Abstract
Antimicrobial peptides in recent years have gained increased interest among scientists, health professionals and the pharmaceutical companies owing to their therapeutic potential. These are low molecular weight proteins with broad range antimicrobial and immuno modulatory activities against infectious bacteria (Gram positive and Gram negative), viruses and fungi. Inability of micro-organisms to develop resistance against most of the antimicrobial peptide has made them as an efficient product which can greatly impact the new era of antimicrobials. In addition to this these peptides also demonstrates increased efficacy, high specificity, decreased drug interaction, low toxicity, biological diversity and direct attacking properties. Pharmaceutical industries are therefore conducting appropriate clinical trials to develop these peptides as potential therapeutic drugs. More than 60 peptide drugs have already reached the market and several hundreds of novel therapeutic peptides are in preclinical and clinical development. Rational designing can be used further to modify the chemical and physical properties of existing peptides. This mini review will discuss the sources, mechanism and recent therapeutic applications of antimicrobial peptides in treatment of infectious diseases.
Keywords: Antimicrobial peptides, Antibiotic resistance, Therapeutic drugs, Infectious diseases, Clinical trials, Immuno modulatory activities
1. INTRODUCTION
Antimicrobial Peptides (AMPs) are low molecular weight proteins with broad spectrum antimicrobial and immuno modulatory activities against infectious bacteria (Gram positive and Gram negative), viruses and fungi [1]. These antimicrobial peptides have been classified according to their physiochemical properties like net charge, secondary structural contents and solubility [2]. AMPs contain both hydrophobic and hydrophilic side chain that enables these molecules to be soluble in aqueous environments [3]. Amongst the most abundant and widespread AMPs in nature, the cationic alpha-helical AMPs are able to perturb the bacterial cytoplasmic membrane causing cell death by osmotic shock. Some of the most important class of AMPs in these groups are cecropin, magainin, the human cathelicidin LL-37, their derivatives and proline rich Antimicrobial Peptides (prAMPs) [4-12]. Besides cationic AMPs, anionic AMPs have also been described [13]. One positive feature of AMPs is their low propensity towards developing resistance which may be attribute to its distinguished mode of action on the plasma membrane, where it natively folds into three dimensional amphiphilic structure that causes bacterial cell disruption, as documented in previous studies [14, 15]. These peptides initially interact with the bacterial cell envelope, and later translocates to cytosol [16, 17]. And unlike common antibiotics, AMPs do not inhibit peptidoglycan synthesis by binding with proteins; rather create pores in membrane forming complex with precursor molecule present in the membrane [18, 19]. Antimicrobial peptides are diverse group of proteins divided into many subgroups on the basis of their amino acid composition and structure and other properties [20, 21]. The secondary structures of these short peptide may have four architectures that include i) α-helical, ii) β-stranded due to the presence of 2 or more disulfide bonds, iii) β-hairpin or loop due to the presence of a single disulfide bond and/or cyclization of the peptide chain, and iv) extended [22]. AMPs participate in the innate immune response by providing rapid first line defence against infection [1]. This review will focus and report various sources of antimicrobial peptides, variety of structure, mechanisms of action and therapeutic interventions of antimicrobial peptides.
2. SOURCES OF AMPS
Discovery of AMPs dates back to 1939, when Dubos extracted an antimicrobial agent from a soil Bacillus strain. After that several AMPs have been discovered from both the prokaryotes and eukaryotes [23-25], frog skin alone is source of more than 300 different AMPs [24]. Recently, scientists from Japan have successfully explained the molecular mechanism of antimicrobial peptides, Bombinins H2 and H4 discovered from skin secretions of frog species Bombina variegate. Both these antimicrobial peptides have shown promising ability to inhibit highly infectious and fatal disease called Leishmaniasis. Mechanism of action of these peptides will enable us to better understand how defence system of the frog has evolved and how this can be implemented in developing antimicrobial peptides against important microbial infections. It was reported that Leishmania affect almost 20 million people worldwide and is the cause of approximately 30,000 deaths each year [26]. Other AMPs discovered are defensin from rabbit leukocytes [27], lectoferrin from cow milk [28], lysosomes of human leukocytes [29] and low molecular weight antimicrobial peptide from human female reproductive tract [30]. Several Bacillus strains producing antimicrobial peptides have been identified which have shown promising inhibitory activity against Shigella, Salmonella, E. coli and Staphylococcus aureus [31-34]. In another study, an antimicrobial peptide reported from Bacillus sp. [31, 32] found to be active against Staphylococcus aureus, Alteromonas sp. strain CCSH174 and Klebsiella pneumoni. An extracellular antimicrobial peptide has also been discovered from Propionibacterium jensenii [35]. Antimicrobial peptides isolated from Pseudomonas [33] showed activity against Shigella, Salmonella, E. coli, Staphylococcus aureus. Another peptide which as shown high inhibitory activity against bio film was discovered in year 2015 [36].
Researchers have also modified lactoferrin of bovine at the N-terminal domain that demonstrated high activity against multidrug-resistant bacteria and Candida [37]. Antimicrobial Peptide Database (APD) contains entry of ~2981 antimicrobial peptides from six kingdoms (335 bacteriocins/peptide antibiotics from bacteria, 4 from archaea, 8 from protists, 13 from fungi, 342 from plants, and 2200 from animals, including some synthetic peptides, in total, more than 5,000 AMPs have been discovered or synthesized till date [38]. More recently, glycocin, a small antimicrobial peptide was discovered from thermophilic bacterium which was found to be stable at relatively high temperatures, the gene encoding glycocin was successfully transformed in E. coli bacterium [39]. Table 1 further summarizes discovery of various AMPs from variety of organisms [40-88].
Table1. List of antimicrobial peptides from different sources.
| AMPs from Insects | |||||
|---|---|---|---|---|---|
| S. No. | Peptide Name | Source | Amino Acid Number | Antimicrobial Activity | References |
| 1 | Acaloleptin | Acalolepta luxuriosa | 71 | G+, G- | [40] |
| 2 | Andropin | Drosophila melanogaster | 34 | G+ | [41] |
| 3 | Apidaecin IA | Apis mellifera | 18 | G- | [42] |
| 4 | Cecropin | Hyalophora cecropia | 37 | G- | [43] |
| 5 | Defensin- α | Aedes aegypti | 40 | G+, G- | [44] |
| 6 | Drosomycin | Drosophila melanogaster | 44 | F | [45] |
| 7 | Holotricin | Holotrichia diomphalia | 43 | G+, G- | [46] |
| 8 | Sapecin- α | Sarcophaga peregrine | 40 | G+, G- | [47] |
| 9 | Tenicin 1 | Tenebrio molitor | 43 | G+, G- | [48] |
| 10 | Thanatin | Podisus maculiventris | 21 | G+, G- | [49] |
| From Humans | |||||
| 1 | Cathelicidins | Human neutrophils | 30 | F, G-, G+ | [50] |
| 2 | Α Defensins | Human neutrophils | 12-80 | F, G-, G+ | [51] |
| 3 | Human Histatin 8 | Homo sapiens | 12 | F, G-, G+ | [52] |
| 4 | LL37 | Neutrophils (Homo sapiens) | 37 | F, G-, G+ | [53] |
| From Animals | |||||
| 1 | Androctonin | Androctonus australis | 25 | F, G-, G+ | [54] |
| 2 | Bactenecin | Bovine Neutrophils | 12 | G-, G+ | [55] |
| 3 | Brevinin | Rana brevipora porsa | 24 | G-, G+ | [56] |
| 4 | Buforin II | Bufo bufo gargarizans | 21 | F, G-, G+ | [57] |
| 5 | Cupiennin | Cupiennius salei | 35 | G-, G+ | [58] |
| 6 | Dermaseptin S1 | Phyllomedusa sauvagii | 34 | G-, G+ | [59] |
| 7 | Lycotoxin | Lycosa carolinensis | 27 | G-, G+ | [60] |
| 8 | Tachyplesins | Tachypleus tridentatus (Horseshoe crab) | 17 | G- | [61] |
| From Plants | |||||
| 1 | Hevein | Latex of rubber trees | 43 | F | [62] |
| 2 | Purothionins | Wheat endosperm | 45 | G+, G- | [63] |
| From Microorganisms | |||||
| 1 | Nisin | Lactococcus lactis | 34 | G+ | [64] |
| 2 | Alamethicin | Trichoderma viride | 20 | G+ | [65] |
| 3 | Enterocin | Enterococcus | 70 | G+, G- | [66] |
| 4 | Hominicin | Staphylococcus hominis MBBL 2-9 | 21 | G+, G- | [67] |
| 5 | Ericin S | Bacillus subtilis | 32 | G+ | [68] |
| 6 | Plantaricin A | Lactobacillus plantarum | 26 | G+, G- | [69] |
| 7 | Carnobacteriocin B2 | Carnobacterium piscicola | 48 | G+, G- | [70] |
| S. No. | Peptide Name | Source | Amino Acid Number | Antimicrobial Activity | References |
| 8 | Leucocin A | Leuconostoc pseudomesenteroides | 37 | G+, G- | [71] |
| 9 | Subtilin | Bacillus subtilis | 32 | G+ | [72] |
| 10 | Pyrularia thionin | Pyrularia pubera | 47 | G+, G- | [73] |
| 11 | Microcin J25 | Escherichia coli AY25 | 21 | G- | [74] |
| 12 | Gramicidin A | Bacillus brevis | 15 | G+, G- | [75] |
| 13 | Pediocin PA-1/ AcH | Pediococcus acidilactici PAC-1.0 | 44 | G+ | [76] |
| 14 | Mesentericin Y105 | Leuconostoc mesenteroides | 37 | G+ | [77] |
| 15 | Carnobacteriocin BM1 | Carnobacterium piscicola LV17B | 43 | G+, G- | [78] |
| 16 | Streptin 1 | Bacillus subtilis A1/3 | 23 | G+ | [79] |
| 17 | Planosporicin | Planomonospora alba | 24 | G+, G- | [80] |
| 18 | Gassericin A | Lactobacillus gasseri LA39 | 58 | G+, G- | [81] |
| 19 | Circularin A | Clostridium beijerinckii ATCC 25752 | 69 | G+, G- | [82] |
| 20 | Divercin V41 | Carnobacterium divergens V41 | 43 | G+ | [83] |
| 21 | Listeriocin 743A | Listeria innocua 743 | 43 | G+ | [84] |
| 22 | Plantaricin C19 | Lactobacillus plantarum C19 | 37 | G+ | [85] |
| 23 | Enterocin P | Enterococcus faecium P13 | 44 | G+ | [86] |
| 24 | Subtilosin A | Bacillus subtilis | 35 | G+, G- | [87] |
| 25 | Plantaricin ASM1 | Lactobacillus plantarum A-1 | 43 | G+ | [85] |
| 26 | Lichenin | Bacillus licheniformis | 12 | G+, G- | [88] |
F – Fungus; G+ - Gram positive; G- - Gram negative.
