Abstract
Immune regulation at the maternal-fetal interface is complex due to conflicting immunological objectives: protection of the fetus from maternal pathogens and prevention of immune-mediated rejection of the semiallogeneic fetus and placenta. Interferon (IFN) signaling plays an important role in restricting congenital infections as well as in the physiology of healthy pregnancies. In this review, we discuss the antiviral and pathogenic effects of type I IFN (IFN-α, IFN-β), type II IFN (IFN-γ), and type III IFN (IFN-λ) during pregnancy, with an emphasis on mouse and non-human primate models of congenital Zika virus infection. In the context of these animal model systems, we examine the role of IFN signaling during healthy pregnancy. Finally, we review mechanisms by which dysregulated type I IFN responses contribute to poor pregnancy outcomes in humans with autoimmune disease, including interferonopathies and systemic lupus erythematosus.
Keywords: Zika virus, pregnancy, interferons, systemic lupus erythematosus
Introduction
Immune regulation at the maternal-fetal interface is complex due to conflicting immunological objectives: protection of the fetus from maternal pathogens and prevention of immune-mediated rejection of the semiallogeneic fetus and placenta. Maternal and fetal tissues are in direct contact where the fetal-derived placenta invades and implants into a layer of specialized maternal endometrial tissue, termed the decidua. The decidua is enriched in maternal leukocytes, particularly natural killer (NK) and Treg cells, which mediate protective and tolerogenic immunity at the maternal-fetal interface. In addition to controlling pathogen transmission from mother to fetus, proper regulation of cytokine signaling, including interferon (IFN) signaling, contributes to the physiology of healthy pregnancy.
In this review, we discuss the antiviral and pathogenic effects of type I, II, and III IFN signaling during pregnancy, with an emphasis on mouse and non-human primate (NHP) models of congenital Zika virus (ZIKV) infection. In the context of these animal model systems, we examine the role of IFN signaling during healthy pregnancy. Finally, we review mechanisms by which dysregulated type I IFN responses contribute to poor pregnancy outcomes in humans with systemic autoimmunity.
IFN Signaling
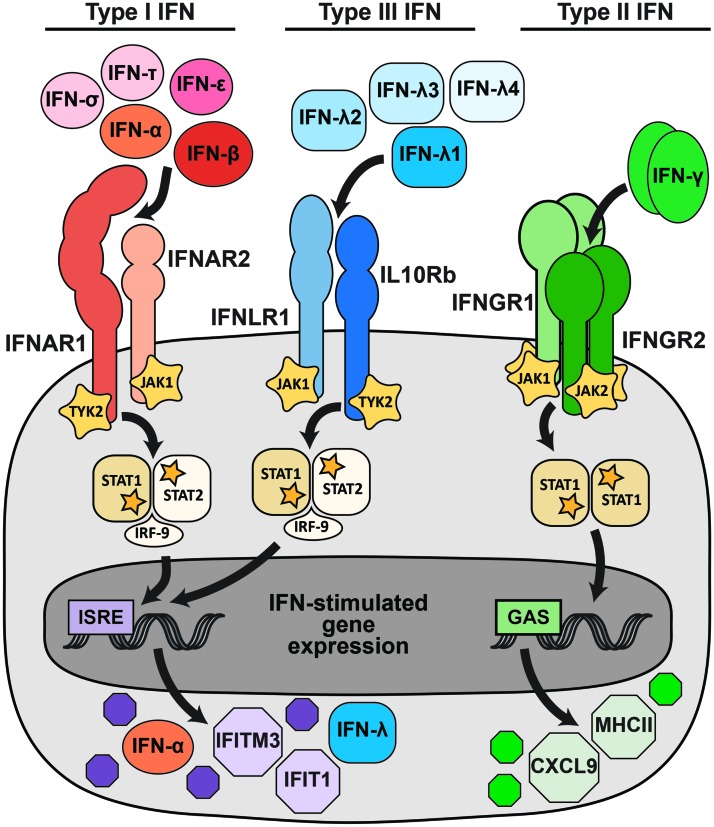
IFNs are cytokines and are divided into three families (type I, type II, and type III) based on sequence homology, evolutionary relatedness, receptor usage, and functional activity (Fig. 1) (5,61,68). In humans, mice, and NHPs, the type I IFN family includes multiple IFN-α subtypes (13 in humans, 14 in mice, and 14 in rhesus macaques) and single IFN-β, IFN-ɛ, IFN-κ, IFN-ω (primates), and IFN-ζ (mice) subtypes (4,5). Additional type I IFNs are found in other mammals, for example multiple IFN-τ genes in ruminants and IFN-δ in swine (57). All type I IFNs signal through the same receptor, IFNAR (comprising two chains, IFNAR1 and IFNAR2). The type II IFN family includes a single member, IFN-γ, which signals as a homodimer through the receptor IFNGR (comprising two copies each of IFNGR1 and IFNGR2) (5). The type III IFN family includes four subtypes in humans and NHPs, IFN-λ1, IFN-λ2, IFN-λ3, and IFN-λ4, although IFN-λ4 is a pseudogene in many human populations (55,91). In mice, the type III IFN family consists only of IFN-λ2 and IFN-λ3; IFN-λ1 is a pseudogene and the genomic region encoding IFN-λ4 is absent. All type III IFNs signal though IFNLR (comprising IFNLR1 and IL10Rβ). Receptor usage distinguishes the three IFN families; so targeting the receptors provides a means to selectively ablate the activity of an entire IFN family. This can be accomplished using transgenic mice lacking a receptor globally or in specific cell types (most commonly Ifnar1−/−, Ifngr1−/−, or Ifnlr1−/−); using receptor-blocking monoclonal antibodies; or targeting the receptors in cell culture (e.g., with knockdown or gene-editing approaches).
FIG. 1.
IFN signaling pathways. Type I, type II, and type III IFNs signal through distinct receptors, but activate overlapping transcriptional programs. More than a dozen type I IFNs, including IFN-α, IFN-β, IFN-ɛ, IFN-δ, and IFN-τ, signal through a heterodimeric receptor comprising IFNAR1 and IFNAR2. A smaller set of type III IFNs (IFN-λ) signal through a heterodimeric receptor comprising IFNLR1 and IL10Rb. The type II IFN family consists only of IFN-γ, which signals as a dimer through a tetrameric receptor comprising IFNGR1 and IFNGR2. Receptor binding activates kinases, including JAK1, JAK2, and TYK2, which phosphorylate STAT1 and STAT2. Phosphorylated STATs dimerize and translocate to the nucleus, where they activate transcription from promoters that contain ISRE (STAT1/2 heterodimers) or GAS (STAT1 homodimers), resulting in the expression of IFN-stimulated genes. The canonical IFN signaling pathways are depicted, but additional signaling pathways also are activated and likely contribute to the specific transcriptional response induced by different IFNs. GAS, γ-activated sites; IFN, interferon; ISRE, IFN-stimulated response elements. Color images are available online.
