Abstract
To date, a variety of delivery systems based on organic or inorganic materials have been investigated. Among them, hydrogels have become one of the most promising field in drug delivery system due to their unique properties. Temperature-sensitive hydrogels, which gelation at physiological temperature, gift the delivery system with excellent spatial and temporal control, and have a widely application in drug delivery, tissue engineering, imaging, and wound dressing. This review provides a brief overview on the concept and classification of temperature-sensitive hydrogels, and covers the application of temperature-sensitive gel systems in delivery of biotherapeutic molecules.
Keywords: Temperature-sensitive, Hydrogels, Biotherapeutic molecules
1. Introduction
With the development of material science and technology, an enormous number of polymers have been carried out for the delivery of biotherapeutics. Compared with other engineered biomaterials, hydrogels have received remarkable attention in the last few decades for its physical properties resemble living tissues, showing good biocompatibility.
Hydrogels, having a three-dimensional cross-linked structure, are composed of hydrophilic polymers with capacity to captivate water without dissolving (Anseth, Bowman and Brannon-Peppas, 1996). The presence of hydrophilic groups in polymers, such as amine (–NH2), carboxylic (–COOH), sulfate (–SO3H) groups, gives hydrogels excellent water lock ability and water imbibition rate of these three-dimensional cross-linked polymers can range from a hundred to several thousand times their own weight (Mathew et al., 2018, Gupta et al., 2002). Interestingly, the rheological mechanical properties of hydrogels storing large volume water are closer to solids rather liquids, which is similar to biological tissue, facilitating hydrogels to mimic natural living tissues (Hoffman, 2012). Chemically or physically cross-linking provides distinctive swelling behavior and three-dimensional porous structure of hydrogels. Hydrogels can reversible swelling and de-swelling, and the swelling properties are highly related to the external environment, such as pH, temperature and ionic concentration, which can in turn contribute to the collapse or phase change (Peppas, 1993, Vinogradov et al., 2004, Ahmed, 2015).
Hydrogels can be classified in a variety of ways, such as according to original source (natural and synthetic polymer), cross-linking method (chemically and physically), physical structure (amorphous, supermolecules, hydrogen bone-gels, semi-crystalline), ionic charge (anionic, cationic, ampholitic, and non-ionic), size (macrogels, microgels, nanogels) and biodegradability (non-degradable and degradable) (Mahinroosta et al., 2018, Nguyen et al., 2015). Formerly, traditional hydrogel researches focus on chemical cross-linking networks to form permanent gels before treating (Buwalda et al., 2014). These types of hydrogels have generally been used for implantable applications by surgery. The use of toxic catalyst and cross-linker in these permanent cross-linked system can damage or denature therapeutic molecules, such as proteins or cells, thereby causing low biocompability (Mamaghani and Kaffashi, 2015, Tomme et al., 2008, Xuan et al., 2013). Moreover, traditional hydrogels are not intelligent response to external environment stimuli and have a weak mechanical strength, causing severely limitation of practical application. Therefore, the development of intelligent gels which response to the environment has become a hot spot.
Smart hydrogels, which can response to factors like temperature, pH, light, etc (Hoffman, 1995, He et al., 2008) have been carried out in many fields such as pharmaceutical materials, biosensors (Buenger et al., 2012), drug delivery (Hoare and Kohane, 2008, Nguyen and Alsberg, 2014), sewage treatment, and soil water conservation. Classification of environment stimulus responsive hydrogels is based on external stimuli type as thermo-sensitive, pH sensitive, electrosensitive, as well as sensitive to light, pressure, ionic strength (Kost and Langer, 2012). Hydrogels, formed de novo in physiological conditions, attract more attention for reducing the risks of surgical implantation such as scarring and infection (Nguyen et al., 2015). Thermo-responsive hydrogels are the best studied polymer systems for the temperature is variable naturally and easy to control. The volume (swelling/de-swelling) and transmittance of thermo-sensitive hydrogels will make corresponding respond intelligently with the variety of temperature.
Temperature-sensitive hydrogels contain both hydrophobic and hydrophilic component in structures generally, and the phenomenon of thermal response is derived from the delicate balance between the hydrophobic and hydrophilic portions of the polymer monomer (Xue et al., 2002, Qiu and Park, 2001). Temperature changes the interaction between hydrophilic and hydrophobic segments in the polymer with water molecules, thus can induce a change in the solubility of the cross-linked network, causing the sol-gel phase transition (Bajpai et al., 2008). The sol phase is defined as a flowing fluid while the gel phase is non-flowing and maintain its integrity. Changing the balance of hydrophilicity and hydrophobicity determines the macroscopic dissolved state of cross-linking network in an aqueous solution (Byeongmoon et al., 2012). Gelation property of thermo-sensitive hydrogels can be achieve by microscopic mechanisms depending on the thermo-sensitive groups. Hydrogels exhibit separation from solution and solid either at lower critical solution temperature (LCST) or at the upper critical solution temperature (UCST) (Schild and Tirrell, 1990, Ishida et al., 2012). The polymer is soluble under the LCST, while it begin to shrink, hydrophobic and insoluble when temperature upon LCST, thus resulting in the formation of gel. In contrast, the hydrogel formed upon cooling of the polymer solution has an upper critical solution temperature (UCST). More specifically, the polymer in the solution near the critical temperature exhibits a phase change from a soluble state (random coil) to an insoluble state (collapse or micelle form). The inherent LCST can be set by altering the ratio of hydrophilic to hydrophobic (Qiu and Park, 2001).
The thermo-sensitive hydrogels have been developed widely due to the controllable release of bioactive agent is sensitive to changes in temperature. Thermo-sensitive gels have many advantages as delivery system. Unlike traditional hydrogels need to be surgically implanted, the temperature-sensitive properties gift the hydrogel injectability for local administration which can avoid first pass metabolism. The temperature-sensitive gel responds to temperature, and the temperature-induced sol-gel transition is safer in the body and more suitable for injectable systems because it does not require any denaturing cross-linking agent. Encapsulation in a flowing state allows the uniform dispersion of therapeutic agent in hydrogels, meanwhile rapid sol-to-gel transition at body temperature avoids initially burst release of the therapeutic agents, thereby controlling the release behavior in a controlled manner. At last, the flowable state of administration imparts the hydrogel shape controllability.
The article reviews the thermosensitive hydrogels and focuses on their application in delivery of bio-therapeutic molecules.
2. Classification of thermosensitive hydrogels
Temperature sensitive hydrogels can be divided into negatively thermo-sensitive and positively thermo-sensitive hydrogels according to their temperature-sensitive structure (Liu et al., 2006). Polymers with LCST can form negatively temperature sensitive hydrogels, the polymers shrink along with the temperature increases. At lower temperature, hydrogen bonding between hydrophilic groups of the polymer chain and water molecules is dominates, leading to the dissolution in water. However, with the temperature increases the hydrophobic interactions among hydrophobic groups become strengthened while the hydrogen bonding becomes weaker, causing gelation. Positively temperature sensitive hydrogels increase their water-solubility as the temperature increases, possessing UCST. LCST of some polymers in water is summarized in Table 1. Some temperature sensitive polymers are described below according to the classification (see Table 2).
Table 1.
The LCST of polymers in aqueous solution (Byeongmoon et al., 2012).
| Polymer | LCST (°C) |
|---|---|
| Poly(N-isoprolylacrylamide), pNiPAAm | ∼32 |
| Poly(ethylene glycol), PEG | ∼120 |
| Poly(propylene glycol), PPG | ∼50 |
| Poly(methacrylic acid), PMAA | ∼75 |
| Poly(vinyl alcohol), PVA | ∼125 |
| Poly(winyl pyrrolidone), PVP | ∼160 |
| Methylcellulose, MC | ∼80 |
Table 2.
