Graphical abstract
Keywords: Sunscreens, Photoprotection, Ultraviolet rays, UVA, UVB, Sun protection factor (SPF), Water resistance, Minimum erythemal dose (MED)
Abstract
The association of sunrays with skin damage have been known since medieval times. The description of the electromagnetic spectrum facilitated the identification of the ultraviolet light spectrum as being responsible for skin damage resulting from prolonged skin exposure. Sunscreens have been used since ancient civilizations with various measures to limit exposure to sun exposure being employed. Awareness of the risks associated with sunrays has been increasing in the last century, and as a result, the science, technologies, and formulation have advanced significantly. The use of sunscreen products continues rising as government health agencies seek to contain increasing cases of UV induced melanomas. Recreational sunbathing and artificial tanning have increased the risk for these diseases significantly. This review article sought to expound the scientific basis of sunscreen use, the classification, formulation, quality control and regulation across the different countries around the world. The literature review was conducted on Google scholar, PubMed, SCOPUS, Cochrane, BMJ, SCIELO among others.
1. Introduction
Repeated exposure of the skin to the sun has the potential to cause both short-term and long-term changes in the structure of the skin. In the short term, repeated exposure results in erythema (reddening) of the skin commonly referred to as sunburns (Moore, 2013). The erythema is followed by activation of melanocytes which increase their rate of melanin production (increased melanization) which darkens the skin appearance otherwise referred to as tanning. Long term effects of repeated exposure include irreversible loss of skin elasticity and may lead to the development of skin cancers, both melanomas and non-melanomas (Harrison and Bergfeld, 2009). The extent of skin damage depends on the duration of exposure, seasonal variations in incident sunrays intensity, geographical location, and host-dependent factors including age, skin color, behavioral factors, immune status among others (Jou and Tomecki, 2014, Rigel, 2008, Harrison and Bergfeld, 2009).
The utilization of sunscreens (also referred to as sunprotectants) for protection against the harmful effects of the sun rays has been increasing over the last few decades. This may have resulted from increased awareness about the potentially harmful effects that arise from repeated exposure to the sun. Increased awareness campaigns by the government(s) have also played a role in the increased uptake (Albert and Ostheimer, 2003). Repeated sun exposure increases the risk of three types of cancer: melanoma, basal cell carcinoma, and squamous cell carcinoma with melanomas causing higher mortality while the non- melanoma skin cancers are associated with higher morbidity and aesthetic skin damage (Schüz and Eid, 2015, Armstrong and Cust, 2017, Craythorne and Al-Niami, 2017). Different clinical studies have shown that regular use of sunscreens can promote skin cancer reduction, especially melanoma and squamous cell carcinoma (Green et al., 2011). Evidence towards the protective role of sunscreens against photoaging has also been established (Hughes et al., 2013).
The formulation and science of sunscreens have also evolved along with improvements in the scientific knowledge and technologies to improve the formulation characteristics in both efficacy, safety and aesthetic appeal. Increased incidence of skin melanomas has attracted regulatory concerns on the quality of sunscreens resulting in higher demands from the authorities regarding the quality of sunscreen products (Jansen et al., 2013). There are also immense economic gains to be realized given the expensive costs of treatment and loss of economic productivity occasioned by individuals suffering from skin cancers (U.D. of H. and H. Services, 2014). The financial burden of non-melanoma in Australia was projected to surpass 700 million dollars highlighting the huge financial burden skin cancers have on the healthcare systems (Gordon and Rowell, 2015).
This article reviews the science behind the use of sunscreens, the historical perspective of sunscreen use, nature and classification of sunscreens, dosage forms and incidental formulation challenges. The science of ultraviolet light and its inherent potential to cause skin damage is discussed. Determination of product effectiveness, regulatory aspects of sunscreen manufacturing and marketing are also discussed.
The use of sunscreens as photoprotectants has evolved significantly over the last few decades. With increasing awareness of the protection afforded by sunscreens against sunburns, skin aging and melanomas, the demand for sunscreen formulations will invariably increase, and there exists a significant opportunity for pharmaceutical industries to fulfill this demand by manufacturing quality, efficacious, safe and aesthetically appealing sunscreen formulations (Svarc, 2015, Tuchinda et al., 2006).
1.1. The basis of sunscreen use
Cosmetics are defined as “articles intended to be rubbed, poured, sprinkled, or sprayed on, introduced into, or otherwise applied to the human body or any part thereof for cleansing, beautifying, promoting attractiveness, or altering the appearance” (FDA, 2018a). Among the commonly used cosmetics are sunscreens. These are formulations that are applied onto the skin surface to protect it from the harmful effects of ultraviolet (UV) light. Repeated exposure of the skin has been associated with a high risk of developing skin cancers. According to cancer research USA, 8 out of 10 cases of melanoma could be prevented through an understanding of the harmful effects of sunlight and how to protect oneself from the harmful rays (Jou and Tomecki, 2014). Of concerns to health agencies around the world is the increase in vacation sunbathing as well as the use of artificial UV sources to induce skin tanning among young whites seeking a darker skin (WHO, 2017).
Sunrays consist of an array of wavelengths ranges that vary in frequency and their energy profiles. The suns electromagnetic spectrum consists of cosmic rays, gamma rays, X-rays, UV rays, microwaves, and radio waves in decreasing order of energy. Among these cosmic, gamma and X rays are effectively filtered out of the earth by the atmosphere and therefore present no potential for causing harm. It is, however, noteworthy that they are the most lethal and exposure would lead to disasters of epic proportions. The UV rays can penetrate the earth' atmosphere as can the rest of the lower energy spectrums. Microwaves and radio waves are not of medical importance as relates to causing skin damage. The focus of this article is thus the UV spectrum of light (Rezende et al., 2014).
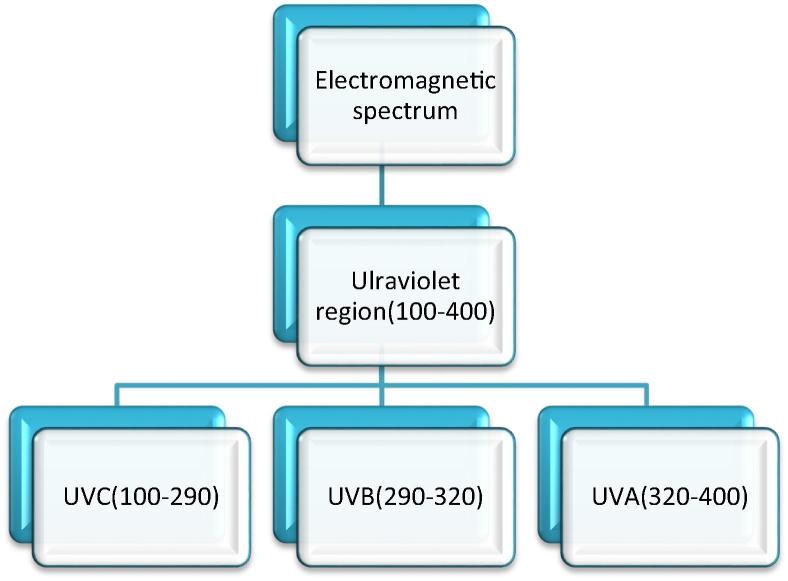
The UV light is part of the visible light and spans the wavelength from 100 to 400 nm as shown in Fig. 1 below. The UV spectrum is further divided into three; 290–320 nm (UVB) and 320–400 nm (UVA) (Moyal and Fourtanier, 2008, Matts, 2006). UVC occupies 100–290 nm of the spectrum; however, it is of no medical importance since it is entirely filtered out by the ozone layer. UVB triggers the production of melanin pigment and stimulates the skin cells to produce a thicker epidermis, resulting in a long-lasting tan. It is also the primary cause of sunburns. The UVA light activates melanin already on the epidermis to produce a short-term tan. It penetrates much deeper into the skin than UVB and can cause long term damage to the skin as well as skin aging characterized by loss of elasticity and wrinkling. Its effects manifest much later compared to the effects of UVB which are acute. UVA light also reacts with skin cells to produce free radicals that are highly active and may indirectly lead to DNA mutations which if unrepaired may lead to cancer (D’Orazio et al., 2013). Individuals with light skin pigmentation suffer comparatively more skin damage from UV because it is relatively easy for UV rays to penetrate the epidermis to damage both keratinocytes and melanocytes in the deeper layers of the epidermis (Harrison and Bergfeld, 2009, D’Orazio et al., 2013, Matsumura and Ananthaswamy, 2004).
