Abstract
Salmonella in pigs is a concern for human foodborne salmonellosis. Dietary fungal fermented products, coated butyrate, and organic acids (OAs) may be promising control strategies. The objectives of this study were (i) to evaluate in vitro binding affinity of Salmonella enterica serovar Typhimurium (S. Typh) and Enteritidis (S. Ent), and enterotoxigenic Escherichia coli (ETEC) F4 or F18 to mannan-rich hydrolyzed copra meal (MCM) and fermented rye (FR) with Agaricus subrufescens; and (ii) to assess MCM and FR efficacy to control in vivo S. Typh shedding when combined with OAs and compared with coated butyrate strategy. A 31-d study included 32 pigs [6.29 ± 0.76 kg BW] individually housed and distributed into four dietary treatments: control diet; OA.BU, 4 kg/t OA plus 6 kg/t coated butyrate; OA.MCM, 4 kg/t OA plus 1 kg/t MCM; and OA.FR, 4 kg/t OA plus 2 kg/t FR. All pigs were challenged for 7 d with 1 mL S. Typh (109 colony forming units daily) at 10 d postweaning. Temperature and fecal samples were collected before and after challenge, and fecal Salmonella shedding quantified. Diarrhea scores were monitored daily and growth performance was evaluated weekly. In vitro, culture with MCM and FR showed significant (P < 0.01) binding affinity for both S. Typh and S. Ent, but not for ETEC F4 and F18. In vivo, pigs fed OA.MCM and OA.FR had lower (P < 0.05) shedding and day 3 peak shedding of S. Typh after infections than pigs fed control and OA.BU diets. Pigs fed OA.FR diet tended to have an 18% increase (P = 0.068) in BW on day 14 post first inoculation compared with control and OA.BU, and 19% increased (P = 0.093) final BW at day 21 compared with control. Diarrhea frequency post infection was overall lower (P = 0.006) for OA.FR (18.9%) than OA.BU (44.8%) and OA.MCM (41.7%) while control (28.7%) was not different. In conclusion, FR and MCM show in vitro-binding affinity to Salmonella enterica serovars Typh and Ent. Feeding FR or MCM combined with OA to nursery pigs reduces the peak and averages S. Typh shedding compared with control. Fermented rye with OA tends to improve pig performance after S. Typh challenge.
Keywords: Agaricus, fermented product, gut health, oligosaccharides, Salmonella, shedding
Introduction
Research for new strategies that promote gut health in pigs is key because there is an urgent need to reduce antibiotic usage in production animals and minimize risk of bacterial resistance against antibiotics. Relevant strategies may focus on reducing risk of pathogenic and zoonotic bacteria in the feed-to-food chain, mitigate postweaning diarrhea, and achieve profitable systems. Salmonellosis, although often asymptomatically in pigs (Andrés-Barranco et al., 2015), is a major human concern globally because contamination can occur at many different levels of the pig production chain [Regulation (EC) No. 2160/2003]. Probabilities of Salmonella shedding and contamination at slaughter are higher when pigs were already infected and(or) colonized at farm (Casanova‐Higes et al., 2017). Prevent shedding load, infection, and colonization will contribute to reduce Salmonella contamination in the food chain. Other important pathogenic bacteria include enterotoxigenic Escherichia coli (ETEC), which cause major economic losses in the swine industry (Nataro and Kaper, 1998). Strains of ETEC are defined for producing heat labile and/or stable enterotoxins (Loos et al., 2012) and expressing adhesion factors such as fimbriae F4 linked to diarrhea and F18 linked to diarrhea and edema disease (Francis, 1999).
Fungal fermented products and their derivatives are described to contain several compounds that may play a role in gastrointestinal health and pathogenic bacteria control (Wisitrassameewong et al., 2012). Microbial enzymes produced by fungal during fermentation will degrade polysaccharides from feed material into indigestible and bioactive oligosaccharides (Ariandi and Meryandini, 2015). Adhesins from some pathogenic Enterobacteriaceae are known to show binding affinity to distinct indigestible oligosaccharides. Wang et al. (2015) demonstrated such affinity for suitable oligosaccharides, i.e., d-mannose showed between 20% and 60% inhibition of bacterial adhesion (E. coli, Vibrio cholerae, Campylobacter jejuni, and Salmonella Typhimurium) to host glycans from HT-29 cells. In vivo, β-1-4 mannobiose reduced Salmonella shedding after infection in broilers (Agunos et al., 2007), and β -galactomannan oligosaccharide reduced subclinical Salmonella infection in fattening pigs (Andrés-Barranco et al., 2015). Among raw materials, rye was found to have a strong binding affinity to pathogenic E. coli (Zhu et al., 2018).
Other bioactive components from fungal fermentation are β-glucans, which are a heterogeneous group of polysaccharides present in cereal grains, fungal cell walls, seaweed, and algae (Akramiene et al., 2007). The immunomodulatory properties of different β-glucans have been demonstrated in vitro (Smiderle et al., 2014; Choi et al., 2016) and in vivo (Samuelsen et al., 2014) to support intestinal health and microbiota balance in pigs (Kogan and Kocher, 2007; Kim et al., 2019). Other promising fungal examples are metabolites derived from edible mushrooms. Products from almond mushroom are reported to contain prophylactic and therapeutic properties including antimicrobial and immunomodulatory (Smiderle et al., 2011; Wisitrassameewong et al., 2012).
In the swine industry, short chain fatty acids or organic acids (OAs) and their salts are used in nursery pig diets to improve performance via effects on digestion and gastrointestinal health (Partanen and Mroz, 1999). Formic and lactic OAs increase stomach barrier function by reducing pH and destabilizing bacterial membranes (Van der Wolf et al., 2001; Zentek et al., 2013). Butyrate, the main source of energy for the epithelial cells (Roediger, 1980; Chapman et al., 1995), is well known to enhance epithelial morphology, homeostasis, and microbiota balance (Biagi et al., 2007; Fang et al., 2014). Additionally, coated butyrate appears promising to control Salmonella. Several studies report in vitro antimicrobial properties and in vivo decreased Salmonella shedding and intestinal colonization (Van Immerseel et al., 2005; Boyen et al., 2008; Guilloteau et al., 2010; De Ridder et al., 2013).
Therefore, fungal fermented products and their derivatives contain several compounds that may reduce pathogenic bacteria load and play a role in gastrointestinal health. OAs can enhance stomach barrier function and are used to manage risk against pathogenic bacteria. Additionally, butyrate may inhibit growth of Salmonella in the intestine. However, a strategic combination of OAs with fungal fermented products has not been previously studied to control Salmonella in pigs exposed for several days.
The objective of this study was to evaluate mannan-rich hydrolyzed copra meal (MCM) and fermented rye (FR) with Agaricus subrufescens to control specific Salmonella and Escherichia coli in vitro, and to assess their efficacy to control in vivo shedding of Salmonella enterica serovar Typhimurium when combined with OA and compared with coated butyrate.
Material and Methods
Diets and additives
A standard experimental diet was used and produced at the Research Feed Plant (Heijen, The Netherlands) without additional additives or medication (Table 1). All diets met or exceeded current nutrient requirement estimates for nursery pigs (NRC, 2012). Spray-dried plasma, antibiotics, and Zn oxide were not included in the diets. Experimental diets were pelleted using a 4-mm die and fed to pigs throughout the experiment. Selko (Trouw Nutrition, Tilburg, the Netherlands) provided all feed additives. The OA blend used in the present experiment was 88% formic acid and 12% lactic acid. Selko-SR Butyrate 30 was the coated butyrate used. The FR used contained ~40% mycelium of A. subrufescens. The MCM used contained 14% β-1,4-mannobiose.
Table 1.
