Abstract
As rodent locomotion becomes a more popular behavioral assay, proper rodent gait analysis becomes more and more important. Gait measures, such as stride length, cycle time, and duty factor, are not independent of one another, making statistical comparisons between groups a tricky endeavor. Instead of identifying the mathematical relationships between a group of locomotor measures, we simply tracked the steps of rodents in x,y,t space. By plotting with respect to the reference limb, we are able to quantify locomotor changes in space, time, and coordination simultaneously. With our technique, we show that the overall locomotion of 77 rats 1 week after a C4/5 right overhemisection injury was significantly different than pre-injury. This difference was maintained in untreated animals for the entire 7 weeks of the study, but how this difference arose changed. Initially, the right forelimb exhibited very abnormal stepping, but eventually reduced its difference from pre-injury levels. Conversely, the left forelimb was initially mildly different from pre-injury, but further deviated from normal stepping as the weeks went on. Our new gait analysis technique helps to show the trade-off between the restoration of function and the spontaneous development of compensatory techniques. When we applied this new analysis technique to 13 mice after a severe controlled cortical impact, we found that their locomotion was no different from 12 sham mice for the entire 4 weeks of the study. We believe that this gait analysis method succinctly addresses the confound of interdependency of gait measures and does so across multiple injury models.
Keywords: gait analysis, multi-dimensional modeling, spinal cord injury, traumatic brain injury
Introduction
Locomotion is becoming a standard behavioral assay in all veins of animal research thanks, in part, to automated gait analysis devices like the CatWalk (Noldus Information Technology Inc, Leesburg, VA), DigiGait (Mouse Specifics Inc, Framingham, MA), MotoRater (TSE Systems Inc, Chesterfield, MO), and TreadScan (Clever Sys Inc, Reston, VA), which quickly and easily provide users with a plethora of locomotor data. A common practice in research to analyze these data is to run individual analysis of variance tests on the means of select measures, such as stride length, cycle time, and duty factor. However, locomotion is not the summation of discrete independent values, but a nuanced coordinated interplay between these multiple moving parts that currently cannot be teased apart using existing approaches.
Many of the measures provided by gait analysis devices are not independent of one another. Cycle time is not independent from duty factor (duty factor = stance time/cycle time); stride length is not independent from stride velocity (stride velocity = stride length/cycle time). If you expand this view to acknowledge that all locomotor measures, from right forelimb stride length to left hindpaw angle, are physically attached to the same moving body, then it stands to reason that the measures reported by gait analysis devices are more likely to be dependent than independent. This is a problem in animal research given that promising treatments may have failed when studies are being reproduced because the confound of gait variable interdependency was not consistently addressed from study to study.
We have focused on developing techniques to address this confound of interdependency of gait variables. Our recent work has expanded upon the early exploratory work on rodent gait analysis from the 1970s,1–3 and found the velocity dependence of common gait variables and how they spontaneously change in a rat model of spinal cord injury (SCI).4 Other groups have repeated this technique to successfully show that gait variables in healthy mice also have velocity dependence,5 and that using this analytical modification better captures the gait signature of murine parkinsonism models.6 However, controlling for only velocity also has statistical limitations. By focusing on the velocity dependence of stride length, it is possible to overlook the phasing dependence of stride length, or the body angle dependence of stride length.
Instead of digging deeper to uncover relationships between all of these measures, we now feel that it is better to take a step back and take a more holistic view. Most, if not all, of the measures exported by gait analysis devices are essentially manipulations of the time (t) and position (x,y) of the recorded paw prints. Stride length = Δx, base of support = Δy, duty factor = (tTO1-tIC1)/(tIC2-tIC1). By plotting the data in its original x,y,t form, the multi-dimensional interdependencies of gait will theoretically become apparent. Essentially, individual variables of locomotion become redundant as the simultaneous change in x,y,t will itself be the measure.
Presented here is a novel technique to quantify the changes in rodent locomotion following a neurological injury. This technique takes the data exported from a commercially available rodent gait analysis device, the CatWalk, and transforms it in a manner to reduce the influence of interdependency among common gait variables. This results in a whole-body view of locomotion, which captures the nuanced coordinated interplay between limbs. With this novel technique, we not only show that rats have impaired gait after an SCI, but also spontaneously develop compensatory techniques 5 weeks after injury. Additionally, we apply our method of gait analysis to demonstrate that a murine model of traumatic brain injury (TBI) does not result in altered gait up to 4 weeks post-injury.
Methods
All animal protocols were approved in advance by Georgetown University Animal Care and Use Committee. All animals were housed in the Georgetown University Division of Comparative Medicine with unlimited access to food and water. At no point were food deprivation or food rewards used as motivators.
Rats and spinal cord injury protocol
One hundred eight adult female Sprague-Dawley rats were used (approximately 5 weeks old, 160- to 220-g range, 186 ± 12 g mean; Taconic Farms, Germantown, NY). The rats are part of our ongoing robotic gait training studies, and we have previously reported a non-linear regression gait analysis of 46 of these animals4 and an irregular conical gait analysis of 74 of these animals.7 Presented here, for the first time, is a novel multi-dimensional gait analysis of 108 animals.
Rats received a right overhemisection injury at the C4–C5 level, which bilaterally ablates the dorsal corticospinal pathway and unilaterally ablates the contralateral rubrospinal pathway. This results in profound asymmetric impairments, with the right side more impaired than the left and the forelimbs more impaired than the hindlimbs. Thus, this model is ideally suited to investigate spontaneous asymmetric gait recovery and development of compensatory techniques. The surgery has been previously described,8,9 but, briefly, rats were anesthetized with 3% isoflurane, a partial C4/C5 laminectomy was done, and iridectomy scissors were used to create a lesions at C4–C5. At the end of the study, all lesion sites were reconstructed from serial cresyl violet sections or magnetic resonance imaging images. We only included the 77 animals with appropriate injuries in post-injury analysis.