Despite using different receptors, type I and type III IFNs share many functional similarities. Both are induced by pattern recognition receptor (PRR) signaling following viral infection, their receptor binding activates STAT1/STAT2 heterodimer formation, and they induce a similar transcriptional program characterized by IFN-stimulated response elements in the promoters of induced genes (5,61,68). Type I and III IFN signaling result in the upregulation of hundreds of IFN-stimulated genes (ISGs), many of which act in a cell-intrinsic manner to restrict viral entry, replication, and spread (101). In addition to these canonical IFN signaling pathways, additional signaling pathways also are activated and likely contribute to the specific transcriptional response induced by different IFNs (61,87). Type I and III IFNs also serve immunomodulatory functions, including priming adaptive immune responses.
Although type I and III IFNs activate similar antiviral transcriptional responses, they have distinct physiological activities largely determined by receptor expression. IFNAR is ubiquitously expressed, but IFNLR expression is greatest on epithelial cells and some immune cell subsets, including neutrophils and NK cells (61). Furthermore, while type I and III IFNs are induced by similar PRR signaling pathways, some stimuli favor production of type III IFNs over type I IFNs. These stimuli include infection of respiratory epithelial cells (19,36,50,82), signaling by the PRR MAVS from peroxisomes rather than mitochondria (80), and signaling by the PRR TLR4 from the plasma membrane rather than endosomes (81). Overall, type III IFN signaling is less potent and less inflammatory than type I IFN and predominates at anatomic barriers, leading to a model wherein type III IFNs provide front-line protection at barrier surfaces, thereby minimizing the activation of the systemic type I IFN response and consequent immune pathology.
While type I and III IFNs are best known for their antiviral activities (61), type II IFN has distinct proinflammatory and immunomodulatory functions that also contribute to the control of viral infections. Type II IFN signaling induces STAT1 homodimers and induced genes are characterized by γ-activated sites (GAS) in their promoters. Type II IFN is produced by T cells and NK cells and functions primarily in later stages of infection by clearing viruses from infected tissues. Crosstalk between the type I and type II responses can impact the outcome of infection. For example, downregulation of IFNGR by type I IFN signaling leads to more severe Listeria monocytogenes infection in Ifnar1−/− mice (95). Indeed, while Ifnar1−/− mice generally have enhanced susceptibility to most viral infections, these mice are protected from infection by many bacterial and protozoan pathogens for which immune control is highly dependent upon type II IFN (13,97).
IFN Signaling During Pregnancy
Pregnancy encompasses multiple developmental stages, including implantation, fetal growth, and parturition, each with unique immunological requirements that have been reviewed elsewhere (73,75,121). Early events in pregnancy, such as blastocyst implantation and subsequent placental invasion, are inflammatory processes that physically degrade and remodel maternal tissue at the site of implantation. In contrast, fetal growth occurs in an anti-inflammatory Th2-type environment that characterizes the majority of pregnancy. Finally, parturition is an inflammatory process with NF-κB signaling contributing to labor induction. Since implantation, fetal growth, and parturition have distinct immunological features, IFNs play different roles at each stage of pregnancy. Accordingly, IFNs may also have distinct effects during viral infection at different gestational stages.
Before implantation, the blastocyst is surrounded by an outer trophectoderm layer that attaches to the maternal endometrium and differentiates into trophoblast layers that constitute the placenta. In mice, type I IFNs are expressed in the trophectoderm of the preimplantation blastocyst, in the decidua following implantation, and in multiple trophoblast layers mid-gestation (115). Accordingly, ISGs, including Irf-8, Iarp, Isg12, and Isg15, are upregulated in the postimplantation decidua (53). IFN-γ also is produced by human trophoblasts early in gestation and in mouse trophoblast giant cells, spongiotrophoblasts, and labyrinth zone by mid-gestation (84,90). Specialized NK cells (CD56bright and CD16−) with reduced cytotoxic potential, termed decidual, uterine, or endometrial NK cells, make up the majority of leukocytes in the maternal decidua and secrete IFN-γ during pregnancy (8,37). IFN-γ signaling at the maternal-fetal interface not only promotes differentiation of decidual NK cells but also facilitates formation of the placenta and maintenance of the decidua (75,121).
In addition to its role promoting the physiology of healthy pregnancy, type II IFN has been associated with adverse fetal outcomes in mouse models of congenital infection. Fetal loss following Toxoplasma gondii and Plasmodium berghei infection is ameliorated in Ifngr1−/− mice (79,103). Likewise, administering an anti-IFN-γ antibody protects dams from fetal loss following Brucella abortus infection (56). Since IFN-γ is a key component of proinflammatory immune responses, fetal loss may be general response to infection and inflammation at the maternal-fetal interface in mice.
The placenta mediates nutrient and waste exchange between mother and fetus as the site of contact between fetal and maternal blood supplies. Maternal blood is delivered to the placenta by spiral arteries, which are remodeled early in pregnancy in a process involving IFN signaling. Mice lacking either type I or type II IFN signaling (Ifng−/−, Ifngr1−/−, Ifnar1−/−, or Stat1−/−) have incomplete spiral artery remodeling, suggesting that both type I and II IFNs contribute to this process (75,117). Nonetheless, these knockout mice all produce healthy pregnancies and can be bred for routine experimental use.
Type I IFNs serve distinct pregnancy functions in other mammals. Ruminants express multiple subtypes of IFN-τ, which signals through IFNAR and induces ISG expression, but is not known to serve a protective antiviral function (31,57). Unlike IFN-αβ, IFN-τ expression is not induced by viral infection, but rather by trophoblast development (33). In trophoblasts, IFN-τ serves as a pregnancy recognition factor that modulates maternal hormonal status before implantation and induces ISGs in the maternal endometrium (43,98). In swine, another type I IFN, IFN-δ, is secreted by peri-implantation conceptuses and serves along with IFN-γ to modulate maternal endometrial gene expression before trophoblast attachment (38,67,100). Primates and rodents do not encode orthologs of IFN-τ or IFN-δ (57), which may reflect their corresponding placental types: ruminant and swine placentas are epitheliochorial and less invasive than the hemochorial placentas of primates and rodents (96). Although congenital ZIKV infection has been studied in swine (23,109,113), these studies have used in utero inoculation and thus do not model transplacental transmission.