List of biotherapeutic molecules delivered by thermo-sensitive hydrogels.
| Biotherapeutic molecules incorporated | Carriers | Significant effects | Reference |
|---|---|---|---|
| Docetaxel | Pluronic F127 and N,N,N-trimethyl chitosan |
|
(Turabee et al. 2019) |
| |||
| Doxorubicin | Chitosan/hyaluronic acid/β-sodium glycerophosphate |
|
(Zhang et al. 2018) |
| |||
| Doxorubicin | Chitosan/β-sodium glycerophosphate/polyethylene glycol |
|
(Han et al. 2018) |
| |||
| Doxorubicin | D-PNAx nanomedicines |
|
(Wan et al. 2016) |
| Diclofenac sodium | Chitosan/β-sodium glycerophosphate |
|
(Qi et al. 2016) |
| Asprin | Poly (N-isopropylacrylamide)/clay (Laponite XLS)/gold nanoparticles (Au-S-S NPs)/caboxymethyl chitosan (CMCTs) |
|
(Chen et al. 2019) |
| Doxorubicin and vaccinia virus vaccine expressing Sig/E7/LAMP-1 | Chitosan |
|
(Han et al. 2008) |
| |||
| Bacillus Calmettee Guérin | Chitosan/β-glycerophosphate /Fe3O4 magnetic nanoparticle |
|
(Zhang et al. 2013) |
| |||
| Luteolin | Hyaluronic acid/poly(N-isopropylacrylamide) |
|
(Kim et al. 2018) |
| Ketoprofen | Chitosan/poly(N-vinylcaprolactam-co-itaconic acid-co-ethylene–glycol dimethacrylate) |
|
(Matos Fonseca et al. 2019) |
| Doxorubicin | Chitosan/poly(N-isopropylacrylamide-co-itaconic acid) |
|
(Fathi et al. 2019) |
| Human adipose tissue-derived stem cells | Chitosan/β-glycerophosphate/collagen |
|
(Song et al. 2017) |
| Induced pluripotent stem cells | Polyethylene glycol-co-poly-ε-caprolactone/collagen-binding peptide |
|
(Wang et al. 2015) |
| Zinc | Chitosan/β-glycerophosphate hydrogel |
|
(Niranjan et al. 2013) |
| Cells | Block poly(-caprolactone) and poly(ethylene glycol) |
|
(Niu et al. 2014) |
| Rabbit chondrocytes | Poly(ethyleneglycol)/poly(ɛ-caprolactone) |
|
(Park et al. 2007) |
| Acellular bone matrix | Poly(ethylene glycol)epoly(ε-capro-lactone)epoly(ethylene glycol) |
|
(Ni et al. 2014) |
| Bone marrow-derived mesenchymal stem cells | Arg-Gly-Asp modified hydroxybutyl chitosan |
|
(Qu et al. 2019) |
| 5-aminosalicylic acid | Levan/N-isopropyl acrylamide |
|
(Osman et al. 2017) |
| Bone marrow-derived mesenchymal stem cells | Poly-NIPAM |
|
(Lei et al. 2018) |
| Dextran | Graphene oxide/oligo (ethylene glycol) methyl ether methacrylate/ 2-(2-methoxyethoxy) ethyl methacrylate |
|
(Cheng et al. 2018) |
| Mouse bone mesenchymal stem cells |
|
||
| Keratinocyte growth factor | Heparin-modified poloxamer |
|
(Xu et al. 2017) |
| Dexamethasone and recombinant human bone morphogenetic protein | Chitosan/β-glycerophosphate |
|
(Mukherjee et al. 2018) |
| Collagen I | Chitosan/β-glycerophosphate |
|
(Tang et al. 2019) |
| Plasmid DNA | Chitosan/Pluronic |
|
(Lee et al. 2009) |
| Nanocomplex of graphene oxide and vascular endothelial growth factor-165 | Polyethylenimine functionalized GO nanosheets/low-modulus methacrylated gelatin |
|
(Paul et al. 2014) |
| Lysozyme | Poly(ethylene glycol)-poly(sulfamethazine carbonate urethane) |
|
(Phan et al. 2017) |
| Insulin | Chitosan/PLGA-PEG-PLGA |
|
(Rong et al. 2019) |
| Insulin | Poly (ε-caprolactone)-b-poly (ethylene glycol)-b-poly (ε-caprolactone) |
|
(Nguyen et al. 2019) |
| Vascular endothelial growth factor | Poly(D,L-lactic-co-glycolic acid)-b-methoxy poly(ethylene glycol) (PLGA-mPEG) block copolymer |
|
(Chen et al. 2018) |
2.1. Negatively sensitive hydrogels
2.1.1. N-Isopropylacrylamide-based system
Hydrogels based on poly (N-isopropylacrylamide) (pNiPAAm) are probably the most extensively studied temperature sensitive systems (Yin et al., 2006, Masamichi et al., 2006, Coughlan et al., 2004). The homopolymer and copolymers of pNiPAAm are often investigated for drug delivery, cell encapsulation and tissue engineering. Polymers of poly(N-isopropylacrylamide) contain a hydrophilic amide group and a hydrophobic isopropyl group with the LCST at about 32 °C, showing a sharp phase transition (Pei et al., 2004). Below the LCST, the polymer exhibits a flexible, extended coil conformation in aqueous solution attributing to the formation of hydrogen bonding between polar groups and water molecules. Above the LCST, the hydrogen bond of the cross-linked network breaks, the pNiPAAm hydrogels discharge the water and shrink rapidly, resulting in the changes from a flowable liquid to a gel-like solid (Schild, 1992, Xue et al., 1992). The LCST of pNiPPAm is closer to the body temperature and can be altered by copolymerizing with other hydrophilic comonomer (Feil et al., 1993). Copolymerization of pNiPAAm with more hydrophilic monomers increases hydrophilicity of the polymer, and the stronger polymer-water interactions cause the increase of LCST. The addition of more hydrophobic monomers results in a lower LCST compared with pNiPAAm (Qiu and Park, 2001). The hydrogels based on pNiPAAm copolymer with acrylic (AA) and propylacrylic acid (PAA) was developed to adjust the LCST closer to body temperature. Although polymers of pNiPAAm have a suitable LCST, the clinical applications of temperature sensitive hydrogels based on pNiPAAm and its derivatives have limitations due to the unbiocompatibility.
2.1.2. Polysaccharides
Cellulose is a natural polysaccharide which is insoluble in water. However, substitute the hydroxyl groups of the cellulose with more hydrophobic groups like methyl or hydroxypropyl gifts the ability of water soluble to the initially insoluble cellulose (Li et al., 2002). Methyl cellulose (MC), with the LCST is 60–80 °C, is a typical example with thermoreversible gel property (Takahashi and Shimazaki, 2001). In order to adjust the LCST of the Methyl cellulose closer to the human body temperature, some other monomer polymers are added. Hydrogels were developed by Liu et al. (2004) via grafting methyl cellulose with N-isopropylacrylamide (NiPAAm) in various ratios to adjust the suitable LCST. A lower percentage of methyl cellulose reduced LCST while LCST increased with higher MC ratio compared with pNiPAAm. They reported that the addition of MC to the NiPAAm polymer enhanced the mechanical strength of the gel without causing syneresis. Due to their higher LCST, cellulose has not been widely used in thermosensitive gels, and almost uses as regulators to regulate the other temperature sensitive system to achieve a suitable LCST.
Chitosan, (1–4)-2-amino-2-deoxy-β-D-glucan, is a polysaccharide obtained from the alkaline hydrolysis of chitin, has many advantages over other natural polymers such as biodegradable and non-toxic (Maite et al., 2013). The chain of chitosan has a lot of amine groups (–NH2) and hydroxyl groups (–OH), which acting as functional groups to react with crosslinking agent (Xiao et al., 2016). The chitosan based thermosensitive system was first introduced by Chenite et al. In 2000 by connecting chitosan with β-glycerophosphate (GP) to alter the gelation temperature (Chenite et al., 2000). It’s a major breakthrough in the development of temperature-sensitive hydrogels on natural polymer. The mixture system can remain a liquid phase at room temperature while gelation after the temperature is increased to a physiological temperature (37 °C), possessing temperature sensitive property. Different molecular weight of chitosan can’t affect the gelation temperature, whereas the concentration of GP directly affects the temperature of gel when the degree of deacetylation and concentration of chitosan maintained. With the increase of GP, the gel temperature decreased (Qi et al., 2015, Anuja and Hema, 2014). Nicolas Anton et al. (Supper et al., 2013) clarified the gelation mechanism of the thermosensitive chitosan/polyol-phosphate system recently. They reported that polyols formed a hydration protective layer around the chitosan chains through weak intermolecular interactions like hydrogen bonding. The increase in temperature destroys the polyol layer and permits the polymer to interact through stronger hydrophobic bonds, thus causing gelation. Bhattarai et al. (Bhattarai et al., 2005) incorporated poly(ethylene glycol) (PEG) into chitosan to form a thermoreversible hydrogel without any additional crosslinkers. Chitosan is one of the most widely used materials in temperature sensitive gel systems. However, it has disadvantages such as low mechanical strength and slow temperature response, which needs to be modified by combination with other materials.
2.1.3. PEO/PPO based system
Pluronic or poloxamers, known as a triblock copolymers poly (ethylene oxide)-b-poly (propylene oxide)-b-poly (ethylene oxide) (PEO-PPO-PEO), has a thermoreversible property which can be changed by adjusting composition, molecular weight and concentration at physiological temperatures. The amphiphilicity of the PEO-PPO-PEO is attributed to the hydrophilic ethylene oxide and hydrophobic propylene oxide in the structure (Jørgensen and Hvidt, 1997). Poloxamers in water exhibit particular gel properties due to differences in physicochemical properties of polyoxyethylene and polyoxypropylene that make up the poloxamer block (Schillén et al., 1993). With the increase of temperature, a micelle structure can be formed, that is the hydrophobic PPO chain is wrapped inside to form an inner core and the relatively hydrophilic PEO is combined with water and wrapped around the hydrophobic structure. More importantly, the above micelle structure is not completely independent. When the solution concentration is higher than the critical micelle concentration (CMC), various forces between the micelles will make the micelle further entangled, piled up and gathered (Rassing and Attwood, 1982). At this point, gelation will occur with arise of temperature, therefore the poloxamer can be used as a temperature-sensitive in situ gel matrix. Hydrogels based on PEO/PPO block copolymer attract more attention for its temperature sensitivity and biocompatibility. However, the copolymer applied in clinical is limited due to the original undegradable in vivo and easy to be diluted by body fluid after injection which can’t achieve long-term effect.
