Abstract
Objective:
The applicability of cystoscopy follow-up protocol that is indicated for low-risk nonmuscle-invasive bladder cancer (NMIBC) in the guidelines was investigated for our population.
Materials and Methods:
Patients who underwent transurethral resection with a diagnosis of primary bladder tumor in our clinic within 10 years with low grade of pathology pTa and follow-up periods of at least 5 years were retrospectively reviewed. Fifty-one patients (39 males and 12 females) who were diagnosed with a low-risk NMIBC, had no recurrence at the 3-month control cystoscopy, and followed up for the first 2 years on 3-month basis with cystoscopy were included in the study.
Results:
The mean age of the patients was 57.37 ± 12.21 years (range: 29–80 years), and the mean duration of recurrence was 25.76 ± 32.45 months. In the cystoscopy follow-ups of 51 patients, up to the 6th month, a total of 12 (24%); up to the 9th month, a total of 21 (41%); up to the 12th month, a total of 30 (59%); up to the 15th month, a total of 36 (71%); up to the 18th month, a total of 36 (71%); up to the 21st month, a total of 39 (77%); and up to the 24th month, a total of 41 (80%) patients were reported to have recurrence. In the case of patients with no recurrence at the 9th month cystoscopy, it was determined that 50% of the patients had recurrence in the first 6 months and 67% in the first 2 years.
Conclusion:
The majority (80%) of recurrences in low-risk NMIBC occurred in the first 2 years. If the follow-up protocol described in the guidelines had been applied, patients with relapses would have a delay of at least 6 months of diagnosis. Therefore, even if there is no recurrence in the low-risk NMIBC at the 3rd and 9th months, it may be more appropriate to follow the cases in the first 2 years with follow-up cystoscopy every 3 months.
Keywords: Cystoscopy, nonmuscle-invasive bladder cancer, recurrence
INTRODUCTION
Bladder cancer (BCa), one of the most frequent types of cancer that the urologists come across, ranks 4th following prostate, lung, and colon cancers in men.[1,2] Approximately 70% of the diagnosed BCas are nonmuscle-invasive BCas (NMIBCs).[3] Standard treatment of NMIBC is the intravesical administration of adjuvant immunotherapeutic or chemotherapeutics based on the risk stratification after transurethral resection (TUR) of bladder tumor.[3] After the TUR of NMIBCs, Ta was detected in 70% of the patients, whereas T1 in 20% and carcinoma in situ in 10%.[3] For NMIBC, subgroups are defined as low-, moderate-, and high-risk disease. Tumors in the low-risk group include noncarcinoma in situ, primary, solitary, Ta, Grade 1, and tumors <3 cm.[2] The most important problem with NMIBC is that 50%–70% of these tumors can recur and sometimes they can progress.[4,5] Due to these biological behaviors, patients with NMIBC need close follow-up.
Today, cystoscopy remains the gold standard in the follow-up of patients with noninvasive BCa. According to the guidelines, patients with low-risk noninvasive BCa should undergo a control cystoscopy at the 3rd month because they have a low risk of recurrence and progression. If there is no recurrence in the control cystoscopy performed at the 3rd month, the next follow-up cystoscopy should be performed in 9–12th months and every year for the next 5 years.[2,6]
In our study, we investigated the feasibility of this cystoscopy follow-up protocol, which is indicated in guidelines in low-risk NMIBC for our society.
MATERIALS AND METHODS
We retrospectively reviewed the data of patients who underwent TUR with primary bladder tumor within 10 years and who had at least 5-year follow-up with low-risk nonmuscle-invasive bladder tumor in our clinic. All patients underwent control cystoscopy at the 3rd month post-TUR. A total of 51 patients, 39 males (76%) and 12 females (24%), who were diagnosed with nonmuscle-invasive bladder tumor; had no recurrence on the 3rd month control cystoscopy, followed with cystoscopy every 3 months for the first 2 years and annually up to the 5th year; and had been followed up for at least 5 years were included in the study. Tumors with the histopathological findings of pTa, low grade, single tumor, and tumor size <3 cm were defined as low-risk nonmuscle-invasive bladder tumors and included in the study.[2]
Patients with the histopathological findings of muscle-invasive bladder tumors, patients with medium- or high-risk nonmuscle-invasive bladder tumors, or carcinoma in situ patients were excluded from the study. The patients included in the study were given a single postoperative intravesical chemotherapy postoperatively after TUR if there were no contraindications and the medicines were available. A single dose of early intravesical chemotherapy with mitomycin C was administered. Single-dose intravesical chemotherapy was not applied to the patients who were suspected to have perforation after TUR or had perforation after TUR and needed bladder irrigation due to macroscopic hematuria. In addition, one dose of early intravesical chemotherapy could not be applied after TUR in all cases due to difficulties or delays in the availability of certain medications from time to time in our country. All patients were applied control cystoscopy with a 17 Fr rigid cystoscope in white light in the lithotomy position.