IFN-ɛ, a type I IFN, is conserved in many mammals, including mice, humans, and NHPs, and is secreted constitutively in the female reproductive tract (27,35,44). In mice, IFN-ɛ is expressed primarily in the uterine endometrium, ovaries, and cervix, and protects against sexually transmitted infections, including HSV-2 and Chlamydia muridarum (35). However, IFN-ɛ levels fluctuate with the estrus cycle rather than being induced downstream of PRR signaling (27). In rhesus macaques, IFN-ɛ is secreted from mucosal epithelial cells in the vagina and cervix, as well as in the lung, foreskin, and small and large intestines (27). A role for IFN-ɛ during pregnancy has not been described, but its expression profile in the female reproductive tract potentially could protect the fetal compartment from ascending infections.
IFN Signaling in Mouse Models of Congenital ZIKV Infection
The 2015–2016 ZIKV outbreak throughout Latin America and the Caribbean led to the discovery that ZIKV infection during pregnancy can produce adverse fetal and neonatal outcomes, including microcephaly, intrauterine growth restriction (IUGR), and vision and hearing loss, as well as miscarriage. Subsequently, models of congenital infection were developed to test vaccines and antivirals as well as to define ZIKV pathogenic mechanisms and antiviral immunity at the maternal-fetal interface (85,88).
Mice have become a common animal model of congenital ZIKV infection (16). Comprehensive comparisons between mouse and human pregnancy have been reviewed elsewhere (6). Like humans, mice have hemochorial, discoid placentas and are an important pregnancy model because they are genetically tractable, cost-effective, and have a short gestation. However, modeling congenital ZIKV infection in mice can be difficult due to physiological differences at the maternal-fetal interface and because their gestational development has inexact parallels with human gestation. The shortened timing of mouse gestation (3 weeks compared to 40 weeks in humans) confounds studies of infection at distinct developmental stages as well as studies of adaptive immune responses during pregnancy. Furthermore, conventional inbred mouse models produce fetuses and placentas that are genetically identical to the dam; this removes the immunologic pressure of supporting invasive, semiallogeneic tissues that exist in outbred models or in humans.
Another complication of mouse models of congenital ZIKV infection is that type I IFN signaling restricts ZIKV replication in mice (59), in part, due to the inability of ZIKV to antagonize murine STAT2 and STING (28,40,41,58). This results in diminished ZIKV replication in immune-competent mice and has led most groups to use IFN-deficient mouse models, including mice lacking the type I and/or type II IFN receptors (e.g., Ifnar1−/− or Ifnar1−/− Ifngr1−/− double knockout), mice with defects in IFN induction or signaling (e.g., Irf3−/− Irf5−/− Irf7−/− or Stat2−/−), or mice treated with IFNAR1-blocking antibody (74). Congenital ZIKV infection has been studied using IFN-deficient mouse models in which maternal ZIKV infection by intravaginal or subcutaneous footpad inoculation results in viral transmission to the fetus, placental damage, and adverse pregnancy outcomes, including IUGR and fetal demise (16,49,51,70,119). IFN-deficient mouse models are valuable because they allow sufficient viral replication to elicit congenital phenotypes, but they limit studies of the specific effects of IFN signaling at the maternal-fetal interface in the context of congenital ZIKV infection. Accordingly, some groups have reported congenital ZIKV infection in wild-type mice, although these models have required high inoculation doses, infection-enhancing antibodies, or direct intrauterine inoculation (22,94,107,110). ZIKV transplacental transmission (but not fetal pathology or loss) also has been reported in transgenic mice expressing human STAT2 (40). Recombinant IFN-λ2 administered to pregnant dams restricted ZIKV transplacental transmission (49) and induced ISGs in both placental and decidual cells (12), consistent with an antiviral effect of type III IFN at the maternal-fetal interface.
The mechanisms by which ZIKV accesses the fetal compartment have yet to be determined and could be affected by the route of inoculation (e.g., subcutaneous vs. intravaginal). Congenital infection also may be impacted by viral strain, as different ZIKV strains produce different pathologic outcomes in nonpregnant mice (59,106,108). Nonetheless, the ability to cause fetal infection and pathology in mice is not a unique property of contemporary ZIKV strains compared to historical ones (48,104,110). Infection at early gestational stages (before E10) results in higher rates of fetal loss and more severe IUGR (49,70,110,119), likely because placentation is incomplete and the placental and fetal tissues are more vulnerable to infection early in development. Fetal and placental pathology generally are more severe in Ifnar1−/− dams compared to wild-type dams treated with an IFNAR1-blocking antibody, consistent with higher viral burdens in Ifnar1−/− dams and suggesting that fetal disease outcomes are driven by maternal viremia (59,70).
An advantage of using mouse models to study congenital infection is the ability to use dams and sires of different IFN receptor genotypes to produce pregnancies with different IFN responsiveness on each side of the maternal-fetal interface, or among fetuses within a single pregnancy, as the placenta is a fetal-derived tissue and each fetus produces its own placenta. For example, crossing an Ifnar1−/− dam and Ifnar1+/− sire yields a pregnancy in which 50% of fetuses (and their associated placentas) are Ifnar1−/− (lack type I IFN signaling) and 50% are Ifnar1+/− (intact type I IFN signaling), within an Ifnar1−/− dam.
Both type I and type III IFN signaling have an antiviral effect at the maternal-fetal interface and limit ZIKV transplacental transmission in mouse models. However, Ifnar1+/− fetuses within Ifnar1−/− dams succumb to ZIKV infection, indicating that fetal and placental type I IFN signaling are not sufficient to restrict congenital ZIKV infection (49,70,118). Somewhat counterintuitively, Ifnar1−/− fetuses within Ifnar1−/− dams exhibited less ZIKV-induced pathology than their Ifnar1+/− littermates, suggesting a detrimental effect of type I IFN signaling on the developing placenta and fetus, and underscoring the delicate balance required of maternal antiviral immunity in the context of congenital infection (118). Uncontrolled viral replication and dysregulated cytokine signaling in Ifnar1−/− mice result in high serum concentrations of type I IFN (60,86), which could contribute to severe infection-induced pathology in Ifnar1+/− fetuses within Ifnar1−/− dams. Moreover, type I IFN-mediated pathology is not a ZIKV-specific effect, as fetal pathology in Ifnar1+/− fetuses can be induced by poly(I:C) (118). Other flaviviruses are able to produce congenital infection and fetal pathology in mice (52,89), so type I IFN-mediated fetal pathology may be a general effect of infection and morbidity in pregnant mice. Conversely, fetal and placental type I IFN signaling may reduce the severity of maternal viral infection (93). Type I IFN induction also is associated with preterm birth in mice following lymphocytic choriomeningitis virus or L. monocytogenes infection, as well as treatment with poly(I:C) or LPS (17). One mechanism for IFN-induced placental pathology could include ISGs that interfere with placental physiology. For example, IFITM proteins inhibit viral infection by interfering with membrane fusion (101), but IFITM expression in the placenta can disrupt cell fusion required for syncytiotrophoblast formation (15,120). Accordingly, mice lacking IFITMs are protected from IFN-induced pregnancy pathology (15).