3. INSIGHTS INTO MECHANISM OF ACTION OF AMPS
Antimicrobial peptides interact with bacterial cell membrane through electrostatic interactions [89] thus making it difficult for bacteria to develop resistance unlike conventional antibiotics [90]. Based on their mode of action, these peptides are classified into membrane acting and non membrane acting peptides. Membrane acting peptides mainly harbour cationic peptides causing membrane disruptions, whereas non membrane peptides are capable of translocation across the membrane without damaging it as also reviewed in previous study [91]. Few antibacterial peptides create trans-membrane pores on the target membrane and include defensin [92], melittin [93], againins [94], and LL-37 [95]. Antimicrobial peptides such as buforin II [82], dermaseptin [96], HNP-1 [97], pleurocidin [98], indolicidin [99], pyrrhocidin [100], and mersacidin [101] these peptides translocates across the cell membrane and disrupt normal cell functioning [102]. Outer membrane of prokaryotic cell is negatively charged owing to presence of lipopolysaccharides or teichoic acid, whereas the outer leaflet of eukaryotic cell consists of zwitterionic phos-phatidylcholine and sphingomyelin phospholipids. Cationic AMPs interact with negatively charged outer microbial membranes via selective interactions [103], and attain well-define secondary structures, makes cell permeable and finally disrupt bacterial membranes [15]. These peptides show dynamics in structure and topologies during their interactions with the microbial cell membranes [104, 105].
AMPs also hamper processes like protein synthesis, nucleic acid synthesis, enzymatic activities, and cell wall synthesis [106-108]. Several factors that include magnitude and charge of the outer membrane, concentration of negatively charged molecules, molecular architecture, and membrane fluidity are essential for the transportation of peptide across the membrane [109]. The membrane fluidity also regulates adsorption and insertion of AMPs into the cell membrane. Malanovic and Lohnerin in year (2016) studied antimicrobial peptides against Gram positive bacteria and found that prior targeting the cytoplasmic membrane these peptides cross the cell wall components such as lipoteichoic acids and peptidoglycan [110]. It was established that highly conserved precursors of cell wall components, especially lipid II are directly targeted by AMPs [111]. Majority of these antimicrobial peptides fold into amphipathic conformations while interacting with membrane [112]. Some of the antimicrobial peptides crosses lipid bilayer, target intracellular components, binds DNA, block enzyme activity, inhibit synthesis of proteins, cell wall, and nucleic acids [113, 114]. AMPs thus displays antibacterial efficacy because of intracellular inhibitory mechanisms. Unfortunately, these aspects remain elusive, various models used to explain the AMP mechanism of action on bacterial membrane are Barrel-stave mechanism [115] Carpet model [116] and Toroidal pore model [117] (Figure 1).
Figure 1.
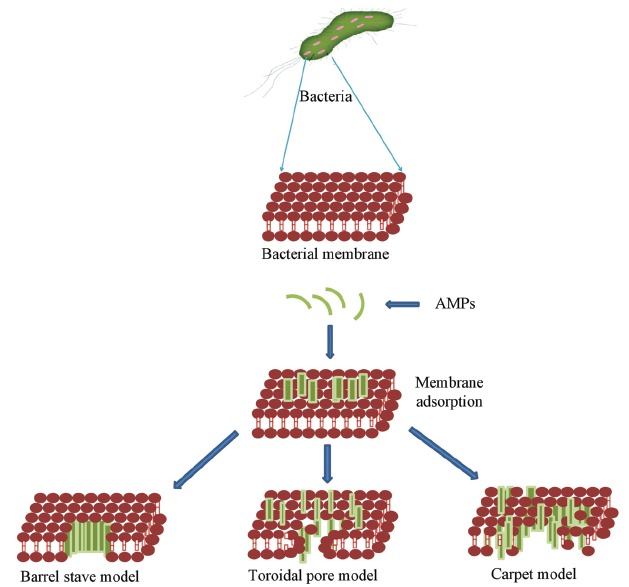
Various models demonstrating mechanism of action of AMPs.
Despite all reported evidences, the mechanism of action and disruption of membranes is not fully understood. Some examples of antimicrobial peptides showing intracellular activities are reported in Table 2 [118-121].
Table 2. Antimicrobial peptide displaying intracellular membrane activities.
| S. No. | AMPs | Intracellular Target | References |
|---|---|---|---|
| 1 | Buforin II, tachyplesin | Binds to DNA | [118] |
| 2 | Pleurocidin, dermaseptin, PR-39, HNP-1, HNP-2, Indolicidin |
Inhibits DNA, RNA and protein synthesis |
[119] |
| 3 | Histatins, pyrrhocoricin, Drosocin, Apidaecin |
Inhibits enzymatic activity | [18] |
| 4 |
N-acetylmuramoyl-L-alanine Amidase |
Activation of autolysin | [120] |
| 5 | PR-39, PR-26, indolicidin, microcin 25 |
Alters cytoplasmic membrane (inhibits septum formation) |
[121] |
| 6 | Mersacidin | Inhibits cell-wall Synthesis | [100] |
4. APPLICATIONS OF AMPS
4.1. Application in Ophthalmology
AMPs are being employed in ophthalmology, a previous study reported use of rabbit alpha defensin (NP-1) against several ocular infections [122]. Recently, a cecropin analogue, Hecate has also shown inhibitory action against many Acanthamoeba species in vitro. Among other tested cecropin analogues SHIVA-11 is widely used against various ocular infections [123]. Table 3 further summarizes examples of peptides and their activity against relevant pathogens in ocular infections.
Table 3. Inhibitory activity of antimicrobial peptides and proteins against relevant pathogens in ocular infections.
| Protein/Peptide | Microorganisms | References |
|---|---|---|
| HB43, HB55, HBPM4 | Staphylococcus aureus | [130] |
| HBCM2, HBCM3, HB14 | Pseudomonas aeruginosa | [131] |
| Lactoferrin | Haemophilus influenzae, Staphylococcus, epidermidis, Pseudomonas spp. | [132] |
| Lactoferricin B | Aspergillus fumigatus, Candida albicans | [14] |
| Mucins | Candida spp., P. aeruginosa | [43, 133] |
| NP-1 | C. albicans, Streptococcus pneumoniae, P. aeruginosa | [134] |
| Protegrin-1 | S. aureus, P. aeruginosa | [135] |
| Shiva-11 | S. aureus, S. pneumoniae, P. aeruginosa | [136] |
| Thiazomycin A | S. aureus | [115] |
| COL-1 | Pseudomonas | [137] |
4.2. Treatment of Local Infections
Several peptides have been used in treating local infections, a peptide NEUPREX (rBPI21, opebacan) is
injectable preparation of rBPI21 used in treatment of paediatric patients undergoing open heart surgery and patients with severe burns [124]. A recombinant peptide HBD-2 is being used in eliminating the infections attained during the use of prosthetics implantation [125]. Peptides derived from amphibian skin e.g. alyteserin, brevinin, ascaphin, pseudin, kassinatuerin and temporin have been effectively used in the treatment of local infections caused by multi-drug resistant strains of bacteria e.g. Acinetobacter baumannii strains, Klebsiella pneumoniae, Escherichia coli, Staphylococcus aureus, Pseudomonas aeruginosa and Candida sp. [126]. P113 is another peptide naturally occurring in saliva [10], which display high in vitro activity against Candida albicans and commonly occurring Gram-positive and Gram-negative pathogens, it is also being used in the form of a mouthwash for the treatment of oral Candidiasis in HIV patients [127]. Pexiganan is first antimicrobial peptide used in the form of an ointment for treating local infection encountered during diabetic foot ulcers [128]. Variants of indolicidin-based peptide, MX-226 and MX-594AN (omiganan pentahydrochloride, 1% gel) are being used in treating infections associated with the use of catheters and against treatment of Acne vulgaris respectively [129]. Another peptide MBI-853NL is being used in preventing infection associated with Methicillin-Resistant Strains (MRSA), it also eliminates their carriage to nasal cavity [122]. IB-367 is a variant of porcine protegrin-1 which is used in treatment of oral mucositis, a side-effect of anticancer therapies with ‘mixed’ infections in the mouth.
5. AMPS IN CLINICAL TRIALS
Due to numerous advantages of antimicrobial peptides such as high potency, efficacy selectivity, broad range targets, potentially low toxicity and low accumulation in tissues, pharmaceutical industries aims to develop them as therapeutic drugs and appropriate clinical trials therefore are
being conducted [138]. More than 60 peptide drugs have reached the market and several hundred novel therapeutic peptides are in preclinical and clinical development [139] (Table 4). Emerging peptide technologies, including multifunctional peptides, cell penetrating peptides and peptide drug conjugates will widen the application of peptides as therapeutics [140]. United States of America dominates the global production and commercialization of peptide drug followed by Europe. Companies like Theravance and Vicuron Pharmaceuticals are the major American companies largely dedicated in the development of peptide antibiotics [141]. However, certain peptides could not have entered clinical trials, MSI-78 (pexiganan acetate, which is a potent antimicrobial peptide designed from Magainin) was a prominent failure that has entered phase III of clinical trials and showed efficacy against diabetic foot ulcer infections [43], however, in July 1999, the FDA disapproved use of Magainin based on inadequacy trial design. Iseganan IB-367, a synthetic protegrin analogue failed Phase III clinical trials as mouth rinse for stomatitis in high risk patients owing to aerosolized Rx in ventilator-associated pneumonia [142]. The trial achieved its secondary endpoint for reduction of pain but did not meet the primary end point for presence of ulceration. Nevertheless, this trial continues to enroll patients for a second phase III trial [143]. Micrologix Biotech Inc. has introduced 3 separate antimicrobial peptides related to indolicidin into clinical trials [144]. The most advanced peptide MBI-226 [115] has entered phase III clinical trials for preventing catheter-related bloodstream infections. According to company press releases and conference presentations, preclinical studies demons-trated that MBI-226 is effective in animal models, it has successfully reduced the skin colonization by variety of bacteria causing catheter-related infections [145], and also demonstrated good antifungal activity against Candida albicans in guinea pig skin [146]. A randomized, double-blind phase I clinical trial in 18 healthy volunteers demonstrated that MBI-226 was safe, well tolerated and eliminated 99.9% of common skin bacteria for prolonged periods [147]. Furthermore, it completely prevented short-term Central Venous Catheter (CVC) colonization Since CVC colonization is a common cause of serious life-threatening infections in hospitalized patients, causing 90% (180,000/year) of bloodstream infections that results in an average of 6.5 additional days of intensive care and up to 50,000 deaths annually [148], Micrologix received fast track status from the FDA and initiated two clinical trials using indolicidin-like peptides for treatment of acute acne (in phaseII clinical trials) and killing MRSA in the nares (in phase Ib trials) [149, 150].
