Abstract
Generally, changes in the metabolic status of cells under conditions like hypoxia and accumulation of lactate can be sensed by various sensing mechanisms, leading to modulation of a number of signal transduction pathways and transcription factors. Several of the proangiogenic cytokines like VEGF, FGF, PDGF, TGF-β, Ang-2, ILs, etc. are secreted by cancer cells, under hypoxic microenvironment. These cytokines bind to their receptors on the endothelial cells and activates a number of signaling pathways including Akt/PIP3, Src, p38/MAPK, Smad2/3, etc., which ultimately results in the proliferation and migration of endothelial cells. Transcription factors that are activated in response to the metabolic status of tumors include HIFs, NF-κb, p53, El-2, and FOXO. Many of these transcription factors has been reported to be regulated by a class of histone deacetylase called sirtuins. Sirtuins are NAD+ dependent histone deacetylases that play pivotal role in the regulation of tumor cell metabolism, proliferation, migration and angiogenesis. The major function of sirtuins include, deacetylation of histones as well as some non-histone proteins like NF-κB, FOXOs, PPAR⋎, PGC1-α, enzymes like acetyl coenzymeA and structural proteins like α tubulin. In the cell, sirtuins are generally considered as the redox sensors and their activities are dependent on the metabolic status of the cell. Understanding the intricate regulatory mechanisms adopted by sirtuins, is crucial in devising effective therapeutic strategies against angiogenesis, metastasis and tumor progression. Keeping this in mind, the present review focuses on the role of sirtuins in the process of tumor angiogenesis and the regulatory mechanisms employed by them.
Keywords: sirtuins, tumor angiogenesis, metabolism, histone deacetylases, endothelial cells, signaling pathways
Introduction
Angiogenesis, the process of formation of new blood vessels from pre-existing ones, is essential for the normal growth, development and wound healing. Apart from this, angiogenesis is also inevitable for tumor growth and metastasis (1–5). The expression and secretion of various modulators of angiogenesis is regulated by microenvironmental factors like hypoxia and accumulation of different metabolites (6–8). Under conditions like hypoxia, a number of signal transduction pathways and transcription factors like PPARα, PGC-1α, AMPK, FOXOs, etc. gets activated (9, 10). The expression and activation of these transcription factors has been reported to be regulated by a class of histone deacetylase called sirtuins or SIRT (11, 12). Sirtuins are NAD+ dependent histone deacetylases that play a vital role in the regulation of metabolism, aging, oncogenesis, angiogenesis and cancer progression (13, 14). It has been reported that SIRT1 can function as a redox sensor, and its activity might be dependent on the overall metabolic status of the cell (15), since it has been shown to regulate the stabilization of transcription factors such as HIF1α under hypoxic conditions (16). Therefore, understanding the regulatory mechanisms employed by sirtuins to modulate tumor angiogenesis is essential for developing effective anti-cancer and anti-angiogenic therapeutic strategies.
Sirtuins: Mechanism of Action and Classification
In mammals, seven homologs of sirtuins, i.e., SIRT1–SIRT7 (17, 18) which were initially described as class III HDACs (Histone deacetylases), are now known as class III KDACS (Lysine deacetylases) (19). The proposed mechanism of Sirtuin deacetylation is reported to be ADPR-peptidyl-imidate (20) where, Sirtuins catalyze NAD+-dependent deacetylation of acetyl lysine, producing nicotinamide, deacetylated lysine, and 2′-O-acetyl-ADP-ribose (21). The major function of sirtuins involve, removal of acetyl groups from the acetyl lysine-modified proteins (22, 23). The reaction gets initiated when, NAD+ binds to the catalytic site of sirtuin, with the C1 of NAD+ getting placed at the channel junction that, houses the acetyl lysine (24). To understand how increasing levels of NAD+ affects sirtuin activity, NAD+ synthesis was enhanced by supplementing different precursors for NAD+ like nicotinic acid to Preiss-Handler pathway and the result showed increased activation of sirtuins and other enzymes which are NAD+ dependent (25). Cellular [NAD+]/[NADH] ratio is reported to control deacetylase activity of the sirtuins where, NAD+ works as activator, and both nicotinamide and reduced nicotinamide adenine dinucleotide (NADH) acts as inhibitors (26, 27). Sirtuins (SIRT1-3, 5, and 7) catalyze deacetylation reaction on lysine residues of target proteins (28) whereas, SIRT4 and SIRT6 catalyze ADP-ribosylation reaction, by transferring ribosyl moiety to the substrates (29). Sirtuins carry out transcriptional repression where acetylated histones H1, H3, and H4 act as substrates (30). In addition, a number of non-histone proteins like nuclear factor-κB (NFκB), forkhead box type O transcription factors (FOXO), peroxisome proliferator-activated receptor ⋎ (PPAR⋎), coactivator 1α (PGC-1α), enzymes like acetyl coenzyme A (CoA) synthetase 2 (AceCS2), and structural proteins, such as α-tubulin are also deacetylated by sirtuins (29). In contrast to other KDACs, whose only function include deacetylation (31), sirtuins can also remove other groups like glutaryl (32), crotonyl (33), succinyl (34), palmitoyl (35), and myristoyl (36) groups. SIRT1-3 is reported to deacylate hydrophobic (butyryl group) and SIRT5, acidic acyl group (Malonyl group) in histones (37, 38). Also, some non-histone proteins like IDH2, MnSOD and TNFα have been reported to be deacylated by sirtuins (39). It is observed that SIRT4 has both, deacylase activity in leucine metabolism and lipoamidase activity in decarboxylation of pyruvate, to generate acetyl CoA (40, 41). The intracellular distribution of sirtuins differs. While SIRT1, 6 and 7 are located within the nucleus, SIRT2 is located in the cytoplasm and SIRT 3, 4, and 5 are located within mitochondria (42). In addition, SIRT1 and SIRT3 are known to shuttle to cytoplasm and nucleus, respectively (43, 44). These findings establish sirtuins as important players in epigenetic gene regulations.
Sirtuins in Endothelial Cell Functions
Different classes of sirtuins have been widely studied in endothelial cell growth and maintenance (45–47). While, blocking the function of SIRT1 reduced endothelial sprout formation, migration and the assembly of primitive vascular network (14) it was observed that, knockdown of SIRT1 altered the levels of sprouting angiogenesis due to reduction of MMP14 expression (48, 49). Potente et al. reported that SIRT1 deacetylates FOXO1, a negative regulator of angiogenesis, as SIRT1- deficient ECs showed abnormal angiogenic behavior due to FOXO1 activity (14). Nutrient deprivation and cellular energy shortage increase the levels of NAD+ and thus the expression and activation of sirtuins (50). It was observed that, endothelial tip cells employ anaerobic glycolysis for generating ATP (Warburg effect) rather than oxidative phosphorylation (51). In addition to promoting endothelial cell proliferation and angiogenesis, this makes ECs more resistant to hypoxia too (52, 53). Recent studies have established that SIRT1 modulates tip and stalk behavior through deacetylation of intracellular domain (NICD) of NOTCH1 in tumor associated endothelial cells (54). Interestingly, sirtuins also regulate, endothelial homeostasis by modulating the endothelial nitric oxide synthase (eNOS) (55). Recent studies reveal that endothelial SIRT1 deficiency, causes fibrosis due to aberrant secretion of ligands of Wnt and Notch pathways, as well as proteolytic fragments of glycocalyx core protein (56). Some studies also reported that SIRT1 can mediate transcriptional repression in association with Hey2 and Hes1 during vascular development (57, 58). In ECs, over expression of SIRT1 prevents cellular senescence, enhances vasodilatory responses, and alleviates aging-induced vascular impairment (59, 60) whereas, SIRT1 deficiency results in reduced migration in response to chemoattractant (14). In addition to SIRT1, SIRT2 is reported to regulate the survival and energy metabolism of ECs. Studies by Zhang et al. demonstrated that SIRT2 inhibition reduce the survival rate of PIEC cells as it causes mitochondrial depolarization (61). SIRT2 is also reported to promote Ang II-induced cytoskeletal remodeling in ECs (62). In addition, SIRT2 knock down studies revealed altered expression of migration associated genes like CALD1 (caldesmon) and CNN2 (calponin) (63, 64). SIRT3 was observed to increase survival of ECs especially during hypoxia through elevating the levels of deacetylation of FOXO3 (65). Recent studies also revealed that in SIRT3 deficient endothelial cells the expression of PFKFB3 was downregulated causing attenuation of glycolysis and angiogenesis (66). SIRT4 however appears to inhibit mononuclear cell adhesion to pulmonary microvascular endothelial cells through repression of E-selectin and VCAM-1 (67). While, angiogenic capacity of endothelial progenitor cells were significantly reduced due to down regulation of CXCR4/JAK2/SIRT5 signaling (68), it was observed that, SIRT6 protects endothelial cells from DNA damage and telomere dysfunction (62, 69). Though various members of the SIRT family has been implicated in the regulation of EC biology, the role of SIRT-1 is the most widely studied ones.
