Abstract
Merkel cell carcinoma (MCC) is a rare, rapidly proliferating skin cancer that commonly metastasizes to regional lymph nodes. We present the case of a 73-year-old woman with a history of MCC and non-Hodgkin B-cell lymphoma who presented with second-degree heart block (Mobitz type II) caused by an interatrial mass. Temporary pacing was required before biopsy, which revealed metastatic MCC. Treatment included permanent pacing, anti–programmed cell death ligand 1 immunotherapy, and radiation to the heart resulting in notable decrease in tumor size and normalized cardiac rhythm.
Abbreviations and Acronyms: CM, cardiac metastases; CT, computed tomography; FDG, [18F]-fluorodeoxyglucose; MCC, Merkel cell carcinoma; MCPyV, Merkel cell polyomavirus; MRI, magnetic resonance imaging; PD-L1, programmed cell death ligand 1; PET, positron emission tomography
Merkel cell carcinoma (MCC) is an aggressive cutaneous skin cancer that primarily affects older adults with lighter skin tones.1,2 Although rare, its incidence continues to increase with the expanding elderly population, and 2835 cases are predicted for 2020 in the United States.1 Risk factors include light skin, older age (mean age at diagnosis, 75 years), and a history of immunosuppression or B-cell malignancy.3 Local recurrence is common along with metastasis to regional lymph nodes. Lesions appear as painless, firm, nontender, shiny, flesh-colored or blue/red intracutaneous nodules that rarely ulcerate or have crusting.2 Most commonly found in sun-exposed areas, lesions can range from 1 cm to over 2 cm. The most common location is the head, neck, and upper limbs including the shoulder. Differential diagnosis should include other benign and malignant lesions found on sun-exposed areas including basal cell carcinoma, keratoacanthoma, amelanotic melanoma, pyogenic granuloma, lipoma, and adnexal tumors.2 The acronym AEIOU can be used to describe the clinical features that suggest a diagnosis of MCC: asymptomatic, expanding rapidly (notable growth in less than 3 months), immunosuppression history (HIV infection, transplant, B-cell malignancy), older than 50 years, and UV light–exposed area in a fair-skinned person.3 The presence of 3 or more of these features increases suspicion for MCC. Tumor biopsy is required to confirm the diagnosis.4 Malignant cells are commonly arranged in nests with frequent mitosis. Immunohistochemical studies reveal features of epithelial and neuroendocrine cells including expression of pancytokeratin, perinuclear punctate cytokeratin 20 immunoreactivity, and epithelial membrane antigen along with chromogranin and synaptophysin.5, 6, 7 Tumorigenesis commonly occurs due to DNA damage from UV exposure. Indeed, many mutations found in malignant cells are characteristic of UV mutagenesis and involve tumor suppressor genes such as TP53.1 Merkel cell polyomavirus (MCPyV) also contributes to malignancy. As part of the normal skin microbiome, MCPyV integrates into the host genome and encodes proteins that target the RB1 tumor suppressor gene, preventing its expression and driving tumor growth. In patients with MCPyV-positive tumors, viral antigen titers can be used to monitor disease recurrence.8,9
TNM staging is used to determine management and prognosis.4,10 Because of the high frequency of metastasis, thorough examination of the skin and regional lymph nodes along with imaging (positron emission tomography [PET] with [18F]-fluorodeoxyglucose [FDG] combined with computed tomography [CT] or magnetic resonance imaging [MRI]) are important in determining stage and management.4 For patients with negative sentinel lymph node biopsy, wide local excision of the lesion is indicated unless not feasible, in which case radiation therapy is used.11,12 If regional lymph nodes are involved, a combination of node dissection and radiation therapy is indicated; alternatively, only one of these approaches may be used.13,14 If distant metastases are found, systemic therapy is required. Metastatic disease unresponsive to systemic therapy warrants local treatment with surgery or radiation.14 In patients at high risk of recurrence, radiation therapy is indicated even in the absence of lymph node involvement or metastasis.14 Features of high-risk disease include primary tumor size greater than 1 cm in any dimension, a head and neck primary tumor, surgical resection margins positive for cancer, lymphovascular invasion, multiple involved nodes, extracapsular lymph node extension, or an immunocompromised host. These patients may also be eligible to enroll in a clinical trial of adjuvant immunotherapy. Avelumab (anti–programmed cell death ligand 1 [PD-L1]), pembrolizumab (anti–programmed cell death protein 1 [PD-1]), and nivolumab (PD-1) have shown antitumor activity in patients with metastatic disease and are indicated in this population.11,12,15, 16, 17, 18, 19, 20, 21 Their efficacy as neoadjuvant treatment, however, remains to be determined.