2.1.4. PEG/PLGA based system
The copolymerization with polyethylene glycol (PEG) and biocompatible polyesters can produce some interesting hydrogel systems. A gel system with thermal response properties can be obtained and the thermal response properties can be improved by adjusting the length of the hydrophobic polyester block and the PEG block appropriately. For the advantages of non-toxic, biocompatible, biodegradable and thermal sensitive, the poly((lactic acid) co-(glycolic acid)) and poly(ethylene glycol) (PLGA-PEG-PLGA) triblock copolymers become the most attracting materials. The PEG/PLGA amphiphilic block copolymers exhibited a single sol-to-gel transition with decreasing of temperature in water, forming micells (Kim and Kim, 2002, Jeong et al., 2000). It is believed that the mechanism of sol-gel transition of a PLGA-PEG-PLGA triblock copolymer is a micelle expansion accompanied by an increase in the number of aggregates driven by hydrophobic forces. Many bioactive drugs have been delivered by thermally sensitive hydrogels based on PEG/PLGA amphiphilic block copolymers.
2.2. Positively temperature sensitive hydrogels
Most natural polymer like gelatin, agarose, amylose, amylopectin and some cellulose derivatives can form a thermoreversible gel phase by lowering the temperature, belonging to the positively temperature sensitive hydrogels. Gelatin is selected as a representative example. Gelatin, possessing thermoreversible properties, is a denatured protein obtained from the partial hydrolysis of collagen (Young et al., 2005). When the temperature is increased above 40 °C, the conformation ranges from a helix to random flexible coil, presenting liquid phase. When the temperature is decreased below UCST (35 °C), some segments of the gelatin molecule are re-spinned into a left-handed helix, and hydrogen bonds are cross-linked between the adjacent three left-handed helical segments to form a stable collagen supercoil structure, thus forming a three-dimensional network (Joly-Duhamel et al., 2002). Water molecule is trapped in the interstices between the supercoiled chains by forming hydrogen bonded combined with the –NH2 groups. Upon the density of crosslink reaches a certain level, the entire system solidifies into a gel. The triple helix conformation in gelatin drives the nucleation and growth of the crystallites during gel formation (Das, 2019). Hydrogels are believed to have a random coiled conformation at higher temperatures, while they begin to form a double helix and an aggregate with the decrease of temperature, which act as a physical junction of gel. Owning a large number of reactive functional groups gift the gelatin ability of modification. Yang and Kao (2006) synthesized a hydrogel composed with gelatin and poly(ethylene glycol)-poly(D,L-lactide)(mPEG-DLLA) block copolymers, which could flow at 37 °C and gel at room temperature.
3. Release mechanism of biotherapeutic molecules from temperature sensitive hydrogels
From various model studies on the possible release mechanisms of compounds from hydrogel, the drug release mechanisms can be classified as: (i) passive diffusion, (ii) erosion of hydrogels, and (iii) Chemical control. The most common release mechanism of thermo-sensitive hydrogels is passive diffusion, and different sizes of biotherapeutic molecules which entrapped in the gel matrix can diffuse freely depending on the size of the mesh of the gel matrix (Amsden, 1998, Canal and Peppas, 2010). The behavior in turn influenced by several parameters, including the degree of cross-linking, the chemical structure of monomer and the intensity of external stimuli. The typical mesh size of temperature-sensitive hydrogels as reported ranges from 5 to 100 nm in swollen state (Mason et al., 2001, Cruise et al., 1998), which is much larger than most small molecule drugs. Thus, the diffusion of these drugs is free in a swollen state, while macromolecules such as oligonucleotides, peptides and proteins will exhibit sustained release unless the structure and mesh size of the swollen hydrogel are properly designed to achieve the desired diffusion rate (Harland and Peppas, 1987). In the case of erosion-controlled mechanism, the release of biotherapeutic molecules is depending on the rate of erosion (Kanjickal and Lopina, 2004). Finally, chemical controlled release is determined by the chemical reactions in the gel matrix, including polymeric chain cleavage by hydrolysis or enzymatic degradation, or reversible/irreversible reactions that occur between the polymer network and the release of the drug (Hamidi et al., 2008).
4. Applications of temperature sensitive hydrogels
Due to the ability of good biocompatibility and mimic the extracellular matrix environment, hydrogels become the potential candidates for many biomedical applications. Typical applications for hydrogels include soft contact lenses, wound healing, tissue engineering, and sensors. To date, hydrogels have been widely developed for significant complicated applications instead of simple contact lenses, particularly in the controlled delivery of therapeutic agents and tissue engineering. The porous structure of hydrogels can be adjusted by cross-linked density, thus the release behavior of different bioactive agent like proteins or drugs can be controlled. Some prominent examples of temperature sensitive hydrogels for delivery of drugs, genes, cells or other therapeutic agents are described below.
4.1. Temperature sensitive hydrogels for drug delivery
Cancer treatment with conventional chemotherapy causes systemic toxicity usually. The use of thermosensitive hydrogels for localized administration can reduce the side effects and maintain the controlled release of drugs at the tumor site, thus increasing its efficacy. The application of chemoimmunotherapy used for cancer therapy has been limited by the toxicity to the healthy cells. To overcome the limitation, localized treatment like hydrogel-based drug delivery systems have been researched to make the therapy more effective with minimal damage to the host immune systems.
Md Hasan Turabee and co-workers (Turabee et al., 2019) developed a thermosensitive hydrogel by mixing pluronic F127 (PF127) and N,N,N-trimethyl chitosan (TMC) to delivery docetaxel (DTX) for the treatment of glioblastoma. The addition of TMC in the gel system increased the porosity of gel network and improved the mechanical strength of PF127, thereby increasing the cumulative of DTX. The thermosensitive hydrogel system showed sustained release of DTX for more than one month and offered a promising strategy for the treatment of brain tumor in vivo. A body temperature sensitivity hydrogel loaded with Doxorubicin (DOX) was developed based on chitosan/hyaluronic acid/β-sodium glycerophosphate system (Zhang et al., 2018). By adjusting the ratio of hyaluronic acid, the mechanical strength improved and the gelation temperature could be tuned at the same time. Meanwhile, the presence of hyaluronic acid inhibited the initial burst release of DOX from the chitosan/β-sodium glycerophosphate system. Han et al. (2018) prepared a self-healing thermosensitive hydrogel based on chitosan/β-sodium glycerophosphate and polyethylene glycol (DF-PEG) loaded with DOX. In a Heps tumor model in mice, gels were injected to control the growth of tumor by intratumoral injection, showing a superior tumor inhibition and relieving cardiotoxicity of DOX.
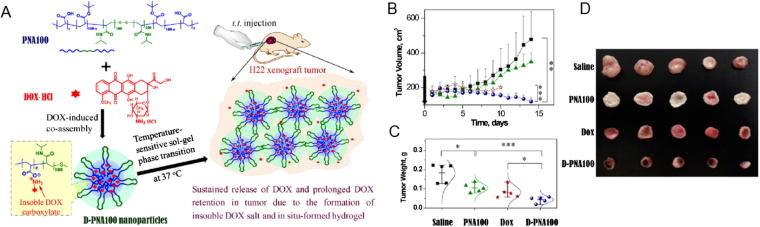
Wan et al. (2016) reported a temperature-sensitive PNAx triblock polymers by co-assembling DOX with nanomedicines (D-PNAx) for regional chemotherapy against liver cancer. Treatment with the temperature sensitive in-situ-forming hydrogels, D-PNAx nanomedicines showed excellent antitumor activity against H22 tumor via intratumoral administration. The treatment of arthritis by intra-articular injection of drugs is considered an effective manner. A schematic representation of this temperature-sensitive system is shown in Fig. 1. A thermo-sensitive hydrogel based on chitosan combined with alginate microspheres was developed to deliver diclofenac sodium to promoting the anti-inflammation effect. The anti-inflammation efficacy was evaluated and confirmed with experimental rheumatoid arthritis in rabbits (Qi et al., 2016) Chen et al. (2019) synthesized a novel tough robust biocompatible and dual pH- and temperature- responsive poly (N-isopropylacrylamide)/clay (Laponite XLS)/gold nanoparticles (Au-S-S NPs)/carboxymethyl chitosan (CMCTs) nanocomposite hydrogel by a facile one-pot method to delivery asprin in a controllable release property.
Fig. 1.
