Abstract
Appropriate aseptic technique is a crucial component of rodent survival surgery. Ease of technique, surgical space constraint, batch surgery, and cost are factors that may affect researcher compliance with appropriate aseptic technique. The first part of this study compared 3 antiseptic preparation agents with the standard triplicate application of povidone-iodine and alcohol. Euthanized mice (n = 40) were shaved on the dorsum, and culture swabs were taken for RODAC plating and bacterial identification. Shaved sites were prepared by using one of the 4 antiseptic preparation agents. Culture samples were obtained immediately and at 20 min after antiseptic preparation. In the 2nd part of the study, 8 mice (n = 2 per group) were prepared for a survival surgical procedure by using one of the 4 antiseptic preparation agents to evaluate whether the antiseptic preparation agents caused skin irritation or impaired healing. Results from this study indicated that all 3 of the antiseptic agents evaluated were equally effective at reducing bacterial populations immediately and at 20 min after preparation. Histopathologic examination of the incision sites revealed signs of normal healing without lesions adjacent to the incision site. We conclude that all 3 of the products evaluated are comparable to traditional povidone–iodine and alcohol as agents for aseptic preparation of surgical sites.
Abbreviation: PA, povidone–iodine and alcohol
Rodents are routinely used to create research surgical models. In accordance with the Guide for the Care and Use of Laboratory Animals, rodent surgical procedures begin with the aseptic preparation of the patient, including hair removal and disinfection of the surgical site.16 Preoperative skin preparation serves to remove soil and microorganisms from the surgical site, reduce the resident microbial count without causing skin irritation, and inhibit rebound microorganism growth.8 Past studies have shown that rodents not only get postprocedural infections but are also used as models of infection.6,21 Although aseptic preparation of rodents is understood as beneficial, aseptic technique is not universally practiced.6 Batch surgeries—a series of survival surgeries performed in succession—small body size, surgical space restraints, and time and cost constraints are all potential limitations to the practice of appropriate aseptic technique.22 The question is: which antiseptic preparation procedures are most effective, efficient, and economical in the preparation of rodents for surgical procedures?
Surgical preparation methods include antiseptic surgical scrubs and solutions. Surgical scrubs contain an ionic detergent and are designed for preoperative preparation of intact skin before surgical incisions.39 Antiseptic agents without the detergent component are termed solutions.39 Triplicate application using alternating antiseptic scrub or solution and rinse has been the long-time standard in the veterinary field for preoperative skin preparation and is also the standard requirement for rodent surgery.3,7,15,26 A recent evaluation of triplicate application of skin antiseptic preparation agents found that skin preparation can be achieved with 1 or 2 applications of antiseptic preparation.7 Another study indicated that a different waterless alcohol-based agent was effective for antiseptic preparation after a single application in dogs.20 This current study further explores alternative antiseptic skin preparation agents for mice that are used in the medical profession.
The human medical field frequently uses skin antiseptic preparation agents that are alcohol-based solutions because they offer the benefit of quick preparation time and sustained and durable broad-spectrum antimicrobial activity.14,34 Alcohol-based prep solutions combine alcohol with povidone-iodine, chlorohexidine or zinc pyrithione providing increased and persistent antimicrobial activity.14 The agents we sought to evaluate include a solution that contains iodine povacrylex (0.7% available iodine) and 74% isopropyl alcohol (w/w; product 1);1,16 a solution with 2% w/v chlorhexidine gluconate in 70% isopropyl alcohol (product 2); and a 4% w/v chlorhexidine gluconate solution used for human patient aseptic skin preparation (product 3).23 Mechanisms of actions vary: product 1 denatures proteins and causes damage to bacterial DNA; product 2 denatures protein and disrupts bacterial membranes, and product 3 disrupts bacterial cell membranes.14 Studies on these 3 products or other similar compounds in veterinary patients is limited. Product 1 was as effective as traditional chlorhexidine gluconate scrub in companion animal preoperative evaluation but has not been evaluated in rodents.10 Product 2 has been used for veterinary patient antisepsis,31 but to our knowledge, efficacy has not been evaluated. Product 3 has not previously been evaluated in veterinary patients.
Although mouse and human skin are similar, several key differences may alter the effectiveness of the antiseptic agents. Both humans and mice have a distinct epidermis, dermis, and subcutis, but human skin is thicker overall.9 Unlike humans, mice have a panniculus carnosus and more hair follicles. The mouse epidermis comprises 2 or 3 layers of keratinocytes (humans have between 5 and 10 layers), resulting in a decreased barrier and greater absorption.9 The immunologic characteristics of human and mouse skin differ also. Human—but not mouse—neutrophil granulocytes produce antimicrobial peptides.9 Most laboratory mice reside in environments that expose surgical sites to bacteria present in feces, and the microorganisms on skin differs between mice and humans.25 For these reasons, it is important to validate the efficacy of these solutions for rodent survival surgery.