Table 4. Peptide antibiotics in clinical trials.
| Peptide | Company | Clinical Trial Phase | Spectrum/Mode of Action | References |
|---|---|---|---|---|
| CZEN-002 | Zengen | Phase I/II | GPB, GNP, Candida. Yeast regulatory mechanisms, Interference by cAMP induction, anti-inflammatory. | [155] |
| Daptomycin | Cubicin | In market | GPB. Depolarisation of membrane potential, inhibition of protein, DNA and RNA synthesis. | [156] |
| EA-230 | Exponential biotherapies | Phase I/II | Anti-inflammatory. Sepsis and renal failure protection. | [157] |
| Pexiganan (MSI-78) | Genaera Corporation | Phase III | Infected diabetic foot ulcers | [158] |
| Omiganan | MIGENIX | Phase II/III | Catheter infections and rosacea | [159, 160] |
| Lytixar (LTX-109) | Lytix Biopharma | Phase I/II | Uncomplicated Gram positive skin infections, impetigo,and nasal colonization with S. aureus | [130] |
| hlF1-11 | AM-Pharma | Phase I/II | Bacteraemia and fungal infections in immunocompromized haematopoetic stem cell transplant recipients | [161] |
| Novexatin (NP-213) | NovaBiotics | Phase II | Onychomycosis (fungal nail infection) | [162] |
| LL-37 | Karolinska Institute | Phase I/II | Hard-to-heal venous leg ulcers | [163] |
| PAC-113 | Demegen | Phase II | Oral candidiasis in HIV seropositive patients | [130] |
| RDP-58 | Genzyme | Post Phase II | Inflammatory bowel disease | [164] |
| MX-594AN | MIgenix | Phase II | Topical treatment for Acne vulgaris | [165] |
| MX-226 | Migenix | Phase III b | Dermatology related infections | [153] |
| HB-1345 | BioMedix | Pre-Phase I | Acne | [166] |
| HB-107 | Biopharmaceuticals | Preclinical | Wound healing | [167] |
| Glutoxim | Pharma BAM | Phase II | Tuberculosis | [168] |
| IMX942 | Inimex | Phase I A | Immunomodulation, Treatment of fevers in chemotherapy patients | [158] |
| DPK-060 | Promore Pharma | Phase II | Treatment of atopic dermatitis | [169] |
| POL7080 | Polyphor Ltd | Phase II | Treatment of non-cystic fibrosis bronchiectasis | [170] |
| SB006 | SpiderBiotech (Italy) | Preclinical | Antiendotoxic activity | [171] |
| PL-5 | China Food and Drug Administration (CFDA) | Phase II | Treatment of skin infections | [172] |
All over, use of AMPs have proven to be successful in treating infections, in fact, antimicrobial peptide have already entered the global market e.g. magainin is being used in treating viral and bacterial diseases [151, 152]. Several studies carried out globally by researchers have provided in depth understanding about antimicrobial peptides mechanism, efficacy, safety, and other related concern and has helped in creating online database service as well as the future potential of AMPs [22, 153, 154]. Incontestable need for new ways to manage infections and the proven importance of peptides in innate immunity should render the investment worthwhile for human medicine. The research investments are needed to bring more peptide antibiotics to the clinic will likely remain substantial in the foreseeable future. As described below, a number of AMPs and AMP derivatives are already at the pre-clinical stage and in clinical trials.
CONCLUSION
Antimicrobial resistance is multifaceted, multi-dimensional, and is second largest cause of deaths in the world. Both the Gram positive and Gram negative bacteria are getting refractory to current armamentarium of antimicrobial drugs. Treatment of bacterial infections caused by MDR strains that include vancomycin-resistant Enterococcus faecium, Enterobacter cloacae (MRSA), XDR strains that include carbapenem-resistant Acinetobacter baumannii, and third generation cephalosporin resistant E. coli, β-lactamase producing Klebsiella pneumonia, carbapenem-resistant Klebsiella pneumoniae, carbapenem-resistant Pseudomonas aeruginosa and Mycobacterium has become quite difficult. All living organisms are constantly threatened by large numbers of microorganisms seeking to exploit the same environmental space. To cope with this substantial microbial threat, most cells produce natural antibiotic like molecules that directly kill or inhibit the growth of foreign microorganisms. The urgent need to obtain new antimicrobials has been driving AMP research. In this respect, AMPs are considered as promising antimicrobial agents for producing new generation antimicrobials. Although there are several obstacles to be overcome for clinical applications, natural and synthetic AMPs are still attractive sources to the pharmaceutical companies. In order to facilitate commercial development of peptide antibiotics, it is reasonable to focus on small peptides.
ACKNOWLEDGEMENTS
Declared none.
List of Abbreviations
- AMPs
Antimicrobial Peptides
- E. coli
Escherichia coli
- APD
Antimicrobial Peptide Database
- sp.
Species
- prAMPs
proline rich Antimicrobial Peptides
- F
Fungus
- G+
Gram positive
- G-
Gram negative
- DNA
Deoxyribonucleic Acid
- RNA
Ribonucleic Acid
- e.g.
exempli gratia
- HIV
Human Immunodeficiency Virus
- MRSA
Methicillin-Resistant Staphylococcus aureus
- P. aeruginosa
Pseudomonas aeruginosa
- C. albicans
Candida albicans
- S. aureus
Staphylococcus aureus
- S. pneumonia
Streptococcus pneumoniae
- FDA
Food and Drug Administration
- CVC
Central Venous Catheter
- cAMP
Cyclic Antimicrobial peptides
- MDR
Multidrug-Resistant
- XDR
Extensive Drug Resistant
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Bardan A., Nizet V., Gallo R.L. Antimicrobial peptides and the skin. Expert Opin. Biol. Ther. 2004;4(4):543–549. doi: 10.1517/14712598.4.4.543. [http://dx.doi.org/10.1517/14712598.4.4.543]. [PMID: 15102603]. [DOI] [PubMed] [Google Scholar]
- 2.Elias P.M., Choi E.H. Interactions among stratum corneum defensive functions. Exp. Dermatol. 2005;14(10):719–726. doi: 10.1111/j.1600-0625.2005.00363.x. [http://dx.doi.org/10.1111/j.1600-0625.2005.00363.x]. [PMID: 16176279]. [DOI] [PubMed] [Google Scholar]
- 3.Bahar A.A. Controlling biofilm and persister cells by targeting cell membranes. Dissertations - ALL. 2015 [Google Scholar]
- 4.Hancock R.E., Rozek A. Role of membranes in the activities of antimicrobial cationic peptides. FEMS Microbiol. Lett. 2002;206(2):143–149. doi: 10.1111/j.1574-6968.2002.tb11000.x. [http://dx.doi.org/10.1111/j.1574-6968.2002.tb11000.x]. [PMID: 11814654]. [DOI] [PubMed] [Google Scholar]
- 5.Kuroda K., Okumura K., Isogai H., Isogai E. The human cathelicidin antimicrobial peptide LL-37 and mimics are potential anticancer drugs. Front. Oncol. 2015;5:144. doi: 10.3389/fonc.2015.00144. [http://dx.doi.org/10.3389/fonc.2015.00144]. [PMID: 26175965]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun E., Belanger C.R., Haney E.F., Hancock R.E. Host defense (antimicrobial) peptides. In: Koutsopoulos S., editor. Peptide Applications in Biomedicine, Biotechnology and Bioengineering. Sawston, United Kingdom: Woodhead Publishing; 2018. pp. 253–285. [https://doi.org/10.1016/C2015-0-02002-0] [Google Scholar]
- 7.Kosikowska P., Lesner A. Antimicrobial peptides (AMPs) as drug candidates: a patent review (2003-2015). Expert Opin. Ther. Pat. 2016;26(6):689–702. doi: 10.1080/13543776.2016.1176149. [http://dx.doi.org/10.1080/13543776.2016.1176149]. [PMID: 27063450]. [DOI] [PubMed] [Google Scholar]
- 8.Haney E.F., Mansour S.C., Hancock R.E. Antimicrobial peptides: an introduction. Methods Mol. Biol. 2017;1548:3–22. doi: 10.1007/978-1-4939-6737-7_1. [http://dx.doi.org/10.1007/978-1-4939-6737-7_1]. [DOI] [PubMed] [Google Scholar]
- 9.Segev-Zarko L.A., Mangoni M.L., Shai Y. Antimicrobial peptides: multiple mechanisms against a variety of targets. In: Wang G., editor. Antimicrobial Peptides: Discovery, Design and Novel Therapeutic Strategies. UK: CAB Inhternational; 2017. p. 119. [Google Scholar]
- 10.Haney E.F., Pletzer D., Hancock R.E. Recent Clinical Techniques, Results, and Research in Wounds. Cham: Springer; 2018. Impact of Host Defense Peptides on Chronic Wounds and Infections. pp. 1–17. [http://dx.doi.org/10.1007/15695_2017_88] [Google Scholar]
- 11.Li W., Tailhades J., O’Brien-Simpson N.M., Separovic F., Otvos L., Jr, Hossain M.A., Wade J.D. Proline-rich antimicrobial peptides: potential therapeutics against antibiotic-resistant bacteria. Amino Acids. 2014;46(10):2287–2294. doi: 10.1007/s00726-014-1820-1. [http://dx.doi.org/10.1007/s00726-014-1820-1]. [PMID: 25141976]. [DOI] [PubMed] [Google Scholar]
- 12.Graf M., Mardirossian M., Nguyen F., Seefeldt A.C., Guichard G., Scocchi M., Innis C.A., Wilson D.N. Proline-rich antimicrobial peptides targeting protein synthesis. Nat. Prod. Rep. 2017;34(7):702–711. doi: 10.1039/c7np00020k. [http://dx.doi.org/10.1039/C7NP00020K]. [PMID: 28537612]. [DOI] [PubMed] [Google Scholar]
- 13.Harris F., Dennison S.R., Phoenix D.A. Anionic antimicrobial peptides from eukaryotic organisms. Curr. Protein Pept. Sci. 2009;10(6):585–606. doi: 10.2174/138920309789630589. [http://dx.doi.org/10.2174/138920309789630589]. [PMID: 19751192]. [DOI] [PubMed] [Google Scholar]
- 14.Nelson K.E., Fleischmann R.D., DeBoy R.T., Paulsen I.T., Fouts D.E., Eisen J.A., Daugherty S.C., Dodson R.J., Durkin A.S., Gwinn M., Haft D.H., Kolonay J.F., Nelson W.C., Mason T., Tallon L., Gray J., Granger D., Tettelin H., Dong H., Galvin J.L., Duncan M.J., Dewhirst F.E., Fraser C.M. Complete genome sequence of the oral pathogenic Bacterium Porphyromonas gingivalis strain W83. J. Bacteriol. 2003;185(18):5591–5601. doi: 10.1128/JB.185.18.5591-5601.2003. [http://dx.doi.org/10.1128/JB.185.18.5591-5601.2003]. [PMID: 12949112]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Da Costa J.P., Cova M., Ferreira R., Vitorino R. Antimicrobial peptides: an alternative for innovative medicines? Appl. Microbiol. Biotechnol. 2015;99(5):2023–2040. doi: 10.1007/s00253-015-6375-x. [http://dx.doi.org/10.1007/s00253-015-6375-x]. [PMID: 25586583]. [DOI] [PubMed] [Google Scholar]
- 16.Santos R.S., Figueiredo C., Azevedo N.F., Braeckmans K., De Smedt S.C. Nanomaterials and molecular transporters to overcome the bacterial envelope barrier: towards advanced delivery of antibiotics. Adv. Drug Deliv. Rev. 2018;136-137:28–48. doi: 10.1016/j.addr.2017.12.010. [https://doi.org/10.1016/j.addr.2017.12.010]. [PMID: 29248479]. [DOI] [PubMed] [Google Scholar]