Sirtuins in Tumor Angiogenesis and their Regulatory Strategies
Expression pattern of sirtuins varies in different types of cancers. While, SIRT1, 4, 5, and 7 have been reported to be upregulated in certain cancers (70–72), the same SIRT1as well as SIRT2 and SIRT6 is shown to be downregulated in breast cancer, hepatic cell carcinoma (73), gliomas, gastric carcinomas (74, 75) and colon adenocarcinoma (76). SIRT1 mainly, mediates heterochromatin formation by deacetylation of histone H1 K26, histone H3 K9 and histone H4 K16, thereby causing deacetylation of non-histone proteins, like transcription factors (E2F1, p53, FOXO, BCL6, p53, Rb), DNA repair proteins and signaling factors (77). SIRT1 mediates regulation of gene expression in response to metabolic status by modulating FOXOs (78). Such a deacetylation of FOXOs by SIRT1 alters various signaling pathways, inhibit apoptosis and regulates mechanisms involved in oxidative stress (79, 80). In general, p53 negatively regulates angiogenesis either, by increasing the production of anti-angiogenic factors or inhibiting pro-angiogenic factors (81). SIRT1 has been reported to regulate neovascularization, through reducing the transcriptional activity of p53 by deacetylation of lysine (45, 46, 82). Apart from SIRT1, SIRT3 and SIRT7 has also been reported to deacetylate p53 thus, negating p53 activity (83, 84). SIRT1 also deacetylates other transcription factors like p73, E2F1, SMAD 7, NFKB and modulate apoptosis and inflammatory responses (45, 46, 85). It was observed that resveratrol, a SIRT1 activator reduced total VEGFR2 expression and inhibited phosphorylation of VEGFR2 by VEGF (86). Also, SIRT1 negatively modulates Delta-like ligand 4 (DLL4)/Notch pathway, inactivates elongation factor2 through activation of ELF2kinase and ultimately inhibits the proliferation and migration of vascular endothelial cells (87, 88). It is reported that SIRT1 deacetylation at K14 and K20 of PH domain is necessary for binding of Akt to PIP3 and further activation during tumor angiogenesis (89, 90). Several reports suggest that, SIRT1 deacetylate eNOS, stimulate its activity and enhance NO production and tumor angiogenesis (91, 92). Also, FOXO1 and FOXO3 have been reported to repress eNOS, suggesting a link between SIRT1, FOXO and eNOS (93). Increase in SIRT1 deacetylase activity and a consecutive HIF2α activity in ECs, results in acidification and reprogramming toward glutamine metabolism during induction of angiogenesis (94, 95). Studies by Kunhiraman et al. and Edatt et al. reveal that glycolytic inhibition using 2-DG at a sublethal concentration increased the expression and activity of SIRT1, causing reduced expression of angiogenesis associated genes like VEGF and MMP9 (96, 97). Contrary to these reports, Portmann et al., Li et al., and Suzuki et al., report that SIRT1 and VEGF expression is positively correlated during hypoxia induced angiogenesis in breast cancer and lung cancer (98–100). It therefore appears that, the SIRT-1 mediated regulation of angiogenesis and factors regulating it, is largely context dependent.
Like SIRT1, SIRT2 also deacetylate proteins like α-tubulin and histones, being co-localized with tubulin (101–103). Hu et al., demonstrated that SIRT2 knockdown prevented STAT3 phosphorylation and translocation to nucleus, thus decreasing the secretion of VEGF (104, 105). In addition, SIRT2 is reported to directly interact with β-catenin thereby altering the expression of genes like MMPs during tumor angiogenesis (106, 107). Also, it is observed that, SerRS (seryl-tRNA synthetase) plays tumor suppressor and anti-angiogenic role by collaborating with SIRT2 to antagonize c-Myc, a known angiogenic and oncogenic gene (108). Another class of sirtuins, SIRT3 is reported to mediate deacetylation of histones, regulate the stability of tubulin polymers (44, 109, 110), mediate induction of uncoupling protein−1 and regulate Acetyl-CoA synthetase activity (111, 112). Contrary to SIRT1 and SIRT2, SIRT3 is reported to have an opposing effect on angiogenesis, as loss of SIRT3 in human breast cancers, resulted in the upregulation of HIF-1α target genes like VEGF and genes involved in glycolysis (113, 114). Interestingly, it was observed that, SIRT3 overexpression reduced angiogenesis by negatively regulating ROS production, glycolysis as well as HIF-1α stabilization, ultimately resulting in a negative regulation of Warburg effect (115). SIRT4, 5, and 6 has been majorly reported to carry out ADP-ribosylation, desuccinylation and demalonylation rather that deacetylation (34, 116–118). ADP-ribosylation, regulate the activity of glutamate dehydrogenase and PARP (119, 120). Studies from our lab and others has demonstrated that PARPs can regulate the VEGF/VEGFR2 signaling circuit by either transactivation of VEGFR2 or poly ADP ribosylating VEGF to reduce its activity (7, 96, 121). Desuccinylation by SIRT5 suppresses the activities of pyruvate dehydrogenase complex and succinate dehydrogenase (117) leading to the accumulation of succinate and mitochondrial reactive oxygen species, thereby activating HIF1α (122). SIRT5 can also cause desuccinylation and negative regulation of S100A10, a protein that regulate invasion and motility (123). Generally, NAD+ levels influence the secretion of various cytokines by inflammatory cells (124). It was found that SIRT6 over expression in pancreatic cancer cells increased TNFα and IL8 production through ADP-ribosylation mediated Ca2+ responses (125) and elevated levels of IL8 led to local inflammation, angiogenesis, and EMT (126). Interestingly, Kawahara et al. demonstrated SIRT6 interaction with RELA subunit of NFκB to regulate the expression of its target genes involved in tumor progression through deacetylation of promoter region (127, 128). SIRT6 is also reported as a corepressor of HIF1α by deacetylating H3K9 causing downregulation of the expression of genes involved in energy metabolism (129). Furthermore, it is observed that SIRT7 can inhibit HIF1α through a mechanism that is independent of its catalytic activity and regulate the expression of downstream genes like VEGF A and erythropoeitin (130). Also, downregulation of SIRT7 during breast cancer lung metastasis, caused activation of TGFβ signaling pathway and angiogenesis (131). Contrary to this, SIRT7 has been reported to promote angiogenic response by modulating endothelial cell function and VEGF like growth factor expression in mice (132). Altogether these contradicting roles played by sirtuins in tumorigenesis and angiogenesis, highlights the epigenetic regulations involved and unravels the therapeutic potential of sirtuin modulators in treatment of tumor progression by targeting tumor angiogenesis (Table 1).
Table 1.
The substrates and pathways regulated by different classes of sirtuins.
| Sirtuin | Enzyme activity | Substrates | Pathway Regulated | References |
|---|---|---|---|---|
| SIRT1 | Deacetylase | Histone, p53, FOXO, Rb, p300, PPARγ, NF-κB, PGC-1α, UCP2, MnSOD, Acetyl-CoAsynthetase 1, Smad7, eNOS | Cell survival, metabolism regulation, lifespan regulation, inflammation, oxidative stress response | (14, 46, 133–142) |
| SIRT2 | Deacetylase | α-tubulin, Histone, FOXO, β-catenin | Cell cycle regulation, nervous system development | (101–103, 107) |
| SIRT3 | Deacetylase | Histone, FOXO3a, Acetyl-CoA synthetase2, MnSOD | Regulation of mitochondrial metabolism, ATP-production fatty acid oxidation | (83, 111, 112, 143) |
| SIRT4 | ADP- ribosyl transferase/Deacylase |
Glutamate dehydrogenase | Regulation of mitochondrial metabolism, insulin secretion | (29, 144) |
| SIRT5 | Deacetylase Demalonylase Desuccinylase |
Cytochrome c, Carbamoyl phosphate synthetase 1 | Apoptosis, urea cycle, regulation of protein-protein interaction, protein stability | (32, 34, 37, 38, 145) |
| SIRT6 | Deacetylase /Deacylase ADP-ribosyl-transferase |
Histone, HIF1α, TNF-α, NFκB | Genome stability, DNA repair | (29, 35, 118, 127) |
| SIRT7 | Deacetylase | Histone, p53 | Regulation of rRNA transcription, cell cycle regulation | (130, 146) |
Post Transcriptional Regulation of Sirtuins: Importance of miRNAs in Tumor Angiogenesis
Along with cytokine and transcriptional factor mediated regulation, precise and effective post transcriptional level regulation are also employed by sirtuins through RNA binding proteins (RBPs) and small non-coding RNA molecules. Micro RNAs are a group of small non-coding RNAs, known as the micro regulators of gene expression. For e.g., miR-34a has been reported to retard endothelial progenitor cell (EPC) mediated angiogenesis by targeting SIRT1 and thereby elevating the levels of acetylated FOXO1, leading to endothelial cell (EC) senescence and cell cycle arrest (147, 148). Similarly, miR-217 has been reported to induce senescence of ECs by modulating the levels of acetylated FOXO1 in a SIRT1 dependent mechanism (149). However, miR-217 has also been reported to promote angiogenesis of Human cytomegalovirus infected endothelial cells by inhibiting SIRT1 and FOXO3A (150). Further, report from our group suggests that miR-106a regulates the expression of MMP9 during cell migration by directly targeting SIRT1 mRNA (151). Our group has also reported that the horizontal transfer of miR-23a from tumor cell colonies can induces angiogenesis by targeting SIRT1 in the recipient endothelial cells (152). Further, miR-212 has been reported to exhibit anti-angiogenic properties, by targeting SIRT1 and Gab1 in endothelial cells (153). SIRT1 has also been reported to inhibit the anti-angiogenic- Notch signaling pathway (54). In addition, TGFβ mediated suppression of SIRT1 expression leading to the activation of Notch signaling pathway in ECs was reported to be depended partly on miR-212 (153). Further, a key micro regulator of angiogenesis and hypoxia responses, miR-138-P_5P has been reported to target SIRT1 (154–156).