Fig. 1.
The electromagnetic spectrum for ultraviolet light. Reproduced from Svobodova et al. (2006). *Wavelength in nm.
Ultraviolet filters also referred to as sunscreens, are the elements present in photo-protector formulas that interfere directly with the incident solar radiation through absorption, reflection or dispersion of energy (Schalka et al., 2014). They are classified into two categories based on their mechanism of action; Chemical or organic sunscreens and mineral-based or inorganic sunscreens. Chemical sunscreens absorb UV light and convert it into heat energy that is then released from the skin. Typical examples of chemical sunscreens include octisalate and avobenzone (Gasparro et al., 1998). The organic sunscreens afford better aesthetics upon application and are therefore more widely accepted, however they carry the potential for systemic absorption therefore sensitivity and untoward effects are more common with this group of sunscreens. Mineral sunscreens also referred to as sunblocks act by reflecting and scattering the UV light thereby protecting the skin. Common examples of mineral sunscreens include titanium dioxide and zinc oxide. Inorganic filters present a minimum potential for allergic sensitization and high photostability and are therefore more appropriate for people with sensitive skin (Chen and Wang, 2016). However, their reflective properties may cause excessive shine and a whitish aspect, limiting their exclusive use to formulas due to low cosmetic acceptance. The efficiency of inorganic filters is related to the size and dispersion of their particles. Currently, existing formulations frequently contain both chemical and mineral sunscreens. Different formulations exist including creams, gels, sprays, and oils. The choice of which is dependent on individual requirements and preferences (Schalka et al., 2014, Robinson, 2017).
1.2. Historical perspective of sunscreen use
There is little literature on the way ancient societies used to shield themselves from the sun. However, sunscreens have been used to mitigate the harmful effects of the sun on the skin since medieval time. In ancient Egypt, women applied various natural products as sunscreens. These include; tirmis, yasmeen, zaytoon, sobar, aquatic lotus oil, almond oil, calcite powder and clay, rice bran extracts among many others. The Greek and other communities living in the Mediterranean, having discovered the harmful effects of the sun, designed special hats to shield themselves from the harmful rays (Trivedi et al., 2017). There is documentary evidence of the use of oil using the Greek Olympics (Cosentino, 2000). Other numerous writings indicate a society well aware of the association of extended skin exposure to the sun and the aging or physical changes. Acidified quinine was used in the 1880s to protect a patient with eczema from harmful UV rays (Urbach, 2001).
Clothing in medieval societies was mainly designed to suit the climatic conditions in which the societies dwelt in. Cave drawings in tropical zones indicate that ancient Egyptians used to cover only certain parts of the body while leaving others exposed. Over time the culture evolved to cover the entire body. These ancient beings must have realized that the warmth of the sun was followed by pain inflamed skin. The Indian and Chinese societies are credited with having invented the umbrella which also dates back to medieval times (Urbach, 2001). The legendary King Arthur is pictured with women covered in wimples. The Tibetans used to smear their skin with tar and herbs while the red Indians covered themselves with red ochre for cosmetic reasons probably unaware of the protective effects against the sun. The Burmese society also used plant extracts as cosmetics way back in 2000 BCE (Goldsberry et al., 2014). In East Africa, the Masai community has a long tradition of smearing red ochre on their hair and face for aesthetic appeal but are oblivious of the protection afforded to their skin. Folklore has it that the Kikuyu community in Kenya used to smear clay over their exposed body parts to shield them from the destructive effects of sunrays as they went about their peasantry farming activities (Ambrose et al., 2016).
In modern times commercial use of sunscreens was first reported in 1928 in the USA following the introduction of an emulsion containing benzyl cinnamate and benzyl salicylate. Formulations containing phenyl salicylate appeared in Australia in the early 1930s. Quinine oleate was used in the USA in the mid-1930s. P- Aminobenzoic acid (PABA) was patented in 1943, and numerous sunscreens containing PABA followed this. The US army developed specifications for sunscreens in the 1950s (Kwan et al., 2014).
1.3. Effects of UV exposure on the skin
Ultraviolet (UV) radiation causes both beneficial and undesirable effects on the skin (Svobodova et al., 2006). The purpose of sun protection is to minimize unwanted effects without affecting the beneficial ones. The effects may present acutely while others develop over prolonged periods (Matsumura and Ananthaswamy, 2004, Soter, 1990). They include tanning, sunburns, photoaging and skin cancer. Tanning refers to the delayed pigmentation of the skin which is considered desirable in many cultures. The practice of cosmetic tanning has gained prominence among young Caucasians with the trend has been increasing with advancements in technologies that make it possible to produce artificial UV light. The WHO has raised the alarm over this practice as it predisposes to skin cancer in the long term (WHO, 2017, O’Sullivan and Tait, 2014). Sunburns refers to dermal erythema arising due to dilatation of superficial blood vessels is a common occurrence following exposure to UV rays. Extreme exposure causes the skin to become painful and edematous with or without blistering (Gilchrest et al., 1981). The most common forms of skin cancer are; basal cell carcinoma, squamous cell carcinoma, and cutaneous malignant melanoma. The first two are grouped together as non-melanomas and are associated with higher morbidity and cause more extensive aesthetic changes on the skin while higher mortality occurs in the malignant melanoma (Madan et al., 2010, Fransen et al., 2012). Exposure to UV radiation is considered to be a significant etiological factor for most forms of cancer (Matts, 2006, Fartasch et al., 2012, Surdu et al., 2013). Photoaging which includes irreversible changes to the skin has been associated with chronic exposure to the sun. It presents as dry skin, rugged furrows, sagging and loss of skin elasticity (Cavinato et al., 2017). This is in contrast to intrinsically aged skin that is pale, finely wrinkled and appears smooth (Farage et al., 2008).
1.4. Use of sunscreens for protection against ultraviolet-induced skin damage
With the advancements in the medical field as well as science in general that came about in the 20th century, it was demonstrated that the UV section of light contributes significantly towards skin damage. Studies in laboratory rodents enabled greater understanding of UV-induced immune depression, carcinogenesis, photodamage and photoaging (Gonzaga, 2009). Animals irradiated with UV demonstrated lesser hypersensitivity, and they failed to reject organ implants, unlike the controls which were not irradiated indicating a reduction in the immunological capacities of the irradiated animals (Schalka et al., 2014). Scientists also observed that the incidence of melanoma was higher in populations where sunbathing is common. More intensive studies confirmed that those who used sunscreens on a routine basis suffered skin damage to a much lesser extent (Soter, 1990). Widespread research has further characterized the causes of skin cancers, and the numerous cancer agencies have included UV rays as one of the significant human carcinogens (El Ghissassi et al., 2009; Newton-Bishop et al., 2011). Public awareness campaigns have since led to greater acceptance and usage of sunscreens. Initial efforts were developed to produce anti UVA products specifically; recently most sunscreens formulations contain both anti-UVA and anti UVB agents (WHO, 2006).
2. Classification of sunscreens and the mechanism of photoprotection
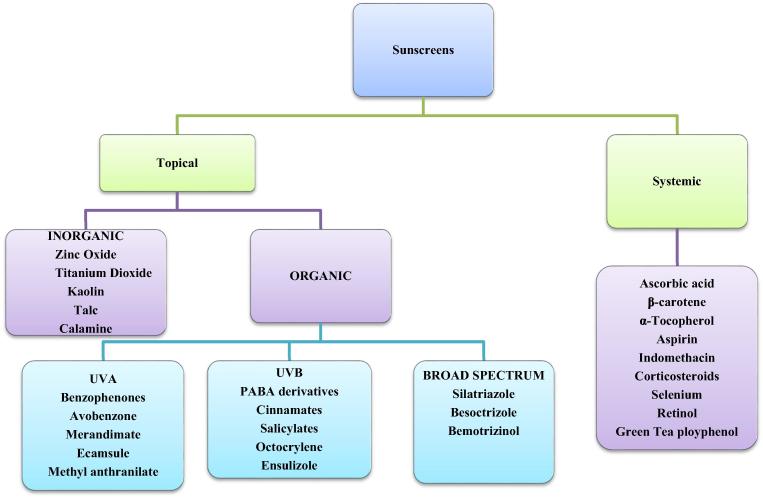
Broadly, sunscreens are classified as either topical or systemic based on the route of administration. Topical sunscreens are further divided into two classes; Organic and inorganic substances based on their mechanism of protection. Inorganic sunscreens are sometimes referred to as sunblocks (Rigel, 2014).