We present the case of a patient with a history of MCC and non-Hodgkin B-cell lymphoma who presented with heart block caused by cardiac metastasis. Biopsy of the interatrial mass revealed metastatic MCC, which was treated with permanent pacing, immunotherapy, and radiation to the heart leading to a notable decrease in the size of the cardiac mass.
Report of Case
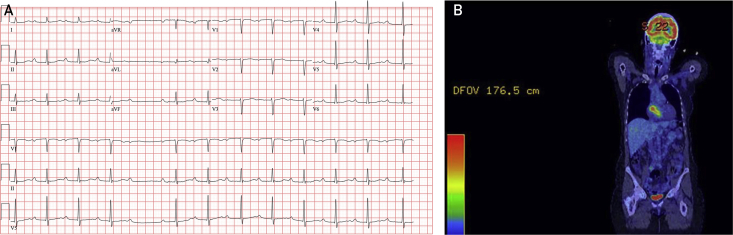
A 73-year-old white woman presented to the emergency department with dyspnea, throat pain, and an apparent awareness of bradycardia. Electrocardiography revealed a second-degree atrioventricular block with a narrowed QRS complex consistent with Mobitz type II and involvement of the bundle branch below the level of the atrioventricular node, requiring temporary pacemaker placement (Figure 1).
Figure 1.
A, Electrocardiogram at presentation shows sinus rhythm with prolonged PR interval, narrow QRS complex, and one dropped ventricular beat suggestive of second-degree atrioventricular block, Mobitz type II, which prompted temporary pacing. B, Coronal PET image. The atrial mass exhibits [18F]-fluorodeoxyglucose avidity up to a maximum standardized uptake value of 7.2. Image also demonstrates physiologic uptake in the brain and bladder. PET = positron emission tomography.
The patient’s medical history was notable for splenic marginal zone lymphoma diagnosed in 2011, for which she initially underwent splenectomy followed by 2 rounds of rituximab therapy (4 doses) for cytopenia due to progressive bone marrow involvement (2013 and 2017). Merkel cell carcinoma was diagnosed in 2017, presenting as a 6-cm axillary mass. Because the mass was not surgically resectable, radiation therapy (60 Gy/30 fractions) and pembrolizumab (4 doses) were administered without notable vascular or neurologic injury. Follow-up imaging revealed complete resolution of the malignancy. Additional medical history included osteopenia, hyperlipidemia, lymphedema of the left arm (postoperative), subclinical hypothyroidism, colon polyp, and coronary artery calcification.
At the current hospitalization, echocardiography revealed a 1.7-cm mass around the aortic root circumference posterior to the aortic valve (Figure 2). Left ventricular ejection fraction was normal at 75%. Subsequent PET-CT revealed moderate FDG avidity throughout the aortic root mass. Considering her previous history of malignancy, this finding could be explained by transformed lymphoma or metastatic MCC. Cardiac MRI confirmed the location of this ill-defined mass measuring approximately 5.3 × 4.3 × 3.4 cm (Figure 2). The mass encased two-thirds of the aortic root posteriorly and laterally without definite intraluminal extension. It also partially encased the right coronary artery. There were papillarylike projections into the right atrial lumen. There was gradual low-level enhancement of the mass on delayed enhancement images. The MRI findings were reported as concerning for lymphoma. These findings were consistent with those seen on whole-body PET (Figure 1), showing moderate FDG avidity throughout the mass with a maximum standardized uptake value of up to 7.2. There was no other evidence of malignancy.
Figure 2.
Imaging of intracardiac mass and guidance for biopsy. A, Atrial mass on magnetic resonance imaging, 4-chamber view showing the mass circumscribing the aortic root. In this view, the mass measures 35.7 mm. B, Initial transthoracic echocardiogram, Mayo format apical 4-chamber view of mass along interatrial septum. C-E, Imaging for biopsy procedural guidance. C, Anteroposterior projection of flouroscopy. D, Intracardiac echocardiogram showing proximity of the mass to the aortic root. E, Transthoracic echocardiogram showing bioptome at the mass at a level where the left atrium is distal to the mass rather than the aortic root. AV = aortic valve; B = bioptome; ECG = external electrocardiogram wire; ICE = intracardiac echocardiogram probe; LA = left atrium; LV = left ventricle; M = mass; PM = right ventricular pacemaker lead; RA = right atrium; RV = right ventricle; TTE = transthoracic echocardiogram probe.
