Abstract
In cervical traumatic spinal cord injury (TSCI), the therapeutic effect of timing of surgery on neurological recovery remains uncertain. Additionally, the relationship between extent of decompression, imaging biomarker evidence of injury severity, and outcome is incompletely understood. We investigated the effect of timing of decompression on long-term neurological outcome in patients with complete spinal cord decompression confirmed on postoperative magnetic resonance imaging (MRI). American Spinal Injury Association (ASIA) Impairment Scale (AIS) grade conversion was determined in 72 AIS grades A, B, and C patients 6 months after confirmed decompression. Thirty-two patients underwent decompressive surgery ultra-early (< 12 h), 25 underwent decompressive surgery early (12–24 h), and 15 underwent decompressive surgery late (> 24–138.5 h) after injury. Age, gender, injury mechanism, intramedullary lesion length (IMLL) on MRI, admission ASIA motor score, and surgical technique were not statistically different among groups. Motor complete patients (p = 0.009) and those with fracture dislocations (p = 0.01) tended to be operated on earlier. Improvement of one grade or more was present in 55.6% of AIS grade A, 60.9% of AIS grade B, and 86.4% of AIS grade C patients. Admission AIS motor score (p = 0.0004) and pre-operative IMLL (p = 0.00001) were the strongest predictors of neurological outcome. AIS grade improvement occurred in 65.6%, 60%, and 80% of patients who underwent decompression ultra-early, early, and late, respectively (p = 0.424). Multiple regression analysis revealed that IMLL was the only significant variable predictive of AIS grade conversion to a better grade (odds ratio, 0.908; confidence interval [CI], 0.862–0.957; p < 0.001). We conclude that in patients with post-operative MRI confirmation of complete decompression following cervical TSCI, pre-operative IMLL, not the timing of surgery, determines long-term neurological outcome.
Keywords: decompression, MRI, outcome, SCI, timing of surgery
Introduction
The pathophysiology of cervical traumatic spinal cord injury (TSCI) is complex, and there remains no effective treatment for this high-impact disorder.1–6 In cervical TSCI, there is disruption of the anatomic integrity of the vertebral column followed by endothelial, neuronal, and axonal damage within fractions of a second of the injury. Continued compression of the spinal cord during the ensuing hours culminates in ischemia, swelling, and hemorrhagic progression of a compressive contusion.7–9 In this scenario, a deleterious cycle of events ensues, in which molecular cascades instigate the upregulation of cationic channels that promote secondary injury and edema, which is visible on magnetic resonance imaging (MRI) within 10 min and ultimately leads to further compression of the injured spinal cord.10,11 The swollen spinal cord becomes compressed circumferentially and longitudinally against the dura mater and rigid bone at the injury epicenter and beyond, resulting in the displacement of cerebrospinal fluid and the effacement of the subarachnoid space across multiple vertebral segments.7,11–13
Compression of the spinal cord against an unyielding spinal canal results in increased intraspinal pressure and reduced perfusion pressure, further jeopardizing blood flow to the spinal cord.14–16 In motor complete TSCI patients, swelling of the spinal cord spreads rostrally and caudally from the injury epicenter in fusiform fashion,8 at a rate of ∼900 μm/h.13,17 Frequently, by the time the victim is transferred to the trauma center, intramedullary lesion length (IMLL), can measure between 40 and 100 mm in length, far beyond the cross-sectional injury epicenter.18–20 Pre-clinical studies indicate that the longer the spinal cord is compressed, the more severe is the parenchymal damage and loss of function.21–24 Together, these findings have encouraged many trauma spine surgeons to recommend early operative intervention as a neuroprotective measure in order to promote neurological improvement.25–30
Ongoing surgical studies on the relationship among imaging biomarkers, surgical strategy, and long-term clinical outcome are shaping the standard of care for these patients.18,28,31–36 Over the past 30 years, overarching evidence has supported spinal cord decompression following trauma, but the question of surgical timing has yet to be established within this evolving paradigm.10,12,32,35,37–41 In addition, although multiple reports have indicated that early anatomical alignment of the spinal column and decompression of the spinal cord followed by internal fixation is neuroprotective, in these studies, decompression of the spinal cord generally has not been verified by post-operative imaging.
Recent efforts have begun to establish the significance of the extent of spinal cord decompression in neurological outcome.16,35,42,43 Data reported by Aarabi and coworkers indicated that standard surgical management of cervical SCI achieves decompression of the entire swollen segment of the spinal cord in only 66% of subjects.35 Additional studies incorporating post-operative MRI indicate that up to 25% of patients may need expansive duraplasty for adequate spinal cord decompression, along with reduction of intraspinal pressure, in order to improve functional outcome.14,15,42,44,45 As a result, studies on the effect of timing of surgical intervention have likely been confounded by incomplete spinal cord decompression, and, therefore, the effect of timing on outcome remains uncertain.28,30,46–48
The null hypothesis was that in patients with subaxial cervical TSCI and post-operative MRI confirmation of decompression, the timing of surgery has no effect on long-term improvement in American Spinal Injury Association (ASIA) Impairment Scale (AIS) grade conversion.
Methods
Design
This study was a retrospective analysis of prospectively collected data.
Cohort
Over a 13-year period, from January 1, 2005 to December 31, 2017, 950 MRI-proven isolated cervical TSCI patients were admitted to this level I trauma center for management. From this cohort, we screened and selected 72 patients who were eligible for this investigation. The inclusion criteria were being ≥16 years of age; Glasgow Coma Scale (GCS) score ≥14; no concurrent life-threatening injury or disease; imaging studies compatible with subaxial cervical spine fracture dislocations; available good quality pre- and post-operative computed tomography (CT) and MRI studies indicating complete spinal cord decompression following surgery;35 and follow-up of at least 6 months after trauma and surgical management. The exclusion criteria were being obtunded, stuporous, and non-testable; having penetrating subaxial TSCI; having upper cervical SCI; a post-operative MRI indicating inadequate spinal cord decompression; non-operative management; having had a cervical CT myelogram and not an MRI as the primary imaging study; dying or being lost to follow-up; or having poor-quality imaging studies. This study was performed after approval from the institutional review board (IRB) of the Human Research Protection Office (HRPO).
Resuscitation, survey, and neurological examination
Patients were transferred to the trauma resuscitation unit (TRU) by emergency medical technicians (EMTs).49 We received intubated and non-intubated patients supine on a backboard with the head and neck secured with a hard collar and chin strap. Median scene or transfer time after the accident was 1 h (mean, 2.3; standard deviation [SD], 3; range, 0.3–15 h). In the TRU, primary and secondary examinations were performed by one of three teams of trauma surgeons who received the patients. Once the patients were medically stable, members of the neurosurgical team (senior resident or nurse practitioners) first examined and then presented them to the attending neurosurgeon. Admission ASIA motor score and AIS grade were determined according to the International Standards for Neurological Classification of Spinal Cord Injury (ISNCSCI).50 ASIA motor scores and AIS grades, which were used for statistical calculation were the ones with no effect from sedatives, analgesics, or mental confusion. In the majority of our patients, the TRU examination was definitive; however, in a minority of patients, the neurological examination within the first 72 h following trauma was used once the sedatives, analgesics and anesthetics were cleared from the patient's body.
Imaging studies
Eligible patients had imaging studies performed when they were medically stable. Cervical spine CT was performed within a median of 2 h (mean, 3.2; SD, 3.1; range, 3.5–15.8 h) and MRI was performed within a median of 5.8 h (mean, 7.2; SD, 5.1; range, 2.4–39.5 h) from the time of the accident. The median time between the accident and MRI was 5 h for the ultra-early patients (mean 5.9 h, range 3–12 h), 6 h for the early patients (mean 6.8 h, range 2.9–14 h), and 6 h for the late patients (mean 10.4 h, range 3.5–39.5 h). Fracture morphology was based on the Harris and coworkers,51 Allen and coworkers,52 and AO Spine53 classification systems. Admission T2-weighted and short T1 inversion recovery (STIR) sequences were used to measure the IMLL and the extent of spinal cord compression/decompression before and after surgery.18,35 An attending trauma neuroradiologist and the principal investigator independently measured the IMLL, and the mean value was taken for statistical analysis.18
Medical management
From 2005 to 2009, 22 of the study patients were administered methylprednisolone following SCI: 30 mg/kg within the first hour and 5.4 mg/kg/h for the next 23 h.54 In 2010, this trauma center stopped the use of steroids for SCI. Patients' mean arterial blood pressure was maintained between 85 and 90 mm Hg for 7 days following trauma.54,55
Traction and surgical intervention
When indicated, we applied traction for the reduction of cervical spine deformities immediately following CT and/or MRI studies.35,56 We set up traction in the TRU, on a Stryker Wedge Turning Frame (Stryker Global Headquarters, Kalamazoo, Michigan) using real-time fluoroscopy. We applied incremental weights of 2–4 kgs per skeletal segment within a maximum of 30–45 min. If this was not successful, we reduced the deformity during surgery. Twenty patients had traction for unilateral (n = 3) or bilateral (n = 14) jumped facets or for compression fracture with >3 mm translation (n = 3). We did not apply skeletal traction in 52 patients for several reasons: no evidence of fracture dislocation (n = 17); flexion or extension injury without translation (n = 20); compression fracture with <3 mm translation (n = 12); or unilateral locked facets (n = 3).
