Abstract
Optimal hygiene management is an essential part of maintaining a high standard of health in conventional pig production systems and for the successful interruption of infection chains. Currently, efficiency assessments on cleaning and disinfection are only performed by visual inspection or are neglected completely. The aim of this study was to evaluate the available methods for on farm monitoring of hygiene, identify critical points in pig pens and use the data obtained for training purposes. In addition to visual inspection by assessing the cleanliness, microbiological swab samples, i.e., aerobic total viable count (TVC), total coliform count, methicillin-resistant Staphylococcus aureus (MRSA), and extended-spectrum β-lactamases-producing bacteria (ESBL), swab samples for ATP as well as protein residues and agar contact plates combined with 3 different culture media, were applied and ranked according to their suitability for livestock farming. Samples were collected on at least 15 critical points from one representative pen on 6 pig fattening farms with various hygiene management practices after cleaning and disinfection. After the first sampling, farmers were trained with their individual results, and sampling was repeated 6 mo after training. Nipple drinkers, feeding tubes (external and inner surface), and troughs (external and inner surface) showed the greatest bacterial loads (TVC: 4.5–6.7 log10 cfu cm-2) and values for ATP and protein residues; therefore, these surfaces could be identified as the most important critical points. Spearman rank correlations (P < 0.01) were found between the different assessment methods, especially for the TVC and ATP (r = 0.82, P < 0.001). For rapid assessment on farms, ATP tests represented an accurate and cost-efficient alternative to microbiological techniques. Training improved cleaning performance as indicated by a lower rating for visual inspection, TVC, ATP, MRSA, and ESBL in the second assessment. The monitoring of cleaning efficiency in pig pens followed by training of the staff constitutes a valuable strategy to limit the spread of infectious diseases and antibiotic-resistant bacteria. Special attention should be paid to the sufficient hygiene of drinkers and feeders.
Keywords: animal health, cleaning, disease prevention, disinfection, method evaluation, pork production
Introduction
Cleaning and disinfection of pens is an integral part of health management in livestock farming. The German law prescribes that pens and equipment for pig farming must be cleaned and disinfected between the housing out and restocking of animals (SchHaltHygV, 1999). However, methods for how sanitation could be monitored systematically are lacking. The most common method, visual inspection, depends on subjective perception and structural conditions such as light intensity or the color of surfaces. To make matters worse, not every soiling or bacterial contamination is visually perceptible; therefore, overestimation of cleanliness is likely (Sherlock et al., 2009). Furthermore, remaining organic material can significantly reduce the effect of applied disinfectants (Ward et al., 2006), which means that the efficacy of disinfection highly depends on the precision of the initial cleaning. How cleaning and disinfection should be carried out is mostly known; however, in practice, thoroughness often suffers from a lack of time. For training and consultancy purposes, easily understandable arguments can help to convince farmers to change their procedure. Changes lead to healthier animals and improve economic factors such as feed efficacy or therapy costs (Banhazi and Santhanam, 2013, Le Floc´h et al., 2014). In addition, decreasing the use of antibiotics reduces the development and spread of livestock-associated antibiotic-resistant bacteria (Gleeson and Collins, 2015). The first objective of this study was to find an appropriate method for assessing hygienic conditions in all-in all-out pig fattening systems. Therefore, different techniques, commonly used in hospital hygiene and food production, namely microbiological swabs, protein- and ATP-rapid tests and different agar contact plates (ACP) were compared. The second objective was to suggest critical points in a pen suitable for routine monitoring after cleaning and disinfection. The third objective was to determine if training and raising awareness of the farmers by identifying individual hygienic condition results and critical points help to improve hygiene status and reduce exposure to pathogenic bacteria.
Materials and methods
This study was conducted in accordance with federal and institutional animal use guidelines (Az. 84 – 02.05.40.16.038), data privacy agreement (University of Bonn, 38/2018) and ethical standards.
Selection of the farms and experimental design
For the study, 6 conventional pig fattening farms, located in northern Germany, with major differences in cleaning and disinfection procedures were chosen. Differences in hygiene management were assessed by a questionnaire before sample collection, containing information about used detergents and disinfectants as well as drying and exposure times. Two samplings were performed on each farm. The purpose of the first was to focus on suitable monitoring methods and identify possible control points. Second sample collection was performed 6 mo after training to survey changes in hygiene management. The samples were taken on each farm after cleaning and disinfection procedures were finished (Table 1) and immediately before restocking. The farm hygiene protocol, especially cleaning and disinfection practices, varied depending on the farm-specific management (Table 1). On each farm, 16 different sampling sites (Table 2) were tested in a representative, but randomly chosen pen: entrance door (inside), back wall, side wall, ceiling, slatted floor, manure area, feeding area, feeding tube (upside), two nipple drinkers from the same pen, trough (outside), trough (inside), two manipulable materials (toys), window sill, and feeding tube (inside). Detailed information on the sampled areas and supplementary notes on the methods used are given in Table 2. Materials and surface roughness of the sampled areas were recorded (Supplementary Table S1). The different methods used and the specific purpose of the methods are given in Supplementary Table S2. For the nipple drinkers, the inner nipple and the outer tube were swabbed in a circular motion. On planar surfaces, samples were taken by wiping the area horizontally and vertically. For every sampling point, an area of 25 cm2 was tested. Swabs were premoistened with sterile physiological saline solution (Oxoid, BR0053, Basingstoke, UK). Each farm was visited twice, so a total of 216 samples were taken from the 6 farms. All samples were stored in chilled insulated boxes (4 to 7 °C) and transported to the laboratory and examined within 24 h.
Table 1.
Differences in hygiene practices of cleaning and disinfection procedures, and number of fattening places on pig fattening farms, depending on the farm
| Use of detergents | Use of disinfection agents | Drying time before disinfection, h | Exposure time to disinfectant agent, h | Change of disinfectant agents | Type of production chain | Number of fattening places | |
|---|---|---|---|---|---|---|---|
| Farm 1 | No | Yes | 4 | 16 | Rarely | Integrated | 1,444 |
| Farm 2 | No | Yes | 0.75 | 4 | Rarely | Contracted | 1,250 |
| Farm 3 | Yes | No | — | — | — | Integrated | 640 |
| Farm 4 | No | Yes | 48 | 24 | Rarely | Contracted | 620 |
| Farm 5 | No | Yes | 12 | 24 | Always | Integrated | 1,120 |
| Farm 6 | Yes | Yes | 6 | 6 | Rarely | Contracted | 3,610 |
Table 2.
