Abstract
Spinal cord injury (SCI) is characterized by vascular disruption leading to ischemia, decreased oxygen delivery, and loss of mitochondrial homeostasis. This mitochondrial dysfunction results in loss of cellular functions, calcium overload, and oxidative stress. Pharmacological induction of mitochondrial biogenesis (MB) may be an effective approach to treat SCI. LY344864, a 5-hydroxytryptamine 1F (5-HT1F) receptor agonist, is a potent inducer of MB in multiple organ systems. To assess the efficacy of LY344864-induced MB on recovery post-SCI, female mice were subjected to moderate force-controlled impactor-induced contusion SCI followed by daily LY344864 administration for 21 days. Decreased mitochondrial DNA and protein content was present in the injury site 3 days post-SCI. LY344864 treatment beginning 1 h after injury attenuated these decreases, indicating MB. Additionally, injured mice treated with LY344864 displayed decreased Evan’s Blue dye accumulation in the spinal cord compared with vehicle-treated mice 7 days after injury, suggesting restoration of vascular integrity. LY344864 also increased locomotor capability, with treated mice reaching a Basso-Mouse Scale score of 3.4 by 21 days, whereas vehicle-treated mice exhibited a score of 1.9. Importantly, knockout of the 5-HT1F receptor blocked LY344864-induced recovery. Remarkably, a similar degree of locomotor restoration was observed when treatment initiation was delayed until 8 h after injury. Furthermore, cross-sectional analysis of the spinal cord 21 days after injury revealed decreased lesion volume with delayed LY344864 treatment initiation, emphasizing the potential clinical applicability of this therapeutic approach. These data provide evidence that induction of MB via 5-HT1F receptor agonism may be a promising strategy for the treatment of SCI.
SIGNIFICANCE STATEMENT
Treatment with LY344864 induces mitochondrial biogenesis in both the naive and injured mouse spinal cord. In addition, treatment with LY344864 beginning after impactor-induced contusion spinal cord injury improves mitochondrial homeostasis, blood–spinal cord barrier integrity, and locomotor function within 7 days. Importantly, similar locomotor results are observed whether treatment is initiated at 1 h after injury or 8 h after injury. These data indicate the potential for pharmacological induction of mitochondrial biogenesis through a 5-hydroxytryptamine 1F agonist as a novel therapeutic approach for spinal cord injury.
Introduction
Traumatic spinal cord injury (SCI) is a debilitating disorder with no meaningful pharmacological therapy. There are approximately 18,000 new cases of SCI documented each year in the United States alone. With an individual cost of care estimated at $3 million, SCI places a tremendous burden on patients, caregivers, and the health care system (Devivo, 2012; Fitzharris et al., 2014). As such, continued research into the development of therapeutics for individuals suffering from SCI remains a necessity.
SCI is composed of the primary injury, or immediate mechanical damage, followed by secondary injury, beginning within seconds and, depending on the severity of the trauma, potentially lasting years (Oyinbo, 2011). Primary injury results in extensive vascular damage, including vasoconstriction and ischemia (Hu et al., 2015, 2016), leading to insufficient oxygen delivery and subsequent mitochondrial dysfunction (Rasbach et al., 2010; Hu et al., 2016). Given that neuronal cells are highly reliant on ATP-driven processes (Castro et al., 1997; Tian et al., 2016), failure to maintain adequate energy production exacerbates secondary injury, resulting in further cell death and dysfunction (Castro et al., 1997; Scholpa et al., 2018, 2019).
Therapeutics aimed at mitigating secondary injury have the potential to limit injury spread and promote the opportunity for recovery (Oyinbo, 2011). Mitochondrial dysfunction post-SCI is essential to the propagation of secondary injury, and evidence suggests that restoration of mitochondrial homeostasis shortly after injury may improve neuronal survival and promote functional recovery (Sullivan et al., 2007; Rabchevsky et al., 2011; Scholpa and Schnellmann, 2017). Multiple studies have targeted mitochondria as a therapeutic strategy following SCI, specifically focusing on consequences of mitochondrial dysfunction, such as increased oxidative damage or altered mitochondrial dynamics (Teng et al., 2004; Patel et al., 2010; Hall, 2011; McEwen et al., 2011; Monaco et al., 2013). We propose that pharmacological induction of mitochondrial biogenesis (MB) is a more comprehensive approach to restore mitochondrial function and promote recovery post-SCI.
MB is a dynamic process of generating new, functional mitochondria that involves a complex network of transcriptional pathways governed by the “master regulator,” peroxisome proliferator–activated receptor y coactivator-1α (PGC-1α) (Rasbach et al., 2010; Wills et al., 2012; Scholpa and Schnellmann, 2017). PGC-1α expression is rapidly decreased in the spinal cord following contusion injury in vivo (Hu et al., 2015; Scholpa et al., 2019), suggesting impaired MB. Interestingly, augmentation of PGC-1α expression post-SCI has been shown to not only improve mitochondrial homeostasis but also reduce lesion volume and improve functional recovery (Hu et al., 2016; Scholpa et al., 2019).
Neuronal 5-hydroxytryptamine (serotonin, 5-HT) receptors are involved in generating and regulating locomotor activity (Ghosh and Pearse, 2015). Following SCI, there is a disruption in descending serotonergic projections in spinal motor areas implicated in locomotor dysfunction (Ghosh and Pearse, 2015). Treatment with exogenous serotonin or a 5-HT analog has been shown to promote locomotor recovery following SCI (Ghosh and Pearse, 2015). A caveat of this approach, however, is the activation of many classes of 5-HT receptors (Ghosh and Pearse, 2015). Through our drug discovery program to ascertain inducers of MB (Beeson et al., 2010), we identified the 5-HT1F receptor as a mediator of MB (Beeson et al., 2010; Rasbach et al., 2010). This receptor, although not fully characterized, is found in the spinal cord and various brain regions (Castro et al., 1997; Tian et al., 2016). In addition, high levels of the 5-HT1F receptor were detected in human vasculature (Nilsson et al., 1999). Though agonism of the 5-HT1F receptor is known to decrease migraines (Mitsikostas and Tfelt-Hansen, 2012; Vila-Pueyo, 2018), the full extent of its role in the central nervous system (CNS), particularly following injury, has not yet been determined.
