Abstract
Lung cancer is still diagnosed at a late stage due to a lack of symptoms. Although there are novel therapies, many patients are still treated with chemotherapy. In an effort to reduce adverse effects associated with chemotherapy, inhaled administration of platinum analogs has been investigated. Inhaled administration is used as a local route in order to reduce the systemic adverse effects; however, this treatment modality has its own adverse effects. In this mini review, we present drugs that were administered as nebulized droplets or dry powder aerosols for non-small-cell lung cancer. We present the adverse effects and methods to overcome them.
Keywords: : 5-fluorouracil, bevacizumab, carboplatin, cisplatin, gemcitabine, inhalation, lung cancer, nitrocamptothecine, non-small-cell lung cancer, paclitaxel, taxol
Practice points.
Every aerosol-delivery system has its advantages and disadvantages.
Inhaled cytotoxic therapies should only be administered in a controlled environment.
The administration, for now, should be done only in medical establishments.
The main contraindication would be a STAGE IV chronic obstruction pulmonary disease diagnosis.
Patients should be evaluated with: lung function tests (spirometry, 6-min walking test and diffusing capacity for carbon monoxide) and imaging techniques.
Low respiratory capacity would mean that the inhaled drug does not reach the lower peripheral parenchyma.
Lung cancer is still diagnosed at an advanced stage in most patients. It has been observed as the leading cause of cancer death for males and females, after prostate and breast cancer [1]. We have novel imaging and endoscopic equipment for diagnosis and staging, such as the positron emission tomography and the radial and convex endobronchial endoscopes [2–4]. Electromagnetic navigation and cone beam computed tomography are also novel, real-time navigation systems for the diagnosis of central and peripheral nodules and masses [5–7]. These endoscopic techniques are minimally invasive and can be performed under a mild anesthesia [8]. Early diagnosis is where all physicians should aim. Several proposals have been made for lung cancer screening protocols with low radiation computed tomography [9]. However, since most of the patients are diagnosed at nonoperable stages, we need to find drugs that are both efficient and have mild side effects. Chemotherapy drugs are known to, in some cases, induce fatigue, nausea, emesis, cardiac complications, neutropenia, anemia, bone pain and allergic shock [10].
Over the past 10 years, there has been a ‘bloom’ in the exploration and development of pharmacogenomics [11]. Based on the genome of the tumor, a series of experiments have investigated several gene expressions and the certain genes, such as EGFR, ALK, BRAF, proto-oncogene tyrosine-protein kinase ROS-1 (ROS-1) and PD-L1, were observed in a high number of patients. Therefore, based on these trials, several drugs targeting these genes were produced [12–14]. Regarding the EGFR, ALK, BRAF and ROS-1 genes, several tyrosine kinase inhibitors were developed [13–16]. However, it was observed that resistance was developed for several patients that used these agents and gene mutations, such as the T790M [16]. Consequently, novel tyrosine kinase inhibitors were developed. These agents have adverse effects, such as pneumonitis, skin rash, orogonitis, emesis and diarrhea, associated with the dosage [17]. A major issue that the industry had to cope with was that these agents were not produced in different dosages, which would allow for efficient control of these adverse effects. In response, certain pharmaceutical companies resolved this issue by producing tablets in different dosages [18].
Immunotherapy is a novel therapy currently based on antagonists for cell surface expressed immune inhibitory molecules. Different drugs are given as first line treatment in non-small-cell lung cancer (NSCLC) based on the expression of PD-L1 ≥50% alone. Alternatively, when PD-L1 is ≤50%, immunotherapy can be administered in combination with chemotherapy [19]. Immunotherapy drugs also have adverse effects, including pneumonitis, orogonitis, vitiligo, dysregulation of the thyroid function, colitis and resurrection of hepatitis [20–22].
All of these therapies are administered intravenously, meaning the drug affects the cancer tissue but at the same time circulates through the whole body inducing adverse effects. Inhaled delivery allows locally high concentrations to be achieved at the tumor bed, while lowering the concentration at peripheral sites. This results in a better patient outcome while minimizing toxicity. Lower drug concentrations would reduce potential toxicity. In order to achieve this concept, several drugs have been used as local therapy in the form of intratumoral chemotherapy, with the use of endoscopic techniques and aerosol administration of chemotherapy [23–28]. Different drugs, such as insulin and antibiotics, have already been tested and demonstrate that the concept of aerosol chemotherapy is feasible [27,29–34]. In the following text, we discuss the methodology of inhaled chemotherapy in NSCLC, the different chemotherapy drugs that have been administered as aerosol and their respective safety profiles.
Airways
Current nebulized aerosol/mist production systems deliver droplets or fine powder to the respiratory system. The drug starts from the mouth and travels to the oropharynx, to the trachea, to the distal airways and finally to the alveoli [35]. The alveoli have a thin wall which is easily permeable and surrounded by small vessels. These small vessels are divisions from the pulmonary arteries. The surface of the alveoli is vast, and it allows for a large quantity of drug to be concentrated in a small period of time. The drug then circulates to the rest of the body. The administration of the drug becomes systemic, but the target site, in this case lung cancer tissue, is reached first. Micrometastasis, or micro-disease, can be controlled in this way [36]. The alveoli are also surrounded by lymphatic drainage, therefore the inhaled drug can act simultaneously to the lymph nodes, treat micrometastasis and act against distant metastasis within the lymph node stations [37].
Defense mechanisms
Mucociliary transport in the central and upper airways and cell mediated transport by alveolar macrophages in the lower airways act as defense mechanisms. However, apart from these mechanisms, we should mention that locally, in the surface of the trachea and then the bronchial tissue, there are different transportation molecules and gene expression in the large and smaller airways [36]. Therefore, when a drug is designed, these factors should be included [38].
Drug production systems
The drug administration can be done with nebulizers (jet or ultrasound) where a mist of aerosol is produced. Air jet nebulizers utilize the Venturi or Bernoulli effect to disperse aerosol droplets. Ultrasonic nebulizers employ piezo-electric transducers to generate aerosols. This is the limit of aerosol dispersion [25–41]. Several drugs, such as insulin and antibiotics, have been tested with this administration method [25,29–31,33,39]. There are also production systems that produce drugs in a form of dry powder.