Defined sampling points and possibility of sampling on pig fattening pens, which partially provide direct animal contact
| Animal contact | Swabs1 | ACP2 | Sampled area, 25 cm2 | |
|---|---|---|---|---|
| Entrance door (inside) | Yes | Yes | Yes | 50 cm height |
| Back wall | Yes | Yes | Yes | 50 cm height |
| Side wall | Yes | Yes | Yes | 50 cm height |
| Ceiling | No | Yes | Yes | Middle of the pen |
| Slatted floor | Yes | Yes | Yes | Middle of the pen |
| Manure area | Yes | Yes | No | 50 cm length, feces corner |
| Feeding area | Yes | Yes | Yes | 10 cm in front of the trough |
| Feeding tube (upside) | No | Yes | Yes | Center above the pen |
| Nipple drinkers | Yes | Yes | No | Inner and outer tube |
| Trough (outside) | Restricted | Yes | Yes | Center, including the fold |
| Trough (inside) | Yes | Yes | Yes | Center, inner side wall |
| Manipulable material | Yes | Yes | Yes | Intensively used area |
| Window sill | No | Yes | Yes | Center |
| Feeding tube (inside) | No | Yes | No | Inner tube |
1Includes all microbiological swabs (aerobic total viable count (TVC), total coliform count (TCC), methicillin-resistant Staphylococcus aureus (MRSA), and extended-spectrum β-lactamases producing bacteria (ESBL)) and swabs for rapid tests for adenosine triphosphate (ATP) and protein.
2Agar contact plates (ACP) including ACP for TVC, Dey Engley agar and violet red bile dextrose agar. ACP were not applicable to all sampling sites because of their shape.
Visual inspection
Before sampling, the visual cleanliness of the area was assessed by at least two persons using a three-score grading system (1 = cleaning was satisfactory, no remaining soiling visible; 2 = cleaning was sufficient, minor soiling visible; 3 = cleaning was unsatisfactory, coarse soiling visible).
Microbiological swab samples
For the microbiological analysis, samples were taken by using sterile moistened flocked swabs with 1 mL of liquid Amies medium (eSwab, Copan, Brescia, Italy). The swabs were well mixed for 30 s to dissolve bacteria quantitatively. From the Amies medium, serial dilution series (1:10) were prepared in sterile saline solution (Oxoid) with 1% tryptone (VWR, Leuven, Belgium) to produce countable results. For aerobic total viable count (TVC), 3 dilution steps were plated with nonselective plate count agar (Merck, Darmstadt, Germany) by pour plating in a dual approach. Plates were stored for 72 h at 30 °C under aerobic conditions. After incubation, all visible colonies were counted as viable numbers of microorganisms, expressed in cfu ⋅ cm-2 or mL-1, from plates containing a minimum of 10 and a maximum of 300 colonies. All microbiological data were log transformed. Additionally, samples were investigated for the number of total coliforms by pour plating with selective Chromocult coliform agar (Merck). After incubation for 24 h at 37 °C, all dark blue to salmon red colonies were counted as total coliform bacteria. For qualitative analysis of methicillin-resistant Staphylococcus aureus (MRSA) and extended-spectrum β-lactamase-producing bacteria (ESBL), selective CHROMagar plates (Mast Group, Reinfeld, Germany) were used. With sterile spatulas, 0.1 mL of the samples was spread on the agar surface without prior dilution. The MRSA plates were incubated for 24 h at 41 °C and afterward, for further increased pigmentation, for another 24 h at room temperature, ESBL plates for 24 h at 41 °C. Pink colonies from MRSA plates were transferred to Columbia sheep blood agar (Mast Group) and incubated for 24 h at 37 °C for confirmation. Light-gray colonies producing characteristic β-hemolysis was counted as resistant S. aureus. All colonies that grew on ESBL agar were considered a positive result for an ESBL builder strain without further identification of the species.
Water samples
Additionally, for microbiological analysis of livestock drinking water, water samples with a volume of 50 mL (stagnating water) were taken. Two samples with equal volume from 1 pen per farm were mixed to generate a pooled sample. Samples were processed analogously to microbiological swab samples.
Sock samples
The pens were tested with sock samples, as routinely used for Salmonella monitoring in broiler houses. Sock samples were taken by covering sterile rubber boots with a disposable cellulose hair net and walking 50 steps through the pen in a serpentine motion including the corners. Socks were transferred to 100 mL of sterile saline solution. After blending with a stomacher for 60 s, the saline solution was analyzed. Microbiological cultivation of TVC was performed analogously to swab samples.
ATP rapid test
For the analysis of ATP content, sterile premoistened ATP swabs were used (CleanTrace Surface ATP Test Swab UXL100, 3M, Neuss, Germany). This test system is based on a bioluminescence reaction, with ATP as a cofactor. After swabbing the targeted area, the ATP test was activated by pushing down the stick handle to remove the membrane and starting the enzymatic reaction by combining all chemical solutions. After 10 s of shaking, the amount of emitted light was measured by a luminometer (NG III, 3M) in relative light units (RLUs). The resulting values are displayed in log10 RLU ⋅ cm-2 or mL-1.
Protein rapid test
Samples were taken by special swabs for a protein rapid test (Clean Trace, 3M). This semiquantitative test system is based on a chemical reaction, resulting in a color change, which depends on the protein content. The test system was activated by pushing down the stick handle and gently shaking to mix the reaction solutions. The results could be obtained visually after 15 min. For a rapid interpretation of the measured protein content, the resulting color change was assessed by a defined 5-score color scheme (1 = no change, 5 = strong change from green to violet).
Microbiological ACPs
All flat surfaces (Table 2) were tested with ACP. Three different commercially available media were used: a nonselective plate count agar for enumeration of TVC, violet red bile dextrose agar (VRBD) for selective cultivation of Enterobacteriaceae and Dey Engley Agar (DE) for cultivation of bacteria after disinfection to neutralize disinfectant residues (HygieneChek, 49404R, 49417R, 49428R, Romerlabs, Butzbach, Germany). All ACPs had a surface of 9 cm2. Bacteria were transferred to the media by gently pressing the agar on the sampling surface. After incubation for 24 h at 30 °C, all grown colonies were counted. Sampling with ACP was only used in the initial sampling before training of the staff.
Hygiene management training and raising awareness
After data analysis and processing of the results from the first sampling, all farm managers were invited for hygiene management training. At least 1 person from each farm participated. The training started with an introductory lecture about basic protocols of hygiene management, the differences in cleaning and disinfection and biological foundations of microbiological contaminations in a 60-min oral presentation. Sampling points were introduced briefly, and participants were asked to guess their own results (one feedback form per farm). The general weak points (as group means) for cleaning and disinfection were noted in a short talk (< 10 min). Subsequently, the individual results were handed out to each farmer in privacy with the possibility to ask questions. To highlight the critical points, the results were presented in bar charts and traffic light-colored for better visualization. Additionally, photos to show soiling were handed out. Farmers were encouraged to compare their individual results voluntarily and suggested measures for improving the hygienic status in a chaired group discussion afterward. Two years after the initial sampling, all farmers were asked again whether they had changed their hygiene protocols in the long-term consequence of the training.
Statistical data analysis
Descriptive statistics were performed with Excel 2016 (Microsoft, Redmond, WA) by calculating percentages. The Wilcoxon signed-rank test was used to estimate differences between sampling points within each sampling technique, as well as training effects comparing the results from the first and second sampling. Correlations were revealed by the Spearman rank correlation procedure of SAS 9.4 (SAS Institute Inc., Cary, NC). The level of significance was set at P < 0.05, with P < 0.01 as highly significant and P < 0.10 as a tendency.