Although Ifnar1−/− mice have worsened disease outcomes in the context of viral infection, Ifnar1−/− mice have reduced bacterial burdens following L. monocytogenes or C. muridarum infection (3,13,76). Accordingly, L. monocytogenes infection is controlled more effectively in pregnant Ifnar1−/− dams compared to wild-type dams, including reduced bacterial burdens in the placenta and spleen as well as reduced fetal reabsorption rates (3).
NHP Models of Congenital ZIKV Infection
NHPs are a more physiologically relevant animal model for studying congenital ZIKV infections as they have singleton pregnancies, a hemochorial discoid placenta, and a long gestation (23 weeks in rhesus macaques). Pregnant rhesus macaques develop prolonged ZIKV viremia compared to nonpregnant macaques (30,65,77), consistent with prolonged viremia observed in ZIKV-infected pregnant women (29,83). Congenital ZIKV infection in rhesus macaques produces disease outcomes corresponding to those observed in humans, including fetal infection, placental pathology, neuropathology, ocular disease, and fetal loss (30,45,65,66,72,77). Rhesus macaques have become the most common NHP model of congenital ZIKV infection, although studies in pregnant pigtail macaques, olive baboons, and marmosets also observed restricted fetal brain growth, viral neuroinvasion, and neuroinflammation (1,2,42,102).
ZIKV infection induces a robust systemic adaptive immune response in both pregnant and nonpregnant NHP characterized by IFN-γ induction, leukocyte expansion, and ZIKV-specific IgG and IgM antibodies (30,45,46,62). During acute ZIKV infection in nonpregnant NHP, peripheral blood mononuclear cells (PBMC) exhibit rapid upregulation of type I and type II IFN, as well as ISGs (e.g., OASL, OAS2, IFIT1, MX1, MX2, and TRIM5) and proinflammatory cytokines and chemokines (e.g., TNFA, IL1, IL18, CCL2, and CCL20) (4). PBMC type I and type III IFN transcript levels correlate with the level of ZIKV viremia (4). ZIKV infection at the maternal-fetal interface also results in robust immune responses in the maternal decidua and in fetal tissues. Following infection, levels of IFN-γ and other proinflammatory cytokines (e.g., IL-2, IL-12, MIF, IL-1B, IL-2, IL-12, and IL-1RA) increased in the fetal blood (45).
During normal pregnancy, immunity in the decidua is tightly regulated to allow trophoblast invasion into decidual tissue and to prevent rejection of the semiallogeneic placenta. Once placentation is complete (the end of the first trimester, ∼3 weeks in rhesus macaques) (25), an anti-inflammatory immune milieu is maintained in the maternal decidua. Decidual leukocytes are dominated by a unique subset of NK cells with reduced cytotoxic potential, as well as Treg cells and M2-like macrophages (121). In ZIKV-infected pregnant rhesus macaques, inflammatory leukocytes, including nonclassical monocyte subsets and CD4+ T cells, were increased in the decidua and placental villous trees compared to uninfected controls late in gestation (135 days) (45). Although classical MHC molecules are not expressed on trophoblasts, Treg and Th2-type immune responses are necessary to prevent rejection of the fetus. Induction of IFN-γ and a proinflammatory Th1-type immune response at the maternal-fetal interface, for example resulting from viral infection, may contribute to placental damage and fetal demise during congenital ZIKV infection.
Th1-type immune responses also may be induced following ZIKV infection in mice. A subset of Th1-type T cells generally is excluded from the decidua during pregnancy due to epigenetic silencing of Cxcl9, Cxcl10, and Ccl5 (121). However, Cxcl9 and Cxcl10 were induced in the maternal decidua of ZIKV-infected mice, further suggesting that ZIKV infection disrupts the immune balance at the maternal-fetal interface (14).
Ex Vivo and Cell Culture Models of Placental Infection
ZIKV infection and IFN responses and production have been modeled using primary placental and decidual tissues cultured ex vivo. Explants from human mid-gestation and term placentas cultured ex vivo constitutively secrete type III IFN (10,18), which is among the mechanisms that render syncytiotrophoblasts refractory to viral infection (11,26). Following ZIKV infection, mid-gestation decidual explants produced type I and type III IFN (112) and placental macrophages produced IFN-α (but not IFN-β or IFN-λ) (92), suggesting additional sources of type I and III IFNs at the maternal-fetal interface. As maternally derived decidual cells respond to type I and type III IFNs, placenta-derived IFNs could induce an antiviral state on both sides of the maternal-fetal interface (18,49). Since type III IFN induces a less potent antiviral response than type I IFN, type III IFN signaling at the maternal-fetal interface may produce an antiviral state without the immune pathology induced by type I IFN (61). Accordingly, IFN-β induced pathological morphological changes (syncytial knots and sprouts) in human mid-gestation chorionic villous explants, whereas IFN-λ3 caused no pathology (118).
Dysregulated IFN Signaling in Autoimmunity and Adverse Pregnancy Outcomes
Women with dysregulated type I IFN signaling (sustained IFN production or impaired receptor downregulation) exhibit poor pregnancy outcomes. These include preeclampsia as well as neurodevelopmental defects similar to those induced by congenital infection, altogether consistent with a role for dysregulated type I IFN responses in placental damage (7,21,69,99). Elevated type I IFN levels during pregnancy may be pathogenic, since diseases involving increased production of type I IFN are associated with miscarriage (21,73). This association is most apparent in patients with systemic lupus erythematosus, a rheumatologic disease characterized by activation of the type I IFN response (9). Transcriptomic analysis of PBMCs from pregnant lupus patients revealed that preeclampsia and other fetal complications were associated with high ISG expression, in contrast to the downregulation of type I IFN responses observed during healthy pregnancy and in uncomplicated lupus pregnancy (47). A subset of patients with lupus develop antiphospholipid antibody syndrome, a disease that is associated with miscarriage, and in which patients produce antibodies that prolong clotting times in laboratory testing, but cause clots in vivo (32). Although lupus patients produce autoantibodies, the potentially pathogenic role of some autoantibodies remains uncertain. Nevertheless, one of the most compelling arguments for the pathogenicity of autoantibodies in lupus is the association between anti-SSA and anti-SSB autoantibodies and the development of neonatal lupus, a disease characterized by rash and other features of lupus in the first few months of life until maternal antibodies are cleared from the neonatal circulation (54). Neonatal lupus also can result in permanent damage to the conduction system of the developing heart, requiring pacemaker placement (39,71,105). Although the presence of maternal anti-SSA and SSB autoantibodies is strongly associated with risk of neonatal lupus, expression of high levels of type I IFN also may contribute to this disease entity (71,78). IFN-α may limit angiogenesis and affect the pathogenesis of preeclampsia in lupus pregnancy (7). In further support of the idea that IFN-α may contribute to neonatal lupus, IFN-α treatment in pregnancy was associated with IUGR and facial rash consistent with neonatal lupus in one case (34).