Surgical management
Patients were decompressed and internally fixed within a median of 12 h (mean, 18.8; SD, 19.4; range, 4.5–138.5 h). Thirty-two (44.5%) patients were operated on in <12 h, 25 (34.7%) were operated on within 12–24 h, and 15 (20.8%) were operated on >24–138.5 h after the trauma. Nine neurosurgeons including four spine fellowship-trained neurosurgeons performed the surgeries. Surgeries included anterior cervical discectomy and fusion (ACDF) in 10, ACDF+laminectomy in 25; anterior cervical corpectomy and fusion (ACCF) in 9; ACCF+laminectomy in 11; and only laminectomy in 17.35,46,57 Post-operative CT and MRI were performed to confirm appropriate technique and to verify spinal cord decompression. Post-operative MRI studies were performed within a median of 34 h (mean, 45.1; SD, 30.8; range, 13.5–148.5 h). Post-operative MRI in the ultra-early group was performed a median of 31.5 h following trauma (mean 34.2, range 13.5–95.5 h). In the early group, the median time following trauma was 29.8 h (mean 42.4 and range 18.8–139.5 h). In the late group, the median time following trauma to MRI was 56.9 h (mean 72.9 and range 39.8–148.5 h). Two spine fellowship-trained neurosurgeons, one fellowship-trained neurotrauma neurosurgeon, a trauma neuroradiologist, and the principal investigator independently verified complete spinal cord decompression on postoperative MRI studies.35 Spinal cord decompression was defined as presence of cerebrospinal fluid in the subarachnoid space around the spinal cord circumferentially in all patients.
Post-operative ICU care and follow-up
The post-operative course in the ICU included deep vein thrombosis (DVT) prophylaxis by enoxaparin (Lovenox®, Sanofi, USA), 30 mg twice daily starting within 24–48 h of admission, and screening by duplex ultrasound for venous thromboembolism (VTE). When needed, patients had early tracheostomy for ventilator support and percutaneous gastroenterostomy for nutrition. When fully weaned from ventilator support, the patients were transferred to rehabilitation centers. While in the ICU, daily neurological examination including digital rectal examination was performed to determine ASIA motor score and evidence for AIS grade conversion. Following discharge, the patients returned at 6 weeks, 3 months, 6 months, and 12 months (or longer) for follow-up neurological examinations. Certified neurologists, rehabilitation specialists, the principal investigator, senior residents, and nurse practitioners performed neurological examinations to document any change in ASIA motor score and AIS grade conversion.18,35
Results
Initial patient characteristics
Age, gender, injury mechanism, IMLL, ASIA motor score, and surgical technique did not differ significantly among the three groups of patients: those with ultra-early (< 12 h), early (12–24 h), or late (> 24–138.5 h) decompression (Table 1). Compared with AIS grade C patients, AIS grade A and B patients were operated on significantly earlier after admission (p = 0.009). Similarly, patients with burst/compression fractures and those with facet dislocations (types A3/A4 and C of the Vaccaro and coworkers AO Spine classification53) were surgically managed earlier than patients with no evidence of fractures and horizontal translation (AO Spine Class types A0, B2, B3) on CT and MRI (p = 0.01)
Table 1.
Baseline Characteristics of the Present Cohort
| Category | <12 h post-trauma | 12-24 h post-trauma | 24-138.5 h post-trauma | Total | p |
|---|---|---|---|---|---|
| Accident (%) | 0.59 | ||||
| Fall | 14 (38.9) | 14 (38.9) | 8 (22.2) | 36 (50) | |
| MVC | 12 (60) | 5 (25) | 3 (15) | 20 (27.8) | |
| Other | 6 (37.5) | 6 (37.5) | 4 (25) | 16 (2.2) | |
| Total | 32 (44.4) | 25 (34.7) | 15 (20.8) | 72 (100) | |
| Gender (%) | 0.89 | ||||
| Male | 26 (43.3) | 21 (35) | 13 (21.7) | 60 (83.3) | |
| Female | 6 (50) | 4 (33.3) | 2 (16.7) | 12 (16.7) | |
| Total | 32 (44.5) | 25 (34.7) | 15 (20.8) | 72 (100) | |
| Age (years): Mean (SD) | 41.8 (18.4) | 49.4 (18.3) | 49.3 (13.2) | 46.0 (17.6) | 0.19 |
| AIS grade (%) | 0.009 | ||||
| AIS A | 13 (48.2) | 11 (40.7) | 3 (11.1) | 27 (37.5) | |
| AIS B | 14 (60.9) | 7 (30.4) | 2 (8.7) | 23 (31.9) | |
| AIS C | 5 (22.7) | 7 (31.8) | 10 (45.5) | 22 (30.6) | |
| ASIA motor score: Mean (SD) | 18.6 (14.4) | 22.0 (15.2) | 24.5 (14.2) | 21.1 (14.6) | 0.40 |
| Morphology (%) | 0.01 | ||||
| A0 | 5 (20) | 12 (48) | 8 (32) | 25 (34.7) | |
| A3/A4/C | 24 (61.5) | 11 (28.2) | 4 (10.3) | 39 (54.2) | |
| B2/B3 | 3 (37.5) | 2 (25) | 3 (37.5) | 8 (11.1) | |
| Total | 32 (44.5) | 25 (34.7) | 15 (20.8) | 72 (100) | |
| IMLL (mm): Mean (SD) | 43.3 (19.5) | 37.5 (17.9) | 30.6 (13.9) | 38.6 (18.4) | 0.07 |
| Surgical technique (%) | 0.32 | ||||
| ACDF | 5 (50) | 2 (20) | 3 (30) | 10 (13.9) | |
| ACDF+Laminectomy | 10 (40) | 11 (44) | 4 (16) | 25 (34.7) | |
| ACCF | 7 (77.8) | 2 (22.2) | 0 (0) | 9 (12.5) | |
| ACCF+Laminectomy | 5 (45.4) | 4 (36.4) | 2 (18.2) | 11 (15.3) | |
| Laminectomy | 5 (29.4) | 6 (35.3) | 6 (35.3) | 17 (23.6) | |
| Total | 32 (44.4) | 25 (34.7) | 15 (20.8) | 72 (100) |
MVC, motor vehicle crash; SD, standard deviation; AIS, American Spinal Injury Association (ASIA) Impairment Scale; IMLL, intramedullary lesion length; ACDF, anterior cervical discectomy and fusion; ACCF, anterior cervical corpectomy and fusion.
