Abstract
Laboratory assessments of aqueous metal toxicity generally demonstrate aquatic insects tolerate relatively high concentrations of metals in aqueous exposures; however, mesocosm experiments and field biomonitoring often indicate effects at relatively low metal concentrations. One hypothesis proposed to reconcile this discrepancy is an increased sensitivity of smaller size classes of organisms. We exposed field colonized benthic communities to aqueous metals in a series of mesocosm experiments. In addition, a novel single-species test system was used to expose first instar, mid-instar, and late instar mayflies (Ephemeroptera, Baetis tricaudatus) to Zn. These experimental approaches tested the hypothesis that small invertebrate size classes are more sensitive than large, mature size classes. Mesocosm results demonstrated strong size-dependent responses of aquatic insects to metals. Smaller organisms generally displayed greater mortality than large, mature individuals, and models were improved when size was included as a predictor of mortality. Size-dependent responses of Baetis spp. occurred in mesocosm experiments and in our single-species test system. The median lethal concentration (LC50) for early instar B. tricaudatus was less than 6% of the previously reported LC50 for late instars. Together, these results suggest that aquatic insect body size is an important predictor of susceptibility to aqueous metals. Toxicity models that account for insect phenology by integrating the natural size progression of organisms have the potential to improve accuracy in predicting effects of metals in the field.
Introduction
In the classic children’s book “Horton Hears a Who!”,1 Horton the elephant discovers a population of microscopic organisms named “Whos”. Other large vertebrates in the Jungle of Nool were oblivious to the existence of such small organisms. After further study and debate these megafauna instated policies protective of small organisms. This is not unlike the current understanding of aquatic communities. Ecological studies and species sensitivity distributions are often limited to macrofauna (>1 mm in size) because of the difficulty associated with sampling meiofauna (∼0.45 to 1 mm in size) and microorganisms (only visible with a microscope). Although all aquatic insects start life as nearly microscopic size classes, most toxicology studies use only larger or older age classes. If smaller age classes are more sensitive to pollution, policies and numeric standards based on traditional toxicity experiments using mature aquatic insects have potential to extirpate entire taxa from streams, even those taxa that are well represented in species sensitivity distributions.
Links between metal pollution and degradation of aquatic communities in streams are well established in the literature.2−8 Laboratory experiments have routinely demonstrated that aquatic insects are tolerant to aqueous metals;6,9−11 however, biomonitoring studies often indicate that aquatic insects are sensitive to metals at relatively low concentrations.2,12−15 This discrepancy in reported metal tolerance may be the result of invertebrate assemblage size structure.16−18 Natural benthic communities contain a diversity of taxa that can widely differ in their rates of development due mainly to phenology (i.e., seasonal environmental cues for development) and voltinism (i.e., number of life cycles per year). These life history traits are spatially and temporally variable, resulting in a diversity of invertebrate developmental sizes within and among different species that differ in response to metal exposure.
Observational studies have often found potential or maximum body size to be a trait associated with taxa present at disturbed or contaminated sites,19−23 with smaller taxa typically being excluded from contaminated sites. Laboratory and mesocosm experiments that have compared early and late life stages of aquatic invertebrates have reported greater sensitivity of smaller size classes.16,17,24−27 In their seminal study of phylogenetic influences on metal sensitivity in aquatic insects, Buchwalter et al.28 controlled for the potential confounding effect of body size on species sensitivity to metals. Despite this evidence, spanning numerous decades, smaller developmental sizes are seldom used in laboratory toxicity tests. We speculate that this is in part due to the lack of aquatic toxicology methods that allow first instars to be assessed and the weak mandate from regulatory agencies to consider early life stages. The research and discussion herewithin was conducted to provide researchers with new tools for studying size dependent toxicity and to provide additional evidence that body size is an important predictor of toxicity across taxa and across toxicants.
Numerous mechanisms have been proposed for the increased sensitivity of small organisms. These differences may in part result from the influence of surface area to volume ratios. The larger surface area to volume ratio exhibited by smaller organisms has potential to increase the accumulation rates of aqueous toxicants. Furthermore, the increased turnover rates of essential ions (K, Na, Cl, H, etc.) exhibited by smaller organisms make them more susceptible to toxicants, such as copper and silver, which adversely affect regulation of specific ions.29 Additionally, lower fat to protein ratios, more rapid accumulation of toxicants in organs, less developed antioxidant systems and less developed physical structures may contribute to the increased sensitivity of smaller organisms.30
Early instar aquatic insects are typically too small to collect in the field or manipulate in the laboratory (Figure 1). Because of this the current understanding of aquatic insect metal sensitivity is based predominately on larger instars. Mesocosm studies have improved predictions of metal sensitivity in the field by integrating naturally colonized communities that contain numerous taxa at differing stages of development, including early instars.15−17 Despite this, few experimental studies directly address the relationship between aquatic insect size and metal sensitivity. This distinction is important because differences in metal sensitivity among aquatic invertebrates are used to generate species sensitivity distributions (SSD) that serve as the basis for deriving water quality standards31,32 but logistic challenges in obtaining, culturing, and/or testing early instars may bias these SSDs. Although many standardized testing procedures encourage the use of early life stages or full life cycle trials for vertebrates (e.g., fish), similar experiments are rarely conducted for aquatic insects.
Figure 1.
Baetis spp. (A) First or early instar 96 to 108 h posthatch from single-species experiments. (B and C): Mid-instars from single-species experiments that were field collected 30 d after egg masses were observed hatching. (D) Late instar typical of field collected organisms in mesocosm communities. Height of T in “TRUST” is ∼800 μm.
Herein, we report the results of a series of mesocosm and laboratory experiments that test the hypothesis that early life stages of aquatic insects are more sensitive to metals than mature, later instars. We tested the following specific hypotheses: (1) metal sensitivity increases as body mass decreases for Ephemeroptera, Plecoptera, and Trichoptera (EPT) species; (2) head capsule width (i.e., body size) and metal concentration is a better predictor of aquatic insect mortality than metal concentration alone; (3) smaller size classes of 4 common aquatic insect species are more susceptible to metal mixtures than larger size classes; and (4) acute median lethal concentrations (LC50 values) for three age classes of Baetis tricaudatus exposed to Zn increase as age class increases (i.e., older age class are less sensitive because they are larger in size).