Patients with and without relapse were recorded during the control cystoscopy. Mean recurrence times were detected. The recurrence rates were recorded in the subsequent control cystoscopy of patients with no recurrence in the control cystoscopy. TUR was applied to the patients with recurrence, and it was recorded irrespective of progression.
Histopathological examination of TUR materials was performed by a single pathologist.
Statistical analysis
All data were analyzed with the SPSS statistics software package (Version 18.0; SPSS Inc., Chicago, IL, USA). Age, gender, smoking status, and intravesical chemotherapy application status of the patients with and without recurrence were compared with t-test and Chi-square statistical methods. Recurrence times were compared with Mann–Whitney U-test. P < 0.05 was considered statistically significant.
RESULTS
The mean age of the 51 patients included in the study was 57.37 ± 12.21 years (range: 29–80 years), the mean follow-up duration was 122.65 ± 38.66 months (range: 63–195 months), and the recurrence interval was 25.76 ± 32.45 months [Table 1]. In the follow-up of the 51 patients, a total of 12 patients (24%) till the 6th month, 21 (41%) till the 9th month, 30 (59%) till the 12th month, 36 (71%) till the 15th month, 36 (71%) till the 18th month, 39 (77%) till the 21st month, 41 (80%) till the 24th month, and 43 patients (84%) till the 5th year were recorded to have relapses [Table 2 and Figure 1].
Table 1.
Distribution and characteristics of male and female patients
| All patients (n=51) | Male patients (n=39; 76%) | Female patients (n=12; 24%) | P | |
|---|---|---|---|---|
| Mean age±SD (minimum-maximum) | 57.37±12.21 (29-80) | 58.03±12.05 (29-80) | 55.25±13.03 (32-76) | 0.497* |
| Mean follow-up period±SD (minimum-maximum) | 122.65±38.66 (63-195) | 118.97±34.93 (63-189) | 134.58±41.13 (65-195) | 0.2* |
| Relapse duration (months), median (IQR) | 12 (12) | 12 (6) | 22,5 (59) | 0.322** |
| Smoking, n (%) | ||||
| Yes | 31 (61) | 28 (72) | 3 (25) | 0.004*** |
| No | 20 (39) | 11 (28) | 9 (75) |
*t-test, **Mann-Whitney U-test, ***Chi-square test. IQR: Interquartile range, SD: Standard deviation
Table 2.
Total recurrence rates by month and distribution of patients receiving single-dose early intravesical chemotherapy
| 6-month relapse | 9-month relapse | 12-month relapse | 24-month relapse | 5-year relapse | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Yes, n (%) | No, n (%) | Yes, n (%) | No, n (%) | Yes, n (%) | No, n (%) | Yes, n (%) | No, n (%) | Yes, n (%) | No, n (%) | |
| Number of patients (%) | 12 (24) | 39 (76) | 21 (41) | 30 (59) | 30 (59) | 21 (41) | 41 (80) | 10 (20) | 43 (84) | 8 (16) |
| Age | ||||||||||
| Mean±SD | 60.7±11.2 | 56.3±12.4 | 59.9±12.0 | 55.6±12.2 | 57.9±12.7 | 56.7±11.7 | 57.4±11.5 | 57.3±15.7 | 57.1±11.9 | 59.0±14.2 |
| P | 0.290* | 0.228* | 0.716* | 0.984* | 0.984* | |||||
| Gender, n (%) | ||||||||||
| Male/female | 9 (75)/3 (25) | 30 (77)/9 (23) | 16 (76)/5 (24) | 23 (77)/7 (23) | 25 (83)/5 (17) | 14 (67)/7 (33) | 33 (80)/8 (20) | 6 (60)/4 (40) | 34 (79)/9 (21) | 5 (63)/3 (38) |
| P | 0.891** | 0.969** | 0.167** | 0.171** | 0.171** | |||||
| Smoking | ||||||||||
| n (%) | 6 (50) | 25 (64) | 13 (62) | 18 (60) | 19 (6) | 12 (57) | 26 (63) | 5 (50) | 26 (61) | 5 (63) |
| P | 0.382** | 0.891** | 0.656** | 0.436** | 0.436** | |||||
| CT | ||||||||||
| n (%) | 1 (8) | 14 (36) | 2 (10) | 13 (43) | 5 (17) | 10 (48) | 9 (22) | 6 (60) | 9 (21) | 6 (75) |
| P | 0.067** | 0.009** | 0.017** | 0.018** | 0.002** | |||||
*t-test, **Chisquare test. CT: Chemotherapy, SD: Standard deviation
Figure 1.
