Abstract
Previously, we reported on the hepatoprotective activity of the total extract of Juniperus sabina L. against CCl4 induced liver toxicity in experimental animals. Biologically directed phytochemical study was conducted to identify the active compounds. Male Wistar rats and the standard drug silymarin were used in the study. Hepatoprotective activity was evaluated via serum biochemical parameters such as aspartate amino transferase (AST), alanine aminotransferase (ALT), gamma glutamyl transpeptidase (GGT), alkaline phosphatase (ALP) and total bilirubin. Tissue parameters including non-protein sulfhydryl groups (NP-SH), malonaldehyde (MDA) and total protein (TP) were also determined. Histopathological study was conducted utilizing Mayer’s hematoxylin stain, Periodic Acid Schiff – Hematoxylin (PAS-H) and Masson trichrome technique on light microscope. Electron microscope images were also generated for the study. The activity of the total extract was trapped to the petroleum ether fraction after liquid-liquid fractionation where 51% reduction in the levels of AST, bilirubin and 44% in the levels of ALT were observed. Chromatographic purification of the petroleum ether fraction resulted in the isolation of nine compounds namely: trans-calamenene (1), cadalene (cadalin) (2), epi-cubenol (3), manool (4), calamenene-10β-ol (5), calamenene-10α-ol (6), 4-epi-abietic acid (7), sandaracopimaric acid (8) and isopimaric acid (9). Compounds 1–3, 5 and 6 are belonging to cadinane sesquiterepenes, while compounds 4, 7–9 were of diterpene skeleton. The major compounds were tested for their hepatoprotective effect. Compounds 3 showed marked improvement in the levels of AST and ALT, compound 4 was effective in improving the levels of AST, ALT, GGT, ALP and bilirubin, while compound 7 showed significant improvement in GGT, ALP and bilirubin levels.
Keywords: Juniperus sabina, Hepatoprotective, Sesquiterpenes, Diterpenes, Electron microscope
1. Introduction
In 2018, the National statistics in the UK ranked liver diseases as one of the major causes of death (UK national statistics). In the US, liver diseases are classified as the second leading cause of mortality among all the digestive diseases (Everhart and Ruhl, 2009). In Saudi Arabia survey revealed that 31.8% of patients suffering from liver diseases concomitantly uses herbs with the physician-prescribed drugs (Al-Zahim et al., 2013). The genus Junipers belonging to the cypress (Cupressaceae) family comprises 50–67 species widely distributed in the Northern Hemisphere (Hampe and Petit, 2010). Junipers are evergreen shrubs or trees with needle or scale like leaves (Ogren, 2015).
Previous phytochemical studies of Junipers species resulted in the isolation of variety of secondary metabolites including phenolic compounds such as lignans, phenylpropanoid, flavonoids, coumarins, in addition to the mevalonate acid pathway product like sesquiterpenes and diterpenes (Seca et al., 2007, Seca and Silva, 2007, Chang et al., 2008, Moujir et al., 2008, Iida et al., 2010, Seca and Silva, 2010, Moujir et al., 2011, Woo et al., 2011, Inatomi et al., 2013). Phytochemical studies of J. sabina resulted in the isolation of α-cedrol, coumasabin, isocupressic acid, skimmin, undulatoside A, hypolaetin 7-O-β-D-xylopyranoside, quercetin 3-O-α-L-rhamnoside, syringin (Zhao et al., 2008), junaphtoic acid, (-)-3-O-demethylyatein (San Feliciano et al, 1991), siderin, coumarsabin and 8-methoxy coumarsabin (De Pascual et al., 1981).
Both J. phoenicea and J. procera were subjected to biologically directed phytochemical study resulted in the identification of hinokiflavone, 4-epi-abietol, sugiol as the most active hepatoprotective components (Alqasoumi and Abdel-Kader, 2012, Alqasoumi et al., 2013). The hot water extract of J. chinensis showed promising anti-obesity effect (Kim et al., 2008). We previously reported on the hepatoprotective effect of J. Sabina total extract of the aerial part against CCl4 induced toxicity in rats (Abdel-Kader et al, 2016).
In this work, detailed biological directed phytochemical study was conducted to identify the active compounds in the plant extract.
2. Materials and methods
2.1. General
Melting points were measured using open capillary tubes Thermosystem FP800 Mettler FP80 central processor supplied with FP81 MBC cell apparatus, and were uncorrected. Ultraviolet absorption were obtained using a Unicum Heyios a UV–Visible spectrophotometer. 1H-, 13C NMR and 2D-NMR experiments were collected using UltraShield Plus 500 MHz (Bruker) (NMR Unite at the College of Pharmacy, Prince Sattam Bin Abdulaziz University) spectrometer operating at 500 MHz for protons and 125 MHz for carbon atoms, respectively. Chemical shift values are reported in δ (ppm) relative to the residual solvent peak, and the coupling constants (J) are reported in Hertz (Hz). X-ray data were collected on a Bruker APEX-II D8 Venture area diffractometerusing graphite monochromatic Mo Kα radiation (λ = 0.71073 Å) at100(2) K. EIMS were obtained using Shimadzu- GC/MS. Silica gel 60/230-400 mesh (EM Science), RP C-18 silica gel 40-63/230-400 mesh (Fluka) were used for column chromatography. TLC were done using silica gel 60 F254 (Merck). Centrifugal preparative TLC (CPTLC) using 2 mm silica gel P254 disc were performed on Chromatotron (Harrison Research Inc. model 7924).
2.2. Plant materials
Aerial parts of Juniperus sabina L. (Cupressaceae) were described earlier (Abdel-Kader et al, 2016).
2.3. Extraction, fractionation and purification
The dried ground aerial parts (1000 g) were extracted till exhaustion by percolation at room temperature with 95% ethanol (10 L). The resulted extract was evaporated in vacuo to leave dark green viscous residue with aromatic odour. Approximately 200 gm of the total extract were dissolved in 1200 ml of 20% aqueous ethanol and fractionated with petroleum ether (500 ml × 3) to yield 45.86 g petroleum ether soluble fraction. The aqueous ethanol fraction was diluted with water to increase water content to 40% and the resulted fraction was fractionated with chloroform (500 ml × 3) to yield 44.93 g of chloroform soluble fraction and 98.54 g of aqueous ethanol soluble fraction.
2.3.1. Petroleum ether fraction
Twenty grams of the petroleum ether soluble fraction were chromatographed on silica gel column (150 × 10 cm i.d., 600 g) eluting with petroleum ether followed by mixtures of petroleum ether/ethyl acetate mixtures in a gradient elution system. Fractions 200 ml each were collected, screened by TLC and similar fraction were collected to yield 4 major fractions (A- D). Fraction A (1 g) eluted with petroleum ether was further purified using silica gel column (45 × 2 cm i.d., 30 g) eluting with petroleum ether followed by mixtures of petroleum ether/ethyl acetate mixtures in a gradient elution system. Fraction 7 afforded 37 mg of 1 as oily liquid. Fraction 9 afforded 5 mg of 2 as oily liquid. Fraction 30 (300 mg) was purified on RP18 silica gel column eluted with 10% water in methanol to afford 75 mg of 3 and 120 mg of 4. Fractions 35–37 (85 mg) afforded 15 mg of 5 and 18 mg of 6.
Fraction B (1.5 g) eluted with 5% ethyl acetate in petroleum ether was further purified using silica gel column (45 × 2 cm i.d., 30 g) eluting with 5% ethyl acetate in petroleum ether followed by Chromatotron (2 mm silica gel GF254 disk, solvent: 5% ethyl acetate in petroleum ether) led to the isolation of compound 7 (190 mg).
Fraction C (2.7 g) eluted with 10% ethyl acetate in petroleum ether was further purified using silica gel column (45 × 2.5 cm i.d., 100 g) eluting with 5% ethyl acetate in petroleum ether. Fraction 21 afforded 130 mg of 8 after crystallization from methanol. Fractions 22–41 were mixture of 8 and 9 (347 mg).
Fraction D (3.2 gm) eluted with 20% ethyl acetate in petroleum ether afforded 250 mg of β-sitosterol after repeated crystallization from methanol.
2.3.2. trans-Calamenene (1)
C15H22, Oily compound. [α]D23 − 49 (c 1.1, CHCl3). 1H- and 13C NMR (CDCl3): Table 1, Table 3. EIMS m/z (%): 202 (29, M+).