Methods
Overview
We examined aquatic insect size distributions from mesocosm studies that exposed natural benthic macroinvertebrate communities to different metal combinations (Cu, Zn, Cd). Macroinvertebrate head capsule widths and body mass are commonly used to estimate invertebrate size.33 We measured the head capsule width of the mayfly Baetis spp., as well as taxa from three other dominant aquatic insect orders (Isoperla spp., Plecoptera, Hydropsyche sp., Trichoptera, and Orthocladiinae, Diptera). A diversity of taxa and body sizes were used to evaluate inter- and intraspecific metal sensitivity. Similarly, average mass of each taxon from each mesocosm experiment was used to estimate sensitivity across metal concentrations. We hypothesized that taxa with lower mass would exhibit a wide range of sensitivity to metals, whereas larger taxa would be consistently tolerant. Lastly, acute Zn toxicity tests were conducted using first instar (<24 h posthatch, originating from field collected eggs) and mid-instar mayflies (∼1 month posthatch, field collected). We then compared results from these early life stages to results from late instars obtained under identical laboratory conditions by Brinkman and Johnston.10
Mesocosm experiments
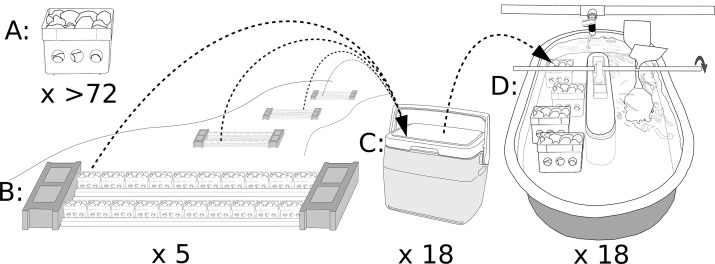
In four previous mesocosm experiments, naturally colonized benthic communities were exposed to different combinations of metals (Cu alone, September 2007; Cu and Zn, October 2007; Cu, Cd, and Zn, August 2010; Cu and Zn, September 2015) at the Colorado State University Stream Research Laboratory (SRL; Fort Collins, Colorado, USA). Details of the SRL design and water chemistry have been described previously.15 In all experiments natural substrate was placed in mesocosm trays (10 cm × 10 cm × 8 cm food storage containers; Figure 2A) with perforations. Trays were fastened to racks (Figure 2B), which are anchored to the benthic zone of reference streams. After ∼30 days of colonization, four trays were randomly selected, assigned to each of the 18 aerated insulated temporary storage tanks (Figure 2C), and then transported to experimental streams (Figure 2D). Experimental streams received flow-through natural water from a mountain reservoir. Toxicants were delivered using peristaltic pumps. Paddle wheels created water velocity similar to lotic ecosystems in Colorado. Mesocosm experiments conducted in 2007–2010 exposed benthic communities from the South Fork of the Michigan River (Gould, Colorado, USA); the experiment conducted in 2015 exposed benthic communities from the Arkansas River (Leadville, Colorado, USA). The 2007–2010 experiments were 10 d exposures, and the 2015 experiment was a 14 d exposure. At the end of each experiment, benthic organisms retained in a 355 μm sieve were preserved in ethanol (80%), and individuals were enumerated and identified to the lowest practical level of taxonomic resolution. Because these experiments used different combinations of metals, cumulative criterion units (CCUs) based on the U.S. Environmental Protection Agency’s hardness-adjusted criteria were used to quantify metal concentrations in the mesocosms (Table S1).15 The 2007 mesocosm experiments exposed aquatic insect communities to a gradient of Cu (0, 1, 2, 3, 6, 12, 25, 50, and 100 CCUs) and Cu + Zn (0, 2, 4, 6, 10, 20, 40, 75, and 150 CCUs) with 2 replicates per treatment level. The 2010 Cu + Zn + Cd mesocosm experiments exposed aquatic insect communities to 0, 3, 6, 12, 25, and 50 CCUs with 3 replicates per treatment level. After evaluating the results from these experiments the 2015 Cu + Zn mesocosm experiment was designed to expose communities to 0, 4, 6, 12, 24, and 52 CCUs (3 replicates per treatment level). Other factors that influence metal toxicity and bioavailability (e.g., pH, dissolved organic carbon) were consistent among treatments and experiments. Because models predicting bioavailability of these metal mixtures were unavailable, hardness-adjusted criteria were used for these analyses. Detailed water chemistry measured in these experiments is listed in Supporting Information (Table S3).
Figure 2.
Stream mesocosm methodologies. Mesocosm trays (A) consisted of food storage containers with 12 perforations that were filled with small cobble. Trays were fastened to racks (B) and anchored to stream beds. Aquatic invertebrate communities were allowed to colonize substrate for ∼30 d. Four mesocosm trays were randomly assigned to small coolers (C) and transported to experimental streams (D).
Head capsule widths of Baetis spp. from 2007 to 10 experiments were measured using a stereomicroscope (Meji EMZ-TR) with a reticle SFW20x eyepiece that provided 0.1 mm resolution. Greater measurement resolution was achieved with the 2015 experiment, which used a high definition microscopy camera (ACCU-SCOPE Excelis Camera AU-600-HD) attached to a stereoscope (Meji EMZ-TR). A stage micrometer (0.01 mm precision) was used to calibrate measurements, and three observations were taken on each individual.
To quantify invertebrate body mass, the wet (preserved in 80% ethanol) mass of every organism from controls of the 2007–2010 experiments was measured. Preserved organisms were placed on dry filter paper on a Buchner funnel for 30 s and weighed (O’Hause GS200D balance; 0.00001 g resolution). Average organism mass of each taxon in controls was calculated and log transformed (ln(mg + 1)). Relative abundance after 10 d of exposure (expressed as a proportion of the mean abundance of the two controls) for each taxon in each experiment was log transformed (ln([abundance]/[average abundance in controls] + 1)) and regressed on log-transformed CCUs (ln(CCU + 1)). The “LM” function in package “car” in R (R Core Team, version 3.5.1) was used to estimate slope.34 The reverse sign of each respective slope estimate was used as a measure of sensitivity for each taxon in each experiment. Lastly, weighted regression (weights = ) was used to regress sensitivity values for taxa from all three mesocosm experiments across average body mass. Naturally colonized communities provided more environmental relevance than artificially constructed communities. However, less abundant taxon typically exhibit greater stochasticity and lower R2 values in dose response models. For this reason, abundance of individuals in each taxon in the controls was used as the weight in the regression analyses to ensure poorly represented taxa did not have a disproportionate influence on the relationship between mass and metal tolerance. Dipterans (true flies), coleopterans (beetles), and noninsect taxa from mesocosms were not consistently represented among experiments and were not included in the analysis.
