Abstract
Background
Split-liver transplantation can be useful in situations of limited donor resources. However, novel preservation methods are required to help the recipient recover from severe ischemic reperfusion injury incurred due to receiving a relatively small liver graft.
Material/Methods
Our experiment was performed using porcine livers without warm ischemia time, assuming a brain-dead organ. We made porcine split-liver grafts by 75% liver resection at the back table and divided the specimens into 4 groups. Group 1 was preserved with simple cold storage after splitting (CS; n=3), Group 2 was preserved with hypothermic perfusion preservation (HMP) after splitting (SBP; n=3), Group 3 was preserved with HMP after splitting under perfusion preservation (SDP; n=4), and Group 4 had the whole liver perfused as control grafts (Whole Liver; n=3). To assess potential methods of preservation and their effects, all grafts were evaluated by an ex vivo isolated liver reperfusion model using diluted autologous blood.
Results
Portal vein pressure resistances during reperfusion were low in Group3 (SDP). Hepatic artery pressure resistances during reperfusion were markedly higher in Group 1(CS) than in the other groups. The levels of AST and LDH were high and increased at 2 h after reperfusion in Group 1 (CS). The histological findings show that the liver cell structure was irregular in Group 1 (CS) but remained regular in Groups 2 (SBP) and 3 (SDP). Histological Suzuki scores were also significantly better in Groups 2 (SBP) and 3 (SDP) compared with Group 1 (CS).
Conclusions
Splitting the liver under machine perfusion preservation may help restore the function and reduce ischemia-reperfusion injury.
MeSH Keywords: Liver Transplantation, Organ Preservation, Reperfusion Injury
Background
As the number of patients waiting for a liver transplant increases, the shortage of donors becomes more serious. It is important to identify all potential transplant donors and avoid unnecessary graft loss [1]. Split-liver transplantation is an important method that can increase the available donor pool. However, the graft-to-recipient weight ratio (GRWR) is an important predictive factor of small-for-size syndrome (SFSS) [2,3]. SFSS is caused by insufficient grafts and leads to severe ischemia-reperfusion injury and excessive postoperative portal blood flow. In clinical practice, only ex situ splitting is available in Japan. Compared to in situ splitting, ex vivo splitting has several limitations, including longer cold ischemia time (including back-table operation). Furthermore, it is more difficult to identify the bile duct and vascular tissues, which results in an increased incidence of bleeding and bile spillage and a higher rate of rewarming injury on the back table.
The major methods of organ storage are simple cold storage (CS) and machine perfusion (MP). Due to its simplicity and low cost, CS is currently being used as a standard organ preservation method. In MP, oxygen, substances, and nutritional elements are provided, and harmful metabolites are washed out to support metabolism. Compared with static CS, MP can restore the liver energy reserve and reverse injury caused by interruption of the adenosine triphosphate (ATP) supply during warm ischemia through the continuous supply of oxygen and nutrients, and also prevents further injury to liver cells during preservation [4]. The use of marginal donor livers is associated with a high risk of primary graft dysfunction or severe ischemic injury and can result in poor outcomes, thus requiring MP [5]. Split-liver grafts may thus be considered marginal grafts because of their small size and the degree of injury incurred due to splitting the liver, especially when utilizing the ex vivo split procedure.
Recently, MP has been reported to be superior to CS in clinical settings [6,7]. Hypothermic machine preservation (HMP) is characterized by practicability and has gradually become the most commonly used approach for preservation of the liver [6,4]. The preclinical work of Dutlowski et al. indicates that even brief HMP prior to transplantation improves mitochondrial and energetic damage, which are associated with cold ischemia [8]. We hypothesize that the application of HMP for a small split-liver graft exerts protective effects against reperfusion injury.
In this study, we simulated and evaluated post-transplant reperfusion by using an extracorporeal reperfusion model. The livers were evaluated by reperfusion with oxygenated diluted autologous blood at 38°C instead of after transplantation. This isolated ex vivo reperfusion model can reduce the need for animal experiments of transplantation. In addition, the beneficial effects of oxygenated HMP for small split porcine livers was evaluated using this model to determine its applicability.
