Abstract
Objective
To evaluate the use of a wearable activity tracker and brief feedback in the workplace to motivate employees to improve activity.
Patients and Methods
A total of 135 adult participants were randomized to 1 of 3 groups: control group (blinded to their step activity), intervention group (received counseling based on their step count), or no step-tracking device group. Participants were recruited from June 27, 2016, through February 21, 2018.
Results
Most of the 135 participants were women (84%), with a mean ± SD age of 42.6±10.1 years. Most participants (96%) completed 11 of the 12 weeks of step counts. Comparing treatment groups at week 12 (end of treatment), the intervention group (vs the control group) had significantly more steps (644.8; P<.01), had an 11.1% increase in step count from baseline (P<.01), was more likely to achieve goal (odds ratio=1.73; P=.02), increased distance traveled per week (0.46 miles; P<.01) and calories burned (90.6; P<.01), and had a decrease in some bioelectrical impedance measurements over time, including a greater loss in body fat mass (–0.90 kg; P=.01), percentage fat (–0.96; P<.01), and visceral fat level (–0.60; P<.01). Finally, the intervention group indicated significantly greater satisfaction with their assigned randomization (89% vs 77%; P=.01) and greater confidence in the effectiveness of their activity tracker (P<.01).
Conclusion
Brief counseling accompanied by use of a step-counting device can improve workplace activity, which, in turn, can increase steps and decrease body fat, including visceral fat.
Trial Registration
clinicaltrials.gov Identifier: NCT02794727
Abbreviations and Acronyms: BIA, bioelectrical impedance analysis; PSS, Perceived Stress Scale; SF-36, 36-Item Short Form Health Survey; SMF, segmental multifrequency; VFA, visceral fat area
Sedentary lifestyle, which has been identified as the new obesity,1, 2, 3 has been associated with an increase in all-cause mortality.4, 5 A recent systematic analysis found a 20% increase in type 2 diabetes in individuals who watched 2 hours or more of television daily.6 Another meta-analysis showed that there was a 5% increase in cardiovascular events with 2 hours of sitting/screen time7; and 5 additional systematic reviews have shown that sedentary behavior is associated with increased risk of colorectal, breast, endometrial, ovarian, and prostate cancer.8 It has been hypothesized that one of the contributing factors for increased sedentary lifestyle is inactivity in the workplace, which has increased throughout the decades.9 Recent attempts to improve workplace activity include sit-stand desks,10 treadmill desks,11 and portable pedal devices.12 Use of these devices could be promoted by emphasizing their health benefits and reducing sedentary time in the workplace.9, 13, 14 The use of portable pedal exercise machines has been studied to help improve activity by increasing energy expenditure.15 The most recent Cochrane review of workplace interventions for reducing sitting at work found that there is low quality of evidence to suggest that these devices reduce workplace sitting.9 There were 2 studies of 12 weeks or greater that had reduced sitting time when providing feedback or counseling in addition to using the devices.
Device- or activy tracker–measured physical activity has been used to study workplace fitness in hundreds of trials. A recent systematic review found that of 132 unique trials (N=15,619 participants), on average, workers accumulated 8124 steps per day.16 One of the primary driving factors in the number of steps per day was the type of work perfomed (eg, postal delivery 16,100 steps vs office workers 6857 steps). To improve physical activityfor staff in our workplace, we conducted a study to determine whether we could improve workplace activity at our medical facility using a wearable activity tracker and combining brief feedback using individualized activity goals. The primary aim was to determine whether monitoring physical activity at work in conjunction with a brief feedback session with a physician could increase workplace activity.
Methods
Study Overview
This study was a randomized controlled trial that measured occupational physical activity. As a proxy for occupational physical activity, participants wore an activity tracker (Fitbit Inc) during their Monday through Friday workdays for 16 weeks. In this study, all the participants wore the activity tracker without the ability to see their total number of steps during the run-in phase (4 weeks). After the run-in phase participants were randomized to 1 of 3 study arms for 12 weeks: in the control group, participants continued to wear the activity tracker for the remaining 12 weeks but also contiued to be blinded to the tracker's output (the display screen was covered with black nail polish so that they were unable to see the number of steps) and did not receive any additional intervention; in the intervention group, participants continued to wear the activity tracker for the remaining 12 weeks but were able to see the number of steps on the display and attended 2 interventional meetings with 1 of the investigators to discuss ways to increase their steps during the workday; and in the no activity tracker group, participants did not wear a tracker for 12 weeks and received no additional intervention. In accordance with the Declaration of Helsinki, this study was reviewed and approved by the Mayo Clinic Institutional Review Board, and written informed consent was obtained from all the study participants.