Whereas the other 58 animals entered training protocols not discussed here, a subset of 19 untrained animals were then tested weekly for an additional 6 weeks starting on post-injury day 11 and ending on post-injury day 46 (hereafter referred to as weeks 2 through 7). Animals were randomly assigned to treatment groups by the MATLAB randperm function (The MathWorks, Inc., Natick, MA) or the drawing of numbers out of a hat. Frequently, not all 19 untrained animals took appropriate steps for each week; therefore, the total N for each week varies from 15 to 19.
Mice and traumatic brain injury protocol
For controlled cortical impact (CCI) surgery, the contusion occurred over the sensory cortex, as previously described.10,11 We compared sham mice (n = 12) to CCI mice (n = 13). Wild-type C57Bl/6 male mice were purchased from The Jackson Laboratory (Bar Harbor, ME) and were 3–4 months of age at the time of injury. CCI mice were administered surgical anesthesia using 4% isoflurane with maintenance in 2%, at flow rate of 1.0–1.5 L/min in freely breathing oxygen. Bupivicaine was administered intradermally to the surgery site, a 10-mm midline incision was made over the skull, the skin and fascia reflected, and a craniotomy performed (4 mm) on the central aspect of the left parietal bone. The impounder tip of a Leica StereoOne Impactor (Leica Microsystems, Wetzlar, Germany) was sterilized, positioned to the surface of the exposed dura, and set to impact the cortical surface at 5.25-m/s velocity and 2-mm tissue deformation. The skull was replaced after injury. Sham mice received isoflurane anesthesia, skin incision, and reflection, but no impact or craniotomy. After injury, the incision was closed with wound clips, anesthesia discontinued, 1 mL of saline administered by intraperitoneal injection, and the mouse placed in a heated cage to maintain normothermia for a 45-min recovery period.
CatWalk protocol
For 3 non-consecutive days, animals were pre-trained on the CatWalk XT10.1 (Noldus Information Technology) gait analysis system before pre-operative overground locomotion was recorded. The Catwalk device consists of a long glass walkway with a green light internally reflecting. When the rodent paw contacts the glass, light is reflected down and recorded by the digital camera. With the software, users assign the recorded prints to identify the time and location of steps. From this point, our custom software (MATLAB; The MathWorks) analyzed the rodent gait. No time, velocity, or directional constraints were placed on the trials as animals were allowed to cross the walkway at their own self-selected walking speed. The trial was considered complete once several walking steps were recorded from each limb. This could be accomplished from as few as one complete pass or from several partial passes. Trotting or galloping steps were omitted. As to not bias the group data toward animals that took more steps, a cap of 15 steps per animal was applied. These 15 steps were not randomly chosen, but were selected from the steps that were most similar to one another. One week after injury, with no retraining the overground locomotion of the animals was reassessed with the CatWalk and weekly thereafter.
Results
Rotation of coordinate frames
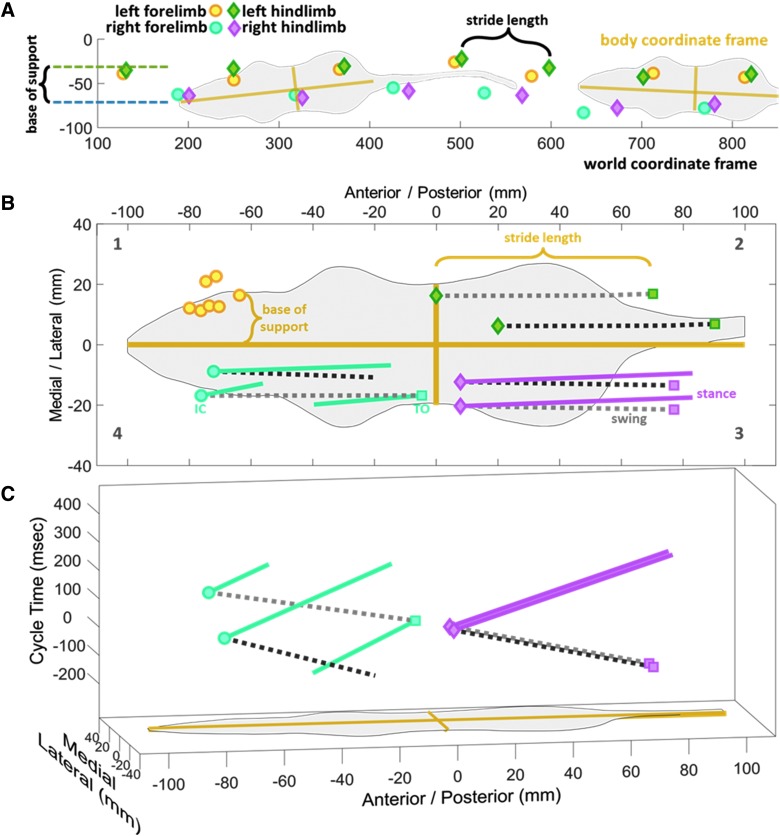
Automated gait analysis systems record the time and location of paw prints as the animals walk. Location is determined by applying a calibrated pixel grid to the field of view. We refer to this pixel grid as the world coordinate frame. Once a cluster of pixels is defined as a paw print, any number of measurements can be made. Figure 1A shows a representative rat walking from right to left with stride length defined as the distance from one print to the next and base of support as the difference between average right y (top to bottom) position and average left y position. This technique works well when the animals walk in perfectly straight lines parallel to the world coordinate frame; however, this does not take into account the natural listing and wandering of a walking rodent. The use of a fixed world coordinate frame can introduce consistent variation and error as the rodent walks with a variable body angle.
FIG. 1.
Translation of CatWalk data into x,y,t space. (A) As a rat crosses the glass walkway, the time and location of the paw prints are recorded in the world coordinate frame. (B) Translating these prints into the body coordinate frame of the rat corrects for errors induced by rats not walking in straight lines parallel to the world coordinate frame. As seen in B2, this has the added benefit of showing the different medial/anterior positioning of two sample steps with equal stride lengths. (C) When the third dimension of time is added, errors in coordination can be seen. Time is expressed as the cycle time of the reference limb (right hindlimb) with initial contact at time 0. During two very similar right hindlimb steps, the right forelimbs are very differently coordinated, even though the right forelimb cycle times and stride lengths may be similar (left-limb data have been omitted from [C] for clarity).