Because the 2007–2010 mesocosm experiments employed a regression experimental design with low replication (n = 2), analysis of covariance (ANCOVA) was used to model survival of Baetis spp. using the predictor variables of metal concentrations (CCU), head capsule width (Instar size) or the interaction (CCU × Instar size). After combining control head capsule distributions among the three experiments, size classes were determined using the “split” function in package “Hmisc”.35 Size class distributions varied among these three experiments due to their different colonization periods and phenological differences; therefore, uneven size class groupings were chosen to allow for absolute size comparisons of Baetis sp. among the three experiments. The number of surviving organisms and the metal concentrations were transformed (ln +1) to satisfy assumptions of parametric statistics. Using the “LM” function, survival was regressed on metal concentration. Akaike Information Criterion (AIC)36 was used to select the model that best predicted mortality based on insect size, metal concentration, and (or) the size × metal interaction. To identify differences in responses to metals among size classes, we used the “estimated marginal means of linear trends” (emtrends) function in package “emmeans”37 and the “multcompLetters” package38 with a Tukey HSD multiple-comparison adjustment.
After observing size dependent effects in the 2007 and 2010 experiments several improvements in experimental design were made. The 2015 mesocosm study was designed with greater replication (n = 3 in each treatment level) to allow use of two-factor ANOVA (package “car”) to test the hypothesis that differences in mortality across metal treatments were determined by insect body size (i.e., head capsule width). Additionally, the improved resolution of head capsule measurements provided by the ACCU-SCOPE digital microscope allowed us to discern six or seven size classes for each taxon, rather than three. Analysis of Variance was used to model the response variable of mortality using the predictor variables of metal concentration, body size class, and their interaction. To separate size classes for each aquatic insect order, the “split” function was used to fit either 6 or 7 size class groupings. Evenly separated size class groupings were used because we wanted to compare size gradient responses to metals among taxa. Data for each size grouping were normalized to proportional mortality by dividing abundance by the average abundance of the three control replicates.
Effects on Early Instar Baetis tricaudatus in the Laboratory
Early life stages of the mayfly B. tricaudatus were exposed to a gradient of Zn concentrations for 96 h. Early instar organisms (mean head capsule width 113.5 μm, SD = 10, n = 7) were obtained by rearing eggs. Egg masses were collected from the Cache la Poudre River (Colorado, USA) in September 2014 substrate (Figure S1). Mid-instar organisms (mean head capsule width 260.1 μm, SD = 25, n = 7) of ∼1 month age, were collected from cobble at the same location using 7.5 mL transfer pipettes (16 November 2014). Early and mid-instar baetids were nearly microscopic and were contained and enumerated using a novel toxicant exposure system that reproduced the natural flows of benthic habitats in high-gradient streams without losing organisms (Figures S2–S5). Importantly, this acute exposure to Zn used the same exposure methodology and dilution water supply as described by Brinkman and Johnston10 for large instars. After initial range-finding experiments for each size, first instars were exposed to 0, 133, 300, 642, 1433, and 3263 μg/L Zn (26 Oct 2014; Table S6). Mid-instar were exposed to 0, 4600, 9380, 20450, 46550, 84800 μg/L Zn (16 Nov 2014). Because phenotypic characteristics used to identify Baetis spp. are not developed until organisms are more mature (late instar), a subsample of surviving organisms from each experiment was preserved for genetic analysis. Ninety six hour LC50 values for first and mid-instar size classes were calculated using a log–logistic regression (“LL.2”) using the dose response model function (“drm”) in package “drc” in R.39 To protect against overfitting and bias, the “mselect” function in “drc” was employed to use Akaike’s information criterion (AIC) to select the most appropriate log–logistic model among 2, 3, and 4 parameter options.
Results
Routine water quality characteristics (pH, hardness, conductivity, temperature) measured in stream mesocosms were similar among the 4 experiments and showed relatively little variation among treatments. Water chemistry in the 2010 and 2015 mesocosm experiments (Cu + Zn + Cd and Cu + Zn) were very similar to experiments conducted in 2007 (Cu + Zn and Cu15), with sourced water representative of oligotrophic headwater streams (Tables S2–S4).
Body mass of aquatic insects in the 2007–2010 mesocosm studies ranged from 0.013 to 36.8 mg (wet weight). Sensitivity to metals for each taxon in each experiment significantly decreased as the average body size for each taxon increased (slope = −0.0806, p < 0.0001; Figure 3). As predicted, smaller taxa had the greatest range in sensitivity to metals, whereas larger taxa were represented only by metal-tolerant organisms. This wedge shaped response distribution contributed to the relatively low r2 (0.31) for this regression.
Figure 3.
Relationship of sensitivity index to body mass (wet weight) of aquatic insect larvae in the 2007 (Cu and Cu + Zn) and 2010 (Cu + Zn+ Cd) mesocosm experiments combined. Sensitivity index equals the reverse sign of the slope of ln([abundance]/[average abundance in controls] + 1) regressed on ln(CCU + 1), where CCU = chronic criterion units for the metals. Diameter of the points reflects average abundance in controls for each taxon at the end of the experiment. Dashed regression line was weighted for average abundance in controls (slope = −0.08052 (±0.01606), p < 0.0001; intercept = 0.24119 (±0.01399), p < 0.0001.) Taxa from Ephemeroptera, Plecoptera, and Trichoptera are color coded orange, blue, and pink, respectively.
Head capsule widths for Baetis spp., the dominant mayfly in the 2007–2010 mesocosm experiments, was an important addition to CCU in predicting mortality (Table S5). Instar size × metal concentration interaction terms were significant for Cu (p = 0.0082) and Cu + Zn + Cd (p = 0.0171) but not for Cu + Zn (p = 0.2395). AIC results support the addition of the interaction term for all ANCOVA models, indicating that size and CCU better explained mortality across treatments compared to CCU or instar size alone (Table 1).