We compared the efficacy of 3 commercial products with the contemporary standard antiseptic preparation of 3 alternating povidone–iodine and 70% isopropyl alcohol wipes (PA) in regard to reduction in bacterial population immediately and at 20 min after preparation of the skin. Our hypothesis was that the 3 alcohol-based preparation agents would be as effective as triplicate application of PA for antiseptic preparation of mice. If these alternative agents prove to be effective, they would offer rodent surgeons alternative antiseptic preparation techniques that are quick, simple, and affordable.
Materials and Methods
Animals.
All procedures described were approved by the Stanford University's Administrative Panel on Laboratory Animal Care and performed in an AAALAC-accredited facility. For part 1, male (n = 20) and female (n = 20) C57BL/6J mice (age, 8 to 10 wk) previously scheduled for euthanasia were used to evaluate the antimicrobial efficacy of the antiseptic agents. For part 2, male and female (n = 2 per group) Balb/c mice (age, 3 mo; Charles River Laboratories, Hollister, CA) were used to evaluate the effect of the antiseptic preparation agents on skin pathology. All mice were housed under a 12:12-h light:dark cycle at a density of 2 to 4 mice per cage in a temperature-controlled vivarium as part of an AAALAC-accredited program. Health surveillance was performed through quarterly testing of dirty-bedding CD1 sentinels (Charles River Laboratories). Sentinels were consistently negative for mouse parvovirus, minute virus of mice, mouse hepatitis virus, enzootic diarrhea of infant mice, Theiler murine encephalomyelitis virus, mites (all species), lice (all species), and pinworms. On an annual schedule, sentinel mice were tested and considered free of Sendai virus, mouse adenovirus types 1 and 2, ectromelia virus, lymphocytic choriomenigitis virus, pneumonia virus of mice, reovirus 3, Mycoplasma pulmonis, Spironucleus muris, and Giardia muris.
Antiseptic prep agents.
Mice were prepared according to manufacturer's instructions or standard rodent surgical guidelines for parts 1 and 2 of the study. For product 1 (Duraprep solution, 3M, St Paul, MN), the sponge was held in downward position, and the operator pressed the cap end of applicator, allowing the solution to flow into the sponge. Beginning at the rostral edge of the shaved fur, the operator painted backward in a single uniform coat of solution on the skin by using light pressure. The solution was allowed to dry thoroughly on the skin for 2 min.
For product 2 (Chloraprep solution, Carefusion, El Paso, TX), the operator tore the pouch to access applicator handle. Beginning at the rostral edge of the shaved fur, the operator used the swab stick to paint caudally, applying a single uniform coat. By using additional swab sticks, this process was repeated twice to uniformly paint the area, after which it was allowed to dry for 1 min.
For product 3 (Hibiclens solution, Molnlye Health Care, Norcross, GA), the operator used a syringe to apply the solution (1 to 3 mL) to the center of surgical site and then used a cotton swab to paint outward to the edge of the shaved area. This process was repeated once. The area then was dried after application by using sterile gauze.
For PA (Povidone–iodine solution, Purdue Products, Stamford, CT, and 70% isopropyl Alcohol USP, Henry Schein, Melville, NY), the operator completed 3 consecutive scrubs, alternating povidone–iodine alternating with gauze soaked in 70% isopropyl alcohol. The agents were applied first to the center of the clipped area and moved outward in concentric circles.
Part 1—evaluating the efficacy of antiseptic preparation agents.
Experimental design.
Mice were divided randomly into 4 groups (products 1, 2, and 3 and PA; n = 10 per group) with equal numbers of males and females per group. Mice were euthanized by using carbon dioxide inhalation and held at room temperature in the preparation area. Hair on the dorsum was removed by using clippers and a no. 50 blade (Professional Animal Trimmer, Wahl, Sterling, IL), and fur was removed with tape. The clipped area was approximately 5 cm in length by 2.5 cm in width. Within 2 h of euthanasia, the 4 skin antiseptics were applied by the same person, who wore clean exam gloves, inside a Biosafety Class II Type A hood. Swabs were obtained at 3 time points (before preparation, immediately after preparation, and at 20 min after preparation) to evaluate antimicrobial efficacy and bacterial identifications.
Antimicrobial efficacy was assessed quantitatively by culturing surface bacteria on RODAC plates containing trypticase soy agar with lecithin and polysorbate 80 (BD BBL TSA with Lec and P80 RODAC, Becton Dickinson, Sparks, MD). A sterile swab stick premoistened with TSB (Tryptic Soy Broth, Hardy Diagnostics, Santa Maria, CA) was swabbed over the shaved area and then rolled on the RODAC plate. The swab was gently streaked over the surface of the plate several times in a zigzag fashion to evenly spread the inoculum across the surface of the agar. All culture plates were incubated aerobically at 35 °C with 5% CO2 and read at 24 and 48 h. For all samples, the colonies of each morphology were counted manually.
Bacterial identification.