Setting
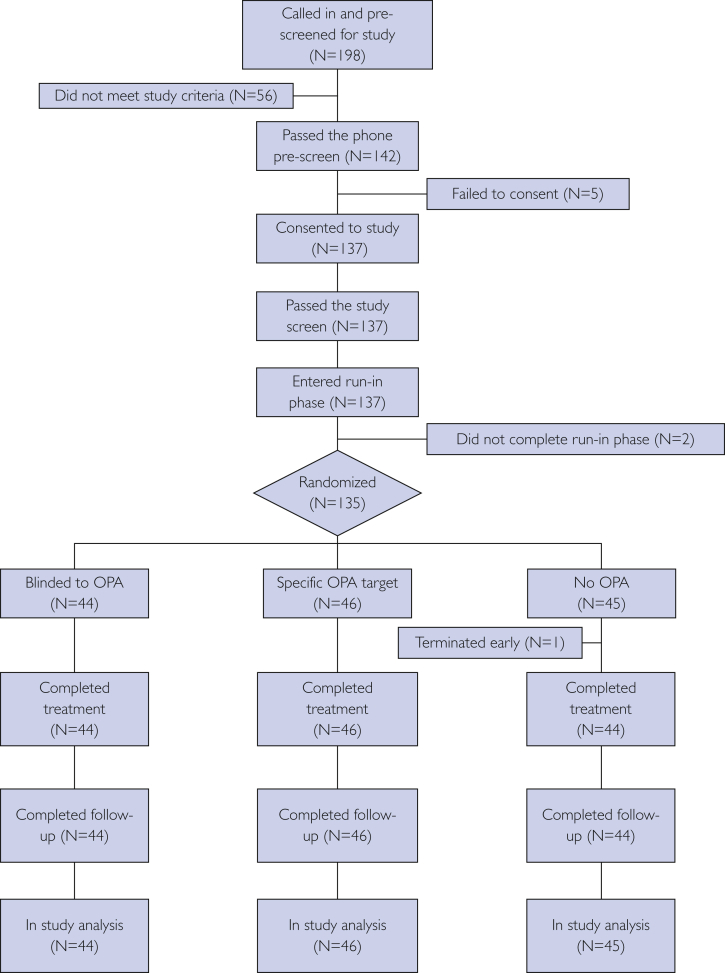
Study participants were primarily employed in the Mayo Clinic Division of General Internal Medicine at Rochester, Minnesota, and were recruited from June 27, 2016, through February 21, 2018. This report is based on all the participants who consented and were randomized to the study. The consort diagram presented in the Figure adheres to consort guidelines on reporting clinical trials.17
Figure.
Participant flow in the study from the first study contact to the last study contact. OPA = occupational physical activity.
Participants
Eligible participants were 18 to 65 years of age, were employed full-time, had not used a wearable activity tracker within 2 weeks of study entry, agreed not to use any other activity tracker during the study, had a stable weight (defined as self-reported weight not changed more than 10% in the past 3 months), were not pregnant at the time of study screening and agreed to not become pregnant during the study, had no history of joint problems that limited free movement, had the ability to participate fully in all aspects of the study, and had no known history of any condition that would preclude study participation, hinder study adherence, or skew data collection as judged by the clinical investigator. Participants were prescreened, attended a face-to-face consent visit, signed the consent form, were screened for the study, and, if they passed the study entry criteria, were enrolled in the study.Once enrolled, participants were provided with their unblinded wearable activity tracker for the 4-week run-in phase.
During the 4-week run-in phase, participants were asked to wear the activity tracker during their Monday through Friday workdays and to remove and store it during nonwork hours. These activity trackers were collected on Friday evenings by study staff; step data were downloaded to a secure server, and the trackers were recharged and returned the following Monday before the beginning of the work schedule for each participant. This schedule continued during the entire 16 weeks of the study.
At the completion of the 4-week run-in phase, participants returned for their baseline visit, during which required study data were collected and randomization took place. After randomization, the particpants’ activity trackers were set up according to the appropriate study arm. The participants continued in the study for an additional 12 weeks after randomization, which included 2 additional study visits where study data were collected (weeks 6 and 12 after randomization). One week after the end of the randomization study visit (week 12 after randomization), participants were asked to complete an end-of-study survey. All the participants received the activity tracker as compensation at the end of the study.
Interventions
Randomization was computer generated and comprised 3 groups:
-
○
Control group—All the participants wore an activity tracker with the digital display covered using black nail polish. Participants were not given any additional instructions regarding their workday activity, and neither were they able to track their steps. Participants continued to use the wearable activity tracker as they did during the 4-week run-in phase for 12 weeks after randomization.
-
○
Intervention group—All the participants wore an activity tracker with the display set on step counts and the nail polish removed, allowing them to view their steps. Participants attended 2 interventional meetings (baseline [randomization] and 6 weeks after randomization) with a study investigator (R.T.H.). During the first 10-minute feedback session participants received a step target of 10% to 25% greater than their baseline steps calculated from the 4-week run-in phase. This range was selected based on past publications for increasing step counts.18, 19 During the following 6 weeks, participants were advised to achieve 10% to 25% more steps than during the baseline 4-week run-in phase. After 6 weeks of the randomization phase, a second 10-minute feedback session was scheduled and the baseline and first 6-week step counts were reviewed and compared with the 10% to 25% goal set during the first feedback session. Participants who achieved the 10% to 25% goal were encouraged to continue to maintain this same goal (they were not asked to increase). Those who did not achieve the 10% to 25% goal were asked to reflect on what strategies they could use to improve step counts. Particicpants continued to use the wearable activity tracker as they did during the 4-week run-in phase for 12 weeks after randomization.
-
○
No activity tracker group—All the participants assigned to this arm did not use an activity tracker during the remaining 12 weeks of the study after randomization. They received no additional instructions regarding their workday activity.
Outcomes
The primary measures included the following: step data, downloaded weekly from participants’ wearable activity trackers; body composition measurements, collected using a medical-grade analyzer (InBody 770; Inbody USA) that used segmental multifrequency bioelectrical impedance analysis (SMF-BIA) for estimating participant body mass index, body composition (total body water, percentage body fat, lean body mass, and resting energy expenditure based on body composition), and visceral fat area (VFA);20 and the 36-Item Short Form Health Survey (SF-36),21 which measures quality of life and captures information about functional health and well-being from the participant’s point of view. The survey measured 8 health domains: physical functioning, role-physical, bodily pain, general health, vitality, social functioning, role-emotional, and mental health. The Perceived Stress Scale (PSS)22 measured the participants' perceptions of their own stress during the past month; and the safety measures included adverse events and concomitant medications.