Table 1.
Selected 1H NMR data (δ ppm, J in parenthese in Hz) for compounds 1–3, 5 and 6.
| Pos | 1 | 2 | 3 | 5 | 6 |
|---|---|---|---|---|---|
| 2 | 7.11 (d, J = 7.5) | 7.32 (d, J = 8.3) | 7.45 (d, J = 8.4) | 7.48 (d, J = 8.4) | |
| 3 | 6.92 (dd, J = 1.5, 7.5) |
7.90, m | 7.00, m | 7.00, m | |
| 5 | 7.01 (d, J = 1.5) | 7.90, m | 5.44 (d, J = 4.4) | 7.00, m | 7.00, m |
| 7 | 2.68, m | – | 1.16, m | 2.58, m | 2.68, m |
| 11 | 2.22, m | 3.71, m | 1.96, m | 1.99, m | 2.01, m |
| 12 | 0.71 (d, J = 7.0) | 1.37 (d, J = 6.8) | 0.80 (d, J = 7.0) | 0.76 (d, J = 6.9) | 0.70 (d, J = 6.6) |
| 13 | 0.99 (d, J = 7.0) | 1.37 (d, J = 6.8) | 0.87 (d, J = 7.0) | 1.04 (d, J = 6.9) | 1.02 (d, J = 6.6) |
| 14 | 1.24 (d, J = 6.7) | 2.63, s | 0.96 (d, J = 6.6) | 1.51, s | 1.48, s |
| 15 | 2.28, s | 2.54, s | 1.70, s | 2.95, s | 2.30, s |
1: 2.74 (m, H-10); 2: 7.19 (d, J = 8.0, H-8), 7.25 (d, J = 8.0, H-8); 5: 1.58 (m, H-10); 6: 1.69 (m, H-6).
Table 3.
13C NMR data (δ ppm) for compounds 1–9.
| Pos | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 139.99 | 131.94 | 72.70 | 38.2 | 140.00 | 141.57 | 39.21 | 38.83 | 38.27 |
| 2 | 126.93 | 123.05 | 26.69 | 19.39 | 128.07 | 128.45 | 19.56 | 17.19 | 18.16 |
| 3 | 126.35 | 124.93 | 22.01 | 42.20 | 126.19 | 125.84 | 38.17 | 36.82 | 37.01 |
| 4 | 134.56 | 134.81 | 133.78 | 33.57 | 136.39 | 136.13 | 43.84 | 46.09 | 47.29 |
| 5 | 128.87 | 127.32 | 122.11 | 55.57 | 126.91 | 126.95 | 49.97 | 44.99 | 48.78 |
| 6 | 140.17 | 140.60 | 48.04 | 24.56 | 139.28 | 138.75 | 24.65 | 25.19 | 24.90 |
| 7 | 43.98 | 131.22 | 41.91 | 39.08 | 43.32 | 43.40 | 121.22 | 121.00 | 35.48 |
| 8 | 21.68 | 125.71 | 24.04 | 148.69 | 19.63 | 20.20 | 134.48 | 135.63 | 136.65 |
| 9 | 31.02 | 121.53 | 31.16 | 57.23 | 37.55 | 38.14 | 51.47 | 52.01 | 50.55 |
| 10 | 32.69 | 131.64 | 49.24 | 39.85 | 70.17 | 70.78 | 35.33 | 35.01 | 36.84 |
| 11 | 32.09 | 28.35 | 26.89 | 17.66 | 30.96 | 30.59 | 23.05 | 20.04 | 18.56 |
| 12 | 17.50 | 24.14 | 15.20 | 41.37 | 17.66 | 17.08 | 27.59 | 36.01 | 34.40 |
| 13 | 21.46 | 24.14 | 21.69 | 73.65 | 21.08 | 21.74 | 145.03 | 36.99 | 37.72 |
| 14 | 22.29 | 19.56 | 15.17 | 145.15 | 31.22 | 30.81 | 122.33 | 46.33 | 129.10 |
| 15 | 21.49 | 22.18 | 23.52 | 111.62 | 21.25 | 21.25 | 34.84 | 150.26 | 148.91 |
| 16 | 28.02 | 20.87 | 109.32 | 110.18 | |||||
| 17 | 106.38 | 21.44 | 21.52 | 26.02 | |||||
| 18 | 33.65 | 29.71 | 17.12 | 16.77 | |||||
| 19 | 21.74 | 183.69 | 185.88 | 185.46 | |||||
| 20 | 14.46 | 12.75 | 15.32 | 15.23 |
2.3.3. Cadalene (cadalin) (2)
C15H18, Oily compound. UV λmax (CHCl3) 232, 311 and 327 nm. 1H- and 13C NMR (CDCl3): Table 1, Table 3. EIMS m/z (%): 198 (34, M+).
2.3.4. epi-Cubenol (3)
C15H26O, Oily compound. [α]D23 − 87 (c 0.9, CHCl3). 1H- and 13C NMR (CDCl3): Table 1, Table 3. EIMS m/z (%): 222 (31, M+).
2.3.5. Manool (4)
C20H34O, crystallin solid. m.p. 51–52 °C. [α]D23 + 56 (c 0.93, CHCl3). 1H- and 13C NMR (CDCl3): Table 2, Table 3. EIMS m/z (%): 290 (11, M+).
Table 2.
Selected 1H NMR data (δ ppm, J in parenthese in Hz) for compounds 4 and 7–9.
| Pos | 4 | 7 | 8 | 9 |
|---|---|---|---|---|
| 7 | 5.43, bs | 5.31 (d, J = 4.3) | 5.22, s | |
| 14 | 5.91 (dd, J = 10.75, 17.35) |
5.78 | ||
| 15 | 5.04 (d, J = 10.75) 5.20 (d, J = 17.35) |
5.81 (dd, J = 10.5, 17.5) |
5.77 (dd, J = 10.2, 17.0) |
|
| 16 | 1.26 s | 0.99 J = 5.0 | 4.86 d J = 10.5 4.92 d J = 17.5 |
4.84 (d, J = 10.2) 4.90 (d, J = 17.0) |
| 17 | 4.48, s 4.80, s |
1.02 (d, J = 5.0) | 0.86, s | 0.83, s |
| 18 | 0.87, s | 1.25, s | 1.26, s | 1.19, s |
| 19 | 0.80, s | |||
| 20 | 0.67, s | 0.71, s | 0.90, s | 1.04, s |
2.3.6. Calamenene-10β-ol (5)
C15H22O, Oily compound. 1H- and 13C NMR (CDCl3): Table 1, Table 3. EIMS m/z (%): 218 (15, M+).
2.3.7. Calamenene-10α-ol (6)
C15H22O, Oily compound. 1H- and 13C NMR (CDCl3): Table 1, Table 3. EIMS m/z (%): 218 (15, M+).
2.3.8. 4-epi-Abietic acid (7)
C20H30O2, gum. UV λmax (MeOH) 232, 241 and 252 nm. 1H- and 13C NMR (CDCl3): Table 2, Table 3. EIMS m/z (%): 302 (6, M+).
2.3.9. Sandaracopimaric acid (8)
C20H30O2, m.p. 168–169 °C. UV λmax (MeOH) 228 nm. 1H- and 13C NMR (CDCl3): Table 2, Table 3. EIMS m/z (%): 302 (23, M+).
2.3.10. Isopimaric acid (9)
C20H30O2, m.p. 160–161 °C. UV λmax (MeOH) 227 nm. 1H- and 13C NMR (CDCl3): Table 2, Table 3. EIMS m/z (%): 302 (19, M+).
2.4. Animals
Male Wistar albino rats (160–180 g) of the same age (8–10 weeks), provided by the Experimental Animal Care Center, College of Pharmacy, Prince Sattam Bin Abdulaziz University, Al-Kharj, KSA, were used. The animals were kept under controlled temperature (22 ± 2 °C), humidity (55%) and light/dark conditions (12/12 h). The animals were provided with Purina chow and free access to drinking water ad libitum (Alqasoumi et al., 2009). The experimental and procedures were approved by the Ethical Committee of Prince Sattam Bin Abdulaziz University.