Composition of the experimental diets (as fed basis)
| Item | Control | OA.BU | OA.MCM | OA.FR |
|---|---|---|---|---|
| Ingredients, % | ||||
| Barley | 23.3 | 23.3 | 23.3 | 23.3 |
| Wheat | 20.0 | 20.0 | 20.0 | 20.0 |
| Corn | 18.0 | 18.0 | 18.0 | 18.0 |
| Wheat bran | 3.0 | 3.0 | 3.0 | 3.0 |
| Soybean meal (crude fiber < 50 g/kg) | 17.3 | 17.3 | 17.3 | 17.3 |
| Potato protein (as <1.0%) | 2.25 | 2.25 | 2.25 | 2.25 |
| dl-Methionine (99%) | 0.2 | 0.2 | 0.2 | 0.2 |
| l-Lysine HCl (98%) | 0.56 | 0.56 | 0.56 | 0.56 |
| L-Threonine (98%) | 0.2 | 0.2 | 0.2 | 0.2 |
| l-Tryptophan (98%) | 0.05 | 0.05 | 0.05 | 0.05 |
| Na bicarbonate | 0.54 | 0.54 | 0.54 | 0.54 |
| Ca carbonate | 0.53 | 0.53 | 0.53 | 0.53 |
| Monocalcium phosphate | 0.96 | 0.96 | 0.96 | 0.96 |
| Salt (NaCl) | 0.37 | 0.37 | 0.37 | 0.37 |
| Lactose | 6.36 | 6.36 | 6.36 | 6.36 |
| Sugar | 2.5 | 2.5 | 2.5 | 2.5 |
| Soybean oil | 2.47 | 2.47 | 2.47 | 2.47 |
| Vitamin E (50% adsorbate) | 0.24 | 0.24 | 0.24 | 0.24 |
| Vitamin–mineral premix | 1.0 | 1.0 | 1.0 | 1.0 |
| Phyzyme1 | 0.01 | 0.01 | 0.01 | 0.01 |
| l-Valine (96.5%) | 0.12 | 0.12 | 0.12 | 0.12 |
| Choline chloride (50%) | 0.03 | 0.03 | 0.03 | 0.03 |
| Organic acid blend (82% formic, 12% lactic) | - | 0.4 | 0.4 | 0.4 |
| Coated butyrate | - | 0.6 | - | - |
| Hydrolyzed copra meal | - | - | 0.2 | - |
| Fermented rye | - | - | - | 0.2 |
| Calculated content, % | ||||
| DM2 | 89.6 | 90 | 89.3 | 89.5 |
| NE3, kcal | 2,425 | 2,425 | 2,425 | 2,425 |
| SID Lys4 | 1.21 | 1.21 | 1.21 | 1.21 |
| SID Met4 | 0.45 | 0.45 | 0.45 | 0.45 |
| SID Met + Cys4 | 0.7 | 0.7 | 0.7 | 0.7 |
| SID Trp4 | 0.23 | 0.23 | 0.23 | 0.23 |
| SID Thr4 | 0.75 | 0.75 | 0.75 | 0.75 |
| CP2 | 17.9 | 17.7 | 17.7 | 17.2 |
| Acid hydrolyzed ether extract2 | 4.3 | 5.1 | 4.7 | 4.6 |
| Crude fiber2 | 3.0 | 3.1 | 3.0 | 3.1 |
| Ash2 | 5.2 | 5.4 | 5.1 | 5.1 |
| Neutral detergent finer | 10.3 | 10.3 | 10.3 | 10.3 |
| Acid detergent fiber | 3.9 | 3.9 | 3.9 | 3.9 |
| Nonstarch polysaccharides | 14.5 | 14.5 | 14.5 | 14.5 |
| Sodium2 | 0.33 | 0.38 | 0.35 | 0.35 |
| Potassium | 0.7 | 0.7 | 0.7 | 0.7 |
| Chloride | 0.39 | 0.39 | 0.39 | 0.39 |
| Magnesium | 0.17 | 0.17 | 0.17 | 0.17 |
| Calcium2 | 0.65 | 0.67 | 0.66 | 0.68 |
| Phosphorus2 | 0.59 | 0.59 | 0.60 | 0.59 |
| Copper, mg/kg | 166 | 166 | 166 | 166 |
| Manganese, mg/kg | 51 | 51 | 51 | 51 |
| Zinc, mg/kg | 126 | 126 | 126 | 126 |
Diets were analyzed for moisture (EC regulation 152/2009, appendix III A), CP (ISO 16634-1:2008), acid hydrolyzed ether extract (EC regulation 152/2009, appendix III H method A), and crude fiber (ISO 6865:2000), and ICP-OES spectrometry (Perkin-Elmer S10, model Avio 200; MA) was used to determine calcium, phosphorus, and sodium (ISO 15510:2008).
Bacterial strains
Salmonalla Typhimurium (S. Typh) strain DT12 (B; O1, 4, 5, 12; Van Winsen et al., 2001) obtained from De Gezondheidsdienst voor Dieren (Deventer, The Netherlands) was used for both the in vitro binding assay and in vivo study. Additionally, the following strains we used in the in vitro binding assays: Salmonella Enteritidis (S. Ent) isolated from infected broiler (Trouw Nutrition Poultry Research Centre, Spain), ETEC F4 (O149:K88acK91; from Wageningen Bioveterinary Research, the Netherlands), and ETEC F18 isolated from infected piglet (Trouw Nutrition Swine Research Centre, the Netherlands).
In vitro-binding affinity assay
Five in vitro binding assays (A, B, C, D, and E) were conducted using the same methodology to assess binding affinity of four different Enterobacteriaceae (S. Typh, S. Ent, ETEC F4, and ETEC F18) to MCM and FR, as described in Table 2. There were some design differences as study A did not test FR and did not include ETEC F18, whereas in study B binding to MCM was not tested. The binding affinities of the bacteria to the feed additives were measured by time required to reach OD600nm of 0.5. Less time indicates that more bacteria adhered to the substrate resulting in less time to reach the OD600nm cutoff.
Table 2.
Design of 5 in vitro assays to test binding affinity of different Enterobacteriaceae to feed additives1 compared with the control2.
| Bacteria | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Salmonella Typhimurium | Salmonella Enteritidis | Escherichia coli F4 | Escherichia coli F18 | ||||||||
| A | Con | FR | Con | FR | Con | FR | Con | |||||
| B | Con | MCM | Con | MCM | Con | MCM | Con | MCM | ||||
| C | Con | MCM | FR | Con | MCM | FR | Con | MCM | FR | Con | MCM | FR |
| D | Con | MCM | FR | Con | MCM | FR | Con | MCM | FR | Con | MCM | FR |
| E | Con | MCM | FR | Con | MCM | FR | Con | MCM | FR | Con | MCM | FR |
1Feed additives at 1% (w/v) were MCM, hydrolyzed copra meal; FR, fermented rye.
2Control, without feed additive.
For each binding assay, different wells of a 96-well microplate were coated with MCM, FR, or BSA as a control in which the binding of different bacteria were tested according to Becker et al. (2007) with some modifications. Briefly, MCM and FR (0.5 mm grinded) were suspended to a final concentration of a 1% (w/v) in PBS suspension. Subsequently, the suspensions were incubated 3 times 2 min in a sonication water bath (Branson 5510) with intermediate vortexing and centrifuged at 460 × g for 5 min. The wells of the microtiterplate (Microlon F plate 655092 Greiner Bio_one B.V.) were coated overnight at 4 °C with 250 µL of supernatant from the different suspensions in duplo. The control wells were coated with 250 µL PBS. After coating, the wells were washed with 300 µL of PBS and subsequently blocked with 300 µL 1% (w/v) BSA [Sigma-Aldrich (A7906)] for 1 h at 4 °C. After blocking the wells, they were washed twice with 300 µL PBS. Consequently, the bacterial suspension was grown to end the logaritmic phase in Brain Heart Infusion broth (BHI; Oxiod). Then washed and resuspended in PBS adjusted to OD600 nm 0.02 and 250 µL added to the wells of the microplate. Bacteria were allowed to adhere at room temperature for 30 min. After adhesion, the plate was washed 3 times with 300 µL of PBS and 250 µL of BHI was added to each well. The plate was incubated in the microplate reader (SpectraMax M2, Molecular Devices Corporation, Silicon Valley, CA) at 37 °C and growth was monitored by measuring OD600nm every 5 min for 18 h with 5 s shaking before each measurement. Therefore, the binding affinities of the bacteria to the feed additives were measured by time required to reach OD600nm of 0.5 cutoff. Blank culture controls for MCM and FR without adding bacteria were also included. These controls show no bacterial growth for >16 h. Bacterial growth was specific measured for the bacteria added.
