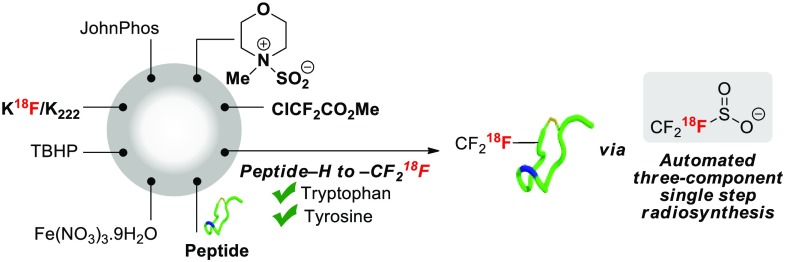
Abstract
18F labeling strategies for unmodified peptides with [18F]fluoride require 18F-labeled prosthetics for bioconjugation more often with cysteine thiols or lysine amines. Here we explore selective radical chemistry to target aromatic residues applying C–H 18F-trifluoromethylation. We report a one-step route to [18F]CF3SO2NH4 from [18F]fluoride and its application to direct [18F]CF3 incorporation at tryptophan or tyrosine residues using unmodified peptides as complex as recombinant human insulin. The fully automated radiosynthesis of octreotide[Trp(2-CF218F)] enables in vivo positron emission tomography imaging.
Positron emission tomography (PET) is a powerful molecular imaging modality for diagnosis, monitoring disease progression, studying biological processes in vivo, and investigating the efficacy of drugs.1−3 Among the radioisotopes employed for the preparation of PET probes, 18F is a widely used and clinically relevant radionuclide.2 Because of its short half-life (t1/2 = 109.7 min), 18F must be incorporated into tracer molecules at a late stage of the synthetic process.4,5 Additional challenges imposed by radiochemistry include low reaction concentration, solvent compatibility, and the fact that cyclotron-produced 18F sources are limited to 18F-fluoride and [18F]F2. These constraints are stringent for biomolecules.
18F-radiolabeled peptides can be used to measure the distribution and pharmacokinetics of peptide-based therapeutics and serve as imaging biomarkers for therapy.6,7 These benefits have encouraged the development of methods for tagging peptides with radioactive functional groups.8−10 Fluorine-18 is incorporated into prefunctionalized peptides via direct C–18F, B–18F, and Si–18F bond formation or chelation with Al–18F.11−14 Alternatively, an 18F-labeled prosthetic group is prepared prior to bioconjugation. To preserve function, this latter conjugation ideally proceeds under mild reaction conditions.15−19 Such strategies require handles with unique reactivity either by, e.g., prior installation of unnatural amino acids or by taking advantage of the inherent reactivity of natural amino acids. To date, the latter has almost exclusively exploited the nucleophilicity of cysteine thiols20 or lysine amines21 to attach the 18F-prosthetic group. Although the structural alteration imposed by the 18F-prosthetic group is typically tolerated, it could alter the efficacy and/or function.1c Therefore, innovative methods that employ [18F]fluoride and target native residues in unmodified peptides with 18F22 or a minimally sized 18F-prosthetic (e.g., [18F]CF3) are of considerable value.
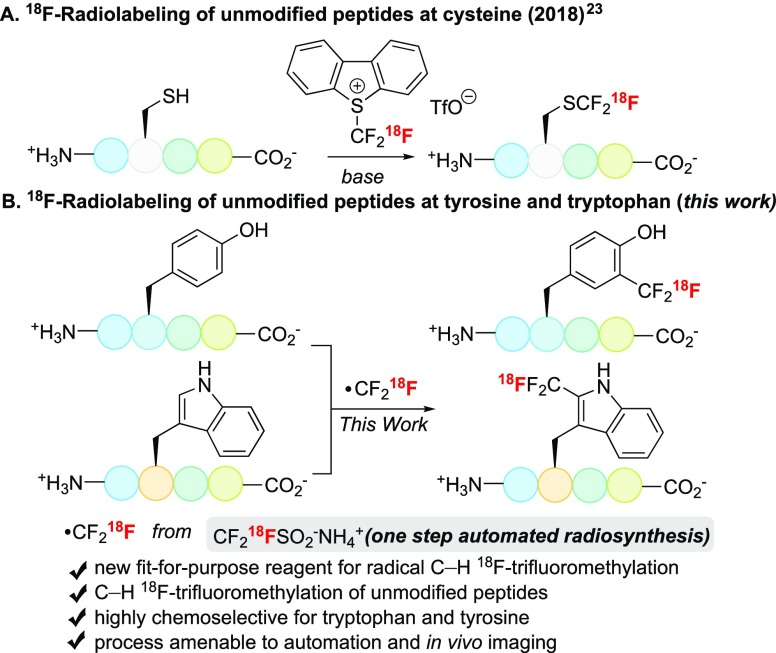
We reported the 18F-trifluoromethylation of native peptides with 5-18F-(trifluoromethyl)dibenzothiophenium trifluoromethanesulfonate, a method modifying cysteine thiols (Figure 1A).23 We also applied tuned radical chemistry to program C–H 19F-trifluoromethylation of aromatic residues in proteins.24a Sodium trifluoromethanesulfinate (NaTFMS, Langlois’ reagent) displayed selective reactivity for tryptophan under redox initiation. Recently, Krska et al. demonstrated that Zn(TFMS)2 (Baran’s reagent), when activated with a stoichiometric oxidant or via visible-light photoredox catalysis, enabled trifluoromethylation of tyrosine in peptides that do not contain tryptophan residues.25 These precedents encouraged us to produce 18F-trifluoromethanesulfinate for selective C–H 18F-trifluoromethylation of these aromatic amino acid residues within unmodified peptides. This approach would generate noncanonical [18F]CF3-tryptophan and -tyrosine residues, a transformation unmatched by alternative 18F labeling methods (Figure 1B).