2.5. Hepatoprotective activity
Rats were divided into four groups' and nine subgroups five animals each. Group I received normal saline and was kept as a control. Groups II- IX received 1.25 ml of CCl4 in liquid paraffin (1:1) per 1 Kg body weight intraperitoneally. Group II received only CCl4 treatment. Group III was treated with 10 mg/kg p.o. (20.7 μmole/ kg) of silymarin (Sigma-Aldrich, St. Louis, MO, USA) (Abdel-Kader et al, 2016). Groups IV was divided into eight sub groups IVa- IVh. Subgroups IVa- IVc were treated with 200 mg/kg body weight of the water, chloroform and petroleum ether soluble fractions. Subgroups IVd- IVg were treated with compounds 3, 4, 7 and 8 at doses of 10, 12, 14 and 14 mg/Kg body weight respectively. Subgroup IVh treated with 18 and 21 mg/Kg body weight of 4 and 7, respectively. Treatment started 5 days prior to CCl4 administration and continued till the end of the experiment. After 48 h, following CC14 administration the animals were sacrificed under ether anesthesia. Blood samples were obtained by heart puncture and the serum was separated for biochemical parameters measurements. The livers were immediately removed and used for determination of tissue parameters. Representative pieces were immersed in10% formalin for fixation necessary for histopathological study.
2.5.1. Determination of enzyme levels
Serum glutamate oxaloacetate transaminase (AST), serum glutamate pyruvate transaminase (ALT), alkaline phosphatase (ALP), gamma glutamyltranspeptidase (GGT) and total bilirubin were determined following the reported methods (Edwards and Bouchier, 1991). The enzyme activities were measured by Reflotron® diagnostic strips (Roche, Basel, Switzerland) and Reflotron® Plus instrument (Roche) (Table 4).
Table 4.
Effect of isolated compounds on the serum levels of liver injury markers in CCl4-intoxicated rats.
| Treatment |
AST (U/L) |
ALT (U/L) |
GGT (U/L) |
ALP (U/L) |
Bilirubin (mg/dl) |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± S.E | % Change | Mean ± S.E | % Change | Mean ± S.E | % Change | Mean ± S.E | % Change | Mean ± S.E | % Change | |
| Control | 98.36 ± 4.48 | 31.55 ± 2.91 | 4.40 ± 0.25 | 332.16 ± 9.48 | 0.59 ± 0.01 | |||||
| CCl4 | 384.66 ± 7.95***a | 300.00 ± 12.02**a | 14.55 ± 0.55*** a | 595.83 ± 11.88*** a | 3.09 ± 0.09*** a | |||||
| Silymarin (10 mg/kg) | 192.00 ± 6.78***b | 50.08 | 98.06 ± 5.14*** b | 67.31 | 7.20 ± 0.24*** b | 50.51 | 425.83 ± 13.93*** b | 28.53 | 1.11 ± 0.09*** b | 63.90 |
| 3 (10 mg/kg) | 225.83 ± 9.77***b | 41.29 | 184.83 ± 5.22*** b | 38.38 | 11.13 ± 0.24*** b | 23.48 | 554.33 ± 6.12* b | 6.88 | 2.76 ± 0.10* b | 10.86 |
| 4 (12 mg/kg) | 245.00 ± 11.89***b | 36.30 | 168.66 ± 5.49***b | 43.77 | 9.48 ± 0.43*** b | 34.82 | 474.83 ± 11.93***b | 20.30 | 1.67 ± 0.18*** b | 45.99 |
| 7 (14 mg/kg) | 290.00 ± 7.42***b | 24.61 | 221.16 ± 8.37*** b | 28.77 | 9.01 ± 0.35*** b | 38.02 | 452.83 ± 12.79*** b | 24.00 | 1.49 ± 0.09*** b | 51.64 |
| 8 (14 mg/kg) | 350.33 ± 9.51*b | 8.92 | 302.83 ± 5.33b | – | 13.38 ± 0.41b | 8.01 | 592.33 ± 8.53b | – | 2.97 ± 0.06b | 4.14 |
| Silymarin (10 mg/kg) | 139.00 ± 7.16***b | 53.15 | 77.62 ± 16.46***b | 65.53 | 6.50 ± 0.30***b | 56.73 | 411.50 ± 25.10***b | 31.01 | 1.04 ± 0.12***b | 64.27 |
| 4 (18 mg/kg) + 7 (21 mg/kg) | 189.50 ± 7.18***b | 36.14 | 142.00 ± 5.78***b | 36.95 | 11.47 ± 0.38***b | 23.62 | 434.50 ± 6.80***b | 27.15 | 1.46 ± 0.03***b | 50.21 |
All values represent mean ± SEM. *p < 0.05; **p < 0.01; ***p < 0.001; ANOVA, followed by Dunnett's multiple comparison test.
As compared with Control group.
As compared with CCl4 only group.
2.5.2. Determination of tissue parameters
Non-protein sulfhydryl groups (NP-SH) were measured following Sedlak and Lindsay (1968) method. The livers were cooled in ice bath. A weight of 200 mg of liver tissues were homogenized in 8 ml of 0.02 M ethylenediaminetetraacetic acid (EDTA). Aliquots of 5 ml of the homogenate were mixed in 15 ml test tubes with 4 ml of distilled water and 1 ml of 50% trichloroacetic acid (TCA). To precipitate protein the tubes were shaken for 10–15 min at intervals and centrifuged at 3000 rpm for 15 min. Two mL of the resulted supernatants were mixed with 4 ml of 0.4 M Tris buffer, pH 8.9 and 0.1 ml of 0.01 M DTNB [5, 5‘-dithio-bis-(2-nitrobenzoic acid)] and the samples were shaken. Five minutes after the addition of DTND the absorbencies were measured at 412 nm comparing with blank with no homogenate (Table 5).
Table 5.
Effect of the isolated compounds on the levels of MDA, NP-SH and Total protein in liver tissue of CCl4-intoxicated rats.
| Treatment | MDA(nmol/g) | NP-SH(nmol/g) | Total protein(g/l) |
|---|---|---|---|
| Control | 1.25 ± 0.03 | 4.40 ± 0.16 | 118.56 ± 2.37 |
| CCl4 | 6.66 ± 0.21*** a | 2.94 ± 0.30** a | 55.48 ± 2.42*** a |
| Silymarin (10 mg/kg) | 2.35 ± 0.11*** b | 4.39 ± 0.16** b | 99.80 ± 2.59*** b |
| 3 (10 mg/kg) | 5.14 ± 0.21*** | 3.60 ± 0.26b | 57.48 ± 1.51b |
| 4 (12 mg/kg) | 4.98 ± 0.33** b | 3.36 ± 0.14b | 65.06 ± 2.58* b |
| 7 (14 mg/kg) | 2.91 ± 0.12*** b | 4.05 ± 0.18* b | 81.03 ± 2.42*** b |
| 8 (14 mg/kg) | 5.61 ± 0.24** b | 3.45 ± 0.15b | 62.67 ± 1.89* b |
| Silymarin (10 mg/kg) | 2.26 ± 0.18*** b | 4.14 ± 0.15*** b | 99.99 ± 3.70*** b |
| 4 (18 mg/kg) + 7 (21 mg/kg | 2.81 ± 0.27*** b | 4.67 ± 0.40*** b | 87.42 ± 3.72*** b |
All values represent mean ± SEM. *p < 0.05; **p < 0.01; ***p < 0.001; ANOVA, followed by Dunnett's multiple comparison test.
As compared with Control group.
As compared with CCl4 only group.
For the measurement of MDA level an aliquots from liver homogenate were incubated with shaking at 37 °C for 3 h, mixed with 1 ml of 10% aqueous TCA and centrifuged at 800 rpm for 10 min. From the supernatants 1 ml from each were mixed with 1 ml aqueous solution of 0.67% 2-thiobarbituric and heated for 10 min on boiling water bath. Mixtures were cooled, mixed with 1 ml distilled water and the absorbance's were measured at 535 nm. The content of MDA (Table 5) (nmol/g wet tissue) were estimated from the calibration curve of MDA solution (Utley et al., 1967).
For determination of the TP portions of the homogenate were mixed with 0.7 ml Lowry’s solution and kept at room temperature for 20 min in dark then 0.1 ml of diluted Folin's reagent were added. Samples were kept for 30 min at room temperature away from light. The absorbance were then measured at 750 nm (Table 5) (Lowry et al., 1951).