Data Analysis
Baseline participant characteristics are summarized for each group using mean ± SD and range for the continuous variables and frequency (percentage) for the categorical variables. Primary outcomes of interest were daily step counts, body composition measurements, and SF-36 and PSS scores. All the outcomes were assessed at a baseline visit before the first week of the study and at selected follow-up visits. Weekly step counts were analyzed as continuous variables, expressed as number of steps and as the percentage change from baseline. In addition, weekly step counts were also analyzed using a binary outcome indicating whether the given participant met his or her step count goal. Distance traveled and number of calories burned were also collected each week. Step count, percentage change in steps, distance traveled, and number of calories burned were all analyzed using linear mixed models with an autoregressive covariance structure taking into account the repeated-measures study design. The baseline value was included as a covariate in these models. Results are reported as point estimates of the effect of the intervention group compared with the control group, with 95% CIs. The difference in the number of participants who increased their physical activity (10%-25% above baseline step counts) between the intervention and control group was analyzed using generalized estimating equations. The result is reported as an odds ratio with a 95% CI. For the BIA measurements, in addition to the baseline value, each participant was tested at weeks 6 and 12 of the study. The change from baseline for each of these time points was calculated, and the groups were compared using a general linear model with the baseline value included as a covariate. The PSS and the SF-36 were completed at baseline and at the end of the 12-week study, with scores calculated according to published guidelines.21, 23 The change from baseline to week 12 was analyzed using a general linear model with the baseline value used as a covariate. The results of these analyses are summarized using point estimates (95% CIs) for the effect of the intervention group.
The sample size for this investigation was chosen after weighing statistical considerations and logistical constraints. In general, a sample size of 45 per group will provide statistical power (2-tailed, α=0.05) of 80% to detect a difference between groups of 0.60 SD.
Results
Study recruitment included word of mouth (82.5%), wait lists (12.8%), and internal flyers (4.7%). Of the 198 participants prescreened, 137 (69%) consented and 135 (98.5%) went on to be randomized (Figure). All the participants except 1 (99%) completed the randomized phase of the study.
Baseline characteristics are reported in Table 1. Most of the participants were female (n=114; 84%), were married (n=93; 69%), and had at least some college education (n=127; 94%), and the mean ± SD age was 42.6±10.1 years.
Table 1.
Baseline Characteristics by Treatment Group
| Characteristic | Treatment group |
|||
|---|---|---|---|---|
| Overall (N=135) | Control (n=44) | Intervention (n=46) | No wearable tracker (n=45) | |
| Age (y) | ||||
| Mean ± SD | 42.6±10.1 | 41.8±10.3 | 40.4±9.7 | 45.5±9.8 |
| Range | 20-61 | 20-57 | 21-59 | 27-61 |
| Sex (No. [%]) | ||||
| Female | 114 (84) | 37 (84) | 38 (83) | 39 (87) |
| Male | 21 (16) | 7 (16) | 8 (17) | 6 (13) |
| Marital status (No. [%]) | ||||
| Never married | 20 (15) | 4 (9) | 11 (24) | 5 (11) |
| Separated/divorced | 19 (14) | 4 (9) | 5 (11) | 10 (22) |
| Married | 93 (69) | 34 (77) | 30 (65) | 29 (64) |
| Other | 3 (2) | 2 (5) | 0 | 1 (2) |
| Race/ethnicity (No. [%]) | ||||
| White, not Hispanic or Latino | 123 (91) | 38 (86) | 42 (91) | 43 (96) |
| Other | 12 (9) | 6 (14) | 4 (9) | 2 (4) |
| Education (No. [%]) | ||||
| High school graduate | 7 (5) | 2 (4) | 4 (9) | 1 (2) |
| Some college | 63 (47) | 17 (39) | 20 (43) | 26 (58) |
| 4-y college degree | 37 (27) | 15 (34) | 13 (28) | 9 (20) |
| Graduate/professional degree | 27 (20) | 10 (23) | 8 (17) | 9 (20) |
| Other | 1 (1) | 0 | 1 (2) | 0 |
| Tobacco use (No. [%]) | ||||
| Never | 94 (70) | 35 (80) | 33 (72) | 26 (58) |
| Former | 33 (24) | 6 (14) | 10 (22) | 17 (38) |
| Current | 8 (6) | 3 (2) | 3 (6) | 2 (4) |
| Alcohol use (No. [%]) | ||||
| Never | 17 (13) | 3 (7) | 4 (9) | 10 (22) |
| Monthly or less | 39 (29) | 11 (25) | 15 (33) | 13 (29) |
| 2-4 times a month | 37 (27) | 15 (34) | 14 (30) | 8 (18) |
| 2-3 drinks a week | 33 (24) | 11 (25) | 11 (24) | 11 (24) |
| ≥4 times a week | 9 (7) | 4 (9) | 2 (4) | 3 (7) |
In the control group, 84% turned in their wearable activity tracker for all 12 time points, and in the intervention group 80% did. The control group had 95% of the individuals turn in their wearable activity tracker for at least 11 of the 12 time points. For the intervention group, 96% turned in the wearable activity tracker for at least 11 of the 12 time points.
The step data were compared for 12 weeks in the control and intervention groups, and the results are shown in Table 2. Steps are reported as the average change in daily number of steps for that week from baseline. The intervention group compared with the control group had an effect estimate of 644.8 steps (P<.01). There was also a significant difference between groups for the percentage change in steps per week (P<.01). For those in the intervention group, a goal was set to increase their step count from baseline by at least 10% (10%-25%). The number of participants in the intervention and control groups who achieved at least an increase of 10% from baseline was calculated. There was a significant difference in the number of individuals who improved at least 10% in step count from baseline between groups (P=.02). The odds ratio for the intervention group compared with the control group was 1.73, which shows an increase in likelihood of an individual achieving the goal if he or she was in the intervention group.