Conclusion: Future Perspectives and Novel Therapeutic Approaches
During the past decade, sirtuins have emerged as critical regulators of endothelial cell behavior and have been directly linked to tumor angiogenesis through multiple signaling pathways and cross-talks (Figure 1). Lack of long-term therapeutic efficacy of current anti-angiogenic strategies requisite for novel angiogenesis inhibitors targeting sirtuins (157, 158). Recent discoveries suggest that employing sirtuin isoform specific modulators is a potent anti-angiogenic strategy. Endothelial microparticles enriched with Sirt6 mRNA induces EC angiogenesis, increases eNOS phosphorylation and prevents release of inflammatory chemokines in diabetic patients (159). Novel approaches like employing various metal (160–162) and inorganic NPs (163–165) have been reported to modulate angiogenesis. Many studies revealed that the shape, size and surface charge of the nanoparticles plays a crucial role in their angiogenic behavior (166, 167). Recently our group has reported that carbon-based nanoparticles (carbon quantum dots) with size <6 nm, inhibit angiogenic process and significantly reduce the expression level of VEGF, VEGFR2, and FGF (168). SirtuinsNano-particle based phytochemicals are reported to regulate sirtuins in cardioprotective treatment strategies (169). So far, no reports are available on the direct correlation with nano particles targeting sirtuins in tumor angiogenesis. Mechanistic studies are under progress on the development of NPs targeting sirtuins and further, tumor angiogenesis. Future studies that unveil the role of potent sirtuin modulators like CQDs at the crossroads of tumor angiogenesis will provide insights for designing novel anti-angiogenic therapies targeting sirtuin.
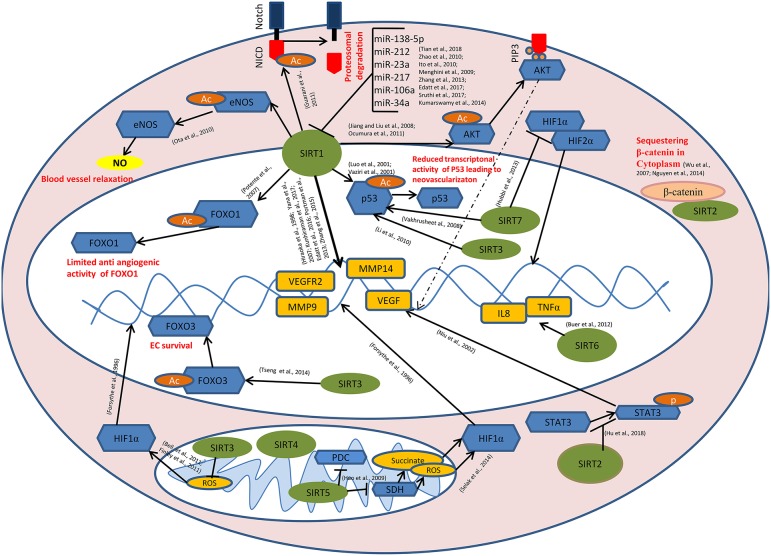
Figure 1.
Role of sirtuins in Tumor Angiogenesis: SIRT1 mediates deacetylation of FOXO1, p53, AKT, eNOS, and the intra cellular domain of the Notch protein (NICD) leading to the reduced anti-angiogenic activity of FOXO1, reduced transcriptional activity of p53, induction of AKT signaling causing the transcriptional activation of pro angiogenic genes, enhanced endothelial NO production causing blood vessel relaxation and disassembly followed by the proteasomal degradation of Notch protein respectively. SIRT1 also modulates the expression of VEGF, VEGFR2, MMP9, MMP14, etc. directly by its histone deacetylase activity. miR-34a, miR-106a, miR-217, miR-23a, miR-212, and miR-138-5p targets SIRT1 at post transcriptional level. SIRT3 and SIRT7 catalyze the deacetylation of p53. SIRT7 inhibits HIF-1α stabilization and hence its nuclear translocation. Binding of SIRT2 with β-catenin leads to the sequestration of β-catenin in the cytoplasm, causing modulation in the expression of β-catenin responsive genes including MMPs. SIRT6 mediates the transcriptional activation of IL8 and TNFα which, in turn positively modulates tumor angiogenesis. SIRT2 inhibits STAT3 phosphorylation and its nuclear translocation. SIRT5 inhibits pyruvate dehydrogenase complex (PDC) and succinate dehydrogenase (SDH) causing the accumulation of succinate and reactive oxygen species (ROS) in the mitochondria, leading to HIF-1α activation. SIRT3 negatively regulates mitochondrial ROS production and hence HIF-1α stabilization. SIRT3 mediates deacetylation of FOXO3, thereby promoting endothelial cell (EC) survival under hypoxia.  - Sirtuins,
- Sirtuins,  - transcription factors/enzymes/signaling molecules,
- transcription factors/enzymes/signaling molecules,  - downstream genes,
- downstream genes,  - acetyl(Ac)/phosphate(p) group,
- acetyl(Ac)/phosphate(p) group,  - β-catenin,
- β-catenin,  - Nitric oxide,
- Nitric oxide,  - Succinate/reactive oxygen species (ROS).
- Succinate/reactive oxygen species (ROS).
Author Contributions
LE and VK contributed to conception and manuscript writing. VR, GR, and SS searched the literature. AP collected data and designed the scheme for the regulation of tumor angiogenesis by sirtuins. LE and VK participated in its coordination and modification. All the authors have read and approved the final manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Financial assistance in the form of fellowships to LE, AP, GR, VR, and SS, received from DST-SERB, DBT, and KSCSTE is gratefully acknowledged.