2.6. Statistical analysis
Analysis of variance (ANOVA) test was used to judge whether the difference between groups is significant or not. Non paired samples such as control and CCl4-treated group were compared for significance using Dunnette's test (Woolson and Clarke, 2002). All the reported values are presented as mean ± S.E.
2.7. Histopathology
The livers samples were dehydrated using water ethanol mixtures till reaching absolute ethanol, cleared and infiltrate by immersion in increasing concentrations of ethanol (70–100%), xylene (3 times, 1hr each) followed by paraffin wax (4 times, 1hr each). The tissues were oriented by hot forceps in moulds and then chilled on cold plates and excess wax were removed. Thin sections (3 μm) were made using rotary microtome (Leitz 1512) and placed onto clean slides. The slides were drained vertically for several minutes and placed onto a warming table at 37–40 °C (Prophet et al., 1994).
2.7.1. Mayer’s hematoxylin stain
The slides were stained in Mayer’s hematoxylin solution for 15 min after deparaffinization, hydration. The slides were then washed in lukewarm running tap water for 15 min then immersed in 80% ethyl alcohol for two minutes and counterstained in eosin-phloxine solution for 2 min. The slides were then washed with 95% ethyl alcohol, absolute ethyl alcohol, and xylene (2 min each) and finally mounted in resinous medium.
2.7.2. Periodic acid schiff – hematoxylin (PAS-H) to study PAS-positive materials
Deparaffinized liver sections were immersed in 1% periodic acid for 10 min, washed with distilled water for 2 min, immersed in Schiff reagent (Product 191203S, BDH Laboratory Supplies, Poole, England) for 10 min, and then washed under running tap water for 10 min. The nuclei were counterstained with Harris’s hematoxylin for 2 min, differentiated in acid alcohol 2 dips, rinsed with tap water 2 dips, and blued in running tap water for 10 min, dehydrated, cleared, and a coverslip mounted with DPX (Product 03600, Loba Chemie Pvt. Ltd., Mumbai, India) (Hamad and Ahmed, 2018).
2.7.3. Masson trichrome technique for connective tissue fibers demonstration (mainly collagen)
Deparaffinized liver sections were stained with Weigert’s iron hematoxylin for 10 min, washed with water, stained in an acid fuchsin solution for 5 min, rinsed rapidly in water, differentiated in 1% phosphomolybdic acid for about 5 min, drained and counterstained with methyl blue, dehydrated, cleared and mounted sections in DPX (Hamad and Ahmed, 2016).
2.8. Electron microscopy
Shortly, Tissue specimens were treated for fixation, dehydration and infiltration using Specimen rotator (Product 15920D, Thermo Fisher Scientific, Carlsbad, CA, USA). Embedding was done by transferring tissues in EMS embedding capsules (Product 69910–05, EMS) to make blocks. Ultramicrotome (Product PT-PC #75,840, RMC Boeckeler Instruments, Inc., Tucson, AZ, USA) were used to obtain ultra thin section of 100–200 nm stained manually in 1% uranyl acetate (Product 93–2840, STREM CHEMICALS, Newburyport, MA, USA). Tissues were then dried and examined under transmission electron microscope (TEM) (Product FEI TECNAI 12, Thermo Fisher Scientific, Hillsboro, Oregon, USA). Detailed procedures are described earlier (Luft, 1961, Woods and Stirling, 2013).
3. Results and discussion
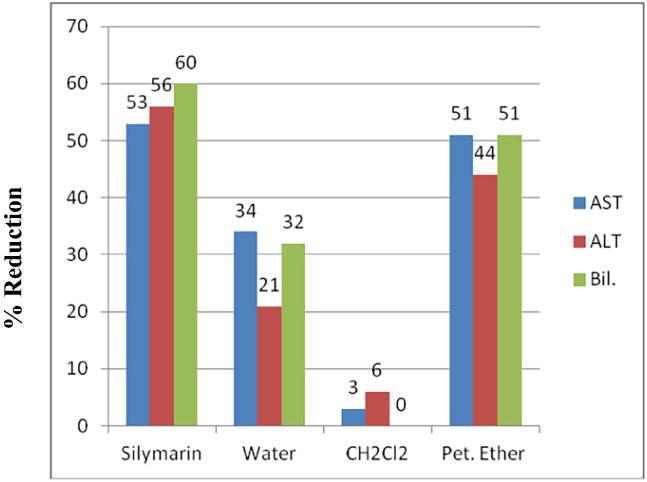
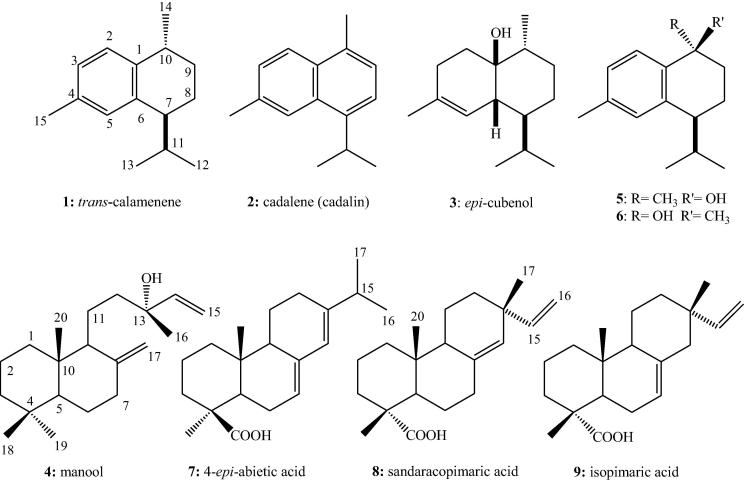
The total extract of J. sabina showed marked hepatoprotective activity (Abdel-Kader et al, 2016). To trace the activity of the plant liquid-liquid fractionation of the total extract was conducted to yield petroleum ether, dichloromethane and water fractions. Rats were treated with the fractions at 200 mg/kg before the administration of CCl4 and three parameters were measured to determine the active fraction(s). The petroleum ether fraction showed the most significant (p < 0.001) and strongest protective effect for the liver cells (Fig. 1). Phytochemical study of the active petroleum ether fraction resulted in the isolation of nine compounds 1–9 including five cadinane sesquiterepenes and four diterpenes (Fig. 2).
Fig. 1.
Effect of JS fractions at 200 mg/kg on reduction of the levels of AST, ALT and bilirubin of CCl4 treated rats.
Fig. 2.
Chemical structures of compounds 1–9.
Compound 1 was identified as trans-calamenene by comparison with reported data (Oyarzún and Garbarino, 1988). However, HSQC experiment indicated that the methyl group at δH 0.71 in the 1H NMR (Table 1) is connected to the carbon at δC 17.50 in the 13C NMR. The methyl group at δH 0.99 in the 1H NMR showed cross peak with the carbon at δC 21.46 in the 13C NMR (Table 3). Based on these facts the assignment of H-12 and H-13 must be revised.
Compound 2 was identified as cadalene (cadalin) based on comparison with literature data (El-Seedi et al, 1994). The carbon data were not assigned in the cited reference. Moreover, the chemical shift at δC 44.3 is apparently not part of the molecules. It is most likely a typographical error or due to impurities. This argument is supported by the data published for 15-hydroxycadalene (Kuo and Yu, 1999).
Compound 3 was identified as epi-cubenol through comparison with reported data for both cubenol and epi-cubenol (Ohta and Hirose, 1967, Oyarzún and Garbarino, 1988). 2D-NMR experiments especially COSY and HSQC enable the exact assignment of all methyl groups in 3. Based on our data the chemical shifts of C-12, C-13 and C-15 must be revised as in Table 3.
Compounds 5 and 6 were identified as calamenene-10β-ol and calamenene-10α-ol respectively. Their 1H- and 13C NMR data (Table 1, Table 3) were identical with the reported values (Kuo and Yu, 1999). Analysis of DEPT 90, COSY, HSQC and HMBC indicated that the carbon resonance at δC 21.25 in both 5 and 6 representing two methyl groups. HMBC showed cross peaks of these methyl protons at δH 2.95, 2.30 with C-3 (δC 126.19, 125.84), C-4 (136.39, 136.13), C-5 (δC 126.91, 126.95) in both 5 and 6 supporting the position of these two methyl groups at C-15. Carbons with chemical shifts δC 30.96 and 30.59 ppm represents two CH rather than CH3 based on DEPT 90 and HSQC experiments. Both H-12, H-13 in 5 and 6 showed strong correlation with that CH groups protons and carbons in COSY and HMBC. Based on the above discussion the literature assignments (Kuo and Yu, 1999) of C-11 and C-15 must be interchanged. Data of C-1, 2 and 4 are consistent with the suggested correction. Compounds 1–3, 5 and 6 are all belonging to the sesquiterpenes with cadinane skeleton.