Results
Critical points in hygiene
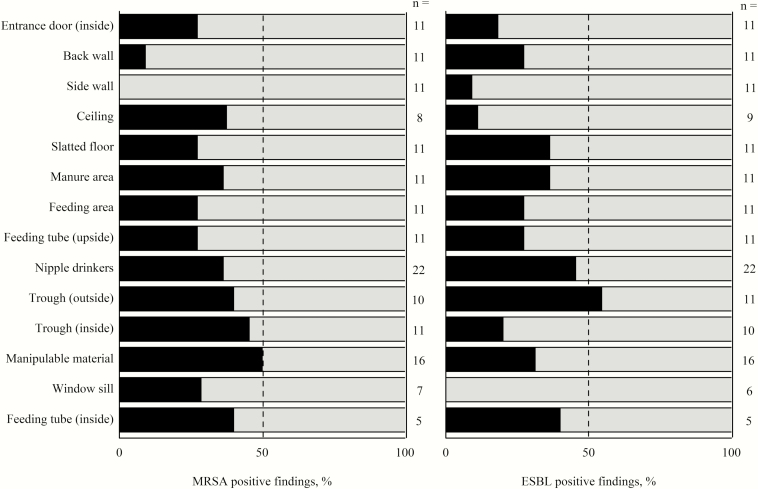
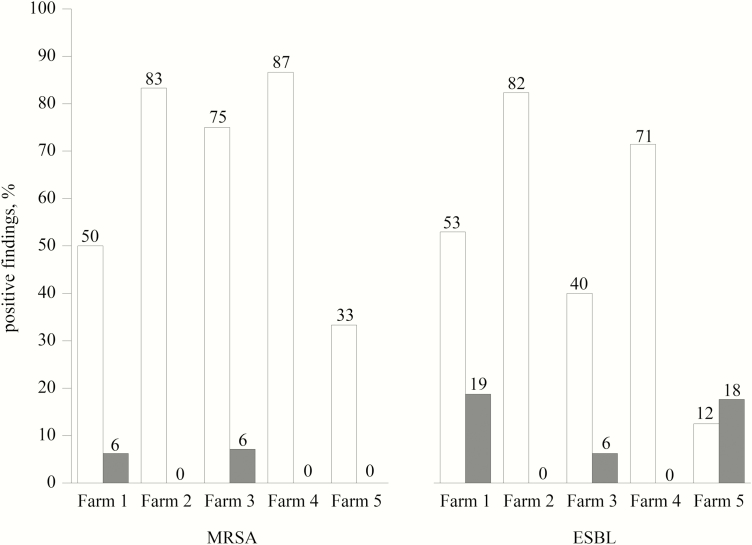
To identify critical points for the cleaning and disinfection of pig fattening farms, the TVC results are presented (Table 3), which are considered to be the gold standard for assessing hygienic conditions. The greatest bacterial loads from TVC swab samples were found for the nipple drinkers, feeding tubes (upside and inside), and troughs (inside and outside) after the first sampling. Entrance doors, back walls, side walls, slatted floors, and manure areas showed the lowest bacterial loads after the first sampling. The median values of total coliform counts (TCCs) fell below the detection limit, except for feeding area, nipple drinkers, troughs (outside), and feeding tube (inside), which indicate hygienic problems in these areas. MRSA were predominantly detected on the sampling points ceiling, manure area, nipple drinkers, trough (inside and outside), manipulable materials, and inside feeding tubes, with the lowest incidence on pen walls (Figure 1). Positive findings for ESBL were detected in at least 40% of the tested inner surfaces of feeding tubes, troughs, and nipple drinkers. On window sills, no ESBL were detectable. For ATP and protein residues obtained results were similar to TVC from swab samples. Visual inspection resulted in highest scores for feeding tubes (inside), troughs (outside and inside), and divergently to the other results manipulable materials (1 and 2) and window sills. The ACPs were not applicable for nipple drinkers and inner surfaces of feeding tubes and could only be applied on flat surfaces. Comparable to TVC results from swab samples, highest loads for TVC ACP and DE ACP could be obtained for the upside surfaces of feeding tubes. In contrast to TVC from swab samples high bacterial loads were also found for feeding area, manipulable materials (1 and 2), window sills and in case of TVC ACP for back walls. The Enterobacteriaceae counts for VRBD ACP were generally low, except for window sills. All farms showed high values for the TVC in animal drinking water samples and varied between 4.5 to 6.1 log10 cfu ∙ mL-1 at the first sampling. Due to the dependence of cleaning status on the sampled areas, no correlations between roughness and cleaning status were calculated.
Table 3.
Minimal, median, and maximal values of TVC, TCC, ATP, and protein residues from swab samples and from different ACP used of the sampled points
| TVC1,3, log10 cfu ∙ cm-2 | TCC2,3, log10 cfu ∙ cm-2 | ATP4 residues, log10 RLU ∙ cm-2 | Protein residues (numeric, 1 to 5) | TVC ACP5, cfu ∙ cm-2 | DE ACP5,6, cfu ∙ cm-2 | VRBD ACP5,7, cfu ∙ cm-2 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample point | Min. | Median8 | Max. | Min. | Median8 | Max. | Min. | Median8 | Max. | Min. | Median8 | Max. | Min. | Median8 | Max. | Min. | Median8 | Max. | Min. | Median8 | Max. |
| Entrance door, inside (n = 6) | 0.4 | 2.9bc | 4.1 | 0.6 | 0.6 | 3.9 | 0.4 | 1.3d | 1.6 | 1 | 2 | 5 | 0.2 | 7.3 | 33.3 | 0.2 | 6.3 | 33.3 | 0 | 0.3 | 1.2 |
| Back wall (n = 6) | 0.8 | 2.2a-c | 4.1 | 0.6 | 0.6 | 1 | 0.9 | 1.2cd | 1.7 | 1 | 1 | 5 | 2.7 | 33.3 | 33.3 | 1.6 | 18 | 33.3 | 0 | 0.2 | 2.9 |
| Side wall (n = 6) | 0.8 | 2.7c | 5.9 | 0.6 | 0.6 | 2.9 | 0.4 | 1.6d | 2.1 | 1 | 3 | 5 | 0.4 | 20.9 | 33.3 | 0.6 | 11.3 | 33.3 | 0 | 0 | 0.1 |
| Ceiling (n = 6) | 1.8 | 3.5 a–c | 5.1 | 0.6 | 0.6 | 3 | 1.4 | 1.5bcd | 2 | 1 | 3 | 5 | 0 | 22.2 | 33.3 | 0.1 | 5.6 | 33.3 | 0 | 0.1 | 0.3 |
| Slatted floor (n = 6) | 1.1 | 3.4a–c | 4.2 | 0.6 | 0.6 | 2.8 | 1.4 | 1.8a–d | 3.4 | 1 | 1.5 | 4 | 1.6 | 22.2 | 33.3 | 0.3 | 21.8 | 33.3 | 0 | 0.4 | 1.2 |