Rare monogenic diseases associated with increased production of type I IFN, known as interferonopathies, also provide insight into contributions of type I IFN to human disease pathogenesis. Some of the classic examples of monogenic interferonopathies include Aicardi-Goutieres Syndrome and STING-associated vasculopathy with onset in infancy (SAVI) (20,21,63). Both of these diseases are caused by mutations that upregulate the type I IFN response downstream of the TREX1-cGAS-STING pathway (24,116). Although these diseases are thought to be mediated by enhanced type I IFN production, this has not been definitively demonstrated in humans with monogenic interferonopathies. However, in an animal model of SAVI (heterozygous STING N153S knockin mice), disease is associated with lethality in pregnant dams and upregulation of ISGs in adult mice, although the mechanism of impaired survival during pregnancy was not determined (111). Autoimmunity in this model of interferonopathy also may reflect effects on adaptive immunity. For example, in addition to upregulation of the type I IFN response, STING N153S mice exhibit severe lung disease mediated primarily by T cells (64,114). Indeed, some features of the disease develop independent of IFNAR1, IRF3, and IRF7 (64), underscoring the complexity of defining the precise contributions of type I IFN in human studies, even when there is an associated type I IFN signature in patient cells.
Summary
Type I, II, and III IFNs serve important roles during pregnancy, both in controlling normal physiology at the maternal-fetal interface and in preventing transmission of maternal pathogens to the fetus. However, excessive or dysregulated IFN signaling may be pathogenic during pregnancies complicated by viral infection or systemic interferonopathies related to autoimmunity. Understanding the balance between protective and pathogenic effects of IFNs during pregnancy may lead to novel therapies to improve outcomes in complicated pregnancies associated with teratogenic infections or excessive production of IFN.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
Research in the authors' laboratories is supported, in part, by R01 AI39512 (H.M.L.) and K08 AR070918 (J.J.M.), and R01 AI143982 (J.J.M.). R.L.C. is supported by T32 AI007419.
References
- 1. Adams Waldorf KM, Nelson BR, Stencel-Baerenwald JE, et al. Congenital Zika virus infection as a silent pathology with loss of neurogenic output in the fetal brain. Nat Med 2018;24:368–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Adams Waldorf KM, Stencel-Baerenwald JE, Kapur RP, et al. Fetal brain lesions after subcutaneous inoculation of Zika virus in a pregnant nonhuman primate. Nat Med 2016;22:1256–1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Agbayani G, Wachholz K, Murphy SP, et al. Type I interferons differentially modulate maternal host immunity to infection by Listeria monocytogenes and Salmonella enterica serovar Typhimurium during pregnancy. Am J Reprod Immunol 2019;81:e13068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Aid M, Abbink P, Larocca RA, et al. Zika virus persistence in the central nervous system and lymph nodes of rhesus monkeys. Cell 2017;169:610–620. e614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Alspach E, Lussier DM, and Schreiber RD. Interferon gamma and its important roles in promoting and inhibiting spontaneous and therapeutic cancer immunity. Cold Spring Harb Perspect Biol 2019;11:a028480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ander SE, Diamond MS, and Coyne CB. Immune responses at the maternal-fetal interface. Sci Immunol 2019;4:eaat6114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Andrade D, Kim M, Blanco LP, et al. Interferon-alpha and angiogenic dysregulation in pregnant lupus patients who develop preeclampsia. Arthritis Rheumatol 2015;67:977–987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ashkar AA, and Croy BA. Interferon-gamma contributes to the normalcy of murine pregnancy. Biol Reprod 1999;61:493–502 [DOI] [PubMed] [Google Scholar]
- 9. Baechler EC, Batliwalla FM, Karypis G, et al. Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc Natl Acad Sci U S A 2003;100:2610–2615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bayer A, Lennemann NJ, Ouyang Y, et al. Type III interferons produced by human placental trophoblasts confer protection against Zika virus infection. Cell Host Microbe 2016;19:705–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bayer A, Lennemann NJ, Ouyang Y, et al. Chromosome 19 microRNAs exert antiviral activity independent from type III interferon signaling. Placenta 2018;61:33–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bierne H, Travier L, Mahlakoiv T, et al. Activation of type III interferon genes by pathogenic bacteria in infected epithelial cells and mouse placenta. PLoS One 2012;7:e39080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Boxx GM, and Cheng G. The roles of type I interferon in bacterial infection. Cell Host Microbe 2016;19:760–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Brown JA, Singh G, Acklin JA, et al. Dengue virus immunity increases Zika virus-induced damage during pregnancy. Immunity 2019;50:751–762. e755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Buchrieser J, Degrelle SA, Couderc T, et al. IFITM proteins inhibit placental syncytiotrophoblast formation and promote fetal demise. Science 2019;365:176–180 [DOI] [PubMed] [Google Scholar]
- 16. Caine EA, Jagger BW, and Diamond MS. Animal models of Zika virus infection during pregnancy. Viruses 2018;10:E598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cappelletti M, Presicce P, Lawson MJ, et al. Type I interferons regulate susceptibility to inflammation-induced preterm birth. JCI Insight 2017;2:e91288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Corry J, Arora N, Good CA, et al. Organotypic models of type III interferon-mediated protection from Zika virus infections at the maternal-fetal interface. Proc Natl Acad Sci U S A 2017;114:9433–9438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Crotta S, Davidson S, Mahlakoiv T, et al. Type I and type III interferons drive redundant amplification loops to induce a transcriptional signature in influenza-infected airway epithelia. PLoS Pathog 2013;9:e1003773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Crow YJ. Type I interferonopathies: mendelian type I interferon up-regulation. Curr Opin Immunol 2015;32:7–12 [DOI] [PubMed] [Google Scholar]
- 21. Crow YJ, and Manel N. Aicardi-Goutieres syndrome and the type I interferonopathies. Nat Rev Immunol 2015;15:429–440 [DOI] [PubMed] [Google Scholar]