Because of the dichotomous treatment of transformed lymphoma involving the heart vs an isolated focus of metastatic MCC (combination chemotherapy vs immune checkpoint blockade, respectively), a biopsy was required for definitive diagnosis before initiation of therapy. The patient was brought to the cardiac catheterization laboratory for transvenous biopsy of the mass in the right atrium with fluoroscopic transthoracic echocardiographic and intracardiac echocardiographic guidance. An 8-F sheath was placed in the right internal jugular vein for intracardiac echocardiography. A 7-F sheath was placed in the right femoral vein for the bioptome. Given the proximity to the aortic root, concurrent transthoracic echocardiography was used to obtain images of the biopsy sites from orthogonal angles (Figure 2). Three tan 0.1- to 0.3-cm samples were obtained without complications. Histologic and immunohistochemical studies confirmed MCC (Figure 3). Given concerns for tumor swelling induced by systemic therapy with an immune checkpoint inhibitor (pseudoprogression) that could further compromise the heart block, the temporary pacemaker was replaced by a permanent single ventricular lead pacemaker before initiation of systemic therapy.
Figure 3.
A-D, Metastatic Merkel cell carcinoma histopathology. Lesional cells demonstrated sheetlike growth with fine nuclear chromatin, scant cytoplasm, and abundant mitotic activity (A and B, hematoxylin-eosin, original magnification ×40 and ×100, respectively). The diagnosis was confirmed with immunoreactivity for CD56 (C, original magnification ×400) and classic perinuclear dotlike reactivity with CK20 (D, original magnification ×400). E, Scan obtained at time of admission when the patient presented with heart block, demonstrating the size and location of the mass around the aortic root. F, Scan 2 months after starting treatment.
Treatment was initiated with systemic anti–PD-L1 immunotherapy (avelumab) and concurrent radiation to the heart. Radiotherapy consisted of 40 Gy given in 5 fractions using intensity modulation and delivered over a week’s time. Treatment was complicated by severe esophagitis restricted to the lower esophagus, requiring percutaneous gastric tube placement for nutrition. Six weeks after starting avelumab and 3 weeks after radiation, CT of the chest with intravenous contrast material revealed a notable reduction in the size of the cardiac mass and diffuse thickening of the wall of the lower esophagus consistent with radiation-induced esophagitis. A follow-up scan 2 months after treatment revealed ongoing decrease in tumor size, with the intracardiac mass measuring 2.6 × 2.1 cm in the greatest dimension compared with 6.2 × 5.3 cm on examination at presentation (Figure 3). Marginal zone B-cell lymphoma, or transformed higher-grade B-cell lymphomas, as well as MCC, rarely involve the heart. To our knowledge, this is the first reported case of MCC metastatic to the heart presenting with conduction abnormalities and successfully treated with combination anti–PD-L1 therapy and radiation to the heart.
Discussion
Merkel cell carcinoma is an aggressive cutaneous neuroendocrine tumor that commonly presents with local recurrence and regional lymph node involvement. Treatment includes excision of the primary lesion and involved lymph nodes with radiation to the tumor base in cases with nodal involvement or inadequate resection.4,13 Metastatic disease is treated with immune checkpoint inhibitors such as avelumab, pembrolizumab, or nivolumab.11,12,15, 16, 17 Although metastasis to the muscle, cartilage, and fascia is common, cardiac metastasis is very rare. In one reported case, an MCC tumor was found in the pericardial fluid of a patient presenting with cardiac tamponade.22 We present the first reported case of MCC leading to compression of the conduction machinery within the heart, causing this patient’s symptoms of dyspnea and bradycardia. Mobitz type II heart block was diagnosed on electrocardiography, with periods of third-degree heart block requiring pacemaker placement (Figure 1).
Echocardiography revealed a mass in the right atrium that was confirmed and further characterized by cardiac MRI (Figure 2). Temporary and eventually permanent pacing was required for cardiovascular stabilization before biopsy and treatment. In the absence of other sites of cancer, a cardiac biopsy was required for diagnosis. Biopsy confirmed MCC recurrence, and the patient was subsequently treated with radiation to the heart and avelumab immunotherapy. After radiation and 2 doses of immunotherapy, the size of the cardiac mass decreased considerably (Figure 3). The patient is doing well and continues with avelumab therapy and symptomatic treatment for radiation-induced esophagitis.