We previously showed that treatment with the specific 5-HT1F agonist LY344864 increases MB in multiple organ systems, including the CNS (Gibbs et al., 2018b; Scholpa et al., 2018). Furthermore, treatment with LY344864 in a mouse model of Parkinson disease increased MB, attenuated neuronal loss, and improved behavioral endpoints (Scholpa et al., 2018). Given these data and the detrimental impact of mitochondrial dysfunction post-SCI, the goal of this study was to assess the therapeutic efficacy of LY344864-induced MB on recovery after SCI.
Methods
Animal Handling and Care.
All mice were purchased from Jackson Laboratories, and female mice (8–10 weeks of age) were used in all experiments. Male and female 5-HT1F receptor knockout (KO) mice (B6N(Cg)-Htr1ftm1.1(KOMP)Vlcg/J, stock no. 024269) were used to generate an in-house breeding colony. For experiments using the KO strain, age-matched females of the suggested wild-type (WT) control line (C57bl/6NJ) were purchased. In all other experiments, female C57bl/6J mice were used. All animals were housed in groups of three to five in temperature-controlled conditions under a light/dark photocycle with unrestricted food and water supplied ad libitum.
LY344864 was obtained from Tocris Bioscience (Ellisville, MO) and dissolved in saline/0.5% DMSO. To determine the MB effect of LY344864 in the spinal cord under physiologic conditions, naïve mice were treated with 2.0 mg/kg LY344864 i.p. once daily for 21 days, followed by euthanasia via isoflurane overdose and subsequent isolation of the T9-13 region of the spinal cord.
In the SCI model, mice were randomized into sham and SCI groups. Animals were anesthetized with 10 mg/kg ketamine and 6 mg/kg xylazine i.p. and continuously monitored for spontaneous breathing. Mice underwent a complete single-level laminectomy at the 10th–12th thoracic vertebrae (T10–12). The vertebral column was clamped and stabilized at the upper thoracic and lumbar levels, and a controlled contusion with a force of 80 kdyn was administered using the Infinite Horizon IH-0400 impactor (Lexington, KY) with the dura intact. Sham mice received laminectomy only. Manual bladder expression was performed twice daily until functional recovery. Injured mice were further randomized into LY344864- or vehicle-treated groups and treated intraperitoneally with either LY344864 or vehicle control beginning 1 or 8 h after injury and continuing daily until euthanasia. A dose of 2 mg/kg LY344864 used in our previous studies (Gibbs et al., 2018a; Scholpa et al., 2018) was continued in these SCI experiments. Groups were euthanized 3, 7, or 21 days post-SCI via isoflurane overdose, and spinal cords were isolated for analysis. All studies were approved by the University of Arizona in accordance with the guidelines set forth by the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Basso Mouse Scale Assessment.
Locomotor capability was assessed using the 10-point (0–9) Basso Mouse Scale (BMS) (Basso et al., 2006) by two trained observers blinded to experimental groups. Each mouse was observed for 3 minutes, with bladder expression taking place prior to assessment. Animals were observed 24 h after surgery and every other day thereafter until euthanasia. Sham animals maintained full locomotor capabilities with a BMS score of 9 throughout the experiment.
RNA Isolation and Quantitative PCR.
The injury site of the spinal cord was collected and total RNA extracted using TRIzol reagent (Invitrogen, Carlsbad, CA) based on the manufacturer’s protocol. cDNA was synthesized using the iScript cDNA Synthesis Kit, and quantitative PCR (qPCR) was performed using 500 ng of cDNA template and SsoAdvanced Universal SYBR Green Supermix (Bio-Rad, Hercules, CA). Fold changes were calculated using the ∆∆Ct method and normalized to the nuclear encoded gene β-actin.
DNA Isolation and qPCR.
The injury site of the spinal cord was collected and total RNA extracted using TRIzol reagent (Invitrogen) based on the manufacturer’s protocol. The cDNA was synthesized using the iScript cDNA Synthesis Kit, and qPCR was performed using 500 ng of cDNA template and SsoAdvanced Universal SYBR Green Supermix (Bio-Rad). Fold changes were calculated using the ΔΔCt method. The primers used can be found in Table 1.
TABLE 1.
| Target | Sense | Antisense |
|---|---|---|
| PGC-1α | 5′-AGGAAATCCGAGCGGAGCTGA-3′ | 5′-GCAAGAAGGCGACACATCGAA-3′ |
| ND1 | 5′-TAG AAC GCA AAA TCT TAG GG-3′ | 5′-TGC TAG TGT GAG TGA TAG GG-3′ |
| β-Actin | 5′-GGGATGTTTGCTCCAACCAA-3′ | 5′-GCGCTTTTGACTCAGGATTTAA-3′ |
| 5-HT1F | 5′-GCCGTGATGATGAGTGTGTC-3′ | 5′-ACTATCCGACTCGCTTGTCT-′3 |
DNA was isolated from the injury and peri-injury sites using the Qiagen DNeasy Blood and Tissue Kit (Valencia, CA) with 5 ng used for qPCR of relative mitochondrial DNA (mtDNA) content. ND1, the mitochondrially encoded NADH dehydrogenase subunit 1 gene, was measured and normalized to the nuclear encoded gene β-actin.
To determine the presence of the 5-HT1F receptor gene, genomic DNA was amplified using Promega 2× PCR Master Mix (Promega, Madison, WI) in accordance with the manufacturer’s protocols. Amplified DNA was separated on a 2.5% agarose gel and visualized by ethidium bromide fluorescence.
Protein Isolation and Immunoblot Analysis.