(A) Schematic illustration of the forming process of the temperature sensitive in situ hydrogels consisting with DOX and PNA for regional chemotherapy; (B) Tumor growth curves of in vivo anti-tumor efficacy against H22 tumor-bearing Balb/C mice; (C) Tumor weight of each group collected at 14 days after treatment; (D) Tumor photographs of each group. (Wan et al., 2016).
Han et al. (2008) developed a chitosan-based thermosensitive hydrogel used as a local anticancer drug delivery system to combine chemotherapy and immunotherapy for cancer treatment. Continuous release of doxorubicin and vaccinia virus vaccine expressing Sig/E7/LAMP-1 could be observed from the thermosensitive injectable hydrogel system via intratumoral injection. The combination did not decrease but rather increased the number of tumor specific CD8+ T cells and improved the efficacy up to 60 days with a synergistic antitumor effect, meanwhile the survival of tumor-bearing mice prolonged dramatically. A magnetic thermosensitive hydrogel as intravesical delivery system loaded Bacillus CalmetteeGuérin (BCG) was developed, which was composed of chitosan (CS), bglycerophosphate (GP) and Fe3O4 magnetic nanoparticle (Fe3O4-MNP) (Zhang et al., 2013). The magnetic in situ hydrogels extended the residence time of BCG in the bladder, thus eliciting stronger Th1 immune response and revealing excellent antitumor efficacy. A Kim et al. (2018) prepared double cross-linked interpenetrating polymer network (IPN) hydrogels by radical polymerization and Michael addition composed of temperature sensitive poly (N-isopropylacrylamide) (PNIPAM) and pH sensitive hyaluronic acid (HA) for transdermal delivery of luteolin. Dual temperature/pH-sensitive hydrogels based on poly(N-vinylcaprolactam-co-itaconic acid-co-ethylene- glycol dimethacrylate) (poly(NVCL-co-IA-co-EGDMA)) were developed to delivery ketoprofen, and seems to be a promising new concept carrier for hydrophobic drugs (Matos Fonseca et al., 2019).
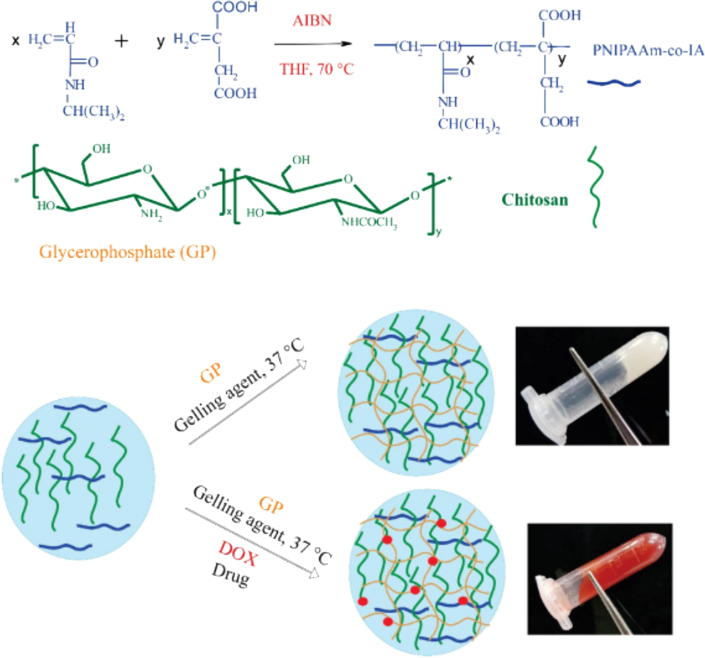
Similarly, dual thermo-and pH-responsive hydrogels were developed and loaded with doxorubicin (DOX) with potential therapy of breast cancer by Marziyeh Fathi (Fathi et al., 2019). The thermo and pH sensitive was achieved by blending synthesis of poly(N-isopropylacrylamide-co-itaconic acid) (PNIAAm-co-IA) with chitosan (CS) through ionic crosslinking using glycerophosphate (GP). The process of the hydrogel preparation is shown in Fig. 2.
Fig. 2.
Schematic illustration for preparation of thermo-sensitive hydrogel loaded with DOX (Fathi et al., 2019).
4.2. Temperature sensitive hydrogels for cell culture and tissue engineering
Hydrogels designed as a scaffolds for tissue engineering application can reflect some characteristics of extracellular matrices such as surface properties, mechanical strength and biodegradability. Mechanical strength is important when hydrogels are applied as implants to replace the diseased or damaged tissue and temperature sensitivity allows the hydrogel form different shapes to meet a variety of damaged tissues. Limitation of maintain long-term survival of the transplanted cells in the host can be overcome by the hydrogel systems.
A chitosan/β-glycerophosphate/collagen (C/GP/Co) hybrid hydrogel with a gelation temperature of 37 °C was developed to encapsulate human adipose tissue-derived stem cells (ADSCs). ADSC in hydrogel maintained a characteristic morphology with high viability over 5 days in vitro and the capability of engineered adipose tissue formation and biocompatibility was demonstrated in SD rats (Song et al., 2017). Wang et al. (2015) developed a polymer hydrogel with temperature-sensitivity to encapsulate, delivery and integrate induced pluripotent stem cells (iPSCs) into infarcted myocardium to restore heart function.
Niranjan et al. (2013) developed a thermo-sensitive hydrogel containing zinc, chitosan and glycerophosphate for bone tissue engineering. The thermo-sensitive hydrogel exhibited geometrically well-defined pores with crystalline nature and shows swelling ability and demonstrated to enhance antibacterial activity and promoted osteoblast differentiation. N poly(3-caprolactone) (PCL) and poly(ethylene glycol) (PEG) used a tissue engineering scaffolds was reported by Xu et al. And presented a highly porous surface area for cell attachment (Niu et al., 2014). Park et al. showed that the PEG/PCL temperature response hydrogel scaffolds retained the morphology of chondrocytes, and enhanced regeneration by serving as a mechanically functional cartilageneous extracellular matrix (ECM) (Park et al., 2007). Meanwhile, Wei et al. reported another PEG/PCL hydrogels with high bone regenerative ability (Ni et al., 2014).
An Arg-Gly-Asp (RGD) modified hydroxybutyl chitosan (HBC) Hydrogel (HBC-RGD) was developed to enhance the adhesion and proliferation of mesenchymal stem cells (BMSCs) within the hydrogel. Qu et al. (2019) reported that the HBC-RGD thermo hydrogel having desired thermosensitivity, biocompatibility and enzymatic degradability in vitro, and could be applied as a potential 3D hydrogel scaffold for cell culture. A thermosensitive hydrogel with more biocompatible was successfully synthesized based on levan cross-linker and N-isopropyl acrylamide (Osman et al., 2017). The delivery system was proven to have good compatibility with mouse fibroblast L929 cells.
A novel biocompatible thermo-sensitive hydrogel to carry BMSCs for full thickness skin wound healing was created (Lei et al., 2018). The results confirmed the thermo sensitive property and showed great promotion of wound closure, reducing the inflammatory responses surrounding the wounds. Cheng et al. (2018) reported an injectable hydrogel which assembled by graphene oxide (GO) and thermo-sensitive nanogels (tNG). The hybrid hydrogel showed its own high antibacterial activity, and demonstrated the responsive drug release behaviour and high cells viability which encapsulated.
4.3. Temperature sensitive hydrogels for protein delivery and gene therapy
Thermosensitive hydrogels have been widely researched in the delivery of proteins or growth factors due to their ability of controllable, accurately, sustained, and localized treatment, to achieve optimal doses at local sites for effective tissue regeneration and repair. Proteins have been attract more and more attention due to their excellent activity. However, protein drugs are susceptible to the environmental conditions (such as temperature, pH and enzymes) which leading to structural changes and loss of activity. The temperature-sensitive hydrogel used as a carrier for the delivery of protein drugs has the following advantages: (i) The protein is dispersed in the three-dimensional network structure uniformly, preventing the aggregation and precipitation and avoiding inactivation; (ii) The hydrogels form a protective film to isolate the proteins from the environment and avoid denaturation of proteins during preparation, storage and delivery; (iii) The proteins in hydrogels release in an controllable and sustained manner. Hydrogel possess a key corss-linking structure for nucleic acid based drugs to release in a controlled manner. Negatively charged nucleic acids like oligonucleotides, siRNA or DNA can be easily incorporated into a weakly crosslinked polyelectrolyte hydrogels or easily loaded into another nanoscale delivery vehicle.