| Manure area (n = 6) | 0.6 | 3.2a–c | 4.9 | 0.6 | 0.6 | 1.6 | 0.9 | 1.7bed | 2.5 | 1 | 4 | 5 | 0.4 | 9.3 | 33.3 | 8.3 | 22.2 | 33.3 | 0 | 0.2 | 33.3 |
| Feeding area (n = 6) | 0.8 | 3.8a–c | 5.1 | 0.6 | 1.1 | 4.1 | 1.1 | 1.9bed | 2.2 | 2 | 3.5 | 4 | 16.7 | 33.3 | 33.3 | 5.6 | 33.3 | 33.3 | 0.8 | 1 | 33.3 |
| Feeding tube, upside (n = 6) | 0.6 | 5a–c | 6.8 | 0.6 | 2 | 2.8 | 0.5 | 2.3a–d | 3.3 | 3 | 4.5 | 5 | 11.1 | 33.3 | 33.3 | 16.7 | 33.3 | 33.3 | 0 | 0.1 | 0.3 |
| Nipple drinker (n = 6) | 4.8 | 5.6ab | 6.3 | 0.6 | 1.1 | 4.1 | 1.7 | 2.7abc | 3.5 | 1 | 5 | 5 | - | - | - | - | - | - | - | - | - |
| Nipple drinker (n = 6) | 3.9 | 5.7ab | 6.9 | 0.6 | 1.6 | 4.1 | 2.3 | 3ab | 3.6 | 4 | 4.5 | 5 | - | - | - | - | - | - | - | - | - |
| Trough, outside (n = 6) | 2.7 | 4.8a–c | 6.8 | 0.6 | 1.1 | 4.1 | 1.4 | 2a–d | 3.8 | 1 | 4 | 5 | 13.9 | 25 | 33.3 | 16.7 | 27.8 | 33.3 | 0 | 0.5 | 33.3 |
| Trough, inside (n = 4) | 1.1 | 5.3a–c | 6.5 | 0.6 | 0.6 | 4.2 | 1.5 | 2.5a–d | 2.7 | 2 | 4 | 5 | 5.1 | 19.2 | 33.3 | 5.9 | 19.6 | 33.3 | 0 | 0.5 | 1.1 |
| Manipulable material 1 (n = 5) | 2.2 | 3.3a–c | 5.7 | 0.6 | 0.6 | 3.8 | 1.2 | 1.9a–d | 2.4 | 1 | 2 | 5 | 22.2 | 33.3 | 33.3 | 22.2 | 33.3 | 33.3 | 0.1 | 0.1 | 0.1 |
| Manipulable material 2 (n = 5) | 1.7 | 4.3a–c | 6.6 | 0.6 | 0.6 | 4.1 | 1.1 | 1.8a–d | 3.2 | 1 | 2 | 4 | 33.3 | 33.3 | 33.3 | 33.3 | 33.3 | 33.3 | 5.6 | 19.4 | 33.3 |
| Window sill (n = 4) | 2.8 | 3.1a–c | 4.1 | 0.6 | 0.6 | 2 | 1.6 | 1.9a–d | 3.9 | 3 | 4 | 5 | 0.3 | 33.3 | 33.3 | 22.2 | 33.3 | 33.3 | 0.1 | 0.4 | 1.7 |
| Feeding tube inside (n = 3) | 5.9 | 6.1a | 8 | 2.7 | 4.1 | 4.1 | 2.6 | 3.6a | 4.2 | 3 | 5 | 5 | - | - | - | - | - | - | - | - | - |
1Aerobic total viable count (TVC) in cfu per cm2.
2Total coliform count (TCC).
3Lower detection limit for TVC and TCC = 0.6 log10 cfu ∙ cm-2.
4Adenosine triphosphate (ATP) in relative light units (RLU) per cm2.
5Upper detection limit for Agar contact plates (ACP) = 33.3 cfu ∙ cm-2.
6Dey Engley Agar (DE).
7Violet red bile dextrose agar (VRBD).
8Median values within a column followed by no common superscript show significant differences (P < 0.05).
Figure 1.
Percentage of positive findings of methicillin-resistant Staphylococcus aureus (MRSA) and extended-spectrum β-lactamase-producing bacteria (ESBL) in relation to the sampling points in pig stables (black = positive findings, gray = negative finding). Interpretation of the results should be considered with care, considering that the number of the samples varied between 5 ≤ n ≥ 22.
Comparison of methods
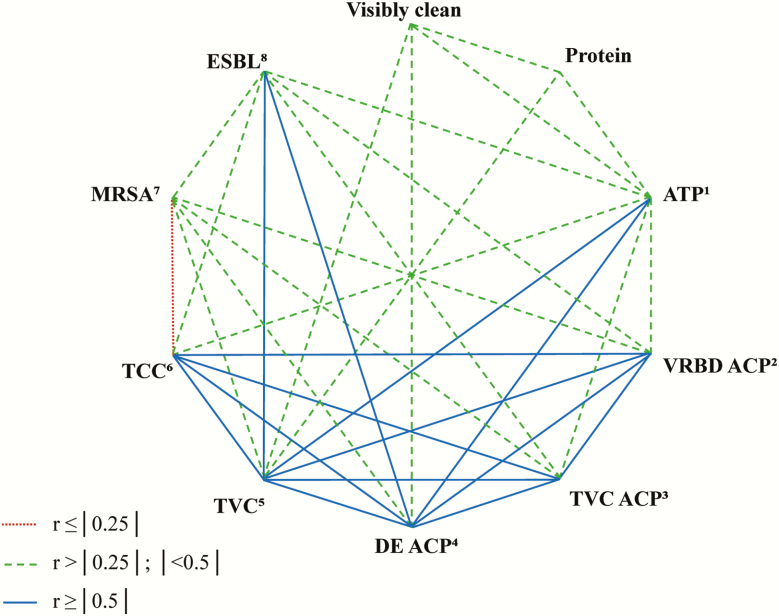
In the dataset of the first sampling, correlations could be found between the different diagnostic methods (Figure 2). Methods are compared with results from TVC of swab samples, which is the commonly used practice for evaluation of hygienic conditions. Aerobic TVC correlated with results from protein tests, ATP residues, ACP, ESBL findings, and visual inspection. For visual inspection, which is commonly used by farmers, correlations with TVC, ATP, MRSA, and ESBL were calculated. Evaluation of the surface roughness of the sample points resulted in correlations with the protein rapid test (r = −0.31, P = 0.002), TVC ACP (r = 0.30, P < 0.02), VRBD ACP (r = 0.42, P = 0.002), and DE ACP (r = 0.29, P = 0.01). Fifty-five percent of the TVC ACP (n = 73) and 48% of the DE ACP (n = 73) were overgrown or unreadable due to dirt particles on the agar surface. For VRBD ACP, 8% were unreadable (n = 52). For the TCC, 109 out of 168 samples were below the lower detection limit (2.0 log10 cfu ∙ mL-1 and 0.6 log10 cfu ∙ cm-2, respectively) due to the sampling technique with swabs. Consequently, the TCC and results from ACP were not further considered.
