Abstract
Anethum graveolens L. (A. graveolens) commonly known as dill, is an essential oil bearing plant extensively being used in traditional system of medicine. However, the reports on the components and biological responses of A. graveolens essential oil (AG-EO) from Saudi Arabia are scarce. The present study was designed to explore the presence of basic constituents and apoptosis induced by AG-EO in HepG2 cells. The constituents in AG-EO was analyzed by Gas chromatography-Mass spectroscopy (GC–MS). Cytotoxicity of AG-EO was measured by MTT assay and cell cycle arrest and apoptosis assays were conducted by using flow cytometer. Based on GC–MS analysis, the main constituents present in AG-EO were carvone (53.130%), dillapole (25.420%), dihydrocarvone 2 (11.350%) and dihydrocarvone 1 (6.260%). A few other minor components were also identified viz. cis-dihydrocarveol (0.690%), limonene (0.580%), isodihydrocarveol (0.370%), myristicin (0.210%) and cis-arsone (0.190%). The cytotoxicity results showed that AG-EO decrease the cell viability and inhibit the cell growth of HepG2 cells in a concentration-dependent manner. The inhibitory activity of AG-EO was found with IC50 = 59.6 ± 5.64. The cell cycle arrest results showed that HepG2 cells exposed to AG-EO exhibited an increase in G2/M and pre-G1 cell population after 24 h exposure. Furthermore, the flow cytometry data revealed the primarily activation of cell death by apoptosis manners in HepG2 cells exposed to AG-EO. Overall, results from this study highlighted the anticancer potential of AG-EO, which could be considered as a new agent for the management of hepatocellular carcinoma.
Keywords: A. graveolens, Essential oil, Hepatocellular carcinoma, Anticancer, Cell cycle arrest, Apoptosis
1. Introduction
A. graveolens, commonly known as dill, is an aromatic herb, belonging to Apiaceae (Umbelliferae) family. The plant has its origin from Mediterranean and Southwest Asia (Singh et al., 2005). Dill plant has a long history of use as a culinary and medicinal herb. The leaves are used in salads and soups, while the seeds are drunk as tea and added to sweets (Babri et al., 2012). The oil from A. graveolens is known to possess anti-bacterial, antifungal and anti-microbial activities (Abed, 2007, Badar et al., 2008, Tian et al., 2011, Chahal et al., 2016). The anti-oxidant (El-Mansouri et al., 2016), antidiabetic (Goodarzi et al., 2016), anticancer (Mohammed et al., 2018), diuretic and anti-hypercholesterolaemic (Sahib et al., 2012), anti-inflammatory & analgesic (Naseri et al., 2012), antibacterial activity (Kaur and Arora, 2010) effect of A. graveolens have also been documented. Traditionally, the seeds of A. graveolens are used as appetizer, carminative, antispasmodic and aphrodisiac agents, by the native people in Saudi Arabia (Youssef, 2013). The phytochemical studies carried out on A. graveolens plant revealed the presence of large number of phytoconstituents such as tannins, flavonoids, triterpenes, coumarins, phenolic acids, capric acid, palmitic acid, stearic acid, oleic acid etc. (Heamalatha et al., 2011, Dahiya and Purkayastha, 2012).
Essential oils are medicinally useful secondary metabolites of volatile nature, produced by plants. It is the need of the hour to scientifically investigate the traditionally used plants, for their composition and biological activities. A. graveolens is one such essential oil bearing traditionally known medicinal plant. The essential oil from fruits of A. graveolens contained compounds such as carvone, limonene, α-phellandrene, pinene, cineole, dillapole etc. (Ishikawa et al., 2002). The seeds of A. graveolens have been extensively used in ethnopharmacological medicine for treating jaundice, rheumatism and gout in Najd region of Saudi Arabia (Al-Asmari et al., 2014). The oil from the aerial parts of A. graveolens was found useful in the management of cancer of uterus (Javadi et al., 2015, Tariq et al., 2017). Some researches (Sathya and Gopalakrishnan, 2013, Mohammed et al., 2018), have reported that the phytochemicals present in A. graveolens exhibited some hepatoprotective effects, and hence, A. graveolens might be used as an herbal medicine against hepatocellular carcinoma. The cytotoxic responses of A. graveolens essential oil against various cancer cell lines such as HeLa (human cervical cancer), Caco-2 (human colon cancer) and MCF-7 (human breast cancer) have been reported (Sharopov et al., 2013). The antiproliferative activity of A. graveolens extract against tumor cell lines MK-1 (human epithelial), Hela (human cervical) and B16F10 (mouse skin melanoma) have also been reported (Nakano et al., 1998).
The essential oil from A. graveolens was found to induce chromosomal aberrations at low toxicity level in human lymphocytes (Lazutka et al 2001). The acute oral toxicity (LD50) of A. graveolens seed oil in rats, mice and rabbits are reported as 4.6 g/kg, >3 g/kg and >5 g/kg, respectively (Opdyke and Letizia, 1982).
The nature and content of secondary metabolites in aromatic plants are widely dependent on environmental factors. The geographic location, seasonal variation and even the method of extraction are known to exert significant effect on the yield and chemical composition of essential oil (Hussein et al., 2015). The chemical composition of essential oil from A. graveolens growing in different geographical regions has been extensively studied. However, till date no scientific communication reports the composition and biological activity of essential oil of A. graveolens seed from Saudi Arabia. Hence, the present study was designed to determine the chemical composition and anti-cancer potential of essential oil of A. graveolens seeds from Saudi Arabia against HepG2, a human hepatocellular carcinoma cell line.
2. Material and methods
2.1. Preparation of essential oil
The seeds of A. graveolens were procured from the local market of Riyadh, Saudi Arabia. The seeds were authenticated by a taxonomist, and a specimen (# 24541) was submitted in the KSU herbarium, Department of Botany & Microbiology. For the extraction of essential oil, the seeds were screened manually and subjected to hydro-distillation method using a Clevenger apparatus. The essential oil collected was dried over sodium sulphate, filtrated, and then stored at 4 °C until tested and analyzed.