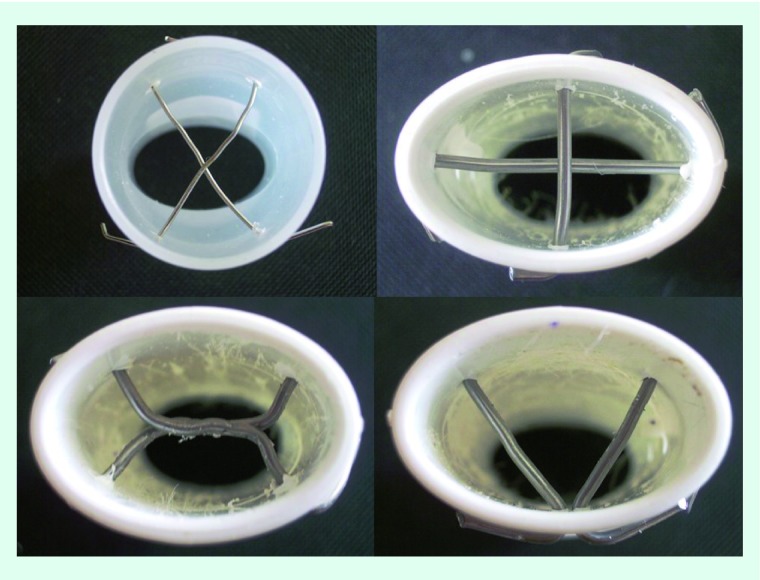
The aerosol production with the nebulizers starts with the initiation of the production system; the patient inhales the aerosol based on his respiratory capacity, usually through a mask with a reservoir attached. A method to enhance the deposition is to attach a mouthpiece in the reservoir [32,40]. The different mouthpiece designs (Figure 1) can be attached either in jet or ultrasonic nebulizers. Refer to the 2014 paper from Zarogoulidis et al. for an image of these nebulizers [30].
Figure 1. . Custom mouthpiece inlets.
Different models of inlets mouthpiece designs that modulated and shrunk the diameter of aerosol droplets or dry powder after leaving the production system. These inlets were previously used with different drugs and production systems, as referred to in the text.
In everyday clinical practice there are aerosol-delivery systems that rely on the respiratory performance of the patients, such as metered dose inhalers and dry powder inhalers [41]. However, this equipment has never been used for the administration of aerosol chemotherapy.
Factors affecting the production & deposition
The particles should not exceed 3 μm in mass median aerodynamic diameter (MMAD) [42]. In the pharmaceutical industry, particle or droplet size is determined by measuring the MMAD with a master sizer equipment or a cascade impactor. The respiratory tract has a high humidity of approximately 99.5%. It is known that all drug particles have salts and absorb water from the surrounding environment. The higher the salt concentration, the higher the droplet expansion [43]. While travelling through the airway branches, the aerosol mist or dry powder particles grow in size, depending on the humidity of the environment. They can grow up to 50% based on the salt concentration. The particle size is very important because larger particles, ≥5 μm, will be deposited in the upper respiratory tract while smaller ones will go deeper [44,45]. Drugs ≤5 μm reach the alveoli, are taken up by alveolar epithelial cells, are carried across the blood stream and also enter the surrounding lymph drainage system [42]. Drugs <5 μum enter the lungs. In order to penetrate to the periphery of the lungs, particles need to be approximately 1 μm in diameter. It should be noted that all droplets and powders have a range of aerodynamic sizes, which facilitates penetration to the periphery; this process is called transcytosis. However, the rule dictating that MMAD must be ≤5 μm only applies to certain drugs. For example, inhaled bronchodilators and corticosteroids act on the large bronchial tubes, so the rule would not apply, but cisplatin has to be absorbed in the alveoli so particles must be ≤5 μm. The electrophysical properties (Z potential) is the most important factor in larger airway branches since the drug interacts with the local transporters and local drug absorption is mostly done with this interaction (Figure 2).
Figure 2. . Droplet measurement equipment.
(A) Z potential measurement equipment; red arrow indicates the place where the solution is inserted, the yellow arrow indicates the computer-processor. (B) Mastersizer system 2000 (Malvern Instruments Ltd, Worcestershire, UK) for aerosol droplet and dry powder measurement.
Adapted with permission from [39] © Ivyspring International Publisher (2018).
It was observed in previous studies that the frequency of breathing and the tidal volume were increased and the inhalation was deeper and the deposition was more efficient proven with scintigraphy (V/Q scan-radiolabeled drug) [46,47]. The additional administration of aerosol bronchodilators and corticosteroids has been observed to also enhance the deposition of the aerosols, regardless of whether or not there is an underlying respiratory disease [48–50]. Bronchodilators are known to dilate airways that are narrowed due to the underlying respiratory disease. On the other hand, inhaled corticosteroids block the initiation of inflammation locally. Based on the principal research of jet nebulizers, it was observed that the main factors affecting the droplet size were: the residual cup design, the flow rate, the drug and its properties, the residual cup loading, the pH and the mouthpiece design [15,51–56]. Moreover, it has been observed that the time of nebulization depends on the volume in the residual cup and inner cup design [40]. In specific, the higher the volume, the shorter the nebulization time [57]. Therefore, one could refill the residual cup with NaCl 0.9% when the volume of the solution reaches half of the initial fill within the residual cup, but this methodology can be applied only once [58]. On the other hand, the factors affecting the ultrasonic nebulizers are different as the equipment works on different principles: the temperature of the piezoelectric crystal, the time of nebulization, the addition of buffer, pH and the drug principles [57–62]. Dry powder cisplatin has been created and it could be possibly administered as local therapy [63–65]. Dry powder has the disadvantage that the particles have two different axis (x,y) and one axis is always larger so they can easily induce cough. It has been previously observed that the inlet of the aerosol production systems also influences the droplet size production [66]. Furthermore, it has been observed that the addition of 5–7% CO2 gas during an aerosol administration increases the tidal volume by 180% and reduces the respiratory frequency. However, this methodology has only been investigated in research studies and thus is not easily applicable in the everyday clinical practice.
Lung disease
During the inhaled insulin study, one of the factors that was thoroughly investigated was the underlying respiratory disease and how this factor affects the inhalation pattern and therefore the deposition and absorption of the inhaled drug. Insulin has been tested both as an aerosol mist and as a dry powder [33,34]. The delivery systems that were created for insulin delivery through the airways had a cut off value of ≤30% of forced expiratory vital capacity. Patients with lower values could not sufficiently inhale the mist/powder and therefore the deposition would not be proper. These patients usually had chronic obstruction pulmonary disease (COPD) with emphysema and/or bronchiectasis. Other patients entered the clinical trials with asthma diagnosis. As far as it has been observed that most patients with asthma have a disease that is well controlled with medication and therefore there was no contraindication. The insulin drug formulations that were administered, first in the trials and then as drugs in the market, were not observed to induce allergic shocks [33]. The main issues that had to be evaluated were the dose administration during lower respiratory tract infection and COPD/asthma exacerbation. Indeed, in these two cases, the best solution was to measure the blood glucose levels and administer the insulin drugs subcutaneously for as long as the symptoms lasted. The insulin dosage that was administered/absorbed was properly controlled during this period of time for the patient [33]. The same principles apply for the administration of aerosol chemotherapy.