Figure 2.
Spearman rank correlations (P ≤ 0.05) for the different techniques used to evaluate hygiene management on pig fattening farms at first sampling. 1Adenosine triphosphate (ATP) with swabs. 2Violet red bile dextrose (VRBD) agar with agar contact plates (ACP). 3Aerobic total viable count (TVC) with ACP. 4Dey Engley (DE) agar with ACP. 5Aerobic TVC with swabs. 6Total coliform count (TCC) with swabs. 7Methcillin-resistant Staphylococcus aureus (MRSA) with swabs. 8Extended-spectrum β-lactamase-producing bacteria (ESBL) with swabs.
Training effect
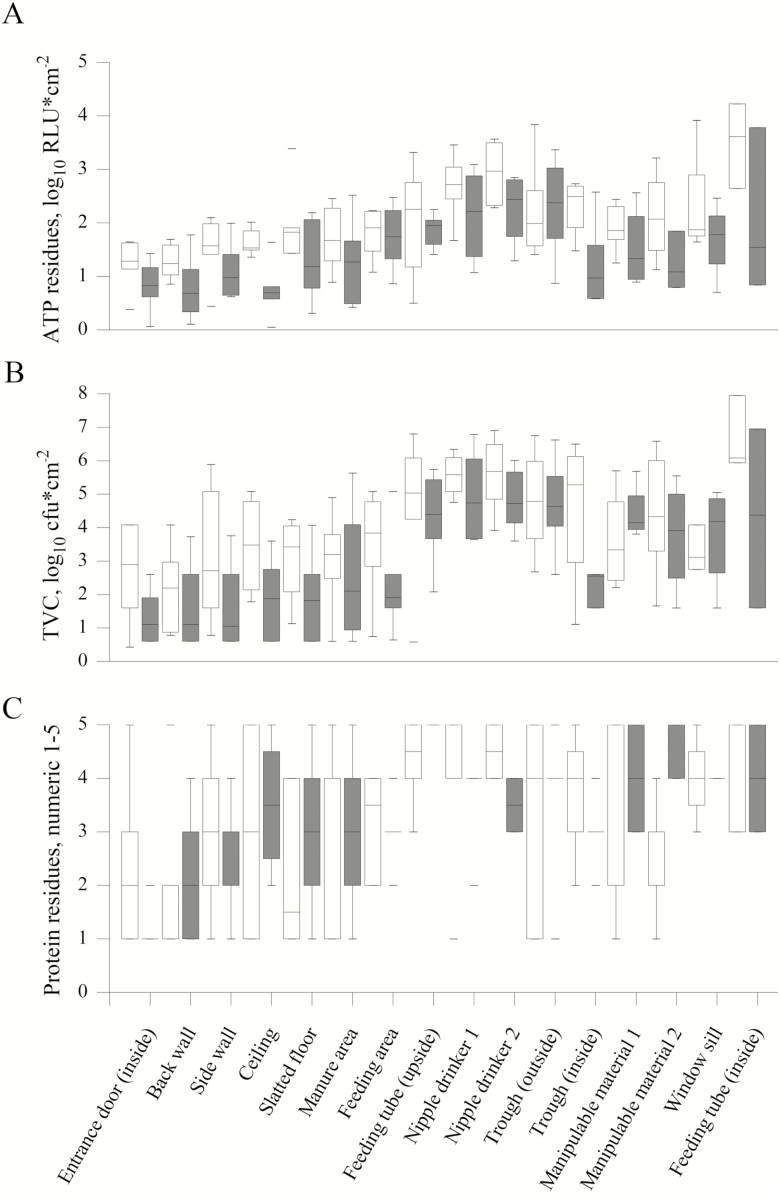
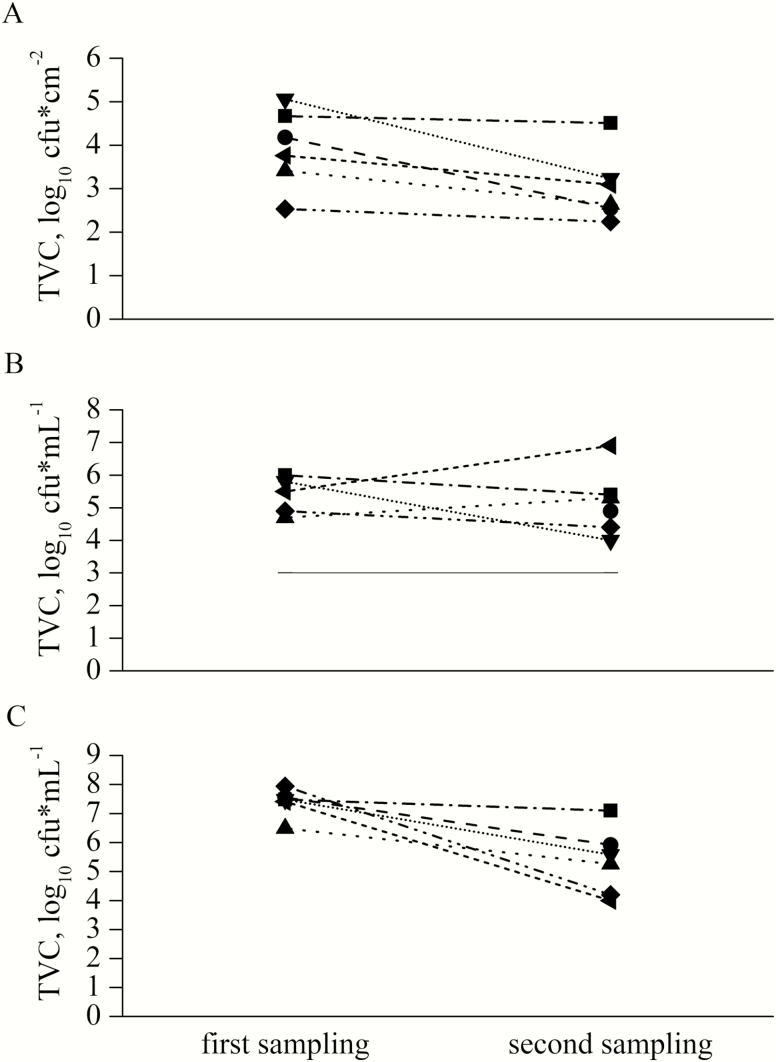
To evaluate the effect of training and improved hygiene management, the results of the different sampling techniques from the first and the second sampling were compared. Most of the results from individual sampling locations for the TVC, ATP, and protein residues showed a decrease with training (Figure 3). A significant training effect in form of a reduction for the single sampling locations between the first and second sampling could only be obtained for the protein residues for one nipple drinker (P < 0.01) and a tendency for ATP residues (P = 0.08) for the nipple drinker. Another tendency was recorded for the TVC of the entrance door which was higher at the first than at the second sampling (P = 0.08). A decrease was found in tendency for the TVC of the swab samples in general (P < 0.07) and the TVC in water samples (P = 0.06) (Figure 4). The TVC for sock samples decreased for all farms between the first and second sampling (P = 0.002) (Figure 4) and for ATP (P < 0.01, not shown). Even after training, the TVC for all water samples still exceeded the recommended value for the microbiological quality of drinking water. The findings for MRSA and ESBL decreased on all tested farms with training (P ≤ 0.02, respectively). Results for MRSA and ESBL from farm 6 were not analyzable. For 3 out of 5 farms, no MRSA could be detected at the second sampling and for 2 out of 5 farms, no ESBL was detected at the second sampling (Figure 5). In the survey for long-term monitoring, farmers that attended the training improved their awareness of critical points for cleaning and disinfection and changed their hygiene management protocols. All farmers regularly point out farm-specific weak points in cleaning to their employees. Four out of 6 farmers reported an extended time for high-pressure cleaning.