Table 2.
Step Data
| Time point | Step counts |
Change (%), mean ± SD |
Achieved goal (No. [%]) |
|||||
|---|---|---|---|---|---|---|---|---|
| Control |
Intervention |
Control | Intervention | Control | Intervention | |||
| No. | Mean ± SD | No. | Mean±SD | |||||
| Baseline | 44 | 5139.1±1808.4 | 46 | 5243.6±1659.7 | ||||
| Δ at week 1 | 43 | –87.0±1059.7 | 46 | 236.0±1042.4 | –1.7±23.3 | 5.7±21.1 | 14 (33) | 16 (35) |
| Δ at week 2 | 44 | –330.8±1573.3 | 45 | 560.3±1497.9 | –3.1±27.5 | 12.4±29.7 | 15 (34) | 24 (53) |
| Δ at week 3 | 43 | –328.4±1136.7 | 46 | 588.2±1274.8 | –5.0±19.9 | 11.9±25.8 | 10 (23) | 21 (46) |
| Δ at week 4 | 42 | –184.8±1370.2 | 45 | 306.7±1302.6 | –2.6±28.4 | 7.4±27.6 | 14 (33) | 15 (33) |
| Δ at week 5 | 44 | –262.1±1662.5 | 46 | 455.1±1302.1 | –1.2±31.5 | 10.6±25.9 | 14 (32) | 20 (43) |
| Δ at week 6 | 42 | –63.1±1237.7 | 45 | 527.7±1494.3 | 0.9±27.4 | 11.3±30.6 | 14 (33) | 18 (40) |
| Δ at week 7 | 43 | –252.9±1440.9 | 46 | 619.2±1208.9 | –4.1±30.7 | 12.8±24.6 | 14 (33) | 24 (52) |
| Δ at week 8 | 43 | –110.7±1524.6 | 44 | 180.3±1586.2 | 0.9±33.5 | 5.3±30.2 | 11 (26) | 14 (32) |
| Δ at week 9 | 42 | –361.9±1655.0 | 44 | 260.9±1755.1 | –4.1±32.8 | 5.7±32.2 | 11 (26) | 18 (41) |
| Δ at week 10 | 43 | –340.7±1782.2 | 44 | 656.1±1510.3 | –4.2±35.1 | 12.2±29.8 | 14 (33) | 23 (52) |
| Δ at week 11 | 44 | –432.8±1445.9 | 46 | 486.6±1406.9 | –6.7±27.5 | 9.4±25.8 | 11 (25) | 23 (50) |
| Δ at week 12 | 43 | –812.0±1561.6 | 44 | –569.1±1723.7 | –13.2±26.0 | –9.3±34.1 | 8 (19) | 11 (25) |
| Effect estimate (95% CI) [P value] | 644.77a (393.3 to 896.2) [<.001] | 11.08a (5.93 to 16.23) [<.001] | 1.73b (1.08 to 2.77) .[022] | |||||
Data were analyzed using a linear mixed model with an autoregressive covariance structure used to account for the repeated measures study design. In all cases, the independent variable was study group (intervention vs control), and the baseline value of the given outcome variable was included as a covariate. The effect estimate corresponds to the estimated difference between study groups (intervention – control).
Data were analyzed using generalized estimating equations to account for the repeated measures study design. The effect estimate corresponds to the odds ratio, with values greater than 1.0 indicating an increased likelihood of achieving goal for the intervention group compared with the control group.
The distance traveled and the number of calories burned were collected by the activity tracker and were reported as the average change in daily distance traveled and calories burned for that specific week from baseline. The changes in mean distance traveled and calories burned for the study groups were compared using baseline as a covariate (Table 3). When the intervention group was compared with the control group, there was a significant increase in both the distance traveled (P<.01) and the number of calories burned (P<.01) for the intervention group.
Table 3.
Distance and Calories
| Time point | Distance |
Calories burned, mean ± SD |
||||
|---|---|---|---|---|---|---|
| Control |
Intervention |
Control | Intervention | |||
| No. | Miles, mean ± SD | No. | Miles, mean ± SD | |||
| Baseline | 44 | 3.48±1.35 | 46 | 3.59±1.11 | 1903.5±1058.8 | 1842.7±364.7 |
| Δ at week 1 | 43 | –0.09±0.74 | 46 | 0.15±0.71 | –67.4±457.3 | 43.2±91.8 |
| Δ at week 2 | 44 | –0.23±1.08 | 45 | 0.38±1.02 | –59.6±261.3 | 66.4±121.0 |
| Δ at week 3 | 43 | –0.25±0.80 | 46 | 0.40±0.88 | –30.3±101.1 | 56.6±131.0 |
| Δ at week 4 | 42 | –0.17±1.00 | 45 | 0.21±0.91 | –66.4±282.5 | 41.2±122.0 |
| Δ at week 5 | 44 | –0.20±1.15 | 46 | 0.31±0.88 | –8.5±161.0 | 42.3±104.8 |
| Δ at week 6 | 42 | –0.07±0.85 | 45 | 0.35±1.04 | –4.5±111.8 | 10.7±152.3 |
| Δ at week 7 | 43 | –0.20±1.02 | 46 | 0.41±0.83 | –21.3±127.1 | 45.7±120.0 |
| Δ at week 8 | 43 | –0.09±1.10 | 44 | 0.12±1.09 | –21.3±121.1 | 25.1±116.9 |
| Δ at week 9 | 42 | –0.26±1.19 | 44 | 0.16±1.17 | –126.9±650.5 | 25.2±135.4 |
| Δ at week 10 | 43 | –0.25±1.26 | 44 | 0.44±1.03 | –111.0±497.7 | 74.3±125.8 |
| Δ at week 11 | 44 | –0.30±1.03 | 46 | 0.33±1.00 | –174.6±1046.8 | 46.1±150.5 |
| Δ at week 12 | 43 | –0.54±1.09 | 44 | –0.39±1.20 | –125.6±170.7 | –108.1±219.8 |
| Effect estimate (95% CI) [P value] | 0.46a (0.29-0.63) [<.001] | 90.64a (32.33-148.95) [.003] | ||||
Data were analyzed using a linear mixed model with an autoregressive covariance structure used to account for the repeated measures study design. In all cases, the independent variable was study group (intervention vs control), and the baseline value of the given outcome variable was included as a covariate. The effect estimate corresponds to the estimated difference between study groups (intervention – control).