In vivo study
The protocol for this experiment was reviewed and approved by the Animal Experiment Committee (DEC) of Utrecht and applied under project license permit number 2014.III.07.063.
Animals, housing, and experimental design
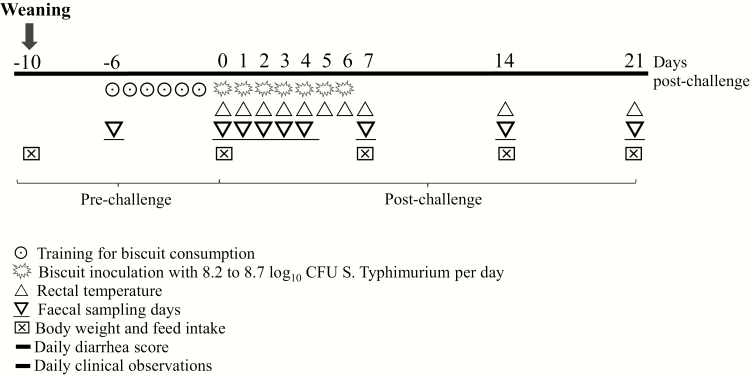
A total of 32 weaning male pigs (Topi × Hypor 24 d of age ± 3 d SD) with an average initial BW of 6.29 ± 0.76 kg were selected from the Swine Research Center of Trouw Nutrition R&D facilities (P.O. Box 220, 5830 AE Boxmeer, the Netherlands). After weaning, all pigs were individually housed (pen size: 1.60 × 1.60 m), and taking into account litter origin, were randomly assigned to 1 of 4 dietary treatments: (i) a control; (ii) 4 kg/t OAs plus 6 kg/t coated butyrate (OA.BU); (iii) 4 kg/t OAs plus 1 kg/t MCM (OA.MCM), and (iv) 4 kg/t OAs plus 2 kg/t FR (OA.FR). The experimental unit was pig and there were 8 replicate pigs per treatment. Each piglet received a feed matrix containing ~9.0 log10 cfus of S. Typh for 7 d consecutive at 10 d postweaning (described below). Pigs were under environmentally control unit for 31 d, as 10 d before (−10 d) and 21 d after the first S. Typh inoculation (day 0) with ad libitum access to feed and water. A summary of the infection model and sampling is presented in Figure 1. Environmental enrichment was provided for each pig. The room was 25 to 27 °C, and daily light was on at 0700 h and off at 1900 h throughout the experiment.
Figure 1.
Summary of infection model with Salmonella Typhimurium for 7 d in nursery pigs and measurements collected.
The inoculum matrix (ladyfinger biscuits) preparation and administration were based on the Litjens et al. (2017) and Van der Wolf et al. (2017) methodology. Briefly, the feed matrix was inoculated with 1 mL of an S. Typh culture of ~9.0 log10 CFU/mL. The piglets received the inoculated feed matrix for 7 d consecutive after 6 d training with non-infected matrix. The required inoculated biscuits were freshly prepared each day before challenge (in the morning), except for weekend days when biscuits were prepared on the Friday before and stored at 4 °C. Salmonella Typh was quantified in the feed matrix after storage for 24 and 48 h at 4 °C to ensure viability of the matrix inoculum (8.2 to 8.7 log10 CFU/piece of feed matrix). Initially, there were two surplus animals per treatment but the day before challenge, 8 piglets were selected for inclusion in the experiment on the basis of the following criteria: (i) no Salmonella detected in feces, (ii) >50 g/d weight gain, (iii) willingness to consume the feed matrix. When more pigs than required animals met all criteria, the BW average animals were selected. Consumption of the feed matrix was in the afternoon and monitored for each individual animal.
Clinical observations and sample collection
A summary of sample collection is presented in Figure 1. Clinical observations and diarrhea score were recorded daily throughout the experiment and rectal temperature before and after the challenge on days 0, 1, 2, 3, 4, 5, 6, 7, 14, and 21 postchallenge. The diarrhea score was visually assessed daily for each pig by a trained evaluator using a scoring system (Van der Wolf et al., 2017) ranging from 0 to 3 (0 = normal feces, 1 = shapeless or loose feces, 2 = diarrhea with thick liquid feces, 3= severe diarrhea as thin watery feces, and 9 = no score possible). Diarrhea incidence was calculated as the percentage of days with a fecal score of 2 and 3 per pen using the following different periods; −6 to 0 d, 1 to 7 d, 8 to 14 d, and 15 to 21 d post first contact to S. Typh. Pigs were weighed at weaning day (−10 d) and 0, 7, 14, and 21 d post challenge. ADG, ADFI, and feed efficiency (G:F) were calculated for each interval and overall. Fecal samples (5 g per pig) were collected at −6, 0, 1, 2, 3, 4, 7, 14, and 21 d post challenge and analyzed quantitatively for S. Typh fecal shedding.
Salmonella shedding
Fecal samples were collected and directly stored at 4 °C and processed within 24 h as described by Litjens et al. (2017). Briefly, 1 g of each fecal sample was diluted 1:10 in buffered peptone water (Oxoid) supplemented with 20 mg/L novobiocin (AppliChem GmbH, Darmstadt, Germany), homogenized using a stomacher, and 10-fold serial dilutions were made in sterile 0.1% peptone physiological salt solution (Tritium Microbiologie B.V.) up to 10–4. Dilutions were surface plated (100 μL) onto Brilliant Green Agar plates with 20 mg/L novobiocin + 40 g/L potassium iodide (Tritium Microbiologie B.V.) and incubated for 21 ± 3 h at 37 °C ± 1 °C for colony counting. For analysis, individual Salmonella counts after challenge were converted into log10 values. The remaining 1:10 suspensions were pre-enriched for 16 to 20 h at 37 °C ± 1 °C. If no Salmonella were found on the counting plates, the pre-enriched samples were analyzed for the presence or absence of Salmonella according to ISO 6579:2002. This method was also used at day −6 before first S. Typh contact to confirm all pigs were Salmonella negative.
Presumptive Salmonella (red/pink) colonies were enumerated and confirmed as Salmonella enterica (spp.) and S. Typh-specific multiplex via quantitative PCR (qPCR) using two randomly selected colonies per sample. The real-time PCR reaction was performed in a CFX96 Real-Time PCR system on a C1000 thermal cycler (Bio-Rad Laboratories Inc., Hercules, CA). The conditions for real-time PCR reaction and the reagent mixes, primers, and probes used were as in Litjens et al. (2017). Positive criterion was set at a cycle threshold smaller than 35. Samples remaining negative were presumed to contain <1 CFU/g feces. Samples were presumed to contain <100 CFU/g feces (as detection limit for quantification) and were included in the data set as “50.” when Salmonella was detected after pre-enrichment.
Statistical analysis
The normality of data was checked on the basis of visual assessment of residual plots (SAS Inst. Inc., Cary, NC). In general, data were analyzed by the MIXED procedure of SAS unless otherwise stated. The time in hours to reach an OD600nm cutoff of 0.5 was analyzed using treatment (control, MCM, and FR) as the main effect, the within study variation included as a random effect, and independent assays (A, B, C, D, and E) were included as a repeated effect for the in vitro assay data. Pig performance data were analyzed using the pig as the experimental unit and the model included treatment (control, OA.BU, OA.MCM, and OA.FR) as the main effect, BW block (pig) as the random effect, and time of measurements included as a repeated measurement. Treatment means were separated by using the LSMEANS statement, PDIFF option, and SIMULATE adjustment for comparison in PROC MIXED. A diarrhea score equal to 9 (no score possible) occurred only at weaning and on 3 d postweaning, and these data were excluded from analysis. Diarrhea incidence was not normally distributed and, therefore, were analyzed using PROC GLIMMIX which included treatment (control, OA.BU, OA.MCM, and OA.FR) as the main effect, BW block (pig) as the random effect, and time of frequency measurements included as a repeated effect. The dist = beta and link = logit functions were used to manage frequency data. Statistical significance and tendency were considered at P ≤ 0.05 and 0.05 ≤ P ≤ 0.10, respectively.