2.1. Organic sunscreens
These are generally aromatic compounds linked with a carbonyl group. They are broadly classified into three categories based on the range of protection; UVB (290–320 nm) and UVA (320–400 nm) and broad-spectrum sunscreens that cover the entire spectrum (290–400 nm) (Gabard, 2009). Examples of organic sunscreens covering UVB include (PABA) and its derivative padimate O. salicylates including octisalate and homosalate, cinnamates including octinoxate and cinoxate, octocrylate, benzsulidone and dibenzoylmenthanes. UVA filters include benzophenones; oxybenzone and sulisobenzone, avobenzone and meradimate, Methyl anthranilanate and ecamsule. Broad spectrum organic filters that cover both UVA and UVB include besoctrizole, silatriazole among others (Tuchinda et al., 2006, Serpone et al., 2007).
2.2. Inorganic sunscreens
These are particles that scatter and reflect UV rays back to the environment. They act as a physical barrier to indent ultraviolet and UV light. The most commonly used particulate sunscreens are titanium dioxide and zinc oxide (Serpone et al., 2007, Dransfield, 2000). They are considered broad spectrum as they cover the entire ultraviolet spectrum. The inorganic sunscreens are also referred to as sunblocks, a term coined from their mechanism of photoprotection (Dransfield, 2000).
2.3. Systemic sunscreens
These are sunscreens that are absorbed into the body and accumulate in the skin affording protection from the UV rays. Common examples under this category are shown in Fig. 2 (Latha et al., 2013). The use of systemic sunscreens for daily routine is minimal, as such the focus of this article ison topical sunscreens as these predominate in the market.
Fig. 2.
Classification of sunscreens. It is adapted from Latha et al. (2013).
2.4. Mechanism of photoprotection
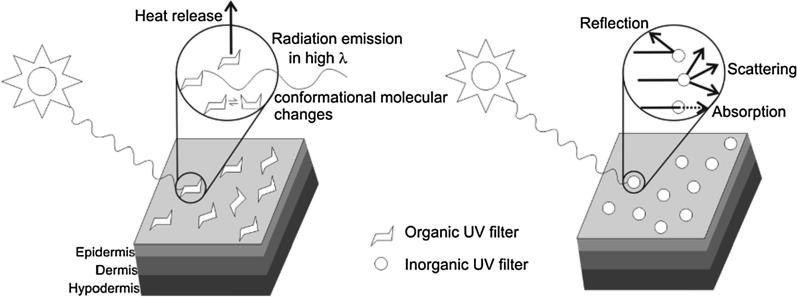
Sunscreens act by preventing and minimizing the damaging effects of the ultraviolet sun rays following exposure to the sun. Sunscreens have been demonstrated to increase the tolerance of the skin to UV exposure. They primarily work through two mechanisms as detailed below. Fig. 3 gives a pictorial perspective of the mechanisms of action stated.
-
(a)
Scattering and reflection of UV energy from the skin surface. Mineral based (Inorganic sunscreens work primarily through this mechanism. They provide a coating that blocks sun rays from penetrating through the skin (Dransfield, 2000).
-
(b)
Absorption of the UV energy by converting it to heat energy thus reducing its harmful effects and reduce the depth through which it can penetrate the skin. Organic sunscreens work primarily through this mechanism (Dransfield, 2000, Lademann et al., 2005, Manaia et al., 2013).
Fig. 3.
Mechanism of action of organic and inorganic sunscreens. Adapted from Manaia et al. (2013).
Multiple organic compounds are usually incorporated into chemical sunscreen agents to achieve protection against a range of the UV spectrum. Inorganic particulates may scatter the microparticles in the upper layers of skin, thereby increasing the optical pathway of photons, leading to absorption of more photons and enhancing the sun protection factor (SPF), this results in high efficiency of the compound (Trivedi et al., 2017).
3. Development of sunscreens
The development of sunscreens requires a thorough understanding of the anatomy and physiology of the skin as well as the physical-chemical properties of the substances that one intends to include in the formulation. The stability of the organic substances and the excipients need to be examined as some exhibit instability on exposure to the UV. Inorganic sunscreens generally have limited stability issues as well as limited toxicity (Tuchinda et al., 2006, Urbach, 2001). The aesthetic appeal of the product must be taken into consideration to promote consumer compliance.
3.1. Properties of an ideal sunscreen
Desirable chemical attributes include; inertness, nonirritant, photostability, and compatibility with other ingredients. Physical characteristics include low viscosity to promote good spreadability, aesthetic appeal, small particle size, waterproof capability, appropriate solubility and non-odorous. Functional attributes include the ability to afford protection across a wide range of wavelength and limited systemic absorption through the skin to minimize sensitization. The products should also be readily available, inexpensive and contaminant free (Manikrao Donglikar and Laxman Deore, 2016).
3.2. Formulation
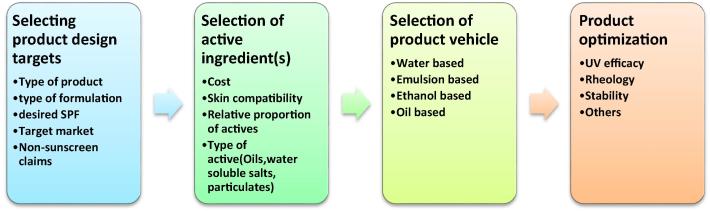
Formulation of sunscreen involves four critical steps; selection of the target product design, choice of active ingredients and the delivery vehicle followed by product optimization as shown in Fig. 4. The primary objective of the formulation expert is to develop a product that forms a continuous film on the skin. Penetration of the organic ingredients into the skin should be minimized (Tanner, 2006). Organic sunscreens are formulated as lotions and light ointments. On application, they form a thin film on the skin surface that affords UV protection. Other formulations include oils, gels, emulsions, mousses (fluid emulsions), aerosols, sticks, and powders. Inorganic sunscreens are more difficult to formulate due to their particulate nature. Traditionally, they were formulated as creams that were sticky, oily and unpleasant to use. Nanomization has allowed spray formulations that form a translucent layer on the skin that affords protection while maintaining the aesthetics of the product. Currently no nanomized spray formulations of sunscreens have been approved for registration owing to safety concerns as these nanoparticles may be inhaled and therefore cause system toxicities. Inorganic sunscreens are formulated as pastes, emulsions, sprays, and ointments. Particle engineering approaches including micronization and nanomization of the particles are done to increase the aesthetic value of the products (Nesseem, 2011). The safety and convenience of the user guide the formulation approach. Any substance with potential skin irritancy and potential allergens must be avoided. Like other skin products, formulation requires the inclusion of adhering agents to promote skin adsorption as well as an appropriate vehicle into which the active substance is dispersed. Patents play an essential role in the development process, and careful consideration must be taken before embarking on product development (Aikens and Dayan, 2016).
Fig. 4.
The process of formulating sunscreen products.
Among the challenges and concerns associated with topical sunscreens formulations involve the photostability of organic filters, broadening the effectiveness spectrum and parameters, incorporating active ingredients, improving cosmetic and sensory aspects, individualizing vehicles. The ideal sunscreen formulation should take into consideration aspects including efficiency for the intended use, the scope of protection spectrum (UVA and UVB), safety and tolerability for topical use, stability, no staining of clothes, adequate cosmetics, pleasant fragrance, resistance to water, spread-ability, high extinction coefficient, and affordable cost. Sunscreen formulations include the main sunscreen agents, excipients specific to the formulation type including an appropriate solvent or vehicle systems. The contents selection is determined by the intended use and the physicochemical nature of the ingredients. Purified water used in product formulation is prepared through reverse osmosis and other established methods of purifying water for industrial use (Tanner, 2006, Nesseem, 2011, Aikens and Dayan, 2016).