Clinical studies
Platinum analogs
A number of studies have been performed, both in humans and in animals, with inhaled platinum analogs. Regarding human trials, the administration was performed with jet-nebulizers as a production system and under strict protection measures such as the high efficiency particulate air (HEPA) filter. In the animal studies, the aerosol was administered while the animals were inside a plastic cage or a plexi-glass chamber [28,64,67–71]. A number of evaluation examinations were utilized in order to check the safety and efficiency of the treatment. These can be summarized in the following: radiographic examinations, ki-67 cell proliferation, blood and urine analysis, pathological findings, high-performance liquid chromatography (HPLC), bronchoalveolar lavage (BALF), terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling assay and recording of Eastern Cooperative Oncology Group common toxicity criteria.
The main adverse effects observed were dose-dependent cough, nausea, fatigue and weight loss. However, the last three adverse effects can be associated with the drugs themselves and not with the route of administration. Moreover, it is important to remember that in most of these trials, a second drug was administered intravenously, and some adverse effects could be attributed to both drugs working synergistically. Several chemotherapy drugs have been administered as aerosol; these include 5-fluorouracil (5-FU), taxanes, doxorubicin, gemcitabine and 9-nitrocamptothecine (9-NC).
5-fluorouracil
5-FU was first used as aerosol almost 30 years ago [72]. The HEPA system and plexi-glass chamber was used and safety was evaluated with blood samples, HPLC and imaging techniques [73–77]. The drug was administered intrabronchially and regression of the tumor was observed. The drug was not delivered intratumorally as in other studies [24]. All patients were evaluated in all their sessions via bronchoscopy. Although the drug was administered as aerosol and it was certainly deposited on the surface of the intrabronchial tumors, a part of it went to the peripheral branches and a part was absorbed by the alveoli to the systematic circulation. We do not have information regarding the systematic circulation of the drug in other studies [28]. However, this was an early study, conducted before intratumoral administration began [78], where the aerosol as a local therapy interacted with the surface of the tumor and was absorbed by the extracellular matrix of the tumor [79]. The adverse effects observed were mainly from the respiratory system and included dose-dependent glossitis and weight loss. 5-FU has been administered either alone or coated with lipids or liposome nanoparticles [75,76].
Taxanes
Taxanes have been administered either alone or in combination with other drugs [80,81]. Cyclosporin A has been also co-administered as a method to augment the efficiency of aerosol taxanes [82]. Safety was evaluated with blood samples, pathology findings, HPLC and V/Q scans. All studies were performed in animals and the administration was done in sealed cages. The drug formulation administered was either coated with liposomes or lipid base. The adverse effects were extreme aggressiveness, weight loss and neurological toxicity.
Doxorubicin
Testing of doxorubicin safety was performed with the respiratory capacity tests, HPLC, V/Q display, imaging techniques, blood samples, pathology findings, confocal laser scanning microscopy and colorimetric assay for cell proliferation. Chest pain, cardiac toxicity, weight loss, wheezing, alveolar hemorrhage, hypoxia and >20% reduction of pulmonary function tests values were observed. Corticosteroids were used to treat these adverse effects. However, when loaded microparticles or liposomes were used to coat the drug, the observed side effects were reduced [83–86]. The administration of the aerosol drug was performed in specially designed cages, chambers or HEPA with hood.
Gemcitabine
Gemcitabine is known to have light systemic side effects when administered intravenously [87]. The aerosol was administered in specially designed chambers and cages. The evaluation of the drug distribution, safety and efficacy were performed with V/Q scan, HPLC, BALF, blood samples, pathology findings and cell proliferation. The most severe adverse effect experienced was acute pulmonary toxicity in the form of neovascularization, pulmonary edema and connective tissue formation. Moreover, emesis, bronchospasm and excessive cough were observed [88–90].
9-nitrocamptothecine
9-NC has been investigated using plastic cages – for animals – and HEPA stations – for humans. The safety was investigated with HPLC, blood and urine samples, imaging techniques, Ki-67 proliferation, pathology samples and platelet–endothelial cell adhesion. No adverse effects were observed when it was administered to the animals; however, cough, pharyngitis and bronchitis were observed in the human studies and they were dose dependent. In all studies, the 9-NC was administered in a liposome form [91,92].
Bevacisumab
Bevacisumab has not been administered as aerosol chemotherapy, but it is a strong antineovascularization drug used to treat NSCLC [93]. It has been used for hereditary hemorrhagic telangiectasia-associated epistaxis in the form of aerosol nasal spray and submucosal injections, and therefore is worth mentioning [94,95]. The concept was to block the local anarchic proliferation of the vessels. The adverse effects were ageusia, anosmia, hypertension, headache, hemorrhage, septal perforation and nasal obstruction. These studies utilized blood samples, epistaxis severity score and custom-made questionnaires with phone contact.
Safety
We have divided this section in to two arms; the first has to do with safety of the surrounding environment during the production and administration of aerosol drugs, using cisplatin as an example, and the second with the adverse effects of these drugs, again focusing on cisplatin, to the body. The proper site of administration of cisplatin for patients would be inside a HEPA filter fan unit with a hood or other capture structure. This system provides clean, micro-filtered air, via a reverse-flow hood, that captures contaminants generated in the work administration area for safe cleanroom release [96]. This system has filters designed to reabsorb the drug that was not inhaled and measure the quantity that is reabsorbed. The masks that have been designed for the clinical studies included in this review did not provide a proper seal around the face, so part of the drug was released in the surrounding environment. In a study by Nygren et. al. [97], wipe sampling from the surrounding area provided information regarding the drug that was not inhaled.