Figure 1.
Direct 18F-trifluoromethylation of native residues in unmodified peptides.
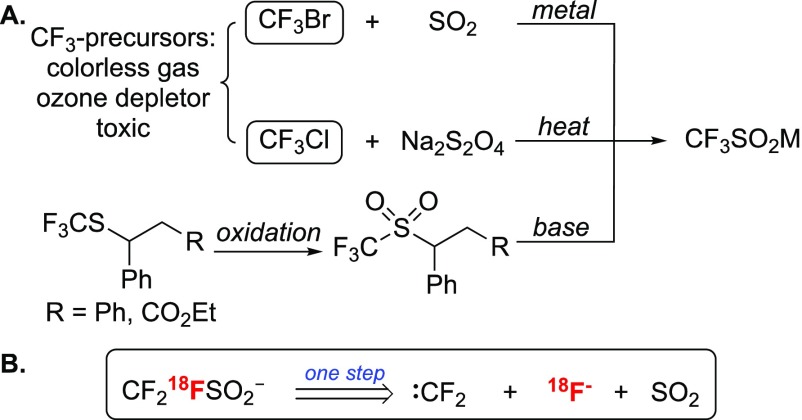
Routes toward trifluoromethanesulfinic acid salts include metal or electroreduction of a mixture of SO2 and CF3Br in N,N-dimethylformamide (DMF),26 treatment of CF3Cl with Na2S2O4,27 or multistep syntheses from trifluoromethylsulfone precursors (Scheme 1A).28 For 18F radiochemistry, these approaches would require a route toward the [18F]CF3 precursor and one or more reactions postlabeling. Our design plan was to construct [18F]CF3SO2– in one step by applying a multicomponent approach that combines 18F-fluoride, a difluorocarbene source, and SO2. The formation of [18F]CF3– from difluorocarbene and [18F]F– is known,29−31 so the challenge was to validate a protocol that couples in situ-generated [18F]CF3– with SO2 (or a surrogate of this gaseous and toxic reagent) (Scheme 1B).
Scheme 1. (A) Multistep Syntheses toward Trifluoromethanesulfinic Acid Salts (M = Metal); (B) Proposed One-Step Radiosynthesis toward 18F-Trifluoromethanesulfinate.
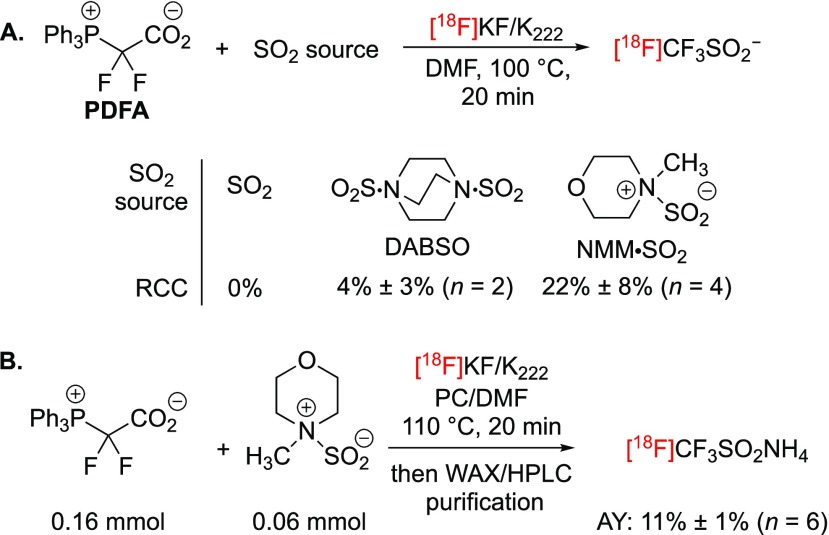
Exploratory studies performed with 19F-fluoride provided useful information.32 The difluorocarbene and SO2 sources were found to be critical in enabling the construction of CF3SO2–. The reaction of 2,2-difluoro-2-(triphenylphosphonio)acetate (PDFA) with either 1,4-diazabicyclo[2.2.2]octane bis(SO2) adduct (DABSO)33 or N-methylmorpholine·SO2 (NMM·SO2) in the presence of KF/K222 in DMF at 100 °C afforded the ammonium salt of CF3SO2– in 31% or 44% yield, respectively, after isolation by semipreparative HPLC. A saturated solution of SO2 in DMF did not lead to product formation, while ClF2CCO2Me in combination with PPh3 was the only alternative difluorocarbene source found to be suitable for this process. For 18F labeling, PDFA was elected as the optimal reagent. In contrast to experiments carried out with fluoride, DABSO and PDFA afforded [18F]CF3SO2K in trace amounts (Scheme 2A). However, the combination of PDFA, NMM·SO2 and [18F]KF/K222 gave [18F]CF3SO2K in 22% radiochemical conversion (RCC). These results encouraged the development of a manual protocol to prepare, purify, and isolate this novel 18F reagent for subsequent use (Scheme 2B). PDFA is thermally unstable and poorly soluble in DMF, so a mixture of this reagent and NMM·SO2 was added as a suspension in a suitable solvent to azeotropically dried 18F-fluoride. Among all solvents tested, propylene carbonate (PC) was best when used with DMF.34 Additional optimization tuning reagents, ratios of various components, and concentrations proved to be beneficial. The optimal process consisted of reacting PDFA (0.16 mmol) and NMM·SO2 (0.06 mmol) with [18F]KF/K222 (up to 10 GBq) in 350 μL of PC/DMF mixture at 110 °C. Initial purification of [18F]CF3SO2– using a weak anion exchange cartridge (WAX) removed most of the unreacted [18F]fluoride and organic byproducts. Elution with a solution of ∼0.4 M ammonium hydroxide in EtOH followed by reversed-phase HPLC purification afforded [18F]CF3SO2NH4 in >99% radiochemical purity. This protocol furnished up to 900 MBq of [18F]CF3SO2NH4 from 10 GBq of [18F]fluoride. The overall non-decay-corrected activity yield (AY) of isolated [18F]CF3SO2NH4 calculated from [18F]fluoride was 11% ± 1% (n = 6, synthesis time = 70 min). The identity of [18F]CF3SO2NH4 was established by HPLC and ESI-MS analysis (m/z calcd for [19F]CF3SO2–, 133.0; found, 133.1).32
Scheme 2. (A) Initial Studies toward the One-Step Synthesis of [18F]CF3SO2–; (B) Radiosynthesis, Purification, and Isolation of [18F]CF3SO2NH4.
