Abstract
Purpose
Circulating tumor cell (CTC) detection methods based on epithelial cell adhesion molecule (EpCAM) have low detection rates in epithelial ovarian cancer (EOC). Meanwhile, folate receptor alpha (FRα) has high expression in EOC cells. We explored the feasibility of combining FRα and EpCAM as CTC capture targets in EOC.
Patients and methods
EpCAM and FRα antibodies were linked to magnetic nanospheres (MNs) using the principle of carbodiimide chemistry. Blood samples from healthy donor spiked with A2780 ovarian cancer cells were used for detecting the capture rate. Ninety-five blood samples from 30 patients with EOC were used for comparing the positive rate of detection when using anti-EpCAM-MNs alone with that when using combination of anti-EpCAM-MNs and anti-FRα-MNs. Samples from 28 patients initially diagnosed with EOC and 20 patients with ovarian benign disease were used for evaluating the sensitivity and specificity of combination of anti-EpCAM-MNs and anti-FRα-MNs.
Results
Regression analysis between the number of recovered and that of spiked A2780 cells revealed yEpCAM = 0.535x (R2 = 0.99), yFRα = 0.901x (R2 = 0.99), and yEpCAM+FRα = 0.928x (R2 = 0.99). In mixtures of A2780 and MCF7 cells, the capture rate was 92% using the combination of anti-EpCAM-MNs and anti-FRα-MNs, exceeding the rate when using anti-EpCAM-MNs or anti-FRα-MNs alone by approximately 20% (P < 0.01). The combination of anti-EpCAM-MNs and anti-FRα-MNs showed a significantly increased positive rate of CTC detection in EOC patients compared with anti-EpCAM-MNs alone (χ2 = 14.45, P < 0.001). Sensitivity values were 0.536 and 0.75 and specificity values were 0.9 and 0.85 when using anti-EpCAM-MNs alone and when using the combination of anti-EpCAM-MNs and anti-FRα-MNs, respectively.
Conclusion
The combination of FRα and EpCAM is feasible as a CTC capture target of CTC detection in patients with EOC.
Keywords: circulating tumor cells, ovarian cancer, epithelial cell adhesion molecule, folate receptor alpha
Introduction
Epithelial ovarian cancer (EOC) is one of the deadliest gynecological malignancies, accounting for 2.5% of all malignancies among females, but 5% of cancer-related deaths in this population.1 Owing to the lack of sensitive signs and symptoms and effective screening methods, most patients are diagnosed at stage III (51%) or IV (29%).1 Although survival has improved with the use of cytoreductive surgery along with platinum- and taxane-based chemotherapy, nearly 80% of patients with ovarian cancer eventually relapse within 5 years.1 Therefore, EOC detection in the early stage and monitoring of tumor progression are extremely important for improving patient outcomes.
Circulating tumor cells (CTCs), which are shed from the tumor into the bloodstream, are emerging as novel diagnostic or prognostic biomarkers for solid tumors, including EOC.2 However, CTCs are extremely rare in an extremely complex matrix of blood, requiring a detection method with high sensitivity and specificity. Some detection methods, such as immunomagnetic separation,3 microfluidic separation,4 filter-based methods,5 and ligand-targeted PCR6 have been developed in recent years. Each detection method has limitations because of heterogeneity of CTCs. Among these platforms, the CellSearch system (Veridex, Raritan, NJ, USA) is the first US Food and Drug Administration (US FDA)-approved test for capturing and enumerating CTCs of breast, prostate, or colorectal cancer using anti-epithelial cell adhesion molecule (EpCAM) coated magnetic beads.3 However, because of low EpCAM expression or epithelial-to-mesenchymal transition (EMT) during metastasis in EOC, EpCAM-based enrichment has low sensitivity for CTC detection in patients with EOC.2 Thus, the selection of highly expressed EOC-specific antigens on the cell surface of CTCs is important for improving the CTC detection rate.
Folate receptor alpha (FRα), which is a glycosylated phosphatidylinositol-anchored glycoprotein that binds to folic acid, has high expression in EOC. Studies have reported that >95% of patients with EOC overexpress FRα on the cell membrane, whereas the receptor is expressed at extremely low levels in most healthy tissues.7,8 Thus, FRα is an ideal immune capture target for CTC detection in patients with EOC.
Combining different immune capture targets can improve the CTC detection rate.9–11 Thus, we combined FRα and EpCAM as capture targets for CTC detection in EOC. In this study, we demonstrated higher efficacy and sensitivity of the combination of EpCAM and FRα as capture targets in EOC cell lines and patients with EOC, suggesting their translational potential for future development in CTC detection methods.
Materials And Methods
Cell Culture
A2780 cell (human EOC), MCF7 cell (human breast cancer), A549 cell (human non-small cell lung cancer), and Jurkat T-cell (human T-cell leukemia) lines were purchased from the China Center for Type Culture Collection. MCF7 cells were cultured in DMEM (Life Technologies, Carlsbad, USA) with 10% FBS (ScienCell, Carlsbad, CA, USA), and the other cells were cultured in RPMI 1640 (Life Technologies) with 10% FBS. All cells were cultured at 37°C in a humidified atmosphere containing 5% CO2.
Flow Cytometry And Immunofluorescence Staining
Flow cytometry was used for analyzing EpCAM and FRα expression on the membrane of A2780 cells. Cells were detached using 0.25% trypsin-EDTA (Life Technologies), after which 1 × 106 cells were resuspended in a 10-mL EP tube and fixed with 4% paraformaldehyde (Servicebio, Wuhan, China). After washing with PBS, cells blocked with 2% BSA (Servicebio) and incubated with anti-EpCAM (SAB4700423, Sigma-Aldrich, St. Louis, MO, USA) or anti-FRα (MAB5646, R&D Systems, Minneapolis, MN, USA) monoclonal antibody for 30 mins at 37°C. After washing with PBST, the cells were incubated with Alexa Fluor® 488-conjugated donkey anti-mouse IgG (H + L) secondary antibody (A21202, Life Technologies). Isotype controls were incubated with irrelevant mouse IgG primary and secondary antibodies, and the negative control was not treated after washing with PBS. The fluorescence signal was detected using three-laser FACSCalibur (BD Biosciences, San Jose, CA, USA) and analyzed using FlowJo software, version 10.0 (TreeStar Inc.).