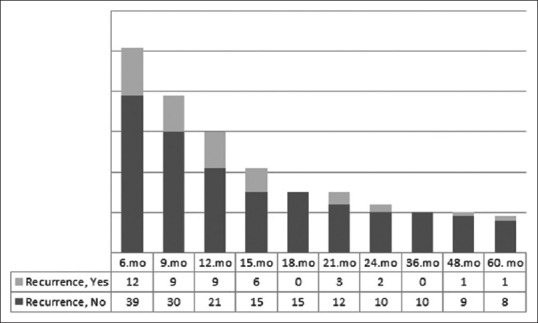
Distribution of recurrence numbers in subsequent follow-ups of patients without recurrence in the month of control cystoscopy
Recurrence was observed in 71% of patients (36 of 51 patients) in the first 15 months and in 80% of patients in the first 2 years. Nearly 67% (20 of 30 patients) of the patients who had no recurrence in the 9th month control cystoscopy were found to have recurrence in the first 2 years [Table 3].
Table 3.
Recurrence rates and mean recurrence times in subsequent follow-up of patients without recurrence
| n/total (%) | Mean relapse duration (months) | |||
|---|---|---|---|---|
| 1st-year relapse | 2nd-year relapse | 3rd-year relapse | ||
| All patients (51 patients) | 30/51 (59) | 41/51 (80) | 41/51 (80) | 25.76±32.45 |
| 6-month control of patients without relapse (n=39 patients) | 24/39 (62) | 29/39 (74) | 30/39 (77) | 31.85±34.98 |
| 9-month control of patients without relapse (n=30 patients) | 18/30 (60) | 20/30 (67) | 21/30 (70) | 38.70±37.32 |
| 12-month control of patients without relapse (n=21 patients) | 11/21 (52) | 11/21 (52) | 12/21 (57) | 50.14±39.52 |
| 15-month control of patients without relapse (n=15 patients) | 5/15 (33) | 5/15 (33) | 7/21 (47) | 64.20±38.60 |
| 24-month control of patients without relapse (n=10 patients) | 0/10 (0) | 1/10 (10) | 2/10 (20) | 85.20±29.09 |
In the control cystoscopy performed at the 6th month of the 51 patients without recurrence in the 3-month control cystoscopy, 12 (24%) patients had recurrence. Nine (23%) of the 39 patients who did not have a relapse in the 6th month control cystoscopy had relapse at the 9th month. Nine (30%) out of the 30 patients without relapse in the 9th month had relapse on the 12th month controls. Six (29%) out of the 21 patients who had no relapse in the 12th month had relapse on the 15th month controls. Three (20%) out of the 15 patients who had no relapse in the 15th month had relapse on the 21st month controls [Figure 1].
Twenty-one (41%) of the 51 patients who had findings of relapse on the 3rd month control cystoscopy showed relapse before the 12th month controls. Similarly, nine (43%) of the 21 patients who did not have relapse on the 12th month control cystoscopy were found to have relapse before the controls on the 24th month [Figure 1].
In 36 (71%) patients who did not receive a single dose of early intravesical chemotherapy, 11 (31%) had recurrence at 6 months, 19 (53%) had recurrence in the first 9 months, and 25 (69%) had recurrence in the first 12 months. Patients who did not receive a single dose of intravesical chemotherapy in the early postoperative period had a statistically higher recurrence rate. Especially when patients who did not receive a single dose of intravesical early chemotherapy (n = 36) were considered, more than half of them (53%) had recurrence prior to the 12th month of control cystoscopy [Tables 2 and 4].