AIS grade conversion
During a period of at least 6 months of follow-up, three patients had regression of their AIS grade compared with at admission. In the ultra-early group, two patients in the AIS grade B group converted to AIS grade A, and in the early group, one patient in the AIS grade C group regressed to AIS grade B (Tables 2–6). The effect of nine independent variables on AIS grade conversion when the spinal cord was completely decompressed on post-operative MRI was analyzed. From the demographic variables, neither age (p = 0.07) nor gender (p = 0.18) had any influence on AIS grade conversion. The mechanism of injury also was without effect on neurological outcome (p = 0.638). Patients with higher ASIA motor score at admission had a better rate of conversion at 6 months (p < 0.0004), but the rate of conversion was not influenced by admission AIS grade (p = 0.058). One reason for lack of congruity between ASIA motor score and AIS grade could be the fact that AIS grade is a composite of ASIA motor score, sensation, and sacral sparing, which could have unknown confounding statistical effects on each other. Regarding the CT scan of the cervical spine, regardless of whether there were no fracture/dislocations (A0) or fracture/dislocation along one or multiple axes (AO Spine classification morphology types A3/A4, B2/B3, C), morphology type did not affect grade conversion (p = 0.11).53 Five different surgical techniques were used for complete decompression of the spinal cord.35 The type of surgical technique did not affect neurological outcome (Fig. 1). Contrary to much of the literature (see Table 7), surgical decompression in any of the three time periods had no effect on improvements in AIS grade conversion (p = 0.424).28,47,58,59 Intramedullary lesion length (IMLL) on T2W or STIR images, as a surrogate end-point biomarker, had the most powerful effect on AIS grade conversion (p = 0.001).18,35,60–62 Compared with patients who had no improvement in AIS grade, patients with grade conversion had significantly shorter IMLL on pre-operative T2W or STIR MRI images. Regression analysis of variables with no effect, marginal effect, or significant effect on AIS grade conversion indicated that IMLL had the most powerful influence on improved grade conversion at 6 months following trauma (Table 6).
Table 2.
AIS Grade Conversion in 32 Patients with Ultra-Early (< 12 h) Decompression
 |
AIS grade regression.
AIS, American Spinal Injury Association (ASIA) Impairment Scale.
Table 3.
AIS Grade Conversion in 25 Patients with Early (12-24 h) Decompression
 |
AIS grade regression.
AIS, American Spinal Injury Association (ASIA) Impairment Scale.
Table 4.
AIS Grade Conversion in 15 Patients with Late (>24-138.5 h) Decompression
| AIS grade at follow-up | ||||||
|---|---|---|---|---|---|---|
| Admission AIS grade | A | B | C | D | E | Total admission |
| AIS grade | ||||||
| A | 1 | 1 | 0 | 1 | 0 | 3 |
| B | 0 | 1 | 0 | 1 | 0 | 2 |
| C | 0 | 0 | 1 | 9 | 0 | 10 |
AIS, American Spinal Injury Association (ASIA) Impairment Scale.
Table 5.
AIS Grade Conversion in Various Categories of the Cohort
| Category | AIS not converted | AIS converted | Total | p value |
|---|---|---|---|---|
| Accident (%) | 0.638 | |||
| Fall | 12 (33.3) | 24 (66.7) | 36 (50) | |
| MVC | 8 (40) | 12 (60) | 20 (27.8) | |
| Other | 4 (25) | 12 (75) | 16 (22.2) | |
| Total | 24 (33.3) | 48 (66.7) | 72 (100) | |
| Gender (%) | 0.18 | |||
| Male | 22 (36.7) | 38 (63.3) | 60 (83.3) | |
| Female | 2 (16.7) | 10 (83.3) | 12 (16.7) | |
| Age (SD) | 40.8 (16.2) | 48.6 (17.9) | 46.0 (17.6) | 0.07 |
| Admission AIS grade (%) | 0.058 | |||
| A | 12 (44.4) | 15 (55.6) | 27 (37.5) | |
| B | 9 (39.1) | 14 (60.9) | 23 (31.9) | |
| C | 3 (13.6) | 19 (86.4) | 22 (30.6) | |
| Total | 24 (33.3) | 48 (66.7) | 72 (100) | |
| Admission ASIA motor score (SD) | 12.7 (12.5) | 25.2 (13.9) | 21.0 (14.6) | 0.0004 |
| Morphology (%) | 0.11 | |||
| A0 (No evidence of fracture dislocation) | 6 (24) | 19 (76) | 25 (34.7) | |
| B2 or B3 (Flexion or extension injury) | 1 (12.5) | 7 (87.5) | 8 (11.1) | |
| A3/4+C (Significant translation in X/Y/Z axes) | 17 (43.6) | 22 (56.4) | 39 (54.2) | |
| Intramedullary lesion length (IMLL) (mm, SD) | ||||
| Admission grade A | 59.3 (20.7) | 35.6 (9.6) | 46.1 (19.4) | 0.002 |
| Admission grade B | 54.2 (19.1) | 31.9 (11.9) | 40.6 (18.4) | 0.003 |
| Admission grade C | 23.9 (17.1) | 27.9 (9.9) | 27.4 (10.6) | 0.472 |
| Total | 53.0 (22.1) | 31.5 (10.7) | 38.6 (18.4) | 0.00001 |
| Surgical intervention (%) | NS | |||
| ACDF | 3 (30) | 7 (70) | 10 (13.9) | |
| ACDF+Laminectomy | 5 (20) | 20 (80) | 25 (34.7) | |
| ACCF | 1 (11.1) | 8 (88.9) | 9 (12.5) | |
| ACCF+Laminectomy | 9(81.8) | 2 (18.2) | 11 (15.3) | |
| Laminectomy | 6 (35.3) | 11 (64.7) | 17 (23.6) | |
| Timing of surgery (%) | 0.424 | |||
| <12 h after trauma | 11 (34.4) | 21 (65.6) | 32 (44.5) | |
| 12-24 h after trauma | 10 (40) | 15 (60) | 25 (34.7) | |
| >24 h after trauma | 3 (20) | 12 (80) | 15 (20.8) | |
| Total | 24 (33.3) | 48 (66.7) | 72 (100) |
AIS, American Spinal Injury Association (ASIA) Impairment Scale; MVC, motor vehicle crash; SD, standard deviation; ACDF, anterior cervical discectomy and fusion; ACCF, anterior cervical corpectomy and fusion.
Table 6.
Multivariate Regression Analysis Comparing the Therapeutic Efficacy of Timing of Surgery Versus Intramedullary Lesion Length (IMLL)
| Outcome | Odds ratio | 95% confidence interval | p value |
|---|---|---|---|
| <12 h trauma-surgery | Referent | - | - |
| 12-24 h trauma-surgery | 0.455 | 0.118-1.752 | 0.25 |
| >24 h trauma-surgery | 0.832 | 0.141-4.88 | 0.83 |
| IMLL (mm) | 0.908 | 0.862-0.957 | 0.001 |
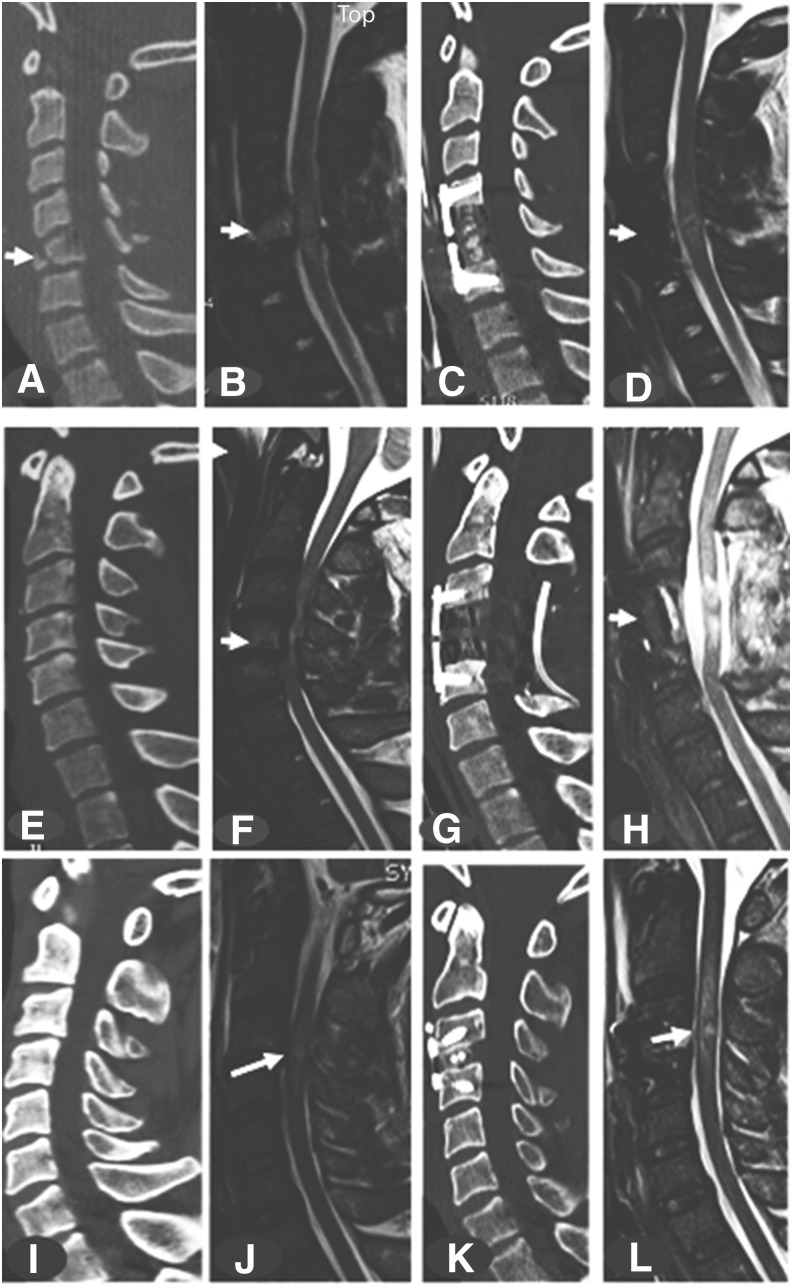
FIG. 1.