Protein was extracted from the injury site of the spinal cord using RIPA buffer (50 mM Tris-HCl, 150 mM NaCl, 0.1% SDS, 0.5% sodium deoxycholate, 1% Triton X-100, pH 7.4) with protease inhibitor cocktail (1:100), mM sodium fluoride, and 1 mM sodium orthovanadate (MilliporeSigma, Burlington, MA). Samples were agitated for 2 h at 4°C and then centrifuged at 14,000g for 20 minutes, and the supernatant was collected. Protein was quantified using a bicinchoninic acid assay, and 10–12 µg protein was separated via electrophoresis using 4%–15% SDS-PAGE gels and then transferred to nitrocellulose membranes (Bio-Rad). Membranes were blocked in 5% milk in Tris-buffered saline/Tween 20 and incubated overnight with primary antibodies with constant agitation at 4°C. Membranes were incubated with the appropriate horseradish peroxidase–conjugated secondary antibody and visualized using chemiluminescence (Thermo Fisher Scientific, Waltham, MA) on a GE ImageQuant LAS4000 (GE Life Sciences, Pittsburgh, PA). Optical density was determined using ImageJ software. Primary antibodies used were as follows: nuclear respiratory factor 2 (Nrf2) (1:500; Santa Cruz Biotechnology, Dallas, TX), PGC-1α (1:1000), mitochondrial transcription factor A (TFAM) (1:1000), NADH:ubiquinone oxidoreductase core subunit S1 (NDUFS1) (1:1000), NADH:ubiquinone oxidoreductase core subunit B8 (NDUFB8) (1:1000), ATP synthase β (ATP Syn β) (1:1000), and α-tubulin (1:1000; Abcam, Cambridge, UK).
Evans Blue Extravasation.
Integrity of the blood–spinal cord barrier (BSCB) was assessed using Evans Blue (EB) dye extravasation (Fang et al., 2015). Evans Blue dye (2%, 0.08 ml) was administered intravenously and allowed to circulate for 30 minutes. Mice were then transcardially perfused with saline, and a 6-mm segment of spinal cord centered at the epicenter was collected. EB dye was extracted in formamide at room temperature overnight, and optical density values of the prepared samples were measured at 620 nm with a microplate reader. EB content was calculated as nanogram dye per milligram tissue using a standard curve.
Lesion Volume Analysis.
For histopathological analysis, spinal cord tissues were processed as described previously (Patel et al., 2017). Briefly, mice were transcardially perfused with 0.1 M PBS followed by 4% paraformaldehyde (PFA). A 1-cm segment of the spinal cord centered on the injury epicenter was removed and postfixed in PFA for 2 h and then washed in 0.2 M phosphate buffer overnight at 4°C. Tissues were then cryoprotected in 20% sucrose with 0.1% sodium azide at 4°C until the spinal cords sank (≥3 days). Spinal cords were trimmed to 6-mm segments centered on the injury sight and frozen in optimal cutting temperature (OCT) compound at −80°C. The entire 6-mm segment was cryosectioned into 10-µm coronal sections, and every section was collected.
Eriochrome cyanine staining for myelin was used to distinguish damaged and spared tissue (Patel et al., 2017). Slides were warmed for 60 minutes at 37°C and then hydrated in dH2O, submerged in acetone for 2 minutes, and rehydrated in dH2O. Slides were exposed to serial dilutions of decreasing concentrations of ethanol and incubated in eriochrome cyanine solution for 30 minutes. Selective myelin staining was obtained by differentiation in 0.3% ammonium hydroxide for 30 seconds. Slides were then exposed to serial dilutions of increasing ethanol concentrations. Analyses were performed in a blinded fashion, with respect to treatment group, using an Olympus microscope (Tokyo, Japan) and ImageJ software. Lesion and spared tissue areas were quantified across 2 mm spinal cord centered on the epicenter at 100-µm intervals using the Cavalieri method (Patel et al., 2017), totaling 21 sections per animal.
Statistical Analysis.
Tissue isolated from a single animal or a single animal’s behavior represented n = 1. Behavioral assays were n = 8 sham mice and n = 10 injured mice per group, and data were analyzed using two-way ANOVA with repeated measures followed by Tukey’s post hoc test. Differences in mtDNA, individual protein expression, and total EB content between all three groups (Sham, SCI + Vehicle, SCI + LY344864) was analyzed using one-way ANOVA. Two-way ANOVA was used to analyze tissue histopathology across the spinal cord. Total histopathological assessments were analyzed using two-tailed student’s t test. In all cases, GraphPad Prism software (La Jolla, CA) was used, and a P < 0.05 was considered indicative of a statistically significant difference between mean values.
Results
Effect of LY344864 on MB in the Naïve Spinal Cord.
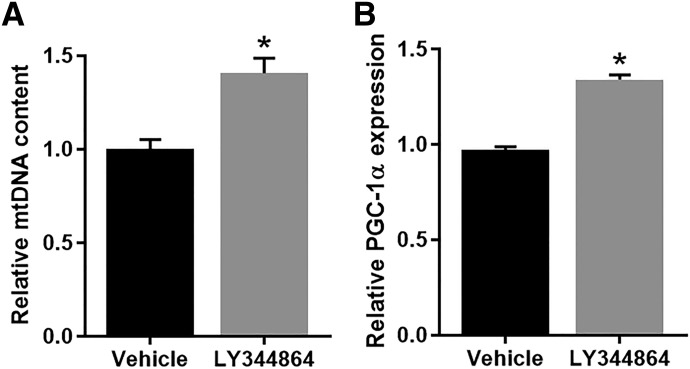
Naïve mice were treated with 2.0 mg/kg LY344864 or vehicle i.p. daily for 21 days. Following treatment, the T9-12 portion of the spinal cord was collected and analyzed for mitochondrial endpoints. LY344864-treated mice displayed a 1.4-fold increase in mtDNA content (Fig. 1A) and 1.3-fold increase in PGC-1α mRNA expression (Fig. 1B) in the spinal cord compared with vehicle-treated mice. It should be noted that 1.3–1.4-fold increases are physiologically relevant (Funk and Schnellmann, 2013; Garrett et al., 2014; Whitaker et al., 2016).
Fig. 1.
Effect of LY344864 on MB in the naïve spinal cord. Naïve mice were exposed to LY344864 (2 mg/kg i.p.) or vehicle daily for 21 days. The spinal cord was extracted and analyzed for mtDNA content (A) and PGC-1α mRNA expression (B). Data represent four mice per group and are expressed as mean ± S.E.M. *P < 0.05 by Student’s t test.
Effect of LY344864 on mtDNA and Mitochondrial Protein Expression Post-SCI.