Regarding the second arm of safety of inhaled chemotherapy, when we evaluate the safety of an inhaled drug, we should consider the respiratory capacity of the patient, which can be evaluated with spirometry and diffusion capacity for carbon monoxide (DLCO). The spirometry can provide us with a short- and long-term evaluation of the respiratory capacity. The main values we have considered are the forced expiratory volume in 1 s, forced vital capacity and peak expiratory flow. The DLCO will provide us, again, with a short- and long-term evaluation of the diffusion capacity of the smaller airways. Another test that has been used is the 6-min walking test, which provides information on both the respiratory capacity and biological status of a patient [28,98]. BALF fluid is another method for early inflammation identification. BALF fluid is actually acquired with bronchoscopy where 180 ml of saline are distilled within the airways and then with suction part of the initial solution is acquired and send to the laboratory [99]. Imaging techniques are necessary for the evaluation, not only for the staging and restaging of NSCLC, but also to provide us with information regarding the lung parenchyma. First, it shows whether we have architectural damage, such as emphysema, bronchiectasis and fibrosis, upon initiation of the therapy. Afterwards, it will provide us with re-evaluation of the possible damage that was induced. As previously described, patients with severe emphysema and bronchiectasis should not be candidates for this method of administration. The main issue incurred during the administration of inhaled chemotherapy was whether fibrosis would occur as a long-term damage, or pneumonitis as a short term [100]. The simple methods of the respiratory evaluation will provide us with information regarding a bronchoconstriction that might occur and inhaled corticoids and bronchodilators can immediately be administered. Bronchitis obliterans can also be presented as an acute or later form of adverse effect. In this case cough and dyspnea is observed. The main radiologic findings are hyperinflation, air trapping and mosaic pattern. The pathology examination usually reveals neutrophil infiltration and granuloma tissue generation [101]. The usual cough observed in COPD patients might occur as persistent and nonproductive either upon initiation of the administration or after. Usually large molecule drugs tend to induce this adverse effect and, in response, inhaled corticoids along with bronchodilators should be administered. This type of cough usually stops after a number of sessions but if the symptom persists then we should definitely stop the inhaled chemotherapy administration [28]. Upon initiation, a patient receiving inhaled chemotherapy should have both respiratory capacity evaluation and imaging evaluation, only in this way upon follow-up the adverse effects will be properly evaluated. Pharyngitis can also be observed in some patients since the inhaled drug passes through the area [36]. Acute pulmonary toxicity can be observed in the form of neovascularization, or even pulmonary edema and connective tissue formation of the surface of the bronchus [88–90].
Regarding dry powder formulations, there is a well-known association between increased morbidity and mortality from cardiopulmonary disease and airborne particulate matter (particles of 3.6 × 106) [102]. We have not commented on other adverse effects, since many of these (e.g., nausea, emesis, neuropathy and fatigue) can also be induced from the therapy itself and not from the interaction of the inhaled drug with the respiratory tract. Based on the previous data presented regarding the adverse effects and safety, we have graded the inhaled chemotherapy drugs as follows, starting from the worse adverse effects presented during administration going to the safest: Taxanes>Doxorubicin>Gemcitabine>Platinum analogs>5-FU>9-NC>Bevacizumab. However; bevacizumab was not administered as aerosol treatment for NSCLC.
Summary
Every aerosol-delivery system has its advantages and disadvantages. Depending on the patients’ underlying respiratory disease and lung function test results, the doctor has to decide which aerosol production system and delivery method is the most appropriate. The production systems of ultrasonic nebulizers and jet-nebulizers do not require the coordination between patient and administration. However, a large quantity of the drug is lost in the surrounding environment. It has been observed that the misuse of dry powder inhalers could lead to the drug being blown into the device. Moreover, local cell structures and cells such as enzymes, beating cilia, mucus, local transporters/genes and macrophages, play a crucial role in the absorption of inhaled drugs as these factors should be considered when designing a drug. Inhaled cytotoxic therapies should only be administered in a controlled environment where the drug that was not inhaled is captured by special filters. Therefore, for now the administration should be done only in medical establishments. Moreover, based on our knowledge from inhaled insulin and inhaled antibiotics, we know that underlying respiratory disease has to be properly evaluated with lung function tests (spirometry, 6-min walking test and DLCO) and imaging techniques such as computed tomography using high resolution pattern. The main contraindication would be a STAGE IV COPD diagnosis, severe uncontrolled asthma and bullous emphysema or extended bronchiectasis. Low respiratory capacity would mean that the inhaled drug does not reach the lower peripheral parenchyma. Moreover, a large concentration of the drug inside emphysema bullaes or large bronchiectasis would possibly induce a nonspecific allergic reaction. It has been previous observed that aerosol compounds have the ability to penetrate both the main lesion and lymph nodes [37]. The same results have been observed with intratumoral injection of platinum analogs in the main lesion and lymph nodes when patients were not fit for inhalation [78]. Aerosol platinum analogs are an efficient treatment and they have presented a fair safety profile compared with other chemotherapy drugs, new studies with novel methods of safety evaluation are needed [103].
Future perspective
The administration of the drug should not be done in a dry or cold environment in order to reduce the possibility of a bronchoconstriction. Breath actuated devices should be chosen since they provide a control release of the drug. Peak expiratory flow devices should be used before any inhaled chemotherapy administration as it has been observed that they are easy to use and they evaluate short term respiratory adverse effects. After the administration, a re-evaluation with a peak flow meter will provide us with a possible adverse effect. Inhaled bronchodilators and corticoids should be used before each session as a method to protect from bronchospasm. Technology allows us to produce different coatings for drug molecules and by doing this modification we might be able to efficiently treat tumors with different gene expression. Drugs will efficiently penetrate the extracellular matrix of different tumors with different gene expressions, as seen in the case of the EGF–gelatin–platinum conjugation where a platinum analog penetrates a tumor expressing epidermal growth factor more efficiently than platinum alone [104]. In case a patient is not fit for aerosol administration, intratumoral platinum analogs could be considered as they have proved efficient as a palliative care in previous studies [24,78]. Custom made evaluation methods (safety and efficacy) must be created for this type of treatment. It is not possible to administer only one agent as therapy, but only as palliative care. In order to utilize this treatment for therapy, we have to test two agents at once, but this means we will not be able to evaluate properly the adverse effects. Moreover, the adverse effects will possibly be more severe with a harsh nonproductive cough, a reduction in pulmonary test values and pneumonitis. We should further evaluate how the aerosol platinum analogs penetrate the lymphatic system and reduce micrometastasis. We could possibly consider aerosol platinum therapy as neoadjuvant or adjuvant treatment as it could have less adverse effects, at least for those patients that are candidates for adjuvant therapy.
Executive summary.
We need more efficient methods for early lung cancer diagnosis.
We need novel drugs for non-small-cell lung cancer.
We need to use these in a more efficient way than current chemotherapy drugs.
Novel aerosol production systems are on the way.
More studies with aerosol chemotherapy drugs are needed in order to observe safety and efficiency.
Platinum analogs are very effective as a local drug administration.