For immunofluorescence, A2780 cells grown on coverslips were fixed using 4% paraformaldehyde for 10 mins, blocked using 2% BSA for 30 mins, and then processed using anti-EpCAM and anti-FRα monoclonal antibodies overnight at 4°C. After thorough washing with PBST (3 × 5 mins), the cells were incubated with Alexa Fluor 488-conjugated donkey anti-mouse IgG (H + L) secondary antibody. Nuclei were visualized using DAPI (Servicebio). After thorough washing with PBST, cells were coverslipped using antifade mounting medium (Servicebio). As a negative control, the primary antibody was omitted, and all other steps were performed as described previously. Fluorescence images were photographed using a fluorescence microscope (BX63, Olympus, Japan).
Construction Of Anti-EpCAM And Anti-FRα Antibody-Modified Magnetic Nanospheres
The principle of carbodiimide chemistry was used for cross-linking the antibodies with magnetic nanospheres (MNs). Specifically, 10 mg of Sera-Mag® speedBeads™ MNs (1 mg/mL, Sigma-Aldrich) were washed twice with PBS (10 mM, pH 6.8) and dispersed into 1000 μL of PBS (10 mM, pH 6.8), after which the samples were incubated with 10 mg/200 μL of EDC (Sigma-Aldrich) and 5 mg/200 μL of NHS (Sigma-Aldrich) for 30 mins to activate the carboxyl groups on the surface of the functional nanospheres. Then, the nanospheres were washed twice with PBS (10 mM, pH 7.2), dispersed in 1000 μL of PBS (10 mM, pH 7.2), and reacted with EpCAM or FRα antibody for 4 hrs with continuous shaking at 37 C. After the reaction was completed, the antibody-modified nanospheres were washed with PBS (10 mM, pH 7.2) and finally stored in PBS (containing 0.05% NaN3 and 1% BSA) at 4°C.
Immunofluorescence was used to verify binding between the antibody and magnetic beads. Specifically, 2 μL of anti-EpCAM-MNs or anti-FRα-MNs was washed twice with PBS, incubated with Alexa Fluor 488-conjugated donkey anti-mouse IgG (H + L) secondary antibody for 30 mins at 37°C, washed with PBST, and coated onto a slide. Fluorescence images were photographed using a fluorescence microscope (BX63, Olympus).
Capture Of Spiked Tumor Cells In PBS, Lysed Blood, And Whole Blood
Hoechst 33342-stained A2780 (EpCAMlow/FRαhigh) cells were added to PBS, lysed blood, and whole blood at a concentration of 200 cells/mL to prepare closely mimicking clinical samples. As controls, immunomagnetic nanospheres (IMNs) were used to capture MCF7 cells (EpCAMhigh/FRαlow), A549 cells (EpCAMlow/FRα−), and Jurkat T-cells (EpCAM−/FRα−) and unmodified MNs were used to capture cells to investigate the specificity of the modified MNs. To prove that high enrichment efficiency was achieved via the capture of EpCAM+ and FRα+ cells, we used a mixture of A2780 and MCF7 cells. Approximately 0.15 mg/mL IMNs were added to the aforementioned samples, and they were incubated at 37°C for 15 mins. Then, they were isolated and washed with a magnetic scaffold. After thorough washing with PBS, the captured cells were counted under a fluorescence microscope. Captured and uncaptured cells were all counted to calculate the capture efficiency. Each experiment was repeated three times.
We tested the ability of IMNs to capture rare A2780 cells in synthetic CTC samples, which were prepared by spiking stained A2780 cells into whole blood at concentrations of 5, 50, 100, 200, and 300 cells/mL. Approximately 0.15 mg/mL IMNs were added to the samples, and they were incubated at 37°C for 15 mins, isolated, and washed with a magnetic scaffold. After thorough washing with PBS, the captured cells were counted under a fluorescence microscope.
ICC Identification And Viability Of The Captured Cells In Mimicked Clinical Samples
A2780 cells were spiked into whole blood at a concentration of approximately 200 cells/mL to prepare mimicking clinical samples. The mimicking clinical samples were incubated with IMNs for 15 mins. After magnetic separation, the captured cells were fixed with 4% paraformaldehyde (10 mins); permeabilized with 0.1% Triton X-100 (10 mins); blocked with 2% BSA (30 mins); and incubated with Alexa Fluor 568-labeled anti-cytokeratin 19 (CK19) monoclonal antibody, Alexa Fluor 488-labeled anti-CD45 monoclonal antibody, and DAPI for 30 mins. After thorough washing with PBS, the cells were coverslipped using antifade mounting medium. Fluorescence images were obtained using a fluorescence microscope BX63 (Figure 1B). Cells with CK19-positive and DAPI-positive but CD45-negative phenotypes were enumerated as CTCs.