(A–D) Midsagittal computed tomography (CT) and magnetic resonance imaging (MRI) of a 19-year-old man involved in an automobile accident who was admitted 30 min later to the trauma resuscitation unit (TRU) with a C5 compression tear-drop fracture (arrow); American Spinal Injury Association (ASIA) motor score of 21, and ASIA Impairment Scale (AIS) grade B; intramedullary lesion length (IMLL) at admission was 27.8 mm. A C5 corpectomy was performed 6 h after the accident, which completely decompressed the spinal cord. MRI 34.5 h after surgery indicated an IMLL of 34.4 mm. One year following the accident, his ASIA motor score was 64 and he was AIS grade D. (E–H) Midsagittal CT and MRI of a 42-year-old man who had a mechanical fall and was admitted 30 min later to the TRU with from spinal stenosis and possible extension injury (arrow); ASIA motor score was 8 and AIS grade was A; IMLL at admission was 42.2 mm. He underwent C4 corpectomy and C3–C5 laminectomy with fusion 13 h after the accident, with complete spinal cord decompression. MRI 23 h after surgery indicated an IMLL of 30.9 mm. Six months following the accident, his ASIA motor score remained 8 and he was AIS grade A. (I–L) Midsagittal CT and MRI of a 53-year-old man who had a mechanical fall and was admitted 10.5 h later to the TRU with a C3/4 extension injury (arrow); ASIA motor score was 33 and AIS grade was C; IMLL at admission was 20.3 mm. He underwent discectomy and fusion at C3/4, 36 h after the accident, with complete spinal cord decompression. MRI 49.8 h after surgery indicated an IMLL of 49.6 mm. Fifty-seven months following the accident, his ASIA motor score was 91 and he was AIS grade D.
Table 7.
Investigations Evaluating the Timing of Decompression on the Therapeutic Effectiveness and Neurological Outcome in Cervical Traumatic Spinal Cord Injury
| Investigator Year Journal |
Design | Cohort | AIS grade | Preop MRI | Timing (hours) | Postop MRI | IMLL | Extent of DEC. | F/UM | TE/AIS grade conversion |
|---|---|---|---|---|---|---|---|---|---|---|
| Vaccaro et al. Spine, 1997 |
PR | 62 | A-D | Yes | ≤72 and >120 | No | No | No | 11.5 | No effect |
| Guest et al. J. Neurosurg., 2002 |
RO | 50 | C-D | Yes | ≤24 and >24 | No | No | No | 36 | Not mentioned |
| Papadopoulos et al. J. Trauma 2002 | PO | 91 | A-D | Yes | <12 and >12 | No | No | No | 33 | Early superior |
| Sapkas and Papadakis J. Orthop. Surg. 2007 | RO | 67 | A-E | Yes | ≤72 and >72 | No | No | No | 48 | No effect |
| Lenehan et al. Spine 2010 | PO | 73 | C-D | Yes | ≤24 and >24 | No | No | No | 12 | No effect |
| Wilson et al. Spinal Cord 2012 | PO | 55 | A-D | Yes | <24 and ≥24 | No | No | No | 3 | Early superior |
| Fehlings et al. PLoS One 2012 | PO | 313 | A-D | Yes | <24 and ≥24 | No | No | No | 6 | Early superior |
| Jug et al. J. Neurotrauma 2015 | PO | 42 | A-C | Yes | ≤8 and 8-24 | No | No | No | 6 | Early superior |
| Dvorak et al. J. Neurotrauma 2015 | PO | 470 | A-D | Yes? | ≤24 and >24 | No | No | No | 3-6 | NM |
| Grassner et al. J. Neurotrauma 2016 | RO | 70 | A-D | Yes | ≤8 and 8-90 | No | No | No | 10 | Early superior |
| Bourassa-Moreau et al. J. Neurotrauma 2016 | PO | 20 | A | Yes | <24-≥24 | No | No | No | 5 | Early superior |
| Mattiassich et al. J. Neurotrauma 2017 | RO | 49 | A-D | Yes | <5 h and ≥5-24 | No | No | No | 6 | Late superior |
| Burke et al. Neurosurgery 2018 |
RO | 48 | A-D | Yes | ≤12,12-24,>24 | No | No | No | ACD | Early superior |
| Kim et al. World Neurosurg. 2018 | RO | 46 | A-D | Yes | ≤48 and >48 | No | No | No | 6 | No effect |
| Sewell et al. World Neurosurg. 2018 | RO | 95 | A-D | Yes | ≤24 and >24 | No | No | No | 6 | No effect |
| Current Study 2019 |
RO | 73 | A-C | Yes | ≤12,12-24,24-138.5 | Yes | Yes | Yes | 6 | No effect |
AIS, American Spinal Injury Association (ASIA) Impairment Scale; MRI, magnetic resonance imaging; IMLL, intramedullary lesion length; ACD, acute care discharge; DEC, decompression; F/U, follow-up; M, months; PO, prospective observational; PR, prospective randomized; RO, retrospective observational; TE, treatment effect.
Discussion
The principal finding of the present study is that, in a cohort of patients with cervical TSCI with pre-operative MRI evidence of spinal cord compression and post-operative MRI evidence of complete decompression, it was the IMLL, not the timing of surgery, that best predicted improved AIS grade conversion.