Results
In vitro-binding affinity
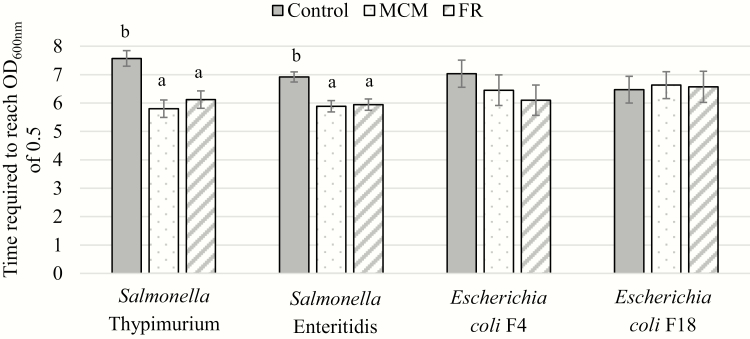
The in vitro results are presented in Figure 2. Culture substrate with additional MCM and FR showed less (P < 0.01) time to growth OD600nm than control culture for both S. Typh and S. Ent. The time (hours) to grow to OD600nm of 0.5 cutoff were 7.57 for control, 5.79 for MCM, and 6.12 for FR on S. Typh and for S. Ent the times were 6.92 for control, 5.88 for MCM, and 5.94 for FR on S. Ent. Less time to grow to OD600nm indicated that more bacteria adhered to the substrate (control, MCM, or FR), which resulted in less time to reach the OD600nm cutoff. There was no effect of culture substrate with treatment for ETEC F4 and F18 species. However, a study interaction was observed among the 5 independent assays (A–E). This interaction indicated that study A had a greater (P < 0.05) and study E tended to have a greater (P < 0.10) binding affinity of FR with ETEC F4 compared with the control. The time to grow to OD600nm of 0.5 cutoff was 7.27 h for FR and 9.17 h for the control in study A (SE = 0.805, P = 0.045), and 5.67 h for FR and 7.11 h for control in study E (SE = 0.922, P = 0.101). The overall variance in the mixed model for ETEC F4 was high and this effect was not consistent across studies.
Figure 2.
In vitro assay to investigate binding affinity of pathogenic Enterobacteriaceae to culture substrate including feed additives1. Results read as time required to reach OD600nm of 0.5. Less time indicates that more bacteria adhered to the substrate (control, MCM, or FR) resulting in it taking less time to reach the OD600nm cutoff. 1Feed additives at a 1% (w/v) were MCM, hydrolyzed copra meal; FR fermented rye. a, bMeans without a common superscript are different (P ≤ 0.01).
In vivo study
Growth performance, rectal temperature, and diarrhea score
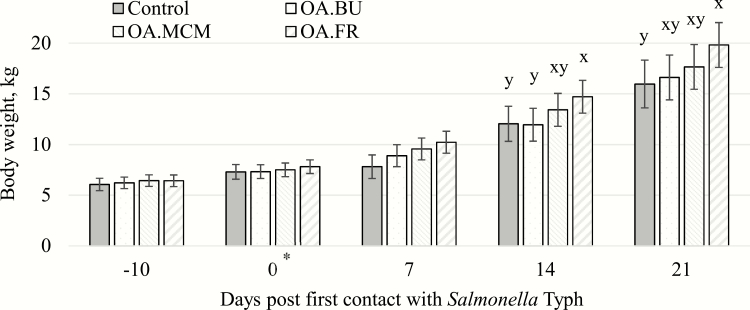
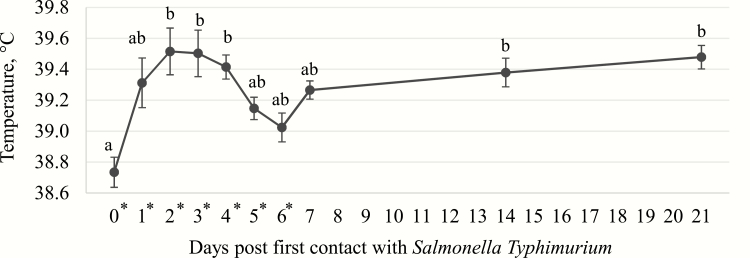
Pigs fed OA.FR at 14 d post challenge (14.7 kg) tended to be heavier (P = 0.068) than those fed OA.BU (11.9 kg) and control (12.0 kg), while OA.MCM was not different (13.4 kg; Figure 3). Similarly, pigs fed the OA.FR (19.8 kg) tended to be heavier (P = 0.093) on 21 d post challenge compared with control (16.0 kg) fed pigs, while pigs fed OA.BU (16.6 kg) and OA.MCM (17.7 kg) were not different. Pigs fed the diet containing OA.FR showed a near tendency (P = 0.101) for higher ADG (641 g) compared with pigs fed control (484 g) and OA.BU (436 g) between days 7 and 14 post first S. Typh contact (Table 3). Rectal temperature was not different amongst pigs fed dietary treatments (Figure 4). All pigs had a higher (P < 0.01) rectal temperature on day 4 post S. Typh challenge.
Figure 3.
BW of weaned pigs (n = 32) challenged (*) at 34 ± 3 d of age (10 d postweaning) with Salmonella Typhimurium (Typh; oral 8.2 to 8.7 log10 CFU per day) for 7 d consecutive (0 to 6 d) and fed four blend dietary treatments. x,yMeans without common superscript showed a tendency for difference at time point (P ≤ 0.10). OA.BU, 4 kg/t organic acids plus coated 6 kg/t butyrate; OA.MCM, 4 kg/t organic acids plus 1 kg/t hydrolyzed copra meal; OA.FR, 4 kg/t organic acids plus 2 kg/t fermented rye.
Table 3.
Weekly performance of weaned pigs (n = 32) challenged (*) at 34 ± d of age 3 d SD (10 d postweaning) with Salmonella Typhimurium (oral 8.2 to 8.7 log10 CFU per day) for 7 d consecutive (0 to 6 d) and distributed in 4 blend dietary treatments
| Control | OA.BU1 | OA.MCM2 | OA.FR3 | SEM4 | P-value | ||
|---|---|---|---|---|---|---|---|
| ADG, g | −10 to 0* | 124 | 111 | 106 | 139 | 16.3 | 0.451 |
| 1 to 7 | 194 | 225 | 295 | 345 | 56.7 | 0.220 | |
| 7 to 14 | 484 | 436 | 552 | 641 | 63.7 | 0.101 | |
| 14 to 21 | 561 | 666 | 606 | 730 | 61.2 | 0.221 | |
| ADFI, g | −10 to 0* | 171 | 155 | 164 | 181 | 17.4 | 0.714 |
| 1 to 7 | 268 | 287 | 313 | 354 | 41.0 | 0.453 | |
| 7 to 14 | 547 | 606 | 654 | 742 | 65.8 | 0.191 | |
| 14 to 21 | 779 | 828 | 874 | 997 | 74.0 | 0.183 | |
| FE4 g/g | −10 to 0* | 0.75 | 0.72 | 0.66 | 0.77 | 0.073 | 0.691 |
| 1 to 7 | 0.74 | 0.72 | 0.91 | 0.97 | 0.103 | 0.162 | |
| 7 to 14 | 0.88 | 0.70 | 0.84 | 0.88 | 0.067 | 0.203 | |
| 14 to 21 | 0.70xy | 0.83x | 0.69y | 0.73xy | 0.046 | 0.086 |
1OA.BU, additional 4 kg/t organic acids plus 6 kg/t coated butyrate.
2OA.MCM, additional 4 kg/t organic acids plus 1 kg/t hydrolyzed copra meal.
3OA.FR, additional 4 kg/t organic acids plus 2 kg/t fermented rye.
4FE, feed efficiency as gram of weight gain divided by gram of feed intake.
x,yMeans without common superscript showed a tendency for difference at time frame (P ≤ 0.10).
Figure 4.
Rectal temperature of weaned pigs (n = 32) challenged at 34 ± 3 d of age (10 d postweaning) with (*) Salmonella Typhimurium (Typh; oral 8.2 to 8.7 log10 CFU per day) for 7 d consecutive (*). a,bMeans without a common superscript are different (P ≤ 0.05).