The most common sunscreen actives; titanium dioxide, zinc oxide, avobenzone, benzophenone 8, octocrylene, and oxybenzone are used. To vary the amount of sun protection, the level of the active ingredient is adjusted. Lademan and group established synergy between organic and organic sunscreens and demonstrated superior efficacy of products comprising of the two compared to those containing only organic or inorganic sunscreens (Lademann et al., 2005). The FDA prescribes the maximum allowable concentration of each ingredient as well as the impurity content. It is common to find sunscreen being co-formulated with other skin products for value addition (Tanner, 2006). A rationally designed and developed product enhances the compliance of the users while affording the necessary protection against the ultraviolet-induced skin damage (Xu et al., 2016).
In the last two decades the adoption of the Quality by Design (QbD) concept has been advocated by the leading regulatory authorities. Embracing this approach includes a scrupulous scientific design of the product, careful selection of materials and process parameters to ensure the achievement of a predefined product quality profile (Mishra et al., 2018). The formulator develops a Quality Target Product Profile (QTTP) that specifies the desired physicochemical and performance attributes of the sunscreen. They then proceed to define the Critical Material Attributes (CMAs) and process parameters required to achieve the QTTP so defined (Fukuda et al., 2018). Risk assessment is conducted to profile areas that may prevent achievement of the desired product quality and appropriate measures are undertaken to address these potential risks. To supplement achievement of the desired product quality, use of design of experiment (DoE) tools could be a valuable guide in optimizing the desirable attributes of the sunscreens (Peres et al., 2017).
3.3. Analysis of the final product
Physicochemical and microbiological characterization of the final product is required to establish its compliance with required quality parameters. The specific tests include the visual analysis, stability testing, pH determination, SPF evaluation, determination of water resistance and its microbiological evaluation.
Physical analysis: Includes organoleptic tests to observe changes in the color, and presence of phase separation in the product.
Stability tests: Colour, phase separation and liquefaction. There should be no color changes nor separation of phases in sunscreen formulations in the stability tests if they are to pass the quality tests. The absence of liquefaction provides strong evidence for the stability of the emulsions (Abdassah et al., 2015). These tests should ascribe to the International Committee on Harmonization (ICH) Q1A-Q1F (Abraham, 2009).
PH determination over time: The pH value of sunscreen stored at different conditions is determined using a digital pH Meter. The pH tests are repeated for multiple emulsions or formulations after a defined period of storage. Ideal pH is around 6.0 which approximates the average PH of the skin. pH changes indicate the occurrence of chemical reactions that indicate the quality of the final product (Smaoui et al., 2017).
Determination of SPF in vitro using spectrophotometry: The in vitro methods are in general of two types. Methods which involve the measurement of absorption or the transmission of UV radiation through sunscreen product films in quartz plates or biomembranes, and methods in which the absorption characteristics of the sunscreens agents are determined based on spectrophotometric analysis of dilute solutions (Mpiana, 2014). A detailed description is given in Section 4.1 below.
Level of water resistance for UVB: This test is conducted by immersion of a volunteer subject in a pool or spa for 40 min with a five-minute rest in between (20-5-20). The procedure is done immediately after the application step described in the SPF determination. To obtain a water resistance label claim of 80 min, four immersions -drying cycles are thus required. After the immersion drying cycles, the SPF determination is then done as per the guidelines. The sunscreen product is considered to be water resistant if it retains no less than 50% of its SPF following immersion (Patrician Poh Agin, 2006).
Microbiological stability: Sunscreens like other topical formulations must be free from any microbial contamination that may render them deleterious to the users (Smaoui, 2012). Common microbiological stability tests for sunscreen products include tests for Streptococcus aureus, Pseudomonas aeroginosa, yeast, and mold. Solutions of the sunscreen product are made an inoculated into the propagation media appropriate for the specific organism being tested, the media is incubated in the at room temperature (25 °C) for specified period after which the number of colonies are enumerated (Smaoui et al., 2017). Preservation systems and strict compliance with good manufacturing practices can mitigate against the introduction of harmful microbes (Smaoui et al., 2017).
Other general quality parameters are formulation type specific and include spreadability, extrudability, texture, viscosity and firmness of gels, creams and lotions, spray characteristics including the spray rate, pattern and droplet sizes, actuation force, spray can leakage among others (Baki and Alexander, 2015).
4. Measurement of the effectiveness of a sunscreen
The effectiveness of a sunscreen is determined by various indices including; Sun protection factor, persistent pigment darkening, immune protection factor among others.
4.1. Sun protection factor
Sun Protection Factor (SPF) refers to the ability of the sunscreen to prevent the development of erythema upon exposure to UV radiation (Mpiana, 2014, SPF, 2017). The SPF value is mainly determined using in vivo approaches but may also employ in vitro spectrophotometric methods as well as in silico ones that employ computer models to predict the SPF value (Osterwalder and Herzog, 2009). Traditionally in vitro test for SPF determination used excised skin from cadavers or laboratory animals, usually albino hairless mice. SPF can also be determined using spectrophotometric methods (Mbanga et al., xxxx, Nobre and Fonseca, 2016, Dutra et al., 2004). Current guidelines prescribe use of human volunteers for in-vivo SPF determination. The sunscreen is carefully applied at the rate 2 mg/cm2 and allowed to dry gradually. The skin areas commonly used are the lower back specifically areas that have not had previous exposure to sunscreens. It is recommended that careful selection of subjects be done with a requirement that they should ideally haven’t had sun exposure or had their skins tanned for at least 90 days prior to enrollment. Other key requirements is lack of skin sensitivity, a Flitzpatrick skin types II, III and IV and who agree to sign an informed consent (D’Orazio et al., 2013, Moyal et al., 2006). Other elements of inclusion and exclusion criterion follow the guidelines for clinical trials (Bayer Inc, 2009, GSK, 2017). Detailed guidelines for the in-vivo SPF determination is outlined in the International sun protection Test Method (ISO, 2010). SPF is expressed as the ratio of the minimal erythemal dose (MED) required to induce erythema on the protected skin and that dose required to induce the same on unprotected skin on the same individual (Osterwalder and Herzog, 2009). The mathematical expression is shown in Formula (1).
| (1) |
Formula 1. Calculation of SPF.
There is a global shift to minimize animal testing in the development of medicines and related products with recent guidelines prohibiting use of animals in experimental studies (Smith, 2015). Notably it has been established that there is a strong in-vitro in-vivo correlation in SPF determination therefore obviating the need for animal studies (Dimitrovska Cvetkovska et al., 2017). As such scientists have developed in vitro techniques that determine SPF. Two approaches have been developed and validated; Measurement of absorption or the transmission of UV radiation through sunscreen product films in quartz plates or membranes and methods in which the absorption characteristics of the sunscreens agents are determined based on spectrophotometric analysis (Dutra et al., 2004, Walters et al., 1997). The tests are relatively inexpensive and rapid to conduct (Sudhahar and Balasubramanian, 2013). The SPF is related to absorbance as per Formula (2);
| (2) |
Formula 2. The relationship between absorbance and SPF (Walters et al., 1997).
Other methods proposed by Mansur et al in 1986 involve spectrophotometric measurement of the absorption characteristics of the sunscreen products may be used with accurate SPF determination (Dutra et al., 2004).
The magnitude of the SPF required for a specific individual is determined by knowledge of the UV climatology, the user’s behavior outdoors and their susceptibility to sunburns (Autier et al., 1999). Different regions have different UV radiation exposures based on their latitudes with the tropics having the highest with the extreme north and south having the least (D’Orazio et al., 2013). The SPF only measures the protection against UVB light. Grading system for SPF ranges from low to high: Low: (SPF 2–15), Medium: (SPF 15–30) High: (SPF 30–50), Highest: (SPF > 50) (Osterwalder and Herzog, 2009). The protection afforded by the sunscreens is usually much less than the SPF indicated due to limited knowledge of their use therefore inaccurate, insufficient and non-uniform application. Recommendations for ideal use include; avoiding sunscreens during the autumn and winter months as there is limited UV exposure. Using sunscreens with SPF above 30 during summer and sunny days is recommended (Draelos, 2006).