Table 4.
Characteristics of patients who received single intravesical chemotherapy in the early postoperative period and who did not receive it
| Single-dose intravesical CT | Yes (n=15) | No (n=36) | P |
|---|---|---|---|
| Age | 57.07±7.82 (43-73) | 57.50±13.73 (29-80) | 0.909* |
| Gender (male/female), n (%) | 10 (66.7)/5 (33.3) | 29 (80.6)/7 (19.4) | 0.287** |
| Smoking, n (%) | 6 (40) | 25 (69,4) | 0.050** |
| Relapse duration (month), median (IQR) | 21 (60) | 9 (9) | 0.004*** |
*t-test, **Chi-square test, ***Mann-Whitney U-test. CT: Chemotherapy, IQR: Interquartile range
Histopathology of Grade 1 pTa was found in patients who had recurrences in our follow-ups (except four patients). Progression was seen in four (8%) patients' follow-ups. The first recurrence in the first case was detected at the 6th month and the histopathology showed pT1 at the 24th month, the first recurrence in the second case was detected at the 9th month and histopathology was pT1 at the 15th month, the first recurrence in the third case was detected at 12 months and histopathology was pT1 at the 60th month; at the 110th month of this case, radical cystectomy was applied because of the pT2 detection in histopathology. Progression was detected in the fourth case, the first recurrence was detected in the 15th month, and histopathology was detected as pT1 at the 24th month.
DISCUSSION
In NMIBC, high-grade recurrence and progression can be seen after TUR.[4,5] For this reason, these tumors need close follow-up. For the follow-up of NMIBC, various bladder tumor markers such as urinary cytology, bladder tumor antigen (BTA), stat and BTA Trak, fluorescent in situ hybridization, cytokeratins, and nuclear matrix protein 22 test have been defined. Urine markers have higher sensitivity in low-grade bladder tumors, but lower specificity compared to urinary cytology. Urinary cytology has a sensitivity of approximately 80% in moderate- and high-risk BCas, whereas in low-risk BCas, it is about 20%.[6,7,8] For follow-ups of NMIBC, cystoscopy remains the gold standard procedure.
NMIBC can be classified as low, intermediate, and high risk regarding recurrence and progression.[2] Different cystoscopic follow-up protocols are recommended according to the risk groups of the patients. Primer, solitary, Ta, Grade 1, tumor <3 cm, and noncarcinoma in situ tumors are grouped as low-risk, NMIBCs.[2] According to the guidelines of the European Association of Urology, for low-risk, NMIBC, cystoscopy should be performed at 3 months. If recurrence is not detected, follow-up cystoscopy is recommended after 9 months and once a year for 5 years.[2] Similarly, according to the American Urological Association/Society of Urologic Oncology Guidelines, it is recommended that the first control cystoscope be performed 3–4 months after the TUR of the bladder tumor. If the initial control cystoscopy is negative, follow-up cystoscopy should be performed after 6–9 months, followed by annual follow-up cystoscopy.[6]
In our patient group included in the study, if the follow-up protocol mentioned in the guidelines had been applied, the diagnosis of 12 (24%) patients at 6 months and 9 (18%) patients at 9 months would be delayed. Of the control cystoscopes between the 3rd and 12th months, 21 (41%) patients were reported to have recurrence. In our study, 50% of the patients who did not have recurrence at the 9th month control on the cystoscope were detected to have recurrence in the following 6 months. If the guideline-recommended cystoscopy follow-up protocol was applied, recurrence in 50% of the patients who had no recurrence at the 9th month would have been delayed. In our study, it was observed that if the guideline-recommended cystoscopy follow-up schedule was used for patients with low-risk, NMIBC in the guideline, there might be a delay in diagnosis in a number of patients.
In a study by Leblanc et al., 55% of the 152 patients with Ta Grade 1 transitional cell carcinoma were reported to have recurrence of indole tumor in follow-up visits on an average for 76 months. In this study, 46% of tumor recurrences were reported within 12 months, 13% between 12 and 24 months, and 27% between 24 and 60 months.[9] In our study also, 59% of the patients were found to have recurrence in the first 12 months. In our study, the majority (80%) of recurrence in low-risk bladder tumors occurred in the first 2 years. The recurrence rate was still high at 67% in the first 2 years, even when the patients without recurrence in the 9th month are focused. For this reason, cystoscopic follow-up on a 3-month basis in the first 2 years is very important in the early diagnosis of recurrences.