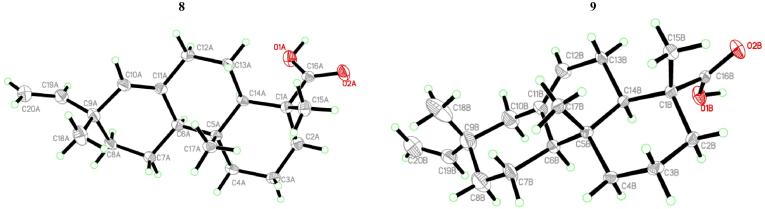
Compounds 8 and 9 were present mainly as mixtures of different proportions. Crystallization from petroleum ether/ethyl acetate provided crystals subjected to single X-ray diffraction analysis (Fig. 3) to establish the two mixture components as the isopimarane diterpene acids, sandaracopimaric acid (8) and isopimaric acid (9). Repeated column chromatography establishes more pure 8 for biological testing while only little amount of pure 9 was obtained for spectral analyses. Data of 8 and 9 were identical with the reported data for the acids as well as the corresponding analogues (Talapatra et al., 1994, Block et al., 2004, El Sawi and Motawe, 2008, Olate et al., 2011).
Fig. 3.
X-ray crystal structure of compounds 8 and 9.
Compounds 4 and 7 were identified as the labdane diterpene, manool and the abitane diterpene acid, 4-epi-abietic acid by comparison of the obtained spectral and physical data with the literature (El Sawi and Motawe, 2008, Olate et al., 2011, Topçu et al., 2013).
Compounds 3, 4, 7 and 8 were isolated in enough yield to test their hepatoprotective effect.
Hepatotoxicity was induced experimentally using CCl4. The endoplasmic reticulum converts carbon tetrachloride into two highly reactive free radicals; trichloromethyl (CCl3) and .Cl3COO consequently conjugated with cellular macromolecules, lipids and proteins with the help of oxygen to induce lipid peroxidation (Snyder and Andrews, 1996). The consequences of this process are the formation of reactive aldehydes that form adducts with proteins (Weber et al., 2003). As a result the endoplasmic reticulum and other cellular membranes become more permeable to Ca2+ resulting in a severe disturbances of calcium homeostasis leading to necrotic cell death (Weber et al., 2003). Treatment of animals with carbon tetrachloride leads to significant increase of transaminases (AST and ALT) and alkaline phosphatase (ALP) levels due to hepatocytes damage (Zafar and Ali, 1998). Severe jaundice was demonstrated by the elevated levels of serum bilirubin (Table 4) (Lin et al, 1997).
The standard drug silymarin at a dose of 10 mg/kg (20.7 μmol/kg) was used as a positive control. The protective effect of silymarin was mediated via scavenging prooxidant free radicals and increasing the intracellular concentration of GSH. In addition, it enhances the cellular membrane permeability to protect against xenobiotics insult. Silymarin also promote the synthesis of ribosomal RNA through stimulating DNA polymerase-I and steroid like action in regulating DNA transcription and stimulation of protein synthesis. These processes lead to regeneration of liver cells (Dehmlow et al., 1996, Saller et al., 2007). All these effects were expressed experimentally by normalization of the biochemical and tissue parameters (Table 4, Table 5).
The tested compounds were used at 20.7 μmol/kg as the dose of silymarin. Compound 8 was inactive and offers no protection against toxicity (Table 4, Table 5). Compound 3 showed marked reduction in the levels of AST, ALT and GGT (41.29, 38.38 and 23.48%) compared with silymarin (50.08, 67.31 and 50.51%) (Table 4). However, its effect on ALP, bilirubin, MDA, NP-SH and total protein was weak. Best results were obtained from groups trated with compounds 4 and 7. Compound 4 was stronger in lowering AST and ALT levels (36.30, 43.77%). However; 7 was superior in the management of GGT, ALP, bilirubin (38.02, 24.00, 51.64%), MDA, NP-SH (2.91 ± 0.12, and 4.05 ± 0.18 nmol/g) and total protein (81.03 ± 2.42 g/l). A combination of 4 and 7 at 18 mg/kg and 21 mg/kg, respectively, were used aiming to effectively improve all the used liver parameters. Significant results (p < 0.001) were obtained from the tissues of this group and were comparable to the standard silymarin in most of the used parameters. However, percent reduction in the levels of AST, ALT and GGT (36.14, 36.95 and 23.62%) were much less that those of the silymarin treated group (53.15, 65.53 and 56.73%).
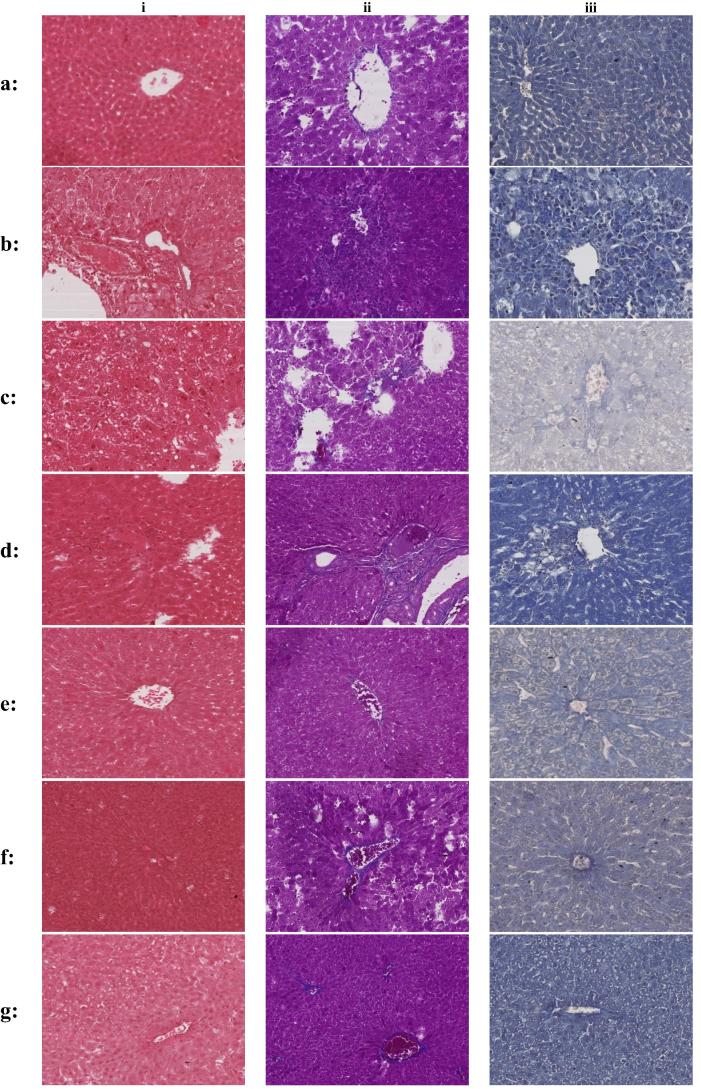
The effect of the tested compounds on the liver condition was monitored also via detailed histopathological examinations using Mayer’s hematoxylin stain, Masson trichrome technique and Periodic Acid Schiff – Hematoxylin (PAS-H) on light microscope (Fig. 4). Masson trichrome stain collaged with blue colour and give an indication about fibrosis due to liver injury. Using PAS-H confirms the presence of glycogen in the liver cells (Krishna, 2013). Tissue specimens were also examined by electron microscopy (Fig. 5).
Fig. 4.