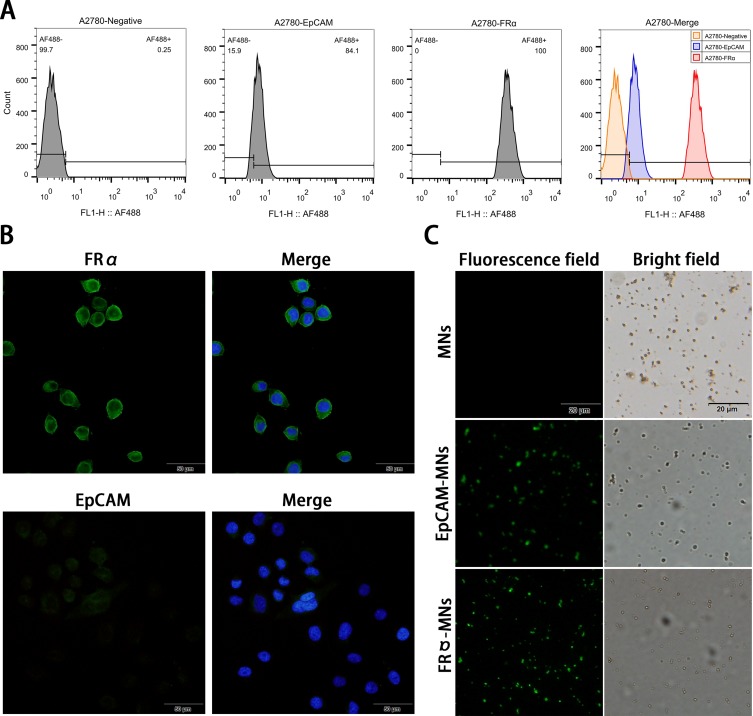
Figure 1.
Expression of EpCAM and FRα in A2780 cells and identification of synthesized MNs. (A) Flow cytometry detection of epithelial cell adhesion molecule (EpCAM) and folate receptor alpha (FRα) expression in A2780 cells. A2780 cells were stained with anti-EpCAM (blue) and anti-FRα antibodies (red) and the negative control was autofluorescent (orange). (B) Immunofluorescence detection of EpCAM and FRα expression in A2780 cells. EpCAM and FRα stained with Alexa Fluor® 488 are green at an excitation wavelength of 488 nm, and nuclei stained with DAPI are blue at an excitation wavelength of 405 nm. (C) Identification of synthesized magnetic nanospheres. The Alexa Fluor 488-conjugated donkey anti-mouse IgG (H + L) secondary antibody specifically bound to antibody-modified magnetic beads (green).
A Live/Dead viability kit was used for analyzing the viability of the captured cells. Cells were stained with calcein-AM and propidium iodide (PI). The final concentration of calcein-AM and PI was 2 and 4 μmol/L, respectively. Then, the cells were observed under a fluorescence microscope, and five fields were randomly selected to calculate the viability rate.
CTC Detection In Patients With EOC
All experimental protocols were approved by the ethics committee of Renmin Hospital of Wuhan University. All experiments were performed in accordance with relevant guidelines and regulations.
Whole blood samples (8 mL) were collected in EDTA tubes (BD Biosciences) and used within 24 hrs. All blood samples were divided into two tubes (4 mL) and captured using anti-EpCAM-MNs or a combination of anti-EpCAM-MNs and anti-FRα-MNs. CTCs were denoted by CK19 and DAPI positivity and CD45 negativity. In total, blood samples were collected from 30 patients with EOC undergoing surgery or chemotherapy and 20 patients with benign ovarian diseases after obtaining informed consent. Among the 30 ovarian cancer patients, blood samples were taken once or up to eight times before surgery or each cycle of chemotherapy, with an average of 3.2 times and a median of 2 times.
Statistical Analysis
Statistical analysis was performed using SPSS software, version 24.0 (SPSS Inc., Armonk, USA). The number of CTCs in different CTC detection method groups was compared using a two-tailed Student’s t-test. A paired chi-square test was used for evaluating the difference between the two detection rates. A two-sided P < 0.05 was considered to be statistically significant.
Results
Expression Of EpCAM And FRα In A2780 Cells And Identification Of Synthesized MNs
EpCAM and FRα expression in A2780 cells were analyzed using flow cytometry. The results illustrated that FRα expression was high in A2780 cells, and the binding rate of the antibody to cells was 99.7%; EpCAM expression was low, and the binding rate of the antibody to cells was 84.1% (Figure 1A). Immunofluorescence also confirmed this result. Specifically, FRα shows high expression on the A2780 cell membrane, whereas EpCAM shows low expression (Figure 1B). The Alexa Fluor 488-conjugated donkey anti-mouse IgG (H + L) secondary antibody specifically bound to antibody-modified MNs, demonstrating successful binding of the antibody to MNs (Figure 1C).
Ability Of IMNs To Capture Target Cells
First, we tested the ability of IMNs to capture A2780 cells in synthetic CTC samples, the capture rate of anti-FRα-MNs to capture A2780 cells was 90.0%, 86.7%, 85.9%, respectively, the capture rate of anti-EpCAM-MNs to capture A2780 cells was 43.4%, 41.9%, 39.1%, respectively, the capture rate of combination of anti-EpCAM-MNs and anti-FRα-MNs to capture A2780 cells was 92.5%, 89.5%, 88.6%, respectively (Figure 2A). The results indicated that anti-FRα-MNs can significantly improve CTC enrichment efficiency compared with anti-EpCAM-MNs (P < 0.001). The efficiency of the test was virtually unaffected by the liquid environment.