4.2. Persistent pigment darkening (PPD)
This measure establishes the ability of the sunscreen to protect against UVA light. The method of determination is similar to that of establishing SPF detailed above (Moyal et al., 2000, Matts et al., 2010, Nash et al., 2006). The level of protection is expressed as the UVA protection factor and expressed as the ratio between the minimal dose required to induce pigmentation (MPD) in the protected skin and the MPD observed on the unprotected skin and is calculated as given below. A consistent study protocol is required to minimize the variability of results across multisite laboratories (Moyal et al., 2006). The mathematical expression for PPD determination is shown in Formula (3) below.
| (3) |
Formula 3. Calculation of UVA protection factor.
Subscript p and u indicate the protected and unprotected skin respectively.
4.3. Immune protection factor
The term immune protection factor (IPF) refers to the ability of sunscreen products to prevent UV-induced immunosuppression. IPF is assessed by complex methods such as the ability of a sunscreen to inhibit either the sensitization or elicitation arm of contact or delayed-type hypersensitivity reactions to allergens such as dinitrochlorobenzene (DNCB) and nickel, respectively. IPF is considered to correlate better with the UVA-protectiveness of sunscreen than with its SPF (Fourtanier et al., 2005).
5. Regulatory requirements
Regulatory agencies seek to safeguard the safety and welfare of consumers using cosmetic products based on sound science. Different jurisdictions classify sunscreens as either therapeutic or cosmetic products. The USA, Australia, and Japan consider sunscreen as medicinal products subject to the strict requirement of manufacture under GMP conditions like other drugs. In the USA, cosmetic manufacturers are required to demonstrate the safety of each of the ingredients incorporated into their final products. The regulations also prescribe the maximum amounts of specific ingredients that have known toxicities while also providing a comprehensive list of substances that should not be included in the formulation (Pirotta, 2015, Benson, 2017). Cosmetic regulation is guided by the federal food drugs and cosmetic act of 1938 (Pirotta, 2015, Cavers, 1939). The sunscreen innovation act is the latest guide guiding the production of sunscreens and established the framework for approval of the next generation of sunscreens. (FDA, 2016) In the European Union (EU), cosmetic products are regulated under the Cosmetic Regulation (EC) No 1223/2009 which came into implementation in July 2013. The EU regulations are the first in the world to impose a complete ban on testing of cosmetic products on animals. Further the regulations proscribe the marketing of cosmetic products containing ingredient(s) tested on animals (Pirotta, 2015). Sunscreens are considered cosmetic products in the EU; however, the quality requirements are equally high. Regulation in emerging markets and developing countries is variable (Kaimal and Abraham, 2011).
5.1. Safety assessment
Before approval is granted for any sunscreen product, evidence towards its safe use must be established. The safety testing of sunscreen products is included in the laws regulating cosmetic products in general for the respective countries or regional jurisdictions where applicable. Cosmetic safety assessment takes into consideration the physicochemical properties of each ingredient included in the sunscreen formulation, as well as its potential to cause harmful effects over short-term, medium-term and long-term use. The toxicological studies aim to investigate both local and systemic effects. The endpoints generally considered include acute toxicity, repeated dose toxicity, skin and eye irritation, skin sensitization, mutagenicity, carcinogenicity, and effects on the reproductive system. Chemicals that may bear potential carcinogenic, mutagenic, or reproductive toxicity (CMR) or those that may persist and accumulate in the body over time are particularly excluded from use in cosmetic products (Smith, 2015). The US food and cosmetic act CFR 21 which is implemented by the Food and Drugs Administration (FDA) declares that cosmetic products whose safety has not be substantiated prior to marketing be deemed misbranded unless the warning that the safety of the product has not been determined be conspicuously included on the principal display panel (Benson, xxxx).
5.2. Labeling requirement
The labeling requirements for sunscreens must comply with the guidelines issued by the specific regulatory authority. Labeling should include the following; Identity of the product and the key ingredients, excipients and their percentage composition. The list of ingredients should be in the order of predominance from the highest to the lowest (Mancebo et al., 2014). The label should include a statement on the name and location of the manufacturer or distributor of the product, cautionary warnings in case a patient is allergic to any of the formulation constituents, optimal storage conditions, appropriate use, frequency, SPF value and Water resistance (Draelos, 2006). The EU cosmetic regulations require that certain ingredients such as nanomaterials to be included in the sunscreen products labelling (Smith, 2015). The EU and the Australia Therapeutics Goods Agency (TGA) do require the inclusion of the products shelf life in the packaging label (Smith, 2015, O’Sullivan and Tait, 2014). In the USA some sunscreen products do not require expiration dating, this is in circumstances where the manufacturer provides documented evidence demonstrating that the product is stable for no less than 3 years (FDA, 2018b, Bergeson, 2019). The FDA does requires the labelling to include the expiration date for sunscreens where the manufacturer cannot provide the stability data to this effect. A monograph reviewing the regulation of sunscreens comes into effect in the year 2019 (FDA, 2018b, Food and Drug Administration, 1999, Sunscreen Drug Products for Pver-the-Counter Human Use, 2001).
6. Controversies associated with sunscreens
Oxybenzone is absorbed systemically following topical application as a component of sunscreens. It is excreted in feces and in urine (Mancebo et al., 2014). Studies have demonstrated that oxybenzone has estrogenic and antiandrogenic activity in laboratory animals therefore potential endocrine disorders for long term usage. The dosage units used for these tests were extremely high and the exposure in humans is significantly much lower. Sunscreens have been associated with environmental contamination with oxybenzone, octocrylene, octinoxate being identified in fresh water. Of major concern is the damage caused to coral reefs with oxybenzone being implicated in coral reef bleaching (Schneider and Lim, 2019). Inorganic sunscreens though considered to be relatively safe can pose potential health risks due to their formulation as nanoparticles which may potentially be absorbed systemically. The inhibitory effect of sunscreen on vitamin D synthesis have also been described (Libon et al., 2017). Other studies show that sunscreen use has no effect on the synthesis of Vitamin D (Hansen et al., 2016). More comprehensive studies are required to establish the accurate association between sunscreen use and vitamin D status.
7. Conclusion
Sunscreens are critical products employed as photoprotectants against the harmful UV rays. Increased awareness about the risk of continuous exposure to the sun and its relation to cancer has increased the demand for sunscreens. Health agencies around the world have taken up the interest in advocating for the appropriate use of sunscreens as this has been demonstrated to afford protection against skin aging, tanning, and melanomas. Regulatory agencies across the world are also coming up with the requisite policies to enhance the oversight on manufacture of quality sunscreen product consistent with emerging scientific knowledge.
Pharmaceutical scientists understand the scientific principles of topical drugs and can formulate sunscreens that comply with all requirements for safety, quality efficacy, and consumer acceptance. The formulation of sunscreen continues to evolve with new technologies enhancing product design and efficacy. Incorporation of quality by design concepts in the manufacture of sunscreen products is being embraced as regulatory authorities reassign the classification of sunscreen from general cosmetics to therapeutic drugs.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abdassah M., Aryani R., Surachman E., Muchtaridi M. In-vitro assessment of effectiveness and photostability Avobenzone in cream formulations by combination ethyl ascorbic acid and alpha tocopherol acetate. J. Appl Pharm. Sci. 2015:070–074. [Google Scholar]
- Abraham, J., 2009. International Conference On Harmonisation Of Technical Requirements For Registration Of Pharmaceuticals For Human Use. In: Brouder, A., Tietje, C. (Eds.), Handb. Transnatl. Econ. Gov. Regimes, Brill, pp. 1041–1054. doi:10.1163/ej.9789004163300.i-1081.897.