Each bacterial isolate was plated onto tryptic soy agar plates containing 5% sheep blood (15 × 100 mm monoplate, Hardy Diagnostics). Isolates were incubated at 35 °C in 5% CO2 for a maximum of 5 d. Subsequently plates were observed for bacterial growth and basic morphologic characteristics, including colony pigmentation, size, and texture. Gram stains were performed to study microscopic morphology. After an incubation period of 18 to 24 h, bacterial growth was emulsified to the specified density in the inoculating fluid. Bacterial identifications were conducted automatically (OmniLog Identification System, Biolog, Hayward, CA). Briefly, the instrument tests a microorganism's chemical sensitivities and its ability to use (that is, oxidize) various carbon sources (GEN III MicroPlate, Biology). All necessary nutrients and biochemicals were prefilled and dried in the provided 96-well microplate. Tetrazolium redox dyes were used to colorimetrically indicate utilization of the carbon sources or resistance to inhibitory chemicals. Each microbe's ability to use the various carbon sources and their chemical sensitivities produced a unique pattern (that is, a ‘phenotypic fingerprint’) for that microbe. The fingerprint data were fed into the software, which searched its extensive database and made an identification.
Part 2—surgical procedure.
Experimental design.
Surgical procedures were performed in a dedicated surgical room. Personnel donned lab coats, surgical masks, disposable hair bonnets, and single-use nitrile gloves. Instruments were autoclaved prior to use and sterilized in a hot bead sterilizer (Fisher Scientific Tools, Foster City, CA) between surgeries. The surgical environment remained between 70 to 74 °F (°C) and 30% to 70% relative humidity. Mice were weighed on a gram scale, and anesthesia was induced by using isoflurane (3% to 5% in O2 at 2 L/min) in a 2-L transparent plastic induction box. Once a loss of righting reflex was observed, mice were placed in ventral recumbency on a disposable pad overlying a circulating warm-water heating pad (Stryker T/Pump, Portage, MI) set to 38 °C and sterile eye lubricant (Puralube, Dechra Pharmaceuticals, Northwich, United Kingdom) was applied to both eyes. Isoflurane was administered at 1.4% to 2% in O2 at 0.7 L/min through a nose cone to maintain a surgical plane of anesthesia throughout the procedure. Mucous membrane color and respiratory pattern were evaluated continuously, and paw withdrawal response was evaluated before and at 5-min intervals. Intraoperative temperature was recorded at the start of the procedure and every 5 min by using PhysioSuite RightTemp monitoring (Kent Scientific, Torrington, CT).
Once anesthetized, each mouse received a dose of carprofen (5 mg/kg SC; Rimadyl, Zoetis, Kalamazoo, MI), with a second dose administered 24 h later. The surgical area was shaved (approximately 2.5 cm × 2.5 cm, on the dorsum) by using clippers and a no. 50 blade (Professional Animal Trimmer, Wahl). Fur was removed by using tape. The shaved area was prepared with 1 of 4 antiseptic preparation agents (according to the technique described earlier and allowing for drying of agents), which were maintained at room temperature.
After presurgical preparation, mice were moved to the surgical station, placed in ventral recumbency on a warm-water heating pad, and draped with a sterile drape (Press N Seal, Glad, Oakland, CA). A circular hole was cut in the drape by using sterile scissors, and a 0.5-cm incision over the dorsum was made by using sterile surgical scissors (Figure 1). The surgical site was closed with a single sterile 7-mm stainless steel wound clip (Reflex 7 Skin Closure System, CellPoint Scientific, Gaithersburg, MD; Figure 2). From the induction of anesthesia, the entire procedure lasted approximately 20 min.
Figure 1.
The draped surgical skin site on the dorsum.
Figure 2.
The surgical site on the dorsum.
Mice recovered from anesthesia in a clean cage lined with paper towels on a warm-water blanket. Females and males were housed postoperatively in same-sex groups. Mice were weighed at 3, 7, and 10 d after surgery and monitored daily for any signs of pain or infection or inflammation at the surgical site. At 10 d postoperatively, mice were euthanized by using CO2 followed by cervical dislocation, and skin and tissues surrounding the surgical site were removed for histopathologic examination.
Tissue collection and histopathology.
Pelts were grossly examined for the presence or absence of wound clips. A section of pelt extending from the rostral scapular region to the base of the tail and laterally to the mid ventral body wall was collected. The pelt was laid flat on cardstock, air dried for 10 s, and immersion-fixed in 10% neutral-buffered formalin for 72 h. After fixation, a linear strip of haired skin was collected perpendicular to the long axis of the wound (as well as hair growth), including approximately 1 to 1.5 cm of tissue lateral to the wound (that is, nonwounded shaved skin exposed to topical preparation solution). A second linear strip of haired skin (perpendicular to hair growth) was collected from the intrascapular region for use as a control. Formalin-fixed tissues were processed routinely, embedded in paraffin, sectioned at 5 μm, and stained with hematoxylin and eosin.