A second error that is introduced when using a fixed world coordinate frame is the generation of phantom strides from impaired limbs. An example of this is in our SCI rats, where the front right limb has a severely reduced range of motion and is only placed on the CatWalk while the less impaired left limb is in swing. While there is weight bearing on this injured right limb, there is minimal active stride being produced. However, by only recording the placement of the right forepaw prints on a fixed frame, the automated software records the movement of the limb across the CatWalk as a stride.
We correct for these errors by translating and rotating the recorded data into the body coordinate frame of the animal (Fig. 1B), as previously reported.4,7 The seven left forepaw prints from Figure 1A are translated to the seven left forepaw initial contacts in section B1, making base of support the distance in the medial/lateral direction (Δy). When the toe off of a single left hindlimb is compared to the subsequent initial contact (section B2), we see that stride length is the difference in anterior/posterior direction (Δx).
Section B2 also shows the translation of two different strides with equal stride lengths. We were unsure whether two stride lengths were truly equal if they occurred at different body positions. And what about if these equal stride lengths happened at different times? Not just different limb cycle times, but at different times in interlimb phasing. Locomotion is not just a bunch of independent spatial and temporal measures. Locomotion is how all limbs move together in space and time. And it should not matter if there is a sophisticated mathematical expression correlating one measure (like right hindpaw stride length) with another (like left forepaw cycle time) or not, locomotor measures are not independent variables; all of the measures generated by automated gait analysis devices are physically attached to the same moving body. In our view, this makes current gait analysis techniques less than ideal.
Reference limb referenced motion
To address these concerns, we have adapted out earlier techniques to better capture the multi-dimensionality of gait. Section B3 of Figure 1 shows the complete step cycle of two right hindlimb steps from toe off (colored square), through swing phase (gray dotted line), to initial contact (colored diamond), and through stance phase (colored line). While these two right hindlimb steps are very similar, we also need to track the motion of the other limbs during the gait cycle of these reference limbs. (The right hindlimb was chosen as the reference limb to be in agreement with our previous work7 and the work of others.12) In Figure 1B, section B4, we see the corresponding right forelimb motion during the respective right hindlimb reference steps. While the right hindlimbs are taking similar steps, one right forelimb transitions from swing to a more medial stance (dark gray) and the other step is finishing a more lateral stance before continuing through a complete swing phase, and a little bit of early stance (light gray); two very different locomotion patterns during two very similar reference limb steps.
The timing of such behavior is just as important as the position, so Figure 1C plots these two sample steps in the third dimension of time. To compare the timing of the two sample steps, we do not measure from their respective start to finish, but from the toe-off to subsequent toe-off of the reference limb. All limb motion within this time frame is then time shifted to put the reference limb initial contact at time 0. If there were differences in right hindlimb cycle time, the magenta lines would have different ranges in the t dimension. If they had different duty factors they would have different proportions of negative/positive times. And if the steps were occurring at different speeds, the slower step would be more open whereas the faster step would be more closed (greater distanced covered in less time). Additionally, the blue right forelimb traces of Figure 1C express the phasing differences in these two steps. Even though the right forelimb steps may have similar stride lengths (Δx), similar base of support(Δy), and similar cycle times (Δt), when plotted in this three-dimensional (3D) space the discoordination is readily apparent. The right fore- and hindlimbs of the dark gray steps are in phase, with swing and stance occurring at the same time, whereas the light gray steps are out of phase.
Traditional techniques of locomotion analysis present different gait measures as independent individual variables, but this approach can lead to problems with interpretation. It is unclear whether it is better to have similar stride lengths but different cycle times, or similar cycle times but different stride lengths, or maybe something in between. Our analysis circumvents this problem by presenting a multi-dimensional measure of locomotion for the entire animal; a measure that equally combines the timing of a step, location of a step, location of the other limbs, and interlimb phasing of a step into one representation of locomotion.
Grouping multi-dimensional data
Above, we have described the analysis of single steps. In order to quantify the many steps from several animals in an experimental group, we modified our earlier technique for grouping steps in a robotic gait training device12 and generated 3D models to represent the multi-dimensional, interlimb, behavior of walking rats. The basic concept is to fit the densest cluster of 68.27% of the data points in x,y,t space with a boundary (MATLAB boundary function, 0.5 shrink factor; The MathWorks), leaving the remaining 31.73% of data points with a 3D residual distance to the boundary. This process is repeated for each limb for every 1% of the reference limb gait cycle. Unlike our previous work, it is important to only group data points of similar swing/stance phases. For instance, at 25% of the reference limb (right hind) gait cycle, two different steps may have their left forelimb at similar locations in space/time. But if one just started stance while the other is finishing up swing, they should not be grouped together, for the phases are distinctly different.
To aid with visual presentation, composite models are constructed by merging the models for each percent of the gait cycle together. Figure 2 represents the composite model throughout the entire gait cycle of 108 naïve female rats. Figure 2A shows the superior view of the rat, and the symmetry of the gait of normal rats is readily apparent. The tightness of the meshes indicates a very consistent stepping pattern with symmetric stride length, no crossing over the midline body axis, and a wider hindlimb base of support than forelimb.
FIG. 2.
Multi-dimensional motion of healthy rats. At each percent of the gait cycle, the steps of 108 healthy rats are fitted with the smallest mesh that contains 68.27% of the data points in x,y,t space. These individual meshes are then merged to form composite models for the entire gait cycle, shown here. Colored meshes represent stance phase and gray represents swing. (A) Healthy gait is consistent (small mesh volumes) with right/left symmetry. (B) By introducing the third dimension of time (right hindlimb cycle time with initial contact at time 0), limb phasing and interlimb coordination can be observed. The fore- and hindlimb pairs are out of phase whereas the diagonal pairs are in phase.