2.2. Identification of AG-EO chemical components by GC–MS analysis
A Perkin Elmer model Clarus 600 T combined with single quadrapole mass spectrometer was used for GC–MS analysis. The chromatographic column was an Elite 5MS column (30 m × 0.25 mm × 0.25 µm film thickness), with high-purity helium as the gas carrier, at a flow rate of 1 mL/min. The injector temperature was 280 °C and it was equipped with a splitless injector at 20:1. The temperature was set initially to 40 °C (held for 2 min), was increased to 150 °C at 5 °C min−1 (held for 2 min), then increased further to 300 °C at 5 °C min−1. The MS ion source temperature was 220 °C and inlet line temperature at 240 °C. The scan range was set at 40–600 mass ranges at 70 eV electron energy and the solvent delay of 4 min. Finally, unknown compounds were identified by comparing the spectra with that of the NIST 2005 (National Institute of Standard and Technology library) and Wiley 2006 library. The total time required for analyzing a single sample was 61 min.
2.3. Chemicals used
Dimethyl sulfoxide (DMSO), MTT dye were purchased from Sigma (St. Louis, Mo., USA). DMEM, fetal bovine serum, gentamycin, HEPES buffer, L-glutamine, and 0.25% Trypsin-EDTA were purchased from Lonza. Propidium iodide flow cytometry and FITC Annexin-V apoptosis detection kits were purchased from BD Biosciences.
2.4. Culture of cells
HepG-2 cells obtained from VACSERA Tissue Culture Unit were grown in DMEM supplemented with 10% FBS, 50 μg/ ml gentamycin and 1% l-glutamine with HEPES buffer. HepG2 cells were cultured in a CO2 incubator at 37 °C/5% CO2 at high humid atmosphere.
2.5. Experimental design
Preparation of A. graveolens essential oil (AG-EO) was done by steam distillation using a clevenger apparatus. The identification of AG-EO chemical components was by GC–MS analysis. Human hepatocellular carcinoma cell line (HepG2) were exposed to a medium containing with various concentrations from the AG-EO (0.5–1000 µg/mL). Then, cytotoxicity was done by MTT assay by assessing the viability of HepG2 cells. Further, HepG2 cells were exposed to AG-EO concentrations (20, 60 and 200 µg/mL) for cell cycle analysis and apoptosis assay using flow cytometer.
2.6. Cytotoxicity assessment by MTT assay in HepG2 cells
HepG2 cells grown in monolayer were trypsinised and 10,000 cells were plated in 96-well culture plates in 0.1 mL culture medium (10% FBS) to attach the cells at the bottom of plate. After overnight incubation, supernatant was taken out and 100 µl samples with various concentrations of AG-EO were added to cells in wells of the microtitre plate. Following treatment for 24 h, medium was distant and 50 μl of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) (5 mg/ml; Sigma, St. Louis, CA, USA) was added to each well. The plates were shaken smoothly then incubated in the dark at 37 °C for an additional 4 h. The reaction was stopped by the addition of 150 μl DMSO (Sigma) and the absorbance of samples at 570 nm was measured with a microplate reader (SunRise, Tecan, USA).
2.7. Cell cycle arrest
The cell cycle arrest in HepG2 cells after the exposure of AG-EO was done using the Propidium iodide flow cytometry kit, BD Biosciences, according to the manufactures protocol. In brief, HepG2 cells were exposed to 20, 60 and 200 µg/mL of AG-EO for 24 h. After exposure, cells were fixed and stained as per instructions provided in the kit and cell cycle arrest was analyzed by flow cytometer (BD FACSCalibur, BD Biosciences, USA).
2.8. Apoptosis assay
To assess the mechanism of HepG2 cell death induced by AG-EO, the apoptosis/necrosis assays were conducted by flow cytometer using FITC Annexin-V apoptosis detection kit, BD, Biosciences. In, brief, HepG2 cells were exposed to 20, 60 and 200 µg/mL of AG-EO for 24 h. The quantity of apoptosis/necrosis cells were analyzed by flow cytometry following the manufactures protocol.
3. Results
3.1. Extraction and chemical composition of A. graveolens essential oil
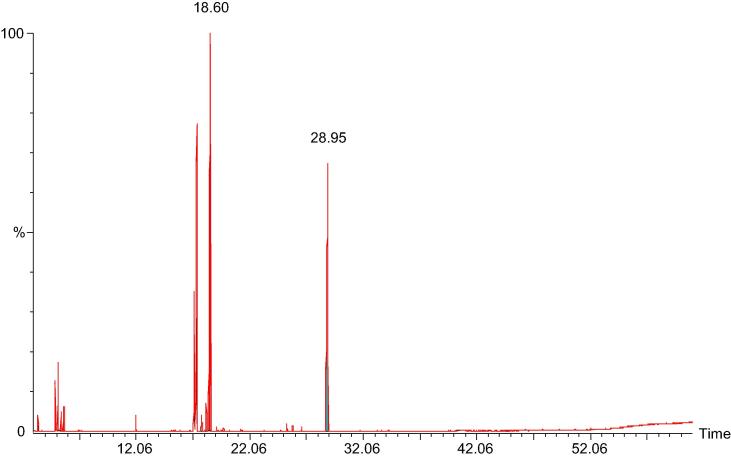
The essential oil of A. graveolens (AG-EO) was obtained by hydrodistillation method. The chemical composition was determined by GC–MS. The results of GC–MS analysis are presented in Fig. 1 and Table 1. In all, 20 different components represented 99.26% of the total oil. The oil mainly contained a mixture of carvone (7, 53.130%), dillapiole (15, 25.420%), dihydrocarvone 2 (4, 11.350%) and dihydrocarvone 1 (3, 6.260%). A few other minor components were also identified viz. cis-dihydrocarveol (6, 0.690%), DL-limonene (1, 0.580%), isodihydrocarveol (5, 0.370%), myristicin (13, 0.210%) and cis-asarone (14, 0.190%).