Material and Methods
Animals
The Institutional Animal Ethics Committee of the Clinical Research Center, Asahikawa Medical University, Japan (permit no. 14172) approved all the experimental procedures. We used domestic pigs (female, crossbred Large-Yorkshire, Landrace, and Duroc hogs, 2–3 months old, 20 kg; Taisetsu-Sanroku-Sya, Asahikawa, Japan) in this study. The recovering procedures were performed under sufficient anesthesia using isoflurane. We intravenously infused potassium chloride and induced cardiac arrest.
Machine perfusion system
The grafts were perfused using our original MP system that we developed independently (Figure 1). In our system, each hepatic artery (HA) and portal vein (PV) was perfused. Details of our system are reported in previous papers [9,10]. Each circuit consisted of a roller-type pump (MasterFlex7520-40; Cole-Parmer, Bunker Court, IL, USA), an electrical flow meter (VN05; Aichi Tokei, Aichi, Japan for PV; FD-SS02; Keyence, Osaka, Japan for HA), a ceramic capacitive pressure sensor (KL76; Nagano Keiki, Nagano, Japan), and an air trap developed in-house. An oxygenator (HP0-06 H-C; Senko Medical Instrument, Tokyo, Japan) was installed in the circuit for the PV and HA [10]. The flow rate of the PV was 0.22 mL/min/g and that of the HA was 0.06 mL/min/g.
Figure 1.
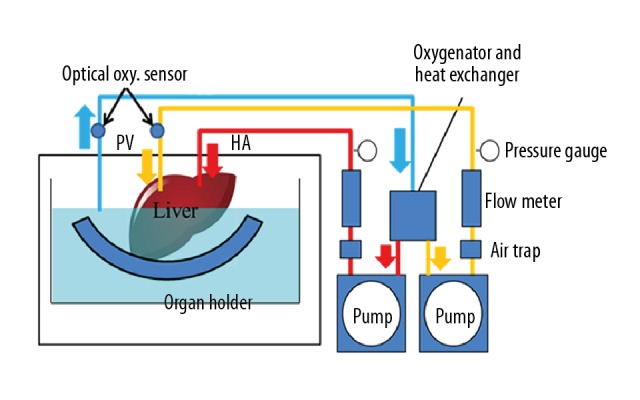
Connection diagram for the machine perfusion device. (Copyright 2018 Yoshikawa R, et al. Reproduced under CC BY-NC-ND 4.0 License: Evaluation Using an Isolated Reperfusion Model for Porcine Liver Donated After Cardiac Death Preserved with Oxygenated Hypothermic Machine Perfusion.Figure 1. Ann Transplant, 2018; 23: 822–27).
Oxygenation
Oxygenated perfusate supplied oxygen to the liver grafts during MP under HMP conditions using an oxygenator. We controlled the oxygen concentrations with the blending volume conditions of oxygen and air using the air blender of the oxygenator. We maintained the condition of the oxygen concentration to hold the minimum dissolved oxygen concentration for the calculation of the oxygen consumption at the outlet port. We calculated the oxygen consumption (mg/min/100 g liver) of the organ as normalized values with the mass of the organ (mg/min/100 g liver) from the difference in the oxygen concentration at the inlet port of the PV circuit and the outlet port positioned near the hepatic vein [10].
Experimental design (Figure 2)
Figure 2.
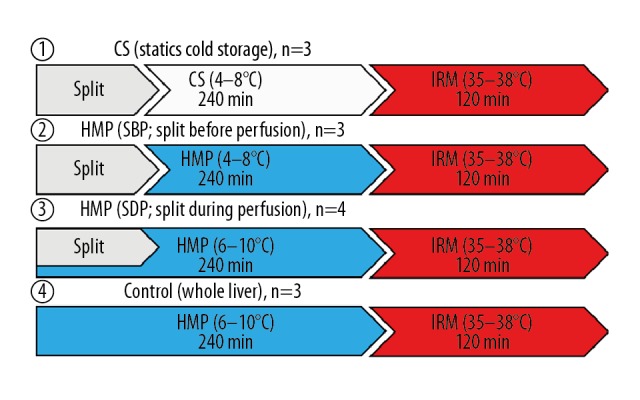
Study design. IRM – isolated ex vivo reperfusion model.