- 22. Cugola FR, Fernandes IR, Russo FB, et al. The Brazilian Zika virus strain causes birth defects in experimental models. Nature 2016;534:267–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Darbellay J, Cox B, Lai K, et al. Zika virus causes persistent infection in porcine conceptuses and may impair health in offspring. EBioMedicine 2017;25:73–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Davidson S, Steiner A, Harapas CR, et al. An update on autoinflammatory diseases: Interferonopathies. Curr Rheumatol Rep 2018;20:38. [DOI] [PubMed] [Google Scholar]
- 25. de Rijk EPCT, and Van Esch E. The macaque placenta—a mini-review. Toxicol Pathol 2008;36:108S–118S [Google Scholar]
- 26. Delorme-Axford E, Donker RB, Mouillet JF, et al. Human placental trophoblasts confer viral resistance to recipient cells. Proc Natl Acad Sci U S A 2013;110:12048–12053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Demers A, Kang G, Ma F, et al. The mucosal expression pattern of interferon-epsilon in rhesus macaques. J Leukoc Biol 2014;96:1101–1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ding Q, Gaska JM, Douam F, et al. Species-specific disruption of STING-dependent antiviral cellular defenses by the Zika virus NS2B3 protease. Proc Natl Acad Sci U S A 2018;115:E6310–E6318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Driggers RW, Ho CY, Korhonen EM, et al. Zika virus infection with prolonged maternal viremia and fetal brain abnormalities. N Engl J Med 2016;374:2142–2151 [DOI] [PubMed] [Google Scholar]
- 30. Dudley DM, Aliota MT, Mohr EL, et al. A rhesus macaque model of Asian-lineage Zika virus infection. Nat Commun 2016;7:12204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ealy AD, and Wooldridge LK. The evolution of interferon-tau. Reproduction 2017;154:F1–F10 [DOI] [PubMed] [Google Scholar]
- 32. Eswaran K, and Rosen SW. Recurrent abortions, thromboses, and a circulating anticoagulant. Am J Obstet Gynecol 1985;151:751–752 [DOI] [PubMed] [Google Scholar]
- 33. Ezashi T, and Imakawa K. Transcriptional control of IFNT expression. Reproduction 2017;154:F21–F31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fritz M, Vats K, and Goyal RK. Neonatal lupus and IUGR following alpha-interferon therapy during pregnancy. J Perinatol 2005;25:552–554 [DOI] [PubMed] [Google Scholar]
- 35. Fung KY, Mangan NE, Cumming H, et al. Interferon-epsilon protects the female reproductive tract from viral and bacterial infection. Science 2013;339:1088–1092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Galani IE, Triantafyllia V, Eleminiadou EE, et al. Interferon-lambda mediates non-redundant front-line antiviral protection against influenza virus infection without compromising host fitness. Immunity 2017;46:875–890. e876. [DOI] [PubMed] [Google Scholar]
- 37. Gaynor LM, and Colucci F. Uterine natural killer cells: functional distinctions and influence on pregnancy in humans and mice. Front Immunol 2017;8:467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Geisert RD, Johnson GA, and Burghardt RC. Implantation and establishment of pregnancy in the pig. Adv Anat Embryol Cell Biol 2015;216:137–163 [DOI] [PubMed] [Google Scholar]
- 39. Goble MM, Dick M, 2nd, McCune WJ, et al. Atrioventricular conduction in children of women with systemic lupus erythematosus. Am J Cardiol 1993;71:94–98 [DOI] [PubMed] [Google Scholar]
- 40. Gorman MJ, Caine EA, Zaitsev K, et al. An immunocompetent mouse model of Zika virus infection. Cell Host Microbe 2018;23:672–685. e676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Grant A, Ponia SS, Tripathi S, et al. Zika virus targets human STAT2 to inhibit type I interferon signaling. Cell Host Microbe 2016;19:882–890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gurung S, Reuter N, Preno A, et al. Zika virus infection at mid-gestation results in fetal cerebral cortical injury and fetal death in the olive baboon. PLoS Pathog 2019;15:e1007507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hansen TR, Sinedino LDP, and Spencer TE. Paracrine and endocrine actions of interferon tau (IFNT). Reproduction 2017;154:F45–F59 [DOI] [PubMed] [Google Scholar]
- 44. Hermant P, Francius C, Clotman F, et al. IFN-epsilon is constitutively expressed by cells of the reproductive tract and is inefficiently secreted by fibroblasts and cell lines. PLoS One 2013;8:e71320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hirsch AJ, Roberts VHJ, Grigsby PL, et al. Zika virus infection in pregnant rhesus macaques causes placental dysfunction and immunopathology. Nat Commun 2018;9:263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hirsch AJ, Smith JL, Haese NN, et al. Zika virus infection of rhesus macaques leads to viral persistence in multiple tissues. PLoS Pathog 2017;13:e1006219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hong S, Banchereau R, Maslow BL, et al. Longitudinal profiling of human blood transcriptome in healthy and lupus pregnancy. J Exp Med 2019;216:1154–1169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Jaeger AS, Murrieta RA, Goren LR, et al. Zika viruses of African and Asian lineages cause fetal harm in a mouse model of vertical transmission. PLoS Negl Trop Dis 2019;13:e0007343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Jagger BW, Miner JJ, Cao B, et al. Gestational stage and IFN-lambda signaling regulate ZIKV infection in utero. Cell Host Microbe 2017;22:366–376. e363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Jewell NA, Cline T, Mertz SE, et al. Lambda interferon is the predominant interferon induced by influenza A virus infection in vivo. J Virol 2010;84:11515–11522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Julander JG, Siddharthan V, Park AH, et al. Consequences of in utero exposure to Zika virus in offspring of AG129 mice. Sci Rep 2018;8:9384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Julander JG, Winger QA, Olsen AL, et al. Treatment of West Nile virus-infected mice with reactive immunoglobulin reduces fetal titers and increases dam survival. Antiviral Res 2005;65:79–85 [DOI] [PubMed] [Google Scholar]
- 53. Kashiwagi A, DiGirolamo CM, Kanda Y, et al. The postimplantation embryo differentially regulates endometrial gene expression and decidualization. Endocrinology 2007;148:4173–4184 [DOI] [PubMed] [Google Scholar]
- 54. Kephart DC, Hood AF, and Provost TT. Neonatal lupus erythematosus: new serologic findings. J Invest Dermatol 1981;77:331–333 [DOI] [PubMed] [Google Scholar]