Footnotes
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Open access
This work is licensed under the Attribution-NonCommercial-NoDerivatives 4.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Choi HCW, Lam KO, Pang HHM, Tsang SKC, Ngan RKC, Lee AWM. Global comparison of cancer outcomes: standardization and correlation with healthcare expenditures. BMC Public Health 19(1), 1065 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zaric B, Stojsic V, Carapic V. et al. Radial endobronchial ultrasound (EBUS) guided suction catheter-biopsy in histological diagnosis of peripheral pulmonary lesions. J. Cancer 7(1), 7–13 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oezkan F, Khan A, Zarogoulidis P. et al. Efficient utilization of EBUS-TBNA samples for both diagnosis and molecular analyses. Onco Targets Ther. 7, 2061–2065 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Groheux D, Quere G, Blanc E. et al. FDG PET-CT for solitary pulmonary nodule and lung cancer: Literature review. Diagn. Interv. Imaging 97(10), 1003–1017 (2016). [DOI] [PubMed] [Google Scholar]
- 5.Hohenforst-Schmidt W, Zarogoulidis P, Vogl T. et al. Cone beam computer tomography (CBCT) in interventional chest medicine – high feasibility for endobronchial realtime navigation. J. Cancer 5(3), 231–241 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hohenforst-Schmidt W, Banckwitz R, Zarogoulidis P. et al. Radiation exposure of patients by cone beam CT during endobronchial navigation – a phantom study. J. Cancer 5(3), 192–202 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zaric B, Stojsic V, Sarcev T. et al. Advanced bronchoscopic techniques in diagnosis and staging of lung cancer. J. Thorac. Dis. 5(Suppl. 4), S359–S370 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hohenforst-Schmidt W, Zarogoulidis P, Huang H. et al. A new and safe mode of ventilation for interventional pulmonary medicine: the ease of nasal superimposed high frequency jet ventilation. J. Cancer 9(5), 816–833 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oudkerk M, Devaraj A, Vliegenthart R. et al. European position statement on lung cancer screening. Lancet Oncol. 18(12), e754–e766 (2017). [DOI] [PubMed] [Google Scholar]
- 10.Livshits Z, Rao RB, Smith SW. An approach to chemotherapy-associated toxicity. Emerg. Med. Clin. North Am. 32(1), 167–203 (2014). [DOI] [PubMed] [Google Scholar]
- 11.Roden DM, McLeod HL, Relling MV. et al. Pharmacogenomics. Lancet 394(10197), 521–532 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Domvri K, Darwiche K, Zarogoulidis P, Zarogoulidis K. Following the crumbs: from tissue samples, to pharmacogenomics, to NSCLC therapy. Transl. Lung Cancer Res. 2(4), 256–258 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Domvri K, Zarogoulidis P, Darwiche K. et al. Molecular targeted drugs and biomarkers in nsclc, the evolving role of individualized therapy. J. Cancer 4(9), 736–754 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Important citation describing how to choose therapy for a non-small-cell lung cancer (NSCLC) patient.
- 14.Tsoulos N, Papadopoulou E, Metaxa-Mariatou V. et al. Tumor molecular profiling of NSCLC patients using next generation sequencing. Oncol. Rep. 38(6), 3419–3429 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zarogoulidis K, Zarogoulidis P, Darwiche K. et al. Treatment of non-small-cell lung cancer (NSCLC). J. Thorac. Dis. 5(Suppl. 4), S389–S396 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cortellini A, Leonetti A, Catino A. et al. Osimertinib beyond disease progression in T790M EGFR-positive NSCLC patients: a multicenter study of clinicians' attitudes. Clin. Transl. Oncol. (2019) (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 17.Zarogoulidis P, Chinelis P, Athanasiadou A. et al. “Liquid elbows” due to afatinib administration. Respir. Med. Case Rep. 22, 64–66 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu CY, Wang CL, Li SH. et al. The efficacy of 40 mg versus dose de-escalation to less than 40 mg of afatinib (Giotrif) as the first-line therapy for patients with primary lung adenocarcinoma harboring favorable epidermal growth factor mutations. Oncotarget 8(57), 97602–97612 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rocco D, Gravara LD, Gridelli C. The new immunotherapy combinations in the treatment of advanced non-small-cell lung cancer: reality and perspectives. Curr. Clin. Pharmacol. (2019) (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zarogoulidis P, Huang H, Tsiouda T. et al. Immunotherapy “Shock” with vitiligo due to nivolumab administration as third line therapy in lung adenocarcinoma. Respir. Med. Case Rep. 22, 283–286 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zarogoulidis P, Athanasiou E, Tsiouda T. et al. Immunotherapy “Shock” a case series of PD-L1 100% and pembrolizumab first-line treatment. Respir. Med. Case Rep. 22, 197–202 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zarogoulidis P, Chinelis P, Athanasiadou A. et al. Possible adverse effects of immunotherapy in non-small cell lung cancer; treatment and follow-up of three cases. Respir. Med. Case Rep. 22, 101–105 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hohenforst-Schmidt W, Zarogoulidis P, Stopek J. et al. Enhancement of intratumoral chemotherapy with cisplatin with or without microwave ablation and lipiodol. Future concept for local treatment in lung cancer. J. Cancer 6(3), 218–226 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• An important clinical trial describing local disease management of NSCLC.
- 24.Hohenforst-Schmidt W, Zarogoulidis P, Darwiche K. et al. Intratumoral chemotherapy for lung cancer: re-challenge current targeted therapies. Drug Des. Devel. Ther. 7, 571–583 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• An important clinical trial describing local disease management of NSCLC.
- 25.Sapalidis K, Zarogoulidis P, Pavlidis E. et al. Aerosol Immunotherapy with or without Cisplatin for metastatic lung cancer non-small cell lung cancer disease: in vivo study. A more efficient combination. J. Cancer 9(11), 1973–1977 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hohenforst-Schmidt W, Zarogoulidis P, Linsmeier B. et al. Enhancement of aerosol cisplatin chemotherapy with gene therapy expressing ABC10 protein in respiratory system. J. Cancer 5(5), 344–350 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• An important study presenting the importance of local gene expression in inhaled therapies for NSCLC.
- 27.Kosmidis C, Sapalidis K, Zarogoulidis P. et al. Inhaled cisplatin for NSCLC: facts and results. Int. J. Mol. Sci. 20(8), E2005 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]; • An important review describing inhalational admnistration of platinum analogs.