Next, we studied the C–H 18F-trifluoromethylation of model peptides containing L-tryptophan and/or L-tyrosine residues using [18F]CF3SO2NH4 and tert-butyl hydroperoxide (TBHP) as the oxidant. In 19F mode, CF3SO2Na is added in large excess (up to ∼200 equiv) to enable C–H trifluoromethylation of peptides and proteins.24,35 These conditions are not compatible with 18F radiochemisty because of the inherent constraints on concentrations for both large peptides and [18F]CF3SO2NH4, the latter being by far the limiting reagent. An additional complication was competitive oxidation of [18F]CF3SO2– to form [18F]CF3SO3– with the initiation oxidant. For 19F-trifluoromethylation, this issue is solved using an excess of CF3SO2Na with respect to TBHP or via slow addition of TBHP to the reaction mixture.36 These solutions are not suitable for 18F labeling because [18F]CF3SO2NH4 is the limiting reagent and operational simplicity is paramount for 18F radiochemistry.
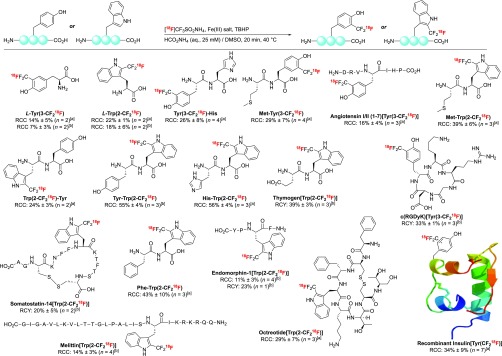
The treatment of L-Tyr with [18F]CF3SO2NH4 and TBHP in AcOH/aqueous ammonium formate did not lead to C–H 18F-trifluoromethylation after 20 min, even at 60 °C.32 Extensive optimization overcame the 18F labeling constraints and led to L-Tyr(3-CF218F) in 14% RCC when the reaction was performed in the presence of TBHP and Fe(NO3)3·9H2O36 in DMSO/aqueous ammonium formate at 40 °C for 20 min (Scheme 3).3218F-trifluoromethylation at C2 was detected in 2% RCC. These two regioisomers are separable by HPLC. The RCC of L-Tyr(3-CF218F) increased to 53% when the reaction was performed at 60 °C. When FeCl3 was used at 40 °C instead of Fe(NO3)3·9H2O, L-Tyr(3-CF218F) was formed in 7% RCC. The C–H 18F-trifluoromethylation of L-Trp was also successful with [18F]CF3SO2NH4 upon activation by either Fe(NO3)3·9H2O or FeCl3 in the presence of TBHP. When these conditions were applied, L-Trp(2-CF218F) was obtained in 22% and 18% RCC, respectively. Two additional regioisomers resulting from competitive 18F labeling at C4 and C7 were also formed, giving a combined RCC of 10% or 9% when Fe(NO3)3·9H2O or FeCl3, respectively, was employed.37
Scheme 3. Substrate Scope of C–H 18F-Trifluoromethylation of Native Aromatic Residues of Peptides.