- 55. Key FM, Peter B, Dennis MY, et al. Selection on a variant associated with improved viral clearance drives local, adaptive pseudogenization of interferon lambda 4 (IFNL4). PLoS Genet 2014;10:e1004681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kim S, Lee DS, Watanabe K, et al. Interferon-gamma promotes abortion due to Brucella infection in pregnant mice. BMC Microbiol 2005;5:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Krause CD, and Pestka S. Cut, copy, move, delete: the study of human interferon genes reveal multiple mechanisms underlying their evolution in amniotes. Cytokine 2015;76:480–495 [DOI] [PubMed] [Google Scholar]
- 58. Kumar A, Hou S, Airo AM, et al. Zika virus inhibits type-I interferon production and downstream signaling. EMBO Rep 2016;17:1766–1775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lazear HM, Govero J, Smith AM, et al. A mouse model of Zika virus pathogenesis. Cell Host Microbe 2016;19:720–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lazear HM, Lancaster A, Wilkins C, et al. IRF-3, IRF-5, and IRF-7 coordinately regulate the type I IFN response in myeloid dendritic cells downstream of MAVS signaling. PLoS Pathog 2013;9:e1003118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Lazear HM, Schoggins JW, and Diamond MS. Shared and distinct functions of type I and type III interferons. Immunity 2019;50:907–923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Li XF, Dong HL, Huang XY, et al. Characterization of a 2016 clinical isolate of Zika virus in non-human primates. EBioMedicine 2016;12:170–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Liu Y, Jesus AA, Marrero B, et al. Activated STING in a vascular and pulmonary syndrome. N Engl J Med 2014;371:507–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Luksch H, Stinson WA, Platt DJ, et al. STING-associated lung disease in mice relies on T cells but not type I interferon. J Allergy Clin Immunol 2019;144:254–266. e258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Magnani DM, Rogers TF, Maness NJ, et al. Fetal demise and failed antibody therapy during Zika virus infection of pregnant macaques. Nature Commun 2018;9:1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Martinot AJ, Abbink P, Afacan O, et al. Fetal neuropathology in Zika virus-infected pregnant female rhesus monkeys. Cell 2018;173:1111–1122. e1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Mathew DJ, Lucy MC, and D Geisert R. Interleukins, interferons, and establishment of pregnancy in pigs. Reproduction 2016;151:R111–R122 [DOI] [PubMed] [Google Scholar]
- 68. Mesev EV, LeDesma RA, and Ploss A. Decoding type I and III interferon signalling during viral infection. Nat Microbiol 2019;4:914–924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Meuwissen ME, Schot R, Buta S, et al. Human USP18 deficiency underlies type 1 interferonopathy leading to severe pseudo-TORCH syndrome. J Exp Med 2016;213:1163–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Miner JJ, Cao B, Govero J, et al. Zika virus infection during pregnancy in mice causes placental damage and fetal demise. Cell 2016;165:1081–1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Miner JJ, and Kim AH. Cardiac manifestations of systemic lupus erythematosus. Rheum Dis Clin North Am 2014;40:51–60 [DOI] [PubMed] [Google Scholar]
- 72. Mohr EL, Block LN, Newman CM, et al. Ocular and uteroplacental pathology in a macaque pregnancy with congenital Zika virus infection. PLoS One 2018;13:e0190617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Mor G, Aldo P, and Alvero AB. The unique immunological and microbial aspects of pregnancy. Nat Rev Immunol 2017;17:469–482 [DOI] [PubMed] [Google Scholar]
- 74. Morrison TE, and Diamond MS. Animal models of Zika virus infection, pathogenesis, and immunity. J Virol 2017;91:e00009–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Murphy SP, Tayade C, Ashkar AA, et al. Interferon gamma in successful pregnancies. Biol Reprod 2009;80:848–859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Nagarajan UM, Prantner D, Sikes JD, et al. Type I interferon signaling exacerbates Chlamydia muridarum genital infection in a murine model. Infect Immun 2008;76:4642–4648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Nguyen SM, Antony KM, Dudley DM, et al. Highly efficient maternal-fetal Zika virus transmission in pregnant rhesus macaques. PLoS Pathog 2017;13:e1006378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Niewold TB, Rivera TL, Buyon JP, et al. Serum type I interferon activity is dependent on maternal diagnosis in anti-SSA/Ro-positive mothers of children with neonatal lupus. Arthritis Rheum 2008;58:541–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Niikura M, Inoue SI, Mineo S, et al. IFNGR1 signaling is associated with adverse pregnancy outcomes during infection with malaria parasites. PLoS One 2017;12:e0185392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Odendall C, Dixit E, Stavru F, et al. Diverse intracellular pathogens activate type III interferon expression from peroxisomes. Nat Immunol 2014;15:717–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Odendall C, Voak AA, and Kagan JC. Type III IFNs are commonly induced by bacteria-sensing TLRs and reinforce epithelial barriers during infection. J Immunol 2017;199:3270–3279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Okabayashi T, Kojima T, Masaki T, et al. Type-III interferon, not type-I, is the predominant interferon induced by respiratory viruses in nasal epithelial cells. Virus Res 2011;160:360–366 [DOI] [PubMed] [Google Scholar]
- 83. Oliveira DB, Almeida FJ, Durigon EL, et al. Prolonged shedding of Zika virus associated with congenital infection. N Engl J Med 2016;375:1202–1204 [DOI] [PubMed] [Google Scholar]
- 84. Paulesu L, Romagnoli R, Cintorino M, et al. First trimester human trophoblast expresses both interferon-gamma and interferon-gamma-receptor. J Reprod Immunol 1994;27:37–48 [DOI] [PubMed] [Google Scholar]
- 85. Pierson TC, and Diamond MS. The emergence of Zika virus and its new clinical syndromes. Nature 2018;560:573–581 [DOI] [PubMed] [Google Scholar]
- 86. Pinto AK, Ramos HJ, Wu X, et al. Deficient IFN signaling by myeloid cells leads to MAVS-dependent virus-induced sepsis. PLoS Pathog 2014;10:e1004086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Platanias LC. Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat Rev Immunol 2005;5:375–386 [DOI] [PubMed] [Google Scholar]
- 88. Platt DJ, and Miner JJ. Consequences of congenital Zika virus infection. Curr Opin Virol 2017;27:1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Platt DJ, Smith AM, Arora N, et al. Zika virus-related neurotropic flaviviruses infect human placental explants and cause fetal demise in mice. Sci Transl Med 2018;10:eaao7090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Platt JS, and Hunt JS. Interferon-gamma gene expression in cycling and pregnant mouse uterus: temporal aspects and cellular localization. J Leukoc Biol 1998;64:393–400 [DOI] [PubMed] [Google Scholar]