The most common cardiac tumors are metastatic and often result from melanoma or primary mediastinal tumors. As better diagnostic and treatment methods improve the longevity of patients with cancer, the incidence of cardiac metastases (CM) is increasing.23 In patients with disseminated disease, the prevalence of CM approaches 10%. Most cases are clinically silent and only found on postmortem examination. When symptomatic, CM can mimic other common cardiovascular diseases such as right-sided heart failure, pulmonary edema, or systolic dysfunction. In severe cases, CM can cause arrhythmia, chest pain, or cardiac tamponade leading to death if not diagnosed and treated aggressively.23 Cardiac metastases should be suspected in patients with cancer who have disseminated disease and present with sudden onset of cardiac or respiratory symptoms. Although MCC with systemic metastasis is treated with immunotherapy, no standard-of-care therapy exists for CM, which is also true of other malignancies that spread to the heart. Although radiation therapy can augment the efficacy of immunotherapy, safely delivering radiation to the heart remains a major challenge because of radiation-induced toxicity.23 Thus, cardiac radiation is often only used in palliative care for CM. The best dose and fractionation must be individualized on a case-by-case basis depending on patient characteristics, tumor type and location, and previous treatment. In the largest retrospective review involving 10 cases of different malignancies, it was found that the maximum response time after receiving cardiac radiation therapy for palliative care was 11 months. Compared with our patient, this cohort received much lower doses and fewer fractions of radiation.23 In the short term, cardiac irradiation can lead to various complications including pericarditis, myocarditis, valve damage, arteritis, ventricular dysfunction, arrhythmia, and esophagitis. Additionally, tumor thrombosis can result and cause outflow obstruction in the lungs or systemic circulation. Pericarditis is the most common complication and presents 2 to 6 months after treatment. Considering the severity of progressive heart block in this patient with MCC in the heart, radiation therapy was warranted to reduce the size of the tumor and improve the efficacy of immunotherapy. Combined with systemic immunotherapy, this treatment has resulted in decreased tumor size and improved cardiac function in this patient.
Acknowledgments
Ms Kazemi and Dr Jain contributed equally to this work.
Footnotes
Potential Competing Interests: The authors report no competing interests.
References
- 1.Paulson K.G., Park S.Y., Vandeven N.A., et al. Merkel cell carcinoma: current US incidence and projected increases based on changing demographics. J Am Acad Dermatol. 2018;78(3):457–463.e2. doi: 10.1016/j.jaad.2017.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heath M., Jaimes N., Lemos B., et al. Clinical characteristics of Merkel cell carcinoma at diagnosis in 195 patients: the AEIOU features. J Am Acad Dermatol. 2008;58(3):375–381. doi: 10.1016/j.jaad.2007.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Albores-Saavedra J., Batich K., Chable-Montero F., Sagy N., Schwartz A.M., Henson D.E. Merkel cell carcinoma demographics, morphology, and survival based on 3870 cases: a population based study. J Cutan Pathol. 2010;37(1):20–27. doi: 10.1111/j.1600-0560.2009.01370.x. [DOI] [PubMed] [Google Scholar]
- 4.Wong H.H., Wang J. Merkel cell carcinoma. Arch Pathol Lab Med. 2010;134(11):1711–1716. doi: 10.5858/2009-0165-RSR2.1. [DOI] [PubMed] [Google Scholar]
- 5.Warner T.F., Uno H., Hafez G.R., et al. Merkel cells and Merkel cell tumors: ultrastructure, immunocytochemistry and review of the literature. Cancer. 1983;52(2):238–245. doi: 10.1002/1097-0142(19830715)52:2<238::aid-cncr2820520209>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 6.Chan J.K., Suster S., Wenig B.M., Tsang W.Y., Chan J.B., Lau A.L. Cytokeratin 20 immunoreactivity distinguishes Merkel cell (primary cutaneous neuroendocrine) carcinomas and salivary gland small cell carcinomas from small cell carcinomas of various sites. Am J Surg Pathol. 1997;21(2):226–234. doi: 10.1097/00000478-199702000-00014. [DOI] [PubMed] [Google Scholar]