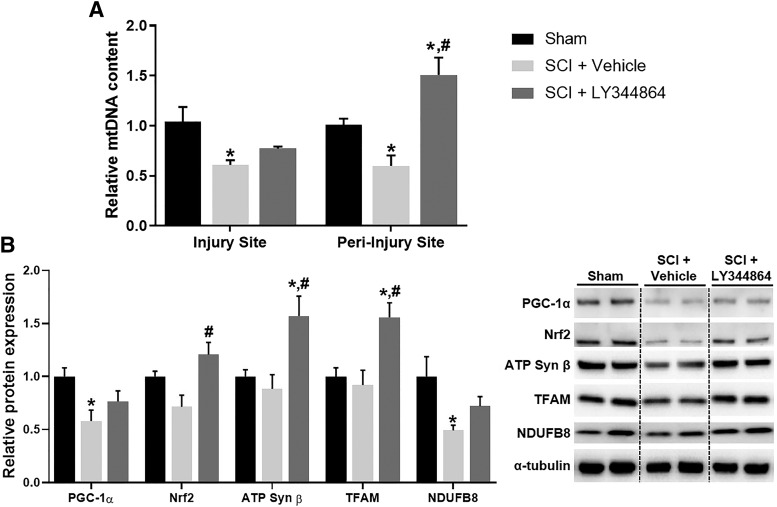
Mice were subjected to SCI followed by daily administration of LY344864 or vehicle i.p. beginning 1 h after injury. By 3 days post-SCI, mtDNA content was reduced approximately 45% in the injury and peri-injury sites compared with sham controls (Fig. 2A). LY344864 administration attenuated this decrease in the injury site and increased mtDNA content to greater than that of sham controls in the peri-injury site.
Fig. 2.
Effect of LY344864 on mtDNA and protein expression 3 days post-SCI. Mice were subjected to moderate SCI using an 80 kdyn force-controlled impactor-induced contusion model followed by daily administration of vehicle or LY344864 (2 mg/kg i.p.) beginning 1 h postinjury and continuing for 3 days. The injury and peri-injury sties were extracted and analyzed for mtDNA content (A). The injury site was also analyzed for mitochondrial protein expression (B). Data represent five mice per group and are expressed as mean ± S.E.M. *P < 0.05 compared with Sham and #P < 0.05 compared with SCI + Vehicle by one-way ANOVA followed by Tukey’s post hoc test.
Immunoblot analysis revealed decreased protein expression of PGC-1α, Nrf2, a transcription factor that controls mitochondrial gene regulation, and electron transport chain subunits ATP Syn β and NADH:ubiquinone oxidoreductase core subunit 8 in the injury site of SCI mice (Fig. 2B), further indicating mitochondrial dysfunction. LY344864 treatment not only attenuated these decreases but also fully restored Nrf2 and ATP Syn β to that of sham levels. Furthermore, LY344864 increased the expression of mitochondrial transcription factor A 1.5-fold compared with sham. These data provide evidence of LY344864-induced MB in the injury site 3 days post SCI.
Effect of LY344864 on EB Dye Accumulation Post-SCI.
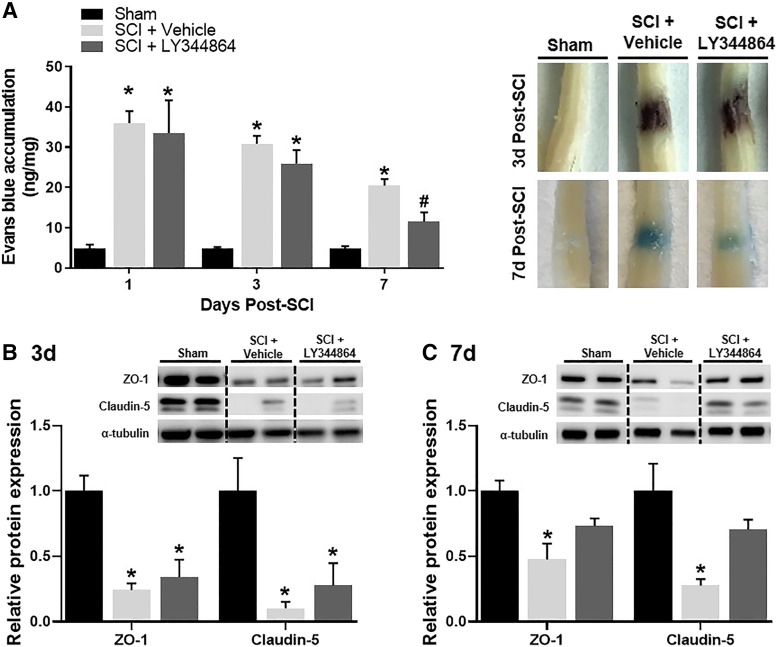
EB dye accumulation was increased in injured mice compared with sham controls beginning 1 day after injury and persisting until 3 days post-SCI, regardless of treatment. Though the degree of EB extravasation was comparable among injured mice for up to 3 days, by day 7, LY344864-treated injured mice displayed decreased dye accumulation compared with vehicle-treated mice (Fig. 3A).
Fig. 3.
Effect of LY344864 on spinal cord EB dye accumulation. Mice were subjected to moderate SCI using an 80 kdyn force-controlled impactor-induced contusion model followed by daily administration of vehicle or LY344864 (2 mg/kg i.p.) beginning 1 h postinjury. Subsets of mice were euthanized 1, 3, and 7 days post-SCI. Prior to euthanasia, mice were intraocularly injected with EB, and the dye was allowed to circulate for 2 h. One centimeter of spinal cord surrounding the injury epicenter was extracted and analyzed for dye accumulation. A subset of mice were transcardially perfused and fixed with 4% PFA and the spinal cords extracted for representative images (A). The injury site was also collected from a subset of mice at 3 days (B) and 7 days (C) post-SCI and analyzed for protein expression. Data represent five mice per group and are expressed as mean ± S.E.M. *P < 0.05 compared with Sham and #P < 0.05 compared with SCI + Vehicle by one-way ANOVA followed by Tukey’s post hoc test.
Expression of the intracellular scaffolding protein zonula occluden protein-1 (ZO-1) and claudin-5, a tight junction protein, was decreased by more than 75% compared with sham at 3 days post-SCI (Fig. 3B). Although decreased expression persisted 7 days after injury in vehicle-treated mice, ZO-1 and claudin-5 were comparable with sham levels in LY344864-treated injured mice (Fig. 3C).
Effect of LY344864 on Locomotor Recovery Post-SCI in Control and 5-HT1F Knockout Mice.