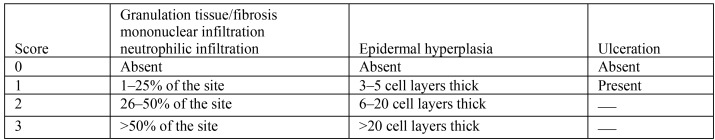
A board-certified veterinary pathologist (KMC) blindly evaluated stained sections at the following 3 locations: surgical wound site, 4-mm lateral to the surgical wound site and intrascapular skin (control). All sections were evaluated for the following histologic criteria: granulation tissue or fibrosis, epidermal hyperplasia, mononuclear infiltration, neutrophilic infiltration, and ulceration. A total score (Figure 3) was generated for each mouse by adding the subscores for each of the evaluated categories. Select sections were Gram-stained when they exhibited any of the following histologic features on H and E: neutrophilic infiltration, ulceration, or obvious intralesional bacteria.
Figure 3.
Histologic scoring criteria for mice and the antiseptic preparation agents.
Statistical analysis.
We conducted Kruskal–Wallis tests to determine the efficacy of each preparation agent in regard to the reduction in colony counts between before preparation to immediately afterward and from before preparation to 20 min after preparation. To determine the effect of the preparation agent on body temperature, a Kruskal–Walllis test was conducted using a sample of 8 mice (that is, 2 per group). Statistical significance was set at a P value of 0.05. All statistical analyses were performed by using JMP 11.0 (SAS Institute, Cary, NC).
Results
Evaluation of preparation agent efficacy.
The total bacterial count per plate before preparation for all skin sites was determined (minimum, 2 cfu; maximum, 1000 cfu; median, 19 cfu; interquartile range, 8.5 to 43.5 cfu). The bacteria present before antiseptic preparation varied, but the most commonly cultured bacteria were Staphylococcus aureus and Enterococcus faecalis (Table 1). Although the samples were taken from euthanized mice, time since euthanasia (data not shown) did not influence the bacterial skin flora isolated. After antiseptic preparation, only negative cultures were obtained.
Table 1.
Skin bacteria recovered before preoperative skin preparation in 40 mice (n = 10 per group)
| Product 1 | Product 2 | Product 3 | PA | Overall no. of positive samples (n = 40) | |
| Enterococcus faecalis | 3 | 7 | 2 | 8 | 20 |
| Micrococcus yunnanensis | 1 | 0 | 0 | 0 | 1 |
| Staphylococcus aureus | 4 | 6 | 8 | 8 | 26 |
| Staphylococcus epidermidis | 1 | 0 | 1 | 0 | 2 |
| Staphylococcus capitis ss capitis | 1 | 0 | 0 | 0 | 1 |
| Streptococcus intermedius | 1 | 0 | 1 | 0 | 2 |
| Streptococcus lutetiensis or infantarius or bovis (group D) | 1 | 0 | 0 | 0 | 1 |
| Escherichia coli | 0 | 1 | 0 | 2 | 3 |
| Streptococcus orisratti | 0 | 1 | 1 | 0 | 2 |
| Lactococcus raffinolactis | 0 | 0 | 1 | 0 | 1 |
| Leuconostoc mesenteroides ss dextranicum | 0 | 0 | 1 | 0 | 1 |
| Streptococcus infantis | 0 | 0 | 1 | 0 | 1 |
| Neisseria perflava | 0 | 0 | 1 | 0 | 1 |
| Lactobacillus fructivorans | 0 | 0 | 0 | 1 | 1 |
Data are given as the number of mice that yielded the indicated organism.
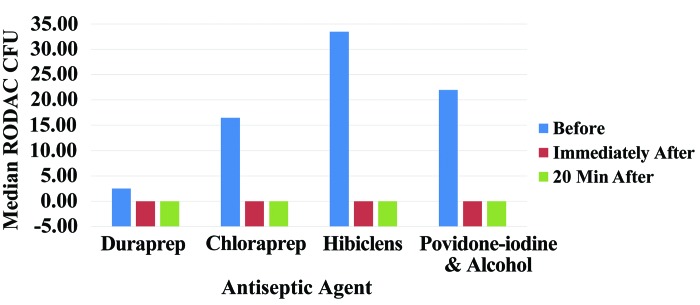
Postantisptic preparation samples (immediate and 20-min postpreparation) indicated no bacterial load. The percentage reductions in colony count from before to immediately after preparation and from before to 20 min after antiseptic preparation were compared for the 4 antiseptic preparation groups (Figure 4). The percentage reduction at immediately after application did not differ significantly (P = 0.212) across the 4 groups. Similarly, the percentage reduction in colony count at 20 min after preparation did not differ significantly (P = 1.00) across the 4 preparation groups. Median percentage reduction at 20 min was 100% for all groups.
Figure 4.
Median colony count (no. of cfu) on RODAC plates for each antiseptic agent group before, immediately after, and at 20 min after skin preparation.
Effect of preparation agent on core body temperature.
Baseline core body temperature at the start of the surgical procedure ranged from 35.5 to 36.5 °C and did not differ significantly (P = 0.06) between groups. The lowest intraoperative core body temperature after antiseptic preparation ranged from 33.0 to 37.5 °C (Table 2) and did not differ significantly (P = 0.212) across the 4 groups.