- 7.Feng H., Shuda M., Chang Y., Moore P.S. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science. 2008;319(5866):1096–1100. doi: 10.1126/science.1152586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carter J.J., Paulson K.G., Wipf G.C., et al. Association of Merkel cell polyomavirus-specific antibodies with Merkel cell carcinoma. J Natl Cancer Inst. 2009;101(21):1510–1522. doi: 10.1093/jnci/djp332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harms K.L., Healy M.A., Nghiem P., et al. Analysis of prognostic factors from 9387 Merkel cell carcinoma cases forms the basis for the new 8th edition AJCC staging system. Ann Surg Oncol. 2016;23(11):3564–3571. doi: 10.1245/s10434-016-5266-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lebbe C., Becker J.C., Grob J.J., et al. European Dermatology Forum (EDF), the European Association of Dermato-Oncology (EADO), and the European Organization for Research and Treatment of Cancer (EORTC). Diagnosis and treatment of Merkel cell carcinoma: European consensus-based interdisciplinary guideline. Eur J Cancer. 2015;51(16):2396–2403. doi: 10.1016/j.ejca.2015.06.131. [DOI] [PubMed] [Google Scholar]
- 11.Harrington C., Kwan W. Outcomes of Merkel cell carcinoma treated with radiotherapy without radical surgical excision. Ann Surg Oncol. 2014;21(11):3401–3405. doi: 10.1245/s10434-014-3757-8. [DOI] [PubMed] [Google Scholar]
- 12.Strom T., Carr M., Zager J.S., et al. Radiation therapy is associated with improved outcomes in Merkel cell carcinoma. Ann Surg Oncol. 2016;23(11):3572–3578. doi: 10.1245/s10434-016-5293-1. [DOI] [PubMed] [Google Scholar]
- 13.Nghiem P., Bhatia S., Daud A., et al. Activity of PD-1 blockade with pembrolizumab as first systemic therapy in patients with advanced Merkel cell carcinoma. Eur J Cancer. 2015;51(suppl 3):S720–S721. Abstract 22LBA. [Google Scholar]
- 14.Topalian S.L., Bhatia S., Kudchadkar R.R., et al. Nivolumab (Nivo) as neoadjuvant therapy in patients with resectable Merkel cell carcinoma (MCC) in CheckMate 358 [abstract] J Clin Oncol. 2018;36(15, suppl):9505. doi: 10.1200/JCO.20.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaufman H.L., Russell J., Hamid O., et al. Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: a multicentre, single-group, open-label, phase 2 trial. Lancet Oncol. 2016;17(10):1374–1385. doi: 10.1016/S1470-2045(16)30364-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaufman H.L., Russell J.S., Hamid O., et al. Updated efficacy of avelumab in patients with previously treated metastatic Merkel cell carcinoma after ≥1 year of follow-up: JAVELIN Merkel 200, a phase 2 clinical trial. J Immunother Cancer. 2018;6(1):7. doi: 10.1186/s40425-017-0310-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nghiem P., Bhatia S., Brohl A.S., et al. Two-year efficacy and safety update from JAVELIN Merkel 200 part A: a registrational study of avelumab in metastatic Merkel cell carcinoma progressed on chemotherapy [abstract] J Clin Oncol. 2018;36(15, suppl):9507. [Google Scholar]
- 18.Nghiem P., Bhatia S., Lipson E.J., et al. Durable tumor regression and overall survival in patients with advanced Merkel cell carcinoma receiving pembrolizumab as first-line therapy. J Clin Oncol. 2019;37(9):693–702. doi: 10.1200/JCO.18.01896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nghiem P.T., Bhatia S., Lipson E.J., et al. PD-1 blockade with pembrolizumab in advanced Merkel-cell carcinoma. N Engl J Med. 2016;374(26):2542–2552. doi: 10.1056/NEJMoa1603702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nghiem P., Bhatia S., Lipson E.J., et al. Durable tumor regression and overall survival (OS) in patients with advanced Merkel cell carcinoma (aMCC) receiving pembrolizumab as first-line therapy [abstract] J Clin Oncol. 2018;36(15, suppl):9506. doi: 10.1200/JCO.18.01896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Topalian S.L., Bhatia S., Hollebecque A., et al. Non-comparative, open-label, multiple cohort, phase 1/2 study to evaluate nivolumab (NIVO) in patients with virus-associated tumors (CheckMate 358): efficacy and safety in Merkel cell carcinoma (MCC) [abstract] Cancer Res. 2017;77(13, suppl):CT074. [Google Scholar]
- 22.Di Loreto M., Francis R. Merkel cell carcinoma cardiac metastasis causing cardiac tamponade. BMJ Case Rep. 2017;2017 doi: 10.1136/bcr-2017-221311. bcr-2017-221311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fotouhi Ghiam A., Dawson L.A., Abuzeid W., et al. Role of palliative radiotherapy in the management of mural cardiac metastases: who, when and how to treat? a case series of 10 patients. Cancer Med. 2016;5(6):989–996. doi: 10.1002/cam4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]