Figure 2B introduces the temporal aspects of gait. As the right hindlimb begins swing (gray mesh) with toe off, the left forelimb is already in swing, while the left hindlimb (green mesh) and right forelimb (blue mesh) are in stance. Before initial contact of the right hindlimb, the right forelimb begins to transition to swing. The overlap of swing and stance phases indicates the variability of healthy gait. Further, 20 msec before right hindlimb initial contact, the right forelimb is sometimes in late stance and sometimes it is in early swing. This limb phasing has previously been represented with polar plots to show the decrease in phasing consistency in bipedal stepping after incomplete thoracic injuries in rats13 or with mean phase intervals to represent the phase shift in limb pairings in the quadrapedal walking of cats after a right thoracic hemisection injury.14
The technique presented here enables us to combine and expand these techniques to simultaneously measure the changing phase shift and consistency. The hindlimbs are out of phase, with periods of initial dual stance around initial contact of the right hindlimb and terminal dual stance with the initial contact and early stance phase of the left hindlimb, as indicated by the small region of green at the top of the left hindlimb trace. This phasing is mirrored in the forelimbs, with the left forelimb initiating swing before toe off of the right hindlimb (region of swing at top of yellow trace).
Quantification of differences in multi-dimensional gait analysis
We have previously described a method for quantifying differences in multi-dimentional gait in our work with rats in a robotic gait training device.15 In brief, each test group consists of a set of data points and a 3D model that encloses 68.27% of the points. The remaining 31.73% of points have a residual distance to the model. The product of this sum of residuals, model volume, and ratio of points outside to inside is the measured error. When comparing experimental groups A and B, 3D model A will be applied to data points B and model B to points A. This will increase the error of both, given that more points will be outside the model, possibly with a greater distance to the model. If healthy animals are assumed to be symmetric, applying the left model to the right data points, and vice versa, will have a minimal increase of error. This increase in error represents the naturally occurring variability in healthy data and the limits of our technique.
When comparing two experimental groups, if the increase in error is more than twice this limit, we considered it statistically significant. For our quadrapedal rodents, this enables us to look at the error of each limb individually, before we sum the four limbs to get an overall relative error score. We apply this method to assess the locomotor deficits and the time course of spontaneous recovery in a rat model of SCI and a mouse model of TBI. By comparing the multi-dimensional error over the course of several weeks, we are able to observe both the recovery of function as well as the development of compensatory techniques. There were no differences found in the pre-injury, nor in the 1 week post-injury, locomotion across experimental sets recorded at different times throughout the years. Therefore, all pre-injury (N = 108) and post-injury (N = 77) rats were binned together. This is consistent with our previous work that used a similar multi-dimensional technique to analyze the stepping of rats in a robotic gait training device.15
Spinal cord injury alters locomotion
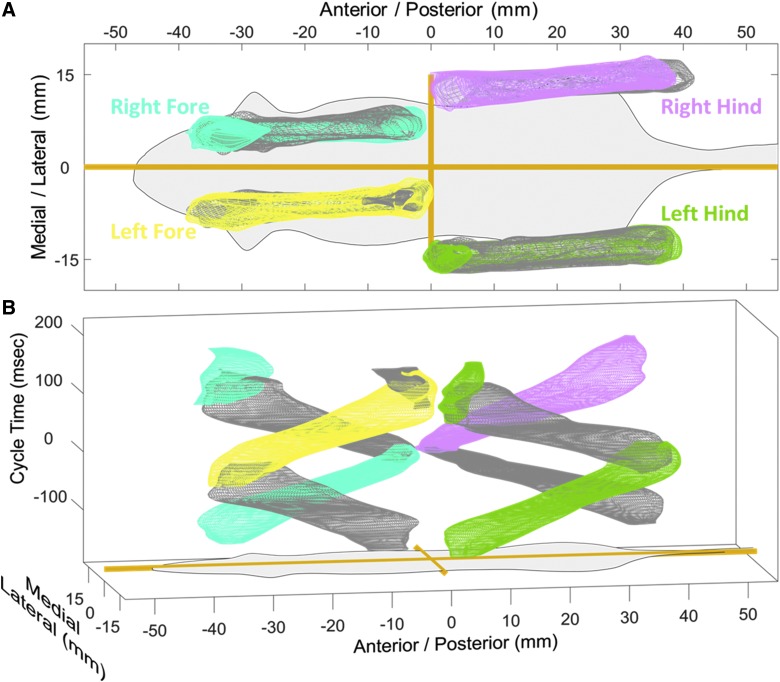
Subsequent to an SCI, impaired locomotion is readily apparent; the animals struggle to make it across the walkway. And traditional gait analysis techniques result in a plethora of measures being significantly different.4,16,17 But, again, we are unsure of how to interpret these differences if locomotor measures are all related and not independent. Figure 3 shows the multi-dimensional gait analysis of 77 rats 1 week after a C4/5 right overhemisection injury. Figure 3A clearly demonstrates that all limbs exhibit a reduced consistency of stepping with a larger volume in medial/lateral direction compared to pre-injury baseline (based on visual inspection). The forelimbs show the expected asymmetric impairment of reduced right forelimb stride length and increased left stride length. And the hindlimbs show a previously unobserved pitch. As the impaired rats walk across the glass, the less impaired left forelimb overcompensates and crosses the midline, resulting in a slanted body axis. When translated into the body coordinate frame, the slanted body axis becomes a slanted limb path.
FIG. 3.
SCI drastically alters multi-dimensional motion. The steps of 77 rats 1 week after a C4/5 right overhemisection injury are fitted with the smallest mesh that contains 68.27% of the data resulting in a multi-dimensional model of gait. Colored meshes represent stance phase and gray represents swing. (A) Impaired consistency (large mesh volumes) and right/left asymmetry is apparent, along with altered body axis. (B) By introducing the third dimension of time (right hindlimb cycle time with initial contact at time 0), complete disruption of limb phasing and interlimb coordination can be observed. SCI, spinal cord injury.