- 28.Zarogoulidis P, Eleftheriadou E, Sapardanis I. et al. Feasibility and effectiveness of inhaled carboplatin in NSCLC patients. Invest. New Drugs 30(4), 1628–1640 (2012). [DOI] [PubMed] [Google Scholar]
- 29.Zarogoulidis P, Kioumis I, Porpodis K. et al. Clinical experimentation with aerosol antibiotics: current and future methods of administration. Drug Des. Devel. Ther. 7, 1115–1134 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zarogoulidis P, Kioumis I, Lampaki S. et al. Optimization of nebulized delivery of linezolid, daptomycin, and vancomycin aerosol. Drug Des. Devel. Ther. 8, 1065–1072 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang H, Zarogoulidis P, Lampaki S. et al. Experimentation with aerosol bonsetan, pirfenidone, treprostinil and sidenafil. J. Thorac. Dis. 6(10), 1411–1419 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zarogoulidis P, Kioumis I, Ritzoulis C. et al. New insights in the production of aerosol antibiotics. Evaluation of the optimal aerosol production system for ampicillin–sulbactam, meropenem, ceftazidime, cefepime and piperacillin–tazobactam. Int. J. Pharm. 455(1-2), 182–188 (2013). [DOI] [PubMed] [Google Scholar]
- 33.Zarogoulidis P, Papanas N, Kouliatsis G, Spyratos D, Zarogoulidis K, Maltezos E. Inhaled insulin: too soon to be forgotten? J. Aerosol Med. Pulm. Drug Deliv. 24(5), 213–223 (2011). [DOI] [PubMed] [Google Scholar]
- 34.Zarogoulidis P, Petridis D, Ritzoulis C. et al. Further experimentation of inhaled; LANTUS, ACTRAPID and HUMULIN with todays' production systems. Int. J. Pharm. 458(1), 39–47 (2013). [DOI] [PubMed] [Google Scholar]
- 35.Schulze M, Wree A. Airway anatomy: relevant structures in emergency medicine. Anaesthesist 66(9), 719–734 (2017). [DOI] [PubMed] [Google Scholar]
- 36.Zarogoulidis P, Chatzaki E, Porpodis K. et al. Inhaled chemotherapy in lung cancer: future concept of nanomedicine. Int. J. Nanomedicine 7, 1551–1572 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zarogoulidis P, Darwiche K, Krauss L. et al. Inhaled cisplatin deposition and distribution in lymph nodes in stage II lung cancer patients. Future Oncol. 9(9), 1307–1313 (2013). [DOI] [PubMed] [Google Scholar]
- 38.Zarogoulidis P, Darwiche K, Yarmus L. et al. Defense mechanisms of the respiratory system and aerosol production systems. Med. Chem. 10(2), 123–136 (2014). [DOI] [PubMed] [Google Scholar]
- 39.Sapalidis K, Zarogoulidis P, Huang H. et al. Inhaled immunotherapy administration for lung cancer; efficient? Certainly possible. J. Cancer 9(6), 1121–1126 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zarogoulidis P, Petridis D, Ritzoulis C. et al. Internal mouthpiece designs as a future perspective for enhanced aerosol deposition. Comparative results for aerosol chemotherapy and aerosol antibiotics. Int. J. Pharm. 456(2), 325–331 (2013). [DOI] [PubMed] [Google Scholar]
- 41.Laube BL, Janssens HM, de Jongh FH. et al. What the pulmonary specialist should know about the new inhalation therapies. Eur. Respir. J. 37(6), 1308–1331 (2011). [DOI] [PubMed] [Google Scholar]; • An important review describing parameters of inhalational therapies.
- 42.Labiris NR, Dolovich MB. Pulmonary drug delivery. Part I: physiological factors affecting therapeutic effectiveness of aerosolized medications. Br. J. Clin. Pharmacol. 56(6), 588–599 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]; • An important review describing parameters of inhalational therapies.
- 43.Bier M, de Graaf J, Zwanikken J, van Roij R. Curvature dependence of the electrolytic liquid–liquid interfacial tension. J. Chem. Phys. 130(2), 024703 (2009). [DOI] [PubMed] [Google Scholar]
- 44.Phipps PR, Gonda I, Anderson SD, Bailey D, Bautovich G. Regional deposition of saline aerosols of different tonicities in normal and asthmatic subjects. Eur. Respir. J. 7(8), 1474–1482 (1994). [DOI] [PubMed] [Google Scholar]
- 45.Swift DL. Aerosols and humidity therapy. Generation and respiratory deposition of therapeutic aerosols. Am. Rev. Respir. Dis. 122(5 Pt 2), 71–77 (1980). [DOI] [PubMed] [Google Scholar]; • An important review describing parameters of inhalational therapies.
- 46.Koshkina NV, Knight V, Gilbert BE, Golunski E, Roberts L, Waldrep JC. Improved respiratory delivery of the anticancer drugs, camptothecin and paclitaxel, with 5% CO2-enriched air: pharmacokinetic studies. Cancer Chemother. Pharmacol. 47(5), 451–456 (2001). [DOI] [PubMed] [Google Scholar]
- 47.Davis JN, Stagg D. Interrelationships of the volume and time components of individual breaths in resting man. J. Physiol. 245(2), 481–498 (1975). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dekhuijzen PN, Vincken W, Virchow JC. et al. Prescription of inhalers in asthma and COPD: towards a rational, rapid and effective approach. Respir. Med. 107(12), 1817–1821 (2013). [DOI] [PubMed] [Google Scholar]
- 49.Mogayzel PJ, Jr., Naureckas ET, Robinson KA. et al. Cystic fibrosis pulmonary guidelines. Chronic medications for maintenance of lung health. Am. J. Respir. Crit. Care Med. 187(7), 680–689 (2013). [DOI] [PubMed] [Google Scholar]
- 50.Chang AB, Marsh RL, Smith-Vaughan HC, Hoffman LR. Emerging drugs for bronchiectasis. Expert Opin. Emerg. Drugs 17(3), 361–378 (2012). [DOI] [PubMed] [Google Scholar]
- 51.Zarogoulidis P, Petridis D, Ritzoulis C. et al. Establishing the optimal nebulization system for paclitaxel, docetaxel, cisplatin, carboplatin and gemcitabine: back to drawing the residual cup. Int. J. Pharm. 453(2), 480–487 (2013). [DOI] [PubMed] [Google Scholar]
- 52.Clay MM, Pavia D, Newman SP, Clarke SW. Factors influencing the size distribution of aerosols from jet nebulisers. Thorax 38(10), 755–759 (1983). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mercer TT, Goddard RF, Flores RL. Effect of auxiliary air flow on the output characteristics of compressed-air nebulizers. Ann. Allergy 27(5), 211–217 (1969). [PubMed] [Google Scholar]