There was a consistent decrease in body fat mass, percentage body fat, and visceral fat level for the intervention group over time (Table 4). The change in BIA estimates of body fat mass, skeletal muscle mass, percentage body fat, and VFA from baseline to week 6 was not significant between groups, but the trend for the intervention group of a gradual increase in skeletal muscle mass continued. For the change from baseline to week 12 there was a significant difference seen between the intervention and control groups for body fat mass (P=.01), percent body fat (P<.01), and VFA (P<.01).
Table 4.
Inbody Measurementsa
| Measurement and time point | Treatment group |
Point estimate (95% CI) [P value]d |
|||
|---|---|---|---|---|---|
| Control (N=44b) | Intervention (N=46b) | No wearable tracker (N=45c) | Intervention vs control | No wearable tracker vs control | |
| Body fat mass (kg) | |||||
| Pre–run-in | 26.2±11.7 | 29.7±15.2 | 33.4±15.3 | ||
| Baseline | 26.2±11.7 | 29.7±14.9 | 33.4±15.5 | ||
| Δ at week 6 | 0.18±1.2 | –0.24±1.1 | 5.2-16±1.3 | –0.43 (–0.93 to 0.07) [.09] | –0.21 (–0.72 to 0.30) [.42] |
| Δ at week 12 | 0.44±1.5 | –0.45±1.5 | 0.44±1.6 | –0.90 (–1.55 to –0.24) [.01] | –0.01 (–0.68 to 0.66) [.98] |
| Skeletal muscle mass (kg) | |||||
| Pre–run-in | 27.3±5.7 | 28.0±6.3 | 28.0±5.3 | ||
| Baseline | 27.4±5.5 | 28.0±6.2 | 28.0±5.5 | ||
| Δ at week 6 | –0.02±0.67 | 0.22±0.64 | 0.20±0.58 | 0.25 (–0.01 to 0.52) [.06] | 0.23 (–0.04 to 0.49) [.09] |
| Δ at week 12 | –0.08±0.63 | 0.17±0.57 | 0.13±0.65 | 0.25 (–0.01 to 0.50) [.06] | 0.21 (–0.05 to 0.47) [.11] |
| Percentage body fat | |||||
| Pre–run-in | 33.8±10.0 | 35.3±8.7 | 38.1±9.4 | ||
| Baseline | 33.6±9.8 | 35.4±8.5 | 37.8±9.6 | ||
| Δ at week 6 | 0.17±1.5 | –0.34±1.3 | –0.24±1.2 | –0.51 (–1.07 to 0.05) [.07] | –0.42 (–0.99 to 0.14) [.14] |
| Δ at week 12 | +0.48±1.7 | –0.48±1.3 | 0.12±1.4 | –0.96 (–1.57 to –0.34) [.003] | –0.34 (–0.97 to 0.29) [.29] |
| Visceral fat level | |||||
| Pre–run-in | 11.6±5.5 | 12.3±5.5 | 13.9±5.3 | ||
| Baseline | 11.5±5.4 | 12.4±5.5 | 13.6±5.4 | ||
| Δ at week 6 | 0.16±0.72 | –0.04±0.64 | –0.09±0.60 | –0.20 (–0.48 to 0.07) [.15] | –0.25 (–0.53 to 0.03) [.08] |
| Δ at week 12 | 0.34±0.91 | –0.26±0.83 | 0.09±0.64 | –0.60 (–0.94 to –0.26) [.001] | –0.25 (–0.59 to 0.10) [.16] |
Data are given as mean ± SD.
One participant had missing information at week 6.
One participant had missing information at week 12.
Data were analyzed separately for the week 6 and week 12 periods using a general linear model. In all cases, the independent variable was study group (intervention vs control vs no step counter), and the baseline value of the given outcome variable was included as a covariate.
The PSS and the mental and physical components of the SF-36 were given to all the participants at the pre–run-in stage, baseline, and week 12. There was no significant difference between the groups for the change in week 12 from baseline for any of the surveys (P>.05) (data not shown).
One serious adverse event was reported that consisted of hospitalization due to unprovoked pulmonary embolism (determined not to be related to the wearable activity tracker or increased physical activity). During the course of the 16-week study, 14 (10%) of the participants reported 15 adverse events, only 1 of which was determined to be related to use of the wearable activity tracker (“light rash on wrist where the wearable activity tracker was worn”).
An end-of-study survey was given to all the participants (data not shown). There was a significant difference in how the groups answered when asked about the overall rating for wearing the activity monitor (P<.01), effectiveness of the wearable activity tracker (P<.01), and satisfaction with the study (P=.01). For overall satisfaction with the study, 89% of the intervention group said that they were "satisfied" or "extremely satisfied" with the study as a whole, and 77% of the control group answered the same.