Figure 3B shows that all limbs exhibit prominent changes in limb phasing and interlimb coordination. By definition, with this method, the reference right hindlimb goes through swing in negative cycle time and stance in positive cycle time, but this motion is much more variable (larger volume) than in the pre-injury baseline. Additionally, after injury the other limbs do not maintain a well-defined separation of swing/stance phase (more gray and colored volumes occupy the same space). In the pre-injury analysis, we observed that when the right hindlimb begins swing, the left forelimb was in sync and also in swing phase. The other diagonal pair, left hindlimb and right forelimb, were ∼180 out of limb phase and in stance. After SCI, there is no consistent coordination. When the right hindlimb begins swing, the forelimbs can be found in just about any phase and any position in body space. The left hindlimb maintains a semblance of shape with the limb moving back as the right hindlimb moves forward, but not having two distinct stance volumes is a clear indication of disrupted coordination.
Spontaneous recovery of spinal cord injury rats is asymmetric
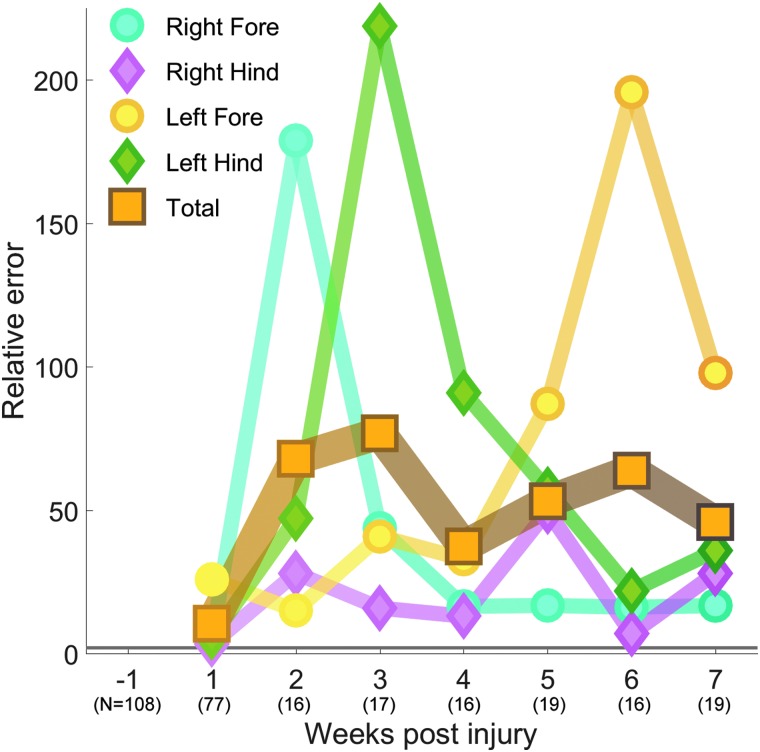
One week after a C4/5 overhemisection injury, the multi-dimensional locomotion of our 77 untrained rats is significantly different from our 108 rats pre-injury with a relative error (RE) of 10.35. This difference increases to a peak at week 3 of RE 76.83 before a decline to RE 45.78 by week 7. The umber line of Figure 4 shows that the locomotion of untrained animals is most different from pre-injury values 3 weeks after injury. When this whole-body error measure is separated into the component limbs, we see that each limb behaves differently. One week after injury, all limbs are equally significantly different from pre-injury, but at week 2 the right forelimb is much more different from pre-injury (RE 178.86) than the other limbs.
FIG. 4.
Asymmetric spontaneous recovery of SCI rats. Over time, some limbs recover whereas others develop compensatory techniques. The more impaired right forelimb begins as the limb with the greatest difference from pre-injury. Over time, the right forelimb becomes much less different, at the expense of the other limbs, particularly the left forelimb. The less impaired limbs, with their greater range of motion and control, take more abnormal steps in order to have the more impaired limb take as normal a step as possible. The gray line at RE 2.0 represents the cutoff for significant difference. RE, relative error; SCI, spinal cord injury.
This difference quickly decreases over time, and for weeks 4 through 7 the RE is <17 (blue line, Fig. 4). The left hindlimb, which was moderately different 1 week after injury (RE 5.82), continues to deviate its multi-dimensional gait patterns away from pre-injury levels, so that by week 3 it is the most different with an RE of 218.69. This difference subsides, and the RE is <35 for weeks 6 and 7 (green line, Fig. 4). After injury, the left forelimb is significantly different from pre-injury levels and maintains a similar level of difference for the first 4 weeks post-injury (RE <41). By the fifth week post-injury, the left forelimb begins to deviate even further away from pre-injury levels, with a peak of RE 195.71 at week 6 (yellow line, Fig. 4). The right hindlimb is the only limb that maintains a consistent amount of difference from pre-injury levels over the 7 weeks of this study (RE <50; red line, Fig. 4).
Cortical impact of the parietal cortex does not alter gait
To further characterize our novel method of gait analysis, we applied it to mice to measure locomotor changes after CCI.
Figure 5A shows the paw placement of the 12 sham-injured mice. Similar to our pre-injury rats, sham mice have a consistent stepping pattern, with right/left symmetry, consistent stride length, and no crossing of the medial/lateral midline. Unlike rats, mice have a greater difference in fore- and hindlimb base of support, with the hindlimbs extending more caudally and even crossing the anterior/posterior midline. Figure 5B shows highly coordinated limb movements, with the reference right hindlimb exhibiting very consistent swing and stance phasing. Like pre-injury rats, sham mice have a predictable gait pattern. The left forelimb of sham mice is in phase with the right hindlimb and the other diagonal pair, the right forelimb and left hindlimb, are also in phase with each other and out of phase with the reference limb.
FIG. 5.