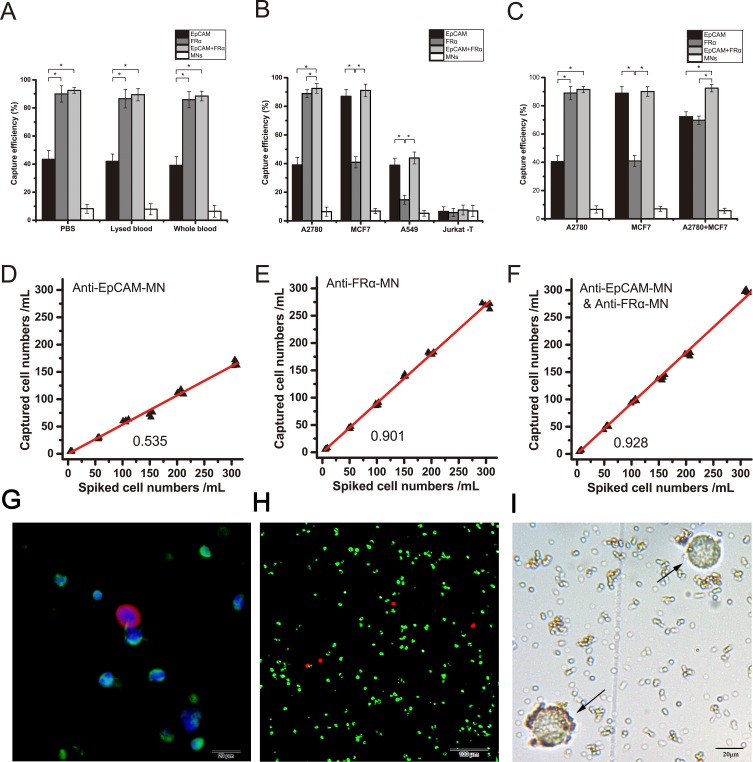
Figure 2.
Efficiencies of anti-EpCAM-MNs and anti-FRα-MNs used alone or in combination to capture target cells. (A) Capture efficiencies of using anti-EpCAM-MNs alone, anti-FRα-MNs alone, or a combination of both to capture A2780 cells in PBS, lysed blood, and whole blood, and unmodified MNs served as a negative control. (B) Capture efficiencies of using anti-EpCAM-MNs alone, anti-FRα-MNs alone, or a combination of both for capturing A2780 cells (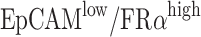 ), MCF7 cells (
), MCF7 cells (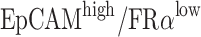 ), A549 cells (
), A549 cells (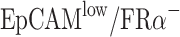 ), and Jurkat T-cells (
), and Jurkat T-cells (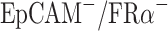 ). (C) Capture efficiencies of anti-EpCAM-MNs (light gray), anti-FRα-MNs (dark gray), combination of anti-EpCAM-MNs and anti-FRα-MNs (white), and unmodified MNs (black) to capture A2780, MCF7, or a mixture of A2780 and MCF7 cells. (D, E, F) The regression analysis plots of recovered vs spiked A2780 cells detected using anti-EpCAM-MNs (D), anti-FRα-MNs (E), and a combination of anti-EpCAM-MNs and anti-FRα-MNs (F). (G) Three-color ICC identified the capture cells. CTCs were CK19-positive (red), DAPI-positive (blue), and CD45-negative (green). (H) Fluorescence microscopic image of the captured cells stained with calcein-AM (green) and propidium iodide (red). (I) Bright field photograph of the captured cells shows that the cells remain intact (arrows). Each experiment was repeated three times. *P < 0.05.
). (C) Capture efficiencies of anti-EpCAM-MNs (light gray), anti-FRα-MNs (dark gray), combination of anti-EpCAM-MNs and anti-FRα-MNs (white), and unmodified MNs (black) to capture A2780, MCF7, or a mixture of A2780 and MCF7 cells. (D, E, F) The regression analysis plots of recovered vs spiked A2780 cells detected using anti-EpCAM-MNs (D), anti-FRα-MNs (E), and a combination of anti-EpCAM-MNs and anti-FRα-MNs (F). (G) Three-color ICC identified the capture cells. CTCs were CK19-positive (red), DAPI-positive (blue), and CD45-negative (green). (H) Fluorescence microscopic image of the captured cells stained with calcein-AM (green) and propidium iodide (red). (I) Bright field photograph of the captured cells shows that the cells remain intact (arrows). Each experiment was repeated three times. *P < 0.05.
Then, we tested the specificity of the detection methods. We used IMNs to capture A2780 cells (EpCAMlow/FRαhigh), MCF7 cells (EpCAMhigh/FRαlow), A549 cells (EpCAMlow/FRα−), and Jurkat T-cells (EpCAM−/FRα−) (Figure 2B). Anti-EpCAM-MNs could capture EpCAM-expressing cells and anti-FRα-MNs could capture FRα-expressing cells, the capture rate was more than 88%. Neither type of MNs could capture Jurkat T-cells, the capture rate was less than 6.6%. Thus, IMNs modified with different antibodies can only capture the target cells of the corresponding antigen. Unmodified MNs could hardly capture target cell including A2780 cells, MCF7 cells, A549 cells and Jurkat T cells, and the capture rate was less than 6.9%. These results demonstrated that IMNs have good specificity.
To demonstrate that high enrichment efficiency was achieved by the capture of EpCAM+ and FRα+ cells, we used a mixture of A2780 cells (EpCAMlow/FRαhigh) and MCF7 cells (EpCAMhigh/FRαlow) cells. In the mixed cell samples, the capture rate was more than 92.5% using a combination of anti-EpCAM-MNs and anti-FRα-MNs, which exceeded the rate when using anti-EpCAM-MNs or anti-FRα-MNs alone by approximately 20% (P < 0.01) (Figure 2C). Therefore, we demonstrated that the enrichment efficiency was improved by the capture of two antigens (EpCAM and FRα) expressed on the cell membrane.
We tested the ability of IMNs to capture rare A2780 cells in synthetic CTC samples. When the concentration of the mixed cells ranges from 5 to 300 cells, the relationship between the numbers of recovered and spiked tumor cells was linear, and the regression analysis produced yEpCAM = 0.535x (R2 = 0.99, 95% CI = 0.523–0.547) (Figure 2D), yFRα = 0.901x (R2 = 0.99, 95% CI = 0.889–0.913) (Figure 2E), and yEpCAM+FRα = 0.928x (R2 = 0.99, 95% CI = 0.914–0.942) (Figure 2F). Therefore, it can be concluded that IMNs can efficiently and specifically capture rare target tumor cells from whole blood.