Diarrhea was already present before the challenge as indicated by 57.4 and 21.4% of pigs had a score greater than or equal to 2 and a score of 3, respectively. The diarrhea incidence was maintained during the S. Typh infection week as indicated by 54.9% of pigs had a score ≥2 and 19.7% of pigs had a score equal to 3. Diarrhea incidence, defined as mild and severe diarrhea (score ≥ 2), tended to be lower (P = 0.100) prior to challenge in pigs fed OA.FR (43.3%) compared with pigs fed OA.MCM (74.0%; Table 4). During the challenge week, diarrhea incidence tended to be lower (P = 0.056) in pigs fed OA.FR (43.3%) or control (43.3%) compared with pigs fed OA.MCM (72.7%). Between days 8 and 14 after first S. Typh inoculation, diarrhea incidence tended to be lower (P = 0.054) in pigs fed OA.FR (21.2%) than pigs fed OA.BU (61.9%). Overall, diarrhea incidence post challenge was lower (P = 0.006) for pigs fed OA.FR (18.9%) than OA.BU (44.8%) and OA.MCM (41.7%), while diarrhea incidence of pigs fed the control (28.7%) was intermediate and not different. No difference in a diarrhea score of 3 was observed for pigs fed different feed additives post first S. Typh inoculation. However, a diarrhea score equal to 3 was lower (P = 0.042) for OA.FR (8.27%) fed pigs compared with OA.BU (15.5%) fed pigs while OA.MCM (13.4%) and control (11.3%) fed pigs were not different over the entire experimental period (i.e., including pre-infection time).
Table 4.
Weekly frequency of days with diarrhea scores1 in weaned pigs (n = 32) challenged (*) at 34 d of age ± 3 d SD (10 d postweaning) with Salmonella Typhimurium (oral 8.2 to 8.7 log10 CFU per day) for 7 d consecutive (0 to 6 d) and distributed in four blend dietary treatments
| Control | OA.BU2 | OA.MCM3 | OA.FR4 | SEM | P-value | |
|---|---|---|---|---|---|---|
| SCORE ≥ 2 | ||||||
| −6 to 0* | 50.6xy | 61.7xy | 74.0y | 43.3x | 8.734 | 0.100 |
| 1 to 7 | 43.3x | 60.3xy | 72.7y | 43.3x | 7.751 | 0.036 |
| 8 to 14 | 46.0xy | 61.9y | 56.6xy | 21.2x | 10.14 | 0.054 |
| 15 to 21 | 17.7 | 33.6 | 17.7 | 0.0 | 10.22 | 0.557 |
| Post infection5 | 28.7ab | 44.8b | 41.7b | 18.9a | 6.393 | 0.003 |
| Overall5 | 32.8ab | 47.3b | 47.2b | 22.2a | 5.553 | 0.006 |
| SCORE 3 | ||||||
| −6 to 0 | 27.2 | 24.3 | 26.3 | 7.7 | 7.791 | 0.253 |
| 1 to 7 | 21.7 | 23.2 | 26.3 | 7.7 | 6.791 | 0.209 |
| 8 to 14 | 17.7 | 14.2 | 1.77 | 0.0 | 5.944 | 0.267 |
| 15 to 21 | 5.31 | 12.4 | 0.0 | 0.0 | 4.555 | 0.572 |
| Post infection5 | 11.2 | 12.8 | 8.69 | 7.04 | 2.945 | 0.152 |
| Overall5 | 11.3ab | 15.5b | 13.4ab | 8.27a | 2.938 | 0.042 |
1Diarrhea score was assessed as 0 = normal feces, 1 = shapeless or loose feces, 2 = thick soft feces as mild diarrhea; and 3 = thin liquid feces as watery severe diarrhea (Wolf et al., 2017).
2OA.BU, additional 4 kg/t organic acids plus 6 kg/t coated butyrate.
3OA.MCM, additional 4 kg/t organic acids plus 1 kg/t hydrolyzed copra meal.
4OA.FR, additional 4 kg/t organic acids plus 2 kg/t fermented rye.
5Overall and regardless of time point.
x,yMeans without common superscript showed a tendency for difference at time frame (P ≤ 0.10).
a,bMeans without a common superscript are different at time frame (P ≤ 0.05).
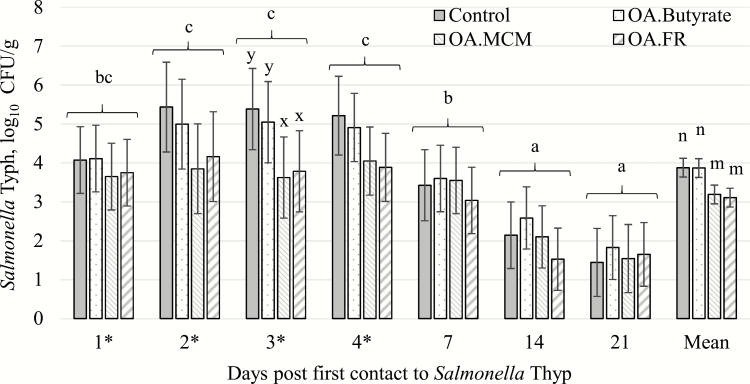
Salmonella shedding
All pigs were negative to Salmonella on days −6 and 0. Salmonella shedding in feces of pigs was detected postchallenge and had a higher (P < 0.05) peak on days 2, 3, and 4 after first inoculation compared with day 7, which was still higher (P < 0.05) than days 14 and 21 (Figure 5). Salmonella Typh fecal shedding counts (log10 CFU/g) for pigs fed OA.MCM (3.62) and OA.FR (3.79) were lower (P < 0.05) than for pigs fed control (5.39) and OA.BUT (5.05) on day 3 post first challenge day. Over the 21-d period post challenge, pigs fed OA.MCM (3.19) and OA.FR (3.11) had reduced (P < 0.05) S. Typh shedding (log10 CFU/g) compared with control (3.88) and OA.BUT (3.87) fed pigs.
Figure 5.
Shedding of Salmonella Typhimurium (presented as least squares means ± SE) in weaned pigs (n = 32) challenged at 34 ± 3 d of age (10 d post weaning) with (*) S. Typhimurium (Typh; oral 8.2 to 8.7 log10 CFU per day) for 7 d consecutive (0 to 6 d) and supplemented with different blend dietary treatments. OA.BU, 4 kg/t organic acids plus 6 kg/t coated butyrate; OA.MCM, 4 kg/t organic acids plus 1 kg/t hydrolyzed copra meal; OA.FR, 4 kg/t organic acids plus 2 kg/t fermented rye. a–cMeans without a common superscript are different among time point (P ≤ 0.05). x,yMeans without a common superscript are different within time point (P ≤ 0.05). m,nMeans without a common superscript are different (P ≤ 0.05).
Discussion
Current measures to prevent pig-related pathogenic zoonotic bacteria, such as Salmonella, in the feed-to-food chain are not sufficient (Baptista et al., 2010; Savall et al., 2016). Furthermore, new and practical strategies for pig producers are required due to the urgent need to reduce antibiotic usage. The present study provided insight for feed additives using in vitro-binding affinity to 2 Salmonella pathogenic species and this insight was used to demonstrate in vivo reduction of S. Typh fecal shedding in nursery pigs.
Oral administration of a matrix containing 8.2- to 8.7-log10 CFU S. Typh (DT12 field strain) to pigs for 7 d consecutive resulted in a detectable and quantifiable fecal Salmonella shedding. Also, fever was detected through an increased rectal temperature in pigs up to day 4 postchallenge, at which point rectal temperature slowly lowered back to normal. Thereafter, the temperature was increasing again to age physiological levels (Sipos et al., 2013). Salmonella shedding during peak days was ~4.5 log10 CFU/g, which is similar to our previous experiments (Litjens et al., 2017; Van der Wolf et al., 2017). Fecal shedding of Salmonella was reduced in pigs fed OA in combination with MCM or FR. This effect was most evident during acute infection and peak of shedding (2 to 4 d post), but not thereafter (7, 14, and 21 d post infection), which suggests a limitation to reduce colonization above a certain threshold (i.e., ~3.5 log10 CFU/g). Reason for such limitation is unknown and could be speculated that may be related to a high load of Salmonalla exposure for several days. These in vivo findings are in agreement with the in vitro experiment that demonstrated binding affinity of both Salmonella strains to culture substrate including MCM and FR. Altogether, blocking adhesion and prevention of colonization during high Salmonella load exposition may be the mode of action of these feed additives.