A temperature-sensitive heparin-modified poloxamer hydrogel with affinity to keratinocyte growth factor (KGF) was developed to deliver KGF and prevent intrauterine adhesion (IUA) by Xu et al. (2017). The solution transited to hydrogel rapidly at 33 °C in uterus cavity, and the KGF released in a sustained release manner for a long time. Meanwhile, the thermo-sensitive hydrogels prolonged the retention of KGF in uterus. The recovery of the injured uterus in morphology and function in rat model demonstrated the successful for IUA treatment with temperature-sensitive hydrogels. A CS/GP hydrogel system was developed as a reservoir to delivery bone morphogenetic protein-2(BMP-2) (Mukherjee et al., 2018). Tang et al. (2019) constructed a thermo-sensitive hydrogel based on chitosan/β-glycerophosphate (CS-GP), which mixing with hydroxypropylcellulose and collagen to improve the adhesion ability, viscosity and biocompatibility. The addition of collagen gifted the hydrogel good cytocompatibility. Jung Im Lee et al. (2009) designed a temperature-sensitive hydrogels based on chitosan/pluronic for gene therapy to enhance local transgene expression. The chemically crosslinking was triggered by photo-irradiated at 37 °C, meanwhile, the release rates of plasmid DNA encapsulated in hydrogel could be controlled by changing the photo-irradiation time and the chitosan content.
A study was carried out to develop an injectable and biocompatible hydrogel which can efficiently deliver a nanocomplex of graphene oxide (GO) and vascular endothelial growth factor-165 (VEGF) pro-angiogenic gene for myocardial therapy (Paul et al., 2014). In order to overcome the limitation of protein delivery system, V.H. Giang Phan and their team (Phan et al., 2017) developed an anionic injectable hydrogel with pH and temperature response properties based on poly(ethylene glycol)-poly(sulfamethazine carbonate urethane) (PEG-PSMCU) copolymers to delivery protein. The gelation time and mechanical strength and viscosity of the hydrogel system could be tunable by varying pH and temperature. The hydrogel was used to encapsulate lysozyme and the results proved the sustained release in SD rats for 7 days. Hydrogels can be used as a vector to delivery insulin. Rong et al. (2019) prepared a new double-controlled hydrogel system to delivery insulin for ocular delivery via subconjunctival injection. The double-controlled release system was prepared by adding insulin-loaded chitosan nanoparticles to PLGA-PEG-PLGA hydrogels and showed good therapy. Meanwhile, Nguyen et al. (2019) researched a pH and temperature responsive hydrogel by poly (ε-caprolactone)-b-poly (ethylene glycol)-b-poly (ε-caprolactone) to delivery insulin.
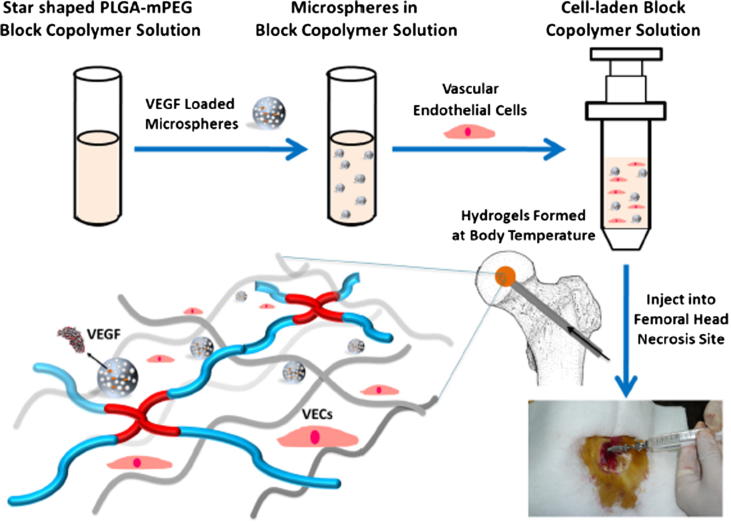
Chen et al. (2018) developed an injectable temperature sensitive hydrogel by star-shaped poly(D,L-lactic-co-glycolic acid)-b-methoxy poly(ethylene glycol) (PLGA-mPEG) block copolymer to load vascular endothelial growth factor (VEGF) for forming neovascularization. The presence of hydrogel delayed the release of VEGF in contrast with micospheres, and the results in vivo suggested the feasibility to apply for the vascularization and bone regeneration of femoral head necrosis. A schematic illustration for preparation of injectable hydrogel is shown in Fig. 3.
Fig. 3.
The preparation process of thermo-sensitive injectable hydrogel with VEGF loaded microspheres by Chen et al. used for vascularization and bone regeneration of femoral head necrosis (Chen et al., 2018).
4.4. Temperature sensitive hydrogels for other application
Thermosensitive gel has an enormous potential in some other application such as wound care, cosmetology etc. Intranasal delivery is a non-invasive and convenient method to provide not only efficient systemic drug delivery but also a potential to bypass the blood-brain barrier (BBB) (Wong and Zuo, 2013). The temperature-sensitive hydrogel was utilized as a delivery system to alter the permeability in nasal cavity (Deshpande et al., 2013). Sultana et al. developed a hydrogel with the ability of temperature gelation for post-surgical peritoneal tissue adhesion prevention (Sultana et al., 2019). Temperature sensitive hydrogel was designed to dissipate heat by mimicking the phenomenon of biological sweating (Pu et al., 2019).
In summary, temperature sensitive hydrogels are excellent carriers for the delivery of various biotherapeutic molecules with the following advantages: (i) Hydrogels which are sensitive to temperature can gel at body temperature and more convenient to administer; (ii) The formed hydrogel matrix can protect the incorporated cells and fragile drugs; (iii) The release of biotherapeutic molecules from hydrogels can be controlled, thereby improving efficacy and reducing side effects. The mechanical strength of the swelling gel, poor temperature sensitivity, and biocompatibility of the polymers are challenges and limitations for temperature sensitive hydrogels used as delivery vehicles for biotherapeutic molecules.
5. Conclusion and future perspectives
The field of thermally responsive hydrogels attract more interests to biomedical scientists and engineers. Overall, this review has highlighted the polymer materials classified by temperature sensitivity and summarized the latest application advances in the field of temperature-sensitive hydrogel systems for biotherapeutic delivery. We introduced the principle of temperature sensitive based on polysaccharides, NiPAAm, PEO/PPO, and PEG/PLGA polymers. Gelation caused by temperature is a mild process which will not damage the encapsulations in hydrogels. Various researches have been carried out to explain the gelation behavior (swelling and de-swelling) of temperature-sensitive hydrogels, propose some new thermosensitive materials based on synthetic and natural polymers, and identify the key factors such as hydrophilic/hydrophobic balance in molecular composition. The shifting temperature within the physiological range, the increase in mechanical properties, and the control and enhancement of biodegradability are major research priorities, making thermosensitive hydrogels an exciting potential for use in biomedical applications. A few points should be emphasized when designing a new temperature-sensitive gelling system for biomedical applications as follows. (a) The choice of the temperature sensitive polymer types will affect the biocompatibility and biodegradability of gel systems. (b) The porosity of the gel system should be adjusted according to the packaged bioactivity agents, thereby regulating the release of drugs and maintaining the activity of cells. (c) The mechanical stiffness of the temperature-sensitive hydrogels formed in situ has a great influence on its practical application. (d) The duration of gelation time should be controlled to decrease the initial burst release of drugs. (e) Appropriate temperature-sensitive materials should be selected according to the route of administration, such as oral, ocular, rectal, vaginal and parenteral, to satisfy on-demand therapeutic release in the body.
In addition, shortcomings of temperature-sensitive hydrogels such as slow temperature response, low mechanical properties, and poor biocompatibility will affect the expansion in application field. Therefore, the development of a successful temperature-sensitive hydrogel delivery system depends on the development of sophisticated material engineering. The future direction of temperature-sensitive hydrogels is to optimize and improve the characteristics of hydrogels to satisfy the treatment needs, and to prepare green smart hydrogels with lower cost, lower toxicity, excellent biocompatibility and biodegradability. Meanwhile, in-depth studies of delivery systems are also needed using in vivo techniques in order to effectively convert a temperature sensitive hydrogel into clinical practice.
Declaration of Competing Interest
The authors declare no conflict of interest.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Yanhua Chen, Email: cdchenyanhua@163.com.
Zhenghong Wu, Email: zhenghongwu66@cpu.edu.cn.