0.535x (R2 = 0.99, 95% CI = 0.523–0.547) (Figure 2D), yFRα = 0.901x (R2 = 0.99, 95% CI = 0.889–0.913) (Figure 2E), and yEpCAM+FRα = 0.928x (R2 = 0.99, 95% CI = 0.914–0.942) (Figure 2F). Therefore, it can be concluded that IMNs can efficiently and specifically capture rare target tumor cells from whole blood.
ICC Identification And Viability Of The Captured Cells
A three-color ICC method including AF488-labeled anti-CD45, AF594-labeled anti-CK19, and DAPI was used for identifying the captured cells. CK19 is a marker for epithelial cells, and CD45 is a marker for white blood cells. CK19-positive (red), DAPI-positive (blue), and CD45-negative cells (green) were enumerated as CTCs (Figure 2G).
Calcein-AM and PI were used for staining cells to analyze the capture ability. Calcein-AM can penetrate the live cell membrane and react with intracellular esterase to form calcein with green fluorescence, whereas PI is a membrane-impermeable nuclear stain that can stain only dead cells with red fluorescence. As shown in Figure 2H, majority of the isolated cells exhibited green fluorescence, and the viability rate was 92%±1.8, indicating that most of the tumor cells remained viable after isolation. The bright field photographs illustrate that the integrity of the cells was good and that the IMNs bound to the surface of the cells (Figure 2I).
CTC Detection In Peripheral Blood Samples From Patients With EOC
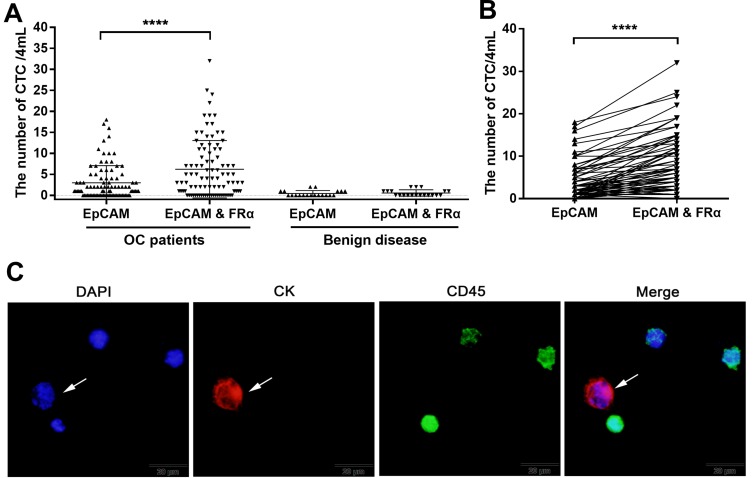
In total, Ninety-five blood samples were collected from 30 patients with EOC undergoing surgery or chemotherapy and 20 patients with benign ovarian diseases after obtaining written informed consent. Patient characteristics are shown in Table 1. Among the 30 ovarian cancer patients, blood samples were taken once or up to eight times before surgery or each cycle of chemotherapy, with an average of 3.2 times and a median of 2 times, each samples were tested using anti-EpCAM-MNs alone or using a combination of anti-EpCAM-MNs and anti-FRα-MNs. CTC levels in the two groups, as measured using each method, are plotted in Figure 3A; the results from the same patient with EOC are shown in Figure 3B. The CTC level in patients with EOC was 0–18 when using anti-EpCAM-MNs and 0–32 when using the combination of anti-EpCAM-MNs and anti-FRα-MNs. The CTC level was 0–2 for patients with benign diseases. The area under the receiver operating characteristic curve (AUC-ROC) analysis indicated that the combination of anti-EpCAM-MNs and anti-FRα-MNs had a higher AUC (0.8011, 95% CI: 0.7212–0.8809, P < 0.001) than anti-EpCAM-MNs alone (0.7208, 95% CI: 0.6209–0.8206, P = 0.002). When the cut-off value was set to 2 (CTC number 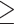 2 is CTC positive and CTC number
2 is CTC positive and CTC number  2 is CTC negative), the highest Youden’s index was achieved. At this cut-off, the positive rate was 48.42% using anti-EpCAM-MNs alone and 67.36% using the combination of anti-EpCAM-MNs and anti-FRα-MNs. A paired chi-square test revealed a significant difference between the two methods (χ2= 14.45, P < 0.001) (Table 2). An image gallery of representative CTCs from patients with EOC is shown in Figure 3C.
2 is CTC negative), the highest Youden’s index was achieved. At this cut-off, the positive rate was 48.42% using anti-EpCAM-MNs alone and 67.36% using the combination of anti-EpCAM-MNs and anti-FRα-MNs. A paired chi-square test revealed a significant difference between the two methods (χ2= 14.45, P < 0.001) (Table 2). An image gallery of representative CTCs from patients with EOC is shown in Figure 3C.
Table 1.
Patient Characteristics
| Characteristic | N | Percent |
|---|---|---|
| Total | 30 | 100.0% |
| Initial | 28 | 93.3% |
| Relapse | 2 | 6.7% |
| Histological type | ||
| Serous carcinoma | 25 | 83.3% |
| Mucinous carcinoma | 2 | 6.7% |
| Clear cell carcinoma | 3 | 10.0% |
| AJCC stage | ||
| I–II | 9 | 30.0% |
| III–IV | 21 | 70.0% |
| Grade | ||
| Low-grade | 7 | 23.3% |
| Borderline | 2 | 6.7% |
| High-grade | 18 | 60.0% |
| Unknown | 3 | 10.0% |
Figure 3.