Table 1. Akaike Information Criterion (AIC) model selection results for model terms in 2007–2010 Mesocosm Experimentsa.
| treatment | model term | AIC | ΔAIC |
|---|---|---|---|
| Cu 0–5.1 CCU | CCU × instar size | 234.64 | 0 |
| CCU, instar size | 244.03 | 9.39 | |
| instar size | 250.36 | 15.72 | |
| CCU | 280.45 | 45.81 | |
| Cu + Zn 0–7.0 CCU | CCU, instar size | 247.88 | 0 |
| CCU × instar size | 249.05 | 1.17 | |
| instar size | 252.96 | 5.08 | |
| CCU | 265.45 | 16.40 | |
| Cu + Zn + Cd 0–12.9 CCU | CCU × instar size | 312.89 | 0 |
| CCU, instar size | 319.18 | 6.29 | |
| instar size | 346.31 | 33.42 | |
| CCU | 368.07 | 55.18 |
ANCOVA was used to test for the responses of Baetis spp. abundances given the model predictors of metals concentration(s) (CCU), instar size, and the CCU × instar size interaction.
In the 2015 mesocosm experiment, survival of Baetis spp. (Ephemeroptera), Orthocladiinae (Diptera), Isoperla (Plecoptera), and Hydropsyche sp. (Trichoptera) decreased with CCU but increased with instar size (p < 0.05, Table 2 and Figure 4). Therefore, including body size in the regression improves model predictions. Additionally, interaction terms (CCU × instar size) for Baetis spp., Orthocladiinae, and Hydropsyche spp. were statistically significant. Body size of the stonefly Isoperla spp. seemed to influence responses to metals, but the interaction term was not significant (p = 0.0762) likely due to high variability among treatments. In general, the greatest mortality was observed for smaller instars (i.e., lower mortality as organisms become larger; Figure 4). Treatment effects for Trichoptera were highly size-dependent, with less than 5% mortality at 53 CCU for the largest instars (>1.05 mm), while the smallest instars (<0.30 mm) had greater than 50% mortality even in the lowest treatment (4 CCU). The slopes describing the body size-survival relationship of Orthocladiinae were similar across treatments, whereas the influence of size for Baetis and Isoperla was more pronounced at the lower metal concentrations due to high or complete mortality in the higher treatments.
Table 2. Two-Factor ANOVA Results from the 2015 Experiments in Which Metal Concentrations (CCU), Instar Size, and CCU × Instar Size Interaction Were Used to Predict Mortality of the Four Dominant Taxa: Baetis spp. (Ephemeroptera), Orthocladiinae (Diptera), Hydropsyche spp. (Trichoptera), and Isoperla spp. (Plecoptera).
| taxa | model term | F-value | P-value |
|---|---|---|---|
| Baetis spp. | CCU treatment | 100.12 | <0.0001 |
| instar size | 14.68 | <0.0001 | |
| CCU treatment × instar size | 4.50 | <0.0001 | |
| Orthocladiinae | CCU treatment | 53.43 | <0.0001 |
| instar size | 17.99 | <0.0001 | |
| CCU treatment × instar size | 2.13 | 0.0076 | |
| Hydropsyche spp. | CCU treatment | 4.56 | 0.0023 |
| instar size | 53.73 | <0.0001 | |
| CCU treatment × instar size | 2.75 | 0.0008 | |
| Isoperla spp. | CCU treatment | 14.75 | <0.0001 |
| instar size | 4.54 | 0.0014 | |
| CCU treatment × instar size | 1.62 | 0.0762 |
Figure 4.
Relationships between mortality and head-capsule size in mesocosm experiments (Cu + Zn) from 30 August to 12 September 2015. Each symbol represents the average proportional mortality of three replicates in each treatment level.
Acute toxicity of Zn to the mayfly Baetis tricaudatus in the single-species experiment decreased as organism size increased (Figure 5; Table S6). LC50 values for first and mid-instar B. tricaudatus were 600.1 (95% confidence interval = 460.5–782.1) μg Zn/L and 6094.3 (95% confidence interval = 4946.2–7509.1) μg Zn/L, respectively. These experiments were conducted in the same laboratory and used the same water sources as Baetis tricaudatus experiments described by Brinkman and Johnston10 who reported LC50 values of 10 020 μg/L. Water quality (Tables S3 and S6) did not differ between these studies.
Figure 5.
Mortality of early instars (light blue squares) and mid-instars (dark blue circles) of Baetis tricaudatus after 96 h exposure to Zn. Results from Brinkman and Johnston10 (red diamonds) are included for a comparison to late instars. (±s.e.; n = 4).
Discussion
We present several lines of evidence that body size of aquatic insects is a strong predictor of metal sensitivity, with greater sensitivity observed in smaller individuals than in larger individuals. The naturally colonized benthic communities used in the mesocosm studies incorporated a diverse size structure within and among taxa. This enabled us to evaluate aquatic insect responses to metals across numerous taxonomic groups and developmental size classes. At metals concentrations in which partial mortality occurred, smaller organisms were consistently more sensitive than larger organisms. Size-dependent responses of Baetis spp., the dominant mayfly in many western streams,40−42 occurred in the four mesocosm experiments and in the single-species toxicity tests. Across all taxa, metal sensitivity was inversely correlated with body mass, with small organisms displaying a wide range of sensitivity to aqueous metals, but large organisms, regardless of species, displaying greater tolerance. Importantly, size-dependent sensitivity occurred even in taxa that are generally considered tolerant to metal exposure. For example, laboratory and field studies have demonstrated that hydropsychid caddisflies are highly tolerant to metals.2,3,43 However, in our study Hydropsyche spp. had the most pronounced size-dependent treatment effects, with greater than 50% mortality of early instars (<0.3 mm) in the lowest metal concentrations (4 CCU).
Consistent with our hypothesis, small size classes had a range of sensitivities to metals, but taxa represented primarily by large size classes (e.g., Drunella spp., Arctopsyche sp., Brachycentrus sp.) were only tolerant. All aquatic macroinvertebrates hatch as small-bodied individuals, and selection against sensitive taxa likely occurs during these early stages of development. Phylogenetic differences in acclimating to stressors is perhaps of greatest importance for early instars.