Light microscope pictures of liver tissues stained with (i) Mayer’s hematoxylin stain (ii): Masson trichrome technique (iii): Periodic Acid Schiff – Hematoxylin (PAS-H). (a) Liver cells of normal control group. (b) Liver cells of CCl4 treated group (i): Degenerative liver tissue, necrosis accompanied by occlusion of blood. (ii): Large amount of collagen fibers taking the blue colour. (iii): Absence of PAS positive materials which indicates illed hepatocyte with very low functioning capacity. (c) Liver cells of CCl4 & silymarin treated group (i): Clear evidence of healing and regaining microanatomical archeticture of liver tissue. Hyperemia still appears in some central veins without complete occlusion (ii): Improvement by lowering the amount of collagen fiber but not completely disappeared (iii): Very few amount near to absence of PAS positive materials. (d) Liver cells of CCl4 & 3 treated group (i): Moderate to high presence of degeneration (D) necrosis (N) accompanied by accumulation of hyaline materials (Hy) among parenchyma of the liver (ii): Moderate presence of collagenfibers (arrows) which indicate healing process did not reach its complete status (iii): Very little to absence of PAS positive materials which indicates hepatocytes could not regain full functioning atatus. (e) Liver cells of CCl4 & 4 treated group i: Regaining the structure of liver tissue but still also show presence of moderate to low necrotic area, degenerative area accompanied by occlusion of thecentral vein by hyaline material (ii): Few and almost normal amount of collagen fibers which confind to the boundaries of central veins and sinusoides wall of the liver (iii): shows few amount of PAS positive material which indicates start of healing of hepatocytes. (f) Liver cells of CCl4 & 7 treated group (i): Wide area of moderate to high degeneration and necrosis accompanied by partial and full occlusion of central veins (ii): Large amount of collagen fibers which indicates weak healing process (iii): Little amount to absence of PAS positive materials. (g) Liver cells of CCl4 & 4& 7 treated group (i): Start of healing process but still areas of degeneration and necrosis are present accompanied by hyperemia that almost occlude the central vein (ii): Moderate amount of collagen fibers (iii): Few amount of PAS positive materials.
Fig. 5.
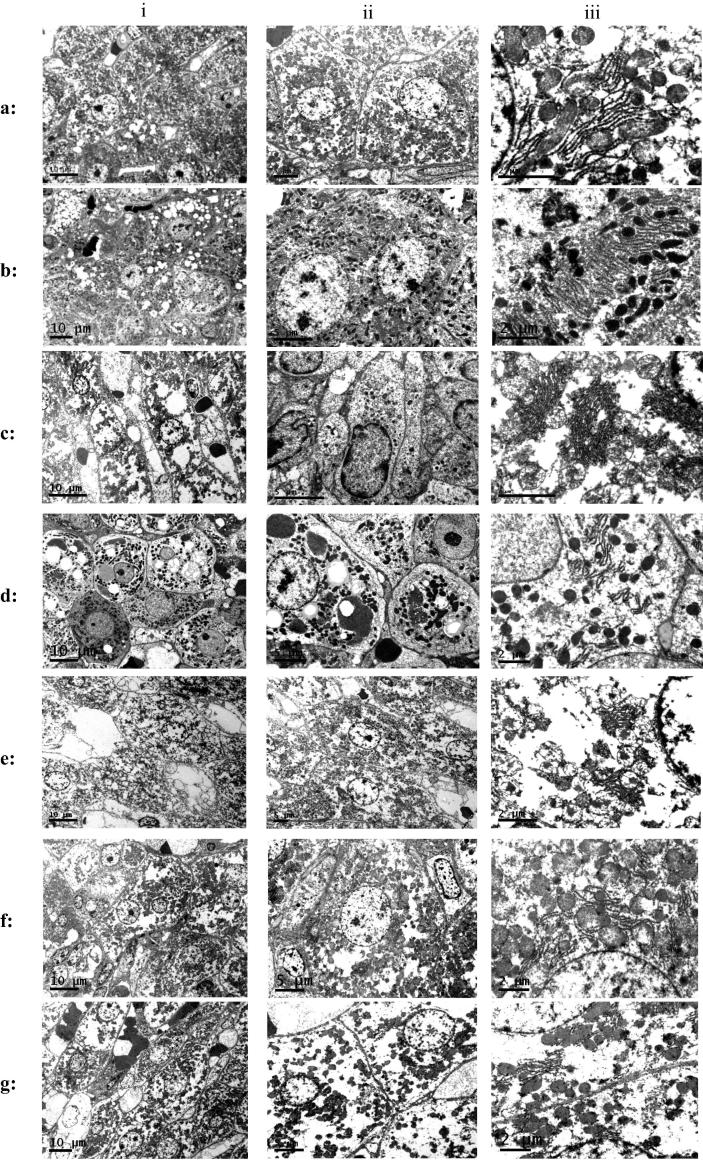
Electron microscope pictures of liver tissues (i): General (ii): Nuclear study (iii): Cytoplasmic study. (a) Liver cells of normal control group. (b) Liver cells of CCl4 treated group (i): Degeneration and necrosis of hepatocytes. Also increased presence of vacuoles (ii): Unusual multiple clumps of chromatin materials (iii): Abnormal pattern of dissolved Golgi apparatus. (c) Liver cells of CCl4 & silymarin treated group (i): Intact cellular and nuclear membrane associated with decreased amount of cytoplasmic vacuolization and normal presence of fat droplets (ii): High healing activity by regaining many normal nuclei without clumps (iii): Normal cytoplasm with normal Golgi apparatus and very few small fat droplets are present in cytoplasm. (d) Liver cells of CCl4 & 3 treated group (i): Intact cytoplasmic and nuclear membranes with absence of nucleus and degeneration associated with increased cytoplasmic vacuolization of larger size (ii): Weak healing process with unusual multiple clumps of chromatin materials inside nucleus, feature of necrosis is indicated by absence of nuclear boundary (iii): abnormal pattern of degenerated Golgi apparatus associated with vacuolization and few fat droplets some of them are large in size. (e) Liver cells of CCl4 & 4 treated group i: Necrosis and cytoplasmic vacuolization associated with very little or almost absence of fat droplets (ii): Unusual multiple clumps of chromatin materials inside nucleus (iii): Abnormal pattern of degenerated Golgi apparatus associated with large vacuolization with absence of fat droplets appear in the cytoplasm. (f) Liver cells of CCl4 & 7 treated group i: Intact cellular and nuclear membrane associated with moderately decreased amount of cytoplasmic vacuolization and presence of fat droplets (ii): Multiple clumps of chromatin materials inside nucleus (iii): Abnormal pattern of degenerated Golgi apparatus associated with vacuolization with absence of fat droplets. (g) Liver cells of CCl4 & 4 & 7 treated group i: Features of healing by intact nuclear and cytoplasmic membrane and normal presence of fat droplets but slightly cytoplasmic vacuolization is also present (ii): Some improvement showing intact nuclear membrane and reducing the amount of abnormal clumps of chromatin materials in the nuclei (iii): Degenerated Golgi apparatus associated with many fat droplets and vacuolization.
Group received CCl4 show (Fig. 4) clear evidence of degenerative liver tissue, necrosis accompanied by occlusion of blood (b-i). Excessive amount of collagen fibers stained blue (b-ii). Absence of PAS positive materials which indicates illed hepatocyte with very low functioning capacity (b-iii). Electron microscope (Fig. 5) study showed degeneration and necrosis of hepatocytes and increased presence of vacuoles (b-i). The nuclei showed unusual multiple clumps of chromatin materials (b-ii) and abnormal pattern of dissolved Golgi apparatus (b-iii). Silymarin adminstration prior to CCl4 provided clear evidence of healing and regaining microanatomical architecture of liver tissue (Fig. 4). However, hyperemia still appears in some central veins without complete occlusion (c-i). The amount of collaged fiber is markedly reduced (c-ii) along withvery few amount near of PAS positive materials was observed (c-iii). Electron microscopy pictures (Fig. 5) showed intact cellular and nuclear membrane associated with decreased amount of cytoplasmic vacuolization and normal presence of fat droplets (c-i), regaining many normal nuclei without clumps (c-ii) and normal cytoplasm with normal Golgi apparatus (c-iii).
Compound 3 treated liver samples showed (Fig. 4) moderate to high presence of degeneration, necrosis accompanied by accumulation of hyaline materials (Hy) among parenchyma of the liver (d-i). In Masson trichrome technique the moderate presence of collagen fibers indicate incomplete healing status (d-ii). Periodic Acid Schiff–Hematoxylin (PAS-H) showed very little to absence of PAS positive materials which indicates hepatocytes could not regain full functioning status (d-iii). Compound 3 Shows some features of healing process (Fig. 5) (intact cytoplasmic and nuclear membranes) but also features of necrosis (absence of nucleus) and degeneration (increased cytoplasmic vacuolization of larger size) are still present (d-i). unusual multiple clumps of chromatin materials inside nucleus (d-ii). Cytoplasm contains abnormal pattern of degenerated Golgi apparatus associated with vacuolization and few fat droplets some of them are large in size (c-iii).