A meta-analysis of pre-clinical studies63,64 on experimental TSCI concluded that neuroprotection may be obtained when decompression is performed early following injury. However, in the studies subjected to meta-analysis, the degree of compression was difficult to compare directly because of important methodological differences, with some studies introducing spacers to narrow the canal diameter, others compressing the cord with aneurysm clips or weights exerting different forces, and others compressing the cord with devices exerting known pressures. In a rodent pre-clinical study by Batchelor and coworkers, a sustained compression force of ∼35 mm Hg quickly resulted in paraplegia.65 In a meta-analysis by the same investigators, early decompression improved behavioral outcome in rodents and non-human primates by up to 35%.64 Although supportive of early decompression, the pre-clinical studies included in Batchelor's meta-analysis were heterogeneous in design, methodology, and timing of decompression, and these studies suffered from low internal validity and reporting bias. Translating data from pre-clinical models to humans has repeatedly proven to be very difficult.63,64
The therapeutic effectiveness of timing (early vs. late) of surgical decompression and its relationship with neurological outcome following cervical TSCI is at equipoise (Table 7).28,30,59,66,67 Heterogeneity in design, methodology, surgical technique, neurological assessment, timing of decompression, number of patients enrolled, length of follow-up, and outcome assessment tools used makes interpretation of the results difficult and the therapeutic effectiveness of timing of decompression uncertain. Notably, most of the studies published during the past 20 years have confirmed pre-operative spinal cord compression by CT or MRI, but seldom has there been a precise, anatomic definition of the term decompression, the purported goal of surgery. In many studies, the surgical technique, including the number of discectomies, corpectomies, or laminectomies in isolation or in combination required to decompress the swollen spinal cord was not well documented.28,30,46,47,68 Almost universally, decompressive surgery was not followed by post-operative MRI to verify complete decompression of a swollen spinal cord.28,29,47,48,58,59,66–73 By contrast, Papadopoulos and coworkers,47 and Sapkas and Papadakis,67 used MRI following closed traction reduction to assess for any residual compression following anatomical realignment and to decide on the need for further spinal cord decompression. In the multi-center prospective observational study by Fehlings and coworkers,28 postoperative MRI was performed only in patients who had neuroworsening following surgical decompression. These studies uniformly used CT scan evidence of injury morphology as a guide for surgical intervention, not the IMLL or the extent of swelling across several skeletal segments present on preoperative MRI. In a recent clinical study, standard surgical approaches as recommended by Dvorak and coworkers57 were successful in decompressing the swollen spinal cord in only 66% of AIS grade A and B patients.35 Another study in AIS grade A, B, and C patients indicated that complete decompression resulted in AIS grade conversion in 58.9% of patients, whereas inadequate decompression resulted in improved AIS grade conversion in only 18.6% of patients.18 Adequate laminectomy is the procedure that is the sine qua non for assured spinal cord decompression.35 In the latter series, almost 74% of patients had to have multi-level laminectomies for adequate decompression of the swollen spinal cord. By contrast, the rate of laminectomy in the randomized study of Vaccaro and coworkers30 was 9.6%, and in the prospective observational study of Jug and coworkers,29 it was 7%. Therefore, the surgical methodology in these two series could have confounded the interpretation of the effect of timing of decompression on neurological outcome. In published articles discussing the timing of decompression and neurological outcome, MRI-documented IMLL, an important predictor of neurological outcome, was not used to guide the extent of surgery required for adequate spinal cord decompression.18,20,61,62,74–78
In addition to imaging biomarkers such as structural MRI, which convey a visual indication of compression, studies have revealed the importance of juxtaluminal pressure measurements inside the parenchyma of the spinal cord.42,43,79 The presence of a swollen spinal cord against nonyielding dura may indicate a requirement for expansive duraplasty.43 In the current study, 100% of the patients had an open subarachnoid space (as a prerequisite inclusion criterion); therefore, the need for a juxtaluminal pressure monitor is less clear. However in a previous report from the University of Maryland, only 7 of 63 patients (11%) with inadequate bony decompression also exhibited effaced subarachnoid space, therefore being candidates for intraspinal pressure monitoring and expansive duraplasty.35 Consideration of intraspinal pressure and spinal cord perfusion pressure and pressure reactivity index may help improve neurological outcomes following inadequate bony decompression performed within the appropriate time window following trauma.22,45,80,81
Apart from structural MRI, which is mandatory for the diagnosis, pre-operative planning, estimation of the degree of spinal cord compression, IMLL, and the extent of spinal cord decompression, quantitative MRI (QMRI) biomarkers including magnetization transfer, relaxation mapping, and diffusion imaging have helped us better understand secondary changes following TSCI at a microstructural level across the entire neuraxis.36, 82–87 QMRI depicts the extent of iron deposition in the spinal cord and brain and the degree of demyelination and degeneration, and it can predict neurological outcome and the degree of neural plasticity.36,83 These studies may be useful as surrogate end-points in trials on the timing of decompression and AIS grade conversion following TSCI.88–90
The present study underscores the emerging importance of injury severity parameters such as IMLL and Brain and Spinal Injury Center (BASIC) score,77 as well as the extent of decompression, in studies involving the timing of decompression. If future multi-center and prospective studies take into account IMLL and completeness of decompression, the actual effect of the timing of decompression in TSCI will likely be clarified.
Limitations of this study include the following.
-
1.
The majority of the motor complete TSCI patients were in the ultra-early and early decompression categories, whereas the reverse was true for motor incomplete patients (AIS grade C) who were in the late decompression category.
-
2.
In a minority of our patients, ISNCSCI examination, which was used for statistical analysis, was completed within 72 h of trauma when the effects of analgesics, sedatives, and anesthesia were absent and there was no mental confusion.
Conclusion
Pre-clinical studies, biological rationale, and recent clinical investigations favor early spinal cord decompression to enhance motor outcome following TSCI. Many of the clinical trials to date have been heterogeneous, biased, and with low internal validity. These studies have been based on standard surgical procedures that rely on pre-operative CT and MRI, with no independent verification of the completeness of spinal cord decompression on post-operative MRI. In our study, when the spinal cord was shown to be decompressed on post-operative MRI, the timing of decompression did not influence AIS grade conversion. Here, we identified intramedullary lesion length as the main predictor of neurological outcome.
Author Disclosure Statement
No competing financial interests exist.
References
- 1. Chen Y., He Y., and DeVivo M.J. (2016). Changing demographics and injury profile of new traumatic spinal cord injuries in the United States, 1972-2014. Arch. Phys. Med. Rehabil. 97, 1610–1619 [DOI] [PubMed] [Google Scholar]
- 2. Curt A., Van Hedel H.J., Klaus D., and Dietz V. (2008). Recovery from a spinal cord injury: significance of compensation, neural plasticity, and repair. J. Neurotrauma 25, 677–685 [DOI] [PubMed] [Google Scholar]