In meta-analyses of randomized clinical trials, in low-risk, NMIBC, single-dose intravesical chemotherapy instillation in the early postoperative period has been reported to significantly reduce tumor recurrence.[2,6,10,11,12] In the present study, statistically higher recurrence rates were detected in patients who did not receive a single dose of intravesical chemotherapy in the early postoperative period. It was observed that tumor recurrence rates were significantly lower and recurrence periods were significantly longer in patients with single intravesical chemotherapy in the early postoperative period. Particularly in 53% of patients who did not receive a single dose of intravesical early chemotherapy, recurrence was observed prior to the control cystoscopy on the 12th month. Therefore, if cystoscopy had been performed according to the standard protocol, diagnosis would be delayed up to 6 months. We, therefore, conclude that follow-ups every 3 months in the first 2 years, especially in patients who did not receive single intravesical chemotherapy in the postoperative period, are important for early diagnosis of recurrences. Especially in developing countries, cystoscopy follow-up protocol of every 3 months in the first 2 years instead of the one defined in guidelines in patients with low-risk nonmuscle-invasive bladder tumors who have not undergone intravesical chemotherapy in the early postoperative period due to the difficulties in obtaining or applying chemotherapy drugs or because of contraindications to chemotherapy, may reduce possible delays in the diagnosis and treatment of relapses. The most important limitations of our study are the low number of patients included and the retrospective nature. A larger group of patients and prospective studies are needed.
BCas with similar histopathological features may exhibit different clinical characteristics. Genetic predisposition plays an important role in the incidence of BCa.[13] Genetic defects such as mutations of fibroblast growth factor receptors 3 (FGFR3) oncogene, P53 gene mutations, and chromosome 9 deletions are important in the prognosis and progression of BCa.[14,15,16,17] FGFR3 oncogene mutations are associated with good prognosis, whereas p53 gene mutations are usually associated with poor prognosis[15,16,17] The incidence and development risk of cancers such as prostate can vary depending on the geographical areas and societies; the incidence of prostate cancer in the USA and Northern Europe is higher, while it is lower in South-East Asia.[18] It is generally accepted that genetics is an important factor in the development, progression, and prognosis of many cancers. NMIBC may have different genetic features, biological behavior, and prognostic properties although they have similar histopathological features. For this reason, the recurrence and progression of NMIBC may also vary depending on the population and geography.
We believe that the determination of cystoscopy follow-up protocols in low-risk NMIBC according to the societies is important for early diagnosis and treatment of recurrences.
CONCLUSION
The majority of recurrences (80%) in low-risk nonmuscle-invasive bladder tumors occurred in the first 2 years. If the cystoscopic follow-up protocol described in the guidelines had been applied, our patients with recurrence would have a delay of at least 6 months of diagnosis. Therefore, we believe that cystoscopy follow-up in the first 2 years every 3 months may be more appropriate even if there is no recurrence in the control cystoscopy performed at 3 and 9 months in patients with low-risk nonmuscle-invasive bladder tumors.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Irani J, Mottet N, Ribal Caparros MJ, Teillac P. New Trends in Bladder Cancer Management. European Urology Supplementes. 2007;6:388–95. [Google Scholar]
- 2.Babjuk M, Böhle A, Burger M, Capoun O, Cohen D, Comperat EM, et al. EAU Guidelines on Non-Muscle-invasive Urothelial Carcinoma of the Bladder: Update 2016. Eur Urol. 2017;71:447–61. doi: 10.1016/j.eururo.2016.05.041. [DOI] [PubMed] [Google Scholar]