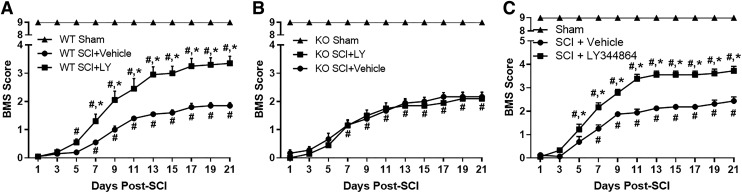
The presence of the 5-HT1F receptor in the spinal cord of female WT mice, and absence in KO mice, was confirmed using PCR amplification of a 163-bp target sequence for the 5-HT1F receptor, with β-actin (69 bp) used as a reference gene (Supplemental Fig. 1). WT (Fig. 4A) and KO (Fig. 4B) mice were subjected to SCI followed by daily LY344864 treatment beginning 1 h after injury. Locomotor capability was assessed using the BMS beginning 24 h after SCI and continuing every alternate day (Basso et al., 2006). As expected, all injured mice displayed paralysis 24 h after injury, and shams maintained normal function throughout the experiment.
Fig. 4.
Effect of LY344864 on recovery post-SCI in C57BL/6J WT and 5-HT1F KO mice. Mice were subjected to moderate SCI using an 80 kdyn force-controlled impactor-induced contusion model followed by daily administration of vehicle or LY344864 (2 mg/kg i.p.) beginning 1 h post-SCI (A and B). Locomotor function was assessed using the Basso Mouse scale beginning 24 h after injury and continuing every alternate day. Mice were also subjected to moderate SCI using an 80 kdyn force-controlled impactor-induced contusion model followed by daily administration of vehicle or LY344864 (2 mg/kg i.p.) beginning 8 h postinjury (C). Data are representative of eight mice per sham group and 10 mice per SCI group and are expressed as mean ± S.E.M. Significance between sham and injured animals is not represented on graph for simplicity. *P < 0.05 compared with SCI + Vehicle and #P < 0.05 compared with day 1 by two-way ANOVA with repeated measures followed by Tukey’s post hoc test.
Vehicle-treated mice of both genotypes displayed improved locomotor function 7 days post-SCI and reached a maximum BMS score of approximately 2 (ankle movement) by 21 days. Injured WT mice treated with LY344864 exhibited recovery by 5 days and an increased BMS score compared with vehicle-treated mice by 7 days post-SCI (1.4 vs. 0.5), reaching a score of 3.5 by 21 days, indicative of dorsal and occasional plantar stepping (Fig. 4A). Conversely, LY344864 had no effect on locomotor recovery in the injured KO mice (Fig. 4B). In WT and KO mice, analysis revealed a significant effect of treatment/injury and days postinjury as well as a significant interaction.
To determine the effect of delaying treatment, daily LY344864 administration was initiated 8 h after SCI and locomotor recovery assessed (Fig. 4C). Similar to that observed when treatment began 1 h post-SCI, LY344864-treated mice displayed an increased BMS score compared with both day 1 and vehicle-treated mice by 5 days after injury, reaching a BMS score of 3.7 by 21 days post-SCI. Again, vehicle-treated mice exhibited recovery compared with day 1 by 7 days and a maximum BMS score of approximately 2. Analysis again revealed a significant effect of treatment/injury and days postinjury as well as a significant interaction.
Effect of LY344864 on Histopathology of Spinal Cord Post-SCI.
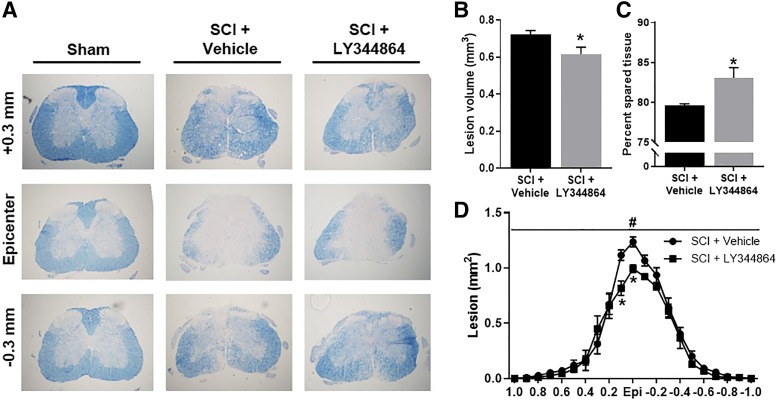
Spinal cord histopathology was assessed following SCI and delayed treatment initiation 21 days after injury using eriochrome cyanine staining for myelin (Fig. 5A). Analysis of 2 mm spinal cord centered on the epicenter revealed decreased total lesion volume (Fig. 5B) and increased tissue sparing (Fig. 5C) in LY344864-treated mice compared with vehicle-treated mice. Cross-sectional analysis also indicated decreased lesion tissue in LY344864-treated mice (Fig. 5D), particularly at the epicenter. Main effects of treatment and spinal level were observed.
Fig. 5.
Effect of LY344864 on spinal cord histopathology post-SCI. Mice were subjected to moderate SCI using an 80 kdyn force-controlled impactor-induced contusion model followed by daily administration of vehicle or LY344864 (2 mg/kg i.p.) beginning 8 h postinjury and continuing for 21 days. Spinal cords were extracted, and evenly spaced tissue sections were stained with eriochrome cyanine (A) and analyzed for lesion volume (B) and spared tissue (C) across 2 mm spinal cord centered on the epicenter. Cross-sectional analysis of lesion (D) across 2 mm spinal cord centered on the epicenter. Data are representative of five mice per group and are expressed as mean ± S.E.M. *P < 0.05 compared with SCI + Vehicle by Student’s t test.