Fig. 1.
GC–MS chromatogram of Anethum graveolens essential oil (AG-EO).
Table 1.
Phyoconstituents identified in Anethum graveolens essential oil (AG-EO).
| # | Name | RT | Area | Area % |
|---|---|---|---|---|
| 1 | DL-limonene | 12.03 | 247,448 | 0.580 |
| 2 | 3-Hexen-1-ol | 15.50 | 46,210 | 0.110 |
| 3 | Dihydrocarvone 1 | 17.18 | 2,678,874 | 6.260 |
| 4 | Dihydrocarvone 2 | 17.40 | 4,859,944 | 11.350 |
| 5 | Isodihydrocarveol | 17.80 | 156,868 | 0.370 |
| 6 | cis-d-dihydrocarveol | 18.23 | 296,645 | 0.690 |
| 7 | d-carvone | 18.60 | 22,742,374 | 53.130 |
| 8 | Isopiperitinone | 19.15 | 191,886 | 0.450 |
| 9 | cis-carvone oxide | 19.34 | 17,835 | 0.040 |
| 10 | Limonene glycol | 21.24 | 25,996 | 0.060 |
| 11 | 2-Allyl-6-methoxyphenol | 21.38 | 20,394 | 0.050 |
| 12 | Germacrene d | 24.78 | 15,009 | 0.040 |
| 13 | 4-Methoxy-6-(2-propenyl)-1,3-benzodioxole | 25.85 | 91,940 | 0.210 |
| 14 | cis-asarone | 26.63 | 79,640 | 0.190 |
| 15 | Dillapiole | 28.95 | 10,881,201 | 25.420 |
| 16 | 1-Methyl-4-(l-methylethenyl)-cyclohexanol | 31.77 | 25,494 | 0.060 |
| 17 | 9-Octadecenoic acid | 40.27 | 27,933 | 0.070 |
| 18 | Triacontane | 43.11 | 17,181 | 0.040 |
| 19 | Dotriacontane | 44.76 | 32,582 | 0.080 |
| 20 | Tritetracontane | 46.33 | 23,985 | 0.060 |
3.2. Cytotoxic effect of A. graveolens essential oil
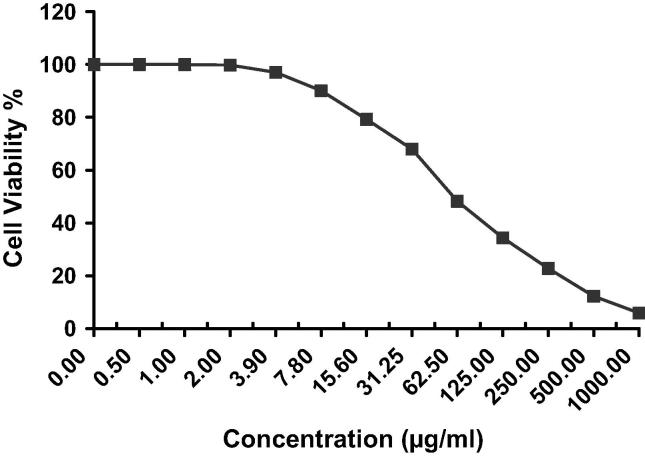
MTT assay was conducted to assess the percent cell viability induced by AG-EO in human hepatocellular carcinoma, HepG2 cell line. The results of the cytotoxic effects of the AG-EO in HepG2 cells are presented in Fig. 2. The results showed a concentration-dependent decrease in the percent cell viability of HepG2 exposed to 0.5–1000 μg/ml concentrations of AG-EO for 24 h. The percent cell viability at concentrations of 7.8, 15.6, 31.25, 62.5, 125, 250, 500 and 1000 μg/ml of AG-EO was found to be 90%, 79%, 67%, 48%, 34%, 22%, 12% and 5% in HepG2 cells, respectively (Fig. 2). However, at 3.9 μg/ml of AG-EO or lower concentrations of AG-EO did not cause any cytotoxic effects on HepG2 cells. The inhibitory activity of AG-EO against human hepatocellular carcinoma cells was found with IC50 = 59.6 ± 5.64 (Table 2) under the experimental conditions. The IC50 value was projected from concentration response curve using Graphpad Prism software (San Diego, CA. USA).
Fig. 2.
Cytotoxicity assessment by MTT assay in HepG2 cells following the exposure of various concentrations of Anethum graveolens essential oil (AG-EO) for 24 h.
Table 2.
Inhibitory activity against Hepatocellular carcinoma cells after the exposure of various concentrations of Anethum graveolens essential oil (AG-EO) for 24 h. The inhibitory concentrations was detected with IC50 = 59.6 ± 5.64 µg/ml.