Discussion
The sedentary workplace environment likely contributes to an overall unhealthy lifestyle for working adults. This study evaluated the use of a wearable activity tracker with brief feedback sessions to improve both activity and body compositon. The major finding was that when participants were able to monitor their physical activity and had 2 brief feedback sessions they were significantly more likely to have more overall steps and to achieve at least a 10% increase than those who only wore an activity tracker but were not able to monitor their steps and did not receive feedback on their activity. In addition, participants who received feedback had improvements in percentage body fat and VFA at 12 weeks compared with those who did not receive this intervention. This approach has the potential to improve the body composition and cardiometabolic outcomes of working adults.
We are aware of a few recently published similar workplace studies that use brief feedback or coaching sessions to improve physical activity. A study recently published evaluated the use of a workplace physical activity program alone or combined with health coaching in 213 employees. Six weeks after the start of the trial, those receiving combined coaching and a physical activity program were more physically active.24 Similarly, a study of software employees (n=46) who underwent a health education program that included physical activity, goal setting, and instruction found that physical activity using an activity tracker was higher during the coaching (median, 9834 steps) than at baseline (median, 6963 steps).25 Similar to the present study, these recent studies suggest that including a coaching session may be beneficial to enhance workplace activity trackers to increase physical activity at work. The present study used physician brief counseling combined with activity trackers to increase activity and evaluate the effect on body composition.
Body composition plays an important role in resting energy expenditure, and fat-free mass may be one of the primary factors.26 The SMF-BIA used in the present study estimated resting energy expenditure based on FFM and equations derived by the company (InBody). The BIA measure, which was used to estimate the VFA in the present study population, was selected over dual-energy x-ray absorptiometry and human measurement of waist circumference for several reasons. Waist circumference has considerable between-examiner and within-examiner variation. In a study by Berker et al27 in 2010 of a population to similar ours (19-58 years of age, >80% female), participants underwent BIA, ultrasonography, and CT to estimate VFA on the same day, in addition to weight, waist circumference, and waist-hip ratio measurements. They concluded that in all the participants, the methods best correlating with VFA by CT were BIA (r=0.870; P<.001), waist circumference (r=0.861; P<.001), body mass index (r=0.843; P<.001), and visceral fat thickness by ultrasonography (r=0.823; P<.001).27 The BIA used in the present study is an SMF-BIA. The current SMF-BIA (Inbody 720) was validated for VFA in a study of 53 participants who had estimates with both CT and SMF-BIA. The VFA estimates with the SMF-BIA had a high correlation (R=0.759) with CT estimates of VFA.28 The SMF-BIA advantages over dual-energy x-ray absorptiometry of rapid, noninvasive, and ease of use make the likelihood of being in clinical use much higher.27, 28, 29, 30 This device is very accurate, takes 2 minutes to run, does not need a technician to operate (thereby removing the interhuman and intrahuman operator variability bias), exposes patients to zero radiation, is less expensive to run (pennies per procedure vs hundreds of dollars), costs less initially ($14,000 vs $50,000+), takes up minimal space vs a whole room, and is much more likely to be used widespread clinically than dual-energy x-ray absorptiometry.
The present data found that use of a wearable activity tracker can be enhanced if it is accompanied by a goal-setting message31 provided in a timely manner (ie, teacheable moment),32 when motivational messaging32 would be most beneficial. The goal-setting message in the study focused on increasing the number of steps achieved at work by at least 10% of the baseline measurements. When comparing across treatment groups at the end of treatment, the intervention group had significantly more steps than the control group (644.8; P<.01), had an 11.1% increase in step count from baseline (P<.01), and were more likely to achieve the 10% or greater goal (odds ratio=1.73; P=.02). In addition, the intervention groups were found to have increased the distance traveled per week (0.46 miles; P<.01) and calories burned (90.6; P<.01). The intervention group also had greater loss in body fat mass (–0.90 kg; P=.01), percentage fat (–0.96; P <.01), and visceral fat level (–0.60; P<.01) at 12 weeks. It makes physiologic sense that these changes would not have been significant at 6 weeks because it would likely take a few months to see body composition changes with 10% to 25% increased activity. In addition it is not suprising that muscle mass was not changed between the groups because the intervention did not consist of resistance exercise recommendations.
Consistent with the current coaching literature, the present study found that participants who were provided with a brief personalized coaching message and who were able to track their steps performed consistently better in increasing the number of steps per day (Table 2). This association was confirmed by another study that showed that employee coaching results in improvement in physical and mental status in a workplace setting.9
Finally, although the small sample size is acceptable for a pilot study, it limits the ability to detect significant differences between groups. In addition, the open-label design limits the study due to patient selection bias,33 participant retention bias,34 and participant performance bias.35 As with any research study with a focus on increasing activity, the present study attracted more female than male participants. Research participants were recruited from a single center and due to the hiring practices for the positions held in the center, they were also more educated (most had some college or more). Because the focus of the study was on increasing activity and participants were required to attend a study visit during scheduled work hours, we did not focus the study on health outcomes, and neither did we collect vital signs or any type of blood work. Another limitation is the lack of body measurements. Past studies have shown lack of consistency in intrastaffing and interstaffing measurements with waist and hip circumference, and, therefore, a decision was made to use body compostion estimates of the BIA rather than body measurement. There are limitations in estimating VFA using whole-body SMF-BIA, such as the likelihood of underestimating abdominal VFA in obese individuals,28 but because we were looking at change from baseline in a population that on average was not obese we feel that these measurements are valid. Wrist-worn activity trackers are accurate when validated to step counts during treadmill step-counting experiments. Wrist-worn trackers may have limitations in more intense activities (running) vs walking36 and in those involving less wrist motion, such as using a desk pedal device.37
Some participants also had scheduling conflicts due to the fact that many of the study face-to-face visits (at consent/baseline, the end of the blind phase [week4], the end of week 10 [week 6 after randomization], and the end of the intervention [week 12 after randomization]) took place during the workday and others could not participate because of other scheduling conflicts, such as extended length of time away from work. Also, the study showed that participants who were able to monitor their own activity and received feedback on this performance were able to make signficiant changes in their physical activity and body composition. Further work should be done to determine how effective self-monitoring without feedback is compared with self-monitoring with feedback. Regardless, this study shows that for a wearable activity tracker to be effective, it needs to be used in an intentional manner.