CTC detection in peripheral blood samples from patients with EOC. (A) CTC levels in 95 blood samples from 30 patients with EOC and 20 patients with benign disease as identified using anti-EpCAM-MNs alone or a combination of anti-EpCAM-MNs and anti-FRα-MNs. (B) Paired comparison shows the number of CTCs detected using anti-EpCAM-MNs alone or a combination of anti-EpCAM-MNs and anti-FRα-MN. (C) Fluorescence microscopic images of cells isolated from patients with EOC and identified using three-color immunofluorescence staining. The cells were stained with Alexa Fluor® 568-labeled anti-CK19 monoclonal antibody (red), Alexa Fluor 488-labeled anti-CD45 monoclonal antibody (green), and DAPI (blue). The Cytokeratin 19 (CK19)-positive (red), DAPI-positive (blue), and CD45-negative cells (green) indicated by the arrows were enumerated as CTCs. ****P < 0.0001.
Table 2.
Paired Chi-Square Test Of The Positive Rate When Using Anti-EpCAM-MNs Alone And That When Using A Combination Of Anti-EpCAM-MNs And Anti-FR-MNs In 95 Blood Samples From 30 Patients With Epithelial Ovarian Cancer
| Anti-EpCAM-MNs | χ2 | P | ||||
|---|---|---|---|---|---|---|
| CTC ≥2 | CTC <2 | Total | ||||
| Anti-EpCAM-MN and anti-FRα-MN | CTC ≥2 | 45 | 19 | 64 | 14.45 | 0.000 |
| CTC <2 | 1 | 30 | 31 | |||
| Total | 46 | 49 | 95 | |||
Abbreviations: CTC, circulating tumor cells; EpCAM, epithelial cell adhesion molecule; FRα, folate receptor alpha; MNs, magnetic nanospheres.
Sensitivity And Specificity Of CTC Detection In Patients With Suspected EOC
Of the 30 patients with EOC, 28 were initially diagnosed with EOC. The number of CTCs tested separately and jointly before surgery in 28 patients with EOC and 20 patients with benign disease is shown in Table 3. The sensitivity and specificity of CTC detection using anti-EpCAM-MNs were 0.536 and 0.9, respectively, and that using the combination of anti-EpCAM-MNs and anti-FRα-MNs was 0.75 and 0.85, respectively. Thus, the combination of anti-EpCAM-MNs and anti-FRα-MNs can improve the sensitivity of CTC detection in patients with newly diagnosed EOC (χ2 = 4.17, P = 0.041). There was no statistical difference with respect to specificity. The Youden’s index was 0.436 when using anti-EpCAM-MNs and 0.600 for the combination of anti-EpCAM-MNs and anti-FRα-MNs.
Table 3.
Sensitivity And Specificity In 28 Patients With Initially Diagnosed Epithelial Ovarian Cancer And 20 Patients With Benign Disease
| Groups | Patients | Healthy Donor | |||||
|---|---|---|---|---|---|---|---|
| Anti-EpCAM-MNs And Anti-FRα-MNs | Anti ≥2-EpCAM-MNs And Anti-FRα-MNs | ||||||
| CTC ≥2 | CTC <2 | Total | CTC | CTC <2 | Total | ||
| Anti-EpCAM-MNs | CTC ≥2 | 15 | 0 | 15 | 0 | 2 | 2 |
| CTC <2 | 6 | 7 | 13 | 3 | 15 | 18 | |
| Total | 21 | 7 | 28 | 3 | 17 | 20 | |
| Groups | Sensitivity | Specificity | Youden’s index | ||||
| Anti-EpCAM-MNs and anti-FRα-MNs | 0.750 | 0.850 | 0.600 | ||||
| Anti-EpCAM-MNs | 0.536 | 0.900 | 0.436 | ||||
Abbreviations: CTC, circulating tumor cells; EpCAM, epithelial cell adhesion molecule; FRα, folate receptor alpha; MNs, magnetic nanospheres.
Discussion
Previous studies have shown that CTCs from the same type of cancer and even from the same patient have extensive molecular and cellular heterogeneity. The CellSearch system remains the only CTC product approved by the FDA. However, because of high heterogeneity of cancer cells, clinical studies showed low sensitivity of EpCAM-based enrichment for CTC detection in patients with EOC. This was mainly due to EMT during metastasis, leading to the loss of more epithelium-like CTCs. FRα is highly expressed in ovarian cancer. It is a potential target for CTC capture of ovarian cancer, so we combined EpCAM and FRα as a capture target for CTCs to improve the detection rate of CTCs. First, in the mimic clinical sample, our results showed that the combined detection method can increase the capture rate of ovarian cancer CTC by capturing two target antigens. In the mixed cell samples of A2780 cells (EpCAMlow/FRαhigh) and MCF7 cells (EpCAMhigh/FRαlow) cells, the capture rate was more than 92.5% using a combination of anti-EpCAM-MNs and anti-FRα-MNs, which exceeded the rate when using anti-EpCAM-MNs or anti-FRα-MNs alone by approximately 20% (P < 0.01). This result showed that the selection of different targets is important for capturing CTCs with different molecular expression characteristics. Studies have shown that there is an increase in the expression of FRα in tumor cells with low expression of EpCAM,12 which is the theoretical basis for the capture of more heterogeneous CTCs by EpCAM combined with FRα as a CTC capture target. EpCAM and FRα appeared to be complementary CTC capture targets, but due to the heterogeneity of the tumor, the combination of EpCAM and FRα may not be able to include all CTCs. More research will need to optimize the capture targets of CTC.