- 17.Sun E., Belanger C.R., Haney E.F., Hancock R.E. Overview of host defense peptides. In: Koutsopoulos S., editor. Peptide Applications in Biomedicine, Biotechnology and Bioengineering. Sawston, United Kingdom: Woodhead Publishing; 2018. p. 253. [Google Scholar]
- 18.Le C.F., Fang C.M., Sekaran S.D. Intracellular targeting mechanisms by antimicrobial peptides. Antimicrob. Agents Chemother. 2017;61(4):e02340–e16. doi: 10.1128/AAC.02340-16. [http://dx.doi.org/10.1128/AAC.02340-16]. [PMID: 28167546]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kumar P., Kizhakkedathu J.N., Straus S.K. Antimicrobial peptides: diversity, mechanism of action and strategies to improve the activity and biocompatibility in vivo. Biomolecules. 2018;8(1):4. doi: 10.3390/biom8010004. [http://dx.doi.org/10.3390/biom8010004]. [PMID: 29351202]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Travkova O.G., Moehwald H., Brezesinski G. The interaction of antimicrobial peptides with membranes. Adv. Colloid Interface Sci. 2017;247:521–532. doi: 10.1016/j.cis.2017.06.001. [http://dx.doi.org/10.1016/j.cis.2017.06.001]. [PMID: 28606715]. [DOI] [PubMed] [Google Scholar]
- 21.Persico M., Mikhaylin S., Doyen A., Firdaous L., Hammami R., Chevalier M., Flahaut C., Dhulster P., Bazinet L. Formation of peptide layers and adsorption mechanisms on a negatively charged cation-exchange membrane. J. Colloid Interface Sci. 2017;508:488–499. doi: 10.1016/j.jcis.2017.08.029. [http://dx.doi.org/10.1016/j.jcis.2017.08.029]. [PMID: 28865343]. [DOI] [PubMed] [Google Scholar]
- 22.Wang G. Antimicrobial peptides: discovery, design and novel therapeutic strategies. UK: CAB Inhternational; 2017. [http://dx.doi.org/10.1079/9781786390394.0000] [Google Scholar]
- 23.Bednarska N.G., Wren B.W., Willcocks S.J. The importance of the glycosylation of antimicrobial peptides: natural and synthetic approaches. Drug Discov. Today. 2017;22(6):919–926. doi: 10.1016/j.drudis.2017.02.001. [http://dx.doi.org/10.1016/j.drudis.2017.02.001]. [PMID: 28212948]. [DOI] [PubMed] [Google Scholar]
- 24.Conlon B.P., Nakayasu E.S., Fleck L.E., LaFleur M.D., Isabella V.M., Coleman K., Leonard S.N., Smith R.D., Adkins J.N., Lewis K. Activated ClpP kills persisters and eradicates a chronic biofilm infection. Nature. 2013;503(7476):365–370. doi: 10.1038/nature12790. [http://dx.doi.org/10.1038/nature12790]. [PMID: 24226776]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andrä J., Berninghausen O., Leippe M. Cecropins, antibacterial peptides from insects and mammals, are potently fungicidal against Candida albicans. Med. Microbiol. Immunol. (Berl.) 2001;189(3):169–173. doi: 10.1007/s430-001-8025-x. [http://dx.doi.org/10.1007/s430-001-8025-x]. [PMID: 11388616]. [DOI] [PubMed] [Google Scholar]
- 26.Sekiya Y., Shimizu K., Kitahashi Y., Ohyama A., Kawamura I., Kawano R. Electrophysiological analysis of membrane disruption by bombinin and its isomer using lipid bilayer system. ACS Applied Bio Mater. 2019;2(4):1542–1548. doi: 10.1021/acsabm.8b00835. [http://dx.doi.org/10.1021/acsabm.8b00835]. [DOI] [PubMed] [Google Scholar]
- 27.Hirsch J.G. Phagocytin: a bactericidal substance from polymorphonuclear leucocytes. J. Exp. Med. 1956;103(5):589–611. doi: 10.1084/jem.103.5.589. [http://dx.doi.org/10.1084/jem.103.5.589]. [PMID: 13319580]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Groves M.L., Peterson R.F., Kiddy C.A. Poliomorphism in the red protein isolated from milk of individual cows. Nature. 1965;207(5000):1007–1008. doi: 10.1038/2071007a0. [http://dx.doi.org/10.1038/2071007a0]. [PMID: 5886923]. [DOI] [PubMed] [Google Scholar]
- 29.Zeya H.I., Spitznagel J.K. Antibacterial and enzymic basic proteins from leukocyte lysosomes: separation and identification. Science. 1963;142(3595):1085–1087. doi: 10.1126/science.142.3595.1085. [http://dx.doi.org/10.1126/science.142.3595.1085]. [PMID: 14068232]. [DOI] [PubMed] [Google Scholar]
- 30.Sharma S., Sethi S., Prasad R., Samanta P., Rajwanshi A., Malhotra S. Characterization of low molecular weight antimicrobial peptide from human female reproductive tract. Indian J. Med. Res. 2011;134(5):679–687. doi: 10.4103/0971-5916.90996. [https://doi.org/10.4103/0971-5916.90996]. [PMID: 22199108]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mora C., Tittensor D.P., Adl S., Simpson A.G., Worm B. How many species are there on Earth and in the ocean? PLoS Biol. 2011;9(8):e1001127. doi: 10.1371/journal.pbio.1001127. [http://dx.doi.org/10.1371/journal.pbio.1001127]. [PMID: 21886479]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baindara P., Mandal S.M., Chawla N., Singh P.K., Pinnaka A.K., Korpole S. Characterization of two antimicrobial peptides produced by a halotolerant Bacillus subtilis strain SK.DU.4 isolated from a rhizosphere soil sample. AMB Express. 2013;3(1):2. doi: 10.1186/2191-0855-3-2. [http://dx.doi.org/10.1186/2191-0855-3-2]. [PMID: 23289832]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hadinegoro S.R., Arredondo-García J.L., Capeding M.R., Deseda C., Chotpitayasunondh T., Dietze R., Muhammad Ismail H.I., Reynales H., Limkittikul K., Rivera-Medina D.M., Tran H.N., Bouckenooghe A., Chansinghakul D., Cortés M., Fanouillere K., Forrat R., Frago C., Gailhardou S., Jackson N., Noriega F., Plennevaux E., Wartel T.A., Zambrano B., Saville M. CYD-TDV Dengue Vaccine Working Group. Efficacy and long-term safety of a dengue vaccine in regions of endemic disease. N. Engl. J. Med. 2015;373(13):1195–1206. doi: 10.1056/NEJMoa1506223. [http://dx.doi.org/10.1056/NEJMoa1506223]. [PMID: 26214039]. [DOI] [PubMed] [Google Scholar]
- 34.Naimah A.K., Al-Manhel A.J.A., Al-Shawi M.J. Isolation, purification and characterization of antimicrobial peptides produced from Saccharomyces boulardii. Int. J. Pept. Res. Ther. 2018;24(3):455–461. [http://dx.doi.org/10.1007/s10989-017-9632-2]. [Google Scholar]
- 35.Holo H., Faye T., Brede D.A., Nilsen T., Ødegård I., Langsrud T. Bacteriocins of propionic acid bacteria. Lait. 2002;82(1):59–68. [http://dx.doi.org/10.1051/lait:2001005]. [Google Scholar]
- 36.De Zoysa G.H., Cameron A.J., Hegde V.V., Raghothama S., Sarojini V. Antimicrobial peptides with potential for biofilm eradication: synthesis and structure activity relationship studies of battacin peptides. J. Med. Chem. 2015;58(2):625–639. doi: 10.1021/jm501084q. [http://dx.doi.org/10.1021/jm501084q]. [PMID: 25495219]. [DOI] [PubMed] [Google Scholar]
- 37.Mishra S.K., Acharya J., Kattel H.P., Koirala J., Rijal B.P., Pokhrel B.M. Metallo-beta-lactamase producing gram-negative bacterial isolates. J. Nepal Health Res. Counc. 2012;10(22):208–213. [PMID: 23281453]. [PubMed] [Google Scholar]
- 38.Zhu M., Liu P., Niu Z.W. A perspective on general direction and challenges facing antimicrobial peptides. Chin. Chem. Lett. 2017;28(4):703–708. [http://dx.doi.org/10.1016/j.cclet.2016.10.001]. [Google Scholar]
- 39.Kaunietis A., Buivydas A., Čitavičius D.J., Kuipers O.P. Heterologous biosynthesis and characterization of a glycocin from a thermophilic bacterium. Nat. Commun. 2019;10(1):1115. doi: 10.1038/s41467-019-09065-5. [http://dx.doi.org/10.1038/s41467-019-09065-5]. [PMID: 30846700]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vogel H., Badapanda C., Knorr E., Vilcinskas A. RNA-sequencing analysis reveals abundant developmental stage-specific and immunity-related genes in the pollen beetle Meligethes aeneus. Insect Mol. Biol. 2014;23(1):98–112. doi: 10.1111/imb.12067. [http://dx.doi.org/10.1111/imb.12067]. [PMID: 24252113]. [DOI] [PubMed] [Google Scholar]
- 41.Abry M.F., Kimenyi K.M., Masiga D., Kulohoma B.W. Comparative genomics identifies male accessory gland proteins in five Glossina species. Wellcome Open Res. 2017;2:73. doi: 10.12688/wellcomeopenres.12445.1. [http://dx.doi.org/10.12688/wellcomeopenres.12445.2]. [PMID: 29260004]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Farouk A.E., Ahamed N.T., AlZahrani O., Alghamdi A., Bahobail A. (2)23-29. Inducible antimicrobial compounds (Halal) production in Honey Bee Larvae (Apis mellifera) from Rumaida, Taif by injecting of various dead microorganisms extracts. J. Appl. Biol. Biotechnol. 2017;5(02):23–29. [http://dx.doi.org/10.7324/JABB.2017.50204]. [Google Scholar]
- 43.Lee J., Lee D.G. Antimicrobial peptides (AMPs) with dual mechanisms: membrane disruption and apoptosis. J. Microbiol. Biotechnol. 2015;25(6):759–764. doi: 10.4014/jmb.1411.11058. [http://dx.doi.org/10.4014/jmb.1411.11058]. [PMID: 25537721]. [DOI] [PubMed] [Google Scholar]
- 44.Price D.P., Schilkey F.D., Ulanov A., Hansen I.A. Small mosquitoes, large implications: crowding and starvation affects gene expression and nutrient accumulation in Aedes aegypti. Parasit. Vectors. 2015;8(1):252. doi: 10.1186/s13071-015-0863-9. [http://dx.doi.org/10.1186/s13071-015-0863-9]. [PMID: 25924822]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Allocca M., Zola S., Bellosta P. 2018. [Google Scholar]
- 46.Thiyonila B., Reneeta N.P., Kannan M., Shantkriti S., Krishnan M. Dung beetle gut microbes: Diversity, metabolic and immunity related roles in host system. Int. J. Sci. Innovs. 2018;1(2):84–91. [Google Scholar]
- 47.Manabe T., Kawasaki K. D-form KLKLLLLLKLK-NH2 peptide exerts higher antimicrobial properties than its L-form counterpart via an association with bacterial cell wall components. Sci. Rep. 2017;7:43384. doi: 10.1038/srep43384. [http://dx.doi.org/10.1038/srep43384]. [PMID: 28262682]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang Y.T., Lee M.R., Lee S.J., Kim S., Nai Y.S., Kim J.S. Tenebrio molitor Gram-negative-binding protein 3 (TmGNBP3) is essential for inducing downstream antifungal Tenecin 1 gene expression against infection with Beauveria bassiana JEF-007. Insect Sci. 2017;25(6):969–977. doi: 10.1111/1744-7917.12482. [PMID: 28544681]. [DOI] [PubMed] [Google Scholar]