Conclusion
Brief counseling accompanied by use of a step-counting device can improve workplace activity/movement, which, in turn, can increase steps and decrease body fat, including visceral fat. These findings are promising as we work to improve the overall health of workers by increasing physical activity, decreasing body fat, and promoting a healthy weight, which seem to be impeded by a significant amount of time in a sedentary workplace. More research should be conducted to determine whether the changes and trends seen in this small pilot study can be sustained over longer periods with more generalizable populations.
Acknowledgments
A special thanks to the exceptional research staff of the Clinical Research Office, Department of Medicine, at Mayo Clinic for their patience and persistence in helping to collect, compile, and organize these data. A special thanks to Marilyn Sloan, Troy Szydel, Bonnie Donelan Dunlap, and Shawn Fokken for their hard work and dedication to this study. We also thank the study participants who participated in this clinical trial, without whom this project would not have been possible.
Footnotes
Grant Support: This study was supported in part by the Mayo Clinic Department of Medicine and by staff support through the Department of Medicine Clinical Research Office. The data entry system used, REDCap, was supported in part by Center for Clinical and Translational Science award UL1 TR000135 from the National Center for Advancing Translational Sciences.
Potential Competing Interests: The authors report no competing interests.
References
- 1.Barnes A.S. Obesity and sedentary lifestyles: risk for cardiovascular disease in women. Tex Heart Inst J. 2012;39(2):224–227. [PMC free article] [PubMed] [Google Scholar]
- 2.Owen N., Sparling P.B., Healy G.N., Dunstan D.W., Matthews C.E. Sedentary behavior: emerging evidence for a new health risk. Mayo Clin Proc. 2010;85(12):1138–1141. doi: 10.4065/mcp.2010.0444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heinonen I., Helajarvi H., Pahkala K., et al. Sedentary behaviours and obesity in adults: the Cardiovascular Risk in Young Finns Study. BMJ Open. 2013;3(3) doi: 10.1136/bmjopen-2013-002901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee J., Dunlop D., Ehrlich-Jones L., et al. Public health impact of risk factors for physical inactivity in adults with rheumatoid arthritis. Arthritis Care Res (Hoboken) 2012;64(4):488–493. doi: 10.1002/acr.21582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Manson J.E., Skerrett P.J., Greenland P., VanItallie T.B. The escalating pandemics of obesity and sedentary lifestyle. A call to action for clinicians. Arch Intern Med. 2004;164(3):249–258. doi: 10.1001/archinte.164.3.249. [DOI] [PubMed] [Google Scholar]
- 6.Grontved A., Hu F.B. Television viewing and risk of type 2 diabetes, cardiovascular disease, and all-cause mortality: a meta-analysis. JAMA. 2011;305(23):2448–2455. doi: 10.1001/jama.2011.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ford E.S., Caspersen C.J. Sedentary behaviour and cardiovascular disease: a review of prospective studies. Int J Epidemiol. 2012;41(5):1338–1353. doi: 10.1093/ije/dys078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Rezende L.F., Rodrigues Lopes M., Rey-Lopez J.P., Matsudo V.K., Luiz Odo C. Sedentary behavior and health outcomes: an overview of systematic reviews. PLoS One. 2014;9(8):e105620. doi: 10.1371/journal.pone.0105620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shrestha N., Kukkonen-Harjula K.T., Verbeek J.H., Ijaz S., Hermans V., Bhaumik S. Workplace interventions for reducing sitting at work. Cochrane Database Syst Rev. 2016;3:CD010912. doi: 10.1002/14651858.CD010912.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rudecki J., Weatherson K., Faulkner G. Evaluating the acceptability of low-cost standing desks in the home environment: an exploratory study. J Phys Act Health. 2019;16(5):375–379. doi: 10.1123/jpah.2018-0575. [DOI] [PubMed] [Google Scholar]
- 11.Bergman F., Wahlstrom V., Stomby A., et al. Treadmill workstations in office workers who are overweight or obese: a randomised controlled trial. Lancet Public Health. 2018;3(11):e523–e535. doi: 10.1016/S2468-2667(18)30163-4. [DOI] [PubMed] [Google Scholar]
- 12.Proenca M., Schuna J.M., Barreira T.V., et al. Worker acceptability of the Pennington Pedal Desk occupational workstation alternative. Work. 2018;60(3):499–506. doi: 10.3233/WOR-182753. [DOI] [PubMed] [Google Scholar]
- 13.Grunseit A.C., Chau J.Y., van der Ploeg H.P., Bauman A. "Thinking on your feet": a qualitative evaluation of sit-stand desks in an Australian workplace. BMC Public Health. 2013;13:365. doi: 10.1186/1471-2458-13-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pronk N.P., Katz A.S., Lowry M., Payfer J.R. Reducing occupational sitting time and improving worker health: the Take-a-Stand Project, 2011. Prev Chronic Dis. 2012;9:E154. doi: 10.5888/pcd9.110323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tudor-Locke C., Schuna J.M., Jr., Frensham L.J., Proenca M. Changing the way we work: elevating energy expenditure with workstation alternatives. Int J Obes (Lond) 2014;38(6):755–765. doi: 10.1038/ijo.2013.223. [DOI] [PubMed] [Google Scholar]
- 16.Prince S.A., Elliott C.G., Scott K., Visintini S., Reed J.L. Device-measured physical activity, sedentary behaviour and cardiometabolic health and fitness across occupational groups: a systematic review and meta-analysis. Int J Behav Nutr Phys Act. 2019;16(1):30. doi: 10.1186/s12966-019-0790-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Consort: Transparent Reporting of Trials. Available at: http://www.consort-statement.org. Accessed January 31, 2019.