Discussion
Given that neuronal cells are highly reliant on ATP-driven processes (Castro et al., 1997; Tian et al., 2016), failure to generate and maintain adequate energy production exacerbates the pathology of secondary injury in SCI and results in further cell dysfunction and death (Castro et al., 1997; Scholpa and Schnellmann, 2017; Scholpa et al., 2019). Mitochondria in the CNS primarily serve to generate energy but can also play a role in the homeostasis and degeneration of neurons (Dubinsky, 2005). Furthermore, pathologic conditions can develop even in the presence of minimal mitochondrial dysfunction (Dubinsky, 2005). Evidence suggests that not only does mitochondrial dysfunction post-SCI contribute to the propagation of secondary injury but also that restoration of mitochondrial homeostasis promptly following injury may improve neuronal survival and promote functional recovery (Sullivan et al., 2007; Rabchevsky et al., 2011; Scholpa and Schnellmann, 2017). By generating new, functional mitochondrial via pharmacological induction of MB, we sought to mitigate the detrimental impact of mitochondrial dysfunction and assess recovery after SCI.
We previously reported that LY344864-induced agonism of the 5-HT1F receptor in renal proximal tubule cells leads to the activation of the Gβγ-Akt-eNOs-sGC-PGC-1α pathway, ultimately resulting in induction of MB (Gibbs et al., 2018b). Additionally, treatment with LY344864 in a mouse model of Parkinson disease increased MB, attenuated neuronal loss, and improved behavioral endpoints (Scholpa et al., 2018). Though we have shown that the 5-HT1F receptor is a potent inducer of MB peripherally and centrally (Rasbach et al., 2010; Garrett et al., 2014; Whitaker et al., 2016; Scholpa et al., 2018), this receptor remains understudied in the spinal cord. Here, we show that 5-HT1F receptor agonism induces MB in the naïve and injured spinal cord. Following SCI, we observed a rapid decrease in the expression of mitochondrial proteins at the injury site, corresponding with previous reports of mitochondrial dysfunction after injury (Dubinsky, 2005; Scholpa et al., 2019). A dose of 2 mg/kg LY344864 used in our previous study induced MB in several organ systems (Gibbs et al., 2018a; Scholpa et al., 2018). As such, this dose was continued in these SCI experiments. Interestingly, LY344864 treatment attenuated these decreases as early as 3 days post-SCI, notably that of PGC-1α, indicating induction of MB and improved mitochondrial homeostasis.
Vascular disruption is a well-established consequence of SCI (Cohen et al., 2009; Patel et al., 2009). The BSCB exists at the capillary level and controls the flux of molecules entering the spinal cord. Studies revealed that restoring BSCB integrity prevents necrosis and apoptosis of neuronal cells after SCI, consequently improving functional endpoints (Wang et al., 2018). Additional studies report maximal BSCB disruption at 24 h post-SCI (Figley et al., 2014). Similarly, we observed permeability increases of the BSCB at 24 h, persisting for 7 days after injury. LY344864-treated mice displayed reduced dye accumulation at 7 days post-SCI, suggesting accelerated recovery of BSCB integrity. Additionally, increased BSCB integrity has been correlated with improved locomotor recovery (Cohen et al., 2009). Functions of the BSCB rely on an intricate network of tight junction proteins, including ZO-1 and claudin-5 (Kumar et al., 2017). Following SCI, we report decreased expression of both ZO-1 and claudin-5, consistent with previous reports (Zhang et al., 2016; Zhou et al., 2017; Kumar et al., 2018). Interestingly, LY344864 treatment attenuated these decreases by 7 days post-SCI. This recovery of tight junction protein expression corroborates our observed decrease in permeability of the BSCB in LY344864-treated animals at 7 days post-SCI. Though we suspect LY344864 treatment induces MB within the endothelial cells composing the BSCB, further work is required to elucidate the pathway by which MB induction facilitates restoration of the BSCB after SCI.
Treatment with LY344864 1 h post-SCI in WT animals resulted in improved locomotor capability by 7 days post-SCI, with mice reaching a BMS score of 3.4 by day 21, corresponding to consistent dorsal to occasional plantar stepping. Vehicle-treated mice, regardless of genotype, depicted a score of only 2 (ankle movement) by day 21. The similar degree of recovery observed in both the vehicle-treated 5-HT1F KO and WT mice suggests that the lack of the 5-HT1F receptor does not affect injury progression or inhibit basal improvement after SCI. Nonetheless, LY344864 had no locomotor effects in the KO mice, indicating that the improved functional recovery observed in WT mice is dependent on the 5-HT1F receptor. In addition, the loss of LY34464 efficacy in the KO mice provides evidence that LY344864 specifically activates its receptor to produce the observed biologic effects.
Remarkably, delaying treatment initiation until 8 h after injury also resulted in improved functional recovery, indicating a potential therapeutic window for 5-HT1F receptor agonism of at least 8 h post-SCI. It has been reported that activation of the 5-HT1F receptor inhibits postsynaptic potentials that trigger spasms after SCI (Murray et al., 2011) and that mitigating serotonergic disruption after SCI can promote locomotor recovery (Ghosh and Pearse, 2015). Therefore, in addition to the MB-related effects, LY344864 treatment after SCI could also attenuate serotonergic disruption or decrease muscle spasms, further enhancing the improved functional behavior observed. In addition to functional improvement, injured mice subjected to the 8-h delay of LY344864 treatment initiation demonstrated decreased lesion volume, particularly at the injury epicenter, which has also been correlated with improved locomotor capability (Patel et al., 2010).
Endogenous serotonin is known to play a stimulatory role in locomotor activity (Sławińska et al., 2014). As such, we cannot preclude the possibility that the locomotor effects observed with LY344864 treatment are in part due to actions on serotonergic systems. The 5-HT1F receptor has yet to be fully characterized. However, given that uninjured KO animals retained full locomotor capabilities and injured KO animals displayed comparable locomotor activity to that of WT vehicle mice, it is unlikely that the 5-HT1F receptor plays a pivotal role in basal locomotor function in this model. In addition, previous research demonstrates that LY344864-induced MB promotes functional recovery in organ systems not directly reliant on the serotonergic system (e.g., renal function) (Gibbs et al., 2018a,b).
Though we know treatment with 2 mg/kg LY344864 induces MB in both male and female mice across several different tissues (Gibbs et al., 2018a; Scholpa et al., 2018), we have not investigated the effects of LY344864 in male mice following SCI. Future studies will explore potential sex-related differences in response to 5-HT1F receptor agonism following SCI.