Table 2.
Lowest intraoperative core body temperature (° C; recorded every 5 min, mean ± 1 SD)
| Antiseptic group | Temperature |
| Product 1 | 34.4 ± 0.42 |
| Product 2 | 34.1 ± 1.48 |
| Product 3 | 34.9 ± 0.85 |
| Povidone–iodine and alcohol | 36.05 ± 0.49 |
Gross evaluation of skin.
At the time of euthanasia, all 8 mice evaluated exhibited hair regrowth within surgical prep regions. Wound clips were present in 6 of the 8 mice. Wound clips were absent in 2 of the 8 mice: one female mouse in the product 2 group and one female mouse from the product 1 group. Wound shape (tented compared with flat) corresponded directly to the presence or absence of wound clips at the time of euthanasia. That is, all mice with intact wound clips had tented surgical wounds, whereas mice lacking wound clips had flat, nonapposed surgical wounds. In all mice, shaved skin that was adjacent to the surgical wound and that received topical antiseptic application was grossly unremarkable.
Skin histopathology.
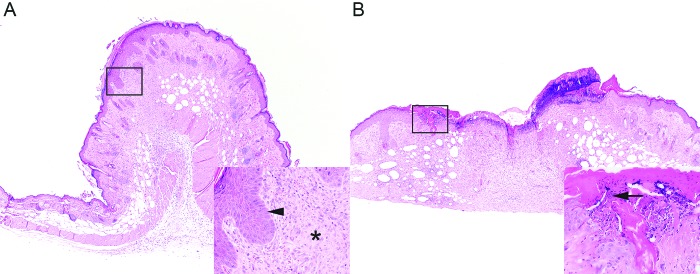
As expected, all mice had histologic lesions at the surgical wound site (Table 3). Generally mice with tented surgical wounds (Figure 5) exhibited various degrees of appropriate wound healing, including granulation tissue or fibrosis, epidermal hyperplasia, and mononuclear infiltrate. Low levels of neutrophilic infiltrates (less than 25% of the surgical site) were present in at least one mouse within each treatment group. Mice lacking wound clips (Figure 2) had higher total scores (F0 and F1), including superficial ulceration (F0) and intralesional bacteria (F0). Overall, higher total scores were seen for female mice, regardless of treatment group. Shaved regions of haired skin adjacent to the surgical wound (width, 4 mm) that received antiseptic application were histologically normal, as were sections of haired skin from the intrascapular regions (controls).
Table 3.
Gross appearance of wound sites and histologic scores for mice receiving topical antiseptic preparations.
| Histologic scores | ||||||||||
| Mouse | Sex | Treatment | Wound clip? | Wound shape | Granulation tissue or fibrosis | Epidermal hyperplasia | Mononuclear infiltrate | Neutrophilic infiltrate | Ulceration | Total score |
| M3 | M | PA | Present | Tented | 1 | 1 | 1 | 0 | 0 | 3 |
| F3 | F | PA | Present | Tented | 2 | 2 | 2 | 1 | 0 | 7 |
| M1 | M | Product 2 | Present | Tented | 1 | 2 | 1 | 0 | 0 | 4 |
| F1 | F | Product 2 | Absent | Flat | 3 | 3 | 2 | 1 | 0 | 9 |
| M0 | M | Product 1 | Present | Tented | 1 | 1 | 1 | 1 | 0 | 4 |
| F0 | F | Product 1 | Absent | Flat | 3 | 2 | 2 | 3 | 1 | 11a |
| M2 | M | Product 3 | Present | Tented | 2 | 2 | 1 | 1 | 0 | 7 |
| F2 | F | Product 3 | Present | Tented | 3 | 3 | 2 | 1 | 0 | 9 |
Intralesional bacteria evident in samples treated with hematoxylin–eosin and Gram stains.
Figure 5.
Histologic features of surgical wounds after the application of topical antiseptics. Regardless of treatment group, (A) mice with tented surgical wounds exhibited appropriate granulation tissue and fibrosis (inset, asterisk) and epidermal hyperplasia (inset, arrowhead). (B) Mice with flat surgical wounds exhibited additional histologic features, including ulceration and intralesional bacteria (inset, arrow). Hematoxylin and eosin stain; magnification, 4× (inset, 40×).
Discussion
Mice have been used as a model of surgical site infections, which are similar to those in humans and other veterinary patients.12,30 Most surgical site infections are the result of skin bacteria contaminating the incision site.19,36 When evaluating antiseptic preparation agents in mice, we sought to consider the spectrum of antimicrobial activity (including immediate and residual) on the skin and allergic or toxic skin reactions in mice.
The differing skin composition and external environmental factors between humans, mice, and other veterinary patients present challenges to effective antiseptic technique.9 Previous studies indicated that mice, like humans, have Staphylococci spp. as the most predominate skin flora.25,38 In our study, S. aureus as the most prevalent bacterial species in mice, rather than S. epidermidis, which is the predominant bacterial species on human skin.25 Overall the skin flora that we identified in mice was similar in composition to human skin, indicating that the antiseptic solutions used in humans would likely be effective in mice.