Figure 3.
The effect of training the farmers of the pig fattening farms is shown by the reduction (time 1, open squares; time 2, filled squares) for adenosine triphosphate (ATP) (A), aerobic TVC (B) and protein values (C) for almost all sampled areas.
Figure 4.
Development of aerobic TVC in swab samples (A), water samples (B), and sock samples (C) from 6 different pig fattening farms before and after training. On farm 2 no water sample was available during first sampling, because water mains were switched off.
Figure 5.
Percentage of positive findings for methicillin-resistant Staphylococcus aureus (MRSA) and extended-spectrum β-lactamase-producing bacteria (ESBL) after the first and the second sampling in relation to the pig fattening farms (time 1, open squares; time 2, filled squares). In the first sampling, samples for ESBL and MRSA from farm 6 were not analyzable.
Discussion
Hygienic critical points
The results show that nipple drinkers must be considered one of the most critical points in cleaning and disinfection procedures as found for nipple drinkers in pig nursery units (Luyckx et al., 2016). By measuring Enterobacteriaceae counts in pig finisher farms, Mannion et al. (2007) showed that feeders and drinkers are more contaminated after sanitation than floors, which is comparable to these results. They suggest that feeders and drinkers are resoiled during power-washing due to the splashing of contaminated water. Gonzalez et al. (2015) demonstrated in their study that the hygiene of feeders and drinkers is afflicted with problems, which could be confirmed in the present investigation. As a result of the insufficient cleaning of drinkers, the analyzed livestock drinking water samples were highly contaminated with bacteria. There is no legally determined upper limit for TVC in livestock drinking water in European law. In Germany, for the biological quality of animal drinking water, a benchmark of 3.0 log10 cfu ∙ mL-1 at 37 °C or of 4.0 log10 cfu ∙ mL-1 at 20 °C is recommended by the Federal Ministry of Food and Agriculture (Kamphues et al., 2007), which was exceeded all analyzed samples (Figure 4B). Pathogenic bacteria from animals of the previous batch can be easily transferred to newly arriving pigs via water intake from insufficiently cleaned drinkers. In a study that analyzed samples from 6 different pig farrow-to-finish farms with ACP, contrary to our results, additionally floors were critical points for cleaning and disinfection. In comparison, wall segments were fairly cleaned and disinfected (Vangroenweghe et al., 2009). The present study resulted in a particularly high contamination of feeders and a lower soiling of walls for TVC from swab samples. The results for the floor differ from those of Vangroenweghe et al. (2009), possibly due to the different sampling techniques. In general, sampling points that are not just in view and require bending down or looking up for visible inspection while cleaning and disinfection seem to be often forgotten. When interpreting the results, it should be considered that the sensitivity of the results is limited due to the small sampled area. It is obvious that a strict cleaning and disinfection protocol is necessary to maintain animal health; however, there are only very few studies available on this topic. The importance of effective cleaning and disinfecting as a substantial step to avoid the carryover of Salmonella in livestock has been demonstrated in several publications (Rose et al., 2000, Carrique-Mas et al., 2009, Gautam et al., 2014; Martelli et al., 2017). This effect can be applied to MRSA and ESBL, which could be considered indicator organisms for resistant bacteria in pig production (Schmithausen et al., 2018). In livestock production, the occurrence of MRSA and ESBL depends, in addition to antibiotic usage, on the amount of dust and feces, and transmission via air to newly arriving pigs seems possible (Venglovský et al., 2011, Friese et al., 2012, Laube et al., 2013). Therefore, appropriate hygiene lowers the risk of colonization with resistant bacteria. In general, hygiene itself has already been suggested as a critical control point for on-farm assessment of pig livestock farms, with visual control at daily intervals (von-Borell et al., 2001). As a part of this, the control of cleaning and disinfection should be included. One possible suggestion is the implementation of so-called hygienogram scores, as already established in poultry farming, to improve routinely performed cleaning and disinfection (Vangroenweghe et al. 2009). In poultry production, hygienograms, which are generated by determining the TVC with ACP, are sampled by a veterinarian or an official body according to a determined protocol (Maertens et al., 2017). The integration of a system similar to hygienogram scores in piggery farm management could possibly improve cleanliness but needs to be further developed, especially considering that greatest bacterial loads were found at sampling points where ACP are not applicable (Table 2). A conceivable possibility would be the combination of a visual inspection and rapid tests to avoid the additional costs of a microbiological examination. Microbiological tests could then be used in cases of recurring health problems and severe illnesses.
Suitable measurement methods
Monitoring methods for sanitation and cleaning must be reliable and sensitive (Turner et al., 2010). For monitoring in livestock farming, excessive sensitiveness can be counterproductive because of the high bacterial load that remains, despite proper sanitation. For example, ACP seem less suitable for hygiene monitoring, even if they did correlate with TVC. Most of the ACP were overgrown, depending on the sampling location, or unreadable because of adhering dirt or dust particles and were therefore excluded from the second sampling. Luyckx et al. (2015) reported similar results when using ACP in broiler houses. They found that ACP sampled from before cleaning were overgrown and noted that enumeration on ACP selective for E. coli allowed fewer countable results compared with enumeration of swab samples. Additionally, ACP are only usable on flat surfaces and are of limited use due to their fixed shape. Rapid tests for ATP and protein are very attractive for on-farm monitoring in contrast to microbiological swabs or ACP because of their short duration. Classical cultural analysis can last up to 72 h and requires high labor costs. Usually, after this time, the new production cycle on fattening farms has already started, meaning it is too late for possible corrective actions. In this study, the results from ATP tests gave highly significant correlations with TVC, but users must be aware that ATP test reports represent more than just remaining microorganisms. Additionally, organic soiling from feed or feces may also lead to high ATP values, as ATP is an energy carrier in all prokaryotic and eukaryotic cells (Sherlock et al., 2009, Pistelok et al., 2016). The reliability of ATP tests depends on possible residues of cleaning or disinfecting agents, which can influence the results and lead to decreased or rarely decreased RLU values (Green et al., 1999, Turner et al., 2010). For routine use of rapid ATP tests, a pass or fail benchmark must be set by the user to allow a correct interpretation of the hygienic status. Other authors have specified that ATP tests have the advantage of more objective information than visual control (Luyckx et al., 2015), which also applies to the protein test; however, due to the costs per test, ~2.90 Euro, it is questionable whether farmers are willing to pay for performance monitoring. Other studies from the hospital sector have shown that visual feedback from rapid tests to the staff increases the thoroughness of cleaning (Goodman et al., 2008), which is possibly transferrable to routinely performed hygiene training for personnel in animal production. Comparing ATP and protein rapid tests, ATP tests better reflect subtle differences than protein tests, in which only roughly different color graduations can be recognized visually.