This is the first report indicating that 5-HT1F receptor activation stimulates MB in SCI and promotes tissue, locomotor activity, and vascular integrity recovery. Importantly, the 5-HT1F receptor agonist lasmiditan has recently been approved by the US Food and Drug Administration for the treatment of migraines and could be repurposed for the treatment of SCI. Finally, the equal efficacy of LY344864 when administered 1 and 8 h after SCI suggests this approach is clinically relevant for the treatment of SCI.
Abbreviations
- 5-HT1F
5-hydroxytryptamine 1F
- ATP Syn β
ATP synthase β
- BMS
Basso Mouse Scale
- BSCB
blood–spinal cord barrier
- CNS
central nervous system
- EB
Evans Blue
- KO
knockout
- MB
mitochondrial biogenesis
- mtDNA
mitochondrial DNA
- Nrf2
nuclear respiratory factor 2
- PFA
paraformaldehyde
- PGC-1α
peroxisome proliferator–activated receptor y coactivator-1α
- qPCR
quantitative PCR
- SCI
spinal cord injury
- WT
wild type
- ZO-1
zonula occluden protein-1
Authorship Contributions
Participated in research design: Simmons, Scholpa, Schnellmann.
Conducted experiments: Simmons, Scholpa, Cleveland.
Performed data analysis: Simmons, Scholpa, Cleveland.
Wrote or contributed to the writing of the manuscript: Simmons, Scholpa, Schnellmann.
Footnotes
This study was supported by the National Institutes of Health National Institute of General Medical Sciences [Grant GM084147] (R.G.S.) and the Biomedical Laboratory Research and Development Program of the Department of Veterans Affairs [BX: 000851] (R.G.S.).
 This article has supplemental material available at jpet.aspetjournals.org.
This article has supplemental material available at jpet.aspetjournals.org.
References
- Basso DM, Fisher LC, Anderson AJ, Jakeman LB, McTigue DM, Popovich PG. (2006) Basso Mouse Scale for locomotion detects differences in recovery after spinal cord injury in five common mouse strains. J Neurotrauma 23:635–659. [DOI] [PubMed] [Google Scholar]
- Beeson CC, Beeson GC, Schnellmann RG. (2010) A high-throughput respirometric assay for mitochondrial biogenesis and toxicity. Anal Biochem 404:75–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro ME, Pascual J, Romón T, del Arco C, del Olmo E, Pazos A. (1997) Differential distribution of [3H]sumatriptan binding sites (5-HT1B, 5-HT1D and 5-HT1F receptors) in human brain: focus on brainstem and spinal cord. Neuropharmacology 36:535–542. [DOI] [PubMed] [Google Scholar]
- Cohen DM, Patel CB, Ahobila-Vajjula P, Sundberg LM, Chacko T, Liu SJ, Narayana PA. (2009) Blood-spinal cord barrier permeability in experimental spinal cord injury: dynamic contrast-enhanced MRI. NMR Biomed 22:332–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devivo MJ. (2012) Epidemiology of traumatic spinal cord injury: trends and future implications. Spinal Cord 50:365–372. [DOI] [PubMed] [Google Scholar]
- Dubinsky JM. (2005) CNS mitochondria in neurodegenerative disorders. Antioxid Redox Signal 7:1089–1091. [DOI] [PubMed] [Google Scholar]
- Fang B, Li XQ, Bi B, Tan WF, Liu G, Zhang Y, Ma H. (2015) Dexmedetomidine attenuates blood-spinal cord barrier disruption induced by spinal cord ischemia reperfusion injury in rats. Cell Physiol Biochem 36:373–383. [DOI] [PubMed] [Google Scholar]
- Figley SA, Khosravi R, Legasto JM, Tseng YF, Fehlings MG. (2014) Characterization of vascular disruption and blood-spinal cord barrier permeability following traumatic spinal cord injury. J Neurotrauma 31:541–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzharris M, Cripps RA, Lee BB. (2014) Estimating the global incidence of traumatic spinal cord injury. Spinal Cord 52:117–122. [DOI] [PubMed] [Google Scholar]
- Funk JA, Schnellmann RG. (2013) Accelerated recovery of renal mitochondrial and tubule homeostasis with SIRT1/PGC-1α activation following ischemia-reperfusion injury. Toxicol Appl Pharmacol 273:345–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett SM, Whitaker RM, Beeson CC, Schnellmann RG. (2014) Agonism of the 5-hydroxytryptamine 1F receptor promotes mitochondrial biogenesis and recovery from acute kidney injury. J Pharmacol Exp Ther 350:257–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh M, Pearse DD. (2015) The role of the serotonergic system in locomotor recovery after spinal cord injury. Front Neural Circuits 8:151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs WS, Collier JB, Morris M, Beeson CC, Megyesi J, Schnellmann RG. (2018a) 5-HT1F receptor regulates mitochondrial homeostasis and its loss potentiates acute kidney injury and impairs renal recovery. Am J Physiol Renal Physiol 315:F1119–F1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs WS, Garrett SM, Beeson CC, Schnellmann RG. (2018b) Identification of dual mechanisms mediating 5-hydroxytryptamine receptor 1F-induced mitochondrial biogenesis. Am J Physiol Renal Physiol 314:F260–F268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall ED. (2011) Antioxidant therapies for acute spinal cord injury. Neurotherapeutics 8:152–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Lang Y, Cao Y, Zhang T, Lu H. (2015) The neuroprotective effect of tetramethylpyrazine against contusive spinal cord injury by activating PGC-1α in rats. Neurochem Res 40:1393–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Lang Y, Zhang T, Ni S, Lu H. (2016) Lentivirus-mediated PGC-1α overexpression protects against traumatic spinal cord injury in rats. Neuroscience 328:40–49. [DOI] [PubMed] [Google Scholar]
- Kumar H, Jo MJ, Choi H, Muttigi MS, Shon S, Kim BJ, Lee SH, Han IB. (2018) Matrix metalloproteinase-8 inhibition prevents disruption of blood-spinal cord barrier and attenuates inflammation in rat model of spinal cord injury. Mol Neurobiol 55:2577–2590. [DOI] [PubMed] [Google Scholar]
- Kumar H, Ropper AE, Lee SH, Han I. (2017) Propitious therapeutic modulators to prevent blood-spinal cord barrier disruption in spinal cord injury. Mol Neurobiol 54:3578–3590. [DOI] [PubMed] [Google Scholar]