In the CTC detection of patients with 95 blood samples collected from 30 patients with EOC and 20 blood samples collected from 20 patients with ovarian benign disease, the results demonstrated that combined FRα as a capture target for immune capture based on EpCAM could significantly increase the capture rate of CTCs. When the threshold for positive CTCs in this study was ≥2, the positive rate for the combined detection method was 67.36%, whereas that for anti-EpCAM-MNs alone was 48.42%, and this difference was statistically significant (χ2 = 14.45, P < 0.001). In patients with suspected EOC, the sensitivity of the combined detection method for CTCs was 75.0%, which was significantly higher than that when using anti-EpCAM-MNs alone (χ2 = 4.17, P = 0.041). The area under the receiver operating characteristic curve (AUC-ROC) analysis indicated that the combination of anti-EpCAM-MNs and anti-FRα-MNs had a higher AUC (0.8011, 95% CI: 0.7212–0.8809, P < 0.001) than anti-EpCAM-MNs alone (0.7208, 95% CI: 0.6209–0.8206, P = 0.002), although the t-test showed no statistically significant difference between the two AUC (P = 0.12), the reason may be that the sample size is insufficient. The low detection rate of CTC detection using EpCAM as a capture target in patients with ovarian cancer in this study is consistent with multiple previous studies. A study used Cellsearch to capture CTC in 29 newly diagnosed ovarian cancer patients, only found that CTC can be detected in 17% of patients.13 Behbakht’s research showed that CTC was enriched in 19/43 (44%) blood specimens prior to the first cycle of chemotherapy in patients with ovarian cancer.14 Poveda’s study showed that 14.4% (31/216) patients with ovarian cancer had 2 or more CTCs prior to the start of therapy.15 Liu’s study found that when the CTC threshold was set to ≥2, 60% (18/30) of ovarian cancer patients in the initial stage had CTC positive, and 58% (28/48) of the ovarian patients who relapsed had CTC positive.16
Intact CTCs can be used for determining functional cellular characteristics17 and performing molecular profiling at the DNA, RNA, and protein levels.18 This might guide personalized treatment selection, particularly after treatment failure. Moreover, CTC analysis enables multiple mutation detection within the same cell, thereby making mapping of the clonal evolution and tumor heterogeneity possible and unraveling potential associations between the mutational status and pathway activation by combining genomic and transcriptomic profiles of CTCs.19,20 Therefore, the CTC detection method should also include the step of nondestructive release. Our research revealed that after separation, CTCs were intact and viable. However, the nondestructive release of CTCs in our method requires further research because antibody/antigen interaction is strong and harsh treatment is needed to release the bound target. An emerging method using aptamers, which can be an alternative to CTC capture using antibodies, is currently under investigation. Some researchers have reported that aptamers can release CTCs without harsh procedures,21 such as the introduction of a complementary sequence to compete with binding with the target,22 removal via thermal denaturation,23 or wash steps using endonucleases.24 However, for antibody/antigen interactions, harsh treatment, such as enzyme degradation,25 is needed to release the bound target.
Although this study was able to capture EpCAM+ cells and FR+ cells, there may be some CTCs with phenotypic characteristic of EpCAM-FR-. Another potential limitation is that one CK19+/DAPI+/CD45- cell is detected in 8% of (8/95) ovarian cancer patients and 25% of healthy people, but it was not confirmed whether this one CK19+/DAPI+/CD45- cell was truly malignant or represented benign circulating epithelial cells. The possible reason may be that we did not strictly request to exclude the first tube blood sample during the collection process and may lead to the incorporation of vascular endothelial cells.
In summary, the results of this exploratory study show for the first time that the combined immunomagnetic separation technique of combined EpCAM and FR can be used to identify and count CTC in certain ovarian cancer patients. The combination of EpCAM and FRα as capture targets can significantly increase the CTC capture rate and prevent low EpCAM-expressing or EMT-converted CTCs from being missed. More experiments are required for subsequent analysis of FRα+ CTCs. Meanwhile, the number of tested samples and follow-up time are being studied to assess the correlation between FRα+ CTCs and clinical outcome.
Conclusion
The combined use of surface markers of EpCAM and FRα as CTC capture targets in EOC can improve the sensitivity of CTC detection. Captured CTCs were intact and viable, and they could be used in subsequent analyses, such as single-cell sequencing. Despite this promising result, further studies are required to assess the clinical validity of this method in patients with EOC.
Acknowledgments
The authors thank the reviewers for their helpful comments on this article. This study was funded by National Natural Science Foundation of China (NNSFC) (81802980, 81770169, and 81670144) and the Young Talent Foundation of Health and Family Planning Commission of Hubei Province (WJ2017Q007).