Single-species laboratory studies with aquatic insects routinely suggest that these organisms are highly tolerant to metals.6,9−11 Laboratory experiments using field-collected aquatic insects (i.e., Drunella doddsii, Ephemerella sp., Cinygmula sp., Lepidostoma sp., and Chloroperlidae) report LC50 ranging from 32,000 to 64,000 μg Zn/L.10 In these studies, larvae were large enough to be collected by hand, and survival was easily assessed without magnification. These LC50 values are orders of magnitude higher than thresholds reported in mesocosm experiments and field studies.2,12,14
Although other environmental factors such as colonization dynamics, drift and emergence propensity, and duration of life cycle likely contribute to laboratory and field discrepancies, our results strongly suggest that the developmental size progression of aquatic insects influences metal sensitivity. These results may also explain, in part, why laboratory experiments typically demonstrate aquatic insects are tolerant to metals, while mesocosm and field studies indicate they are quite sensitive. The physical and chemical cues that influence the phenology of macroinvertebrates in the field likely affect their spatiotemporal sensitivity to contaminants. For example, environmental cues such as degree days, streamflow, and day length influence hatching, adult aquatic insect emergence, diapause, and secondary production.44−46 Seasonal fluctuations in metal concentrations may co-occur with the presence of sensitive or tolerant life stages, and changes in water chemistry may affect certain life stages of some taxa but not others based on their timing of development.
Our findings have important implications for biomonitoring studies designed to assess effects of contaminants. Field studies typically use sampling procedures that retain only large benthic organisms (e.g., 500 or 350 μm mesh) because smaller mesh sizes clog easily, slow sampling speed, and are often impractical to use. Early instars are not retained in these samples, so effects of metals and other stressors may be underestimated in the field. Sampling small age classes in nature and conducting toxicity trials with small age classes is difficult. This is especially true for the interstitial spaces between gravel and cobble substrate. Innovations in sampling procedures that collect early instars (e.g., smaller mesh sizes) have the potential to improve ecotoxicological studies.
Observational studies have demonstrated that large maximal body size is a trait commonly associated with taxa at contaminated sites19−23 In contrast, Pomeranz et al.47 found abundance of both large and small organisms was reduced by aqueous metals. This could possibly suggest smaller organisms are more susceptible and larger organisms did not have the energy needed to mature to a large size or that large bodied predators were lost when small organisms were extirpated. Observational studies are limited in addressing these relationships because immigration and emigration are not controlled, whereas our experiments measured the direct effects of toxicity on survival controlling for the influence of immigration. It is possible that maximal body size predicts which taxa can immigrate and survive at a site, but minimal body size at a site might better explain which species can actually complete their full life cycle.
Benthic survey comparisons in the Rocky Mountains have demonstrated the influence of insect phenology on metal sensitivity along elevation gradients and among seasons.16,17 Although we generally observed greater mortality in less mature instars, the results were complicated by the concurrent emergence of larger organisms during our experiments. For example, Baetis spp. in the October 2007 experiment was dominated by late instars. It is possible that some of the lower abundances in larger size classes that we attributed to larval mortality were at least partly the result of adult emergence, which was not quantified in these experiments. Toxicity models need to better incorporate early instar sizes and differentiate sensitivity throughout an organism’s life cycle. Moreover, linking invertebrate phenology to the temporal changes of contaminant concentrations in the field will better characterize exposure outcomes.
Standard testing guidelines(e.g., those from the American Society for Testing and Materials48 and the U.S. Environmental Protection Agency31,49) have long noted the importance of using early life stages in toxicity tests. These same guidelines limit “acceptable” mortality in controls to 5–10%, a requirement likely intended to limit the risk of erroneously determining a toxic effect when none exists. Starting in the early twentieth century, ecologists have used the concept of survivorship curves (Figure 6) to describe the natural rates of mortality throughout an organism’s lifespan.50,51 Fish and aquatic insects generally display a type III survivorship curve, with high mortality in early life stages (dashed box in Figure 6) and a lower mortality in later life stages (solid box in Figure 6). High mortality in early life stages can be attributed to predation, limited resources, competition, and the stochastic mortality commonly observed in r-selected species. For example, Willis and Hendricks52 conducted a comprehensive study of the population dynamics of the caddisfly Hydropysche slossonae in an undisturbed river and observed first instar mortality approaching 93%. These high rates of natural mortality would be unacceptable in the current testing guidance (e.g., those from the American Society for Testing and Materials48 and the U.S. Environmental Protection Agency31,49). This, illustrates the challenges associated with developing test protocols for aquatic insects that balance environmental realism and laboratory control. Early instar toxicity tests are rarely attempted or the results are excluded from criteria/guideline derivation data sets. More research is needed to characterize background mortality of early instars of aquatic insects, so benchmark “acceptable” control mortality can be established for early life stages.
Figure 6.
Conceptual survivorship curves are commonly used by ecologists to characterize life history traits. Fish and insects generally occupy a type III curve, whereas longer lived species such as large mammals typically occupy a type I curve. The dashed box includes early, more sensitive life stages; the solid box represents larger, more tolerant age classes. Mortality in controls similar to that in the dashed box would be deemed unacceptable in standardized testing guidelines, but it is common in natural aquatic communities.
