biosciences. [Internet]. 2018. C57BL/6 Mice | Black 6 inbred mouse strain. [Cited 27 October 2018]. Available at: https://www.taconic.com/mouse-model/black-6-b6ntac
- 39.Touma C, Palme R, Sachser N. 2004. Analyzing corticosterone metabolites in fecal samples of mice: a noninvasive technique to monitor stress hormones. Horm Behav 45:10–22. 10.1016/j.yhbeh.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 40.Van Loo PLP, Van Zutphen LFM, Baumans V. 2003. Male management: Coping with aggression problems in male laboratory mice. Lab Anim 37:300–313. 10.1258/002367703322389870. [DOI] [PubMed] [Google Scholar]
- 41.Wong D, Makowska IJ, Weary DM. 2012. Rat aversion to isoflurane versus carbon dioxide. Biol Lett 9:1–4. 10.1098/rsbl.2012.1000. [DOI] [PMC free article] [PubMed] [Google Scholar]