- Aikens P. Formulation of sunscreens in the United States. In: Dayan N., editor. Handb. Formul. Dermal Appl. John Wiley & Sons, Inc.; Hoboken, NJ, USA: 2016. pp. 589–609. 10.1002/9781119364221.ch21. [Google Scholar]
- Albert M.R., Ostheimer K.G. The evolution of current medical and popular attitudes toward ultraviolet light exposure: part 3. J. Am. Acad. Dermatol. 2003;49:1096–1106. doi: 10.1016/s0190-9622(03)00021-5. [DOI] [PubMed] [Google Scholar]
- Ambrose, S., Zipkin, A., Gakii, M., Lundstrom, C., 2016. The 81st Annual Meeting of the Society for American Archaeology, Ethnography and archaeometry of red ochre use by the Maasai and Samburu in Kenya.
- Armstrong B.K., Cust A.E. Sun exposure and skin cancer, and the puzzle of cutaneous melanoma. Cancer Epidemiol. 2017;48:147–156. doi: 10.1016/j.canep.2017.04.004. [DOI] [PubMed] [Google Scholar]
- Autier P., Dore J.-F., Negrier S., Lienard D., Panizzon R., Lejeune F.J., Guggisberg D., Eggermont A.M.M. Sunscreen use and duration of sun exposure: a double-blind, randomized trial. JNCI J. Natl. Cancer Inst. 1999;91:1304–1309. doi: 10.1093/jnci/91.15.1304. [DOI] [PubMed] [Google Scholar]
- Baki Gabriella, Alexander Kenneth S. Introd. Cosmet. Formul. Technol. John Wiley & Sons, Inc.; 2015. Sun care products; pp. 273–309. [Google Scholar]
- Bayer Inc, 2009. Determination of Sun Protection in Sunscreen Formulas (Study SR09-15)(P08236). https://clinicaltrials.gov/ct2/show/NCT01001975 (accessed May 20, 2019).
- Benson, L., 2017. A Guide to United States Cosmetic Products Compliance Requirements, 49.
- Benson, L., n.d. A Guide to United States Cosmetic Products Compliance Requirements, 49.
- Bergeson, n.d. FDA OTC Sunscreen Drug Products Proposed Rule Coming. https://www.natlawreview.com/article/fda-will-publish-proposed-rule-otc-sunscreen-drug-products (accessed June 12, 2019).
- Cavers D.F. The food, drug, and Cosmetic Act of 1938: its legislative history and its substantive provisions. Law Contemp. Probl. 1939;6:2. [Google Scholar]
- Cavinato M., Waltenberger B., Baraldo G., Grade C.V.C., Stuppner H., Jansen-Dürr P. Plant extracts and natural compounds used against UVB-induced photoaging. Biogerontology. 2017;18:499–516. doi: 10.1007/s10522-017-9715-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L.L., Wang S.Q. Nanotechnology in photoprotection. Nanosci. Dermatol. 2016:229–236. [Google Scholar]
- Cosentino F. The use of oil in the Greek Olympics. J. Olymp. Hist. 2000;8:47–48. [Google Scholar]
- Craythorne E., Al-Niami F. Skin cancer. Medicine (Baltimore) 2017;45:431–434. [Google Scholar]
- D’Orazio J., Jarrett S., Amaro-Ortiz A., Scott T. UV radiation and the skin. Int. J. Mol. Sci. 2013;14:12222–12248. doi: 10.3390/ijms140612222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitrovska Cvetkovska A., Manfredini S., Ziosi P., Molesini S., Dissette V., Magri I., Scapoli C., Carrieri A., Durini E., Vertuani S. Factors affecting SPF in vitro measurement and correlation with in vivo results. Int. J. Cosmet. Sci. 2017;39:310–319. doi: 10.1111/ics.12377. [DOI] [PubMed] [Google Scholar]
- Draelos Z.D. Compliance and sunscreens. Dermatol. Clin. 2006;24:101–104. doi: 10.1016/j.det.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Dransfield G.P. Inorganic sunscreens. Radiat. Prot. Dosimetry. 2000;91:271–273. [Google Scholar]
- Dutra E.A., Oliveira D.A.G.da C., Kedor-Hackmann E.R.M., Santoro M.I.R.M. Determination of sun protection factor (SPF) of sunscreens by ultraviolet spectrophotometry. Rev. Bras. Ciênc. Farm. 2004;40:381–385. [Google Scholar]
- El Ghissassi F., Baan R., Straif K., Grosse Y., Secretan B., Bouvard V., Benbrahim-Tallaa L., Guha N., Freeman C., Galichet L., Cogliano V. A review of human carcinogens—Part D: radiation. Lancet Oncol. 2009;10:751–752. doi: 10.1016/s1470-2045(09)70213-x. [DOI] [PubMed] [Google Scholar]
- Farage M.A., Miller K.W., Elsner P., Maibach H.I. Intrinsic and extrinsic factors in skin ageing: a review. Int. J. Cosmet. Sci. 2008;30:87–95. doi: 10.1111/j.1468-2494.2007.00415.x. [DOI] [PubMed] [Google Scholar]
- Fartasch M., Diepgen T.L., Schmitt J., Drexler H. The relationship between occupational sun exposure and non-melanoma skin cancer. Dtsch. Aerzteblatt Online. 2012 doi: 10.3238/arztebl.2012.0715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FDA, 2016. Sunscreen Innovation Act: Section 586C(c) Advisory Committee Process Guidance for Industry, 12.
- FDA, 2018a. Federal food drug and cosmetic act.
- FDA, 2018b. CFR - Code of Federal Regulations Title 21. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=211.137 (accessed May 18, 2019).
- Food and Drug Administration, 1999. Sunscreen Drug Products For Over-The-Counter Human Use; FinalMonograph. https://www.govinfo.gov/content/pkg/FR-1999-05-21/pdf/99-12853.pdf.
- Fourtanier A., Moyal D., Maccario J., Compan D. Measurement of sunscreen immune protection factors in humans: a consensus paper. J. Invest. Dermatol. 2005;125:403–409. doi: 10.1111/j.0022-202X.2005.23857.x. [DOI] [PubMed] [Google Scholar]
- Fransen M., Karahalios A., Sharma N., English D.R., Giles G.G., Sinclair R.D. Non-melanoma skin cancer in Australia. Med. J. Aust. 2012;197:565–568. doi: 10.5694/mja12.10654. [DOI] [PubMed] [Google Scholar]
- Fukuda I.M., Pinto C.F.F., Moreira C. dos S., Saviano A.M., Lourenço F.R. Design of Experiments (DoE) applied to Pharmaceutical and Analytical Quality by Design (QbD) Braz. J. Pharm. Sci. 2018;54 [Google Scholar]
- Gabard, Bernard, 2009. Sun protection and sunscreens. In: Handb. Cosmet. Sci. Technol., third ed., pp. 323–332.
- Gasparro F.P., Mitchnick M., Nash J.F. A review of sunscreen safety and efficacy. Photochem. Photobiol. 1998;68:243–256. [PubMed] [Google Scholar]
- Gilchrest B.A., Soter N.A., Stoff J.S., Mihm M.C. The human sunburn reaction: histologic and biochemical studies. J. Am. Acad. Dermatol. 1981;5:411–422. doi: 10.1016/s0190-9622(81)70103-8. [DOI] [PubMed] [Google Scholar]
- Goldsberry A., Dinner A., Hanke C.W. Thanaka: traditional Burmese sun protection. J. Drugs Dermatol. JDD. 2014;13:306–307. [PubMed] [Google Scholar]
- Gonzaga E.R. Role of UV light in photodamage, skin aging, and skin cancer: importance of photoprotection. Am. J. Clin. Dermatol. 2009;10:19–24. doi: 10.2165/0128071-200910001-00004. [DOI] [PubMed] [Google Scholar]
- Gordon L.G., Rowell D. Health system costs of skin cancer and cost-effectiveness of skin cancer prevention and screening: a systematic review. Eur. J. Cancer Prev. 2015;24:141–149. doi: 10.1097/CEJ.0000000000000056. [DOI] [PubMed] [Google Scholar]
- Green A.C., Williams G.M., Logan V., Strutton G.M. Reduced melanoma after regular sunscreen use: randomized trial follow-up. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2011;29:257–263. doi: 10.1200/JCO.2010.28.7078. [DOI] [PubMed] [Google Scholar]
- GSK, 2017. Determination of the Sun Protection Factor (SPF) of a Cosmetic Daily De-fence Skin Cream. https://clinicaltrials.gov/ct2/show/NCT03136107 (accessed May 20, 2019).