- 49.Duwadi D., Shrestha A., Yilma B., Kozlovski I., Sa-Eed M., Dahal N., Jukosky J. Identification and screening of potent antimicrobial peptides in arthropod genomes. Peptides. 2018;103:26–30. doi: 10.1016/j.peptides.2018.01.017. [http://dx.doi.org/10.1016/j.peptides.2018.01.017]. [PMID: 29501691]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sheehan G., Bergsson G., McElvaney N.G., Reeves E.P., Kavanagh K. The human cathelicidin antimicrobial peptide LL-37 promotes the growth of the pulmonary pathogen Aspergillus fumigatus. Infect. Immun. 2018;86(7):e00097. doi: 10.1128/IAI.00097-18. [http://dx.doi.org/10.1128/IAI.00097-18]. [PMID: 29712727]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schaal J.B., Maretzky T., Tran D.Q., Tran P.A., Tongaonkar P., Blobel C.P. Macrocyclic θ-defensins suppress tumor necrosis factor-α (TNF-α) shedding by inhibition of TNF-α converting enzyme. J. Biol. Chem. 2018;293(8):2725–2734. doi: 10.1074/jbc.RA117.000793. [https://doi.org/10.1074/jbc.RA117.000793]. [PMID: 29317500]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Khurshid Z., Najeeb S., Mali M., Moin S.F., Raza S.Q., Zohaib S. Histatin peptides: Pharmacological functions and their applications in dentistry. Saudi Pharm. J. 2017;25(1):25–31. doi: 10.1016/j.jsps.2016.04.027. [https://doi.org/10.1016/j.jsps.2016.04.027]. [PMID: 28223859]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Baxter A.A., Lay F.T., Poon I.K.H., Kvansakul M., Hulett M.D. Tumor cell membrane-targeting cationic antimicrobial peptides: novel insights into mechanisms of action and therapeutic prospects. Cell. Mol. Life Sci. 2017;74(20):3809–3825. doi: 10.1007/s00018-017-2604-z. [http://dx.doi.org/10.1007/s00018-017-2604-z]. [PMID: 28770291]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Panteleev P.V., Balandin S.V., Ivanov V.T., Ovchinnikova T.V. A therapeutic potential of animal β-hairpin antimicrobial peptides. Curr. Med. Chem. 2017;24(17):1724–1746. doi: 10.2174/0929867324666170424124416. [http://dx.doi.org/10.2174/0929867324666170424124416]. [PMID: 28440185]. [DOI] [PubMed] [Google Scholar]
- 55.Young-Speirs M., Drouin D., Cavalcante P.A., Barkema H.W., Cobo E.R. Host defense cathelicidins in cattle: types, production, bioactive functions and potential therapeutic and diagnostic applications. Int. J. Antimicrob. Agents. 2018;51(6):813–821. doi: 10.1016/j.ijantimicag.2018.02.006. [http://dx.doi.org/10.1016/j.ijantimicag.2018.02.006]. [PMID: 29476808]. [DOI] [PubMed] [Google Scholar]
- 56.Savelyeva A., Ghavami S., Davoodpour P., Asoodeh A., Łos M.J. Anticancer Genes. London, UK: Springer; 2014. An overview of Brevinin superfamily: structure, function and clinical perspectives. pp. 197–212. [http://dx.doi.org/10.1007/978-1-4471-6458-6_10] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sun T., Zhan B., Gao Y. A novel cathelicidin from Bufo bufo gargarizans Cantor showed specific activity to its habitat bacteria. Gene. 2015;571(2):172–177. doi: 10.1016/j.gene.2015.06.034. [http://dx.doi.org/10.1016/j.gene.2015.06.034]. [PMID: 26091834]. [DOI] [PubMed] [Google Scholar]
- 58.Upadhyay R.K. Spider venom toxins, its purification, solubilization, and antimicrobial activity. Int. J. Green Pharmacy. 2018;12(Suppl. 1):S200–S2014. [http://dx.doi.org/10.22377/ijgp.v12i01.1620]. [Google Scholar]
- 59.Belmadani A., Semlali A., Rouabhia M. Dermaseptin‐S1 decreases Candida albicans growth, biofilm formation and the expression of hyphal wall protein 1 and aspartic protease genes. J. Appl. Microbiol. 2018;125(1):72–83. doi: 10.1111/jam.13745. [https://doi.org/10.1111/jam.13745]. [DOI] [PubMed] [Google Scholar]
- 60.Tahir H.M., Zaheer A., Khan A.A., Abbas M. Antibacterial potential of venom extracted from wolf spider, Lycosa terrestris (Araneae: Lycosiade). Indian J. Anim. Sci. 2018;52(2):286–290. [http://dx.doi.org/10.18805/ijar.v0iOF.8484]. [Google Scholar]
- 61.Kuzmin D.V., Emelianova A.A., Kalashnikova M.B., Panteleev P.V., Ovchinnikova T.V. Effect of N- and C-terminal modifications on cytotoxic properties of antimicrobial peptide tachyplesin I. Bull. Exp. Biol. Med. 2017;162(6):754–757. doi: 10.1007/s10517-017-3705-2. [http://dx.doi.org/10.1007/s10517-017-3705-2]. [PMID: 28429216]. [DOI] [PubMed] [Google Scholar]
- 62.Coulen S.C., Sanders J.P., Bruins M.E. Valorisation of proteins from rubber tree. Waste Biomass Valoriz. 2017;8(4):1027–1041. [http://dx.doi.org/10.1007/s12649-016-9688-9]. [Google Scholar]
- 63.Thao H.T., Lan N.T.N., Mau C.H. Overexpression of VrPDF1 gene confers resistance to weevils in transgenic mung bean plant. Peer J. Preprints. 2017 e3264v2. [Google Scholar]
- 64.Mills S., Griffin C., O’Connor P.M., Serrano L.M., Meijer W.C., Hill C., Ross R.P. A multibacteriocin cheese starter system, comprising nisin and lacticin 3147 in Lactococcus lactis, in combination with plantaricin from Lactobacillus plantarum. Appl. Environ. Microbiol. 2017;83(14):e00799–e17. doi: 10.1128/AEM.00799-17. [http://dx.doi.org/10.1128/AEM.00799-17]. [PMID: 28476774]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Su Z., Leitch J.J., Abbasi F., Faragher R.J., Schwan A.L., Lipkowski J. EIS and PM-IRRAS studies of alamethicin ion channels in a tethered lipid bilayer. J. Electroanal. Chem. (Lausanne Switz.) 2018;812:213–220. [Google Scholar]
- 66.Braïek O.B., Morandi S., Cremonesi P., Smaoui S., Hani K., Ghrairi T. Biotechnological potential, probiotic and safety properties of newly isolated enterocin-producing Enterococcus lactis strains. LWT. 2018;92:361–370. [http://dx.doi.org/10.1016/j.lwt.2018.02.045]. [Google Scholar]
- 67.Ebrahimipour G.H., Khosravibabadi Z., Sadeghi H., Aliahmadi A. Isolation, partial purification and characterization of an antimicrobial compound, produced by Bacillus atrophaeus. Jundishapur J. Microbiol. 2014;7(9):e11802. doi: 10.5812/jjm.11802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sharma G., Dang S., Gupta S., Gabrani R. Antibacterial activity, cytotoxicity, and the mechanism of action of bacteriocin from Bacillus subtilis GAS101. Med. Princ. Pract. 2018;27(2):186–192. doi: 10.1159/000487306. [http://dx.doi.org/10.1159/000487306]. [PMID: 29402863]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jiang H., Tang X., Zhou Q., Zou J., Li P., Breukink E., Gu Q. Plantaricin NC8 from Lactobacillus plantarum causes cell membrane disruption to Micrococcus luteus without targeting lipid II. Appl. Microbiol. Biotechnol. 2018;102(17):7465–7473. doi: 10.1007/s00253-018-9182-3. [https://doi.org/10.1007/s00253-018-9182-3]. [PMID: 29982926]. [DOI] [PubMed] [Google Scholar]
- 70.Hammi I., Delalande F., Belkhou R., Marchioni E., Cianferani S., Ennahar S. Maltaricin CPN, a new class IIa bacteriocin produced by Carnobacterium maltaromaticum CPN isolated from mould-ripened cheese. J. Appl. Microbiol. 2016;121(5):1268–1274. doi: 10.1111/jam.13248. [http://dx.doi.org/10.1111/jam.13248]. [PMID: 27489131]. [DOI] [PubMed] [Google Scholar]
- 71.Chen Y.S., Wu H.C., Kuo C.Y., Chen Y.W., Ho S., Yanagida F. Leucocin C-607, a novel bacteriocin from the multiple-bacteriocin-producing Leuconostoc pseudomesenteroides 607 isolated from Persimmon. Probiotics Antimicrob. Proteins. 2018;10(2):148–156. doi: 10.1007/s12602-017-9359-6. [https://doi.org/10.1007/s12602-017-9359-6]. [PMID: 29177756]. [DOI] [PubMed] [Google Scholar]
- 72.Singh R., Miriyala S.S., Giri L., Mitra K., Kareenhalli V.V. Identification of unstructured model for subtilin production through Bacillus subtilis using hybrid genetic algorithm. Process Biochem. 2017;60:1–12. [http://dx.doi.org/10.1016/j.procbio.2017.06.005]. [Google Scholar]
- 73.Guzmán-Rodríguez J.J., Ochoa-Zarzosa A., López-Gómez R., López-Meza J.E. Plant antimicrobial peptides as potential anticancer agents. BioMed Res. Int. 2015:2015735087. doi: 10.1155/2015/735087. [http://dx.doi.org/10.1155/2015/735087]. [PMID: 25815333]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhao N., Pan Y., Cheng Z., Liu H. Lasso peptide, a highly stable structure and designable multifunctional backbone. Amino Acids. 2016;48(6):1347–1356. doi: 10.1007/s00726-016-2228-x. [http://dx.doi.org/10.1007/s00726-016-2228-x]. [PMID: 27074719]. [DOI] [PubMed] [Google Scholar]
- 75.Muhammad S.A., Ali A., Naz A., Hassan A., Riaz N. Saeed-ul-Hassan, S. A new broad-spectrum peptide antibiotic produced by Bacillus brevis strain MH9 isolated from Margalla Hills of Islamabad, Pakistan. Int. J. Pept. Res. Ther. 2016;22(2):271–279. [http://dx.doi.org/10.1007/s10989-015-9508-2]. [Google Scholar]
- 76.Araújo C., Muñoz-Atienza E., Poeta P., Igrejas G., Hernández P.E., Herranz C., Cintas L.M. Characterization of Pediococcus acidilactici strains isolated from rainbow trout (Oncorhynchus mykiss) feed and larvae: safety, DNA fingerprinting, and bacteriocinogenicity. Dis. Aquat. Organ. 2016;119(2):129–143. doi: 10.3354/dao02992. [http://dx.doi.org/10.3354/dao02992]. [PMID: 27137071]. [DOI] [PubMed] [Google Scholar]
- 77.Arakawa K., Yoshida S., Aikawa H., Hano C., Bolormaa T., Burenjargal S., Miyamoto T. Production of a bacteriocin-like inhibitory substance by Leuconostoc mesenteroides subsp. dextranicum 213M0 isolated from Mongolian fermented mare milk, Airag. Anim. Sci. J. 2016;87(3):449–456. doi: 10.1111/asj.12445. [http://dx.doi.org/10.1111/asj.12445]. [PMID: 26388181]. [DOI] [PubMed] [Google Scholar]
- 78.Tulini F.L., Lohans C.T., Bordon K.C., Zheng J., Arantes E.C., Vederas J.C., De Martinis E.C. Purification and characterization of antimicrobial peptides from fish isolate Carnobacterium maltaromaticum C2: Carnobacteriocin X and carnolysins A1 and A2. Int. J. Food Microbiol. 2014;173:81–88. doi: 10.1016/j.ijfoodmicro.2013.12.019. [http://dx.doi.org/10.1016/j.ijfoodmicro.2013.12.019]. [PMID: 24412962]. [DOI] [PubMed] [Google Scholar]
- 79.Bosma T.U.S. Bacterial surface display and screening of thioetherbridge- containing peptides. U.S. Patent No. 9,651,558. 2017
- 80.Gajalakshmi P. Selective isolation and characterization of rare actinomycetes adopted in glacier soil of Manali ice point and its activity against Mycobacterium spp. J. Microbiol. Biotechnol. Res. 2017;7(5):1–10. [http://dx.doi.org/10.24896/jmbr.2017751]. [Google Scholar]