References
- 1.Stephenson JA, Goddard JC, Taan OA, Dennison AR, Morgan B. Tumour angiogenesis: a growth area—from John Hunter to Judah Folkman and beyond. J Cancer Res. (2013) 2013:895019 10.1155/2013/895019 [DOI] [Google Scholar]
- 2.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. (2000) 407:249–57. 10.1038/35025220 [DOI] [PubMed] [Google Scholar]
- 3.Folkman J. Tumor angiogenesis: from bench to bedside. In: Marmé D, Fusenig N. editors. Tumor Angiogenesis. Berlin; Heidelberg: Springer; (2008). p. 3–28. [Google Scholar]
- 4.Hall AP. The role of angiogenesis in cancer. Comp Clin Path. (2005) 13:95–9. 10.1007/s00580-004-0533-3 [DOI] [Google Scholar]
- 5.Hunter J. (1861). Essays and Observations on Natural History, Anatomy, Physiology, and Geology. London: Van Voorst. [Google Scholar]
- 6.Ohno-Matsui K, Hirose A, Yamamoto S, Saikia J, Okamoto N, Gehlbach P, et al. Inducible expression of vascular endothelial growth factor in adult mice causes severe proliferative retinopathy and retinal detachment. Am J Pathol. (2002) 160:711–9. 10.1016/S0002-9440(10)64891-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kumar VBS, Viji RI, Kiran MS, Sudhakaran PR. Endothelial cell response to lactate: implication of PAR modification of VEGF. J Cell Physiol. (2007) 211:477–85. 10.1002/jcp.20955 [DOI] [PubMed] [Google Scholar]
- 8.Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD, et al. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol. (1996) 16:4604–13. 10.1128/MCB.16.9.4604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kumar VBS, Binu S, Soumya SJ, Haritha K, Sudhakaran PR. Regulation of vascular endothelial growth factor by metabolic context of the cell. Glycoconjugate J. (2014) 31–7, 427–434. 10.1007/s10719-014-9547-5 [DOI] [PubMed] [Google Scholar]
- 10.Jensen KS, Binderup T, Jensen KT, Therkelsen I, Borup R, Nilsson E, et al. FoxO3A promotes metabolic adaptation to hypoxia by antagonizing Myc function. EMBO J. (2011) 30:4554–70. 10.1038/emboj.2011.323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maher JC, Wangpaichitr M, Savaraj N, Kurtoglu M, Lampidis TJ. Hypoxia-inducible factor-1 confers resistance to the glycolytic inhibitor 2-deoxy-D-glucose. Mol Cancer Ther. (2007) 6:732–41. 10.1158/1535-7163.MCT-06-0407 [DOI] [PubMed] [Google Scholar]
- 12.Balaiya S, Ferguson LR, Chalam KV. Evaluation of sirtuin role in neuroprotection of retinal ganglion cells in hypoxia. Investig Ophthalmol Vis Sci. (2012) 53:4315–22. 10.1167/iovs.11-9259 [DOI] [PubMed] [Google Scholar]
- 13.Balcerczyk A, Pirola L. Therapeutic potential of activators and inhibitors of sirtuins. Biofactors. (2010) 36:383–93. 10.1002/biof.112 [DOI] [PubMed] [Google Scholar]
- 14.Potente M, Ghaeni L, Baldessari D, Mostoslavsky R, Rossig L, Dequiedt F, et al. SIRT1 controls endothelial angiogenic functions during vascular growth. Genes Dev. (2007) 21:2644–58. 10.1101/gad.435107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aguilar AL, Ranjit S, Stringari C, OrozcoSR, Gratton E, Sassone CP. Spatial dynamics of SIRT1 and the subnuclear distribution of NADH species. Proc Natl Acad Sci USA. (2016) 113:12715–20. 10.1073/pnas.1609227113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rane S, He M, Sayed D, Vashistha H, Malhotra A, Sadoshima J, et al. Downregulation of miR-199a derepresses hypoxia-inducible factor-1α and Sirtuin 1 and recapitulates hypoxia preconditioning in cardiac myocytes. Circ Res. (2009) 104:879–86. 10.1161/CIRCRESAHA.108.193102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frye RA. Characterization of five human cDNAs with homology to the yeast SIR2 gene: Sir2-like proteins (sirtuins) metabolize NAD and may have protein ADP-ribosyltransferase activity. Biochem Biophys Res Commun. (1999) 260:273–9. 10.1006/bbrc.1999.0897 [DOI] [PubMed] [Google Scholar]
- 18.Frye RA. Phylogenetic classification of prokaryotic and eukaryotic Sir2-like proteins. Biochem Biophys Res Commun. (2000) 273:793–8. 10.1006/bbrc.2000.3000 [DOI] [PubMed] [Google Scholar]
- 19.Ruijter AJM, van Gennip AH, Caron HN, Kemp S, van Kuilenburg ABP. Histone deacetylases (HDACs): characterization of the classical HDAC family. Biochem J. (2003) 370:737–49. 10.1042/bj20021321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sauve AA, Wolberger C, Schramm VL, Boeke JD. The biochemistry of sirtuins. Annu Rev Biochem. (2006) 75:435–65. 10.1146/annurev.biochem.74.082803.133500 [DOI] [PubMed] [Google Scholar]
- 21.Jackson MD, Denu JM. Structural identification of 2′-and 3′-O-acetyl-ADP-ribose as novel metabolites derived from the Sir2 family of β-NAD+-dependent histone/protein deacetylases. J Biol Chem. (2002) 277:18535–44. 10.1074/jbc.M200671200 [DOI] [PubMed] [Google Scholar]
- 22.Imai SI, Armstrong CM, Kaeberlein M, Guarente L. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature. (2000) 403:795–800. 10.1038/35001622 [DOI] [PubMed] [Google Scholar]
- 23.Landry J, Sutton A, Tafrov ST, Heller RC, Stebbins J, Pillus L, et al. The silencing protein SIR2 and its homologs are NAD-dependent protein deacetylases. Proc Natl Acad Sci USA. (2000) 97:5807–11. 10.1073/pnas.110148297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Min J, Landry J, Sternglanz R, Xu RM. Crystal structure of a SIR2 homolog–NAD complex. Cell. (2001) 105:269–79. 10.1016/S0092-8674(01)00317-8 [DOI] [PubMed] [Google Scholar]
- 25.Frothingham R, Meeker OCWA, Talbot EA, George JW, Kreuzer KN. Identification, cloning, and expression of the Escherichia coli pyrazinamidase and nicotinamidase gene, pncA. Antimicrob Agents Chemother. (1996) 40:1426–31. 10.1128/AAC.40.6.1426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anderson RM, Bitterman KJ, Wood JG, Medvedik O, Sinclair DA. Nicotinamide and PNC1 govern lifespan extension by calorie restriction in Saccharomyces cerevisiae. Nature. (2003) 423:181–5. 10.1038/nature01578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin SJ, Defossez PA, Guarente L. Requirement of NAD and SIR2 for life-span extension by calorie restriction in Saccharomyces cerevisiae. Science. (2000) 289:2126–8. 10.1126/science.289.5487.2126 [DOI] [PubMed] [Google Scholar]
- 28.Vaquero A. The conserved role of sirtuins in chromatin regulation. Int J Dev Biol. (2009) 53–3, 303–322. 10.1387/ijdb.082675av [DOI] [PubMed] [Google Scholar]
- 29.Yamamoto H, Schoonjans K, Auwerx J. Sirtuin functions in health and disease. Mol Endocrinol. (2007) 21:1745–55. 10.1210/me.2007-0079 [DOI] [PubMed] [Google Scholar]
- 30.Liou GG, Tanny JC, Kruger RG, Walz T, Moazed D. Assembly of the SIR complex and its regulation by O-acetyl-ADP-ribose, a product of NAD-dependent histone deacetylation. Cell. (2005) 121:515–27. 10.1016/j.cell.2005.03.035 [DOI] [PubMed] [Google Scholar]
- 31.Madsen AS, Olsen CA. Profiling of substrates for zinc-dependent lysine deacylase enzymes: HDAC3 exhibits decrotonylase activity in vitro. Angew Chem. (2012) 124:9217–21. 10.1002/ange.201203754 [DOI] [PubMed] [Google Scholar]
- 32.Tan M, Peng C, Anderson KA, Chhoy P, Xie Z, Dai L, et al. Lysine glutarylation is a protein posttranslational modification regulated by SIRT5. Cell Metab. (2014) 19:605–17. 10.1016/j.cmet.2014.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bao X, Wang Y, Li X, Li X, Liu Z, Yang T, et al. Identification of ‘erasers' for lysine crotonylated histone marks using a chemical proteomics approach. Elife. (2014) 3:e02999. 10.7554/eLife.02999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Du J, Zhou Y, Su X, Yu JJ, Khan S, Jiang H, et al. Sirt5 is a NAD-dependent protein lysine demalonylase and desuccinylase. Science. (2011) 334:806–9. 10.1126/science.1207861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jiang H, Khan S, Wang Y, Charron G, He B, Sebastian C, et al. SIRT6 regulates TNF-α secretion through hydrolysis of long-chain fatty acyl lysine. Nature. (2013) 496:110–3. 10.1038/nature12038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Feldman JL, Baeza J, Denu JM. Activation of the protein deacetylase SIRT6 by long-chain fatty acids and widespread deacylation by mammalian sirtuins. J Biol Chem. (2013) 288:31350–6. 10.1074/jbc.C113.511261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Feldman JL, Kristin E, Dittenhafer-Reed KN, Thelen JN, Ito A, Yoshida M, et al. Kinetic and structural basis for acyl-group selectivity and NAD+ dependence in sirtuin-catalyzed deacylation. Biochemistry. (2015) 54:3037–50. 10.1021/acs.biochem.5b00150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peng C, Lu Z, Xie Z, Cheng Z, Chen Y, Tan M, et al. The first identification of lysine malonylation substrates and its regulatory enzyme. Mol Cell Proteomics. (2011) 10:M111.012658. 10.1074/mcp.M111.012658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen B, Zang W, Wang J, Huang Y, He Y, Yan L, et al. The chemical biology of sirtuins. Chem Soc Rev. (2015) 44:5246–64. 10.1039/C4CS00373J [DOI] [PubMed] [Google Scholar]