Distinct, commonly used feed ingredients and their bran fractions are known to also possess an affinity to bind pathogenic bacteria. However, this may not always result in a biological benefit to the animal. Zhu et al. (2018) demonstrated that wheat, corn, oats, barley, rye, soybean meal, and sweet whey powder have affinity to bind ETEC F4, while only rye, oats, and wheat reduced ETEC F4 adhesion to IPEC-J2 cells. Thus, binding affinity does not always translate to reduced risk of pathogenic adhesion, whereas in vivo bacterial shedding and colonization explain the actual susceptibility to disease (De Ridder et al., 2013). The blocking of adhesion was not evaluated in the present study but shedding after infection was assessed in vivo.
Less fecal Salmonella shedding is indicative of less Salmonella colonization in the intestine and reduced severity of infection (Knetter et al., 2015; Casanova‐Higes et al., 2017). However, lymph node tissue was not measured in this study to confirm colonization. Nonetheless, MCM and FR binding affinity taken together with a lower shedding suggest bioactivity to reduce Salmonella infection. More research is needed to elucidate the effect of MCM and FR on actual colonization of tissues. Adhesion of ETEC to FR was unclear and only observed for 1 study and a near tendency for another 1 out of 5 assays. This was unexpected since Zhu et al. (2018) reported ETEC F4 having 17.3% binding affinity and 9.02% blocking adhesion to nonfermented rye. Data from ETEC F4 assays showed greater variance than for Salmonella without a clear explanation, hence, ETEC binding affinity to FR remains unclear.
From the present results, MCM and FR effects on Salmonella shedding should be attributed only when combined with the OA blend; however, it is noteworthy that OA combined with coated butyrate (OA.BU) did not reduce Salmonella shedding. The lack of intervention from OA.BU was partly expected since additional OA showed only numerical reduction of shedding (Van der Wolf et al., 2017). However, OA.BU results contrasts with Boyen et al. (2008), who reported a decreased colonization and shedding in challenged pigs with S. Typh when supplemented with 2 g/kg coated butyrate. Furthermore, De Ridder et al. (2013) observed a reduction of positive pigs as Salmonella shedding or having positive intestinal tissues feeding 3 g/kg coated butyrate. These experiments used a single day challenge model inoculating all animals with 107 CFU/mL or 2 out of 8 pigs per pen inoculated with 109 CFU/mL. Whereas the present study used a 7-d challenge with 109 CFU/mL per day resulting in higher infection pressure compared with the abovementioned experiments, thus, comparison across challenge studies is difficult.
The mode of action of most short chain fatty acids in the gastrointestinal tract is linked to pH-lowering and antimicrobial anion toxicity properties (Van der Wolf et al., 2001). Indeed, Salmonella seroprevalence can be controlled with acidifiers used in water or feed under field conditions (Van der Wolf et al., 2001; Van der Heijden et al., 2005). For coated butyrate, bioactivity is more complex and includes downregulation of Salmonella virulence, renewal of intestine necrotic areas, and a reduced inflammatory response (Van Immerseel et al., 2005; Boyen et al., 2008; Hamer et al., 2008). Although unknown intestinal tissue morphplogy, inflammation, and virulence of Salmonella herein, growth performance and shedding were not influenced in the present use of OA.BUT (including formic and lactic acids plus coated butyrate). Our 7-d oral administration of Salmonella is the main difference compared with a single inoculum dose in the abovementioned literature, which may explain the varying outcome. Differently, fermented or enzymatically hydrolyzed product as FR and MCM, respectively, did show a reduction of Salmonella shedding during the 1-wk inoculation. These findings are important since under field conditions carrier pigs can shed and expose pen mates to high Salmonella loads for extended periods (Griffith et al., 2006).
The MCM and FR include indigestible polysaccharides and oligosaccharides from fungal enzyme hydrolysis of copra meal and rye, which may be promoting the bioactivity against Salmonella. Wang et al. (2015) demonstrated blocking adhesion of S. Typh to HT-29 cells for fucoidan (71.4%, β-1, 4-mannose, GlcUA, Gal, and α-1,3-fucose), tara gum (62.1%, β-1, 4-mannose, α-1, and 6-galactose), and guar gum (54.5%, β-1, 4-mannose, and α-1,6-galactose) oligosaccharides. Copra meal includes ~40% to 45% of mannan-polysaccharides from a total 61% carbohydrate content (Saittagaroon et al., 1983). Industrial hydrolysis into mannan-oligosaccharides (i.e., mannose, mannobiose, mannotriose, etc.) is feasible with fungal β-mannanases (i.e., Actinomycetes from the Streptomycetes group; Ademark et al., 1998; Ariandi and Meryandini, 2015). The oligosaccharide content is ~14% β-1,4-mannobiose in MCM. Hydrolyzed copra meal which contained 11.4% β-1,4-mannobiose and was supplemented at 1 g/kg feed in broilers reduced Salmonella colonization, caecal carriage, and fecal shedding after infection (Agunos et al., 2007). Such effects were accompanied with immunomodulatory properties including IgA production. Furthermore, β-1,4-mannobiose increased in vitro Salmonella-killing activity in chicken macrophages (Ibuki et al., 2011). In a colitis pig model, it was reported that β-1,4-mannobiose downregulated innate T helper pro-inflammatory pathways which maintained intestinal permeability and histological morphology (Ibuki et al., 2014). Altogether, this suggests a mode of action more complex than bacterial-binding affinity. Rye fermented with A. subrufescens poly-oligosaccharide composition is not reported; however, use of nonfermented rye already shows promising binding affinity to pathogenic E. coli (Zhu et al., 2018). Further investigation is needed to better elucidate the binding affinity, shedding reduction, and other health promoting potential associated with FR.
The observed diarrhea before Salmonella challenge increased variance and might interfere with dietary treatment intervention and caution must be used when interpreting the results. It was confirmed, however, that Salmonellosis was not the cause of diarrhea prechallenge because pigs tested negative for S. Typh pre challenge. Dietary treatment did influence diarrhea outcome. Furthermore, whether mild diarrhea may interact with Salmonella shedding outcome is not clear. Higher intestinal transit may reduce chances of Salmonella colonization or derived inflammation may increase Salmonella colonization. In fact, research of Salmonella challenge interaction with postweaning diarrhea is lacking. Because all treatments had between 43% and 74% postweaning and prechallenge diarrhea, none included mortality, and performance differences were not observed, the outcome reported herein is relevant.
Although the diarrhea incidence was altered, results are worthy of discussion since postweaning diarrhea is a common commercial problem that is difficult to fully explain because it is multifactorial. Pigs fed OA.FR diets tended to have a low frequency of diarrhea before Salmonella challenge, and had lower diarrhea postchallenge and overall compared with pigs fed OA.BU. Additionally, OA.FR fed pigs tended to have a greater final BW. This fungal itself (also known as almond mushroom or sun mushroom) and extracts of it are reported to have several bioactive properties, i.e., tumor suppressor, immune modulatory, antimicrobial, antiviral, antioxidant, and anti-allergy (Wisitrassameewong et al., 2012) and these bioactive components within FR may have improved intestinal health of these pigs and this may have led to an improved BW. Furthermore, ~40% of FR is mycelium of A. subrufescens that grow during the fermentation of the rye, of which β -glucan polysaccharides are important active components in A. subrufescens (Kogan and Kocher, 2007; Ohno et al., 2011). β -Glucans are recognized by distinct cell receptors (i.e., dectin, TLR-2, TLR-4, and CR3) which are known to promote gut health via immunomodulatory properties demonstrated in vitro (Smiderle et al., 2014; Choi et al., 2016) and in vivo (Samuelsen et al., 2014; Kim et al., 2019). Phenolic compounds (i.e., as gallic acid, syringic acid, and pyrogallol) in the mycelia from FR may also include valuable antioxidant properties (Carvajal et al., 2012). Nonetheless, to our knowledge, this is the first time that a derivate product of A. subrufescens rye fermentation was used in a Salmonella challenge and in a pig model. Therefore, further studies to evaluate FR composition and bioactivity are needed to elucidate its mode of action and potential effects on the gastrointestinal health of pigs.