- 91. Prokunina-Olsson L. Genetics of the human interferon lambda region. J Interferon Cytokine Res 2019. [Epub ahead of print]; DOI: 10.1089/jir.2019.0043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Quicke KM, Bowen JR, Johnson EL, et al. Zika virus infects human placental macrophages. Cell Host Microbe 2016;20:83–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Racicot K, Aldo P, El-Guindy A, et al. Cutting edge: fetal/placental type I IFN can affect maternal survival and fetal viral load during viral infection. J Immunol 2017;198:3029–3032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Rathore APS, Saron WAA, Lim T, et al. Maternal immunity and antibodies to dengue virus promote infection and Zika virus-induced microcephaly in fetuses. Sci Adv 2019;5:eaav3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Rayamajhi M, Humann J, Penheiter K, et al. Induction of IFN-alphabeta enables Listeria monocytogenes to suppress macrophage activation by IFN-gamma. J Exp Med 2010;207:327–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Roberts RM, Green JA, and Schulz LC. The evolution of the placenta. Reproduction 2016;152:R179–R189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Rossi M, Castiglioni P, Hartley MA, et al. Type I interferons induced by endogenous or exogenous viral infections promote metastasis and relapse of leishmaniasis. Proc Natl Acad Sci U S A 2017;114:4987–4992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Sanchez JM, Mathew DJ, Passaro C, et al. Embryonic maternal interaction in cattle and its relationship with fertility. Reprod Domest Anim 2018;53 Suppl 2:20–27 [DOI] [PubMed] [Google Scholar]
- 99. Sanchis A, Cervero L, Bataller A, et al. Genetic syndromes mimic congenital infections. J Pediatr 2005;146:701–705 [DOI] [PubMed] [Google Scholar]
- 100. Sang Y, Bergkamp J, and Blecha F. Molecular evolution of the porcine type I interferon family: subtype-specific expression and antiviral activity. PLoS One 2014;9:e112378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Schoggins JW. Interferon-stimulated genes: what do they all do? Annu Rev Virol 2019. [Epub ahead of print]; DOI: 10.1146/annurev-virology-092818-015756 [DOI] [PubMed] [Google Scholar]
- 102. Seferovic M, Sanchez-San Martin C, Tardif SD, et al. Experimental Zika virus infection in the pregnant common marmoset induces spontaneous fetal loss and neurodevelopmental abnormalities. Sci Rep 2018;8:6851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Senegas A, Villard O, Neuville A, et al. Toxoplasma gondii-induced foetal resorption in mice involves interferon-gamma-induced apoptosis and spiral artery dilation at the maternofoetal interface. Int J Parasitol 2009;39:481–487 [DOI] [PubMed] [Google Scholar]
- 104. Setoh YX, Peng NY, Nakayama E, et al. Fetal brain infection is not a unique characteristic of Brazilian Zika viruses. Viruses 2018;10:E541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Shimizu T, Ino T, Nishimoto K, et al. Advanced atrioventricular block in a neonate with lupus erythematosus and anti-SS-A antibodies. Pediatr Cardiol 1988;9:121–124 [DOI] [PubMed] [Google Scholar]
- 106. Smith DR, Sprague TR, Hollidge BS, et al. African and Asian Zika virus isolates display phenotypic differences both in vitro and in vivo. Am J Trop Med Hyg 2018;98:432–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Szaba FM, Tighe M, Kummer LW, et al. Zika virus infection in immunocompetent pregnant mice causes fetal damage and placental pathology in the absence of fetal infection. PLoS Pathog 2018;14:e1006994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Tripathi S, Balasubramaniam VR, Brown JA, et al. A novel Zika virus mouse model reveals strain specific differences in virus pathogenesis and host inflammatory immune responses. PLoS Pathog 2017;13:e1006258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Udenze D, Trus I, Berube N, et al. The African strain of Zika virus causes more severe in utero infection than Asian strain in a porcine fetal transmission model. Emerg Microb Infect 2019;8:1098–1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Vermillion MS, Lei J, Shabi Y, et al. Intrauterine Zika virus infection of pregnant immunocompetent mice models transplacental transmission and adverse perinatal outcomes. Nat Commun 2017;8:14575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Warner JD, Irizarry-Caro RA, Bennion BG, et al. STING-associated vasculopathy develops independently of IRF3 in mice. J Exp Med 2017;214:3279–3292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Weisblum Y, Oiknine-Djian E, Vorontsov OM, et al. Zika virus infects early- and midgestation human maternal decidual tissues, inducing distinct innate tissue responses in the maternal-fetal interface. J Virol 2017;91:pii: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Wichgers Schreur PJ, van Keulen L, Anjema D, et al. Microencephaly in fetal piglets following in utero inoculation of Zika virus. Emerg Microb Infect 2018;7:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Wu J, Chen YJ, Dobbs N, et al. STING-mediated disruption of calcium homeostasis chronically activates ER stress and primes T cell death. J Exp Med 2019;216:867–883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Yamamoto Y, Kurohmaru M, and Hayashi Y. Localization of type I interferon in murine trophoblast and decidua during decidual formation. J Reprod Fertil 1992;95:559–565 [DOI] [PubMed] [Google Scholar]
- 116. Yan N. Immune diseases associated with TREX1 and STING dysfunction. J Interferon Cytokine Res 2017;37:198–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Yockey LJ, and Iwasaki A. Interferons and proinflammatory cytokines in pregnancy and fetal development. Immunity 2018;49:397–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Yockey LJ, Jurado KA, Arora N, et al. Type I interferons instigate fetal demise after Zika virus infection. Sci Immunol 2018;3:eaao1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Yockey LJ, Varela L, Rakib T, et al. Vaginal exposure to Zika virus during pregnancy leads to fetal brain infection. Cell 2016;166:1247–1256. e1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Zani A, Zhang L, Kenney A, et al. IFITMs inhibit cell fusion mediated by trophoblast syncytins. BioArxiv 2019:DOI: 10.1101/713032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Zhou JZ, Way SS, and Chen K. Immunology of uterine and vaginal mucosa. Trends Immunol 2018;39:355. [DOI] [PMC free article] [PubMed] [Google Scholar]