Reagents and conditions: peptide (0.03 mmol), TBHP (2 or 4 equiv), and [a] Fe(NO3)3·9H2O (2 equiv) or [b] FeCl3 (2 equiv). The synthesis time for the 18F-labeled peptide from [18F]CF3SO2NH4 was 90 min.32
A series of dipeptides was evaluated, focusing on feasibility and selectivity (Scheme 3).32 For reactions leading to more than one 18F-labeled product, identification was made by comparison of HPLC traces with fully characterized references prepared independently. The dipeptides Tyr-Trp and Trp-Tyr underwent [18F]CF3 incorporation exclusively at Trp, a result corroborating our previous studies.24 For Tyr-Trp, 18F labeling experiments performed with [18F]CF3SO2NH4 and TBHP with either Fe(NO3)3·9H2O or FeCl3 gave 40% or 55% RCC, respectively. For the dipeptide Phe-Trp, 18F-trifluoromethylation occurred at Trp, affording Phe-Trp(2-CF218F) in 43% RCC. [18F]CF3 incorporation on Trp occurred at C2, C4, and C7 (C2 major), while 18F labeling on Phe was not observed. 18F labeling at His was not detected for either Tyr-His or His-Trp. Met oxidation was minimized for the 18F-trifluoromethylation of Met-Trp or Met-Tyr by decreasing the TBHP:Fe(III) ratio (1:1). Oxidative dimerization of cysteine by disulfide formation is unavoidable.24,25
Next, we studied biologically relevant peptides of increasing complexity. The dipeptide immunomodulator thymogen (oglufanide)38 was 18F-trifluoromethylated at Trp with an isolated radiochemical yield (RCY) calculated from [18F]CF3SO2NH4 of 39%. Endomorphin-1, a tetrapeptide associated with Alzheimer’s disease,39,40 underwent Trp-selective 18F labeling in 23% RCY, and somatostatin-14, a cyclic tetradecapeptidic hormone with a broad inhibitory effect on endocrine secretion, was 18F-labeled in 20% RCY.41 The 18F-trifluoromethylations of melittin,42 a 26-residue venom peptide, and octreotide,43 an octapeptide that mimics natural somatostatin, were equally successful (14% RCC and 29% RCC, respectively). Tyrosine-containing peptides were examined next. Angiotensin fragment 1–7, a peptide with anti-inflammatory properties,44,45 and c(RGDyK), a peptide ligand of integrin αvβ3 receptors,46 both underwent 18F labeling at Tyr in 16% and 33% RCC, respectively. The C–H 18F-trifluoromethylation of a much larger peptide, recombinant human insulin (MW = 5808 Da), was also considered.47 This experiment was carried out with insulin (5.2 μmol), Fe(NO3)3·9H2O (5.8 equiv), and TBHP (11.5 equiv) in DMSO/aqueous ammonium formate and afforded [18F]CF3-insulin in 34% overall RCC as a mixture of four products resulting from [18F]CF3 incorporation at all tyrosine residues. The main site of 18F-trifluoromethylation was at chain A residue Y19, a result consistent with the report of Krska et al.25
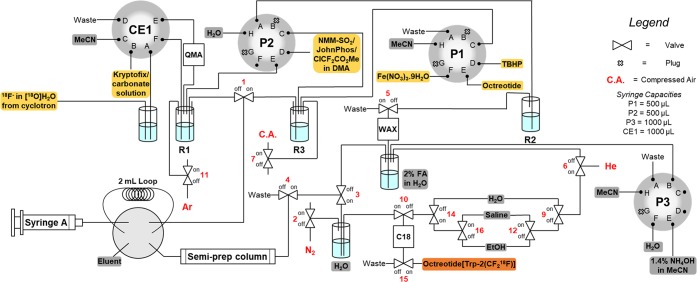
To date, automated radiosyntheses have focused on small molecules but rarely on peptides.48 To demonstrate translational applicability, we developed a fully automated radiosynthesis of octreotide[Trp(2-CF218F)] on the Advion NanoTek microfluidic synthesis system (Figure 2).32 The automated radiosynthesis of [18F]CF3SO2NH4 required optimization of selected steps. The addition of the suspension of PDFA and NMM·SO2 in PC/DMF to a vial containing [18F]KF was not compatible with automation. This issue was solved by changing the difluorocarbene source to ClF2CCO2Me, a reagent activated with (2-biphenyl)di-tert-butylphosphine (JohnPhos), and the solvent to DMA; no change was required for NMM·SO2. With these modifications, starting from up to 45 GBq of [18F]fluoride, [18F]CF3SO2NH4 was produced in up to 6% ± 1% activity yield (non-decay-corrected, n = 2) after semipreparative HPLC (Am = 1.13 GBq/μmol, synthesis time = 40 min). Removal of HPLC solvents was necessary to afford dry [18F]CF3SO2NH4 required for peptide 18F labeling. This critical drying step also required extensive modification. For automation, [18F]CF3SO2NH4 was trapped on a WAX cartridge and subsequently eluted with NH4OH in MeCN (1.4%) followed by evaporation.
Figure 2.
Automated radiosynthesis of octreotide[Trp(2-CF218F)] from [18F]fluoride on the Advion NanoTek microfluidic synthesis system.
Successful C–H 18F-trifluoromethylation in the presence of Fe(NO3)3·9H2O (4 equiv) and TBHP (8 equiv) afforded up to 69 MBq of octreotide[Trp(2-CF218F)] (n = 3, Am = 0.28 ± 0.08 GBq/μmol) after purification by HPLC. The total synthesis time from [18F]fluoride to octreotide[Trp(2-CF218F)] was 133 min. This automated protocol enabled an in vivo PET imaging experiment with this [18F]CF3-peptide on naïve Sprague–Dawley rats, a preliminary study suggesting excretion via the gastrointestinal pathway and the kidneys.32,49−51
In conclusion, we have reported the first protocol enabling direct 18F labeling of unmodified peptides at tryptophan and tyrosine residues (with high selectivity for tryptophan) via direct C–H 18F-trifluoromethylation. This method is a new tool to accelerate the discovery of 18F-peptides as imaging agents as well as the development of peptide-based drugs. The strategy required the novel 18F reagent [18F]CF3SO2NH4, which was prepared in one step from [18F]fluoride, a difluorocarbene reagent, and a source of SO2. The iron salt was essential to overcome the difficulties arising from [18F]CF3SO2NH4 being the limiting reagent, thereby enabling C–H 18F-trifluoromethylation of peptides as complex as insulin. The automated radiosynthesis of octreotide[Trp(2-CF218F)] from [18F]fluoride enabled in vivo PET imaging. This major milestone, unrivaled by known methods making use of minimally sized labeled prosthetics,23,52,53 sets the stage for in-depth investigations of clinically relevant peptides. In view of the number of reactions relying on Langlois-type reagents, [18F]CF3SO2NH4 could expand considerably the radiochemical space for PET applications beyond the peptides described herein.