- 3. Krueger H., Noonan V.K., Trenaman L.M., Joshi P., and Rivers C.S. (2013). The economic burden of traumatic spinal cord injury in Canada. Chronic diseases and injuries in Canada 33, 113–122 [PubMed] [Google Scholar]
- 4. Selvarajah S., Hammond E.R., Haider A.H., Abularrage C.J., Becker D., Dhiman N., Hyder O., Gupta D., Black J.H., 3rd, and Schneider E.B. (2014). The burden of acute traumatic spinal cord injury among adults in the united states: an update. J. Neurotrauma 31, 228–238 [DOI] [PubMed] [Google Scholar]
- 5. Kwon B.K., Tetzlaff W., Grauer J.N., Beiner J., and Vaccaro A.R. (2004). Pathophysiology and pharmacologic treatment of acute spinal cord injury. Spine J. 4, 451–464 [DOI] [PubMed] [Google Scholar]
- 6. Rowland J.W., Hawryluk G.W., Kwon B., and Fehlings M.G. (2008). Current status of acute spinal cord injury pathophysiology and emerging therapies: promise on the horizon. Neurosurg. Focus 25, E2. [DOI] [PubMed] [Google Scholar]
- 7. Kurland D., Hong C., Aarabi B., Gerzanich V., and Simard J.M. (2012). Hemorrhagic progression of a contusion after traumatic brain injury: a review. J. Neurotrauma 29, 19–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Balentine J.D. (1978). Pathology of experimental spinal cord trauma. I. The necrotic lesion as a function of vascular injury. Lab. Invest. 39, 236–253 [PubMed] [Google Scholar]
- 9. Dohrmann G.J., Wagner F.C. Jr., and Bucy P.C. (1972). Transitory traumatic paraplegia: electron microscopy of early alterations in myelinated nerve fibers. J. Neurosurg. 36, 407–415 [DOI] [PubMed] [Google Scholar]
- 10. Bilgen M., Abbe R., Liu S.J., and Narayana P.A. (2000). Spatial and temporal evolution of hemorrhage in the hyperacute phase of experimental spinal cord injury: in vivo magnetic resonance imaging. Magn. Reson. Med. 43, 594–600 [DOI] [PubMed] [Google Scholar]
- 11. Simard J.M., Tsymbalyuk O., Ivanov A., Ivanova S., Bhatta S., Geng Z., Woo S.K., and Gerzanich V. (2007). Endothelial sulfonylurea receptor 1-regulated NC Ca-ATP channels mediate progressive hemorrhagic necrosis following spinal cord injury. J. Clin. Invest. 117, 2105–2113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ahuja C.S., Wilson J.R., Nori S., Kotter M.R.N., Druschel C., Curt A., and Fehlings M.G. (2017). Traumatic spinal cord injury. Nat. Rev. Dis. Primers 3, 17018. [DOI] [PubMed] [Google Scholar]
- 13. Aarabi B., Simard J.M., Kufera J.A., Alexander M., Zacherl K.M., Mirvis S.E., Shanmuganathan K., Schwartzbauer G., Maulucci C.M., Slavin J., Ali K., Massetti J., and Eisenberg H.M. (2012). Intramedullary lesion expansion on magnetic resonance imaging in patients with motor complete cervical spinal cord injury. Journal of neurosurgery. Spine 17, 243–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chen S., Smielewski P., Czosnyka M., Papadopoulos M.C., and Saadoun S. (2017). Continuous monitoring and visualization of optimum spinal cord perfusion pressure in patients with acute cord injury. J. Neurotrauma 34, 2941–2949 [DOI] [PubMed] [Google Scholar]
- 15. Gallagher M.J., Hogg F.R.A., Zoumprouli A., Papadopoulos M.C., and Saadoun S. (2019). Spinal cord blood flow in patients with acute spinal cord injuries. J. Neurotrauma 36, 919–929 [DOI] [PubMed] [Google Scholar]
- 16. Hogg F.R.A., Gallagher M.J., Chen S., Zoumprouli A., Papadopoulos M.C., and Saadoun S. (2018). Predictors of intraspinal pressure and optimal cord perfusion pressure after traumatic spinal cord injury. Neurocrit. Care. [Epub ahead of print; DOI: 10.1007/s12028-018-0616-7] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Le E., Aarabi B., Hersh D.S., Shanmuganathan K., Diaz C., Massetti J., and Akhtar-Danesh N. (2015). Predictors of intramedullary lesion expansion rate on MR images of patients with subaxial spinal cord injury. J. Neurosurg. Spine, 22, 611–621 [DOI] [PubMed] [Google Scholar]
- 18. Aarabi B., Sansur C.A., Ibrahimi D.M., Simard J.M., Hersh D.S., Le E., Diaz C., Massetti J., and Akhtar-Danesh N. (2017). Intramedullary lesion length on postoperative magnetic resonance imaging is a strong predictor of ASIA impairment scale grade conversion following decompressive surgery in cervical spinal cord injury. Neurosurgery 80, 610–620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Miyanji F., Furlan J.C., Aarabi B., Arnold P.M., and Fehlings M.G. (2007). Acute cervical traumatic spinal cord injury: MR imaging findings correlated with neurologic outcome--prospective study with 100 consecutive patients. Radiology 243, 820–827 [DOI] [PubMed] [Google Scholar]
- 20. Schaefer D.M., Flanders A., Northrup B.E., Doan H.T., and Osterholm J. (1989). Magnetic resonance imaging of acute cervical spine trauma. Spine 14, 1090–1095 [DOI] [PubMed] [Google Scholar]
- 21. Carlson G.D., Gordon C.D., Oliff H.S., Pillai J.J., and Lamanna J.C. (2003). Sustained spinal cord compression: part I: time-dependent effect on long-term pathophysiology. J. Bone Joint Surg. Am. 85-A, 86–94 [PubMed] [Google Scholar]
- 22. Saadoun S., Chen S., and Papadopoulos M.C. (2017). Intraspinal pressure and spinal cord perfusion pressure predict neurological outcome after traumatic spinal cord injury. J. Neurol. Neurosurg. Psychiatry 88, 452–453 [DOI] [PubMed] [Google Scholar]
- 23. Kobrine A.I., Evans D.E., and Rizzoli H. (1978). Correlation of spinal cord blood flow and function in experimental compression. Surg. Neurol. 10, 54–59 [PubMed] [Google Scholar]
- 24. Kobrine A.I., Evans D.E., and V., R.H. (1979). Experimental acute balloon compression of the spinal cord. Factors affecting disappearance and return of the spinal evoked response. J. Neurosurg. 51, 841–845 [DOI] [PubMed] [Google Scholar]
- 25. Anderson K.K., Tetreault L., Shamji M.F., Singh A., Vukas R.R., Harrop J.S., Fehlings M.G., Vaccaro A.R., Hilibrand A.S., and Arnold P.M. (2015). Optimal timing of surgical decompression for acute traumatic central cord syndrome: a systematic review of the literature. Neurosurgery 77, Suppl. 4, S15–32 [DOI] [PubMed] [Google Scholar]
- 26. Cadotte D.W., Singh A., and Fehlings M.G. (2010). The timing of surgical decompression for spinal cord injury. F1000 Med. Rep. 2, 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fehlings M.G., Sekhon L.H., and Tator C. (2001). The role and timing of decompression in acute spinal cord injury: what do we know? What should we do? Spine 26, S101–110 [DOI] [PubMed] [Google Scholar]
- 28. Fehlings M.G., Vaccaro A., Wilson J.R., Singh A., D, W.C., Harrop J.S., Aarabi B., Shaffrey C., Dvorak M., Fisher C., Arnold P., Massicotte E.M., Lewis S., and Rampersaud R. (2012). Early versus delayed decompression for traumatic cervical spinal cord injury: results of the Surgical Timing in Acute Spinal Cord Injury Study (STASCIS). PloS one 7, e32037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jug M., Kejzar N., Vesel M., Al Mawed S., Dobravec M., Herman S., and Bajrovic F.F. (2015). Neurological recovery after traumatic cervical spinal cord injury is superior if surgical decompression and instrumented fusion are performed within 8 hours versus 8 to 24 hours after injury: a single center experience. J. Neurotrauma 32, 1385–1392 [DOI] [PubMed] [Google Scholar]
- 30. Vaccaro A.R., Daugherty R.J., Sheehan T.P., Dante S.J., Cotler J.M., Balderston R.A., Herbison G.J., and Northrup B.E. (1997). Neurologic outcome of early versus late surgery for cervical spinal cord injury. Spine 22, 2609–2613 [DOI] [PubMed] [Google Scholar]
- 31. Levi A.D., Anderson K.D., Okonkwo D.O., Park P., Bryce T.N., Kurpad S.N., Aarabi B., Hsieh J., and Gant K. (2019). Clinical outcomes from a multi-center study of human neural stem cell transplantation in chronic cervical spinal cord injury. J. Neurotrauma 36, 891–902 [DOI] [PubMed] [Google Scholar]
- 32. Ter Wengel P.V., De Witt Hamer P.C., Pauptit J.C., van der Gaag N.A., Oner F.C., and Vandertop W.P. (2019). Early surgical decompression improves neurological outcome after complete traumatic cervical spinal cord injury: a meta-analysis. J. Neurotrauma 36, 835–844 [DOI] [PubMed] [Google Scholar]
- 33. Fehlings M.G., Nakashima H., Nagoshi N., Chow D.S., Grossman R.G., and Kopjar B. (2016). Rationale, design and critical end points for the Riluzole in Acute Spinal Cord Injury Study (RISCIS): a randomized, double-blinded, placebo-controlled parallel multi-center trial. Spinal Cord 54, 8–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kucher K., Johns D., Maier D., Abel R., Badke A., Baron H., Thietje R., Casha S., Meindl R., Gomez-Mancilla B., Pfister C., Rupp R., Weidner N., Mir A., Schwab M.E., and Curt A. (2018). First-in-man intrathecal application of neurite growth-promoting anti-nogo-a antibodies in acute spinal cord injury. Neurorehabil. Neural Repair 32, 578–589 [DOI] [PubMed] [Google Scholar]