Conclusion
Hydrolyzed copra meal and fermented rye feed additives showed in vitro binding affinity to S. Typh and S. Ent. Feed additive blends including MCM or FR combined with OA reduced peak shedding and mean shedding of S. Typh in nursery pigs under a 7-d challenge evaluated for 21 d. Shedding was not influenced by the use of coated butyrate with OA blend. Fermented rye combined with OA fed to pigs tended to improve BW compared with control and coated butyrate with OA (to 14 d post first inoculation) and to control (as final BW at 21 d post first inoculation). In addition, FR combined with OA shows the greatest potential to reduce frequency of postweaning diarrhea and 7 d post Salmonella infection.
Acknowledgments
Research was subsidized by the European Union and European Regional Development Fund. Authors would like to thank Scelta Mushrooms BV (Venlo, The Netherlands) providing fermented rye.
Conflict of interest statementAll authors declare no conflict of interest.
Literature Cited
- Ademark P., Varga A., Medve J., Harjunpää V., Drakenberg T., Tjerneld F., and Stålbrand H.. . 1998. Softwood hemicellulose-degrading enzymes from Aspergillus niger: purification and properties of a beta-mannanase. J. Biotechnol. 63:199–210. doi: 10.1016/s0168-1656(98)00086-8 [DOI] [PubMed] [Google Scholar]
- Agunos A., Ibuki M., Yokomizo F., and Mine Y.. . 2007. Effect of dietary beta1-4 mannobiose in the prevention of Salmonella enteritidis infection in broilers. Br. Poult. Sci. 48:331–341. doi: 10.1080/00071660701370442 [DOI] [PubMed] [Google Scholar]
- Akramiene D., Kondrotas A., Didziapetriene J., and Kevelaitis E.. . 2007. Effects of beta-glucans on the immune system. Medicina (Kaunas). 43:597–606. doi: 10.3390/medicina43080076 [DOI] [PubMed] [Google Scholar]
- Andrés-Barranco S., Vico J. P., Grilló M. J., and Mainar-Jaime R. C.. . 2015. Reduction of subclinical Salmonella infection in fattening pigs after dietary supplementation with a ß-galactomannan oligosaccharide. J. Appl. Microbiol. 118:284–294. doi: 10.1111/jam.12713 [DOI] [PubMed] [Google Scholar]
- Ariandi Y., and Meryandini A.. . 2015. Enzymatic hydrolysis of copra meal by mannanase from Streptomyces sp. BF3.1 for the production of mannooligosaccharides. HAYATI J. Biosci. 22:79–86. doi: 10.4308/hjb.22.2.79 [DOI] [Google Scholar]
- Baptista F. M., Dahl J., and Nielsen L. R.. . 2010. Factors influencing Salmonella carcass prevalence in Danish pig abattoirs. Prev. Vet. Med. 95:231–238. doi: 10.1016/j.prevetmed.2010.04.007. [DOI] [PubMed] [Google Scholar]
- Becker P. M., Galletti S., Roubos-van den Hil P. J., and van Wikselaar P. G.. . 2007. Validation of growth as measurand for bacterial adhesion to food and feed ingredients. J. Appl. Microbiol. 103:2686–2696. doi: 10.1111/j.1365-2672.2007.03524.x. [DOI] [PubMed] [Google Scholar]
- Biagi G., Piva A., Moschini M., Vezzali E., and Roth F. X.. . 2007. Performance, intestinal microflora, and wall morphology of weanling pigs fed sodium butyrate. J. Anim. Sci. 85:1184–1191. doi: 10.2527/jas.2006-378. [DOI] [PubMed] [Google Scholar]
- Boyen F., Haesebrouck F., Vanparys A., Volf J., Mahu M., Van Immerseel F., Rychlik I., Dewulf J., Ducatelle R., and Pasmans F.. . 2008. Coated fatty acids alter virulence properties of Salmonella Typhimurium and decrease intestinal colonization of pigs. Vet. Microbiol. 132:319–327. doi: 10.1016/j.vetmic.2008.05.008. [DOI] [PubMed] [Google Scholar]
- Carvajal A. E. S., Koehnlein E. A., Soares A. A., Eler G. J., Nakashima A. T., Bracht A., and Peralta R. M.. . 2012. Bioactives of fruiting bodies and submerged culture mycelia of Agaricus brasiliensis (A. blazei) and their antioxidant properties. LWT-Food Sci. Technol. 46:493–499. doi: 10.1016/j.lwt.2011.11.018 [DOI] [Google Scholar]
- Casanova‐Higes A., Andrés‐Barranco S., and Mainar‐Jaime R.. . 2017. Influence of on‐farm pig salmonella status on salmonella shedding at slaughter. Zoonoses Public Health. 64:328–336. doi: 10.1111/zph.12301 [DOI] [PubMed] [Google Scholar]
- Chapman M. A., Grahn M. F., Hutton M., and Williams N. S.. . 1995. Butyrate metabolism in the terminal ileal mucosa of patients with ulcerative colitis. Br. J. Surg. 82:36–38. doi: 10.1002/bjs.1800820115 [DOI] [PubMed] [Google Scholar]
- Choi E. Y., Lee S. S., Hyeon J. Y., Choe S. H., Keum B. R., Lim J. M., Park D. C., Choi I. S., and Cho K. K.. . 2016. Effects of β-glucan on the release of nitric oxide by macrophages stimulated with lipopolysaccharide. Asian-Australas. J. Anim. Sci. 29:1664–1674. doi: 10.5713/ajas.16.0418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- CVB 2006. Tabellenboek Veevoeding 2006 voedernormen Varkens en voederwaarden voedermiddelen voor Varkens, ed. The Netherlands:Federatie Nederlandse Diervoederketen. [Google Scholar]
- De Ridder L., Maes D., Dewulf J., Pasmans F., Boyen F., Haesebrouck F., Méroc E., Butaye P., and Van der Stede Y.. . 2013. Evaluation of three intervention strategies to reduce the transmission of Salmonella Typhimurium in pigs. Vet. J. 197:613–618. doi: 10.1016/j.tvjl.2013.03.026 [DOI] [PubMed] [Google Scholar]
- Fang C. L., Sun H., Wu J., Niu H. H., and Feng J.. . 2014. Effects of sodium butyrate on growth performance, haematological and immunological characteristics of weanling piglets. J. Anim. Physiol. Anim. Nutr. (Berl.). 98:680–685. doi: 10.1111/jpn.12122 [DOI] [PubMed] [Google Scholar]
- Francis D. H. 1999. Colibacillosis in pigs and its diagnosis. Swine Health Prod. 7:241–244. [Google Scholar]
- Griffith R. W., Schwartz K. J., and Meyerholz D. K.. . 2006. Salmonella. In: Straw B. E., Zimmerman J. J., D’Allaire S., and Taylor D. J., 9th ed Diseases of swine. Ames (IA): Blckwaell Publishing; p. 739–754. [Google Scholar]
- Guilloteau P., Martin L., Eeckhaut V., Ducatelle R., Zabielski R., and Van Immerseel F.. . 2010. From the gut to the peripheral tissues: the multiple effects of butyrate. Nutr. Res. Rev. 23:366–384. doi: 10.1017/S0954422410000247 [DOI] [PubMed] [Google Scholar]
- Hamer H. M., Jonkers D., Venema K., Vanhoutvin S., Troost F. J., and Brummer R. J.. . 2008. Review article: the role of butyrate on colonic function. Aliment. Pharmacol. Ther. 27:104–119. doi: 10.1111/j.1365-2036.2007.03562.x [DOI] [PubMed] [Google Scholar]
- Ibuki M., Fukui K., Kanatani H., and Mine Y.. . 2014. Anti-inflammatory effects of mannanase-hydrolyzed copra meal in a porcine model of colitis. J. Vet. Med. Sci. 76:645–651. doi: 10.1292/jvms.13-0424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibuki M., Kovacs-Nolan J., Fukui K., Kanatani H., and Mine Y.. . 2011. β 1-4 mannobiose enhances Salmonella-killing activity and activates innate immune responses in chicken macrophages. Vet. Immunol. Immunopathol. 139:289–295. doi: 10.1016/j.vetimm.2010.10.011 [DOI] [PubMed] [Google Scholar]
- Kim K., Ehrlich A., Perng V., Chase J. A., Raybould H., Li X., Atwill E. R., Whelan R., Sokale A., and Liu Y.. . 2019. Algae-derived β-glucan enhanced gut health and immune responses of weaned pigs experimentally infected with a pathogenic E. coli. Anim. Feed Sci. Technol. 248:114–125. doi: 10.1016/j.anifeedsci.2018.12.004 [DOI] [Google Scholar]