| Concentrations of AG-EO (µg/ml) | % Inhibition | (±) S.D. |
|---|---|---|
| 1000 | 94.14 | 1.21 |
| 500 | 87.74 | 1.39 |
| 250 | 77.30 | 1.57 |
| 125 | 65.67 | 2.42 |
| 62.5 | 51.77 | 4.08 |
| 31.25 | 32.04 | 4.51 |
| 15.6 | 20.76 | 4.49 |
| 7.8 | 9.89 | 2.81 |
| 3.9 | 2.94 | 1.58 |
| 2 | 0.24 | 0.21 |
| 1 | 0.00 | 0.00 |
| 0.5 | 0.00 | 0.00 |
| 0 | 0 | 0.00 |
3.3. Cell cycle arrest by flow cytometry
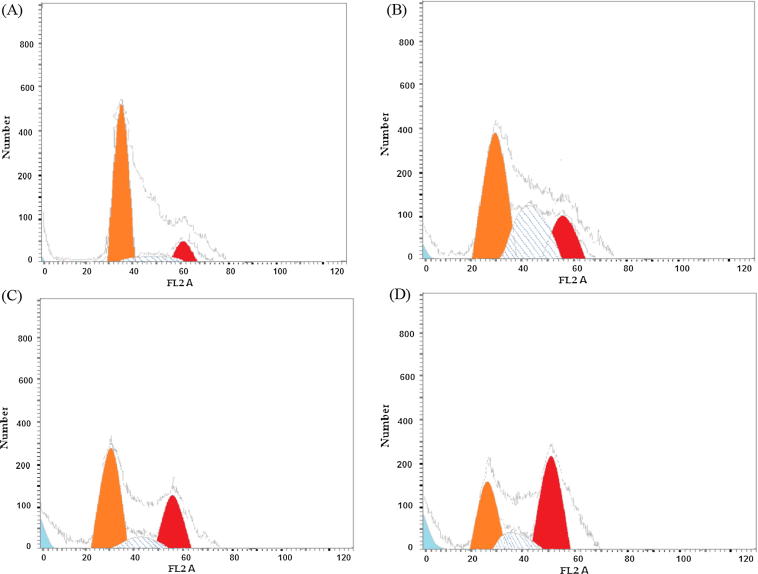
To evaluate the role of AG-EO in the cell death (anticancer/cytotoxic potential) in HepG2 cells, the cell cycle arrest analysis was conducted. The results of the cell cycle arrest are presented in Fig. 3 and Table 3. The results showed that HepG2 cells exposed to AG-EO exhibited an increase in G2/M and pre-G1 cell population in a concentration dependent manner after 24 h exposure. HepG2 cells exposed to AG-EO at 20, 60 and 200 μg/ml clearly exhibited cell death as shown by an appearance of 8.31%, 12.69 and 21.36% increase in pre-G1 phase, respectively as compared to the appearance of 1.02% in untreated control. Further, a concentration dependent increase of cell population in G2/M arrest were also observed in HepG2 cells exposed to AG-EO at 20–200 μg/ml concentrations. After exposure of AG-EO at 20, 60 and 200 μg/ml of AG-EO, an increase of 17.33%, 31.76% and 44.59%, respectively in G2/M cell population were observed in HepG2 cells as compared to untreated control cell population (6.15%) (Table 3).
Fig. 3.
Cell cycle analysis in HepG2 cells exposed to Anethum graveolens essential oil (AG-EO) for 24 h. Representative flow cytometric image exhibiting changes in the progression of cell cycle. [A]: Control; [B]: 20 μg/ml; [C]: 60 μg/ml; [D]: 200 μg/ml.
Table 3.
Percentage of cells arrested in different phases of cell cycle after the exposure of Anethum graveolens essential oil (AG-EO) in HepG2 for 24 h.
| Concentrations of AG-EO (μg/ml) | % G0-G1 | % S | % G2/M | % Pre-G1 |
|---|---|---|---|---|
| 20 | 56.24 | 26.43 | 17.33 | 8.31 |
| 60 | 47.19 | 21.05 | 31.76 | 12.69 |
| 200 | 32.57 | 22.84 | 44.59 | 21.36 |
| Untreated Control | 63.42 | 29.41 | 6.15 | 1.02 |
3.4. Apoptosis assessment induced by A. graveolens essential oil in HepG2 cells
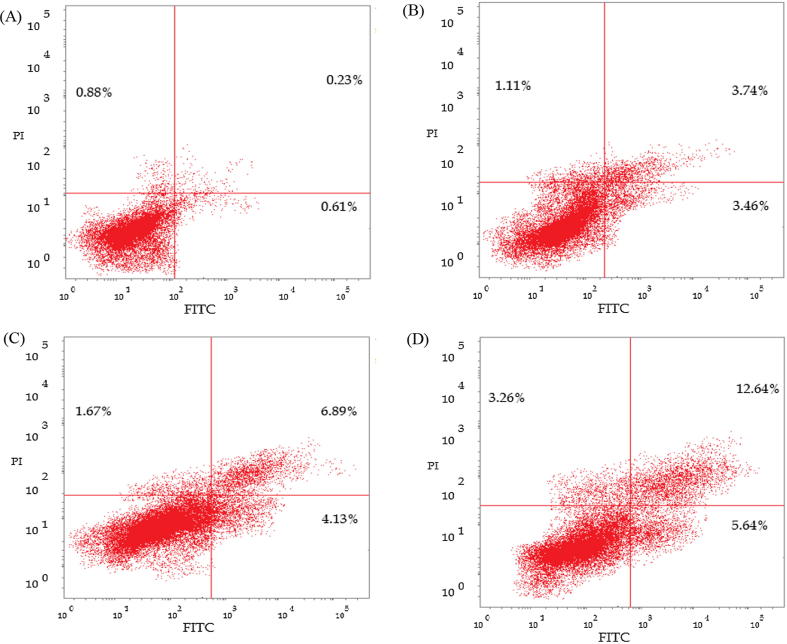
In order to assess the mechanism of cell death induced by AG-EO in HepG2 cells, the apoptosis assay was conducted using flow cytometric analysis. The apoptotic cell death results obtained by flow cytometer are shown in Fig. 4 and Table 4. Here, the data are shown in scatter plot, which characterizes the fluorescence generated by FITC and PI staining. The flow cytometry data clearly confirmed that AG-EO primarily initiate cell death by starting early and late apoptotic manners by coincidently convert into the necrotic cell death. Based on the staining, more than 98% cells were alive in untreated control with 0.88%, 0.23% and 0.61% cells in early, late and necrotic stages. HepG2 cells exposed to 20, 60 and 200 µg/ml of AG-EO concentrations exhibited in the induction of necrotic cell death, which represented by a shift of 1.11%, 1.68%, and 3.26% of the cell population into the upper left quadrant that showed necrotic cell death (Fig. 4). HepG2 cells exposed to 20, 60 and 200 µg/ml of AG-EO concentrations induced early and late apoptosis with an increase of 3.46%, 4.13% and 5.64% in lower right quadrant and 3.74%, 6.89% and 12.46% in upper right quadrant, respectively (Table 4).
Fig. 4.