- 81.Maldonado-Barragán A., Caballero-Guerrero B., Martín V., Ruiz-Barba J.L., Rodríguez J.M. Purification and genetic characterization of gassericin E, a novel co-culture inducible bacteriocin from Lactobacillus gasseri EV1461 isolated from the vagina of a healthy woman. BMC Microbiol. 2016;16(1):37. doi: 10.1186/s12866-016-0663-1. [https://doi.org/10.1186/s12866-016-0663-1]. [PMID: 26969428]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Perez R.H., Ishibashi N., Inoue T., Himeno K., Masuda Y., Sawa N. Functional analysis of genes involved in the biosynthesis of enterocin NKR-5-3B, a novel circular bacteriocin. J. Bacteriol. 2016;198(2):291–300. doi: 10.1128/JB.00692-15. [https://doi.org/10.1128/JB.00692-15]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Brillet-Viel A., Pilet M.F., Courcoux P., Prévost H., Leroi F. Optimization of growth and bacteriocin activity of the food bioprotective Carnobacterium divergens V41 in an animal origin protein free medium. Front. Mar. Sci. 2016;3:128. [http://dx.doi.org/10.3389/fmars.2016.00128]. [Google Scholar]
- 84.Wan X., Li R., Saris P.E., Takala T.M. Genetic characterisation and heterologous expression of leucocin C, a class IIa bacteriocin from Leuconostoc carnosum 4010. Appl. Microbiol. Biotechnol. 2013;97(8):3509–3518. doi: 10.1007/s00253-012-4406-4. [http://dx.doi.org/10.1007/s00253-012-4406-4]. [PMID: 23053070]. [DOI] [PubMed] [Google Scholar]
- 85.Wang Y., Shang N., Qin Y., Zhang Y., Zhang J., Li P. The complete genome sequence of Lactobacillus plantarum LPL-1, a novel antibacterial probiotic producing class IIa bacteriocin. J. Biotechnol. 2018;266:84–88. doi: 10.1016/j.jbiotec.2017.12.006. [http://dx.doi.org/10.1016/j.jbiotec.2017.12.006]. [PMID: 29229543]. [DOI] [PubMed] [Google Scholar]
- 86.Le T.N., Do T.H., Nguyen T.N., Tran N.T., Enfors S.O., Truong H. Expression and simple purification strategy for the generation of anti-microbial active Enterocin P from Enterococcus faecium expressed in Escherichia coli ER2566. Iranian J. Biotechnol. 2014;12(4):17–25. [http://dx.doi.org/10.15171/ijb.1154]. [Google Scholar]
- 87.Venturina D.H., Villegas L.C., Perez M.T.M., Elegado F.B. Isolation and identification of subtilosin A-producing Bacillus subtilis from mongo sprouts, silage and soil samples in the Philippines. Asia Life Sci. 2016;25(1):123–136. [Google Scholar]
- 88.Bhat S.G. Modelling and computational sequence analysis of a bacteriocin Isolated from Bacillus licheniformis strain BTHT. Int. J. Comp. Biol. 2018;7(1):29–34. [http://dx.doi.org/10.34040/IJCB.7.1.2018.93]. [Google Scholar]
- 89.Hollmann A., Martinez M., Maturana P., Semorile L.C., Maffia P.C. Antimicrobial peptides: Interaction with model and biological membranes and synergism with chemical antibiotics. Front Chem. 2018;6:204. doi: 10.3389/fchem.2018.00204. [http://dx.doi.org/10.3389/fchem.2018.00204]. [PMID: 29922648]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pfalzgraff A., Brandenburg K., Weindl G. Antimicrobial peptides and their therapeutic potential for bacterial skin infections and wounds. Front. Pharmacol. 2018;9:281. doi: 10.3389/fphar.2018.00281. [http://dx.doi.org/10.3389/fphar.2018.00281]. [PMID: 29643807]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hancock R.E.W., Patrzykat A. Clinical development of cationic antimicrobial peptides: from natural to novel antibiotics. Curr. Drug Targets Infect. Disord. 2002;2(1):79–83. doi: 10.2174/1568005024605855. [http://dx.doi.org/10.2174/1568005024605855]. [PMID: 12462155]. [DOI] [PubMed] [Google Scholar]
- 92.Shafee T.M., Lay F.T., Phan T.K., Anderson M.A., Hulett M.D. Convergent evolution of defensin sequence, structure and function. Cell. Mol. Life Sci. 2017;74(4):663–682. doi: 10.1007/s00018-016-2344-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sun D., Forsman J., Woodward C.E. Molecular simulations of melittin-induced membrane pores. J. Phys. Chem. B. 2017;121(44):10209–10214. doi: 10.1021/acs.jpcb.7b07126. [http://dx.doi.org/10.1021/acs.jpcb.7b07126]. [PMID: 29035531]. [DOI] [PubMed] [Google Scholar]
- 94.Strandberg E., Zerweck J., Wadhwani P., Reichert J., Bürck J., Ulrich A.S. Molecular mechanism of synergy between the antimicrobial peptides PGLa and magainin 2 in membranes. Biophys. J. 2018;114(3):452a–453a. doi: 10.1038/s41598-017-12599-7. [http://dx.doi.org/10.1016/j.bpj.2017.11.2502]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Xhindoli D., Pacor S., Benincasa M., Scocchi M., Gennaro R., Tossi A. The human cathelicidin LL-37--A pore-forming antibacterial peptide and host-cell modulator. Biochim. Biophys. Acta. 2016;1858(3):546–566. doi: 10.1016/j.bbamem.2015.11.003. [http://dx.doi.org/10.1016/j.bbamem.2015.11.003]. [PMID: 26556394]. [DOI] [PubMed] [Google Scholar]
- 96.Belmadani A., Semlali A., Rouabhia M. Dermaseptin‐S1 decreases Candida albicans growth, biofilm formation and the expression of hyphal wall protein 1 and aspartic protease genes. J. Appl. Microbiol. 2018;125(1):72–83. doi: 10.1111/jam.13745. [https://doi.org/10.1111/jam.13745]. [PMID: 29476689]. [DOI] [PubMed] [Google Scholar]
- 97.Beadell B., Powell T.R., Berton R., Porter E. The antimicrobial peptides HNP-1 and HBD-2 act against Mycobacterium smegmatis independent from their chirality. Southern California Conferences for Undergraduate Research; 2017. [Google Scholar]
- 98.Zhang M., Wei W., Sun Y., Jiang X., Ying X., Tao R., Ni L. Pleurocidin congeners demonstrate activity against Streptococcus and low toxicity on gingival fibroblasts. Arch. Oral Biol. 2016;70:79–87. doi: 10.1016/j.archoralbio.2016.06.008. [http://dx.doi.org/10.1016/j.archoralbio.2016.06.008]. [PMID: 27341459]. [DOI] [PubMed] [Google Scholar]
- 99.Tsai C.W., Lin Z.W., Chang W.F., Chen Y.F., Hu W.W. Development of an indolicidin-derived peptide by reducing membrane perturbation to decrease cytotoxicity and maintain gene delivery ability. Colloids Surf. B Biointerfaces. 2018;165:18–27. doi: 10.1016/j.colsurfb.2018.02.007. [http://dx.doi.org/10.1016/j.colsurfb.2018.02.007]. [PMID: 29448216]. [DOI] [PubMed] [Google Scholar]
- 100.Wuerth K. Doctoral dissertation: University of British Columbia. 2017. Combating Pseudomonas aeruginosa lung infections using synthetic host defense peptides. [Google Scholar]
- 101.Baindara P., Kapoor A., Korpole S., Grover V. Cysteine-rich low molecular weight antimicrobial peptides from Brevibacillus and related genera for biotechnological applications. World J. Microbiol. Biotechnol. 2017;33(6):124. doi: 10.1007/s11274-017-2291-9. [http://dx.doi.org/10.1007/s11274-017-2291-9]. [PMID: 28534113]. [DOI] [PubMed] [Google Scholar]
- 102.Lohner K. Membrane-active antimicrobial peptides as template structures for novel antibiotic agents. Curr. Top. Med. Chem. 2017;17(5):508–519. [http://dx.doi.org/10.2174/1568026616666160713122404]. [PMID: 28117020]. [PubMed] [Google Scholar]
- 103.Sani M.A., Separovic F. How membrane-active peptides get into lipid membranes. Acc. Chem. Res. 2016;49(6):1130–1138. doi: 10.1021/acs.accounts.6b00074. [http://dx.doi.org/10.1021/acs.accounts.6b00074]. [PMID: 27187572]. [DOI] [PubMed] [Google Scholar]
- 104.Haney E.F., Mansour S.C., Hancock R.E. Antimicrobial peptides: an introduction. Antimicrobial Peptides. New York, NY: Humana Press; 2017. pp. 3–22. [http://dx.doi.org/10.1007/978-1-4939-6737-7_1] [DOI] [PubMed] [Google Scholar]
- 105.Mingeot-Leclercq M.P., Décout J.L. Bacterial lipid membranes as promising targets to fight antimicrobial resistance, molecular foundations and illustration through the renewal of aminoglycoside antibiotics and emergence of amphiphilic aminoglycosides. MedChemComm. 2016;7(4):586–611. [http://dx.doi.org/10.1039/C5MD00503E]. [Google Scholar]
- 106.Cudic M., Otvos L., Jr Intracellular targets of antibacterial peptides. Curr. Drug Targets. 2002;3(2):101–106. doi: 10.2174/1389450024605445. [http://dx.doi.org/10.2174/1389450024605445]. [PMID: 11958294]. [DOI] [PubMed] [Google Scholar]
- 107.Krizsan A., Volke D., Weinert S., Sträter N., Knappe D., Hoffmann R. Insect-derived proline-rich antimicrobial peptides kill bacteria by inhibiting bacterial protein translation at the 70S ribosome. Angew. Chem. Int. Ed. Engl. 2014;53(45):12236–12239. doi: 10.1002/anie.201407145. [http://dx.doi.org/10.1002/anie.201407145]. [PMID: 25220491]. [DOI] [PubMed] [Google Scholar]
- 108.Mansour S.C., Pena O.M., Hancock R.E. Host defense peptides: front-line immunomodulators. Trends Immunol. 2014;35(9):443–450. doi: 10.1016/j.it.2014.07.004. [http://dx.doi.org/10.1016/j.it.2014.07.004]. [PMID: 25113635]. [DOI] [PubMed] [Google Scholar]
- 109.Claro B., Bastos M., Garcia-Fandino R. Design and applications of cyclic peptides. In: Koutsopoulos S., editor. Peptide Applications in Biomedicine, Biotechnology and Bioengineering. Sawston, United Kingdom: Woodhead Publishing; 2018. pp. 87–129. [https://doi.org/10.1016/B978-0-08-100736-5.00004-1] [Google Scholar]
- 110.Malanovic N., Lohner K. Antimicrobial peptides targeting gram-positive bacteria. Pharmaceuticals (Basel) 2016;9(3):59. doi: 10.3390/ph9030059. [http://dx.doi.org/10.3390/ph9030059]. [PMID: 27657092]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Shagaghi N., Palombo E.A., Clayton A.H.A., Bhave M. Antimicrobial peptides: biochemical determinants of activity and biophysical techniques of elucidating their functionality. World J. Microbiol. Biotechnol. 2018;34(4):62. doi: 10.1007/s11274-018-2444-5. [http://dx.doi.org/10.1007/s11274-018-2444-5]. [PMID: 29651655]. [DOI] [PubMed] [Google Scholar]
- 112.Matsuzaki K. Why and how are peptide-lipid interactions utilized for self-defense? Magainins and tachyplesins as archetypes. Biochim. Biophys. Acta. 1999;1462(1-2):1–10. doi: 10.1016/s0005-2736(99)00197-2. [http://dx.doi.org/10.1016/S0005-2736(99)00197-2]. [PMID: 10590299]. [DOI] [PubMed] [Google Scholar]
- 113.Falanga A., Galdiero S. 2017. Emerging therapeutic agents on the basis of naturally occurring antimicrobial peptides. [Google Scholar]