- Hansen L., Tjønneland A., Køster B., Brot C., Andersen R., Lundqvist M., Christensen J., Olsen A. Sun exposure guidelines and serum Vitamin D status in Denmark: The Status D Study. Nutrients. 2016;8:266. doi: 10.3390/nu8050266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison S.C., Bergfeld W.F. Ultraviolet light and skin cancer in athletes. Sports Health. 2009;1:335–340. doi: 10.1177/1941738109338923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes M.C.B., Williams G.M., Baker P., Green A.C. Sunscreen and prevention of skin aging: a randomized trial. Ann. Intern. Med. 2013;158:781. doi: 10.7326/0003-4819-158-11-201306040-00002. [DOI] [PubMed] [Google Scholar]
- ISO, 2010. ISO 24444:2010(en), Cosmetics — Sun protection test methods — In vivo determination of the sun protection factor (SPF). https://www.iso.org/obp/ui/#iso:std:iso:24444:ed-1:v1:en (accessed May 20, 2019).
- Jansen R., Osterwalder U., Wang S.Q., Burnett M., Lim H.W. Photoprotection: sunscreen development, efficacy, and controversies. J. Am. Acad. Dermatol. 2013;69 doi: 10.1016/j.jaad.2013.08.022. 867.e1-867.e14. [DOI] [PubMed] [Google Scholar]
- Jou P.C., Tomecki K.J. Sunscreens in the United States: current status and future outlook. Adv. Exp. Med. Biol. 2014;810:464–484. [PubMed] [Google Scholar]
- Kaimal S., Abraham A. Sunscreens. Indian J. Dermatol. Venereol. Leprol. 2011;77:238. doi: 10.4103/0378-6323.77480. [DOI] [PubMed] [Google Scholar]
- Kwan Y.H., Tung Y.K., Kochhar J.S., Li H., Poh A.-L., Kang L. Handb. Cosmeceutical Excip. Their Safeties. Woodhead Publishing; 2014. 1 - History of cosmeceutics; pp. 1–5. [Google Scholar]
- Lademann J., Schanzer S., Jacobi U., Schaefer H., Pflücker F., Driller H., Beck J., Meinke M., Roggan A., Sterry W. Synergy effects between organic and inorganic UV filters in sunscreens. J. Biomed. Opt. 2005;10:014008. doi: 10.1117/1.1854112. [DOI] [PubMed] [Google Scholar]
- Latha M.S., Martis J., Shobha V., Sham Shinde R., Bangera S., Krishnankutty B., Bellary S., Varughese S., Rao P., Naveen Kumar B.R. Sunscreening agents. J. Clin. Aesthetic Dermatol. 2013;6:16–26. [PMC free article] [PubMed] [Google Scholar]
- Libon F., Courtois J., Le Goff C., Lukas P., Fabregat-Cabello N., Seidel L., Cavalier E., Nikkels A.F. Sunscreens block cutaneous vitamin D production with only a minimal effect on circulating 25-hydroxyvitamin D. Arch. Osteoporos. 2017;12:66. doi: 10.1007/s11657-017-0361-0. [DOI] [PubMed] [Google Scholar]
- Madan V., Lear J.T., Szeimies R.-M. Non-melanoma skin cancer. The Lancet. 2010;375:673–685. doi: 10.1016/S0140-6736(09)61196-X. [DOI] [PubMed] [Google Scholar]
- Manaia E.B., Kaminski R.C.K., Corrêa M.A., Chiavacci L.A. Inorganic UV filters. Braz. J. Pharm. Sci. 2013;49:201–209. [Google Scholar]
- Mancebo S.E., Hu J.Y., Wang S.Q. Sunscreens. Dermatol. Clin. 2014;32:427–438. doi: 10.1016/j.det.2014.03.011. [DOI] [PubMed] [Google Scholar]
- Manikrao Donglikar M., Laxman Deore S. Sunscreens: a review. Pharmacogn. J. 2016;8:171–179. [Google Scholar]
- Matsumura Y., Ananthaswamy H.N. Toxic effects of ultraviolet radiation on the skin. Toxicol. Appl. Pharmacol. 2004;195:298–308. doi: 10.1016/j.taap.2003.08.019. [DOI] [PubMed] [Google Scholar]
- Matts P.J. Solar ultraviolet radiation: definitions and terminology. Dermatol. Clin. 2006;24:1–8. doi: 10.1016/j.det.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Matts P.J., Alard V., Brown M.W., Ferrero L., Gers-Barlag H., Issachar N., Moyal D., Wolber R. The COLIPA in vitro UVA method: a standard and reproducible measure of sunscreen UVA protection. Int. J. Cosmet. Sci. 2010;32:35–46. doi: 10.1111/j.1468-2494.2009.00542.x. [DOI] [PubMed] [Google Scholar]
- Mbanga, L., Mulenga, M., Mpiana, P.T., Bokolo, K., Mumbwa, M., Mvingu, K., n.d. Determination of Sun Protection Factor (SPF) of Some body creams and lotions marketed in Kinshasa by ultraviolet spectrophotometry. Int. J. Adv. Res. Chem. Sci. 7.
- Mishra V., Thakur S., Patil A., Shukla A. Quality by design (QbD) approaches in current pharmaceutical set-up. Expert. Opin. Drug Deliv. 2018;15:737–758. doi: 10.1080/17425247.2018.1504768. [DOI] [PubMed] [Google Scholar]
- Moore C. UVB radiation generates sunburn pain and affects skin by activating epidermal TRPV4 ion channels and triggering endothelin-1 signaling. Med. Sci. 2013:11. doi: 10.1073/pnas.1312933110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyal D., Chardon A., Kollias N. UVA protection efficacy of sunscreens can be determined by thepersistent pigment darkening (PPD) method. Photodermatol. Photoimmunol. Photomed. 2000;16:250–255. doi: 10.1034/j.1600-0781.2000.160603.x. [DOI] [PubMed] [Google Scholar]
- Moyal D.D., Fourtanier A.M. Broad-spectrum sunscreens provide better protection from solar ultraviolet–simulated radiation and natural sunlight–induced immunosuppression in human beings. J. Am. Acad. Dermatol. 2008;58:S149–S154. doi: 10.1016/j.jaad.2007.04.035. [DOI] [PubMed] [Google Scholar]
- Moyal D., Wichrowski K., Tricaud C. In vivo persistent pigment darkening method: a demonstration of the reproducibility of the UVA protection factors results at several testing laboratories. Photodermatol. Photoimmunol. Photomed. 2006;22:124–128. doi: 10.1111/j.1600-0781.2006.00223.x. [DOI] [PubMed] [Google Scholar]
- Mpiana P.T. Determination of Sun Protection Factor (SPF) of Some Body Creams and Lotions Marketed in Kinshasa by Ultraviolet Spectrophotometry. Int. J. Adv. Res. Chem. Sci. 2014;1:7–13. [Google Scholar]
- Nash J.F., Tanner P.R., Matts P.J. Ultraviolet A radiation: testing and labeling for sunscreen products. Dermatol. Clin. 2006;24:63–74. doi: 10.1016/j.det.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Nesseem D. Formulation of sunscreens with enhancement sun protection factor response based on solid lipid nanoparticles. Int. J. Cosmet. Sci. 2011;33:70–79. doi: 10.1111/j.1468-2494.2010.00598.x. [DOI] [PubMed] [Google Scholar]
- Newton-Bishop J.A., Chang Y.-M., Elliott F., Chan M., Leake S., Karpavicius B., Haynes S., Fitzgibbon E., Kukalizch K., Randerson-Moor J., Elder D.E., Bishop D.T., Barrett J.H. Relationship between sun exposure and melanoma risk for tumours in different body sites in a large case-control study in a temperate climate. Eur. J. Cancer. 2011;47:732–741. doi: 10.1016/j.ejca.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobre R., Fonseca A.P. Determination of sun protection factor by Uv-Vis spectrophotometry. Health Care Curr. Rev. 2016;4 [Google Scholar]
- O’Sullivan N.-A., Tait C.P. Tanning bed and nail lamp use and the risk of cutaneous malignancy: a review of the literature. Australas. J. Dermatol. 2014;55:99–106. doi: 10.1111/ajd.12145. [DOI] [PubMed] [Google Scholar]
- Osterwalder U., Herzog B. Sun protection factors: world wide confusion. Br. J. Dermatol. 2009;161:13–24. doi: 10.1111/j.1365-2133.2009.09506.x. [DOI] [PubMed] [Google Scholar]
- Patrician Poh Agin Water resistance and extended wear sunscreens. Dermatol. Clin. 2006;24:75–79. doi: 10.1016/j.det.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Peres D.D., Ariede M.B., Candido T.M., de Almeida T.S., Lourenço F.R., Consiglieri V.O., Kaneko T.M., Velasco M.V.R., Baby A.R. Quality by design (QbD), Process Analytical Technology (PAT), and design of experiment applied to the development of multifunctional sunscreens. Drug Dev. Ind. Pharm. 2017;43:246–256. doi: 10.1080/03639045.2016.1236809. [DOI] [PubMed] [Google Scholar]
- Pirotta, Giulio, 2015. An overview of sunscreen regulations in the world, ResearchGate. https://www.researchgate.net/publication/283515177_An_overview_of_sunscreen_regulations_in_the_world (accessed February 18, 2019).