- McEwen ML, Sullivan PG, Rabchevsky AG, Springer JE. (2011) Targeting mitochondrial function for the treatment of acute spinal cord injury. Neurotherapeutics 8:168–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsikostas DD, Tfelt-Hansen P. (2012) Targeting to 5-HT1F receptor subtype for migraine treatment: lessons from the past, implications for the future. Cent Nerv Syst Agents Med Chem 12:241–249. [DOI] [PubMed] [Google Scholar]
- Monaco EA, III, Weiner GM, Friedlander RM. (2013) Randomized-controlled trial of minocycline for spinal cord injury shows promise. Neurosurgery 72:N17–N19. [DOI] [PubMed] [Google Scholar]
- Murray KC, Stephens MJ, Rank M, D’Amico J, Gorassini MA, Bennett DJ. (2011) Polysynaptic excitatory postsynaptic potentials that trigger spasms after spinal cord injury in rats are inhibited by 5-HT1B and 5-HT1F receptors. J Neurophysiol 106:925–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson T, Longmore J, Shaw D, Pantev E, Bard JA, Branchek T, Edvinsson L. (1999) Characterisation of 5-HT receptors in human coronary arteries by molecular and pharmacological techniques. Eur J Pharmacol 372:49–56. [DOI] [PubMed] [Google Scholar]
- Oyinbo CA. (2011) Secondary injury mechanisms in traumatic spinal cord injury: a nugget of this multiply cascade. Acta Neurobiol Exp (Warsz) 71:281–299. [DOI] [PubMed] [Google Scholar]
- Patel CB, Cohen DM, Ahobila-Vajjula P, Sundberg LM, Chacko T, Narayana PA. (2009) Effect of VEGF treatment on the blood-spinal cord barrier permeability in experimental spinal cord injury: dynamic contrast-enhanced magnetic resonance imaging. J Neurotrauma 26:1005–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel SP, Cox DH, Gollihue JL, Bailey WM, Geldenhuys WJ, Gensel JC, Sullivan PG, Rabchevsky AG. (2017) Pioglitazone treatment following spinal cord injury maintains acute mitochondrial integrity and increases chronic tissue sparing and functional recovery. Exp Neurol 293:74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel SP, Sullivan PG, Lyttle TS, Rabchevsky AG. (2010) Acetyl-L-carnitine ameliorates mitochondrial dysfunction following contusion spinal cord injury. J Neurochem 114:291–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabchevsky AG, Patel SP, Springer JE. (2011) Pharmacological interventions for spinal cord injury: where do we stand? How might we step forward? Pharmacol Ther 132:15–29. [DOI] [PubMed] [Google Scholar]
- Rasbach KA, Funk JA, Jayavelu T, Green PT, Schnellmann RG. (2010) 5-hydroxytryptamine receptor stimulation of mitochondrial biogenesis. J Pharmacol Exp Ther 332:632–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholpa NE, Lynn MK, Corum D, Boger HA, Schnellmann RG. (2018) 5-HT1F receptor-mediated mitochondrial biogenesis for the treatment of Parkinson’s disease. Br J Pharmacol 175:348–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholpa NE, Schnellmann RG. (2017) Mitochondrial-based therapeutics for the treatment of spinal cord injury: mitochondrial biogenesis as a potential pharmacological target. J Pharmacol Exp Ther 363:303–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholpa NE, Williams H, Wang W, Corum D, Narang A, Tomlinson S, Sullivan PG, Rabchevsky AG, Schnellmann RG. (2019) Pharmacological stimulation of mitochondrial biogenesis using the Food and Drug Administration-approved β2-adrenoreceptor agonist formoterol for the treatment of spinal cord injury. J Neurotrauma 36:962–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sławińska U, Miazga K, Jordan LM. (2014) The role of serotonin in the control of locomotor movements and strategies for restoring locomotion after spinal cord injury. Acta Neurobiol Exp (Warsz) 74:172–187. [DOI] [PubMed] [Google Scholar]
- Sullivan PG, Krishnamurthy S, Patel SP, Pandya JD, Rabchevsky AG. (2007) Temporal characterization of mitochondrial bioenergetics after spinal cord injury. J Neurotrauma 24:991–999. [DOI] [PubMed] [Google Scholar]
- Teng YD, Choi H, Onario RC, Zhu S, Desilets FC, Lan S, Woodard EJ, Snyder EY, Eichler ME, Friedlander RM. (2004) Minocycline inhibits contusion-triggered mitochondrial cytochrome c release and mitigates functional deficits after spinal cord injury. Proc Natl Acad Sci USA 101:3071–3076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian B, Wang XL, Huang Y, Chen LH, Cheng RX, Zhou FM, Guo R, Li JC, Liu T. (2016) Peripheral and spinal 5-HT receptors participate in cholestatic itch and antinociception induced by bile duct ligation in rats. Sci Rep 6:36286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vila-Pueyo M. (2018) Targeted 5-HT1F therapies for migraine. Neurotherapeutics 15:291–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Wu Y, Han W, Li J, Xu K, Li Z, Wang Q, Xu K, Liu Y, Xie L, et al. (2018) Hydrogen sulfide ameliorates blood-spinal cord barrier disruption and improves functional recovery by inhibiting endoplasmic reticulum stress-dependent autophagy. Front Pharmacol 9:858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker RM, Corum D, Beeson CC, Schnellmann RG. (2016) Mitochondrial biogenesis as a pharmacological target: a new approach to acute and chronic diseases. Annu Rev Pharmacol Toxicol 56:229–249. [DOI] [PubMed] [Google Scholar]
- Wills LP, Trager RE, Beeson GC, Lindsey CC, Peterson YK, Beeson CC, Schnellmann RG. (2012) The β2-adrenoceptor agonist formoterol stimulates mitochondrial biogenesis. J Pharmacol Exp Ther 342:106–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Wang J, Gu Z, Zhang Q, Zheng H. (2016) Effect of lycopene on the blood-spinal cord barrier after spinal cord injury in mice. Biosci Trends 10:288–293. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Wu Y, Liu Y, He Z, Zou S, Wang Q, Li J, Zheng Z, Chen J, Wu F, et al. (2017) The cross-talk between autophagy and endoplasmic reticulum stress in blood-spinal cord barrier disruption after spinal cord injury. Oncotarget 8:1688–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]