- 114.Savini F., Bobone S., Roversi D., Mangoni M.L., Stella L. From liposomes to cells: Filling the gap between physicochemical and microbiological studies of the activity and selectivity of host‐defense peptides. Peptide Sci. 2018;110(5):e24041. [https://doi.org/10.1002/pep2.24041]. [Google Scholar]
- 115.Shabir U., Ali S., Magray A.R., Ganai B.A., Firdous P., Hassan T., Nazir R. Fish antimicrobial peptides (AMP’s) as essential and promising molecular therapeutic agents: A review. Microb. Pathog. 2018;114:50–56. doi: 10.1016/j.micpath.2017.11.039. [https://doi.org/10.1016/j.micpath.2017.11.039]. [PMID: 29180291]. [DOI] [PubMed] [Google Scholar]
- 116.Han J., Zhao S., Ma Z., Gao L., Liu H., Muhammad U. The antibacterial activity and modes of LI–F type antimicrobial peptides against Bacillus cereus in vitro. J. Appl. Microbiol. 2017;123(3):602–614. doi: 10.1111/jam.13526. [https://doi.org/10.1111/jam.13526]. [DOI] [PubMed] [Google Scholar]
- 117.Phoenix D.A., Dennison S.R., Harris F. Bacterial resistance to host defence peptides. In: Epand R.M., editor. Host Defense Peptides and Their Potential as Therapeutic Agents. Switzerland: Springer; 2016. pp. 161–204. [http://dx.doi.org/10.1007/978-3-319-32949-9_7] [Google Scholar]
- 118.Carrera M., Böhme K., Gallardo J.M., Barros-Velázquez J., Cañas B., Calo-Mata P. Characterization of foodborne strains of Staphylococcus aureus by shotgun proteomics: functional networks, virulence factors and species-specific peptide biomarkers. Front. Microbiol. 2017;8:2458. doi: 10.3389/fmicb.2017.02458. [http://dx.doi.org/10.3389/fmicb.2017.02458]. [PMID: 29312172]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nagarajan K., Marimuthu S.K., Palanisamy S., Subbiah L. Peptide therapeutics versus superbugs: highlight on current research and advancements. Int. J. Pept. Res. Ther. 2018;24(1):19–33. [http://dx.doi.org/10.1007/s10989-017-9650-0]. [Google Scholar]
- 120.Gordon Y.J., Romanowski E.G., McDermott A.M. A review of antimicrobial peptides and their therapeutic potential as anti-infective drugs. Curr. Eye Res. 2005;30(7):505–515. doi: 10.1080/02713680590968637. [http://dx.doi.org/10.1080/02713680590968637]. [PMID: 16020284]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mirski T., Niemcewicz M., Bartoszcze M., Gryko R., Michalski A. Utilisation of peptides against microbial infections - a review. Ann. Agric. Environ. Med. 2017;25(2):205–210. doi: 10.26444/aaem/74471. [http://dx.doi.org/10.26444/aaem/74471]. [PMID: 29936826]. [DOI] [PubMed] [Google Scholar]
- 122.Levy O. Antimicrobial proteins and peptides: anti-infective molecules of mammalian leukocytes. J. Leukoc. Biol. 2004;76(5):909–925. doi: 10.1189/jlb.0604320. [http://dx.doi.org/10.1189/jlb.0604320]. [PMID: 15292276]. [DOI] [PubMed] [Google Scholar]
- 123.Warnke P.H., Voss E., Russo P.A., Stephens S., Kleine M., Terheyden H., Liu Q. Antimicrobial peptide coating of dental implants: biocompatibility assessment of recombinant human beta defensin-2 for human cells. Int. J. Oral Maxillofac. Implants. 2013;28(4):982–988. doi: 10.11607/jomi.2594. [http://dx.doi.org/10.11607/jomi.2594]. [PMID: 23869355]. [DOI] [PubMed] [Google Scholar]
- 124.Conlon J.M., Sonnevend A. Clinical applications of amphibian antimicrobial peptides. J. Med. Sci. 2011;4(2):62–72. [http://dx.doi.org/10.2174/1996327001104020062]. [Google Scholar]
- 125.Shin S.H., Lee Y.S., Shin Y.P., Kim B., Kim M.H., Chang H.R., Jang W.S., Lee I.H. Therapeutic efficacy of halocidin-derived peptide HG1 in a mouse model of Candida albicans oral infection. J. Antimicrob. Chemother. 2013;68(5):1152–1160. doi: 10.1093/jac/dks513. [http://dx.doi.org/10.1093/jac/dks513]. [PMID: 23302580]. [DOI] [PubMed] [Google Scholar]
- 126.Migoń D., Neubauer D., Kamysz W. Hydrocarbon stapled antimicrobial peptides. Protein J. 2018;37(1):2–12. doi: 10.1007/s10930-018-9755-0. [http://dx.doi.org/10.1007/s10930-018-9755-0]. [PMID: 29330644]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yu K., Lo J.C., Yan M., Yang X., Brooks D.E., Hancock R.E., Lange D., Kizhakkedathu J.N. Anti-adhesive antimicrobial peptide coating prevents catheter associated infection in a mouse urinary infection model. Biomaterials. 2017;116:69–81. doi: 10.1016/j.biomaterials.2016.11.047. [http://dx.doi.org/10.1016/j.biomaterials.2016.11.047]. [PMID: 27914268]. [DOI] [PubMed] [Google Scholar]
- 128.Greber K.E., Dawgul M. Antimicrobial peptides under clinical trials. Curr. Top. Med. Chem. 2017;17(5):620–628. doi: 10.2174/1568026616666160713143331. [http://dx.doi.org/10.2174/1568026616666160713143331]. [PMID: 27411322]. [DOI] [PubMed] [Google Scholar]
- 129.Sachdeva S. Peptides as ‘Drugs’: the journey so far. Int. J. Pept. Res. Ther. 2017;23(1):49–60. [http://dx.doi.org/10.1007/s10989-016-9534-8]. [Google Scholar]
- 130.Mohammad H., Thangamani S., Seleem M.N. Antimicrobial peptides and peptidomimetics - potent therapeutic allies for staphylococcal infections. Curr. Pharm. Des. 2015;21(16):2073–2088. doi: 10.2174/1381612821666150310102702. [http://dx.doi.org/10.2174/1381612821666150310102702]. [PMID: 25760338]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Dawgul M., Maciejewska M., Jaskiewicz M., Karafova A., Kamysz W. Antimicrobial peptides as potential tool to fight bacterial biofilm. Acta Pol. Pharm. 2014;71(1):39–47. [PMID: 24779193]. [PubMed] [Google Scholar]
- 132.Tsou Y.A., Tung Y.T., Wu T.F., Chang G.R.L., Chen H.C., Lin C.D., Lai C.H., Chen H.L., Chen C.M. Lactoferrin interacts with SPLUNC1 to attenuate lipopolysaccharide-induced inflammation of human nasal epithelial cells via down-regulated MEK1/2-MAPK signaling. Biochem. Cell Biol. 2017;95(3):394–399. doi: 10.1139/bcb-2016-0047. [http://dx.doi.org/10.1139/bcb-2016-0047]. [PMID: 28178421]. [DOI] [PubMed] [Google Scholar]
- 133.Samad T., Co J.Y., Witten J., Ribbeck K. Mucus and mucin environments reduce the efficacy of polymyxin and fluoroquinolone antibiotics against Pseudomonas aeruginosa. ACS Biomater. Sci. Eng. 2019;5(3):1189–1194. doi: 10.1021/acsbiomaterials.8b01054. [http://dx.doi.org/10.1021/acsbiomaterials.8b01054]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Daliri E.B.M., Lee B.H., Oh D.H. Current trends and perspectives of bioactive peptides. Crit. Rev. Food Sci. Nutr. 2018;58(13):2273–2284. doi: 10.1080/10408398.2017.1319795. [http://dx.doi.org/10.1080/10408398.2017.1319795]. [PMID: 28604060]. [DOI] [PubMed] [Google Scholar]
- 135.Kumar P., Kizhakkedathu J.N., Straus S.K. Antimicrobial peptides: Diversity, mechanism of action and strategies to improve the activity and biocompatibility in vivo. Biomolecules. 2018;8(1):4. doi: 10.3390/biom8010004. [http://dx.doi.org/10.3390/biom8010004]. [PMID: 29351202]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Woodburn K.W., Chiang C.M., Jaynes J., Clemens L.E. 2019. Designed Antimicrobial Peptides: A New Horizon. [Google Scholar]
- 137.Kolar S.S.N., Luca V., Baidouri H., Mannino G., McDermott A.M., Mangoni M.L. Esculentin-1a (1-21) NH 2: a frog skin-derived peptide for microbial keratitis. Cell. Mol. Life Sci. 2015;72(3):617–627. doi: 10.1007/s00018-014-1694-0. [https://doi.org/10.1007/s00018-014-1694-0]. [PMID: 25086859]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Bach H. A New Era without. Antibiotics (Basel) 2018:1. [Google Scholar]
- 139.Lau J.L., Dunn M.K. Therapeutic peptides: Historical perspectives, current development trends, and future directions. Bioorg. Med. Chem. 2018;26(10):2700–2707. doi: 10.1016/j.bmc.2017.06.052. [http://dx.doi.org/10.1016/j.bmc.2017.06.052]. [PMID: 28720325]. [DOI] [PubMed] [Google Scholar]
- 140.Raucher D., Ryu J.S. Cell-penetrating peptides: strategies for anticancer treatment. Trends Mol. Med. 2015;21(9):560–570. doi: 10.1016/j.molmed.2015.06.005. [http://dx.doi.org/10.1016/j.molmed.2015.06.005]. [PMID: 26186888]. [DOI] [PubMed] [Google Scholar]
- 141.Ben Lagha A., Haas B., Gottschalk M., Grenier D. Antimicrobial potential of bacteriocins in poultry and swine production. Vet. Res. (Faisalabad) 2017;48(1):22. doi: 10.1186/s13567-017-0425-6. [http://dx.doi.org/10.1186/s13567-017-0425-6]. [PMID: 28399941]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Garbacz K., Kamysz W., Piechowicz L. Activity of antimicrobial peptides, alone or combined with conventional antibiotics, against Staphylococcus aureus isolated from the airways of cystic fibrosis patients. Virulence. 2017;8(1):94–100. doi: 10.1080/21505594.2016.1213475. [http://dx.doi.org/10.1080/21505594.2016.1213475]. [PMID: 27450039]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Huque M., Röhmel J. Multiplicity problems in clinical trials: a regulatory perspective. In: Bretz F., Tamhane A.C., editors. Multiple testing problems in pharmaceutical statistics. USA: CRC Press; 2009. pp. 1–34. [http://dx.doi.org/10.1201/9781584889854-c1] [Google Scholar]
- 144.Sandreschi S., Piras A.M., Batoni G., Chiellini F. Perspectives on polymeric nanostructures for the therapeutic application of antimicrobial peptides. Nanomedicine (Lond.) 2016;11(13):1729–1744. doi: 10.2217/nnm-2016-0057. [http://dx.doi.org/10.2217/nnm-2016-0057]. [PMID: 27348155]. [DOI] [PubMed] [Google Scholar]
- 145.Riool M., de Breij A., Drijfhout J.W., Nibbering P.H., Zaat S.A.J. Antimicrobial peptides in biomedical device manufacturing. Front Chem. 2017;5:63. doi: 10.3389/fchem.2017.00063. [http://dx.doi.org/10.3389/fchem.2017.00063]. [PMID: 28971093]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Żelechowska P., Agier J., Brzezińska-Błaszczyk E. Endogenous antimicrobial factors in the treatment of infectious diseases. Cent. Eur. J. Immunol. 2016;41(4):419–425. doi: 10.5114/ceji.2016.65141. [http://dx.doi.org/10.5114/ceji.2016.65141]. [PMID: 28450805]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Mohamed M.F.K. Doctoral dissertation, Purdue University: USA. 2016. Targeting multi-drug resistant pathogens with novel antimicrobial peptides. [Google Scholar]
- 148.Mealy N.E., Bayes M. Annual Update 2003/2004-Treatment of Dermatological Disorders. Drugs Future. 2004;29(4):393. [Google Scholar]
- 149.Tasiemski A., Salzet M., Gaill F.U.S. Patent No. 8. Antimicrobial peptides. 2014 652,514.