Antiseptic technique is critical to performing rodent survival surgical procedures.27 Surgical infections are important control both from the perspectives of animal welfare and promoting accurate research outcomes. In rodents, the number of bacteria within a surgical site is associated the risk of postprocedural infection.2,6 Culture samples taken after antiseptic preparation can be used to predict the possibility of postprocedural infections.28 The results of our current study suggest that all 3 of the commercial products we evaluated were as effective as PA for the antiseptic preparation of mice for surgery. Efficacy was defined as the absence of all bacteria at the immediate and 20-min postapplication time points.
Rodents can quickly develop hypothermia during anesthetic surgical procedures, potentially significantly affecting recovery5 and increasing the risk of surgical site infection through impairment of immune function.18,33 Past studies have indicated that excessive use of alcohol-based skin antiseptics can predispose animals to hypothermia.29 Using fewer repeated applications of antiseptic agents or single application of a waterless alcohol-based scrub can help mitigate loss of core body temperature.7 Our results indicated no significant difference between antiseptics on core body temperature. All survival-surgery mice showed similar high and low body temperatures. Unlike previous studies, we used a surgical drape to help maintain core body temperature and sterility during the procedure.7,35
Because the dermis is substantially thicker in humans than in mice, we felt it was important to evaluate whether application of any of the evaluated antiseptic agents had negative effects on skin physiology.11 However, histopathologic evaluation revealed no evidence of contact dermatitis, skin irritation, or other postoperative complications associated with any of the antiseptic agents. Differences in wound healing at the surgical site were seen in 2 mice that lacked wound clips, but this difference can be explained by second-intention wound healing.13 With second-intention healing, wounds heal through the formation of granulation tissue, contraction, and reepithelization, and increased care is needed to prevent infection.15 Second-intention healing was not restricted to a specific antiseptic agent, and the skin adjacent to the surgical site lacked histologic abnormalities, indicating that none of the antiseptic agents resulted in direct irritation or toxicity of the skin.
Cost may have a significant influence on the choice of aseptic methods for rodent surgery. In this study, we followed the manufacturer's recommendations and used all of the 3 sponges in a packet for a single preparation site. Skin preparation with product 1 was most expensive, at $4.60 per preparation. Product 2 was $1.60 per preparation, and the applicator sponges were smaller than those for product 1. The potential reuse of applicators for products 1 and 2 for purposes of cost savings should be further evaluated in a follow-up study. Product 3 was the least expensive, at $0.10/preparation when using 6 mL of solution per mouse. To evaluate PA, we used prepackaged 3 swab-stick povidone–iodine packets and individually packaged alcohol pads, resulting in a cost of $0.42 per preparation. These prepackaged swab sticks and pads are more expensive than purchasing bottles of povidone–iodine and alcohol, but in our experience researchers who value ease of use frequently elect to purchase these packaged options. Considering both ease of use and cost, researcher compliance may be highest with product 3.
Although our data have shown that all 3 commercial antiseptic agents used are effective, several limitations should be noted. Utilization of alcohol-based preparations is limited in terms of anatomic location of use. We evaluated the efficacy of the antiseptic agents specifically on the dorsum of the mouse. The composition of the skin differs between haired areas, the ear, and the tail.37 Because of potential irritation, alcohol-based products are contraindicated for use in mucous membranes, such as the anal mucosa.14,24 Alcohol can be absorbed in small amounts through the dermis and potentially can affect the biology of the animal. One study4 indicated that occluded application of 70% isopropanol to a 4.3-cm2 area of a shaved area of rat skin resulted in maximal absorption (males, 0.19 μmol/g; females, 0.24 μmol/g) at 4 h after application. It is unlikely that brief dermal contact of alcohol without occlusion would cause significant absorption. In our study, there was no evidence of behavioral changes, CNS depression, narcosis, or gastritis in mice undergoing survival surgery, but researchers who consider using alcohol-based antiseptics should consider all potential effects of alcohol absorption on their studies. There have been reports of operating room fires as a result of alcohol-based skin preparation agent use.17,32 This risk can be avoided by following manufacturer's recommendations regarding drying time and avoiding pooling.17
Our findings indicate that all 3 alcohol-based preparation agents are as effective as triplicate application of PA for the antiseptic preparation of mice. These alternative antiseptic agents offer ease of application, and the use of product 3 potentially can decrease cost, resulting in improved compliance during rodent surgeries.
Acknowledgments
We thank the Veterinary Service Center Diagnostic Laboratory and the Comparative Medicine Animal Histology Service Center for their support and assistance with bacterial culture testing and slide preparation, respectively. We thank Amy Vasquez for her assistance with statistical analysis.