Training effect
A training effect could be observed when comparing the results from the first sampling with the second sampling. The values for TVC, ATP, and protein residues decreased numerical for almost all sampled areas. Especially for flat surfaces, such as walls, floors, and the inner surface of the troughs, the TVC value dropped below 3 log10 cfu ∙ cm-2, which could be seen as a general target value in the prophylactic disinfection in animal houses, depending on the type of material (Böhm, 1998). However, the suitability of the target value is limited to sample points with a defined surface area and is not suitable for sock samples. To define a target value for sock samples, further investigations are needed. Only 2 out of 6 farmers in our study used detergent for cleaning, while the others cleaned the stables with water and high pressure only. By training, farmers should be made aware that cleaning with detergents prepares stables optimally for subsequent disinfection (Hancox et al., 2013). To improve hygienic conditions on farms and enhance animal health, changes in the attitude toward management practices are fundamental (Becton, 2006, Gleeson and Collins, 2015). To convince farmers of the importance of proper hygiene management, persuasive arguments are needed, which should include not only economic aspects such as greater productivity but also the improvement of animal welfare (Pastorelli, 2012, Banhazi and Santhanam, 2013, Le Floc´h et al., 2014, Gosling, 2018). Time is a key factor that influences the thoroughness of cleaning and disinfection, which emphasizes the importance of knowledge of farm-specific weak points in sanitation (Gosling, 2014). A possibility for improving hygiene management could be the development of a farm-specific hygiene protocol in consultation with a supervising veterinarian; with this protocol, the individual work would be checked off by the individual carrying out the work, similar to already existing protocols in the food industry known as the control of self-monitoring. A regular in-house training, perhaps guided by a specific consultant and with a possible turnaround of once a year, represents a conceivable opportunity for improvement. Correct and successful cleaning and disinfection always rely on the proficiency of the person performing the work (Carrique-Mas et al., 2009, Martelli et al., 2017, Gosling, 2018). Targeted training with monitoring results can help to increase efficiency and prevent from becoming inattentive due to routine. An alternative for improving sanitary status lies in outsourcing to professional cleaning contractors, as is common in poultry production. In several studies, cleaning and disinfection performed by professional cleaning companies was better than that by farm staff (Vangroenweghe et al., 2009, Maertens et al., 2017). Rapid tests may help farmers monitor the performance of professional cleaning companies. This question should be clarified in further studies. In conclusion, the awareness of the importance of hygiene in livestock production should be enhanced. The results of this study supported the long-term objective to enable farmers developing farm-specific solutions and continuously improving their hygiene management by providing monitoring methods and suggestions for the training.
Conflict of interest statement
None declared.
Supplementary Material
Acknowledgments
The project was part of the research project MarkiT (FKZ: 748655/1), which was supported by funds of the Federal Ministry of Food and Agriculture (BMEL) based on a decision of the Parliament of the Federal Republic of Germany via the Federal Office for Agriculture and Food (BLE) under the innovation support program. We gratefully acknowledge the participation of the farm managers and staff, EDEKA Nord (S. Weber & S. Erdmann, Valluhn, Germany) and ZNVG (A. Münster, L. Kruse, C. Gripp & L. Guckenberger, Neumünster, Germany) and thank for the support with selecting farms and hosting the training.
Literature cited
- Banhazi T., and Santhanam B.. . 2013. Practical evaluation of cleaning methods that could be implemented in livestock building. In: Aland A., Banhazi T., editors, Livestock housing: modern management to ensure optimal health and welfare of farm animals. Wageningen, The Netherlands:Wageningen Academic Publishers; p. 355–376. doi: 10.3920/978-90-8686-771-4 [DOI] [Google Scholar]
- Becton L. 2006. Management of antibiotic-free pigs. Allen D. Leman swine conference, 2016, p. 145–146. [Google Scholar]
- Böhm R. 1998. Disinfection and hygiene in the veterinary field and disinfection of animal houses and transport vehicles. Int. Biodeterior. Biodegrad. 41:217–224. doi: 10.1016/S0964-8305(98)00030-4 [DOI] [Google Scholar]
- von-Borell E., Bockisch F.-J., Büscher W., Hoy S., Krieter J., Müller C., Parvizi N., Richter T., Rudovsky A., Sundrum A., and Van den Weghe H.. . 2001. Critical control points for on-farm assessment of pig housing. Livest. Prod. Sci. 72:177–184. doi: 10.1016/S0301-6226(01)00278-0 [DOI] [Google Scholar]
- Carrique-Mas J. J., Marin C., Breslin M., McLaren I., and Davies R.. . 2009. A comparison of the efficacy of cleaning and disinfection methods in eliminating Salmonella spp. from commercial egg laying houses. Avian Pathol. 38:419–424. doi: 10.1080/03079450903193768 [DOI] [PubMed] [Google Scholar]
- Friese A., Schulz J., Hoehle L., Fetsch A., Tenhagen B. A., Hartung J., and Roesler U.. . 2012. Occurrence of MRSA in air and housing environment of pig barns. Vet. Microbiol. 158:129–135. doi: 10.1016/j.vetmic.2012.01.019 [DOI] [PubMed] [Google Scholar]
- Gautam R., Lahodny G. Jr, Bani-Yaghoub M., Morley P. S., and Ivanek R.. . 2014. Understanding the role of cleaning in the control of Salmonella Typhimurium in grower-finisher pigs: a modelling approach. Epidemiol. Infect. 142:1034–1049. doi: 10.1017/S0950268813001805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleeson B. L., and Collins A. M.. . 2015. Under what conditions is it possible to produce pigs without using antimicrobials? Animal Prod. Sci. 55:1424–1431. doi: 10.1071/AN15271 [DOI] [Google Scholar]
- Gonzalez M., Lainez M., Vega S., Ingresa-Capaccioni S., Marco-Jimenez F., and Marin C.. . 2015. Sources for salmonella contamination during pig production in eastern Spain. J. Anim. Vet. Sci.. 2(5):37–42. [Google Scholar]