Acknowledgments
Financial support was provided by the Agency for Science, Technology and Research (A*STAR, Singapore, to C.W.K.), the BBSRC (BB/M016757/1 and BB/P026311/1), European Union Horizon 2020 Marie Skłodowska-Curie Grant Agreement 675071 (to M.I.), the Swiss National Science Foundation (P2BSP2_178609 to P.G.I.), CRUK (C5255/A16466), the Medical Research Council (MR/R01695X/1), and the EPSRC (EP/L025604/1, NS/A000024/1, and EP/L015838/1). The authors thank Dr. M. Tredwell for helpful discussions.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacs.9b11709.
Detailed experimental procedures, characterization of new compounds, automation protocol, and in vivo experiments (PDF)
Author Present Address
⊥ C.W.K: Institute of Chemical and Engineering Sciences, A*STAR, 1 Pesek Road, Jurong Island, 627833 Singapore.
Author Contributions
# C.W.K. and O.T. contributed equally.
The authors declare no competing financial interest.
Supplementary Material
References
- a Phelps M. E. Positron emission tomography provides molecular imaging of biological processes. Proc. Natl. Acad. Sci. U. S. A. 2000, 97, 9226–9233. 10.1073/pnas.97.16.9226. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Matthews P. M.; Rabiner E. A.; Passchier J.; Gunn R. N. Positron emission tomography molecular imaging for drug development. Br. J. Clin. Pharmacol. 2012, 73, 175–186. 10.1111/j.1365-2125.2011.04085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Krishnan H. S.; Ma L.; Vasdev N.; Liang S. H. 18F-Labeling of sensitive biomolecules for positron emission tomography. Chem. - Eur. J. 2017, 23, 15553–15577. 10.1002/chem.201701581. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Willmann J. K.; van Bruggen N.; Dinkelborg L. M.; Gambhir S. S. Molecular imaging in drug development. Nat. Rev. Drug Discovery 2008, 7, 591–607. 10.1038/nrd2290. [DOI] [PubMed] [Google Scholar]
- a Miller P. W.; Long N. J.; Vilar R.; Gee A. D. Synthesis of 11C, 18F, 15O and 13N radiolabels for positron emission tomography. Angew. Chem., Int. Ed. 2008, 47, 8998–9033. 10.1002/anie.200800222. [DOI] [PubMed] [Google Scholar]; b Jacobson O.; Kiesewetter D. O.; Chen X. Fluorine-18 radiochemistry, labeling strategies and synthetic routes. Bioconjugate Chem. 2015, 26, 1–18. 10.1021/bc500475e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Ametamey S. M.; Honer M.; Schubiger P. A. Molecular imaging with PET. Chem. Rev. 2008, 108, 1501–1516. 10.1021/cr0782426. [DOI] [PubMed] [Google Scholar]; b Deng X.; Rong J.; Wang L.; Vasdev N.; Zhang L.; Josephson L.; Liang S. H. Chemistry for positron emission tomography: recent advances in 11C-, 18F-, 13N-, 15O-labeling reactions. Angew. Chem., Int. Ed. 2019, 58, 2580–2605. 10.1002/anie.201805501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole E. L.; Stewart M. N.; Littich R.; Hoareau R.; Scott P. J. H. Radiosyntheses using fluorine-18: the art of late-stage fluorination. Curr. Top. Med. Chem. 2014, 14, 875–900. 10.2174/1568026614666140202205035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks A. F.; Topczewski J. J.; Ichiishi N.; Sanford M. S.; Scott P. J. H. Late-stage [18F]fluorination: new solutions to old problems. Chem. Sci. 2014, 5, 4545–4553. 10.1039/C4SC02099E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sah B. R.; Burger I. A.; Schibli R.; Friebe M.; Dinkelborg L.; Graham K.; Borkowski S.; Bacher-Stier C.; Valencia R.; Srinivasan A.; Hany T. F.; Mu L.; Wild P. J.; Schaefer N. G. Dosimetry and First Clinical Evaluation of the New 18F-Radiolabeled Bombesin Analogue BAY 864367 in Patients with Prostate Cancer. J. Nucl. Med. 2015, 56, 372–378. 10.2967/jnumed.114.147116. [DOI] [PubMed] [Google Scholar]
- Marik J.; Sutcliffe J. L. Click for PET: rapid preparation of [18F]fluoropeptides using CuI catalyzed 1,3-dipolar cycloaddition. Tetrahedron Lett. 2006, 47, 6681–6684. 10.1016/j.tetlet.2006.06.176. [DOI] [Google Scholar]
- Richter S.; Wuest F. 18F-Labeled peptides: the future is bright. Molecules 2014, 19, 20536–20556. 10.3390/molecules191220536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson I. M.; Scott P. J. H.; Thompson S. Clinical applications of radiolabeled peptides for PET. Semin. Nucl. Med. 2017, 47, 493–523. 10.1053/j.semnuclmed.2017.05.007. [DOI] [PubMed] [Google Scholar]