Multi-dimensional motion of sham mice. The steps of 12 sham-injured mice are fitted with the smallest mesh that contains 68.27% of the data resulting in a multi-dimensional model of healthy gait. Colored meshes represent stance phase and gray represents swing. (A) Sham mouse gait is consistent (small mesh volumes) with right/left symmetry. The forelimb base of support is narrower than the hindlimbs. (B) By introducing the third dimension of time (right hindlimb cycle time with initial contact at time 0), limb phasing and interlimb coordination can be observed. The fore- and hindlimb pairs are out of phase whereas the diagonal pairs are in phase.
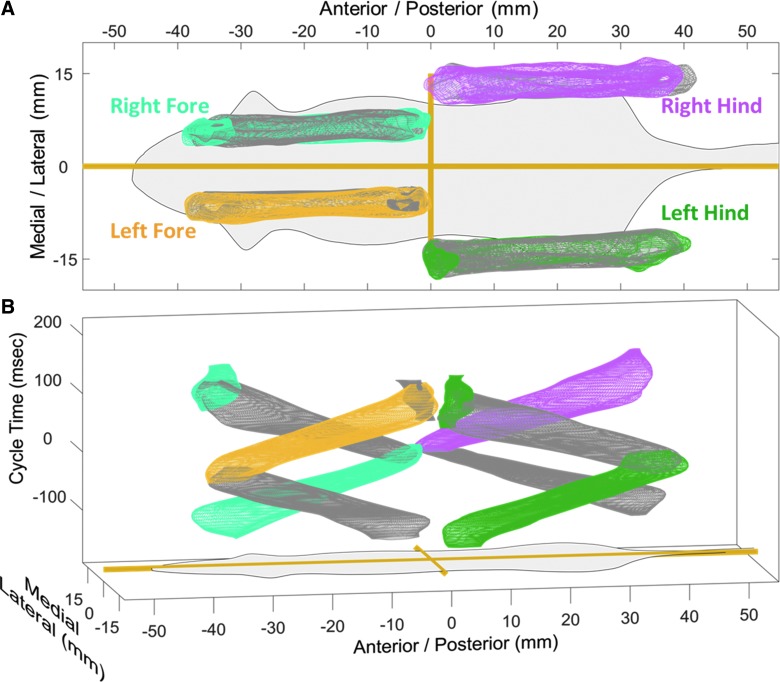
We also quantified the multi-dimensional gait of CCI mice at 1 week post-injury (Fig. 6). The 4-mm impact is centered over the sensory cortex of the mice and leads to the formation of a visible lesion with widespread tissue loss.10,11 These mice have previously been shown to be unimpaired in spontaneous exploration of a novel arena, but display fine motor coordination deficits on a beam-walk test.10,11 Using our transformation, we could observe no noticeable differences between the sham mice and TBI mice. Injured mice maintain right/left symmetry, consistent stepping, and solid intra- and interlimb coordination.
FIG. 6.
TBI does not alter multi-dimensional motion. The steps of 13 mice 1 week after a CCI injury are fitted with the smallest mesh that contains 68.27% of the data resulting in a multi-dimensional model of gait. Colored meshes represent stance phase, and gray represents swing. (A) Stride lengths, base of support, and symmetry are remarkably similar to sham mice. (B) By introducing the third dimension of time (right hindlimb cycle time with initial contact at time 0), the lack of change in limb phasing and interlimb coordination can be observed. CCI, controlled cortical impact; TBI, traumatic brain injury.
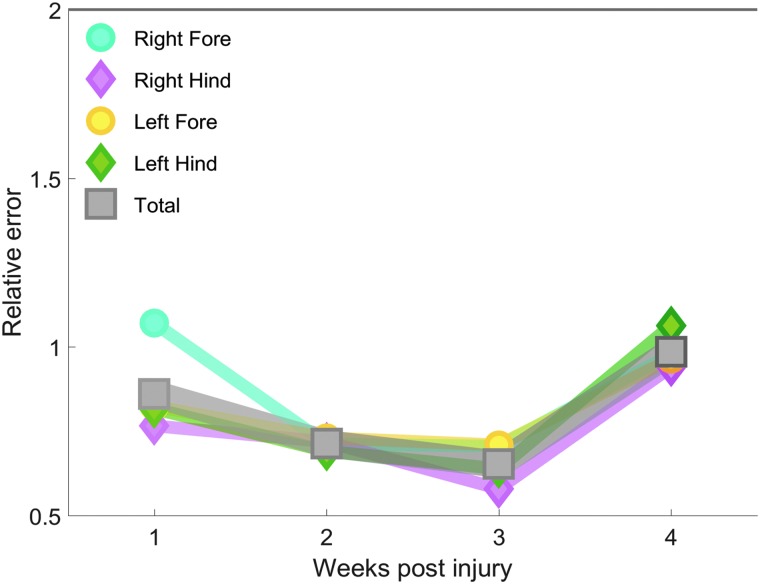
We continued to follow the CCI and sham mice for 4 weeks post-injury to determine how TBI alters multi-dimensional locomotion over time. Relative to the sham mice, we found that there was no difference in gait after CCI. One week post-injury, the multi-dimensional locomotion of TBI mice is not different from sham-injured mice (RE 0.86). This lack of change is maintained over the next 3 weeks as the CCI mice continue to display similar locomotor ability as the sham mice (Fig. 7). When whole-body error measure is separated into the component limbs, we see that the limbs behave similarly at all time points, and there is no difference between injury groups. At no point does the RE of any limb or whole-body measure exceed 1.10 (significance occurs at values >2).
FIG. 7.
No change in mouse gait after TBI. For up to 4 weeks after a CCI injury, there is no significant difference in multi-dimensional gait measures between injured (N = 13) and sham (N = 12) animals. This is true of both total composite gait as well as individual limbs. The gray line at RE 2.0 represents the cutoff for significant difference. CCI, controlled cortical impact; RE, relative error; TBI, traumatic brain injury.