Flow cytometry analysis of apoptosis in HepG2 cells exposed to various concentrations of treated with various concentrations of Anethum graveolens essential oil (AG-EO). The scatter plots show total, early apoptosis, late apoptotic and necrotic cells following 24 h exposure. [A]: Control; [B]: 20 μg/ml; [C]: 60 μg/ml; [D]: 200 μg/ml.
Table 4.
Percent early apoptosis, late apoptotic and necrotic cells in HepG2 following 24 h exposure to various concentrations of Anethum graveolens essential oil (AG-EO).
| Concentrations of AG-EO (μg/ml) | Apoptosis |
|||
|---|---|---|---|---|
| Total | Early | Late | Necrosis | |
| 20 | 8.31 | 3.46 | 3.74 | 1.11 |
| 60 | 12.69 | 4.13 | 6.89 | 1.67 |
| 200 | 21.36 | 5.64 | 12.46 | 3.26 |
| Untreated Control | 1.72 | 0.88 | 0.23 | 0.61 |
4. Discussion
The natural phytochemicals are well recognized for their health benefits and minimal side effects. A. graveolens is one such traditionally known medicinal plant. The anticancer effect of A. graveolens for the treatment of cancer of uterus and some hepatoprotective activity has been reported (Javadi et al., 2015, Tariq et al., 2017, Sathya and Gopalakrishnan, 2013). In the present study the chemical composition and anti-cancer potential of essential oil of A. graveolens seeds from Saudi Arabia against HepG2, a human hepatocellular carcinoma cell line was evaluated. The essential oil of A. graveolens (AG-EO), obtained by hydrodistillation method was chemically analyzed by GC–MS. The results of this study are in agreement with the previous reports where major compound of dill seed essential oil was carvone whereas dillapiole and trans-dihydrocarvone were present in appreciable amounts (Chahal et al., 2017). The AG-EO from Uzbekistan and Algeria contained 73.61% and 31.04% carvone respectively (Yili et al., 2009, Khaldi et al., 2015). Asraf et al. (1977) reported 52.25% carvone and 28.28% dillapiole in AG-EO from Pakistan. The main components of AG-EO from Tzakistan were carvone (51.7%) and trans-dihydrocarvone (14.7%) (Sharopov et al., 2013). The AG-EO from Iran was found rich in carvone, 30.2%, trans dihydrocarvone, 11.7% and dillapiole, 11.5% (Khani and Basavand, 2013). The broad variations seen in the relative amounts of the main components of the essential oils of A. graveolens from different parts, can be attributed to different geographic location, seasonal variation, genetic variation, conditions of growth and even the method of extraction.
Further, we used MTT assay to assess the cytotoxic/antiproliferative effects of AG-EO in HepG2 cell line. The results of the cytotoxic effects of the AG-EO in HepG2 cells are presented in Fig. 2 and Table 2. The MTT assay commonly use endpoint to assess the cytotoxicity, introduced into the cells and reduced mitochondria dependent reaction to yield a blue color formazan product (Mosmann, 1983) that indicates mitochondrial integrity as a measurement of cell viability (Maioli et al., 2009). Our results clearly showed the ability of HepG2 cells to reduce MTT to the formazan derivative after exposure of AG-EO that indicates cytotoxic/antiproliferative activity. Our results showed that AG-EO with increasing concentrations decreases the cell viability of HepG2 cells after 24 h of treatment. A concentration dependent cytotoxic effect of A. graveolens essential oil against HeLa (human cervical cancer), Caco-2 (human colon cancer) and MCF-7 (human breast cancer) has been reported (Sharopov et al., 2013) with the IC50 values 67 μg/ml for MCF-7, 93 μg/ml for HeLa and 216 μg/ml for Caco-2 cell lines. The methanolic extract of A. graveolens is also known to inhibit the proliferation of cancer cells such as L1210 (mouse leukemia), MK-1 (human epithelial), Hela (human cervical) and B16F10 (mouse skin melanoma) have also been reported (Nakano et al., 1998, Goun et al., 2002). The cytotoxic response observed in this study might be due to the presence of compounds carvone and limonene (Zheng et al., 1992).
These results are in well agreements with the other reports showing decrease in the cell viability of WISH, MCF-7, HEp-2 and Vero cell lines with increasing concentrations of fenugreek seed oil after 24 h exposure (Al-Oqail et al., 2013). The in vitro cytotoxic activities of essential oil obtained from seeds of Moringa oleifera against HeLA, L929, Caco-2, MCF-7 and HepG2 cell lines have been previously reported (El-Sayed et al., 2015). In other study, Oliveira et al. (2015), have also reported cytotoxic potential of different essential oils against various cancer cell lines, such as HT29 human colon carcinoma, MCF-7 human breast adenocarcinoma, HeLa human cervical adenocarcinoma, human glioblastomas and HepG2 human hepatocellular carcinoma.
To evaluate the role of AG-EO in the cell death (anticancer/cytotoxic potential) in HepG2 cells, the cell cycle arrest analysis was conducted. The results of the cell cycle arrest are presented in Fig. 3 and Table 3. The results obtained here clearly suggest that AG-EO induced G2/M and pre-G1 cell population arrest in HepG2 cells after 24 h exposure. The increase in pre-G1 peak in cell cycle arrest indicating the upregulation of apoptosis pathway. This could be due to the changes in the mitochondrial and lysosomal activities (Al-Sheddi et al., 2015). Furthermore, the increased population in G2/M cell cycle following the exposure of AG-EO in HepG2 cells might be due to the possibility of DNA damage and failure of repair mechanism in the cells. It is already reported that DNA repair mechanism(s) in the cells are very well maintained and highly DNA damage could leads to cell cycle arrest and cell death (Ferreira et al., 2002, Ravi et al., 2010). As shown in Fig. 3, AG-EO treated HepG2 cells showed higher cell count in G2/M and pre-G1 phase, which indicate AG-EO induced cell cycle arrest. This kind of cell cycle arrest have also been reported in HSC-3 human oral squamous carcinoma cells after the exposure of Cinnamomum cassia essential oil and its constituents (Chang et al., 2017). Seal et al. (2012) have also reported that vapor of volatile oil compounds acquired from seeds of Litsea cubeba induced cell death via cell cycle arrest in human non-small cell lung cancer (NSCLC) and human lung carcinoma (A-549) cell lines.