- Morris O.; Fairclough M.; Grigg J.; Prenant C.; Mcmahon A. A review on the approaches to 18F radiolabelling affinity peptides and proteins. J. Labelled Compd. Radiopharm. 2019, 62, 4–23. 10.1002/jlcr.3634. [DOI] [PubMed] [Google Scholar]
- Thompson S.; Onega M.; Ashworth S.; Fleming I. N.; Passchier J.; O’Hagan D. A two-step fluorinase enzyme mediated 18F labelling of an RGD peptide for positron emission tomography. Chem. Commun. 2015, 51, 13542–13545. 10.1039/C5CC05013H. [DOI] [PubMed] [Google Scholar]
- Perrin D. M. [18F]-Organotrifluoroborates as radioprosthetic groups for PET imaging: from design principles to preclinical applications. Acc. Chem. Res. 2016, 49, 1333–1343. 10.1021/acs.accounts.5b00398. [DOI] [PubMed] [Google Scholar]
- a Bernard-Gauthier V.; Wängler C.; Schirrmacher E.; Kostikov A.; Jurkschat K.; Wängler B.; Schirrmacher R. 18F-labeled silicon-based fluoride acceptors: potential opportunities for novel positron emitting radiopharmaceuticals. BioMed Res. Int. 2014, 2014, 454503. 10.1155/2014/454503. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Cleeren F.; Lecina J.; Bridoux J.; Devoogdt N.; Tshibangu T.; Xavier C.; Bormans G. Direct fluorine-18 labeling of heat-sensitive biomolecules for positron emission tomography imaging using the Al18F-RESCA method. Nat. Protoc. 2018, 13, 2330–2347. 10.1038/s41596-018-0040-7. [DOI] [PubMed] [Google Scholar]
- Laverman P.; McBride W. J.; Sharkey R. M.; Goldenberg D. M.; Boerman O. C. Al18F labeling of peptides and proteins. J. Labelled Compd. Radiopharm. 2014, 57, 219–223. 10.1002/jlcr.3161. [DOI] [PubMed] [Google Scholar]
- a Marik J.; Sutcliffe J. L. Click for PET: rapid preparation of [18F]fluoropeptides using CuI catalyzed 1,3-dipolar cycloaddition. Tetrahedron Lett. 2006, 47, 6681–6684. 10.1016/j.tetlet.2006.06.176. [DOI] [Google Scholar]; b Meyer J. P.; Adumeau P.; Lewis J. S.; Zeglis B. M. Click chemistry and radiochemistry: the first 10 years. Bioconjugate Chem. 2016, 27, 2791–2807. 10.1021/acs.bioconjchem.6b00561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Z.; Gouverneur V.; Davis B. G. Enhanced aqueous Suzuki-Miyaura coupling allows site-specific polypeptide 18F-labeling. J. Am. Chem. Soc. 2013, 135, 13612–13615. 10.1021/ja4049114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson O.; Yan X.; Ma Y.; Niu G.; Kiesewetter D. O.; Chen X. Novel method for radiolabeling and dimerizing thiolated peptides using 18F-hexafluorobenzene. Bioconjugate Chem. 2015, 26, 2016–2020. 10.1021/acs.bioconjchem.5b00278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Way J. D.; Bergman C.; Wuest F. Sonogashira cross-coupling reaction with 4-[18F]fluoroiodobenzene for rapid 18F-labelling of peptides. Chem. Commun. 2015, 51, 3838–3841. 10.1039/C5CC00182J. [DOI] [PubMed] [Google Scholar]
- Chiotellis A.; Sladojevich F.; Mu L.; Müller Herde A.; Valverde I. E.; Tolmachev V.; Schibli R.; Ametamey S. M.; Mindt T. L. Novel chemoselective 18F-radiolabeling of thiol-containing biomolecules under mild aqueous conditions. Chem. Commun. 2016, 52, 6083–6086. 10.1039/C6CC01982J. [DOI] [PubMed] [Google Scholar]
- Chalker J. M.; Bernardes G. J. L.; Lin Y. A.; Davis B. G. Chemical modification of proteins at cysteine: opportunities in chemistry and biology. Chem. - Asian J. 2009, 4, 630–640. 10.1002/asia.200800427. [DOI] [PubMed] [Google Scholar]
- Clark J.; O’Hagan D. Strategies for radiolabelling antibody, antibody fragments and affibodies with fluorine-18 as tracers for positron emission tomography (PET). J. Fluorine Chem. 2017, 203, 31–46. 10.1016/j.jfluchem.2017.08.001. [DOI] [Google Scholar]
- For recent reports on 18F-labeling of peptides, see:; a Britton R.; Yuan Z.; Nodwell M.; Yang H.; Malik N.; Merkens H.; Benard F.; Martin R.; Schaffer P. Site-selective, Late-Stage C–H 18F-fluorination on unprotected peptides for positron emission tomography imaging. Angew. Chem., Int. Ed. 2018, 57, 12733–12736. 10.1002/anie.201806966. [DOI] [PubMed] [Google Scholar]; b Rickmeier J.; Ritter T. Site-specific deoxyfluorination of small peptides with [18F]fluoride. Angew. Chem., Int. Ed. 2018, 57, 14207–14211. 10.1002/anie.201807983. [DOI] [PubMed] [Google Scholar]
- Verhoog S.; Kee C. W.; Wang Y.; Khotavivattana T.; Wilson T. C.; Kersemans V.; Smart S.; Tredwell M.; Davis B. G.; Gouverneur V. 18F-Trifluoromethylation of unmodified peptides with 5-18F-(trifluoromethyl)dibenzothiophenium trifluoromethanesulfonate. J. Am. Chem. Soc. 2018, 140, 1572–1575. 10.1021/jacs.7b10227. [DOI] [PubMed] [Google Scholar]