Discussion
Gait analysis is a powerful, but complex, tool that helps researchers to quantify behavioral differences between test groups. Commercially available automated gait analysis systems enable researchers to quickly and easily gather enormous amounts of data. In traditional gait analysis, multiple interdependent factors are studied, but analyzed and reported as independent factors. This can lead to the generation of errors such as reporting the same finding multiple times. A comparison would be to report the radius, diameter, and circumference of different marbles—if one factor is different, then all of the other dependent factors would also most likely be different too. More specific to gait analysis, it is not appropriate to report differences in both ankle angle and toe location because the ankle angle determines the location of the toes—the two variables are not independent.
Over the years, we have attempted several techniques to address these concerns of interdependence. The behavior of one limb will affect the behavior of the others, simultaneously in both time and space. Presented here is a novel gait analysis method that aims to embrace this multi-dimensional interdependency of rodent gait rather than isolating individual parameters. Our previous work showed that by rotating the data out of the world coordinate frame and into the coordinate frame of the animal, we are able to reduce the influence of the animals not walking in straight lines parallel to the coordinate frame of the software4.
In this work, we have extended our techniques to keep these data as whole as possible and not break it up into discrete values such as right forelimb stride length or left hindlimb duty factor. When a step is looked at as the simple change of x,y,t of all four limbs as the reference limb progresses from toe off to subsequent toe off, our multi-dimensional measure appears. This helps maintain the link between time and space in gait analysis. As we tried to show in Figure 1C, a traditional measure like stride length (Δx) and base of support (Δy) may be similar, but if it occurs at different times in the reference limbs step cycle (Δt), is it really a similar step? If only limb spatial measures are reported and not interlimb temporal measures, one may falsely conclude that the steps are similar.
We appreciate the fact that locomotion is not just the timing and location of paw prints. Including ventral body measures, such as truncal deviations captured from devices like the Motorater, would have given us more data points; however, having more, or different, measures does not address the issue that locomotor measures are dependent upon one another. The technique presented here can be adapted to quantify errors in the 3D space of protraction angle, knee angle, and truncal deviation. Expanding this even further, while we believe it can be done, N-dimensional cluster analysis of gait variables is outside the scope of this study.
We applied our technique to assess the locomotor recover of rats after a C4/5 right overhemisection injury. It is of no surprise that our technique revealed significant changes after injury, as this has been previously reported.16,17 What our technique enables us to do is track the restoration of function and the development of compensatory techniques while minimizing the confound of measuring multiple dependent variables. The right forelimb is the most severely impaired limb, so it stands to reason that it has the greatest difference from pre-injury levels after injury.
At first glance, it is a little surprising that the peak difference is 2 weeks after injury instead of 1, but this is most likely attributable to the high variability after injury. As the stepping becomes more consistent 2 weeks after injury, the relative error increases. However, as the weeks go on, the right forelimb does get more like pre-injury. On the other hand, for the first 4 weeks post-injury, the left forelimb is moderately different from pre-injury before dramatically increasing its difference for the last 3 weeks of the study. We consider this not a delayed response to injury, but the development of a compensatory technique. The less impaired limbs, with their greater control and range of motion, take more abnormal steps to enable the more impaired limbs to take a more normal step. This is a trade-off between all the limbs that takes weeks to unfold. A trade-off that is not readily apparent with traditional gait analysis techniques, but much clearer with our multi-dimensional analysis.
The behavior of the hindlimbs muddies this theory a bit. The left hindlimb is the most different from pre-injury 3 weeks after injury. This could be interpreted as either a delayed response to injury or a compensatory technique that develops only to resolve as the left forelimb compensatory technique emerges. The right hindlimb is the reference limb, so phase differences will not be present, only space and time differences. Thus, it is not surprising that the right hindlimb exhibits the least amount of differences. There are still significant differences in space and time, but no apparent compensatory techniques develop.
The CCI mouse model of TBI is extensively used to model contusion injury, and the behavioral and motor consequences of this type of injury are well documented by many groups, including ours.10,11 Injury severity in this model has a linear relationship in both cognitive and motor domains, but not in affective disorder domains.11 We used a 2-mm impact depth in this study, which we have previously shown to have a strong impact on learning and memory, and on striatum-mediated torsoflexion in the mice.11 CCI mice also have acute impairments in other motor-mediated tasks, such as the rotarod test, and chronic impairments in tests of fine motor coordination, such as the beamwalk and gridwalk tests10,18,19; however tests of locomotor ability and exploration in novel chambers show that the mice are not impaired in their ability to walk given that their locomotion distance in a novel arena remains similar to sham mice.10
The literature surrounding the use of CatWalk as a tool to detect motor impairments after TBI has both negative and positive findings. A report by Wang and colleagues showed that CCI mice had reduced stance phase and paw-print area compared to sham mice, with significant differences from 1 to 4 weeks post-TBI.20 However our data better conform to a more recent report that CCI rats do not have impairments in gait at 2 or 4 weeks post-TBI when measuring paw-print area, stride length, stand duration, mean swing, swing speed, step cycle, or gait regularity, support, speed, cadence, or couplings.21 A study of CCI in mice found significant deficits in the grid-walk test at 1 and 32 days post-TBI, but only acute impairments in the CatWalk.19 CatWalk measures that were significantly decreased at 1 day post-TBI were cadence, swing speed, and swing—but this was only an acute change, and there were no significant differences at 32d post-TBI.
In conclusion, rodent locomotion is a multi-dimensional behavior that requires multi-dimensional measures to accurately assess the differences between groups. Because of the interdependence of limb motion, and the limitations of the coordinate frame, traditional techniques may report different gait measures for a slow animal that wanders compared to a fast animal that walks straight—even if they are the same animal. We have developed a technique to simplify these multiple measures into a single variable that embraces the nuanced coordinated interplay between multiple moving limbs. By plotting all steps in x,y,t space, we were able to show that rats develop compensatory techniques to overcome the locomotor deficits after an SCI. We also conclude that mice do not exhibit locomotor changes in our model of TBI.
Acknowledgments
We thank Barbara Bregman for a critical review of the manuscript. We also thank the following for help with CatWalk limb classifying: Samantha Hughes, Brittany Kania, Alex Luu, Lindsay Jeffries, Kathryn Veltman, Dan Lagalante, Anya Dabic, and Kevin Jansen.