- Rezende S.G., Dourado J.G., Amorim De Lino F.M., Vinhal D.C., Silva E.C., Gil E.D.S. Methods used in evaluation of the sun protection factor(SPF) of sunscreens. Rev. Eletrônica Farmácia. 2014;11 [Google Scholar]
- Rigel D.S. Cutaneous ultraviolet exposure and its relationship to the development of skin cancer. J. Am. Acad. Dermatol. 2008;58:S129–S132. doi: 10.1016/j.jaad.2007.04.034. [DOI] [PubMed] [Google Scholar]
- Rigel Darrel S. Sunscreens and self-tanners. Cosmeceuticals Cosmet. Sci. 2014:252–260. [Google Scholar]
- Robinson, Julia, 2017. Science of sunscreen. Pharm. J. http://www.pharmaceutical-journal.com/news-and-analysis/infographics/science-of-sunscreen/20203013.article (accessed October 16, 2017).
- Schalka S., Steiner D., Ravelli F.N., Steiner T., Terena A.C., Marçon C.R., Ayres E.L., Addor F.A.S., Miot H.A., Ponzio H., Duarte I., Neffá J., da Cunha J.A.J., Boza J.C., Samorano L.de P., Corrêa M.de P., Maia M., Nasser N., Leite O.M.R.R., Lopes O.S., Oliveira P.D., Meyer R.L.B., Cestari T., dos Reis V.M.S., Rego V.R.P.de A. Brazilian consensus on photoprotection. An. Bras. Dermatol. 2014;89:1–74. doi: 10.1590/abd1806-4841.20143971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider S.L., Lim H.W. Review of environmental effects of oxybenzone and other sunscreen active ingredients. J. Am. Acad. Dermatol. 2019;80:266–271. doi: 10.1016/j.jaad.2018.06.033. [DOI] [PubMed] [Google Scholar]
- Schüz N., Eid M. Int. Encycl. Soc. Behav. Sci. Elsevier; 2015. Sun exposure and skin cancer prevention; pp. 696–700. [DOI] [Google Scholar]
- Serpone N., Dondi D., Albini A. Inorganic and organic UV filters: their role and efficacy in sunscreens and suncare products. Inorganica Chim. Acta. 2007;360:794–802. [Google Scholar]
- Smaoui Slim. Cosmetic emulsion from virgin olive oil: Formulation and bio-physical evaluation. Afr. J. Biotechnol. 2012;11 [Google Scholar]
- Smaoui S., Ben Hlima H., Ben Chobba I., Kadri A. Development and stability studies of sunscreen cream formulations containing three photo-protective filters. Arab. J. Chem. 2017;10:S1216–S1222. [Google Scholar]
- Smith R. Core EU Legis. Macmillan Education UK; London: 2015. Regulation (EC) No 764/2008 of the European Parliament and of the Council; pp. 183–186. [DOI] [Google Scholar]
- Soter N.A. Acute effects of ultraviolet radiation on the skin. Semin. Dermatol. 1990;9:11–15. [PubMed] [Google Scholar]
- C. for D.E. and FDA, Sun Protection Factor (SPF), 2017. https://www.fda.gov/aboutfda/centersoffices/officeofmedicalproductsandtobacco/cder/ucm106351.htm (accessed October 19, 2017).
- Sudhahar, V., Balasubramanian, V., 2013. Sun production factor (SPF) determination of marketed sunscreen formulation by In-Vitro method using UV-VIS spectrophotometer, 4.
- Sunscreen Drug Products for Over-the-Counter Human Use, 2001. Final Monograph; Partial Stay; Final Rule, Fed. Regist. https://www.federalregister.gov/documents/2001/12/31/01-32086/sunscreen-drug-products-for-over-the-counter-human-use-final-monograph-partial-stay-final-rule (accessed May 19, 2019).
- Surdu S., Fitzgerald E.F., Bloom M.S., Boscoe F.P., Carpenter D.O., Haase R.F., Gurzau E., Rudnai P., Koppova K., Févotte J., Leonardi G., Vahter M., Goessler W., Kumar R., Fletcher T. Occupational exposure to ultraviolet radiation and risk of non-melanoma skin cancer in a multinational European study. PLoS ONE. 2013;8:e62359. doi: 10.1371/journal.pone.0062359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svarc, Federico, 2015. A brief illustrated history on sunscreens and sun protection, ResearchGate. https://www.researchgate.net/publication/283002256_A_brief_illustrated_history_on_sunscreens_and_sun_protection (accessed February 16, 2019).
- Svobodova A., Walterova D., Vostalova J. Ultraviolet induced alteration to the skin. Biomed. Pap. 2006;150:25–38. doi: 10.5507/bp.2006.003. [DOI] [PubMed] [Google Scholar]
- Tanner P.R. Sunscreen product formulation. Dermatol. Clin. 2006;24:53–62. doi: 10.1016/j.det.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Trivedi M., Murase J. Titanium dioxide in sunscreen. In: Janus M., editor. Appl Titan. Dioxide. InTech; 2017. [Google Scholar]
- Tuchinda C., Lim H.W., Osterwalder U., Rougier A. Novel emerging sunscreen technologies. Dermatol. Clin. 2006;24:105–117. doi: 10.1016/j.det.2005.09.003. [DOI] [PubMed] [Google Scholar]
- U.D. of H. and H. Services, The Surgeon General’s Call to Action to Prevent Skin Cancer., Office of the Surgeon General (US), 2014. https://www.ncbi.nlm.nih.gov/books/NBK247176/ (accessed May 18, 2019). [PubMed]
- Urbach F. The historical aspects of sunscreens. J. Photochem. Photobiol. B. 2001;64:99–104. doi: 10.1016/s1011-1344(01)00202-0. [DOI] [PubMed] [Google Scholar]
- Walters C., Keeney A., Wigal C.T., Johnston C.R., Cornelius R.D. The spectrophotometric analysis and modeling of sunscreens. J. Chem. Educ. 1997;74:99. [Google Scholar]
- WHO, 2006. WHO | Solar ultraviolet radiation: Global burden of disease from solar ultraviolet radiation, WHO. http://www.who.int/uv/publications/solaradgbd/en/ (accessed November 16, 2017).
- WHO, 2017. WHO | Artificial tanning devices: public health interventions to manage sunbeds, WHO. http://www.who.int/uv/publications/artificial-tanning-devices/en/ (accessed November 16, 2017).
- Xu S., Kwa M., Agarwal A., Rademaker A., Kundu R.V. Sunscreen product performance and other determinants of consumer preferences consumer evaluations of commercial sunscreen consumer evaluations of commercial sunscreen. JAMA Dermatol. 2016;152:920–927. doi: 10.1001/jamadermatol.2016.2344. [DOI] [PubMed] [Google Scholar]