Recent advancements in full life cycle tests using parthenogenetic mayflies53−55 has enabled assessment of first instar mayflies, full life cycle trials, and have potential to include more sensitive species at early life stages.53 The novel single-species toxicity test methodology presented in this paper, along with the ability to genetically identify species before diagnostic morphological characteristics develop, offers an alternative technique to test the sensitivity of early instars for species that are not parthenogenetic or not able to be cultured in the laboratory. The toxicity test method incorporates flow in a way that better simulates hyporheic hydrologic processes (e.g., exchange of dissolved oxygen and water, and toxicant replenishment) and enables handling and enumeration of small early instars. These methods may prove helpful during the early life stages of complete life cycle tests using lotic species. Although this method routinely produced acceptable control survival (94–100%), success may be limited to species that oviposit in clusters (pads) and have higher rates of survival in early age classes. This experiment was only possible after a decade of efforts to culture numerous mayfly species, in which Baetis was found to be the most tolerant of laboratory conditions. Although these methods produced an acute LC50 value for early instars at 6% of the value obtained from late instars, even surrogate test species like this might routinely fail to represent the sensitivity of aquatic invertebrates found in natural communities.56 This stark limitation implies the need to develop more innovative testing methods and/or ways to incorporate stream mesocosm results into the development of water quality guidelines and criteria.57
Our results demonstrate that aquatic insect body size is a strong predictor of susceptibility to metals. Size-dependent responses occurred among multiple aquatic insect orders, with smaller invertebrates generally displaying greater susceptibility to metals than in larger, mature invertebrates These conclusions agree with numerous laboratory experiments showing that smaller or younger age classes of aquatic insects are more sensitive. Powlesland and George24 found the median effect concentration for first instar Chironomus riparius (Diptera) to be half that of second instars when exposed to nickel. Regardless of duration of exposure, (24, 48, 96 h) mortality of Agapetus fuscipes (Trichoptera) was greatest for first instars exposed to cadmium.25 Additionally, crucial sublethal behaviors were altered in early instars. Diamond et al.26 found smaller sizes classes of the heptageniid mayfly Stenonema modestum to be more sensitive to sodium chloride. Despite these studies, toxicity models and risk assessment rarely considers body size or phenology. The addition of body size in our models improved model fit compared to metal concentration alone. Toxicity models that account for the sensitive life stages of aquatic insects have the potential to improve the accuracy in predicting effects of contaminants in the field.
Further study into the mechanisms of toxicity at the cellular, biochemical, and physical level may begin to explain why small sized organisms are susceptible. The results presented here imply body size is inversely correlated to sensitivity to toxicants. However, many other characteristics and life processes are correlated to age and each might add to toxic stress. These are not limited to hatching, molting, emergence, growth rates, competition, predator avoidance, prey/forage abundance, seasonal timing, voltinism. These also may help explain why early age classes are more sensitive than older age classes.
All aquatic insects hatch as nearly microscopic organisms and small size classes were consistently the most sensitive in our experiments. Failure to characterize sensitivity of early size classes may lead to gross overestimation of tolerance. To paraphrase Horton in reference to Who-ville, “an [insect’s] an [insect] no matter how small.”1
Acknowledgments
Funding for this research was provided by the National Institute of Environmental Health Sciences (1R01ES020917-01) and the US Fish and Wildlife Service Federal Aid Project F-243-R22. We thank Boris C. Kondratieff for the insight and edits. We thank Joseph S. Meyer for his critiques and for adding the “Horton the elephant” references. S. F. Brinkman and W. D. Johnston for many years refining and improving techniques used to culture mayflies in the laboratory. Lastly, we acknowledge Dr. Seuss for being so prescient.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.est.9b04089.
Hardness-adjusted criterion values for mesocosm studies, average Cu, Zn, and Cd concentrations with CCU calculations, average and range of water chemistry parameters for mesocosm experiments, average water chemistry values of dilution water to SRL, ANCOVA and multiple comparison results, survival and Zn concentrations from 2014 single-species trials using Baetis tricaudatus, Baetis tricaudatus egg mass image, image of exposure tube used in single-species testing, illustration of exposure tubes placed under dissecting scope, schematic of exposure tube mechanics, and picture of exposure system. (PDF)
Author Contributions
The role of first author was shared equally by P.C. and C.J.K.
The authors declare no competing financial interest.
Supplementary Material
References
- Dr. Seuss . Horton Hears a Who!; Random House: New York, NY, USA, 1954. [Google Scholar]
- Clements W. H.; Carlisle D. M.; Lazorchak J. M.; Johnson P. C. Heavy metals structure benthic communities in Colorado mountain streams. Ecological Applications 2000, 10 (2), 626–638. 10.1890/1051-0761(2000)010[0626:HMSBCI]2.0.CO;2. [DOI] [Google Scholar]
- Mebane C. A. Testing Bioassessment Metrics: Macroinvertebrate, Sculpin, and Salmonid Responses to Stream Habitat, Sediment, and Metals. Environ. Monit. Assess. 2001, 67 (3), 293–322. 10.1023/A:1006306013724. [DOI] [PubMed] [Google Scholar]
- Maret T. R.; Cain D. J.; MacCoy D. E.; Short T. M. Response of benthic invertebrate assemblages to metal exposure and bioaccumulation associated with hard-rock mining in northwestern streams, USA. Journal of the North American Benthological Society 2003, 22 (4), 598–620. 10.2307/1468356. [DOI] [Google Scholar]
- Cain D. J.; Luoma S. N.; Wallace W. G. Linking metal bioaccumulation of aquatic insects to their distribution patterns in a mining-impacted river. Environ. Toxicol. Chem. 2004, 23 (6), 1463–1473. 10.1897/03-291. [DOI] [PubMed] [Google Scholar]
- Brix K. V.; Deforest D. K.; Burger M.; Adams W. J. Assessing the relative sensitivity of aquatic organisms to divalent metals and their representation in toxicity datasets compared to natural aquatic communities. Hum. Ecol. Risk Assess. 2005, 11 (6), 1139–1156. 10.1080/10807030500278628. [DOI] [Google Scholar]