- 40.Anderson KA, Huynh FK, Fisher WK, Stuart JD, Peterson BS, Douros JD, et al. SIRT4 is a lysine deacylase that controls leucine metabolism and insulin secretion. Cell Metab. (2017) 25:838–55. 10.1016/j.cmet.2017.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mathias RA, Greco TM, Oberstein A, Budayeva HG, Chakrabarti R, Rowland EA, et al. Sirtuin 4 is a lipoamidase regulating pyruvate dehydrogenase complex activity. Cell. (2014) 159:1615–25. 10.1016/j.cell.2014.11.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jing H, Lin H. Sirtuins in epigenetic regulation. Chem Rev. (2015) 115:2350–75. 10.1021/cr500457h [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jin Q, Yan T, Ge X, Sun C, Shi X, Zhai Q. Cytoplasm-localized SIRT1 enhances apoptosis. J Cell Physiol. (2007) 213:88–97. 10.1002/jcp.21091 [DOI] [PubMed] [Google Scholar]
- 44.Scher MB, Vaquero A, Reinberg D. SirT3 is a nuclear NAD+-dependent histone deacetylase that translocates to the mitochondria upon cellular stress. Genes Dev. (2007) 21:920–8. 10.1101/gad.1527307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vaziri H, Dessain SK, Eaton EN, Imai SI, Frye RA, Pandita TK, et al. hSIR2SIRT1 functions as an NAD-dependent p53 deacetylase. Cell. (2001) 107:149–59. 10.1016/S0092-8674(01)00527-X [DOI] [PubMed] [Google Scholar]
- 46.Luo J, Nikolaev AY, Imai SI, Chen D, Su F, Shiloh A, et al. Negative control of p53 by Sir2α promotes cell survival under stress. Cell. (2001) 107:137–48. 10.1016/S0092-8674(01)00524-4 [DOI] [PubMed] [Google Scholar]
- 47.Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y, et al. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. (2004) 303:2011–5. 10.1126/science.1094637 [DOI] [PubMed] [Google Scholar]
- 48.Hiraoka N, Allen E, Apel IJ, Gyetko MR, Weiss SJ. Matrix metalloproteinases regulate neovascularization by acting as pericellular fibrinolysins. Cell. (1998) 95:365–77. 10.1016/S0092-8674(00)81768-7 [DOI] [PubMed] [Google Scholar]
- 49.Yana I, Sagara H, Takaki S, Takatsu K, Nakamura K, Nakao K, et al. Crosstalk between neovessels and mural cells directs the site-specific expression of MT1-MMP to endothelial tip cells. J Cell Sci. (2007) 120:1607–14. 10.1242/jcs.000679 [DOI] [PubMed] [Google Scholar]
- 50.Cantó C, Houtkooper RH, Pirinen E, Youn DY, Oosterveer MH, Cen Y, et al. The NAD+ precursor nicotinamide riboside enhances oxidative metabolism and protects against high-fat diet-induced obesity. Cell Metab. (2012) 15:838–47. 10.1016/j.cmet.2012.04.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Culic O, Gruwel ML, Schrader J. Energy turnover of vascular endothelial cells. Am J Physiol Cell Physiol. (1997) 273:205–13. 10.1152/ajpcell.1997.273.1.C205 [DOI] [PubMed] [Google Scholar]
- 52.De BK, Georgiadou M, Schoors S, Kuchnio A, Wong BW, Cantelmo AR, et al. Role of PFKFB3-driven glycolysis in vessel sprouting. Cell. (2013) 154:651–63. 10.1016/j.cell.2013.06.037 [DOI] [PubMed] [Google Scholar]
- 53.Fitzgerald GA, Soro AI, De Bock K. The Warburg effect in endothelial cells and its potential as an anti-angiogenic target in cancer. Front Cell Dev Biol. (2018) 6:100–17. 10.3389/fcell.2018.00100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Guarani V, Deflorian G, Franco CA, Krüger M, Phng LK, Bentley K, et al. Acetylation-dependent regulation of endothelial Notch signalling by the SIRT1 deacetylase. Nature. (2011) 473:234–8. 10.1038/nature09917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wallerath T, Li H, Ambrust UG, Schwarz PM, Förstermann U. A blend of polyphenolic compounds explains the stimulatory effect of red wine on human endothelial NO synthase. Nitric Oxide. (2005) 12:97–104. 10.1016/j.niox.2004.12.004 [DOI] [PubMed] [Google Scholar]
- 56.Lipphardt M, Dihazi H, Müller GA, Goligorsky M. Fibrogenic secretome of sirtuin 1-deficient endothelial cells: Wnt, notch and glycocalyx rheostat. Front Physiol. (2018) 9:1325. 10.3389/fphys.2018.01325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Takata T, Ishikawa F. Human Sir2-related protein SIRT1 associates with the bHLH repressors HES1 and HEY2 and is involved in HES1-and HEY2-mediated transcriptional repression. Biochem Biophys Res Commun. (2003) 301:250–7. 10.1016/S0006-291X(02)03020-6 [DOI] [PubMed] [Google Scholar]
- 58.Fischer A, Schumacher N, Maier M, Sendtner M, Gessler M. The Notch target genes Hey1 and Hey2 are required for embryonic vascular development. Genes Dev. (2004) 18:901–11. 10.1101/gad.291004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Guo S, Long M, Li X, Zhu S, Zhang M, Yang Z. Curcumin activates autophagy and attenuates oxidative damage in EA. hy926 cells via the Akt/mTOR pathway. Mol Med Rep. (2016) 13:2187–93. 10.3892/mmr.2016.4796 [DOI] [PubMed] [Google Scholar]
- 60.Guo L, Zhou SR, Wei XB, Liu Y, Chang XX, Liu Y, et al. Acetylation of mitochondrial trifunctional protein α-subunit enhances its stability to promote fatty acid oxidation and is decreased in nonalcoholic fatty liver disease. Mol Cell Biol. (2016) 36:2553–67. 10.1128/MCB.00227-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang J, Wang C, Nie H, Wu D, Ying W. SIRT2 plays a significant role in maintaining the survival and energy metabolism of PIEC endothelial cells. Int J Physiol Pathophysiol Pharmacol. (2016) 8:120. [PMC free article] [PubMed] [Google Scholar]
- 62.D'Onofrio N, Servillo L, Giovane A, Casale R, Vitiello M, Marfella R, et al. Ergothioneine oxidation in the protection against high-glucose induced endothelial senescence: involvement of SIRT1 and SIRT6. Free Radic Biol Med. (2016) 96:211–22. 10.1016/j.freeradbiomed.2016.04.013 [DOI] [PubMed] [Google Scholar]
- 63.Liu J, Wu X, Wang X, Zhang Y, Bu P, Zhang Q, et al. Global gene expression profiling reveals functional importance of Sirt2 in endothelial cells under oxidative stress. Int J Mol Sci. (2013) 14:5633–49. 10.3390/ijms14035633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yang D, Lee J, Yang X, Komarova Y. SIRT2 stabilizes endothelial adherens junctions. FASEB J. (2019) 33:682–8. [Google Scholar]
- 65.Tseng AHH, Wu LH, Shieh SS, Wang DL. SIRT3 interactions with FOXO3 acetylation, phosphorylation and ubiquitinylation mediate endothelial cell responses to hypoxia. Biochem J. (2014) 464:157–68. 10.1042/BJ20140213 [DOI] [PubMed] [Google Scholar]
- 66.He X, Zeng H, Chen ST, Roman RJ, Aschner JL, Didion S, et al. Endothelial specific SIRT3 deletion impairs glycolysis and angiogenesis and causes diastolic dysfunction. J Mol Cell Cardiol. (2017) 112:104–13. 10.1016/j.yjmcc.2017.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen Y, Wang H, Luo G, Dai X. SIRT4 inhibits cigarette smoke extracts-induced mononuclear cell adhesion to human pulmonary microvascular endothelial cells via regulating NF-κB activity. Toxicol Lett. (2014) 226:320–7. 10.1016/j.toxlet.2014.02.022 [DOI] [PubMed] [Google Scholar]
- 68.Yu BB, Zhi H, Zhang XY, Liang JW, He J, Su C, et al. Mitochondrial dysfunction-mediated decline in angiogenic capacity of endothelial progenitor cells is associated with capillary rarefaction in patients with hypertension via downregulation of CXCR4/JAK2/SIRT5 signaling. EBioMedicine. (2019) 42:64–75. 10.1016/j.ebiom.2019.03.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cardus A, Uryga AK, Walters G, Erusalimsky JD. SIRT6 protects human endothelial cells from DNA damage, telomere dysfunction, and senescence. Cardiovasc Res. (2012) 97:571–9. 10.1093/cvr/cvs352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stünkel W, Peh BK, Tan YC, Nayagam VM, Wang X, Tellez MS, et al. Function of the SIRT1 protein deacetylase in cancer. Biotechnol J Healthcare Nutr Technol. (2007) 2:1360–8. 10.1002/biot.200700087 [DOI] [PubMed] [Google Scholar]