References
- Ahmed E.M. Hydrogel: preparation, characterization, and applications: a review. J. Adv. Res. 2015;6:105–121. doi: 10.1016/j.jare.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amsden B. Solute diffusion in hydrogels. Polym. Gels Networks. 1998;6:13–43. [Google Scholar]
- Anseth K.S., Bowman C.N., Brannon-Peppas L. Mechanical properties of hydrogels and their experimental determination. Biomaterials. 1996;17:1647–1657. doi: 10.1016/0142-9612(96)87644-7. [DOI] [PubMed] [Google Scholar]
- Anuja N., Hema N. Formulation and evaluation of thermosensitive biogels for nose to brain delivery of doxepin. Biomed. Res. Int. 2014. 2014:847547. doi: 10.1155/2014/847547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajpai A.K., Shukla S.K., Bhanu S., Kankane S. Responsive polymers in controlled drug delivery. Prog. Polym. Sci. 2008;33:1088–1118. [Google Scholar]
- Bhattarai N., Matsen F.A., Zhang M. PEG-grafted chitosan as an injectable thermoreversible hydrogel. Macromol. Biosci. 2005;5:107–111. doi: 10.1002/mabi.200400140. [DOI] [PubMed] [Google Scholar]
- Buenger D., Topuz F., Groll J. Hydrogels in sensing applications. Prog. Polym. Sci. 2012;37:1678–1719. [Google Scholar]
- Buwalda S.J., Boere K.W.M., Dijkstra P.J., Feijen J., Vermonden T., Hennink W.E. Hydrogels in a historical perspective: from simple networks to smart materials. J. Control Release. 2014;190:254–273. doi: 10.1016/j.jconrel.2014.03.052. [DOI] [PubMed] [Google Scholar]
- Byeongmoon J., Sung Wan K., Han B.Y. Thermosensitive sol-gel reversible hydrogels. Adv. Drug. Del. Rev. 2012;64:154–162. [Google Scholar]
- Jeong Byeongmoon, Kibbey Merinda R., Birnbaum Jerome C.a., Won YouYeon, Gutowska Anna. Thermogelling biodegradable polymers with hydrophilic backbones: PEG-g-PLGA. Macromolecules. 2000;33:8317–8322. [Google Scholar]
- Canal T., Peppas N.A. Correlation between mesh size and equilibrium degree of swelling of polymeric networks. J. Biomed. Mater. Res. 2010:23. doi: 10.1002/jbm.820231007. [DOI] [PubMed] [Google Scholar]
- Chen D., Zhang C., Huo H., Ji C., Sun M., Nie L. Injectable temperature-sensitive hydrogel with VEGF loaded microspheres for vascularization and bone regeneration of femoral head necrosis. Mater. Lett. 2018;229:138–141. [Google Scholar]
- Chen Y., Kang S., Yu J., Wang Y., Zhu J., Hu Z. Tough robust dual responsive nanocomposite hydrogel as controlled drug delivery carrier of asprin. J. Mech. Behav. Biomed. Mater. 2019;92:179–187. doi: 10.1016/j.jmbbm.2019.01.017. [DOI] [PubMed] [Google Scholar]
- Cheng W., Chen Y., Teng L., Lu B., Ren L., Wang Y. Antimicrobial colloidal hydrogels assembled by graphene oxide and thermo-sensitive nanogels for cell encapsulation. J. Colloid Interface Sci. 2018;513:314–323. doi: 10.1016/j.jcis.2017.11.018. [DOI] [PubMed] [Google Scholar]
- Chenite A., Chaput C., Wang D., Combes C., Buschmann M.D., Hoemann C.D., Leroux J.C., Atkinson B.L., Binette F., Selmani A. Novel injectable neutral solutions of chitosan form biodegradable gels in situ. Biomaterials. 2000;21:2155–2161. doi: 10.1016/s0142-9612(00)00116-2. [DOI] [PubMed] [Google Scholar]
- Coughlan D.C., Quilty F.P., Corrigan O.I. Effect of drug physicochemical properties on swelling/deswelling kinetics and pulsatile drug release from thermoresponsive poly(N -isopropylacrylamide) hydrogels. J. Controll. Release Off. J. Controll. Release Soc. 2004;98:97–114. doi: 10.1016/j.jconrel.2004.04.014. [DOI] [PubMed] [Google Scholar]
- Cruise G.M., Scharp D.S., Hubbell J.A. Characterization of permeability and network structure of interfacially photopolymerized poly(ethylene glycol) diacrylate hydrogels. Biomaterials. 1998;19:1287. doi: 10.1016/s0142-9612(98)00025-8. [DOI] [PubMed] [Google Scholar]
- Das N. Biodegradable Hydrogels for Controlled Drug Delivery[M] Cellulose-Based Superabsorbent Hydrogels. 2019 [Google Scholar]
- Deshpande S.T., Lahoti S.R., Dhamecha D.L., Rajendra V.B., Dehghan M.H.G., Puranik P.K. Enhanced iontophoretic delivery of sumatriptan succinate from thermosensitive gel using chemical enhancers. Indian J. Pharm. Educ. 2013;47:27–33. [Google Scholar]
- Fathi M., Alami-Milani M., Geranmayeh M.H., Barar J., Erfan-Niya H., Omidi Y. Dual thermo-and pH-sensitive injectable hydrogels of chitosan/(poly(N-isopropylacrylamide-co-itaconic acid)) for doxorubicin delivery in breast cancer. Int. J. Biol. Macromol. 2019;128:957–964. doi: 10.1016/j.ijbiomac.2019.01.122. [DOI] [PubMed] [Google Scholar]
- Feil H., You H.B., Feijen J., Kim S.W. Effect of comonomer hydrophilicity and ionization on the lower critical solution temperature of N-isopropylacrylamide copolymers. Macromolecules. 1993;26:2496–2500. [Google Scholar]
- Gupta P., Vermani K., Garg S. Hydrogels: from controlled release to pH-responsive drug delivery. Drug. Discovery Today. 2002;7:569–579. doi: 10.1016/s1359-6446(02)02255-9. [DOI] [PubMed] [Google Scholar]
- Hamidi Mehrdad, Azadi Amir, Rafiei Pedram. Hydrogel nanoparticles in drug delivery. Adv. Drug. Deliv. Rev. 2008;60:1638–1649. doi: 10.1016/j.addr.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Han H.D., Song C.K., Park Y.S., Noh K.H., Kim J.H., Hwang T., Kim T.W., Shin B.C. A chitosan hydrogel-based cancer drug delivery system exhibits synergistic antitumor effects by combining with a vaccinia viral vaccine. Int. J. Pharm. 2008;350:27–34. doi: 10.1016/j.ijpharm.2007.08.014. [DOI] [PubMed] [Google Scholar]
- Han X., Meng X., Wu Z., Wu Z., Qi X. Dynamic imine bond cross-linked self-healing thermosensitive hydrogels for sustained anticancer therapy via intratumoral injection. Mater. Sci. Eng. C Mater. Biol. Appl. 2018;93:1064–1072. doi: 10.1016/j.msec.2018.08.064. [DOI] [PubMed] [Google Scholar]
- Harland R.S., Peppas N.A. Solute diffusion in swollen membranes. Polym. Bull. 1987;18:553–556. [Google Scholar]
- He C., Kim S.W., Lee D.S. In situ gelling stimuli-sensitive block copolymer hydrogels for drug delivery. J. Control Release. 2008;127:189–207. doi: 10.1016/j.jconrel.2008.01.005. [DOI] [PubMed] [Google Scholar]
- Hoare T.R., Kohane D.S. Hydrogels in drug delivery: progress and challenges ☆. Polymer. 2008;49:1993–2007. [Google Scholar]
- Hoffman A.S. “Intelligent” polymers in medicine and biotechnology. Artif. Organs. 1995;19:645–664. doi: 10.1111/j.1525-1594.1995.tb02359.x. [DOI] [PubMed] [Google Scholar]
- Hoffman A.S. Hydrogels for biomedical applications. Ann. N. Y. Acad. Sci. 2012;64:18–23. doi: 10.1111/j.1749-6632.2001.tb03823.x. [DOI] [PubMed] [Google Scholar]
- Ishida K., Uno T., Itoh T., Kubo M. Synthesis and property of temperature-responsive hydrogel with movable cross-linking points. Macromolecules. 2012;45:6136–6142. [Google Scholar]
- Joly-Duhamel C., Hellio D., Djabourov M. All gelatin networks: 1. Biodiversity and physical chemistry†. Langmuir. 2002;18:7208–7217. [Google Scholar]
- Jørgensen E.B., Hvidt S. Effects of salts on the micellization and gelation of a triblock copolymer studied by rheology and light scattering. Macromolecules. 1997;30:2355–2364. [Google Scholar]
- Kanjickal D.G., Lopina S.T. Modeling of drug release from polymeric delivery systems – a review. Crit. Rev. Ther. Drug. 2004;21:345. doi: 10.1615/critrevtherdrugcarriersyst.v21.i5.10. [DOI] [PubMed] [Google Scholar]