References
- 1.3M. 2018. [Internet]. 3M DuraPrep Surgical Solution [product label]. [Cited 22 June 2018]. Available at: https://www.3m.com/3M/en_US/company-us/all-3m-products/~/3M-DuraPrep-Surgical-Solution-Iodine-Povacrylex-0-7-available-iodine-and-Isopropyl-Alcohol-74-w-w-Patient-Preoperative-Skin-Preparation-8630-26-mL/?N=5002385+3293500411&rt=rud.
- 2.Badia JM, Torres JM, Tur C, Sitges-Serra A. 1996. Saline wound irrigation reduces the postoperative infection rate in guinea pigs. J Surg Res 63:457–459. 10.1006/jsre.1996.0292. [DOI] [PubMed] [Google Scholar]
- 3.Bernal J, Baldwin M, Gleason T, Kuhlman S, Moore G, Talcott M. 2009. Guidelines for rodent survival surgery. J Invest Surg 22:445–451. 10.3109/08941930903396412. [DOI] [PubMed] [Google Scholar]
- 4.Boatman RJ, Perry LG, Fiorica LA, English JC, Kapp RW, Jr, Bevan C, Tyler TR, Banton MI, Wright GA. 1998. Dermal absorption and pharmacokinetics of isopropanol in the male and female F-344 rat. Drug Metab Dispos 26:197–202. [PubMed] [Google Scholar]
- 5.Caro AC, Hankenson FC, Marx JO. 2013. Comparison of thermoregulatory devices used during anesthesia of C57BL/6 mice and correlations between body temperature and physiologic parameters. J Am Assoc Lab Anim Sci 52:577–583. [PMC free article] [PubMed] [Google Scholar]
- 6.Cooper DM, McIver R, Bianco R. 2000. The thin blue line: a review and discussion of aseptic technique and postprocedural infections in rodents. Contemp Top Lab Anim Sci 39:27–32. [PubMed] [Google Scholar]
- 7.Del Valle JM, Fisk EA, Noland EL, Pak D, Zhang J, Crim MJ, Lawrence FR, Hankenson FC. 2018. Comparison of aqueous and alcohol-based agents for presurgical skin preparation methods in mice. J Am Assoc Lab Anim Sci 57:401–414. 10.30802/AALAS-JAALAS-17-000128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fossum TW. 2019. Small animal surgery, 5 ed. St Louis (MO): Elsevier Mosby. [Google Scholar]
- 9.Gerber PA, Buhren BA, Schrumpf H, Homey B, Zlotnik A, Hevezi P. 2014. The top skin-associated genes: a comparative analysis of human and mouse skin transcriptomes. Biol Chem 395:577–591. 10.1515/hsz-2013-0279. [DOI] [PubMed] [Google Scholar]
- 10.Gibson KL, Donald AW, Hariharan H, McCarville C. 1997. Comparison of 2 presurgical skin preparation techniques. Can J Vet Res 61:154–156. [PMC free article] [PubMed] [Google Scholar]
- 11.Gudjonsson J, Johnston A, Dyson M, Valdimarsson H, Elder J. 2007. Mouse models of psoriasis. J Invest Dermatol 127:1292–1308. 10.1038/sj.jid.5700807 [DOI] [PubMed] [Google Scholar]
- 12.Håkansson J, Björn C, Lindgren K, Sjöström E, Sjöstrand V, Mahlapuu M. 2014. Efficacy of the Novel topical antimicrobial agent PXL150 in a mouse model of surgical site infections. Antimicrob Agents Chemother 58:2982–2984. 10.1128/AAC.00143-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Han S-K. 2015. Basics of wound healing, p 1–37. Innovations and advances in wound healing. Berlin, Heidelberg: Springer Berlin Heidelberg. [Google Scholar]
- 14.Hemani ML, Lepor H. 2009. Skin preparation for the prevention of surgical site infection: which agent is best? Rev Urol 11:190–195. [PMC free article] [PubMed] [Google Scholar]
- 15.Hoogstraten-Miller SL, Brown PA. 2008. Techniques in aseptic rodent surgery. Curr Protoc Imunol 82: 1.12.1-1–1.12-14. 10.1002/0471142735.im0112s82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Institute for Laboratory Animal Research. 2011. Guide for the care and use of laboratory animals, 8th ed. Washington (DC): National Academies Press. [Google Scholar]
- 17.Jones EL, Overbey DM, Chapman BC, Jones TS, Hilton SA, Moore JT, Robinson TN. 2017. Operating room fires and surgical skin preparation. J Am Coll Surg 225:160–165. 10.1016/j.jamcollsurg.2017.01.058. [DOI] [PubMed] [Google Scholar]
- 18.Kurz A, Sessler DI, Lenhardt R. 1996. Perioperative normothermia to reduce the incidence of surgical-wound infection and shorten hospitalization. Study of wound infection and temperature group. N Engl J Med 334:1209–1216. [DOI] [PubMed] [Google Scholar]
- 19.Lowbury EJ, Lilly HA, Bull JP. 1960. Disinfection of the skin of operation sites. Br Med J 2:1039–1044. 10.1136/bmj.2.5205.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maxwell EA, Bennett RA, Mitchell MA. 2018. Efficacy of application of an alcohol-based antiseptic hand rub or a 2% chlorhexidine gluconate scrub for immediate reduction of the bacterial population on the skin of dogs. Am J Vet Res 79:1001–1007. 10.2460/ajvr.79.9.1001. [DOI] [PubMed] [Google Scholar]
- 21.McRipley RJ, Whitney RR. 1976. Characterization and quantitation of experimental surgical-wound infections used to evaluate topical antibacterial agents. Antimicrob Agents Chemother 10:38–44. 10.1128/AAC.10.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mendenhall V, Baran S, Johnson E, Perret-Gentil M. [Internet]. 2010. Aseptic technique in rodent surgery: Why should I pay attention? [Cited 11 February 2019]. Available at: https://www.laboratoryequipment.com/article/2010/03/aseptic-technique-rodent-surgery-why-should-i-pay-attention.