Treatment with compound 4 showed (Fig. 4) start of healing process by regaining the structure of liver tissue but still also show presence of moderate to low necrotic, degenerative areas accompanied by occlusion of the central vein by hyaline material (e-i). Almost normal amount of collagen fibers which confind to the boundaries of central veins and sinusoides wall of the liver were observed (e-ii). Few amount of PAS positive material was observed indicating the start but not complete healing of hepatocytes (e-iii). In the electron microscope pictures (Fig. 5) showed features of degeneration, necrosis and cytoplasmic vacuolization associated with very little or almost absence of fat droplets (e-i). very week healing process associated with unusual multiple clumps of chromatin materials inside nucleus (e-ii). Cytoplasm contains abnormal pattern of degenerated Golgi apparatus associated with large vacuolization (e-iii).
Moderate healing activity was evident (Fig. 4) by partial regaining the normal liver structure with slight hyperemia, necrosis and degeneration accompanied by partial occlusion of central veins (f-i) as a result of compound 7 treatment. Considerable amount of collagen fibers (f-ii) and very little positive materials (d-iii) both indicated injured hepatocytes. Compound 7 shows (Fig. 5) intact cellular and nuclear membrane associated with moderately decreased amount of cytoplasmic vacuolization and presence of fat droplets (f-i). Still have unusual multiple clumps of chromatin materials inside nucleus (f-ii). Cytoplasm contains abnormal pattern of degenerated Golgi apparatus associated with vacuolization (f-iii).
Combination of 4 and 7 at 18 and 21 mg/kg respectively resulted in moderate improvement (Fig. 4) by regaining the microanatomical structure of liver tissue, but still shows area of necrosis, degeneration, hyperemia and occlusion with hyaline material (g-i). Moderate amount of collagen fibers (g-ii) and few amount of PAS positive materials (g-iii) indicates some degree of healing. Electron microscope picture (Fig. 5) shows features of healing by intact nuclear and cytoplasmic membrane and normal presence of fat droplets but slightly cytoplasmic vacuolization is also present (g-i). some improvement by intact nuclear membrane and reducing the amount of abnormal clumps of chromatin materials in the nuclei (g-ii). Cytoplasm still showing some degenerated Golgi apparatus (g-iii).
In support of our results, manool (4) expressed significant protection against chromosomal damage induced by methyl methanesulfonate in HepG2 cell line (Nicolella et al, 2014). The isomer of compound 7, abietic acid was previously reported to have potent hepatoprotective effect against lipopolysaccharide induced liver injury in BALB/c mice (Ramnath et al, 2016).
4. Conclusion
The hepatoprotective activity of the total extract was trapped to the petroleum ether fraction after liquid-liquid fractionation. Chromatographic purification of the petroleum ether fraction resulted in the isolation of nine compounds namely: trans-calamenene (1), cadalene (cadalin) (2), epi-cubenol (3), manool (4), calamenene-10β-ol (5), calamenene-10α-ol (6), 4-epi-abietic acid (7), sandaracopimaric acid (8) and isopimaric acid (9). Compounds 3, 4 and 7 showed significant improvement in certain liver parameters. Combinations of the most active two compounds 4 and 7 at 18 and 21 mg/kg respectively resulted in moderate improvement by regaining the microanatomical structure, moderate amount of collagen fibers and few amount of PAS positive materials. Electron microscope picture shows features of healing by intact nuclear and cytoplasmic membrane and normal presence of fat droplets but slightly cytoplasmic vacuolization is also present. The amount of abnormal clumps of chromatin materials in the nuclei were reduced. However, cytoplasm still showing some degenerated Golgi apparatus.
Declaration of Competing Interest
The authors declare that there is no conflict of interest.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abdel-Kader M.S., Alanazi M.T., Bin Saeedan A.S., Al-Saikhan F.I., Hamad A.M. Hepatoprotective and nephroprotective activities of Juniperus sabina L. aerial parts. J. Pharm. Pharmacog. Res. 2016;5(1):29–39. [Google Scholar]
- Alqasoumi S.I., Abdel-Kader M.S. Terpenoids from Juniperus procera with hepatoprotective activity. Pak. J. Pharm. Sci. 2012;25(2):315–322. [PubMed] [Google Scholar]
- Alqasoumi S.I., El Tahir K.E.H., AlSheikh A.M., Abdel-Kader M.S. Hepatoprotective effect and safety studies of Juniperus phoenicea. Alex. J. Pharm. Sci. 2009;23(2):81–88. [Google Scholar]
- Alqasoumi S.I., Farraj A.I., Abdel-Kader M.S. Study of the hepatoprotective effect of Juniperus phoenicea constituents. Pak. J. Pharm. Sci. 2013;26(5):999–1008. [PubMed] [Google Scholar]
- Al-Zahim A.A., Al-Malki N.Y., Al-Abdulkarim F.M., Al-Sofayan S.A., Abunab H.A., Abdo A.A. Use of alternative medicine by Saudi liver disease patients attending a tertiary care center: prevalence and attitudes. Saudi J. Gastroenterol. 2013;19(2):75–80. doi: 10.4103/1319-3767.108477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block S., Baccelli C., Tinant B., Van Meervelt L., Rozenberg R., Habib J.L., Llabrès G., De Pauw-Gillet M.C., Quetin-Leclercq J. Diterpenes from the leaves of Croton zambesicus. Phytochemistry. 2004;65(8):1165–1171. doi: 10.1016/j.phytochem.2004.02.023. [DOI] [PubMed] [Google Scholar]
- Chang C.-I., Chen W.-C., Shao Y.-Y., Yeh G.-R., Yang N.-S., Chiang W., Kuo Y.-H. A new labdane-type diterpene from the bark of Juniperus chinensis Linn. Nat. Prod. Res. 2008;22(13):1158–1162. doi: 10.1080/14786410601132444. [DOI] [PubMed] [Google Scholar]
- Dehmlow C., Murawski N., de Groot H. Scavenging of reactive oxygen species and inhibition of arachidonic acid metabolism by silybinin in human cells. Life Sci. 1996;52:1591–1600. doi: 10.1016/0024-3205(96)00134-8. [DOI] [PubMed] [Google Scholar]
- De Pascual J., San Feliciano A., del Corral J.M.M., Barrero A.F., Rubio M., Muriel L. 2,5-Dimethylcoumarins from leaves of Juniperus sabina. Phytochemistry. 1981;20(12):2778–2779. [Google Scholar]
- Edwards C.R.W., Bouchier I.A.D. Churchill Livingstone Press; UK: 1991. Davidson’s Principles and Practice Medicine. [Google Scholar]
- El Sawi S.A., Motawe H.M. Labdane, pimarane and abietane diterpenes from the fruits of Juniperus phoenicea L. growing in Egypt and their activities against human liver carcinoma. Can. J. Pure Appl. Sci. 2008;2(1):115–122. [Google Scholar]
- El-Seedi H., Ghia F., Torssell K.B.G. Cadinane sesquiterpenes from Siparuna macrotepala. Phytochemistry. 1994;35(6):1495–1497. [Google Scholar]
- Everhart J.E., Ruhl C.E. Burden of digestive diseases in the United States part III: liver, biliary tract, and pancreas. Gastroenterology. 2009;136:1134–1144. doi: 10.1053/j.gastro.2009.02.038. [DOI] [PubMed] [Google Scholar]
- Hamad A.M., Ahmed H.G. Association of connective tissue fibers with estrogen expression in breast lesions among Sudanese females. Int. Clin. Pathol. J. 2016;2(5):97–102. [Google Scholar]