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Torre LA, Trabert B, DeSantis CE, et al. Ovarian cancer statistics, 2018. CA Cancer J Clin. 2018;68(4):284–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Giannopoulou L, Kasimir-Bauer S, Lianidou ES. Liquid biopsy in ovarian cancer: recent advances on circulating tumor cells and circulating tumor DNA. Clin Chem Lab Med. 2018;56(2):186–197. doi: 10.1515/cclm-2017-0019 [DOI] [PubMed] [Google Scholar]
- 3.Dementeva N, Kokova D, Mayboroda OA. Current methods of the Circulating Tumor Cells (CTC) analysis: a brief overview. Curr Pharm Des. 2017;23(32):4726–4728. doi: 10.2174/1381612823666170616082608 [DOI] [PubMed] [Google Scholar]
- 4.Burinaru TA, Avram M, Avram A, et al. Detection of circulating tumor cells using microfluidics. ACS Comb Sci. 2018;20(3):107–126. doi: 10.1021/acscombsci.7b00146 [DOI] [PubMed] [Google Scholar]
- 5.Rawal S, Ao Z, Datar RH, Agarwal A. Microfilter-based capture and release of viable circulating tumor cells. Methods Mol Biol. 2017;1634:93–105. doi: 10.1007/978-1-4939-7144-2_7 [DOI] [PubMed] [Google Scholar]
- 6.Chen X, Zhou F, Li X, et al. Folate receptor-positive circulating tumor cell detected by LT-PCR-based method as a diagnostic biomarker for non-small-cell lung cancer. J Thorac Oncol. 2015;10(8):1163–1171. doi: 10.1097/JTO.0000000000000606 [DOI] [PubMed] [Google Scholar]
- 7.Kalli KR, Oberg AL, Keeney GL, et al. Folate receptor alpha as a tumor target in epithelial ovarian cancer. Gynecol Oncol. 2008;108(3):619–626. doi: 10.1016/j.ygyno.2007.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oseledchyk A, Andreou C, Wall MA, Kircher MF. Folate-targeted surface-enhanced resonance raman scattering nanoprobe ratiometry for detection of microscopic ovarian cancer. ACS Nano. 2017;11(2):1488–1497. doi: 10.1021/acsnano.6b06796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen L, Peng M, Li N, et al. Combined use of EpCAM and FRalpha enables the high-efficiency capture of circulating tumor cells in non-small cell lung cancer. Sci Rep. 2018;8(1):1188. doi: 10.1038/s41598-018-19391-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu S, Tian Z, Zhang L, et al. Combined cell surface carbonic anhydrase 9 and CD147 antigens enable high-efficiency capture of circulating tumor cells in clear cell renal cell carcinoma patients. Oncotarget. 2016;7(37):59877–59891. doi: 10.18632/oncotarget.10979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weissenstein U, Schumann A, Reif M, Link S, Toffol-Schmidt UD, Heusser P. Detection of circulating tumor cells in blood of metastatic breast cancer patients using a combination of cytokeratin and EpCAM antibodies. BMC Cancer. 2012;12:206. doi: 10.1186/1471-2407-12-206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lou J, Ben S, Yang G, et al. Quantification of rare circulating tumor cells in non-small cell lung cancer by ligand-targeted PCR. PLoS One. 2013;8(12):e80458. doi: 10.1371/journal.pone.0080458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lou E, Vogel RI, Teoh D, et al. Assessment of circulating tumor cells as a predictive biomarker of histology in women with suspected ovarian cancer. Lab Med. 2018;49(2):134–139. doi: 10.1093/labmed/lmx084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Behbakht K, Sill MW, Darcy KM, et al. Phase II trial of the mTOR inhibitor, temsirolimus and evaluation of circulating tumor cells and tumor biomarkers in persistent and recurrent epithelial ovarian and primary peritoneal malignancies: a gynecologic oncology group study. Gynecol Oncol. 2011;123(1):19–26. doi: 10.1016/j.ygyno.2011.06.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poveda A, Kaye SB, McCormack R, et al. Circulating tumor cells predict progression free survival and overall survival in patients with relapsed/recurrent advanced ovarian cancer. Gynecol Oncol. 2011;122(3):567–572. doi: 10.1016/j.ygyno.2011.05.028 [DOI] [PubMed] [Google Scholar]
- 16.Liu JF, Kindelberger D, Doyle C, Lowe A, Barry WT, Matulonis UA. Predictive value of circulating tumor cells (CTCs) in newly-diagnosed and recurrent ovarian cancer patients. Gynecol Oncol. 2013;131(2):352–356. doi: 10.1016/j.ygyno.2013.08.006 [DOI] [PubMed] [Google Scholar]
- 17.Baccelli I, Schneeweiss A, Riethdorf S, et al. Identification of a population of blood circulating tumor cells from breast cancer patients that initiates metastasis in a xenograft assay. Nat Biotechnol. 2013;31(6):539–544. doi: 10.1038/nbt.2576 [DOI] [PubMed] [Google Scholar]
- 18.Heitzer E, Auer M, Gasch C, et al. Complex tumor genomes inferred from single circulating tumor cells by array-CGH and next-generation sequencing. Cancer Res. 2013;73(10):2965–2975. doi: 10.1158/0008-5472.CAN-12-4140 [DOI] [PubMed] [Google Scholar]
- 19.Brouwer A, De Laere B, Peeters D, et al. Evaluation and consequences of heterogeneity in the circulating tumor cell compartment. Oncotarget. 2016;7(30):48625–48643. doi: 10.18632/oncotarget.8015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van Berckelaer C, Brouwers AJ, Peeters DJ, Tjalma W, Trinh XB, van Dam PA. Current and future role of circulating tumor cells in patients with epithelial ovarian cancer. Eur J Surg Oncol. 2016;42(12):1772–1779. doi: 10.1016/j.ejso.2016.05.010 [DOI] [PubMed] [Google Scholar]
- 21.Zamay AS, Zamay GS, Kolovskaya OS, Zamay TN, Berezovski MV. Aptamer-based methods for detection of circulating tumor cells and their potential for personalized diagnostics. Adv Exp Med Biol. 2017;994:67–81. doi: 10.1007/978-3-319-55947-6_3 [DOI] [PubMed] [Google Scholar]
- 22.Zhang Z, Chen N, Li S, Battig MR, Wang Y. Programmable hydrogels for controlled cell catch and release using hybridized aptamers and complementary sequences. J Am Chem Soc. 2012;134(38):15716–15719. doi: 10.1021/ja307717w [DOI] [PubMed] [Google Scholar]
- 23.Zhu J, Nguyen T, Pei R, Stojanovic M, Lin Q. Specific capture and temperature-mediated release of cells in an aptamer-based microfluidic device. Lab Chip. 2012;12(18):3504–3513. doi: 10.1039/c2lc40411g [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nair SV, Witek MA, Jackson JM, et al. Enzymatic cleavage of uracil-containing single-stranded DNA linkers for the efficient release of affinity-selected circulating tumor cells. Chem Commun. 2015;51(15):3266–3269. doi: 10.1039/c4cc09765c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sheng W, Ogunwobi OO, Chen T, et al. Capture, release and culture of circulating tumor cells from pancreatic cancer patients using an enhanced mixing chip. Lab Chip. 2014;14(1):89–98. doi: 10.1039/c3lc51017d [DOI] [PMC free article] [PubMed] [Google Scholar]