- 71.Bradbury CA, Khanim FL, Hayden R, Bunce CM, White DA, Drayson MT, et al. Histone deacetylases in acute myeloid leukaemia show a distinctive pattern of expression that changes selectively in response to deacetylase inhibitors. Leukemia. (2005) 19:1751–9. 10.1038/sj.leu.2403910 [DOI] [PubMed] [Google Scholar]
- 72.Kozako T. New strategy of adult T-cell leukemia treatment targeted for anti-tumor immunity and a longevity gene-encoded protein. Yakugaku Zasshi. (2011) 131:1061–72. 10.1248/yakushi.131.1061 [DOI] [PubMed] [Google Scholar]
- 73.Wang RH, Sengupta K, Li C, Kim HS, Cao L, Xiao C, et al. Impaired DNA damage response, genome instability, and tumorigenesis in SIRT1 mutant mice. Cancer Cell. (2008) 14:312–23. 10.1016/j.ccr.2008.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Inoue T, Hiratsuka M, Osaki M, Yamada H, Kishimoto I, Yamaguchi S, et al. SIRT2, a tubulin deacetylase, acts to block the entry to chromosome condensation in response to mitotic stress. Oncogene. (2007) 26:945–57. 10.1038/sj.onc.1209857 [DOI] [PubMed] [Google Scholar]
- 75.Lennerz V, Fatho M, Gentilini C, Frye RA, Lifke A, Ferel D, et al. The response of autologous T cells to a human melanoma is dominated by mutated neoantigens. Proc Natl Acad Sci USA. (2005) 102:16013–8. 10.1073/pnas.0500090102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sebastián C, Zwaans BMM, Silberman DM, Gymrek M, Goren A, Zhong L, et al. The histone deacetylase SIRT6 is a tumor suppressor that controls cancer metabolism. Cell. (2012) 151:1185–99. 10.1016/j.cell.2012.10.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Alcaín FJ, Villalba JM. Sirtuin inhibitors. Expert Opin Ther Pat. (2009) 19:283–94. 10.1517/13543770902755111 [DOI] [PubMed] [Google Scholar]
- 78.Cantó C, Auwerx J. Caloric restriction, SIRT1 and longevity. Trends Endocrinol Metab. (2009) 20:325–31. 10.1016/j.tem.2009.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tsai KL, Sun YJ, Huang CY, Yang JY, Hung MC, Hsiao CD. Crystal structure of the human FOXO3a-DBD/DNA complex suggests the effects of post-translational modification. Nucleic Acids Res. (2007) 35:6984–94. 10.1093/nar/gkm703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Daitoku H, Hatta M, Matsuzaki H, Aratani S, Ohshima T, Miyagishi M, et al. Silent information regulator 2 potentiates Foxo1-mediated transcription through its deacetylase activity. Proc Natl Acad Sci USA. (2004) 101:10042–7. 10.1073/pnas.0400593101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Teodoro JG, Evans SK, Green MR. Inhibition of tumor angiogenesis by p53: a new role for the guardian of the genome. J Mol Med. (2007) 85:1175–86. 10.1007/s00109-007-0221-2 [DOI] [PubMed] [Google Scholar]
- 82.Lynch CJ, Shah ZH, Allison SJ, Ahmed SF, Ford J, Warnock LJ, et al. SIRT1 undergoes alternative splicing in a novel auto-regulatory loop with p53. PLoS ONE. (2010) 5:e13502. 10.1371/journal.pone.0013502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Li SD, Banck M, Mujtaba S, Zhou MM, Sugrue MM, Walsh MJ. p53-induced growth arrest is regulated by the mitochondrial SirT3 deacetylase. PLoS ONE. (2010) 5:e10486. 10.1371/journal.pone.0010486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Vakhrusheva O, Smolka C, Gajawada P, Kostin S, Boettger T, Kubin T, et al. Sirt7 increases stress resistance of cardiomyocytes and prevents apoptosis and inflammatory cardiomyopathy in mice. Circ Res. (2008) 102:703–10. 10.1161/CIRCRESAHA.107.164558 [DOI] [PubMed] [Google Scholar]
- 85.Cheng HL, Mostoslavsky R, Saito S, Manis JP, Gu Y, Patel P, et al. Developmental defects and p53 hyperacetylation in Sir2 homolog (SIRT1)-deficient mice. Proc Natl Acad Sci USA. (2003) 100:10794–9. 10.1073/pnas.1934713100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang H, He S, Spee C, Ishikawa K, Hinton DR. SIRT1 mediated inhibition of VEGF/VEGFR2 signaling by Resveratrol and its relevance to choroidal neovascularization. Cytokine. (2015) 76:549–52. 10.1016/j.cyto.2015.06.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Khan AA, Dace DS, Ryazanov AG, Kelly J, Apte RS. Resveratrol regulates pathologic angiogenesis by a eukaryotic elongation factor-2 kinase-regulated pathway. Am J Pathol. (2010) 177:481–92. 10.2353/ajpath.2010.090836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Xie M, Liu M, He CS. SIRT1 regulates endothelial Notch signaling in lung cancer. PLoS ONE. (2012) 7:e45331. 10.1371/journal.pone.0045331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jiang BH, Liu LZ. AKT signaling in regulating angiogenesis. Curr Cancer Drug Targets. (2008) 8:19–26. 10.2174/156800908783497122 [DOI] [PubMed] [Google Scholar]
- 90.Okumura N, Yoshida H, Kitagishi Y, Murakami M, Nishimura Y, Matsuda S. PI3K/AKT/PTEN signaling as a molecular target in leukemia angiogenesis. Adv Hematol. (2012) 2012:843085. 10.1155/2012/843085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ota H, Eto M, Ogawa S, Iijima K, Akishita M, Ouchi Y. SIRT1/eNOS axis as a potential target against vascular senescence, dysfunction and atherosclerosis. J Atheroscler Thromb. (2010) 17:431–5. 10.5551/jat.3525 [DOI] [PubMed] [Google Scholar]
- 92.Mattagajasingh I, Kim CS, Naqvi A, Yamamori T, Hoffman TA, Jung SB, et al. SIRT1 promotes endothelium-dependent vascular relaxation by activating endothelial nitric oxide synthase. Proc Natl Acad Sci USA. (2007) 104:14855–60. 10.1073/pnas.0704329104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Potente M, Urbich C, Sasaki KI, Hofmann WK, Heeschen C, Aicher A, et al. Involvement of Foxo transcription factors in angiogenesis and postnatal neovascularization. J Clin Invest. (2005) 115:2382–92. 10.1172/JCI23126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fukumura D, Xu L, Chen Y, Gohongi T, Seed B, Jain RK. Hypoxia and acidosis independently up-regulate vascular endothelial growth factor transcription in brain tumors in vivo. Cancer Res. (2001) 61:6020–4. [PubMed] [Google Scholar]
- 95.Corbet C, Draoui N, Polet F, Pinto A, Drozak X, Riant O, et al. The SIRT1/HIF2α axis drives reductive glutamine metabolism under chronic acidosis and alters tumor response to therapy. Cancer Res. (2014) 74:5507–19. 10.1158/0008-5472.CAN-14-0705 [DOI] [PubMed] [Google Scholar]
- 96.Kunhiraman H, Edatt L, Thekkeveedu S, Poyyakkara A, Raveendran V, Kiran MS, et al. 2-Deoxy Glucose modulates expression and biological activity of VEGF in a SIRT-1 dependent mechanism. J Cell Biochem. (2017) 118:252–62. 10.1002/jcb.25629 [DOI] [PubMed] [Google Scholar]
- 97.Edatt L, Haritha K, Sruthi TV, Aswini P, Sameer Kumar VB. 2-Deoxy glucose regulate MMP-9 in a SIRT-1 dependent and NFkB independent mechanism. Mol Cell Biochem. (2016) 423–2, 197–206. 10.1007/s11010-016-2837-4 [DOI] [PubMed] [Google Scholar]
- 98.Portmann S, Fahrner R, Lechleiter A, Keogh A, Overney S, Laemmle A, et al. Antitumor effect of SIRT1 inhibition in human HCC tumor models in vitro and in vivo. Mol Cancer Ther. (2013) 12:499–508. 10.1158/1535-7163.MCT-12-0700 [DOI] [PubMed] [Google Scholar]
- 99.Li C, Wang L, Zheng L, Zhan X, Xu B, Jiang J, et al. SIRT1 expression is associated with poor prognosis of lung adenocarcinoma. Onco Targets Ther. (2015) 8:977. 10.2147/OTT.S82378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Suzuki K, Hayashi R, Ichikawa T, Imanishi S, Yamada T, Inomata M, et al. SRT1720, a SIRT1 activator, promotes tumor cell migration, and lung metastasis of breast cancer in mice. Oncol Rep. (2012) 27:1726–32. 10.3892/or.2012.1750 [DOI] [PubMed] [Google Scholar]
- 101.Dryden SC, Nahhas FA, Nowak JE, Goustin AS, Tainsky MA. Role for human SIRT2 NAD-dependent deacetylase activity in control of mitotic exit in the cell cycle. Mol Cell Biol. (2003) 23:3173–85. 10.1128/MCB.23.9.3173-3185.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.North BJ, Marshall BL, Borra MT, Denu JM, Verdin E. The human Sir2 ortholog, SIRT2, is an NAD+-dependent tubulin deacetylase. Mol Cell. (2003) 11:437–44. 10.1016/S1097-2765(03)00038-8 [DOI] [PubMed] [Google Scholar]