In order to assess the mechanism of cell death induced by AG-EO in HepG2 cells, the apoptosis assay was conducted using flow cytometric analysis. The apoptotic cell death results obtained by flow cytometer are shown in Fig. 4 and Table 4. The programed cell death or apoptosis is hemostatic process, which controls the cell population of normal tissue and in different kind of cancer diseases. Apoptosis occurs due to the DNA break down, cleavage in proteins and over expressions of caspases activate proteolytic cascade (Elmore, 2007). Herein, we observed that AG-EO induced apoptotic and necrotic cell death in HepG2 cells after 24 h exposure. Phytoconstituets from plants have been proven as an alternate method in the induction of apoptotic cell death mediated through different pathways (Lahlou, 2013, Srivastava et al., 2016). These cytotoxic agents inhibit cell survival via cell cycle arrest and apoptotic induction in cancer cells through non-polar or covalent binding to DNA (Palchaudhuri and Hergenrother, 2007). The results from this study exhibited that AG-EO is able to arrest the cell cycle in a concentration dependent manner consequently leads to apoptosis. The cell cycle arrest induction to avert the cell proliferation is effective way used by the cytotoxic agents. These cytotoxic agents play a role in anticancer mechanism through arresting the G0/G1 and G2/M phases in that way inhibiting the growth of cells and leading the apoptosis cell death (Joe et al., 2002, Keyvani-Ghamsari et al., 2017). Subsequently, the exposure of HepG2 cells with different concentrations of AG-EO, results clearly demonstrated that AG-EO has the potential to arrest HepG2 cells at G2/M and pre-G1 phase. Thus, in present study, AG-EO induced apoptosis may be due to the cell cycle arrest that blocked the DNA synthesis and inhibit the growth of the HepG2 cells. These results are in agreements with the recent reports that exhibited apoptosis cell death after the exposure of various essential oil obtained from plants in different cancer cells (Chang et al., 2017, Jamali et al., 2018).
5. Conclusion
Our results revealed the presence of 20 different components representing 99.26% of the total A. graveolens essential oil. The oil mainly contained a mixture of carvone, dillapole, dihydrocarvone 2 and dihydrocarvone 1. A few other minor components were also identified viz. cis-dihydrocarveol, limonene, isodihydrocarveol, myristicin and cis-arsone. Further, the results also demonstrated that the AG-EO has antiproliferative/anticancer potential against human hepatocellular carcinoma cells. A concentration- dependent decrease in the cell viability of HepG2 cells were observed after AG-EO exposure. The AG-EO was also found to induce cell cycle arrest and apoptosis in HepG2 cells. The AG-EO treated HepG2 cell death certainly indicated the role of cell cycle arrest at pre-G1 and G2/M phases. This study also provided a new understanding into the mechanism(s) of action of AG-EO induced apoptosis in HepG2 cells. Therefore, AG-EO could be considered as a new agent for the management of hepatocellular carcinoma.
Declaration of Competing Interest
The authors hereby declare that there are no conflicts of interest.
Acknowledgement
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University for funding this work through the Undergraduate Student’s Research Support Program, Project no. (URSP-3-17-13).
Footnotes
Peer review under responsibility of King Saud University.
References
- Abed K.F. Antimicrobial activity of essential oils of some medicinal plants from Saudi Arabia. Saudi J. Biol. Sci. 2007;14:53–60. [Google Scholar]
- Al-Asmari A.K., Al-Elaiwi A.M., Athar M.T., Tariq M., Al Eid A., Al-Asmary S.M. A review of hepatoprotective plants used in saudi traditional medicine. Evid. Based Complement. Altern. Med. 2014;2014:890842. doi: 10.1155/2014/890842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Oqail M.M., Farshori N.N., Al-Sheddi E.S., Musarrat J., Al-Khedhairy A.A., Siddiqui M.A. In vitro cytotoxic activity of seed oil of fenugreek against various cancer cell lines. Asian Pac. J. Cancer Prev. 2013;14(3):1829–1932. doi: 10.7314/apjcp.2013.14.3.1829. [DOI] [PubMed] [Google Scholar]
- Al-Sheddi E.S., Al-Oqail M.M., Saquib Q., Siddiqui M.A., Musarrat J., Al-Khedhairy A.A., Farshori N.N. Novel all trans-retinoic acid derivatives: cytotoxicity, inhibition of cell cycle progression and induction of apoptosis in human cancer cell lines. Molecules. 2015;20(5):8181–8197. doi: 10.3390/molecules20058181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asraf M., Aziz J., Bhatty M.K. Studies on the essential oils of the Pakistani species of the family Umbelliferae. Part VI. Anethum graveolens (Dill, Sowa) seed oil. Pakistan J. Sci. Ind. Res. 1977;20(1):52–54. [Google Scholar]
- Babri R.A., Khokhar I., Mahmood Z., Mahmud S. Chemical composition and insecticidal activity of the essential oil of Anethum graveolens L. Sci. Int. 2012;24(4):453–455. [Google Scholar]
- Badar N., Arshad M., Farooq U. Characteristics of Anethum graveolens (Umbelliferae) seed oil: extraction, composition, and antimicrobial activity. Int. J. Agri. Biol. 2008;10:329–332. [Google Scholar]