- 54.Kendrick AH, Smith EC, Wilson RS. Selecting and using nebuliser equipment. Thorax 52(Suppl. 2), S92–S101 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Newman SP, Pellow PG, Clay MM, Clarke SW. Evaluation of jet nebulisers for use with gentamicin solution. Thorax 40(9), 671–676 (1985). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sterk PJ, Plomp A, van de Vate JF, Quanjer PH. Physical properties of aerosols produced by several jet- and ultrasonic nebulizers. Bull. Eur. Physiopathol. Respir. 20(1), 65–72 (1984). [PubMed] [Google Scholar]
- 57.Ferron GA, Kerrebijn KF, Weber J. Properties of aerosols produced with three nebulizers. Am. Rev. Respir. Dis. 114(5), 899–908 (1976). [DOI] [PubMed] [Google Scholar]
- 58.Steckel H, Eskandar F. Factors affecting aerosol performance during nebulization with jet and ultrasonic nebulizers. Eur. J. Pharm. Sci. 19(5), 443–455 (2003). [DOI] [PubMed] [Google Scholar]
- 59.Lourenco RV, Cotromanes E. Clinical aerosols II. Therapeutic aerosols. Arch. Intern. Med. 142(13), 2299–2308 (1982). [DOI] [PubMed] [Google Scholar]
- 60.Niven RW, Ip AY, Mittelman S, Prestrelski SJ, Arakawa T. Some factors associated with the ultrasonic nebulization of proteins. Pharm. Res. 12(1), 53–59 (1995). [DOI] [PubMed] [Google Scholar]
- 61.Dennis JH, Stenton SC, Beach JR, Avery AJ, Walters EH, Hendrick DJ. Jet and ultrasonic nebuliser output: use of a new method for direct measurement of aerosol output. Thorax 45(10), 728–732 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.O'Callaghan C, Barry PW. The science of nebulised drug delivery. Thorax 52(Suppl. 2), S31–S44 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Levet V, Rosiere R, Merlos R. et al. Development of controlled-release cisplatin dry powders for inhalation against lung cancers. Int. J. Pharm. 515(1-2), 209–220 (2016). [DOI] [PubMed] [Google Scholar]
- 64.Levet V, Merlos R, Rosiere R, Amighi K, Wauthoz N. Platinum pharmacokinetics in mice following inhalation of cisplatin dry powders with different release and lung retention properties. Int. J. Pharm. 517(1-2), 359–372 (2017). [DOI] [PubMed] [Google Scholar]
- 65.Lee HY, Mohammed KA, Goldberg EP, Kaye F, Nasreen N. Cisplatin loaded albumin mesospheres for lung cancer treatment. Am. J. Cancer Res. 5(2), 603–615 (2015). [PMC free article] [PubMed] [Google Scholar]
- 66.Darwiche K, Zarogoulidis P, Baehner K. et al. Assessment of SHOX2 methylation in EBUS-TBNA specimen improves accuracy in lung cancer staging. Ann. Oncol. 24(11), 2866–2870 (2013). [DOI] [PubMed] [Google Scholar]
- 67.Wittgen BP, Kunst PW, Perkins WR, Lee JK, Postmus PE. Assessing a system to capture stray aerosol during inhalation of nebulized liposomal cisplatin. J. Aerosol Med. 19(3), 385–391 (2006). [DOI] [PubMed] [Google Scholar]
- 68.Selting K, Waldrep JC, Reinero C. et al. Feasibility and safety of targeted cisplatin delivery to a select lung lobe in dogs via the AeroProbe intracorporeal nebulization catheter. J. Aerosol Med. Pulm. Drug Deliv. 21(3), 255–268 (2008). [DOI] [PubMed] [Google Scholar]
- 69.Wittgen BP, Kunst PW, van der Born K. et al. Phase I study of aerosolized SLIT cisplatin in the treatment of patients with carcinoma of the lung. Clin. Cancer Res. 13(8), 2414–2421 (2007). [DOI] [PubMed] [Google Scholar]
- 70.Anderson K, Lawson KA, Simmons-Menchaca M, Sun L, Sanders BG, Kline K. Alpha-TEA plus cisplatin reduces human cisplatin-resistant ovarian cancer cell tumor burden and metastasis. Exp. Biol. Med. 229(11), 1169–1176 (2004). [DOI] [PubMed] [Google Scholar]
- 71.El-Gendy N, Berkland C. Combination chemotherapeutic dry powder aerosols via controlled nanoparticle agglomeration. Pharm. Res. 26(7), 1752–1763 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tatsumura T, Yamamoto K, Murakami A, Tsuda M, Sugiyama S. New chemotherapeutic method for the treatment of tracheal and bronchial cancers–nebulization chemotherapy. Gan No Rinsho 29(7), 765–770 (1983). [PubMed] [Google Scholar]
- 73.Wattenberg LW, Wiedmann TS, Estensen RD. Chemoprevention of cancer of the upper respiratory tract of the Syrian golden hamster by aerosol administration of difluoromethylornithine and 5-fluorouracil. Cancer Res. 64(7), 2347–2349 (2004). [DOI] [PubMed] [Google Scholar]
- 74.Tatsumura T, Koyama S, Tsujimoto M, Kitagawa M, Kagamimori S. Further study of nebulisation chemotherapy, a new chemotherapeutic method in the treatment of lung carcinomas: fundamental and clinical. Br. J. Cancer 68(6), 1146–1149 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hitzman CJ, Wattenberg LW, Wiedmann TS. Pharmacokinetics of 5-fluorouracil in the hamster following inhalation delivery of lipid-coated nanoparticles. J. Pharm. Sci. 95(6), 1196–1211 (2006). [DOI] [PubMed] [Google Scholar]
- 76.Hitzman CJ, Elmquist WF, Wiedmann TS. Development of a respirable, sustained release microcarrier for 5-fluorouracil II: In vitro and in vivo optimization of lipid coated nanoparticles. J. Pharm. Sci. 95(5), 1127–1143 (2006). [DOI] [PubMed] [Google Scholar]
- 77.Hitzman CJ, Elmquist WF, Wattenberg LW, Wiedmann TS. Development of a respirable, sustained release microcarrier for 5-fluorouracil I: in vitro assessment of liposomes, microspheres, and lipid coated nanoparticles. J. Pharm. Sci. 95(5), 1114–1126 (2006). [DOI] [PubMed] [Google Scholar]
- 78.Celikoglu F, Celikoglu SI, Goldberg EP. Intratumoural chemotherapy of lung cancer for diagnosis and treatment of draining lymph node metastasis. J. Pharm. Pharmacol. 62(3), 287–295 (2010). [DOI] [PubMed] [Google Scholar]
- 79.Faiyazuddin M, Mujahid M, Hussain T. et al. Aerodynamics and deposition effects of inhaled submicron drug aerosol in airway diseases. Recent Pat. Inflamm. Allergy Drug Discov. 7(1), 49–61 (2013). [PubMed] [Google Scholar]