- Kim A.R., Lee S.L., Park S.N. Properties and in vitro drug release of pH- and temperature-sensitive double cross-linked interpenetrating polymer network hydrogels based on hyaluronic acid/poly (N-isopropylacrylamide) for transdermal delivery of luteolin. Int. J. Biol. Macromol. 2018;118:731–740. doi: 10.1016/j.ijbiomac.2018.06.061. [DOI] [PubMed] [Google Scholar]
- Kim Y.J., Kim S.W. Controlled drug delivery from injectable biodegradable triblock copolymer. ACS. Sympos. 2002;833:300–311. [Google Scholar]
- Kost J., Langer R. Responsive polymeric delivery systems. Adv. Drug Deliv. Rev. 2012;64:327–341. doi: 10.1016/s0169-409x(00)00136-8. [DOI] [PubMed] [Google Scholar]
- Lee J.I., Kim H.S., Yoo H.S. DNA nanogels composed of chitosan and Pluronic with thermo-sensitive and photo-crosslinking properties. Int. J. Pharm. 2009;373:93–99. doi: 10.1016/j.ijpharm.2009.01.016. [DOI] [PubMed] [Google Scholar]
- Lei Z., Singh G., Min Z., Shixuan C., Xu K., Pengcheng X., Xueer W., Yinghua C., Lu Z., Lin Z. Bone marrow-derived mesenchymal stem cells laden novel thermo-sensitive hydrogel for the management of severe skin wound healing. Mater. Sci. Eng. C Mater. Biol. Appl. 2018;90:159–167. doi: 10.1016/j.msec.2018.04.045. [DOI] [PubMed] [Google Scholar]
- Li L., Shan H., Yue C.Y., Lam Y.C., Tam K.C., Hu X. Thermally induced association and dissociation of methylcellulose in aqueous solutions. Langmuir. 2002;18:7291–7298. [Google Scholar]
- Liu W., Zhang B., Lu W.W., Li X., Zhu D., De Yao K., Wang Q., Zhao C., Wang C. A rapid temperature-responsive sol-gel reversible poly(N-isopropylacrylamide)-g-methylcellulose copolymer hydrogel. Biomaterials. 2004;25:3005–3012. doi: 10.1016/j.biomaterials.2003.09.077. [DOI] [PubMed] [Google Scholar]
- Liu Y.Y., Shao Y.H., Jian L. Preparation, properties and controlled release behaviors of pH-induced thermosensitive amphiphilic gels. Biomaterials. 2006;27:4016–4024. doi: 10.1016/j.biomaterials.2006.02.042. [DOI] [PubMed] [Google Scholar]
- Mahinroosta M., Jomeh Farsangi Z., Allahverdi A., Shakoori Z. Hydrogels as intelligent materials: a brief review of synthesis, properties and applications. Mater. Today Chem. 2018;8:42–55. [Google Scholar]
- Maite A.P., Leyre P.Á., Luis Carlos C.I., Issa K. Biodegradable chitosan nanogels crosslinked with genipin. Carbohyd. Polym. 2013;94:836–842. doi: 10.1016/j.carbpol.2013.01.082. [DOI] [PubMed] [Google Scholar]
- Mamaghani P.Y., Kaffashi B. Synthesis, characterization, and viscoelastic behavior of thermothickening poly (N-isopropylacrylamide-methacrylicacide-vinylpyrrolidone) nanogels as an injectable biocompatible drug carrier. Int. J. Polym. Mater. 2015;64:55–63. [Google Scholar]
- Masamichi N., Teruo O., Takanari M., Fukashi K., Kiyotaka S., Masayuki Y. Molecular design of biodegradable polymeric micelles for temperature-responsive drug release. J. Control Release. 2006;115:46–56. doi: 10.1016/j.jconrel.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Mason M.N., Metters A.T., Bowman C.N., Anseth K.S. Predicting controlled-release behavior of degradable PLA-PEG-PLA hydrogels. Macromolecules. 2001;34:4630–4635. [Google Scholar]
- Mathew A.P., Uthaman S., Cho K.H., Cho C.S., Park I.K. Injectable hydrogels for delivering biotherapeutic molecules. Int. J. Biol. Macromal. 2018 doi: 10.1016/j.ijbiomac.2017.11.113. S0141813017334244. [DOI] [PubMed] [Google Scholar]
- Matos Fonseca J., Fatima Medeiros S., Alves G.M., Santos D.M.D., Campana-Filho S.P., Santos A.M.D. Chitosan microparticles embedded with multi-responsive poly(N-vinylcaprolactam-co-itaconic acid-co-ethylene-glycol dimethacrylate)-based hydrogel nanoparticles as a new carrier for delivery of hydrophobic drugs. Colloids Surf. B Biointerfaces. 2019;175:73–83. doi: 10.1016/j.colsurfb.2018.11.042. [DOI] [PubMed] [Google Scholar]
- Mukherjee D., Azamthulla M., Santhosh S., Dath G., Ghosh A., Natholia R., Anbu J., Teja B.V., Muzammil K.M. Development and characterization of chitosan-based hydrogels as wound dressing materials. J. Drug. Deliv. Sci. Tec. 2018 S1773224718301916. [Google Scholar]
- Nguyen D.T., Phan V.H.G., Lee D.S., Thambi T., Huynh D.P. Bioresorbable pH- and temperature-responsive injectable hydrogels-incorporating electrosprayed particles for the sustained release of insulin. Polym. Degrad. Stabil. 2019;162:36–46. [Google Scholar]
- Nguyen M.K., Alsberg E. Bioactive factor delivery strategies from engineered polymer hydrogels for therapeutic medicine. Prog. Polym. Sci. 2014;39:1235–1265. doi: 10.1016/j.progpolymsci.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen Q.V., Dai P.H., Park J.H., Lee D.S. Injectable polymeric hydrogels for the delivery of therapeutic agents: a review. Eur. Polym. J. 2015;72 S0014305715001512. [Google Scholar]
- Ni P., Ding Q., Fan M., Liao J., Qian Z., Luo J., Li X., Luo F., Yang Z., Wei Y. Injectable thermosensitive PEG-PCL-PEG hydrogel/acellular bone matrix composite for bone regeneration in cranial defects. Biomaterials. 2014;35:236–248. doi: 10.1016/j.biomaterials.2013.10.016. [DOI] [PubMed] [Google Scholar]
- Niranjan R., Koushik C., Saravanan S., Moorthi A., Vairamani M., Selvamurugan N. A novel injectable temperature-sensitive zinc doped chitosan/beta-glycerophosphate hydrogel for bone tissue engineering. Int. J. Biol. Macromol. 2013;54:24–29. doi: 10.1016/j.ijbiomac.2012.11.026. [DOI] [PubMed] [Google Scholar]
- Niu Y., Chen K.C., He T., Yu W., Huang S., Xu K. Scaffolds from block polyurethanes based on poly(varepsilon-caprolactone) (PCL) and poly(ethylene glycol) (PEG) for peripheral nerve regeneration. Biomaterials. 2014;35:4266–4277. doi: 10.1016/j.biomaterials.2014.02.013. [DOI] [PubMed] [Google Scholar]
- Osman A., Oner E.T., Eroglu M.S. Novel levan and pNIPA temperature sensitive hydrogels for 5-ASA controlled release. Carbohyd. Polym. 2017;165:61–70. doi: 10.1016/j.carbpol.2017.01.097. [DOI] [PubMed] [Google Scholar]
- Park J.S., Woo D.G., Sun B.K., Chung H.M., Im S.J., Choi Y.M., Park K., Huh K.M., Park K.H. In vitro and in vivo test of PEG/PCL-based hydrogel scaffold for cell delivery application. J. Control Release. 2007;124:51–59. doi: 10.1016/j.jconrel.2007.08.030. [DOI] [PubMed] [Google Scholar]
- Paul A., Hasan A., Kindi H.A., Gaharwar A.K., Rao V.T., Nikkhah M., Shin S.R., Krafft D., Dokmeci M.R., Shum-Tim D., Khademhosseini A. Injectable graphene oxide/hydrogel-based angiogenic gene delivery system for vasculogenesis and cardiac repair. ACS Nano. 2014;8:8050–8062. doi: 10.1021/nn5020787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei Y., Chen J., Yang L., Shi L., Tao Q., Hui B., Li J. The effect of pH on the LCST of poly(N-isopropylacrylamide) and poly(N-isopropylacrylamide-co-acrylic acid) J. Biomater. Sci. Polym. Ed. 2004;15:585–594. doi: 10.1163/156856204323046852. [DOI] [PubMed] [Google Scholar]
- Peppas N.A. Preparation, structure and diffusional behavior of hydrogels in controlled release. Adv. Drug. Del. Rev. 1993;11:1–35. [Google Scholar]
- Phan V.H.G., Thambi T., Gil M.S., Lee D.S. Temperature and pH-sensitive injectable hydrogels based on poly(sulfamethazine carbonate urethane) for sustained delivery of cationic proteins. Polymer. 2017;109:38–48. [Google Scholar]
- Pu S., Su J., Li L., Wang H., Chen C., Hu X. Bioinspired sweating with temperature sensitive hydrogel to passively dissipate heat from high-end wearable electronics. Energ. Convers. Manage. 2019;180:747–756. [Google Scholar]
- Qi F.D., Jing Q.Y., Hong L., Cheng S.L., Xi G.C., Qiu X.J., Jing L., Liu Y. Biological evaluation of chitosan-based in situ-forming hydrogel with low phase transition temperature. J. Appl. Polym. Sci. 2015:132. [Google Scholar]