- 18.Puig-Ribera A., McKenna J., Gilson N., Brown W.J. Change in work day step counts, wellbeing and job performance in Catalan university employees: a randomised controlled trial. Promot Educ. 2008;15(4):11–16. doi: 10.1177/1025382308097693. [DOI] [PubMed] [Google Scholar]
- 19.Bravata D.M., Smith-Spangler C., Sundaram V., et al. Using pedometers to increase physical activity and improve health: a systematic review. JAMA. 2007;298(19):2296–2304. doi: 10.1001/jama.298.19.2296. [DOI] [PubMed] [Google Scholar]
- 20.Anderson L.J., Erceg D.N., Schroeder E.T. Utility of multifrequency bioelectrical impedance compared with dual-energy x-ray absorptiometry for assessment of total and regional body composition varies between men and women. Nutr. Res. 2012;32(7):479–485. doi: 10.1016/j.nutres.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 21.Ware J.E., Kosinski M., Keller S.D. The Health Institute, New England Medical Center; Boston, MA: 1994. SF-36 Physical and Mental Health Summary Scales: A User's Manual. [Google Scholar]
- 22.Cohen S., Kamarck T., Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385–396. [PubMed] [Google Scholar]
- 23.Ware J.E., Sherbourne C.D. The MOS 36-item short-form health survey (SF-36), I: conceptual framework and item selection. Med. Care. 1992;30(6):473–483. [PubMed] [Google Scholar]
- 24.Krebs S., Baaken A., Wurst R., Goehner W., Fuchs R. effects of a worksite group intervention to promote physical activity and health: the role of psychological coaching. https://doi.org/10.1111/aphw.12170 [published online June 18, 2019]. Appl Psychol Health Well Being. [DOI] [PubMed]
- 25.Mathew V., Akkilagunta S., Kumar D., Lakshminarayanan S., Kar S.S. Effectiveness of pedometer-based walking program to improve physical activity of workers in a software industry: an experimental study. Int J Prev Med. 2019;10:49. doi: 10.4103/ijpvm.IJPVM_378_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hills A.P., Mokhtar N., Byrne N.M. Assessment of physical activity and energy expenditure: an overview of objective measures. Front Nutr. 2014;1:5. doi: 10.3389/fnut.2014.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berker D., Koparal S., Isik S., et al. Compatibility of different methods for the measurement of visceral fat in different body mass index strata. Diagn Interv Radiol. 2010;16(2):99–105. doi: 10.4261/1305-3825.DIR.2749-09.1. [DOI] [PubMed] [Google Scholar]
- 28.Ogawa H., Fujitani K., Tsujinaka T., et al. InBody 720 as a new method of evaluating visceral obesity. Hepatogastroenterology. 2011;58(105):42–44. [PubMed] [Google Scholar]
- 29.Nagai M., Komiya H., Mori Y., Ohta T., Kasahara Y., Ikeda Y. Development of a new method for estimating visceral fat area with multi-frequency bioelectrical impedance. Tohoku J Exp Med. 2008;214(2):105–112. doi: 10.1620/tjem.214.105. [DOI] [PubMed] [Google Scholar]
- 30.Park K.S., Lee D.H., Lee J., et al. Comparison between two methods of bioelectrical impedance analyses for accuracy in measuring abdominal visceral fat area. J Diabetes Complications. 2016;30(2):343–349. doi: 10.1016/j.jdiacomp.2015.10.014. [DOI] [PubMed] [Google Scholar]
- 31.Westland H., Sluiter J., Te Dorsthorst S., et al. Patients' experiences with a behaviour change intervention to enhance physical activity in primary care: a mixed methods study. PLoS One. 2019;14(2):e0212169. doi: 10.1371/journal.pone.0212169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mills A.L., Pierce J.P. Using teachable moments to improve nutrition and physical activity in patients. Am Fam Physician. 2008;77(11):1510–1511. [PubMed] [Google Scholar]
- 33.Juni P., Altman D.G., Egger M. Systematic reviews in health care: assessing the quality of controlled clinical trials. BMJ. 2001;323(7303):42–46. doi: 10.1136/bmj.323.7303.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Halpern S.D. Evaluating preference effects in partially unblinded, randomized clinical trials. J Clin Epidemiol. 2003;56(2):109–115. doi: 10.1016/s0895-4356(02)00598-x. [DOI] [PubMed] [Google Scholar]
- 35.Rucker G. A two-stage trial design for testing treatment, self-selection and treatment preference effects. Stat Med. 1989;8(4):477–485. doi: 10.1002/sim.4780080411. [DOI] [PubMed] [Google Scholar]
- 36.Alinia P., Cain C., Fallahzadeh R., Shahrokni A., Cook D., Ghasemzadeh H. How accurate is your activity tracker? a comparative study of step counts in low-intensity physical activities. JMIR Mhealth Uhealth. 2017;5(8):e106. doi: 10.2196/mhealth.6321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reddy R.K., Pooni R., Zaharieva D.P., et al. Accuracy of wrist-worn activity monitors during common daily physical activities and types of structured exercise: evaluation study. JMIR Mhealth Uhealth. 2018;6(12):e10338. doi: 10.2196/10338. [DOI] [PMC free article] [PubMed] [Google Scholar]