- Herbst D. B.; Medhurst R. B.; Black N. J. Long-term effects and recovery of streams from acid mine drainage and evaluation of toxic metal threshold ranges for macroinvertebrate community reassembly. Environ. Toxicol. Chem. 2018, 37 (10), 2575–2592. 10.1002/etc.4217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornberger M. I.; Luoma S. N.; Johnson M. L.; Holyoak M. Influence of remediation in a mine-impacted river: metal trends over large spatial and temporal scales. Ecological Applications 2009, 19 (6), 1522–1535. 10.1890/08-1529.1. [DOI] [PubMed] [Google Scholar]
- Brinkman S. F.; Johnston W. D. Acute toxicity of aqueous copper, cadmium, and zinc to the mayfly Rhithrogena hageni. Arch. Environ. Contam. Toxicol. 2008, 54 (3), 466–472. 10.1007/s00244-007-9043-z. [DOI] [PubMed] [Google Scholar]
- Brinkman S. F.; Johnston W. D. Acute toxicity of zinc to several aquatic species native to the Rocky Mountains. Arch. Environ. Contam. Toxicol. 2012, 62 (2), 272–281. 10.1007/s00244-011-9698-3. [DOI] [PubMed] [Google Scholar]
- Mebane C. A.; Dillon F. S.; Hennessy D. P. Acute toxicity of cadmium, lead, zinc, and their mixtures to stream-resident fish and invertebrates. Environ. Toxicol. Chem. 2012, 31 (6), 1334–1348. 10.1002/etc.1820. [DOI] [PubMed] [Google Scholar]
- Clements W. H. Small-scale experiments support causal relationships between metal contamination and macroinvertebrate community responses. Ecological Applications 2004, 14 (3), 954–967. 10.1890/03-5009. [DOI] [Google Scholar]
- Buchwalter D. B.; Cain D. J.; Clements W. H.; Luoma S. N. Using biodynamic models to reconcile differences between laboratory toxicity tests and field biomonitoring with aquatic insects. Environ. Sci. Technol. 2007, 41 (13), 4821–4828. 10.1021/es070464y. [DOI] [PubMed] [Google Scholar]
- Schmidt T. S.; Clements W. H.; Mitchell K. A.; Church S. E.; Wanty R. B.; Fey D. L.; Verplanck P. L.; San Juan C. A. Development of a new toxic-unit model for the bioassessment of metals in streams. Environ. Toxicol. Chem. 2010, 29 (11), 2432–2442. 10.1002/etc.302. [DOI] [PubMed] [Google Scholar]
- Clements W. H.; Cadmus P.; Brinkman S. F. Responses of aquatic insects to Cu and Zn in stream microcosms: understanding differences between single species tests and field responses. Environ. Sci. Technol. 2013, 47 (13), 7506–7513. 10.1021/es401255h. [DOI] [PubMed] [Google Scholar]
- Kiffney P. M.; Clements W. H. Size-dependent response of macroinvertebrates to metals in experimental streams. Environ. Toxicol. Chem. 1996, 15 (8), 1352–1356. 10.1002/etc.5620150814. [DOI] [Google Scholar]
- Clark J. L.; Clements W. H. The use of in situ and stream microcosm experiments to assess population- and community-level responses to metals. Environ. Toxicol. Chem. 2006, 25 (9), 2306–2312. 10.1897/05-552.1. [DOI] [PubMed] [Google Scholar]
- Kotalik C. J.; Clements W. H. Stream mesocosm experiments show significant differences in sensitivity of larval and emerging adults to metals. Environ. Sci. Technol. 2019, 53 (14), 8362–8370. 10.1021/acs.est.9b00883. [DOI] [PubMed] [Google Scholar]
- Archaimbault V.; Usseglio-Polatera P.; Vanden Bossche J. P. Functional differences among benthic macroinvertebrate communities in reference streams of same order in a given biogeographic area. Hydrobiologia 2005, 551 (1), 171–182. 10.1007/s10750-005-4459-9. [DOI] [Google Scholar]
- Statzner B.; Bady P.; Doledec S.; Scholl F. Invertebrate traits for the biomonitoring of large European rivers: an initial assessment of trait patterns in least impacted river reaches. Freshwater Biol. 2005, 50 (12), 2136–2161. 10.1111/j.1365-2427.2005.01447.x. [DOI] [Google Scholar]
- Doledec S.; Statzner B. Invertebrate traits for the biomonitoring of large European rivers: an assessment of specific types of human impact. Freshwater Biol. 2008, 53 (3), 617–634. 10.1111/j.1365-2427.2007.01924.x. [DOI] [Google Scholar]
- Statzner B.; Bis B.; Doledec S.; Usseglio-Polatera P. Perspectives for biomonitoring at large spatial scales: a unified measure for the functional composition on invertebrate communities in European running waters. Basic Appl. Ecol. 2001, 2 (1), 73–85. 10.1078/1439-1791-00039. [DOI] [Google Scholar]
- Statzner B.; Beche L. A. Can biological invertebrate traits resolve effects of multiple stressors on running water ecosystems?. Freshwater Biol. 2010, 55 (1), 80–119. 10.1111/j.1365-2427.2009.02369.x. [DOI] [Google Scholar]
- Powlesland C.; George J. Acute and chronic toxicity of nickel to larvae of Chironomus riparis (Meigen). Environ. Pollut., Ser. A 1986, 42 (1), 47–64. 10.1016/0143-1471(86)90044-9. [DOI] [Google Scholar]
- McCahon C. P.; Whiles A. J.; Pascoe D. The toxicity of cadmium to different larval instars of the trichopteran larvae Agapetusfuscipes (Curtis) and the importance of life-cycle information to the design of toxicity tests. Hydrobiologia 1989, 185 (2), 153–162. 10.1007/BF00010812. [DOI] [Google Scholar]
- Diamond J. M.; Winchester E. L.; Mackler D. G.; Gruber D. Use of the mayfly Stenonemamodestum (Heptageniidae) in subacute toxicity assessments. Environ. Toxicol. Chem. 1992, 11 (3), 415–425. 10.1002/etc.5620110316. [DOI] [Google Scholar]
- Stuhlbacher A.; Bradley M. C.; Naylor C.; Calow P. Variation in the development of cadmium resistance in Daphnia magna (Straus) effect of temperature, nutrition, age and genotype. Environ. Pollut. 1993, 80 (2), 153–158. 10.1016/0269-7491(93)90141-A. [DOI] [PubMed] [Google Scholar]
- Buchwalter D. B.; Cain D. J.; Martin C. A.; Xie L.; Luoma S. N.; Garland T. Jr. Aquatic insect ecophysiological traits reveal phylogenetically based differences in dissolved cadmium susceptibility. Proc. Natl. Acad. Sci. U. S. A. 2008, 105 (24), 8321–8326. 10.1073/pnas.0801686105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosell M.; Nielsen C.; Bianchini A. Sodium turnover rate determines sensitivity to acute copper and silver exposure in freshwater animals. Comp. Biochem. Physiol., Part C: Toxicol. Pharmacol. 2002, 133, 287–303. 10.1016/S1532-0456(02)00085-6. [DOI] [PubMed] [Google Scholar]
- Mohammed A.Why are early life stages of aquatic organisms more sensitive to toxicants than adults? New Insights into Toxicity and Drug Testing; IntechOpen, 2013; pp 49–62. [Google Scholar]
- Stephan C. E.; Mount D. I.; Hanson D. J.; Gentile J. R.; Chapman G. A.; Brungs W. A.. Guidelines for Deriving Numerical Standards for the Protection of Aquatic Organisms and Their Uses, PB85-227049; U.S. Environmental Protection Agency, U.S. EPA Office of Water: Washington, D.C., 1985. [Google Scholar]
- Carsten von der Ohe P.; Liess M. Relative sensitivity distribution of aquatic invertebrates to organic and metal compounds. Environ. Toxicol. Chem. 2004, 23 (1), 150–156. 10.1897/02-577. [DOI] [PubMed] [Google Scholar]
- Benke A. C.; Huryn A. D.; Smock L. A.; Wallace J. B. Length-mass relationships for freshwater macroinvertebrates in North America with particular reference to the southeastern United States. Journal of the North American Benthological Society 1999, 18 (3), 308–343. 10.2307/1468447. [DOI] [Google Scholar]
- Fox J.; Weisberg S.. An R Companion to Applied Regression; SAGE Publications: Thousand Oaks, CA, 2011. [Google Scholar]
- Harrell F. E. Jr.; Dupont M. C. Hmisc Package, R Package, version 2006: 2–0. https://CRAN.R-project.org/package=Hmisc.