- a Imiołek M.; Karunanithy G.; Ng W. L.; Baldwin A. J.; Gouverneur V.; Davis B. G. Selective radical trifluoromethylation of native residues in proteins. J. Am. Chem. Soc. 2018, 140, 1568–1571. 10.1021/jacs.7b10230. [DOI] [PMC free article] [PubMed] [Google Scholar]; For a recent example of (perfluoro)alkylation of histidine in unprotected peptides, see:; b Noisier A. M.; Johansson M. J.; Knerr L.; Hayes M. A.; Drury W. J. III; Valeur E.; Malins L. R.; Gopalakrishnan R. Late-Stage Functionalization of Histidine in Unprotected Peptides. Angew. Chem., Int. Ed. 2019, 58, 19096–19102. 10.1002/anie.201910888. [DOI] [PubMed] [Google Scholar]
- Ichiishi N.; Caldwell J. P.; Lin M.; Zhong W.; Zhu X.; Streckfuss E. C.; Kim H.-Y. Y.; Parish C. A.; Krska S. W. Protecting group free radical C-H trifluoromethylation of peptides. Chem. Sci. 2018, 9, 4168–4175. 10.1039/C8SC00368H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folest J.-C.; Nédélec J.-Y.; Périchon J. Electrochemical synthesis of trifluoromethane sulfinic acid salt from CF3Br and SO2. Synth. Commun. 1988, 18, 1491–1494. 10.1080/00397918808081305. [DOI] [Google Scholar]
- Cao H. P.; Chen Q. Y. Practical and efficient synthesis of perfluoroalkyl iodides for perfluoroalkyl chlorides via modified sulfinatodehalogenation. J. Fluorine Chem. 2007, 128, 1187–1190. 10.1016/j.jfluchem.2007.04.018. [DOI] [Google Scholar]
- Langlois B. R.; Billard T.; Mulatier J.-C.; Yezeguelian C. A new preparation of trifluoromethanesulfinate salts. J. Fluorine Chem. 2007, 128, 851–856. 10.1016/j.jfluchem.2007.04.012. [DOI] [Google Scholar]
- Huiban M.; Tredwell M.; Mizuta S.; Wan Z.; Zhang X.; Collier T. L.; Gouverneur V.; Passchier J. A broadly applicable [18F]trifluoromethylation of aryl and heteroaryl iodides for PET imaging. Nat. Chem. 2013, 5, 941–944. 10.1038/nchem.1756. [DOI] [PubMed] [Google Scholar]
- Zheng J.; Wang L.; Lin J. H.; Xiao J. C.; Liang S. H. Difluorocarbene-derived trifluoromethylthiolation and [18F]trifluoromethylthiolation of aliphatic electrophiles. Angew. Chem., Int. Ed. 2015, 54, 13236–13240. 10.1002/anie.201505446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J.; Cheng R.; Lin J. H.; Yu D. H.; Ma L.; Jia L.; Zhang L.; Wang L.; Xiao J. C.; Liang S. H. An unconventional mechanistic insight into SCF3 formation from difluorocarbene: preparation of 18F-labeled α-SCF3 carbonyl compounds. Angew. Chem., Int. Ed. 2017, 56, 3196–3200. 10.1002/anie.201611761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For details, see the Supporting Information.
- Emmett E. J.; Willis M. C. The development and application of sulfur dioxide surrogates in synthetic organic chemistry. Asian J. Org. Chem. 2015, 4, 602–611. 10.1002/ajoc.201500103. [DOI] [Google Scholar]
- The conversion of CF3SO2– into CF3SSCF3 induced by PPh3 is a side reaction that is not observed in the presence of DMF. See:Yang Y.; Xu L.; Yu S.; Liu X.; Zhang Y.; Vicic D. A. Triphenylphosphine-mediated deoxygenative reduction of CF3SO2Na and its application for trifluoromethylthiolation of aryl iodides. Chem. - Eur. J. 2016, 22, 858–863. 10.1002/chem.201504790. [DOI] [PubMed] [Google Scholar]
- Ji Y.; Brueckl T.; Baxter R. D.; Fujiwara Y.; Seiple I. B.; Su S.; Blackmond D. G.; Baran P. S. Innate C-H trifluoromethylation of heterocycles. Proc. Natl. Acad. Sci. U. S. A. 2011, 108, 14411–14415. 10.1073/pnas.1109059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langlois B. R.; Laurent E.; Roidot N. Trifluoromethylation of aromatic compounds with sodium trifluoromethanesulfinate under oxidative conditions. Tetrahedron Lett. 1991, 32, 7525–7528. 10.1016/0040-4039(91)80524-A. [DOI] [Google Scholar]
- Kuttruff C. A.; Haile M.; Kraml J.; Tautermann C. S. Late-stage functionalization of drug-like molecules using diversinates. ChemMedChem 2018, 13, 983–987. 10.1002/cmdc.201800151. [DOI] [PubMed] [Google Scholar]
- Deigin V. I.; Semenets T. N.; Zamulaeva I. A.; Maliutina Y. V.; Selivanova E. I.; Saenko A. S.; Semina O. V. The effect of the EW dipeptide optical and chemical isomers on the CFU-S population in intact and irradiated mice. Int. Immunopharmacol. 2007, 7, 375–382. 10.1016/j.intimp.2006.11.010. [DOI] [PubMed] [Google Scholar]