- 103.Michishita E, Park JY, Burneskis JM, Barrett JC, Horikawa I. Evolutionarily conserved and nonconserved cellular localizations and functions of human SIRT proteins. Mol Biol Cell. (2005) 16:4623–35. 10.1091/mbc.e05-01-0033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hu F, Sun X, Li G, Wu Q, Chen Y, Yang X, et al. Inhibition of SIRT2 limits tumour angiogenesis via inactivation of the STAT3/VEGFA signalling pathway. Cell Death Dis. (2018) 10:9–23. 10.1038/s41419-018-1260-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Niu G, Wright KL, Huang M, Song L, Haura E, Turkson J, et al. Constitutive Stat3 activity up-regulates VEGF expression and tumor angiogenesis. Oncogene. (2002) 21:2000–8. 10.1038/sj.onc.1205260 [DOI] [PubMed] [Google Scholar]
- 106.Wu B, Crampton SP, Hughes CCW. Wnt signaling induces matrix metalloproteinase expression and regulates T cell transmigration. Immunity. (2007) 26:227–39. 10.1016/j.immuni.2006.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nguyen P, Lee S, Leins DL, Trepel J, Smart DDK. SIRT2 interacts with β-catenin to inhibit Wnt signaling output in response to radiation-induced stress. Mol Cancer Res. (2014) 12:1244–53. 10.1158/1541-7786.MCR-14-0223-T [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Shi Y, Xu X, Zhang Q, Fu G, Mo Z, Wang GS, et al. tRNA synthetase counteracts c-Myc to develop functional vasculature. Elife. (2014) 3:e02349. 10.7554/eLife.02349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Khalek WA, Cortade F, Ollendorff V, Lapasset L, Tintignac L, Chabi B, et al. SIRT3, a mitochondrial NAD+-dependent deacetylase, is involved in the regulation of myoblast differentiation. PLoS ONE. (2014) 9:e114388. 10.1371/journal.pone.0114388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wu K, Wang L, Chen Y, Pirooznia M, Singh K, Wälde S, et al. GCN5L1 interacts with αTAT1 and RanBP2 to regulate hepatic α-tubulin acetylation and lysosome trafficking. J Cell Sci. (2018) 131:jcs221036. 10.1242/jcs.221036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Schwer B, Bunkenborg J, Verdin RO, Andersen JS, Verdin E. Reversible lysine acetylation controls the activity of the mitochondrial enzyme acetyl-CoA synthetase 2. Proc Natl Acad Sci USA. (2006) 103:10224–9. 10.1073/pnas.0603968103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Shi T, Wang F, Stieren E, Tong Q. SIRT3, a mitochondrial sirtuin deacetylase, regulates mitochondrial function and thermogenesis in brown adipocytes. J Biol Chem. (2005) 280:13560–7. 10.1074/jbc.M414670200 [DOI] [PubMed] [Google Scholar]
- 113.Bell EL, Emerling BM, Ricoult SJH, Guarente L. SirT3 suppresses hypoxia inducible factor 1α and tumor growth by inhibiting mitochondrial ROS production. Oncogene. (2011) 30:2986–96. 10.1038/onc.2011.37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Finley LWS, Carracedo A, Lee J, Souza A, Egia A, Zhang J, et al. SIRT3 opposes reprogramming of cancer cell metabolism through HIF1α destabilization. Cancer Cell. (2011) 19:416–28. 10.1016/j.ccr.2011.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Schumacker PT. SIRT3 controls cancer metabolic reprogramming by regulating ROS and HIF. Cancer Cell. (2011) 19:299–300. 10.1016/j.ccr.2011.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Liszt G, Ford E, Kurtev M, Guarente L. Mouse Sir2 homolog SIRT6 is a nuclear ADP-ribosyltransferase. J Biol Chem. (2005) 280:21313–20. 10.1074/jbc.M413296200 [DOI] [PubMed] [Google Scholar]
- 117.Hao HX, Khalimonchuk O, Schraders M, Dephoure N, Bayley JP, Kunst H, et al. SDH5, a gene required for flavination of succinate dehydrogenase, is mutated in paraganglioma. Science. (2009) 325:1139–42. 10.1126/science.1175689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sauve AA. Sirtuin chemical mechanisms. Biochim Biophys Acta. (2010) 1804:1591–603. 10.1016/j.bbapap.2010.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Herrero YA, Bakhit SMA, Franke P, Weise C, Schweiger M, Jorcke D, et al. Regulation of glutamate dehydrogenase by reversible ADP-ribosylation in mitochondria. EMBO J. (2001) 20:2404–12. 10.1093/emboj/20.10.2404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Cantó C, Sauve AA, Bai P. Crosstalk between poly (ADP-ribose) polymerase and sirtuin enzymes. Mol Aspects Med. (2013) 34:1168–201. 10.1016/j.mam.2013.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mathews MT, Berk BC. PARP-1 inhibition prevents oxidative and nitrosative stress–induced endothelial cell death via transactivation of the VEGF receptor 2. Arterioscler Thromb Vasc Biol. (2008) 28:711–7. 10.1161/ATVBAHA.107.156406 [DOI] [PubMed] [Google Scholar]
- 122.Selak MA, Armour SM, MacKenzie ED, Boulahbel H, Watson GD, Mansfield KD, et al. Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-α prolyl hydroxylase. Cancer Cell. (2005) 7:77–85. 10.1016/j.ccr.2004.11.022 [DOI] [PubMed] [Google Scholar]
- 123.Wang C, Zhang C, Li X, Shen J, Xu Y, Shi H, et al. CPT 1A-mediated succinylation of S100A10 increases human gastric cancer invasion. J Cell Mol Med. (2019) 23:293–305. 10.1111/jcmm.13920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Van GF, Gallí M, Gueydan C, Kruys V, Prevot PP, Bedalov A, et al. Intracellular NAD levels regulate tumor necrosis factor protein synthesis in a sirtuin-dependent manner. Nat Med. (2009) 15:206–10. 10.1038/nm.1906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bauer I, Grozio A, Lasigliè D, Basile G, Sturla L, Magnone M, et al. The NAD+-dependent histone deacetylase SIRT6 promotes cytokine production and migration in pancreatic cancer cells by regulating Ca2+ responses. J Biol Chem. (2012) 287:40924–37. 10.1074/jbc.M112.405837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Fernando RI, Castillo MD, Litzinger M, Hamilton DH, Palena C. IL-8 signaling plays a critical role in the epithelial–mesenchymal transition of human carcinoma cells. Cancer Res. (2011) 71:5296–306. 10.1158/0008-5472.CAN-11-0156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kawahara TLA, Michishita E, Adler AS, Damian M, Berber E, Lin M, et al. SIRT6 links histone H3 lysine 9 deacetylation to NF-κB-dependent gene expression and organismal life span. Cell. (2009) 136:62–74. 10.1016/j.cell.2008.10.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Basseres DS, Baldwin AS. Nuclear factor-κB and inhibitor of κB kinase pathways in oncogenic initiation and progression. Oncogene. (2006) 25:6817–30. 10.1038/sj.onc.1209942 [DOI] [PubMed] [Google Scholar]
- 129.Zhong L, D'Urso A, Toiber D, Sebastian C, Henry RE, Vadysirisack DD, et al. The histone deacetylase Sirt6 regulates glucose homeostasis via Hif1α. Cell. (2010) 140:280–93. 10.1016/j.cell.2009.12.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hubbi ME, Hu H, Gilkes DM, Semenza GL. Sirtuin-7 inhibits the activity of hypoxia-inducible factors. J Biol Chem. (2013) 288:20768–75. 10.1074/jbc.M113.476903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Tang X, Shi L, Xie N, Liu Z, Qian M, Meng F, et al. SIRT7 antagonizes TGF-β signaling and inhibits breast cancer metastasis. Nat Commun. (2017) 8:318. 10.1038/s41467-017-00396-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Rokutanda T, Izumiya Y, Araki S, Hanatani S, Bober E, Braun T, et al. Sirt7 regulates endothelial cell functions and promotes angiogenic response in mice model of hindlimb ischemia. Circulation. (2012) 126:A10980. [Google Scholar]
- 133.Nemoto S, Fergusson MM, Finkel T. SIRT1 functionally interacts with the metabolic regulator and transcriptional coactivator PGC-1α. J Biol Chem. (2005) 280:16456–60. 10.1074/jbc.M501485200 [DOI] [PubMed] [Google Scholar]
- 134.Yeung F, Hoberg JE, Ramsey CS, Keller MD, Jones DR, Frye RA, et al. Modulation of NF-κB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. (2004) 23:2369–80. 10.1038/sj.emboj.7600244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Fulco M, Schiltz RL, Iezzi S, King MT, Zhao P, Kashiwaya Y, et al. Sir2 regulates skeletal muscle differentiation as a potential sensor of the redox state. Mol Cell. (2003) 12:51–62. 10.1016/S1097-2765(03)00226-0 [DOI] [PubMed] [Google Scholar]