Funding Information
This work has been funded in part by NIH/NCMRR (R00HD067339 PI Neckel), NIH/NICHD (T32HD007459 Bregman), NIH/NINDS (R01NS051656 Bregman), NIDRR (H133P100015 Bregman).
Author Disclosure Statement
No competing financial interests exist.
References
- 1. Heglund N., Taylor C., and McMahon T. (1974). Scaling stride frequency and gait to animal size: mice to horses. Science 186, 1112–1113 [DOI] [PubMed] [Google Scholar]
- 2. Taylor R.C. (1978). Why change gaits? Recruitment of muscles and muscle fibers as a function of speed and gait. Am. Zool. 18, 153–161 [Google Scholar]
- 3. Hruska R., and Silbergeld E. (1979). Abnormal locomotion in rats after bilateral intrastriatal injection of kainic acid. Life Sci. 25, 181–193 [DOI] [PubMed] [Google Scholar]
- 4. Neckel N., Dai H., and Bregman B. (2013). Quantifying changes following spinal cord injury with velocity dependent locomotor measures. J. Neurosci. Methods 214, 27–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Batka R., Brown T., Mcmillan K., Meadows R., Jones K., and Haulcomb M. (2014). The need for speed in rodent locomotion analyses. Anat. Rec. 297, 1839–1864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Broom L., Ellison B., Worley A., Wagenaar L., Sörberg E., Ashton C., Bennett D., Buchman A., Saper C., Shih L., and Hausdorff J., (2017). A translational approach to capture gait signatures of neurological disorders in mice and humans. Sci. Rep. 7, 3225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Neckel N. (2015). Methods to quantify the velocity dependence of common gait measurements from automated rodent gait analysis devices. J. Neurosci. Methods 253, 244–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bregman B., Kunkel-Bagden E., Reier P., Dai H., McAtee M., and Gao D. (1993). Recovery of function after spinal cord injury: mechanisms underlying transplant-mediated recovery of function differ after spinal cord injury in newborn and adult rats. Exp. Neurol, 123, 3–16 [DOI] [PubMed] [Google Scholar]
- 9. Lynskey J.V., Sandhu F.A., Dai H.N., McAtee M., Slotkin J.R., MacArthur L., and Bregman B.S. (2006). Delayed intervention with transplants and neurotrophic factors supports recovery of forelimb function after cervical spinal cord injury in adult rats. J. Neurotrauma 23, 617–634 [DOI] [PubMed] [Google Scholar]
- 10. Loane D., Pocivavsek A., Moussa C., Thompson R., Matsuoka Y., Faden A., Rebeck G., and Burns M. (2009). Amyloid precursor protein secretases as therapeutic targets for traumatic brain injury. Nat. Med. 15, 377–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Washington P., Forcelli P., Wilkins T., Zapple D., Parsadanian M., and Burns M. (2012). The effect of injury severity on behavior: a phenotypic study of cognitive and emotional deficits after mild, moderate, and severe controlled cortical impact injury in mice. J. Neurotrauma 29, 2283–2296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Frigon A., D'Angelo G., Thibaudier Y., Hurteau M., Telonio A., Kuczynski V., and Dambreville C. (2014). Speed-dependent modulation of phase variations on a step-by-step basis and its impacton the consistency of interlimb coordination during quadrupedal locomotion in intact adult cats. J. Neurophysiol. 111,1885–1902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Maier I., Ichiyama R., Courtine G., Schnell L., Lavrov I., Edgerton V., and Schwab M. (2009). Differential effects of anti-Nogo-A antibody treatment and treadmill training in rats with incomplete spinal cord injury. Brain 132, 1426–1440 [DOI] [PubMed] [Google Scholar]
- 14. Thibaudier Y., Hurteau M., Dambreville C., Chraibi A., Goetz L., and Frigon A. (2017). Interlimb coordination during tied-belt and transverse split-belt locomotion before and after an incomplete spinal cord injury. J. Neurotrauma 34,1751–1765 [DOI] [PubMed] [Google Scholar]
- 15. Neckel N. (2017). Novel spatiotemporal analysis of gait changes in body weight supported treadmill trained rats following cervical spinal cord injury. J. Neuroeng. Rehabil. 14, 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dai H., MacArthur L., McAtee M., Hockenbury N., Das P., and Bregman B. (2011). Delayed rehabilitation with task-specific therapies improves forelimb function after a cervical spinal cord injury. Restor. Neurol. Neurosci. 29, 91–103 [DOI] [PubMed] [Google Scholar]
- 17. Dai H., MacArthur L., McAtee M., Hockenbury N., Tidwell J., McHugh B., Mansfield K., Finn T., Hamers F., and Bregman B. (2009). Activity-based therapies to promote forelimb use after a cervical spinal cord injury. J. Neurotrauma 26, 1719–1732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chen Y., Mao H., Yang K., Abel T., and Meaney D. (2014). A modified controlled cortical impact technique to model mild traumatic brain injury mechanics in mice. Front. Neurol. 5, 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cline M., Yumul J., Hysa L., Murra D., Garwin G., Cook D., Ladiges W., Minoshima S., and Cross D. (2017). Novel application of a radial water tread maze can distinguish cognitive deficits in mice with traumatic brain injury. Brain Res. 1657, 140–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang G., Shi Y., Jiang X., Leak R., Hu X., Wu Y., Pu H., Li W., Tang B., Wang Y., and Gao Y. (2015). HDAC inhibition prevents white matter injury by modulating microglia/macrophage polarization through the GSK3β/PTEN/Akt axis. Proc. Natl. Acad. Sci. U. S. A. 112, 2853–2858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schönfeld L., Jahanshahi A., Lemmens E., Schipper S., Dooley D., Joosten E., Temel Y., and Hendrix S. (2017). Long-term motor deficits after controlled cortical impact in rats can be detected by fine motor skill tests but not by automated gait analysis. J. Neurotrauma 34, 505–516 [DOI] [PubMed] [Google Scholar]