- Goodman E. R., Platt R., Bass R., Onderdonk A. B., Yokoe D. S., and Huang S. S.. . 2008. Impact of an environmental cleaning intervention on the presence of methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci on surfaces in intensive care unit rooms. Infect. Control Hosp. Epidemiol. 29:593–599. doi: 10.1086/588566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosling R. J. 2018. A review of cleaning and disinfection studies in farmin environments. Livestock. 23(5):232–237. doi: 10.12968/live/2018.23.5.232 [DOI] [Google Scholar]
- Gosling R. J., Martelli F., Wintrip A., Sayers A. R., Wheeler K., and Davies R. H.. . 2014. Assessment of producers´response to Salmonella biosecurity issues and uptake of advice on laying hen farms in England and Wales. Br. Poultry Sci. 55(5):559–568. doi: 10.1080/00071668.2014.949620 [DOI] [PubMed] [Google Scholar]
- Green T. A., Russell S. M., and Fletcher D. L.. . 1999. Effect of chemical cleaning agents and commercial sanitizers on ATP bioluminescence measurements. J. Food Prot. 62:86–90. doi: 10.4315/0362-028x-62.1.86. [DOI] [PubMed] [Google Scholar]
- Hancox L. R., Le Bon M., Dodd C. E., and Mellits K. H.. . 2013. Inclusion of detergent in a cleaning regime and effect on microbial load in livestock housing. Vet. Rec. 173:167. doi: 10.1136/vr.101392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamphues J., Böhm R., Flachowsky G., Lahrssen-Wiederholt M., Meyer U., and Schenkel H.. . 2007. Empfehlungen zur Beurteilung der hygienischen Qualität von Tränkwasser für Lebensmittel liefernde Tiere unter Berücksichtigung der gegebenen rechtlichen Rahmenbedingungen. Landbauforsch. Völk. 3(57):255–272. [Google Scholar]
- Laube H., Friese A., von Salviati C., Guerra B., Käsbohrer A., Kreienbrock L., and Roesler U.. . 2013. Longitudinal monitoring of extended-spectrum-beta-lactamase/AmpC-producing Escherichia coli at German broiler chicken fattening farms. Appl. Environ. Microbiol. 79:4815–4820. doi: 10.1128/AEM.00856-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Floc´h N., Knudsen C., Gidenne T., Montagne L., Merlot E., and Zemb O.. . 2014. Impact of feed restriction on health, digestion and faecal microbiota of growing pigs housed in good or poor hygiene conditions. Animal. 8(10):1632–1642. doi: 10.1017/S1751731114001608 [DOI] [PubMed] [Google Scholar]
- Luyckx K., Dewulf J., Van Weyenberg S., Herman L., Zoons J., Vervaet E., Heyndricks M., and De Reu K.. . 2015. Comparison of sampling procedures and microbiological and non-microbiological parameters to evaluate cleaning and disinfection in broiler houses. Poult. Sci. 94:740–749. doi: 10.3382/ps/pev019 [DOI] [PubMed] [Google Scholar]
- Luyckx K., Millet S., Van Weyenberg S., Herman L., Heyndrickx M., Dewulf J., and De Reu K.. . 2016. A 10-day vacancy period after cleaning and disinfection has no effect on the bacterial load in pig nursery units. BMC Vet. Res. 12:236. doi: 10.1186/s12917-016-0850-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maertens H., De Reu K., Van Weyenberg S., Van Coillie E., Meyer E., Van Meirhaeghe H., Van Immerseel F., Vandenbroucke V., Vanrobaeys M., and Dewulf J.. . 2017. Evaluation of the hygienogram scores and related data obtained after cleaning and disinfection of poultry houses in Flanders during the period 2007 to 2014. Poultry Sci. 97:620–627. doi: 10.3382/ps/pex327 [DOI] [PubMed] [Google Scholar]
- Mannion C., Lynch P. B., Egan J., and Leonard F. C.. . 2007. Efficacy of cleaning and disinfection on pig farms in Ireland. Vet. Record. 61:371–375. doi: 10.1136/vr.161.11.371 [DOI] [PubMed] [Google Scholar]
- Martelli F., Lambert M., Butt P., Cheney T., Tatone F. A., Callaby R., Rabie A., Gosling R. J., Fordon S., Crocker G., . et al. 2017. Evaluation of an enhanced cleaning and disinfection protocol in Salmonella contaminated pig holdings in the United Kingdom. PLoS One 12:e0178897. doi: 10.1371/journal.pone.0178897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastorelli H., Le Floc’h N., Merlot E., Meunier-Salaün M. C., van Milgen J., and Montagne L.. . 2012. Sanitary housing conditions modify the performance and behavioural response of weaned pigs to feed- and housing-related stressors. Animal 6:1811–1820. doi: 10.1017/S1751731112001231 [DOI] [PubMed] [Google Scholar]
- Pistelok F., Pohl A., Stuczyński T., and Wiera B.. . 2016. Using ATP tests for assessment of hygiene risks. Ecol. Chem. Eng. 23 (2):259–270. doi: 10.1515/eces-2016-0018 [DOI] [Google Scholar]
- Rose N., Beaudeau F., Drouin P., Toux J. Y., Rose V., and Colin P.. . 2000. Risk factors for Salmonella persistence after cleansing and disinfection in French broiler-chicken houses. Prev. Vet. Med. 44:9–20. doi: 10.1016/s0167-5877(00)00100-8 [DOI] [PubMed] [Google Scholar]
- Schmithausen R. M., Schulze-Geisthövel S. V., Heinemann C., Bierbaum G., Exner M., Petersen B., and Steinhoff-Wagner J.. . 2018. Reservoirs and transmission pathways of resistant indicator bacteria in the biotope pig stable and along the food chain: a review from a one health perspective. Sustainability. 10:1–26. doi: 10.3390/su1011396730607262 [DOI] [Google Scholar]
- Schweinehaltungshygieneverordnung (SchHaltHygV). 1999 in der Fassung und Bekanntmachung vom 2 April 2014 (BGBI. I S. 326), die zuletzt durch Artikel 134 des Gesetzes vom 29. März 2017 (BGBI I S. 626) geändert worden ist. [Google Scholar]
- Sherlock O., O’Connell N., Creamer E., and Humphreys H.. . 2009. Is it really clean? An evaluation of the efficacy of four methods for determining hospital cleanliness. J. Hosp. Infect. 72:140–146. doi: 10.1016/j.jhin.2009.02.013. [DOI] [PubMed] [Google Scholar]
- Turner D. E., Daugherity E. K., Altier C., and Maurer K. J.. . 2010. Efficacy and limitations of an ATP-based monitoring system. J. Am. Assoc. Lab. Anim. Sci. 49:190–195. [PMC free article] [PubMed] [Google Scholar]
- Vangroenweghe F., Heylen P., Arijs D., and Castryck F.. . 2009. Hygienograms for evaluation of cleaning and disinfection protocols in pig facilities. Safe pork 2009 – Quebec City, Quebec Canada; p. 220–223. doi: 10.31274/safepork-180809-846 [DOI] [Google Scholar]
- Venglovský J., Gregová G., Kmet´ V., and Sasáková N.. . 2011. Detection of airborne microorganisms and antibiotic resistance from animal housing facilities. Proc. XVth International Congress of the International Society for Animal Hygiene, Vienna, Austria Brno (Czech Republic): Tribun EU s.r.o.; p. 813–815. [Google Scholar]
- Ward P. J., Fasenko G. M., Gibson S., and McMullen L. M.. . 2006. A microbiological assessment of on-farm food safety cleaning methods in broiler barns. J. Appl. Poult. Res. 15:326–332. doi: 10.1093/japr.15.2.326 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.