- 80.Hershey AE, Kurzman ID, Forrest LJ. et al. Inhalation chemotherapy for macroscopic primary or metastatic lung tumors: proof of principle using dogs with spontaneously occurring tumors as a model. Clin. Cancer Res. 5(9), 2653–2659 (1999). [PubMed] [Google Scholar]
- 81.Koshkina NV, Golunski E, Roberts LE, Gilbert BE, Knight V. Cyclosporin A aerosol improves the anticancer effect of paclitaxel aerosol in mice. J. Aerosol Med. 17(1), 7–14 (2004). [DOI] [PubMed] [Google Scholar]
- 82.Knight V, Koshkina NV, Golunski E, Roberts LE, Gilbert BE. Cyclosporin A aerosol improves the anticancer effect of paclitaxel aerosol in mice. Trans. Am. Clin. Climatol. Assoc. 115, 395–404 (2004). [PMC free article] [PubMed] [Google Scholar]
- 83.Azarmi S, Tao X, Chen H. et al. Formulation and cytotoxicity of doxorubicin nanoparticles carried by dry powder aerosol particles. Int. J. Pharm. 319(1-2), 155–161 (2006). [DOI] [PubMed] [Google Scholar]
- 84.Otterson GA, Villalona-Calero MA, Sharma S. et al. Phase I study of inhaled Doxorubicin for patients with metastatic tumors to the lungs. Clin. Cancer Res. 13(4), 1246–1252 (2007). [DOI] [PubMed] [Google Scholar]
- 85.Otterson GA, Villalona-Calero MA, Hicks W. et al. Phase I/II study of inhaled doxorubicin combined with platinum-based therapy for advanced non-small cell lung cancer. Clin. Cancer Res. 16(8), 2466–2473 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Garbuzenko OB, Saad M, Pozharov VP, Reuhl KR, Mainelis G, Minko T. Inhibition of lung tumor growth by complex pulmonary delivery of drugs with oligonucleotides as suppressors of cellular resistance. Proc. Natl Acad. Sci. USA 107(23), 10737–10742 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang L, Wang ZH, Chen XQ. et al. First-line combination of gemcitabine, oxaliplatin, and L-asparaginase (GELOX) followed by involved-field radiation therapy for patients with stage IE/IIE extranodal natural killer/T-cell lymphoma. Cancer 119(2), 348–355 (2013). [DOI] [PubMed] [Google Scholar]
- 88.Rodriguez CO, Jr, Crabbs TA, Wilson DW. et al. Aerosol gemcitabine: preclinical safety and in vivo antitumor activity in osteosarcoma-bearing dogs. J. Aerosol. Med. Pulm. Drug Deliv. 23(4), 197–206 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gagnadoux F, Le Pape A, Urban T. et al. Safety of pulmonary administration of gemcitabine in rats. J. Aerosol Med. 18(2), 198–206 (2005). [DOI] [PubMed] [Google Scholar]
- 90.Min R, Li T, Du J, Zhang Y, Guo J, Lu WL. Pulmonary gemcitabine delivery for treating lung cancer: pharmacokinetics and acute lung injury aspects in animals. Can. J. Physiol. Pharmacol. 86(5), 288–298 (2008). [DOI] [PubMed] [Google Scholar]
- 91.Koshkina NV, Waldrep JC, Roberts LE, Golunski E, Melton S, Knight V. Paclitaxel liposome aerosol treatment induces inhibition of pulmonary metastases in murine renal carcinoma model. Clin. Cancer Res. 7(10), 3258–3262 (2001). [PubMed] [Google Scholar]
- 92.Riedel SB, Fischer SM, Sanders BG, Kline K. Vitamin E analog, alpha-tocopherol ether-linked acetic acid analog, alone and in combination with celecoxib, reduces multiplicity of ultraviolet-induced skin cancers in mice. Anticancer Drugs 19(2), 175–181 (2008). [DOI] [PubMed] [Google Scholar]
- 93.Reinmuth N, Bryl M, Bondarenko I. et al. PF-06439535 (a bevacizumab biosimilar) compared with reference bevacizumab (avastin®), both plus paclitaxel and carboplatin, as first-line treatment for advanced non-squamous non-small-cell lung cancer: a randomized, double-blind study. BioDrugs 33(5), 555–570 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chen St, Karnezis T, Davidson TM. Safety of intranasal Bevacizumab (Avastin) treatment in patients with hereditary hemorrhagic telangiectasia-associated epistaxis. Laryngoscope 121(3), 644–646 (2011). [DOI] [PubMed] [Google Scholar]
- 95.Amedee RG. Efficacy of intranasal bevacizumab (avastin) treatment in patients with hereditary hemorrhagic telangiectasia-associated epistaxis. Am. J. Rhinol. Allergy 25(5), 368 (2011). [DOI] [PubMed] [Google Scholar]
- 96.Wang WH. Validation of the integrity of a HEPA filter system. Health Phys. 85(Suppl. 5), S101–S107 (2003). [DOI] [PubMed] [Google Scholar]
- 97.Nygren O. Wipe sampling as a tool for monitoring aerosol deposition in workplaces. J. Environ. Monit. 8(1), 49–52 (2006). [DOI] [PubMed] [Google Scholar]
- 98.Xu J, He S, Han Y, Pan J, Cao L. Effects of modified pulmonary rehabilitation on patients with moderate to severe chronic obstructive pulmonary disease: a randomized controlled trail. Int. J. Nurs. Sci. 4(3), 219–224 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 99.Chan YL, Wang B, Chen H. et al. Pulmonary inflammation induced by low dose particulate matter exposure in mice. Am. J. Physiol. Lung Cell Mol. Physiol. 317(3), L424–L430 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zarogoulidis P, Giraleli C, Karamanos NK. Inhaled chemotherapy in lung cancer: safety concerns of nanocomplexes delivered. Ther. Deliv. 3(9), 1021–1023 (2012). [DOI] [PubMed] [Google Scholar]
- 101.Prasad R, Gupta P, Singh A, Goel N. Drug induced pulmonary parenchymal disease. Drug Discov. Ther. 8(6), 232–237 (2014). [DOI] [PubMed] [Google Scholar]
- 102.Pope CA, 3rd, Burnett RT, Thun MJ. et al. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. JAMA 287(9), 1132–1141 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rosiere R, Berghmans T, De Vuyst P, Amighi K, Wauthoz N. The position of inhaled chemotherapy in the care of patients with lung tumors: clinical feasibility and indications according to recent pharmaceutical progresses. Cancers 11(3), E329 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tseng CL, Su WY, Yen KC, Yang KC, Lin FH. The use of biotinylated-EGF-modified gelatin nanoparticle carrier to enhance cisplatin accumulation in cancerous lungs via inhalation. Biomaterials 30(20), 3476–3485 (2009). [DOI] [PubMed] [Google Scholar]