- Chahal K.K., Monika, Kataria D., Singh R. Antifungal potential of dill seed essential oil and its constituents. Indian J. Ecol. 2016;43(2):903–906. [Google Scholar]
- Chahal K.K., Monika, Kumar A., Bhardwaj U., Kaur R. Chemistry and biological activities of Anethum graveolens L. (dill) essential oil: a review. J. Pharmacogn Phytochem. 2017;6(2):295–306. [Google Scholar]
- Chang W.L., Cheng F.C., Wang S.P., Chou S.T., Shih Y. Cinnamomum cassia essential oil and its major constituent cinnamaldehyde induced cell cycle arrest and apoptosis in human oral squamous cell carcinoma HSC-3 cells. Environ. Toxicol. 2017;32(2):456–468. doi: 10.1002/tox.22250. [DOI] [PubMed] [Google Scholar]
- Dahiya P., Purkayastha S. Phytochemical analysis and antibacterial efficacy of dill seed oil against multi-drug resistant clinical isolates. Asian J. Pharm. Clin. Res. 2012;5(2):62–64. [Google Scholar]
- El Mansouri L., Bousta D., Balouiri M., Ouedrhiri W., Elyoubi-El Hamsas A. Antioxidant activity of aqueous seed extract of Anethum graveolens L. Int. J. Pharm. Sci. Res. 2016;7:1219–1223. [Google Scholar]
- Elmore S. Apoptosis: a review of programmed cell death. Toxicol. Pathol. 2007;35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sayed E.A., Sharaf-Eldin M.A., Wadaan M. In vitro evaluation of cytotoxic activities of essential oil from Moringa oleifera seeds on HeLa, HepG2, MCF-7, CACO-2 and L929 cell lines. Asian Pac. J. Cancer Prev. 2015;16(11):4671–4675. doi: 10.7314/apjcp.2015.16.11.4671. [DOI] [PubMed] [Google Scholar]
- Ferreira C.G., Epping M., Kruyt F.A., Giaccone G. Apoptosis: target of cancer therapy. Clin. Cancer Res. 2002;8:2024–2034. [PubMed] [Google Scholar]
- Goodarzi M.T., Khodadadi I., Tavilani H., Abbasi Oshaghi E. The role of Anethum graveolens L. (Dill) in the management of diabetes. J Trop. Med. 2016;1–11:1098916. doi: 10.1155/2016/1098916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goun E.A., Petrichenko V.M., Solodnikov S.U., Suhinina T.V., Kline M.A., Cunningham G., Nguyen C., Miles H. Anticancer and antithrombin activity of Russian plants. J. Ethnopharmacol. 2002;81(3):337–342. doi: 10.1016/s0378-8741(02)00116-2. [DOI] [PubMed] [Google Scholar]
- Heamalatha S., Swarnalatha S., Divya M., Gandhi Lakshmi R., Ganga Devi A., Gomathi E. Pharmacognostical, pharmacological, investigation on Anethum graveolens linn: a review. Res. J. Pharm. Biol. Chem. Sci. 2011;2(4):564–574. [Google Scholar]
- Hussein A.H., Said-Al Ahl Atef M., Abou Dahab M., El-Shahat N., Abou-Zeid M.S., Nabila A.Y., Naguib M.A. Essential oils of Anethum graveolens L. Chemical composition and their antimicrobial activities at vegetative, flowering and fruiting stages of development. Int. J. Plant Sci. Ecol. 2015;1(3):98–102. [Google Scholar]
- Ishikawa T., Kudo M., Kitajima J. Water-soluble constituents of dill. Chem. Pharm. Bull. 2002;50(4):501–507. doi: 10.1248/cpb.50.501. [DOI] [PubMed] [Google Scholar]
- Jamali T., Kavoosi G., Safavi M., Ardestani S.K. In-vitro evaluation of apoptotic effect of OEO and thymol in 2D and 3D cell cultures and the study of their interaction mode with DNA. Sci. Rep. 2018;8(1):15787. doi: 10.1038/s41598-018-34055-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javadi B., Iranshahy M., Emami S.A. Anticancer plants in Islamic traditional medicine. In: Saad M., editor. Complementary Therapies for the Body, Mind and Soul. In Tech; Croatia: 2015. p. 119. [Google Scholar]
- Joe A.K., Liu H., Suzui M., Vural M.E., Xiao D., Weinstein I.B. Resveratrol induces growth inhibition, S-phase arrest, apoptosis, and changes in biomarker expression in several human cancer cell lines. Clin. Cancer Res. 2002;8:893–903. [PubMed] [Google Scholar]
- Kaur G.J., Arora D.S. Bioactive potential of Anethum graveolens, Foeniculum vulgare and Trachyspermum ammi belonging to the family Umbelliferae –Current status. J. Med. Plants Res. 2010;4:87–94. [Google Scholar]
- Keyvani-Ghamsari S., Rabbani-Chadegani A., Sargolzaei J., Shahhoseini M. Effect of irinotecan on HMGB1, MMP9 expression, cell cycle, and cell growth in breast cancer (MCF-7) cells. Tumor Biol. 2017;39(4) doi: 10.1177/1010428317698354. 1010428317698354. [DOI] [PubMed] [Google Scholar]
- Khaldi A., Meddah B., Moussaoui A., Sonnet P., Akermy M.M. Chemical composition and antifungal activity of essential oil of Anethum graveolens L. from Southwestern Algeria (Bechar) J. Chem. Pharm. Res. 2015;7(9):615–620. [Google Scholar]
- Khani A., Basavand F. Chemical composition and insecticide activity of essential oil from dill seeds. Int. J. Agric. 2013;3(3):489–494. [Google Scholar]
- Lahlou M. The success of natural products in drug discovery. Pharmacol. Pharm. 2013;4:17–31. [Google Scholar]
- Lazutka J.R., Mierauskien J., Slapšyt G., Dedonyt V. Genotoxicity of dill (Anethum graveolens L.), peppermint (Mentha× piperita L.) and pine (Pinus sylvestris L.) essential oils in human lymphocytes and Drosophila melanogaster. Food Chem. Toxicol. 2001;39(5):485–492. doi: 10.1016/s0278-6915(00)00157-5. [DOI] [PubMed] [Google Scholar]