- 23.Molnlycke. [Internet]. 2018. Hibiclens. A 4% chlorhexidine gluconate solution for patient skin antisepsis. [product label]. [Cited 22 June 2018]. Available at: https://www.molnlycke.us/products-solutions/hibiclens/
- 24.Müller CSL, Hubner W, Thieme-Ruffing S, Pföhler C, Vogt T, Volk T, Gärtner BC, Bialas P. 2017. Pre- and perioperative aspects of dermatosurgery. J Dtsch Dermatol Ges 15:117–146. [DOI] [PubMed] [Google Scholar]
- 25.Nagase N, Sasaki A, Yamashita K, Shimizu A, Wakita Y, Kitai S, Kawano J. 2002. Isolation and species distribution of staphylococci from animal and human skin. J Vet Med Sci 64:245–250. 10.1292/jvms.64.245. [DOI] [PubMed] [Google Scholar]
- 26.Osuna DJ, DeYoung DJ, Walker RL. 1990. Comparison of 3 skin preparation techniques in the dog. Part 1: Experimental trial. Vet Surg 19:14–19. 10.1111/j.1532-950X.1990.tb01136.x. [DOI] [PubMed] [Google Scholar]
- 27.Pritchett-Corning KR, Mulder GB, Luo Y, White WJ. 2011. Principles of rodent surgery for the new surgeon. J Vis Exp 2011:2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Psaila JV, Wheeler MH, Crosby DL. 1977. The role of plastic wound drapes in the prevention of wound infection following abdominal surgery. Br J Surg 64:729–732. 10.1002/bjs.1800641012. [DOI] [PubMed] [Google Scholar]
- 29.Redrobe S. 2002. Soft tissue surgery of rabbits and rodents. Seminars in Avian and Exotic Pet Medicine 11:231–245. [Google Scholar]
- 30.Rittenhouse S, Singley C, Hoover J, Page R, Payne D. 2006. Use of the surgical wound infection model to determine the efficacious dosing regimen of retapamulin, a novel topical antibiotic. Antimicrob Agents Chemother 50:3886–3888. 10.1128/AAC.00183-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roberts C. 2014. Reducing surgical site infections. Veterinary Nursing Journal 28:211–217. 10.1111/vnj.12045. [DOI] [Google Scholar]
- 32.Rocos B, Donaldson LJ. 2012. Alcohol skin preparation causes surgical fires. Ann R Coll Surg Engl 94:87–89. 10.1308/003588412X13171221501221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sessler DI. 1997. Mild perioperative hypothermia. N Engl J Med 336:1730–1737. [DOI] [PubMed] [Google Scholar]
- 34.Sidhwa F, Itani KM. 2015. Skin preparation before surgery:options and evidence. Surg Infect (Larchmt) 16:14–23. [DOI] [PubMed] [Google Scholar]
- 35.Skorupski AM, Zhang J, Ferguson D, Lawrence F, Hankenson FC. 2017. Quantification of induced hypothermia from aseptic scrub applications during rodent surgery preparation. J Am Assoc Lab Anim Sci 56:562–569. [PMC free article] [PubMed] [Google Scholar]
- 36.Stevens DA. 1974. Infection control in the hospital. A report on workshop discussions. West J Med 120:267–269. [PMC free article] [PubMed] [Google Scholar]
- 37.Sundberg JP, Nanney LB, Fleckman P, King LE. 2012. Skin and adnexa. Chapter 23. p 433–455. In: Treuting PM, Dintzis SM. Comparative anatomy and histology. San Diego (CA): Academic Press. [Google Scholar]
- 38.Tavakkol Z, Samuelson D, deLancey Pulcini E, Underwood RA, Usui ML, Costerton JW, James GA, Olerud JE, Fleckman P. 2010. Resident bacterial flora in the skin of C57BL/6 mice housed under SPF conditions. J Am Assoc Lab Anim Sci 49:588–591. [PMC free article] [PubMed] [Google Scholar]
- 39.Trott AT. 2012. Wound cleansing and irrigation, Chapter 7. p 73–81. In: Trott AT. Wounds and lacerations: Emergency care and closure, 4th ed Philadelphia (PA): Elsevier–Saunders. [Google Scholar]