- Zadina J. E.; Hackler L.; Ge L. J.; Kastin A. J. A potent and selective endogenous agonist for the μ-opiate receptor. Nature 1997, 386, 499–502. 10.1038/386499a0. [DOI] [PubMed] [Google Scholar]
- Frydman-Marom A.; Convertino M.; Pellarin R.; Lampel A.; Shaltiel-Karyo R.; Segal D.; Caflisch A.; Shalev D. E.; Gazit E. Structural basis for inhibiting β-amyloid oligomerization by a non-coded β-creaker-substituted endomorphin analogue. ACS Chem. Biol. 2011, 6, 1265–1276. 10.1021/cb200103h. [DOI] [PubMed] [Google Scholar]
- Vale W.; Grant G.; Rivier J.; Monahan M.; Amoss M.; Blackwell R.; Burgus R.; Guillemin R. Synthetic polypeptide antagonists of the hypothalamic luteinizing hormone releasing factor. Science 1972, 176, 933–934. 10.1126/science.176.4037.933. [DOI] [PubMed] [Google Scholar]
- Raghuraman H.; Chattopadhyay A. Melittin: a membrane-active peptide with diverse functions. Biosci. Rep. 2007, 27, 189–223. 10.1007/s10540-006-9030-z. [DOI] [PubMed] [Google Scholar]
- Pauwels E.; Cleeren F.; Tshibangu T.; Koole M.; Serdons K.; Dekervel J.; Van Cutsem E.; Verslype C.; Van Laere K.; Bormans G.; Deroose C. M. Al18F-NOTA-octreotide: first comparison with 68Ga-DOTATATE in a neuroendocrine tumour patient. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 2398–2399. 10.1007/s00259-019-04425-1. [DOI] [PubMed] [Google Scholar]
- El-Hashim A. Z.; Renno W. M.; Raghupathy R.; Abduo H. T.; Akhtar S.; Benter I. F. Angiotensin-(1–7) inhibits allergic inflammation, via the MAS1 receptor, through suppression of ERK1/2- and NF-kB-dependent pathways. Br. J. Pharmacol. 2012, 166, 1964–1976. 10.1111/j.1476-5381.2012.01905.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silveira K. D.; Coelho F. M.; Vieira A. T.; Sachs D.; Barroso L. C.; Costa V. V.; Bretas T. L.; Bader M.; de Sousa L. P.; da Silva T. A.; dos Santos R. A.; Simões e Silva A. C.; Teixeira M. M. Anti-inflammatory effects of the activation of the angiotensin-(1–7) receptor, MAS, in experimental models of arthritis. J. Immunol. 2010, 185, 5569–5576. 10.4049/jimmunol.1000314. [DOI] [PubMed] [Google Scholar]
- Cai W.; Zhang X.; Wu Y.; Chen X. A thiol-reactive 18F-labeling agent, N-[2-(4-18F-fluorobenzamido)ethyl]maleimide, and synthesis of RDG peptide-based tracer for PET imaging of αvβ3 integrin expression. J. Nucl. Med. 2006, 47, 1172–1180. [PMC free article] [PubMed] [Google Scholar]
- Guenther K. J.; Yoganathan S.; Garofalo R.; Kawabata T.; Strack T.; Labiris R.; Dolovich M.; Chirakal R.; Valliant J. F. Synthesis and in Vitro Evaluation of 18F- and 19F-Labeled Insulin: A New Radiotracer for PET-based Molecular Imaging Studies. J. Med. Chem. 2006, 49, 1466–1474. 10.1021/jm0509344. [DOI] [PubMed] [Google Scholar]
- Davis R. A.; Drake C.; Ippisch R. C.; Moore M.; Sutcliffe J. L. Fully automated peptide radiolabeling from [18F]fluoride. RSC Adv. 2019, 9, 8638–8649. 10.1039/C8RA10541C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guhlke S.; Wester H. J.; Bruns C.; Stöcklin G. (2-[18F]Fluoropropionyl-(D)phe1)-octreotide, a potential radio-pharmaceutical for quantitative somatostatin receptor imaging with PET: synthesis, radiolabeling, in vitro validation and biodistribution in mice. Nucl. Med. Biol. 1994, 21, 819–825. 10.1016/0969-8051(94)90161-9. [DOI] [PubMed] [Google Scholar]
- Wester H. J.; Brockmann J.; Rösch F.; Wutz W.; Herzog H.; Smith-Jones P.; Stolz B.; Bruns C.; Stöcklin G. PET-pharmacokinetics of 18F-octreotide: a comparison with 67Ga-DFO- and 86Y∼DTPA-octreotide. Nucl. Med. Biol. 1997, 24, 275–286. 10.1016/S0969-8051(97)00039-5. [DOI] [PubMed] [Google Scholar]
- Anderson C. J.; Pajeau T. S.; Edwards W. B.; Sherman E. L. C.; Rogers B. E.; Welch M. J. In vitro and in vivo evaluation of copper-64-octreotide conjugates. J. Nucl. Med. 1995, 36, 2315–2325. [PubMed] [Google Scholar]
- Zhao W.; Lee H. G.; Buchwald S. L.; Hooker J. M. Direct 11CN-labeling of unprotected peptides via palladium-mediated sequential cross-coupling reactions. J. Am. Chem. Soc. 2017, 139, 7152–7155. 10.1021/jacs.7b02761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skogh A.; Friis S. D.; Skrydstrup T.; Sandström A. Palladium-catalyzed aminocarbonylation in solid-phase peptide synthesis: a method for capping, cyclization, and isotope labeling. Org. Lett. 2017, 19, 2873–2876. 10.1021/acs.orglett.7b01068. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.