- Knetter S. M., Bearson S. M., Huang T. H., Kurkiewicz D., Schroyen M., Nettleton D., Berman D., Cohen V., Lunney J. K., Ramer-Tait A. E., . et al. 2015. Salmonella enterica serovar Typhimurium-infected pigs with different shedding levels exhibit distinct clinical, peripheral cytokine and transcriptomic immune response phenotypes. Innate Immun. 21:227–241. doi: 10.1177/1753425914525812 [DOI] [PubMed] [Google Scholar]
- Kogan G., and Kocher A.. . 2007. Role of yeast cell wall polysaccharides in pig nutrition and health protection. Livest Sci. 109:161–165. doi: 10.1016/j.livsci.2007.01.134 [DOI] [Google Scholar]
- Litjens R., Oudshoorn A. K., and Roubos-van den Hil P. J.. . 2017. Technical note: development of a feed matrix as inoculum in Salmonella infection studies in piglets. J. Anim. Sci. 95:2891–2897. doi: 10.2527/jas.2016.0696. [DOI] [PubMed] [Google Scholar]
- Loos M., Geens M., Schauvliege S., Gasthuys F., van der Meulen J., Dubreuil J. D., Goddeeris B. M., Niewold T., and Cox E.. . 2012. Role of heat-stable enterotoxins in the induction of early immune responses in piglets after infection with enterotoxigenic Escherichia coli. PLoS One 7:e41041. doi: 10.1371/journal.pone.0041041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nataro J. P., and Kaper J. B.. . 1998. Diarrheagenic Escherichia coli. Clin. Microbiol. Rev. 11:142–201. doi: 10.1128/CMR.11.1.142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council 2012. Nutrient requirements of swine, 10th rev. ed Washington, DC: National Academic Press. [Google Scholar]
- Ohno S., Sumiyoshi Y., Hashine K., Shirato A., Kyo S., and Inoue M.. . 2011. Phase I clinical study of the dietary supplement, Agaricus blazei Murill, in cancer patients in remission. Evid. Based Complement. Alternat. Med. 2011:192381. doi: 10.1155/2011/192381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partanen K. H., and Mroz Z.. . 1999. Organic acids for performance enhancement in pig diets. Nutr. Res. Rev. 12:117–145. doi: 10.1079/095442299108728884 [DOI] [PubMed] [Google Scholar]
- Roediger W. E. 1980. Role of anaerobic bacteria in the metabolic welfare of the colonic mucosa in man. Gut 21:793–798. doi: 10.1136/gut.21.9.793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saittagaroon S., Kawakishi S., and Namiki M.. . 1983. Characterisation of polysaccharides of copra meal. J. Sci. Food Agric. 34:855–860. doi: 10.1002/jsfa.2740340813 [DOI] [Google Scholar]
- Samuelsen A. B., Schrezenmeir J., and Knutsen S. H.. . 2014. Effects of orally administered yeast-derived beta-glucans: a review. Mol. Nutr. Food Res. 58:183–193. doi: 10.1002/mnfr.201300338. [DOI] [PubMed] [Google Scholar]
- Savall J. F., Bidot C., Leblanc-Maridor M., Belloc C., and Touzeau S.. . 2016. Modelling Salmonella transmission among pigs from farm to slaughterhouse: interplay between management variability and epidemiological uncertainty. Int. J. Food Microbiol. 229:33–43. doi: 10.1016/j.ijfoodmicro.2016.03.020. [DOI] [PubMed] [Google Scholar]
- Sipos W., Wiener S., Entenfellner F., and Sipos S.. . 2013. Physiological changes of rectal temperature, pulse rate and respiratory rate of pigs at different ages including the critical peripartal period. Vet. Med. Austria. 100:93–98. [Google Scholar]
- Smiderle F. R., Baggio C. H., Borato D. G., Santana-Filho A. P., Sassaki G. L., Iacomini M., and Van Griensven L. J.. . 2014. Anti-inflammatory properties of the medicinal mushroom Cordyceps militaris might be related to its linear (1→3)-β-D-glucan. PLoS One 9:e110266. doi: 10.1371/journal.pone.0110266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smiderle F. R., Ruthes A. C., van Arkel J., Chanput W., Iacomini M., Wichers H. J., and Van Griensven L. J.. . 2011. Polysaccharides from Agaricus bisporus and Agaricus brasiliensis show similarities in their structures and their immunomodulatory effects on human monocytic THP-1 cells. BMC Complement. Altern. Med. 11:58. doi: 10.1186/1472-6882-11-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Heijden M., Van Dam H., Niewerth D., and Frankena K.. . 2005. Effectiveness of Salmonella control strategies in fattening pigs. Proc. SafePork. 2005:145–148. doi: 10.31274/safepork-180809-736 [DOI] [Google Scholar]
- Van Immerseel F., Boyen F., Gantois I., Timbermont L., Bohez L., Pasmans F., Haesebrouck F., and Ducatelle R.. . 2005. Supplementation of coated butyric acid in the feed reduces colonization and shedding of Salmonella in poultry. Poult. Sci. 84:1851–1856. doi: 10.1093/ps/84.12.1851 [DOI] [PubMed] [Google Scholar]
- Van der Wolf P., Van Schie F., Elbers A., Engel B., Van der Heijden H., Hunneman W., and Tielen M.. . 2001. Epidemiology: administration of acidified drinking water to finishing pigs in order to prevent salmonella infections. Vet. Q. 23:121–125. doi: 10.1080/01652176.2001.9695097 [DOI] [PubMed] [Google Scholar]
- Wang S., Wang J., Mou H., Luo B., and Jiang X.. . 2015. Inhibition of adhesion of intestinal pathogens (Escherichia coli, Vibrio cholerae, Campylobacter jejuni, and Salmonella Typhimurium) by common oligosaccharides. Foodborne Pathog. Dis. 12:360–365. doi: 10.1089/fpd.2014.1835 [DOI] [PubMed] [Google Scholar]
- van Winsen R. L., van Nes A., Keuzenkamp D., Urlings H. A., Lipman L. J., Biesterveld S., Snijders J. M., Verheijden J. H., and van Knapen F.. . 2001. Monitoring of transmission of Salmonella enterica serovars in pigs using bacteriological and serological detection methods. Vet. Microbiol. 80:267–274. doi: 10.1016/s0378-1135(01)00313-3 [DOI] [PubMed] [Google Scholar]
- Wisitrassameewong K., Karunarathna S. C., Thongklang N., Zhao R., Callac P., Moukha S., Férandon C., Chukeatirote E., and Hyde K. D.. . 2012. Agaricus subrufescens: a review. Saudi J. Biol. Sci. 19:131–146. doi: 10.1016/j.sjbs.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Wolf P. J., Wientjes J. G. M., Heuvelink A. E., Veldhuis A. M. B., van Hees H. M. J., and Roubos-van den Hil P. J.. . 2017. Development of a Salmonella Typhimurium challenge model in weaned pigs to evaluate effects of water and feed interventions on fecal shedding and growth performance. J. Anim. Sci. 95:2879–2890. doi: 10.2527/jas.2016.1136 [DOI] [PubMed] [Google Scholar]
- Zentek J., Ferrara F., Pieper R., Tedin L., Meyer W., and Vahjen W.. . 2013. Effects of dietary combinations of organic acids and medium chain fatty acids on the gastrointestinal microbial ecology and bacterial metabolites in the digestive tract of weaning piglets. J. Anim. Sci. 91:3200–3210. doi: 10.2527/jas.2012-5673. [DOI] [PubMed] [Google Scholar]
- Zhu Y., González-Ortiz G., Solà-Oriol D., López-Colom P., and Martín-Orúe S. M.. . 2018. Screening of the ability of natural feed ingredients commonly used in pig diets to interfere with the attachment of ETEC K88 (F4) to intestinal epithelial cells. Anim. Feed Sci. Technol. 242:111–119. doi: 10.1016/j.anifeedsci.2018.06.005 [DOI] [Google Scholar]