- 136.Bordone L, Motta MC, Picard F, Robinson A, Jhala US, Apfeld J, et al. Sirt1 regulates insulin secretion by repressing UCP2 in pancreatic beta cells. PLoS Biol. (2006) 4:e31. 10.1371/journal.pbio.0040031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Picard F, Kurtev M., Chung N., Topark-Ngarm A., Senawong T., Machado D. O. R., et al. Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR. Nature. (2004) 429:771–6. 10.1038/nature02583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zhang W, Huang Q, Zeng Z, Wu J, Zhang Y, Chen Z. Sirt1 inhibits oxidative stress in vascular endothelial cells. Oxid Med Cell Longev. (2017) 2017:7543973. 10.1155/2017/7543973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Nisoli E, Tonello C, Cardile A, Cozzi V, Bracale R, Tedesco L, et al. Calorie restriction promotes mitochondrial biogenesis by inducing the expression of eNOS. Science. (2005) 310:314–7. 10.1126/science.1117728 [DOI] [PubMed] [Google Scholar]
- 140.Sahar S, Masubuchi S, Mahan KE, Vollmer S, Galla L, Ceglia N, et al. Circadian control of fatty acid elongation by SIRT1 protein-mediated deacetylation of acetyl-coenzyme A synthetase 1. J Biol Chem/. (2014) 289:6091–7. 10.1074/jbc.M113.537191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zerr P, Zerr KP, Huang J, Tomcik M, Sumova B, Distler O, et al. Sirt1 regulates canonical TGF-β signalling to control fibroblast activation and tissue fibrosis. Ann Rheum Dis. (2016) 75:226–33. 10.1136/annrheumdis-2014-205740 [DOI] [PubMed] [Google Scholar]
- 142.Patel VB, Preedy VR. (Eds.). Handbook of Nutrition, Diet, and Epigenetics. Cham: Springer; (2019). [Google Scholar]
- 143.Hirschey MD, Shimazu T, Goetzman E, Jing E, Schwer B, Lombard DB, et al. SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation. Nature. (2010) 464:121–5. 10.1038/nature08778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Han Y, Zhou S, Coetzee S, Chen A. SIRT4 and its roles in energy and redox metabolism in health, disease and during exercise. Front Physiol. (2019) 10:1006. 10.3389/fphys.2019.01006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Schlicker C, Gertz M, Papatheodorou P, Kachholz B, Becker CFW, Steegborn C. Substrates and regulation mechanisms for the human mitochondrial sirtuins Sirt3 and Sirt5. J Mol Biol. (2008) 382:790–801. 10.1016/j.jmb.2008.07.048 [DOI] [PubMed] [Google Scholar]
- 146.Barber MF, Michishita KE, Xi Y, Tasselli L, Kioi M, Moqtaderi Z, et al. SIRT7 links H3K18 deacetylation to maintenance of oncogenic transformation. Nature. (2012) 487:114–8. 10.1038/nature11043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Zhao T, Li J, Chen AF. MicroRNA-34a induces endothelial progenitor cell senescence and impedes its angiogenesis via suppressing silent information regulator 1. American J Physiol Endocrinol Metab. (2010) 299:E110–16. 10.1152/ajpendo.00192.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ito T, Yagi S, Yamakuchi M. MicroRNA-34a regulation of endothelial senescence. Biochem Biophys Res Commun. (2010) 398:735–40. 10.1016/j.bbrc.2010.07.012 [DOI] [PubMed] [Google Scholar]
- 149.Menghini R, Casagrande V, Cardellini M, Martelli E, Terrinoni A, Amati F, et al. MicroRNA 217 modulates endothelial cell senescence via silent information regulator. Circulation. (2009) 120:1524–32. 10.1161/CIRCULATIONAHA.109.864629 [DOI] [PubMed] [Google Scholar]
- 150.Zhang S, Liu L, Wang R, Tuo H, Guo Y, Yi L, et al. MicroRNA-217 promotes angiogenesis of human cytomegalovirus-infected endothelial cells through downregulation of SIRT1 and FOXO3A. PLoS ONE. (2013) 8:e83620. 10.1371/journal.pone.0083620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Edatt L, Maurya AK, Raji G, Kunhiraman H, Kumar VBS. MicroRNA106a regulates matrix metalloprotease 9 in a sirtuin-1 dependent mechanism. J Cell Physiol. (2018) 233:238–48. 10.1002/jcp.25870 [DOI] [PubMed] [Google Scholar]
- 152.Sruthi TV, Edatt L, Raji GR, Kunhiraman H, Shankar SS, Shankar V, et al. Horizontal transfer of miR-23a from hypoxic tumor cell colonies can induce angiogenesis. J Cell Physiol. (2018) 233:3498–514. 10.1002/jcp.26202 [DOI] [PubMed] [Google Scholar]
- 153.Kumarswamy R, Volkmann I, Beermann J, Napp LC, Jabs O, Bhayadia R, et al. Vascular importance of the miR-212/132 cluster. Eur Heart J. (2014) 35:3224–31. 10.1093/eurheartj/ehu344 [DOI] [PubMed] [Google Scholar]
- 154.Zhang Y, Du X, Li W, Sang H, Qian A, Sun L, et al. Resveratrol improves endothelial progenitor cell function through miR-138 by targeting focal adhesion kinase (FAK) and promotes thrombus resolution in vivo. Med Sci Monit. (2018) 24:951. 10.12659/MSM.906116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zheng Z, Zhao B. Astragalus polysaccharide protects hypoxia-induced injury by up-regulation of miR-138 in rat neural stem cells. Biomed Pharmacother. (2018) 102:295–301. 10.1016/j.biopha.2018.03.040 [DOI] [PubMed] [Google Scholar]
- 156.Tian F, Yuan C, Yue H. MiR-138/SIRT1 axis is implicated in impaired learning and memory abilities of cerebral ischemia/reperfusion injured rats. Exp Cell Res. (2018) 367:232–40. 10.1016/j.yexcr.2018.03.042 [DOI] [PubMed] [Google Scholar]
- 157.Vernucci E, Tomino C, Molinari F, Limongi D, Aventaggiato M, Sansone L, et al. Mitophagy and oxidative stress in cancer and aging: focus on sirtuins and nanomaterials. Oxid Med Cell Longev. (2019) 2019:6387357. 10.1155/2019/6387357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Christovam AC, Theodoro V, Mendonça FAS, Esquisatto MAM, Santos GMT, Amaral MEC. Activators of SIRT1 in wound repair: an animal model study. Arch Dermatol Res. (2019) 311:193–201. 10.1007/s00403-019-01901-4 [DOI] [PubMed] [Google Scholar]
- 159.Jing T, Ya SK, Xue JW, Han JH, Yan L, Yi AY, et al. Sirt6 mRNA-incorporated endothelial microparticles (EMPs) attenuates DM patient-derived EMP-induced endothelial dysfunction. Oncotarget. (2017) 8:114300–13. 10.18632/oncotarget.23259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Arvizo RR, Saha S, Wang E, Robertson JD, Bhattacharya R, Mukherjee P. Inhibition of tumor growth and metastasis by a self-therapeutic nanoparticle. Proc Natl Acad Sci USA. (2013) 110:6700–5. 10.1073/pnas.1214547110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Yang T, Yao Q, Cao F, Liu Q, Liu B, Wang XH. Silver nanoparticles inhibit the function of hypoxia-inducible factor-1 and target genes: insight into the cytotoxicity and antiangiogenesis. Int J Nanomed. (2016) 11:6679. 10.2147/IJN.S109695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Li W, Zhao X, Du B, Li X, Liu S, Yang XY, et al. Gold nanoparticle–mediated targeted delivery of recombinant human endostatin normalizes tumour vasculature and improves cancer therapy. Sci Rep. (2016) 6:30619. 10.1038/srep30619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Xu Y, Wen Z, Xu Z. Chitosan nanoparticles inhibit the growth of human hepatocellular carcinoma xenografts through an antiangiogenic mechanism. Anticancer Res. (2009) 29:5103–9. [PubMed] [Google Scholar]
- 164.Jin H, Pi J, Yang F, Wu C, Cheng X, Bai H, et al. Ursolic acid-loaded chitosan nanoparticles induce potent anti-angiogenesis in tumor. Appl Microbiol Biotechnol. (2016) 100:6643–52. 10.1007/s00253-016-7360-8 [DOI] [PubMed] [Google Scholar]
- 165.Hu H, You Y, He L, Chen T. The rational design of NAMI-A-loaded mesoporous silica nanoparticles as antiangiogenic nanosystems. J Mater Chem B. (2015) 3:6338–46. 10.1039/C5TB00612K [DOI] [PubMed] [Google Scholar]
- 166.Arvizo RR, Rana S, Miranda OR, Bhattacharya R, Rotello VM, Mukherjee P. Mechanism of anti-angiogenic property of gold nanoparticles: role of nanoparticle size and surface charge. Nanomed Nanotechnol Biol Med. (2011) 7:580–7. 10.1016/j.nano.2011.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Shi X, Zhou K, Huang F, Wang C. Interaction of hydroxyapatite nanoparticles with endothelial cells: internalization and inhibition of angiogenesis in vitro through the PI3K/Akt pathway. Int J Nanomed. (2017) 12:5781–95. 10.2147/IJN.S140179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Shereema TM, Sruthi TV, Kumar VBS, Rao TP, Shankar SS. Angiogenic profiling of synthesized carbon quantum dots. Biochemistry. (2015) 54:6352–6. 10.1021/acs.biochem.5b00781 [DOI] [PubMed] [Google Scholar]
- 169.Treviño NS, Rivas GG. Regulation of sirtuin-mediated protein deacetylation by cardioprotective phytochemicals. Oxid Med Cell Longev. (2017) 2017:1750306 10.1155/2017/1750306 [DOI] [PMC free article] [PubMed] [Google Scholar]