- Maioli E., Torricelli C., Fortino V., Carlucci F., Tommassini V., Pacini A. Critical appraisal of the MTT assay in the presence of rottlerin and uncouplers. Biol. Proced. Online. 2009;11(1):227–240. doi: 10.1007/s12575-009-9020-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammed F.A., Elkadya A.I., Syeda F.Q., Mirzaa M.B., Hakeema K.R., Alkarima S. Anethum graveolens (dill) – A medicinal herb induces apoptosis and cell cycle arrest in HepG2 cell line. J. Ethnopharmacol. 2018;219:15–22. doi: 10.1016/j.jep.2018.03.008. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Nakano Y., Matsunaga H., Saita T., Mori M., Katano M., Okabe H. Antiproliferative constituents in Umbelliferae plants II. Screening for polyacetylenes in some Umbelliferae plants and isolation of panaxynol and falcarindiol from the root of Heracleum moellendorffii. Biol. Pharm. Bull. 1998;21(3):257–261. doi: 10.1248/bpb.21.257. [DOI] [PubMed] [Google Scholar]
- Naseri M., Mojab F., Khodadoost M., Kamalinejad M., Davati A., Choopani R., Hasheminejad A., Bararpoor Z., Shariatpanahi S., Emtiazy M. The study of anti-inflammatory activity of oil-based dill (Anethum graveolens L.) extract used topically in formalin-induced inflammation male rat paw. Iranian J. Pharm. Res. 2012;11(4):1169–1174. [PMC free article] [PubMed] [Google Scholar]
- Oliveira P.F., Alves J.M., Damasceno J.L., Oliveira R.A., Dias Júnior H., Crotti A.E., Tavares D.C. Cytotoxicity screening of essential oils in cancer cell lines. Rev. Bras. Farmacogn. 2015;25(2):183–188. [Google Scholar]
- Opdyke D.L.J., Letizia C. Dill seed oil Indian. Fragrance raw materials monographs. Food Chem. Toxicol. 1982;20(4):673–674. doi: 10.1016/0015-6264(77)90086-4. [DOI] [PubMed] [Google Scholar]
- Palchaudhuri R., Hergenrother P.J. DNA as a target for anticancer compounds: methods to determine the mode of binding and the mechanism of action. Curr. Opin. Biotechnol. 2007;18:497–503. doi: 10.1016/j.copbio.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Ravi S., Chiruvella K.K., Rajesh K., Prabhu V., Raghavan S.C. 5-Isopropylidene-3-ethyl rhodanine induce growth inhibition followed by apoptosis in leukemia cells. Eur. J. Med. Chem. 2010;45:2748–2752. doi: 10.1016/j.ejmech.2010.02.054. [DOI] [PubMed] [Google Scholar]
- Sahib A., Mohammad I., Al-Gareeb A. Effects of Anethum graveolens leave powder on lipid profile in hyperlipidemic patients. Spatula DD. 2012;2:153–158. [Google Scholar]
- Sathya V., Gopalakrishnan V.K. Virtual screening of potential drug-like inhibitors from medicinal plants against hbxip of hepatocellular carcinoma. Int. J. Pharm. Pharm. Sci. 2013;5(2):535–539. [Google Scholar]
- Seal S., Chatterjee P., Bhattacharya S., Pal D., Dasgupta S., Kundu R., Mukherjee S., Bhattacharya S., Bhuyan M., Bhattacharyya P.R., Baishya G. Vapor of volatile oils from Litsea cubeba seed induces apoptosis and causes cell cycle arrest in lung cancer cells. PLoS One. 2012;7(10):e47014. doi: 10.1371/journal.pone.0047014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharopov F.S., Wink M., Gulmurodov I.S., Isupov S.I., Zhang H.J., Setzer W.N. Composition and bioactivity of the essential oil of Anethum graveolens L. from Tajikistan. Int. J. Med. Arom. Plants. 2013;3(2):125–130. [Google Scholar]
- Singh G., Maurya S., de Lampasona M.P., Catalan C. Chemical constituents, antimicrobial investigations, and antioxidative potentials of Anethum graveolens L. essential oil and acetone extract. J. Food Sci. 2005;70(4):208–215. [Google Scholar]
- Srivastava S., Somasagara R.R., Hegde M., Nishana M., Tadi S.K., Srivastava M., Choudhary B., Raghavan S.C. Quercetin, a natural flavonoid interacts with DNA, arrests cell cycle and causes tumor regression by activating mitochondrial pathway of apoptosis. Sci. Rep. 2016;6:24049. doi: 10.1038/srep24049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tariq A., Sadia S., Pan K., Ullah I., Mussarat S., Sun F., Abiodun O.O., Batbaatar A., Li Z., Song D., Xiong Q., Ullah R., Khan S., Basnet B.B., Kumar B., Islam R., Adnan M. A systematic review on ethnomedicine of anti-cancer plants. Phytother. Res. 2017;31(2):202–264. doi: 10.1002/ptr.5751. [DOI] [PubMed] [Google Scholar]
- Tian J., Ban X., Zeng H., Huang B., He J., Wang Y. In vitro and in vivo activity of essential oil from dill (Anethum graveolens L.) against fungal spoilage of cherry tomatoes. Food Cont. 2011;22:1992–1999. [Google Scholar]
- Yili A., Aisa H.A., Maksimov V.V., Veshkurova O.N., Salikhov Sh.I. Chemical composition and antimicrobial activity of essential oil from seeds of Anethum graveolens growing in Uzbekistan. Chem. Nat. Comp. 2009;45(2):280–281. [Google Scholar]
- Youssef R.S.A. Medicinal and non-medicinal uses of some plants found in the middle region of Saudi Arabia. J. Med. Plants Res. 2013;7(34):2501–2513. [Google Scholar]
- Zheng G.Q., Kenney P.M., Lam L.K. Anethofuran, carvone, and limonene: potential cancer chemoprotective agents from dill weed oil and caraway oil. Planta Med. 1992;58(04):338–341. doi: 10.1055/s-2006-961480. [DOI] [PubMed] [Google Scholar]