- 35. Aarabi B., Olexa J., Chryssikos T., Galvagno S.M., Hersh D.S., Wessell A., Sansur C., Schwartzbauer G., Crandall K., Shanmuganathan K., Simard J.M., Mushlin H., Kole M., Le E., Pratt N., Cannarsa G., Lomangino C.D., Scarboro M., Aresco C., and Curry B. (2019). Extent of spinal cord decompression in motor complete (American Spinal Injury Association Impairment Scale Grades A and B) traumatic spinal cord injury patients: post-operative magnetic resonance imaging analysis of standard operative approaches. J. Neurotrauma 36, 862–876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Seif M., Curt A., Thompson A.J., Grabher P., Weiskopf N., and Freund P. (2018). Quantitative MRI of rostral spinal cord and brain regions is predictive of functional recovery in acute spinal cord injury. NeuroImage Clin. 20, 556–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wilson J.R., Tetreault L.A., Kwon B.K., Arnold P.M., Mroz T.E., Shaffrey C., Harrop J.S., Chapman J.R., Casha S., Skelly A.C., Holmer H.K., Brodt E.D., and Fehlings M.G. (2017). Timing of decompression in patients with acute spinal cord injury: a systematic review. Global Spine J. 7, 95s–115s [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hackney D.B., Ford J.C., Markowitz R.S., Hand C.M., Joseph P.M., and Black P. (1994). Experimental spinal cord injury: MR correlation to intensity of injury. J. Comput. Assist. Tomogr. 18, 357–362 [DOI] [PubMed] [Google Scholar]
- 39. Simard J.M., Popovich P.G., Tsymbalyuk O., Caridi J., Gullapalli R.P., Kilbourne M.J., and Gerzanich V. (2013). MRI evidence that glibenclamide reduces acute lesion expansion in a rat model of spinal cord injury. Spinal Cord 51, 823–827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Collignon F., Martin D., Lenelle J., and Stevenaert A. (2002). Acute traumatic central cord syndrome: magnetic resonance imaging and clinical observations. J. Neurosurg. 96, 29–33 [DOI] [PubMed] [Google Scholar]
- 41. Mihai G., Nout Y.S., Tovar C.A., Miller B.A., Schmalbrock P., Bresnahan J.C., and Beattie M.S. (2008). Longitudinal comparison of two severities of unilateral cervical spinal cord injury using magnetic resonance imaging in rats. J. Neurotrauma 25, 1–18 [DOI] [PubMed] [Google Scholar]
- 42. Phang I., and Papadopoulos M.C. (2015). Intraspinal pressure monitoring in a patient with spinal cord injury reveals different intradural compartments: Injured Spinal Cord Pressure Evaluation (ISCoPE) Study. Neurocrit. Care [Epub ahead of print; DOI: 10.1007/s12028-015-0153-6] [DOI] [PubMed] [Google Scholar]
- 43. Phang I., Werndle M.C., Saadoun S., Varsos G., Czosnyka M., Zoumprouli A., and Papadopoulos M.C. (2015). Expansion duroplasty improves intraspinal pressure, spinal cord perfusion pressure, and vascular pressure reactivity index in patients with traumatic spinal cord injury: injured spinal cord pressure evaluation study. J. Neurotrauma 32, 865–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chen S., Gallagher M.J., Hogg F., Papadopoulos M.C., and Saadoun S. (2018). Visibility graph analysis of intraspinal pressure signal predicts functional outcome in spinal cord injured patients. J. Neurotrauma 35, 2947–2956 [DOI] [PubMed] [Google Scholar]
- 45. Saadoun S., and Papadopoulos M.C. (2016). Spinal cord injury: is monitoring from the injury site the future? Crit. Care 20, 308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Vale F.L., Burns J., Jackson A.B., and Hadley M.N. (1997). Combined medical and surgical treatment after acute spinal cord injury: results of a prospective pilot study to assess the merits of aggressive medical resuscitation and blood pressure management. J. Neurosurg. 87, 239–246 [DOI] [PubMed] [Google Scholar]
- 47. Papadopoulos S.M., Selden N.R., Quint D.J., Patel N., Gillespie B., and Grube S. (2002). Immediate spinal cord decompression for cervical spinal cord injury: feasibility and outcome. J. Trauma 52, 323–332 [DOI] [PubMed] [Google Scholar]
- 48. Burke J.F., Yue J.K., Ngwenya L.B., Winkler E.A., Talbott J.F., Pan J.Z., Ferguson A.R., Beattie M.S., Bresnahan J.C., Haefeli J., Whetstone W.D., Suen C.G., Huang M.C., Manley G.T., Tarapore P.E., and Dhall S.S. (2018). Ultra-early (<12 hours) surgery correlates with higher rate of american spinal injury association impairment scale conversion after cervical spinal cord injury. Neurosurgery 185, 199–203 [DOI] [PubMed] [Google Scholar]
- 49. Maryland Institute for Emergency Medical Services Systems. (2015). Maryland Institute for Emergency Medical Services Systems. https://www.m.emss.org
- 50. American Spinal Injury Association (1992). International Standards for Neurological and Functional Classification of Spinal Cord Injury. Chicago: American Spinal Injury Association/International Spinal Cord Society [Google Scholar]
- 51. Harris J.H. Jr., Edeiken-Monroe B., and Kopaniky D.R. (1986). A practical classification of acute cervical spine injuries. Orthop. Clin. North Am. 1, 15–30 [PubMed] [Google Scholar]
- 52. Allen B.L. Jr., Ferguson R.L., Lehmann T.R., and O'Brien R.P. (1982). A mechanistic classification of closed, indirect fractures and dislocations of the lower cervical spine. Spine 7, 1–27 [DOI] [PubMed] [Google Scholar]
- 53. Vaccaro A.R., Koerner J.D., Radcliff K.E., Oner F.C., Reinhold M., Schnake K.J., Kandziora F., Fehlings M.G., Dvorak M.F., Aarabi B., Rajasekaran S., Schroeder G.D., Kepler C.K., and Vialle L.R. (2015). AOSpine subaxial cervical spine injury classification system. Eur. Spine J. [Epub ahead of print; DOI: 10.1007/s00586-015-3831-3] [DOI] [PubMed] [Google Scholar]
- 54. Hadley M.N., Walters B.C., Grabb P.A., Oyesiku N.M., Prezybylski G.J., Resnick D.K., Ryken T.C., and Mielke D.H. (2002). Guidelines for the Management of Acute Cervical Spine and Spinal Cord injuries. Neurosurgery 50, S1–S199 [DOI] [PubMed] [Google Scholar]
- 55. Walters B.C., Hadley M.N., Hurlbert R.J., Aarabi B., Dhall S.S., Gelb D.E., Harrigan M.R., Rozelle C.J., Ryken T.C., and Theodore N. (2013). Guidelines for the management of acute cervical spine and spinal cord injuries: 2013 update. Neurosurgery 60, Suppl. 1, 82–91 [DOI] [PubMed] [Google Scholar]
- 56. Lee A.S., MacLean J.C., and Newton D.A. (1994). Rapid traction for reduction of cervical spine dislocations. J Bone Joint Surg. Br. 76, 352–356 [PubMed] [Google Scholar]
- 57. Dvorak M.F., Fisher C.G., Fehlings M.G., Rampersaud Y.R., Oner F.C., Aarabi B., and Vaccaro A.R. (2007). The surgical approach to subaxial cervical spine injuries: an evidence-based algorithm based on the SLIC classification system. Spine 32, 2620–2629 [DOI] [PubMed] [Google Scholar]
- 58. Grassner L., Wutte C., Klein B., Mach O., Riesner S., Panzer S., Vogel M., Buhren V., Strowitzki M., Vastmans J., and Maier D. (2016). Early decompression (< 8 h) after traumatic cervical spinal cord injury improves functional outcome as assessed by spinal cord independence measure after one year. J. Neurotrauma 33, 1658–1666 [DOI] [PubMed] [Google Scholar]
- 59. Mattiassich G., Gollwitzer M., Gaderer F., Blocher M., Osti M., Lill M., Ortmaier R., Haider T., Hitzl W., Resch H., and Aschauer-Wallner S. (2017). Functional outcomes in individuals undergoing very early (< 5 h) and early (5–24 h) surgical decompression in traumatic cervical spinal cord injury: analysis of neurological improvement from the Austrian Spinal Cord Injury Study. J. Neurotrauma 34, 3362–3371 [DOI] [PubMed] [Google Scholar]
- 60. Aarabi B., Alexander M., Mirvis S.E., Shanmuganathan K., Chesler D., Maulucci C., Iguchi M., Aresco C., and Blacklock T. (2011). Predictors of outcome in acute traumatic central cord syndrome due to spinal stenosis. J. Neurosurg. Spine 14, 122–130 [DOI] [PubMed] [Google Scholar]
- 61. Boldin C., Raith J., Fankhauser F., Haunschmid C., Schwantzer G., and Schweighofer F. (2006). Predicting neurologic recovery in cervical spinal cord injury with postoperative MR imaging. Spine 31, 554–559 [DOI] [PubMed] [Google Scholar]