- Hamad A.M., Ahmed H.G. Association of some carbohydrates with estrogen expression in breast lesions among Sudanese females. J. Histotechnol. 2018;41(1):2–9. [Google Scholar]
- Hampe A., Petit R.J. Cryptic forest refugia on the ‘Roof of the World’. New Phytologist. 2010;185(1):5–7. doi: 10.1111/j.1469-8137.2009.03112.x. [DOI] [PubMed] [Google Scholar]
- Iida N., Inatomi Y., Murata H., Murata J., Lang F.A., Tanaka T., Nakanishi T., Inada A. New phenylpropanoid glycosides from Juniperus communis var. depressa. Chem. Pharm. Bull. 2010;58(5):742–746. doi: 10.1248/cpb.58.742. [DOI] [PubMed] [Google Scholar]
- Inatomi Y., Murata H., Inada A., Nakanishi T., Lang F.A., Murata J., Iinuma M. New glycosides of acetophenone derivatives and phenylpropanoids from Juniperus occidentalis. J. Nat. Med. 2013;67(2):359–368. doi: 10.1007/s11418-012-0694-3. [DOI] [PubMed] [Google Scholar]
- Kim S.-J., Jung J.Y., Kim H.W., Park T. Anti-obesity effects of Juniperus chinensis extract are associated with increased AMP-activated protein kinase expression and phosphorylation in the visceral adipose tissue of rats. Biol. Pharm. Bull. 2008;31(7):1415–1421. doi: 10.1248/bpb.31.1415. [DOI] [PubMed] [Google Scholar]
- Krishna M. Role of special stains in diagnostic liver pathology. Clin. Liver Dis. 2013;2(S1):S8–S10. doi: 10.1002/cld.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo Y.-H., Yu M.-T. Four new Sesquiterpenes from the Heartwood of Juniperus formosana var.concolor. Chem. Pharm. Bull. 1999;47(7):1017–1019. [Google Scholar]
- Lin C.C., Shieh D.E., Yen M.N. Hepatoprotective effects of fractions Ban-zhi-lian of experimental liver injuries in rats. J. Ethnopharmacol. 1997;56(3):193–200. doi: 10.1016/s0378-8741(97)00026-3. [DOI] [PubMed] [Google Scholar]
- Lowry O.H., Rosebrough N.J., Farr A.L., Randall R.J. Protein measurement with the Folin-phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Luft J.H. Improvements in epoxy resin embedding methods. J. Biophys. Biochem. Cytol. 1961;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moujir L.M., Seca A.M.L., Araujo L., Silva A.M.S., Barreto M.C. A new natural spiro heterocyclic compound and the cytotoxic activity of the secondary metabolites from Juniperus brevifolia leaves. Fitoterapia. 2011;82(2):225–229. doi: 10.1016/j.fitote.2010.10.001. [DOI] [PubMed] [Google Scholar]
- Moujir L.M., Seca A.M.L., Silva A.M.S., Barreto M.C. Cytotoxic activity of diterpenes and extracts of Juniperus brevifolia. Planta Med. 2008;74(7):751–753. doi: 10.1055/s-2008-1074529. [DOI] [PubMed] [Google Scholar]
- Nicolella H.D., de Oliveira P.F., Munari C.C., Costa G.F.D., Moreira M.R., Veneziani R.C.S., Tavares D.C. Differential effect of manool – a diterpene from Salvia officinalis, on genotoxicity induced by methyl methanesulfonate in V79 and HepG2 cells. Food Chem. Toxicol. 2014;72:8–12. doi: 10.1016/j.fct.2014.06.025. [DOI] [PubMed] [Google Scholar]
- Ogren T.L. Ten Speed Press, CA; Berkeley: 2015. The Allergy-Fighting Garden: Stop Asthma and Allergies with Smart Landscaping; pp. 131–133. [Google Scholar]
- Ohta Y., Hirose Y. The structure of cubenol and epi-cubenol. Tetrahedron Lett. 1967:2073. [Google Scholar]
- Olate V.R., Usandizaga O.G., Schmeda-Hirschmann G. Resin diterpenes from Austrocedrus chilensis. Molecules. 2011;16(12):10653–10667. doi: 10.3390/molecules161210653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyarzún M.L., Garbarino J.A. Sesquiterpenoids from Pilgerodendron uvífera. Phytochemistry. 1988;27(4):1121–1123. [Google Scholar]
- Prophet E.P., Mills B., Arrington J.B., Sobin L.H. 2nd ed. American Registry of Pathology; Washington DC: 1994. Laboratory Methods in Histology. [Google Scholar]
- Ramnath M.G., Thirugnanasampandan R., Mathusalini S., Mohan P.S. Hepatoprotective and cytotoxic activities of abietic acid from Isodon wightii (Bentham) H Hara. Pharmacognosy Res. 2016;8(3):206–208. doi: 10.4103/0974-8490.182920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saller R., Melzer J., Rechling J., Brignoli R., Meier R. An updated systematic review of the pharmacology of silymarin. Forsch Komplementarmed. 2007;14:70–80. doi: 10.1159/000100581. [DOI] [PubMed] [Google Scholar]
- San Feliciano A., del Corral J.M.M., Gordaliza M., Castro A. Acidic and phenolic lignans from Juniperus sabina. Phytochemistry. 1991;30(10):3483–3485. [Google Scholar]
- Seca A.M.L., Silva A.M.S., Bazzocchi I.L., Jimenez I.A. Diterpene constituents of leaves from Juniperus brevifolia. Phytochemistry. 2007;69(2):498–505. doi: 10.1016/j.phytochem.2007.07.026. [DOI] [PubMed] [Google Scholar]
- Seca A.M.L., Silva A.M.S. The chemical composition of the Juniperus genus (1970–2004) In: Govil J.N., Sing V.K., Bhardwaj R., editors. Recent Progress in Medicinal. Studium Press Pvt. Ltd., India; Plants, 16: Phytomedicines: 2007. pp. 401–522. [Google Scholar]
- Seca A.M.L., Silva A.M.S. A new 4',7-epoxy-8,3'-oxyneolignan from the acetone extract of Juniperus brevifolia leaves. Phytochem. Lett. 2010;3(3):126–128. [Google Scholar]
- Sedlak J., Lindsay R.H. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman's reagent. Anal. Biochem. 1968;25:192–205. doi: 10.1016/0003-2697(68)90092-4. [DOI] [PubMed] [Google Scholar]
- Snyder R., Andrews L.S. Toxic effects of solvents and vapors. In: Klassen C.D., editor. Toxicology: the Basic Science of Poisons. McGraw-Hill; New York: 1996. [Google Scholar]
- Talapatra S.K., Polley M., Talapatra B. Calliphyllin, a new diterpene from the leaves of Callicarpa macrophylla. J. Indian Chem. Soc. 1994;71:527–532. [Google Scholar]
- Topçu G., Öztürk M., Tuba Kuşman T. Terpenoids, essential oil composition, fatty acid profile, and biological activities of Anatolian Salvia fruticosa Mill. Turk. J. Chem. 2013;37:619–632. [Google Scholar]
- UK national statistics, http://www.statistics.gov.uk/ [consulted 16-03-2019].
- Utley H.G., Bernheim F., Hochstein P. Effect of sulfhydryl reagents on peroxidation in microsomes. Arch. Biochem. Biophys. 1967;118:29–32. [Google Scholar]
- Weber L.W., Boll M., Stampfl A. Hepatotoxicity and mechanism of action of haloalkanes: carbon tetrachloride as a toxicological model. CRC Cr. Rev. Toxicol. 2003;33(2):105–136. doi: 10.1080/713611034. [DOI] [PubMed] [Google Scholar]
- Woo K.W., Choi S.U., Park J.C., Lee K.R. A new lignan glycoside from Juniperus rigida. Arch. Pharm. Res. 2011;34(12):2043–2049. doi: 10.1007/s12272-011-1206-9. [DOI] [PubMed] [Google Scholar]
- Woods A.E., Stirling J.W. Transmission electron microscopy. In: Bancroft J.D., Layton C., Suvarna S.K., editors. Theory and practice of histological techniques. 7th ed. Churchill; Livingstone: 2013. [Google Scholar]
- Woolson R.F., Clarke W.R. 2nd ed. John Wiley & Sons. Inc.; New York: 2002. Statistical Methods for the Analysis of Biomedical Data. [Google Scholar]
- Zafar R., Ali S.M. Anti-hepatotoxic effects of root and root callus extracts of Cichorium intybus L. J. Ethnopharmacol. 1998;63:227–231. doi: 10.1016/s0378-8741(98)00087-7. [DOI] [PubMed] [Google Scholar]
- Zhao J., Yan M., Huang Y., Liu T., Zhao Y. Study on chemical constituents from Juniperus sabina L. Zhongguo Yaoxue Zazhi (Beijing, China) 2008;43(19):1461–1463. [Google Scholar]