- 150.Cal P.M., Matos M.J., Bernardes G.J. Trends in therapeutic drug conjugates for bacterial diseases: a patent review. Expert Opin. Ther. Pat. 2017;27(2):179–189. doi: 10.1080/13543776.2017.1259411. [http://dx.doi.org/10.1080/13543776.2017.1259411]. [PMID: 27828733]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ostroumova O.S., Efimova S.S., Malev V.V. Modifiers of membrane dipole potentials as tools for investigating ion channel formation and functioning. Int. Rev. Cell Mol. Biol. 2015;315:245–297. doi: 10.1016/bs.ircmb.2014.12.001. [http://dx.doi.org/10.1016/bs.ircmb.2014.12.001]. [PMID: 25708465]. [DOI] [PubMed] [Google Scholar]
- 152.Deslouches B., Di Y.P. Antimicrobial peptides with selective antitumor mechanisms: prospect for anticancer applications. Oncotarget. 2017;8(28):46635–46651. doi: 10.18632/oncotarget.16743. [http://dx.doi.org/10.18632/oncotarget.16743]. [PMID: 28422728]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Felício M.R., Silva O.N., Gonçalves S., Santos N.C., Franco O.L. Peptides with dual antimicrobial and anticancer activities. Front Chem. 2017;5:5. doi: 10.3389/fchem.2017.00005. [http://dx.doi.org/10.3389/fchem.2017.00005]. [PMID: 28271058]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wade H.M., Darling L.E., Elmore D.E. Systematic analysis of hybrid antimicrobial peptides. Biophys. J. 2018;114(3):453. [http://dx.doi.org/10.1016/j.bpj.2017.11.2503]. [Google Scholar]
- 155.Ghosh C., Haldar J. Membrane-active small molecules: designs inspired by antimicrobial peptides. ChemMedChem. 2015;10(10):1606–1624. doi: 10.1002/cmdc.201500299. [http://dx.doi.org/10.1002/cmdc.201500299]. [PMID: 26386345]. [DOI] [PubMed] [Google Scholar]
- 156.Cortes-Penfield N., Oliver N.T., Hunter A., Rodriguez-Barradas M. Daptomycin and combination daptomycin-ceftaroline as salvage therapy for persistent methicillin-resistant Staphylococcus aureus bacteremia. Infect. Dis. (Lond.) 2018;50(8):643–647. doi: 10.1080/23744235.2018.1448110. [http://dx.doi.org/10.1080/23744235.2018.1448110]. [PMID: 29508663]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Gagliardini E., Benigni A., Perico N. Pharmacological induction of kidney regeneration. In: Orlando G., Remuzzi G., Williams D.F., editors. Kidney Transplantation, Bioengineering and Regeneration. Cambridge, MA, United States: Academic Press; 2017. pp. 1025–1037. [http://dx.doi.org/10.1016/B978-0-12-801734-0.00074-6] [Google Scholar]
- 158.Jepson A.K., Schwarz-Linek J., Ryan L., Ryadnov M.G., Poon W.C. What Is the ‘Minimum Inhibitory Concentration’(MIC) of Pexiganan Acting on Escherichia coli?-A Cautionary Case Study. Adv. Exp. Med. Biol. 2016;915:33–48. doi: 10.1007/978-3-319-32189-9_4. [https://doi.org/10.1007/978-3-319-32189-9_4]. [PMID: 27193536]. [DOI] [PubMed] [Google Scholar]
- 159.Ng S.M.S., Teo S.W., Yong Y.E., Ng F.M., Lau Q.Y., Jureen R., Hill J., Chia C.S.B. Preliminary investigations into developing all-D Omiganan for treating Mupirocin-resistant MRSA skin infections. Chem. Biol. Drug Des. 2017;90(6):1155–1160. doi: 10.1111/cbdd.13035. [http://dx.doi.org/10.1111/cbdd.13035]. [PMID: 28581672]. [DOI] [PubMed] [Google Scholar]
- 160.Ross J.E., Jones R.N., Rhomberg P.R., Fritsche T.R. In vitro activity of omiganan pentahydrochloride against> 1,600 clinical trial isolates; 2007. p. 433. 45th Infectious Diseases Society of America Annual Meeting, San Diego, [Google Scholar]
- 161.Morici P., Fais R., Rizzato C., Tavanti A., Lupetti A. Inhibition of Candida albicans biofilm formation by the synthetic lactoferricin derived peptide hLF1-11. PLoS One. 2016;11(11):e0167470. doi: 10.1371/journal.pone.0167470. [http://dx.doi.org/10.1371/journal.pone.0167470]. [PMID: 27902776]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Javia A., Amrutiya J., Lalani R., Patel V., Bhatt P., Misra A. Antimicrobial peptide delivery: an emerging therapeutic for the treatment of burn and wounds. Ther. Deliv. 2018;9(5):375–386. doi: 10.4155/tde-2017-0061. [http://dx.doi.org/10.4155/tde-2017-0061]. [PMID: 29681237]. [DOI] [PubMed] [Google Scholar]
- 163.De Lorenzi E., Chiari M., Colombo R., Cretich M., Sola L., Vanna R. Evidence that the human innate immune peptide LL-37 may be a binding partner of Abeta and inhibitor of fibril assembly. Biophys. J. 2018;114(3):393a. doi: 10.3233/JAD-170223. [http://dx.doi.org/10.1016/j.bpj.2017.11.2174]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Menko A.S. Patent. 8. Method to treat and prevent posterior capsule opacification. 2015 999,370.
- 165.Moorthy N.S.H.N., Pratheepa V., Manivannan E. Natural product derived drugs for immunological and inflammatory diseases. Nat. Prod. Clin. Trials. 2018;1:1–31. [Google Scholar]
- 166.Döşler S. Antimicrobial peptides: Coming to the end of antibiotic era, the most promising agents. Ist. J. Pharm. 2017;47(2):72–76. [http://dx.doi.org/10.5152/IstanbulJPharm.2017.0012]. [Google Scholar]
- 167.Mangoni M.L., McDermott A.M., Zasloff M. Antimicrobial peptides and wound healing: biological and therapeutic considerations. Exp. Dermatol. 2016;25(3):167–173. doi: 10.1111/exd.12929. [http://dx.doi.org/10.1111/exd.12929]. [PMID: 26738772]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Krutetskaya Z.I., Melnitskaya A.V., Antonov V.G., Nozdrachev A.D. Lipoxygenases modulate the effect of glutoxim on Na+ transport in the frog skin epithelium. Dokl. Biochem. Biophys. 2017;474(1):193–195. doi: 10.1134/S1607672917030073. [http://dx.doi.org/10.1134/S1607672917030073]. [PMID: 28726099]. [DOI] [PubMed] [Google Scholar]
- 169.Harvey A., Edrada-Ebel R., Quinn R.J. The re-emergence of natural products for drug discovery in the genomics era. Nat. Rev. Drug Discov. 2015;14:111–129. doi: 10.1038/nrd4510. [http://dx.doi.org/10.1038/nrd4510]. [DOI] [PubMed] [Google Scholar]
- 170.Butler M.S., Blaskovich M.A., Cooper M.A. Antibiotics in the clinical pipeline at the end of 2015. J. Antibiot. (Tokyo) 2017;70(1):3–24. doi: 10.1038/ja.2016.72. [http://dx.doi.org/10.1038/ja.2016.72]. [PMID: 27353164]. [DOI] [PubMed] [Google Scholar]
- 171.Giuliani A., Pirri G., Nicoletto S. Antimicrobial peptides: an overview of a promising class of therapeutics. Open Life Sci. 2007;2(1):1–33. [http://dx.doi.org/10.2478/s11535-007-0010-5]. [Google Scholar]
- 172.Feng Q., Huang Y., Chen M., Li G., Chen Y. Functional synergy of α-helical antimicrobial peptides and traditional antibiotics against Gram-negative and Gram-positive bacteria in vitro and in vivo. Eur. J. Clin. Microbiol. Infect. Dis. 2015;34(1):197–204. doi: 10.1007/s10096-014-2219-3. [http://dx.doi.org/10.1007/s10096-014-2219-3]. [PMID: 25169965]. [DOI] [PubMed] [Google Scholar]