- 62. Farhadi H.F., Kukreja S., Minnema A., Vatti L., Gopinath M., Prevedello L., Chen C., Xiang H., and Schwab J.M. (2018). Impact of admission imaging findings on neurological outcomes in acute cervical traumatic spinal cord injury. J. Neurotrauma. [DOI] [PubMed] [Google Scholar]
- 63. Furlan J.C., Noonan V., Cadotte D.W., and Fehlings M.G. (2011). Timing of decompressive surgery of spinal cord after traumatic spinal cord injury: an evidence-based examination of pre-clinical and clinical studies. J. Neurotrauma 28, 1371–1399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Batchelor P.E., Wills T.E., Skeers P., Battistuzzo C.R., Macleod M.R., Howells D.W., and Sena E.S. (2013). Meta-analysis of pre-clinical studies of early decompression in acute spinal cord injury: a battle of time and pressure. PloS one 8, e72659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Batchelor P.E., Kerr N.F., Gatt A.M., Cox S.F., Ghasem-Zadeh A., Wills T.E., Sidon T.K., and Howells D.W. (2011). Intracanal pressure in compressive spinal cord injury: reduction with hypothermia. J. Neurotrauma 28, 809–820 [DOI] [PubMed] [Google Scholar]
- 66. Sewell M.D., Vachhani K., Alrawi A. and Williams R. (2018). Results of early and late surgical decompression and stabilization for acute traumatic cervical spinal cord injury in patients with concomitant chest injuries. World Neurosurg. 118, e161–e165 [DOI] [PubMed] [Google Scholar]
- 67. Sapkas G.S., and Papadakis S.A. (2007). Neurological outcome following early versus delayed lower cervical spine surgery. J. Orthop. Surg. (Hong Kong) 15, 183–186 [DOI] [PubMed] [Google Scholar]
- 68. Dvorak M.F., Noonan V.K., Fallah N., Fisher C.G., Finkelstein J., Kwon B.K., Rivers C.S., Ahn H., Paquet J., Tsai E.C., Townson A., Attabib N., Bailey C.S., Christie S.D., Drew B., Fourney D.R., Fox R., Hurlbert R.J., Johnson M.G., Linassi A.G., Parent S., and Fehlings M.G. (2015). The influence of time from injury to surgery on motor recovery and length of hospital stay in acute traumatic spinal cord injury: an observational Canadian cohort study. J. Neurotrauma 32, 645–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Bourassa-Moreau E., Mac-Thiong J.M., Li A., Ehrmann Feldman D., Gagnon D.H., Thompson C., and Parent S. (2016). Do patients with complete spinal cord injury benefit from early surgical decompression? Analysis of neurological improvement in a prospective cohort study. J. Neurotrauma 33, 301–306 [DOI] [PubMed] [Google Scholar]
- 70. Guest J., Eleraky M.A., Apostolides P.J., Dickman C.A., and Sonntag V.K. (2002). Traumatic central cord syndrome: results of surgical management. J. Neurosurg. 97, 25–32 [DOI] [PubMed] [Google Scholar]
- 71. Lenehan B., Fisher C.G., Vaccaro A., Fehlings M., Aarabi B., and Dvorak M.F. (2010). The urgency of surgical decompression in acute central cord injuries with spondylosis and without instability. Spine 35, Suppl. 21, S180–S186 [DOI] [PubMed] [Google Scholar]
- 72. Wilson J.R., Singh A., Craven C., Verrier M.C., Drew B., Ahn H., Ford M., and Fehlings M.G. (2012). Early versus late surgery for traumatic spinal cord injury: the results of a prospective Canadian cohort study. Spinal Cord 50, 840–843 [DOI] [PubMed] [Google Scholar]
- 73. Kim M., Hong S.K., Jeon S.R., Roh S.W., and Lee S. (2018). Early (</ = 48 hours) versus late (>48 hours) surgery in spinal cord injury: treatment outcomes and risk factors for spinal cord injury. World Neurosurg. 118, e513–e525 [DOI] [PubMed] [Google Scholar]
- 74. Schaefer D.M., Flanders A.E., Osterholm J., and Northrup B.E. (1992). Prognostic significance of magnetic resonance imaging in the acute phase of cervical spine injury. J. Neurosurg. 76, 218–223 [DOI] [PubMed] [Google Scholar]
- 75. Flanders A.E., Schaefer D.M., Doan H.T., Mishkin M.M., Gonzalez C.F., and Northrup B.E. (1990). Acute cervical spine trauma: correlation of MR imaging findings with degree of neurologic deficit. Radiology 177, 25–33 [DOI] [PubMed] [Google Scholar]
- 76. Flanders A.E., Spettell C.M., Friedman D.P., Marino R.J., and Herbison G.J. (1999). The relationship between the functional abilities of patients with cervical spinal cord injury and the severity of damage revealed by MR imaging. AJNR. Am. J. Neuroradiol. 20, 926–934 [PMC free article] [PubMed] [Google Scholar]
- 77. Talbott J.F., Whetstone W.D., Readdy W.J., Ferguson A.R., Bresnahan J.C., Saigal R., Hawryluk G.W., Beattie M.S., Mabray M.C., Pan J.Z., Manley G.T., and Dhall S.S. (2015). The Brain and Spinal Injury Center score: a novel, simple, and reproducible method for assessing the severity of acute cervical spinal cord injury with axial T2-weighted MRI findings. J. Neurosurg. Spine, 1–10 [DOI] [PubMed] [Google Scholar]
- 78. Farhadi H.F., Minnema A.J., Talbott J.F., and Aarabi B. (2018). Letter to the Editor: What has been learned from magnetic resonance imaging examination of the injured human spinal cord: a Canadian perspective. J. Neurotrauma. [DOI] [PubMed] [Google Scholar]
- 79. Phang I., Mada M., Kolias A.G., Newcombe V.F., Trivedi R.A., Carpenter A., Hawkes R.C., and Papadopoulos M.C. (2016). Magnetic resonance imaging of the codman microsensor transducer used for intraspinal pressure monitoring: findings from the Injured Spinal Cord Pressure Evaluation Study. Spine 41, E605–610 [DOI] [PubMed] [Google Scholar]
- 80. Saadoun S., Bell B.A., Verkman A.S., and Papadopoulos M.C. (2008). Greatly improved neurological outcome after spinal cord compression injury in AQP4-deficient mice. Brain 131, 1087–1098 [DOI] [PubMed] [Google Scholar]
- 81. Saadoun S., Werndle M.C., Lopez de Heredia L., and Papadopoulos M.C. (2016). The dura causes spinal cord compression after spinal cord injury. Br. J. Neurosurg. 30, 582–584 [DOI] [PubMed] [Google Scholar]
- 82. Freund P., Friston K., Thompson A.J., Stephan K.E., Ashburner J., Bach D.R., Nagy Z., Helms G., Draganski B., Mohammadi S., Schwab M.E., Curt A., and Weiskopf N. (2016). Embodied neurology: an integrative framework for neurological disorders. Brain 139, 1855–1861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Freund P., Weiskopf N., Ashburner J., Wolf K., Sutter R., Altmann D.R., Friston K., Thompson A., and Curt A. (2013). MRI investigation of the sensorimotor cortex and the corticospinal tract after acute spinal cord injury: a prospective longitudinal study. Lancet Neurol. 12, 873–881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Mohammadi S., Freund P., Feiweier T., Curt A., and Weiskopf N. (2013). The impact of post-processing on spinal cord diffusion tensor imaging. Neuroimage 70, 377–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Seif M., Ziegler G. and Freund P. (2018). Progressive ventricles enlargement and cerebrospinal fluid volume increases as a marker of neurodegeneration in patients with spinal cord injury: a longitudinal magnetic resonance imaging study. J. Neurotrauma. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Shanmuganathan K., Gullapalli R.P., Zhuo J., and Mirvis S.E. (2008). Diffusion tensor MR imaging in cervical spine trauma. AJNR Am. J. Neuroradiol. 29, 655–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Shanmuganathan K., Zhuo J., Chen H.H., Aarabi B., Adams J., Miller C., Menakar J., Gullapalli R.P., and Mirvis S.E. (2017). Diffusion tensor imaging parameter obtained during acute blunt cervical spinal cord injury in predicting long-term outcome. J. Neurotrauma 34, 2964–2971 [DOI] [PubMed] [Google Scholar]
- 88. Nout Y.S., Mihai G., Tovar C.A., Schmalbrock P., Bresnahan J.C., and Beattie M.S. (2009). Hypertonic saline attenuates cord swelling and edema in experimental spinal cord injury: a study utilizing magnetic resonance imaging. Crit. Care Med. 37, 2160–2166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Salegio E.A., Bresnahan J.C., Sparrey C.J., Camisa W., Fischer J., Leasure J., Buckley J., Nout-Lomas Y.S., Rosenzweig E.S., Moseanko R., Strand S., Hawbecker S., Lemoy M.J., Haefeli J., Ma X., Nielson J.L., Edgerton V.R., Ferguson A.R., Tuszynski M.H., and Beattie M.S. (2016). A unilateral cervical spinal cord contusion injury model in non-human primates (Macaca mulatta). J. Neurotrauma 33, 439–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Chou P.C., Shunmugavel A., El Sayed H., Desouki M.M., Nguyen S.A., Khan M., Singh I., and Bilgen M. (2011). Preclinical use of longitudinal MRI for screening the efficacy of S-nitrosoglutathione in treating spinal cord injury. J. Magn. Reson. imaging 33, 1301–1311 [DOI] [PubMed] [Google Scholar]