- 3.Kirkali Z, Chan T, Manoharan M, Algaba F, Busch C, Cheng L, et al. Bladder cancer: Epidemiology, staging and grading, and diagnosis. Urology. 2005;66:4–34. doi: 10.1016/j.urology.2005.07.062. [DOI] [PubMed] [Google Scholar]
- 4.Soloway MS. It is time to abandon the “superficial” in bladder cancer. Eur Urol. 2007;52:1564–5. doi: 10.1016/j.eururo.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 5.Whelan P. The treatment of non muscle invasive bladder cancer with intravesical chemotherapy and immunotherapy. Eur Urol Suppl. 2007;6:568–71. [Google Scholar]
- 6.Chang SS, Boorjian SA, Chou R, Clark PE, Daneshmand S, Konety BR, et al. Diagnosis and treatment of non-muscle invasive bladder cancer: AUA/SUO guideline. J Urol. 2016;196:1021–9. doi: 10.1016/j.juro.2016.06.049. [DOI] [PubMed] [Google Scholar]
- 7.Karakiewicz PI, Benayoun S, Zippe C, Lüdecke G, Boman H, Sanchez-Carbayo M, et al. Institutional variability in the accuracy of urinary cytology for predicting recurrence of transitional cell carcinoma of the bladder. BJU Int. 2006;97:997–1001. doi: 10.1111/j.1464-410X.2006.06036.x. [DOI] [PubMed] [Google Scholar]
- 8.van Rhijn BW, van der Poel HG, van der Kwast TH. Urine markers for bladder cancer surveillance: A systematic review. Eur Urol. 2005;47:736–48. doi: 10.1016/j.eururo.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 9.Leblanc B, Duclos AJ, Bénard F, Côté J, Valiquette L, Paquin JM, et al. Long-term followup of initial ta grade 1 transitional cell carcinoma of the bladder. J Urol. 1999;162:1946–50. doi: 10.1016/S0022-5347(05)68075-5. [DOI] [PubMed] [Google Scholar]
- 10.Sylvester RJ, Oosterlinck W, van der Meijden AP. A single immediate postoperative instillation of chemotherapy decreases the risk of recurrence in patients with stage ta T1 bladder cancer: A meta-analysis of published results of randomized clinical trials. J Urol. 2004;171:2186–90. doi: 10.1097/01.ju.0000125486.92260.b2. quiz 2435. [DOI] [PubMed] [Google Scholar]
- 11.Abern MR, Owusu RA, Anderson MR, Rampersaud EN, Inman BA. Perioperative intravesical chemotherapy in non-muscle-invasive bladder cancer: A systematic review and meta-analysis. J Natl Compr Canc Netw. 2013;11:477–84. doi: 10.6004/jnccn.2013.0060. [DOI] [PubMed] [Google Scholar]
- 12.Perlis N, Zlotta AR, Beyene J, Finelli A, Fleshner NE, Kulkarni GS, et al. Immediate post-transurethral resection of bladder tumor intravesical chemotherapy prevents non-muscle-invasive bladder cancer recurrences: An updated meta-analysis on 2548 patients and quality-of-evidence review. Eur Urol. 2013;64:421–30. doi: 10.1016/j.eururo.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 13.Burger M, Catto JW, Dalbagni G, Grossman HB, Herr H, Karakiewicz P, et al. Epidemiology and risk factors of urothelial bladder cancer. Eur Urol. 2013;63:234–41. doi: 10.1016/j.eururo.2012.07.033. [DOI] [PubMed] [Google Scholar]
- 14.Pollard C, Smith SC, Theodorescu D. Molecular genesis of non-muscle-invasive urothelial carcinoma (NMIUC) Expert Rev Mol Med. 2010;12:e10. doi: 10.1017/S1462399410001407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lindgren D, Liedberg F, Andersson A, Chebil G, Gudjonsson S, Borg A, et al. Molecular characterization of early-stage bladder carcinomas by expression profiles, FGFR3 mutation status, and loss of 9q. Oncogene. 2006;25:2685–96. doi: 10.1038/sj.onc.1209249. [DOI] [PubMed] [Google Scholar]
- 16.Ecke TH, Sachs MD, Lenk SV, Loening SA, Schlechte HH. TP53 gene mutations as an independent marker for urinary bladder cancer progression. Int J Mol Med. 2008;21:655–61. [PubMed] [Google Scholar]
- 17.Esrig D, Elmajian D, Groshen S, Freeman JA, Stein JP, Chen SC, et al. Accumulation of nuclear p53 and tumor progression in bladder cancer. N Engl J Med. 1994;331:1259–64. doi: 10.1056/NEJM199411103311903. [DOI] [PubMed] [Google Scholar]
- 18.Mottent N, Bellmunt J, Bolla M, Briers E, Cumberbatch MG, De Santis M, et al. EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part 1: Screening, Diagnosis, and Local Treatment with Curative. Intent Eur Urol. 2017;71:618–29. doi: 10.1016/j.eururo.2016.08.003. [DOI] [PubMed] [Google Scholar]