- Burnham K. P.; Anderson D. R. Multimodel inference: understanding AIC and BIC in model selection. Sociological Methods & Research 2004, 33 (2), 261–304. 10.1177/0049124104268644. [DOI] [Google Scholar]
- Lenth R.Estimated marginal means, aka least-squares means, 2018. https://github.com/rvlenth/emmeans.
- Graves S.; Piepho H. P.; Selzer L.; Dorai-Jai S.. Multcomp View: Visualizations of paired comparisons, R Package version 0.1–5; 2012.
- Ritz C.; Baty F.; Streibig J. C.; Gerhard D. Dose-response analysis using R. PLoS One 2015, 10, e0146021 10.1371/journal.pone.0146021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward J. V.; Kondratieff B. C.; Zuellig R. E.. An Illustrated Guide to the Mountain Stream Insects of Colorado; University Press of Colorado: Boulder, Colorado, USA, 2002. [Google Scholar]
- Merritt R. W.; Cummins K. W.; Berg M. B.. An Introduction to the Aquatic Insects of North America; Kendall Hunt, 2008. [Google Scholar]
- McCafferty W. P.; Randolph R. P.; Jacobus L. M.. Mayflies of the Intermountain West, Memoirs of the American Entomological Institute 85; American Entomological Institute, 2012; pp 1–317. [Google Scholar]
- Cain D. J.; Luoma S. N. Metal exposures to native populations of the caddisfly Hydropsyche (Trichoptera: Hydropsychidae) determined from cytosolic and whole body metal concentrations. Hydrobiologia 1998, 386 (1–3), 103–117. 10.1023/A:1003583117293. [DOI] [Google Scholar]
- Benke A. C. A modification of the Hynes method for estimating secondary production with particular significance for multivoltine populations. Limnol. Oceanogr. 1979, 24 (1), 168–171. 10.4319/lo.1979.24.1.0168. [DOI] [Google Scholar]
- Vannote R. L.; Sweeney B. W. Geographic analysis of thermal equilibria: a conceptual model for evaluating the effect of natural and modified thermal regimes on aquatic insect communities. Am. Nat. 1980, 115 (5), 667–695. 10.1086/283591. [DOI] [Google Scholar]
- Peckarsky B. L.; Taylor B. W.; Caudill C. C. Hydrologic and behavioral constraints on oviposition of stream insects: implications for adult dispersal. Oecologia 2000, 125 (2), 186–200. 10.1007/s004420000446. [DOI] [PubMed] [Google Scholar]
- Pomeranz J. P. F.; Warburton H. J.; Harding J. S. Anthropogenic mining alters macroinvertebrate size spectra in streams. Freshwater Biol. 2019, 64 (1), 81–92. 10.1111/fwb.13196. [DOI] [Google Scholar]
- American Society for Testing and Materials (ASTM) . Standards on aquatic toxicology and hazard evaluation 1993, ASTM Publication Code 03–547093–16. [Google Scholar]
- U.S. Environmental Protection Agency, U.S. EPA Office of Water Methods for Measuring the Acute Toxicity of Effluents and Receiving Waters to Freshwater and Marine Organisms (4303T); U.S. Environmental Protection Agency: Washington, DC, 2002. [Google Scholar]
- Deevey E. S. Jr. Life tables for natural populations of animals. Q. Rev. Biol. 1947, 22 (4), 283–314. 10.1086/395888. [DOI] [PubMed] [Google Scholar]
- Pearl R.; Miner J. R. Experimental studies on the duration of life XIV The comparative mortality of certain lower organisms. Q. Rev. Biol. 1935, 10 (1), 60–79. 10.1086/394476. [DOI] [Google Scholar]
- Willis L. D. Jr.; Hendricks A. C. Life history, growth, survivorship, and production of Hydropsycheslossonae in Mill Creek, Virginia. Journal of the North American Benthological Society 1992, 11 (3), 290–303. 10.2307/1467649. [DOI] [Google Scholar]
- Xie L.; Funk D. H.; Buchwalter D. B. Trophic transfer of Cd from natural periphyton to the grazing mayfly Centroptilumtriangulifer in a life cycle test. Environ. Pollut. 2010, 158 (1), 272–277. 10.1016/j.envpol.2009.07.010. [DOI] [PubMed] [Google Scholar]
- Xie L.; Buchwalter D. B. Cadmium exposure route affects antioxidant responses in the mayfly Centroptilumtriangulifer. Aquat. Toxicol. 2011, 105 (3–4), 199–200. 10.1016/j.aquatox.2011.06.009. [DOI] [PubMed] [Google Scholar]
- Soucek D. J.; Dickinson A. Full-life chronic toxicity of sodium salts to the mayfly Neocloeontriangulifer in tests with laboratory cultured food. Environ. Toxicol. Chem. 2015, 34 (9), 2126–2137. 10.1002/etc.3038. [DOI] [PubMed] [Google Scholar]
- Cairns J. Jr. The myth of the most sensitive species. BioScience 1986, 36 (10), 670–672. 10.2307/1310388. [DOI] [Google Scholar]
- Buchwalter D. B.; Clements W. H.; Luoma S. N. Modernizing water quality criteria in the United States: a need to expand the definition of acceptable data. Environ. Toxicol. Chem. 2017, 36 (2), 285–291. 10.1002/etc.3654. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.