- Qi X., Qin X., Yang R., Qin J., Li W., Luan K., Wu Z., Song L. Intra-articular administration of chitosan thermosensitive in situ hydrogels combined with diclofenac sodium-loaded alginate microspheres. J. Pharm. Sci. 2016;105:122–130. doi: 10.1016/j.xphs.2015.11.019. [DOI] [PubMed] [Google Scholar]
- Qiu Y., Park K. Environment-sensitive hydrogels for drug delivery. Adv. Drug. Del. Rev. 2001;53:321–339. doi: 10.1016/s0169-409x(01)00203-4. [DOI] [PubMed] [Google Scholar]
- Qu C., Bao Z., Zhang X., Wang Z., Ren J., Zhou Z., Tian M., Cheng X., Chen X., Feng C. A thermosensitive RGD-modified hydroxybutyl chitosan hydrogel as a 3D scaffold for BMSCs culture on keloid treatment. Int. J. Biol. Macromol. 2019;125:78–86. doi: 10.1016/j.ijbiomac.2018.12.058. [DOI] [PubMed] [Google Scholar]
- Rassing J., Attwood D. Ultrasonic velocity and light-scattering studies on the polyoxyethylene—polyoxypropylene copolymer Pluronic F127 in aqueous solution. Int. J. Pharmaceut. 1982;13:47–55. [Google Scholar]
- Rong X., Yang J., Ji Y., Zhu X., Lu Y., Mo X. Biocompatibility and safety of insulin-loaded chitosan nanoparticles/ PLGA-PEG-PLGA hydrogel (ICNPH) delivered by subconjunctival injection in rats. J. Drug. Deliv. Sci. Tec. 2019;49:556–562. [Google Scholar]
- Schild G.H. Poly (N-isopropylacrylamide): experiment, theory and application. Prog. Polym. Sci. 1992;17:163–249. [Google Scholar]
- Schild H.G., Tirrell D.A. Microcalorimetric detection of lower critical solution temperatures in aqueous polymer solutions. J. Phys. Chem. 1990;94:4352–4356. [Google Scholar]
- Schillén K., Glatter O., Brown W. Characterization of a PEO-PPO-PEO block copolymer system. Prog. Colloid Polym. Sci. 1993;93:66–71. [Google Scholar]
- Song K., Li L., Yan X., Zhang W., Zhang Y., Wang Y., Liu T. Characterization of human adipose tissue-derived stem cells in vitro culture and in vivo differentiation in a temperature-sensitive chitosan/beta- glycerophosphate/collagen hybrid hydrogel. Mater. Sci. Eng. C Mater. Biol. Appl. 2017;70:231–240. doi: 10.1016/j.msec.2016.08.085. [DOI] [PubMed] [Google Scholar]
- Sultana T., Van Hai H., Abueva C., Kang H.J., Lee S.-Y., Lee B.-T. TEMPO oxidized nano-cellulose containing thermo-responsive injectable hydrogel for post-surgical peritoneal tissue adhesion prevention. Mat. Sci. Eng. C. 2019 doi: 10.1016/j.msec.2019.03.110. [DOI] [PubMed] [Google Scholar]
- Supper S., Anton N., Seidel N., Riemenschnitter M., Schoch C., Vandamme T. Rheological study of chitosan/polyol-phosphate systems: influence of the polyol part on the thermo-induced gelation mechanism. Langmuir. 2013;29:10229–10237. doi: 10.1021/la401993q. [DOI] [PubMed] [Google Scholar]
- Takahashi, M. Shimazaki, M., 2001. Thermoreversible gelation and phase separation in aqueous methyl cellulose solutions.
- Tang B., Shan J., Yuan T., Xiao Y., Liang J., Fan Y., Zhang X. Hydroxypropylcellulose enhanced high viscosity endoscopic mucosal dissection intraoperative chitosan thermosensitive hydrogel. Carbohyd. Polym. 2019;209:198–206. doi: 10.1016/j.carbpol.2018.12.103. [DOI] [PubMed] [Google Scholar]
- Tomme S.R.V., Storm G., Hennink W.E. In situ gelling hydrogels for pharmaceutical and biomedical applications. Int. J. Pharmaceut. 2008;355:1–18. doi: 10.1016/j.ijpharm.2008.01.057. [DOI] [PubMed] [Google Scholar]
- Turabee M.H., Jeong T.H., Ramalingam P., Kang J.H., Ko Y.T. N, N, N-trimethyl chitosan embedded in situ Pluronic F127 hydrogel for the treatment of brain tumor. Carbohyd. Polym. 2019;203:302–309. doi: 10.1016/j.carbpol.2018.09.065. [DOI] [PubMed] [Google Scholar]
- Vinogradov S.V., Batrakova E.V., Kabanov A.V. Nanogels for oligonucleotide delivery to the brain. Bioconjug. Chem. 2004;15:50–60. doi: 10.1021/bc034164r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan J., Geng S., Zhao H., Peng X., Zhou Q., Li H., He M., Zhao Y., Yang X., Xu H. Doxorubicin-induced co-assembling nanomedicines with temperature-sensitive acidic polymer and their in-situ-forming hydrogels for intratumoral administration. J. Control Release. 2016;235:328–336. doi: 10.1016/j.jconrel.2016.06.009. [DOI] [PubMed] [Google Scholar]
- Wang X., Chun Y.W., Zhong L., Chiusa M., Balikov D.A., Frist A.Y., Lim C.C., Maltais S., Bellan L., Hong C.C., Sung H.J. A temperature-sensitive, self-adhesive hydrogel to deliver iPSC-derived cardiomyocytes for heart repair. Int. J. Cardiol. 2015;190:177–180. doi: 10.1016/j.ijcard.2015.04.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong Y.C., Zuo Z. Brain disposition and catalepsy after intranasal delivery of loxapine: role of metabolism in PK/PD of intranasal CNS drugs. Pharm. Res. 2013;30:2368–2384. doi: 10.1007/s11095-013-1080-x. [DOI] [PubMed] [Google Scholar]
- Xiao C., You R., Fan Y., Zhang Y. Tunable functional hydrogels formed from a versatile water-soluble chitosan. Int. J. Biol. Macromol. 2016;85:386–390. doi: 10.1016/j.ijbiomac.2016.01.006. [DOI] [PubMed] [Google Scholar]
- Xu H.L., Xu J., Zhang S.S., Zhu Q.Y., Jin B.H., ZhuGe D.L., Shen B.X., Wu X.Q., Xiao J., Zhao Y.Z. Temperature-sensitive heparin-modified poloxamer hydrogel with affinity to KGF facilitate the morphologic and functional recovery of the injured rat uterus. Drug. Deliv. 2017;24:867–881. doi: 10.1080/10717544.2017.1333173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xuan Y., Emilia B., Todd H., Cranston E.D. Injectable polysaccharide hydrogels reinforced with cellulose nanocrystals: morphology, rheology, degradation, and cytotoxicity. Biomacromolecules. 2013;14:4447–4455. doi: 10.1021/bm401364z. [DOI] [PubMed] [Google Scholar]
- Xue S.W., Hoffman A.S., Yager P. Synthesis and characterization of thermal reversible macroporous poly(N-isopropylacrylamide) hydrogels. J. Polym. Sci. Pt. A. 1992;30:2121–2129. [Google Scholar]
- Xue W., Hamley I.W., Huglin M.B. Rapid swelling and deswelling of thermoreversible hydrophobically modified poly(N -isopropylacrylamide) hydrogels prepared by freezing polymerisation. Polymer. 2002;43:5181–5186. [Google Scholar]
- Yang H.U., Kao W.J. Thermoresponsive gelatin/monomethoxy poly(ethylene glycol)-poly(d, l-lactide) hydrogels : formulation, characterization, and antibacterial drug delivery. Pharm. Res. 2006;23:205. doi: 10.1007/s11095-005-8417-z. [DOI] [PubMed] [Google Scholar]
- Yin X., Hoffman A.S., Stayton P.S. Poly(N-isopropylacrylamide-co-propylacrylic acid) copolymers that respond sharply to temperature and pH. Biomacromolecules. 2006;7:1381–1385. doi: 10.1021/bm0507812. [DOI] [PubMed] [Google Scholar]
- Young S., Wong M., Tabata Y., Mikos A.G. Gelatin as a delivery vehicle for the controlled release of bioactive molecules. J. Control. Release. 2005;109:256–274. doi: 10.1016/j.jconrel.2005.09.023. [DOI] [PubMed] [Google Scholar]
- Zhang D., Sun P., Li P., Xue A., Zhang X., Zhang H., Jin X. A magnetic chitosan hydrogel for sustained and prolonged delivery of Bacillus Calmette-Guerin in the treatment of bladder cancer. Biomaterials. 2013;34:10258–10266. doi: 10.1016/j.biomaterials.2013.09.027. [DOI] [PubMed] [Google Scholar]
- Zhang W., Jin X., Li H., Zhang R.R., Wu C.W. Injectable and body temperature sensitive hydrogels based on chitosan and hyaluronic acid for pH sensitive drug release. Carbohyd. Polym. 2018;186:82–90. doi: 10.1016/j.carbpol.2018.01.008. [DOI] [PubMed] [Google Scholar]