Our experiment was performed using porcine livers without warm ischemia time, assuming a brain-dead organ. We made porcine split-liver grafts by 75% liver resection at the back table and divided the specimens into 4 groups. In Group 1, grafts were preserved with CS for 4 h after splitting (CS; n=3). In Group 2, grafts were preserved with HMP for 4 h after splitting (SBP; n=3). In Group 3, grafts were preserved with HMP after splitting under perfusion preservation (SDP; n=4). In Group 4, whole livers were perfused for 4 h as control grafts (Whole Liver; n=3). The grafts were perfused for 4 h with UW-gluconate solution (Adenine: 5 mmol/L, Calcium Chloride: 0.5 mmol/L, Dextrose: 10 mmol/L, Glutathione: 3 mmol/L, HEPES: 10 mmol/L, Hydroxyethyl Starch, Magnesium Gluconate: 5 mmol/L, Mannitol: 30 mmol/L, Potassium Phosphate: 25 mmol/L, Ribose: 5 mmol/L, Sodium Gluconate: 80 mmol/L, Sodium Hydroxide, osmolarity: 300 mOsm, pH: 7.4; Belzer MPS UW Machine Perfusion Solution; Bridge to Life, Columbia, SC, USA).
Isolated ex vivo reperfusion model
After MP preservation, the liver function was evaluated by an isolated liver reperfusion model [11]. Following the preservation, all organs were rinsed with 500 mL of cold Euro-Collins solution. The grafts were then reperfused with oxygenated diluted autologous blood at 38°C. The autologous blood was diluted with saline (40%), dextran (20%), Calcicol (5%), sodium bicarbonate, heparin sodium, and potassium. The hematocrit was maintained at around 10–12%. The oxygenator was adjusted so that the pO2 was 150–200 mmHg and the pCO2 was 30–50 mmHg. HA perfusion was set at 70 mL/min. Perfusion of the PV was maintained in a flow-constant manner (200 mL/min) [11].
Evaluation methods
Aspartate aminotransferase (AST), lactate dehydrogenase (LDH), and hyaluronic acid were measured from perfusate every 30 min.
In addition to observing hematoxylin and eosin-stained specimens, we used the Suzuki classification [12] to evaluate ischemia-reperfusion injury, which consists of 3 parameters — sinusoidal congestion, vacuolization of hepatocyte cytoplasm, and parenchymal necrosis – scored on a scale from 0 to 4.
Statistical analyses
We presented the results as the mean±standard deviation. We performed statistical analysis using the Microsoft Excel 2013 software program (Microsoft, Redmond, WA, USA). We compared the results using a one-way analysis of variance (ANOVA) and the Steel-Dwass test. We performed a postmortem analysis using Bonferroni’s modification when significant differences were detected, defined as p value less than 0.05.
Results
Pressures in the PV and HA during perfusion preservation (Figures 3, 4)
Figure 3.

PV pressure during perfusion. PV – portal vein.
Figure 4.

HA pressure during perfusion. HA – hepatic artery.
In Group 2 (Split Before Perfusion [SBP]), the PV pressure was 4.50±1.25 mmHg at 120 min of perfusion preservation and 4.78±1.17 mmHg at 240 min. In Group 4 (Whole Liver), the PV pressure was 2.83±0.45 mmHg at 120 min of perfusion preservation and 2.69 ± 0.50 mmHg at 240 min. The PV pressure tended to be higher in Group 2 (SBP) than in Group 4 (Whole Liver). In Group 3 (Split During Perfusion [SDP]), the PV pressure was 2.77±0.80 mmHg at 120 min of perfusion preservation and 3.20±1.44 mmHg at 240 min. The PV pressure in Group 3 (SDP) was highest at the start of perfusion preservation among the 3 groups, but at 60 min of perfusion preservation, it was similar to that in Group 4 (Whole Liver).
In Group 2 (SBP), the HA pressure was 49.83±35.52 mmHg at 120 min of perfusion preservation and 48.53±27.26 mmHg at 240 min, showing the highest pressure among the 3 groups after 60 min of perfusion preservation. In Group 4 (Whole Liver), the HA pressure was 22.97±4.27 mmHg at 120 min of perfusion preservation and 23.86±4.30 mmHg at 240 min, showing the lowest pressure among the 3 groups during perfusion preservation. In Group 3 (SDP), the HA pressure was 37.35±6.63 mmHg at 120 min of perfusion preservation and 36.58±6.75 mmHg at 240 min, showing the highest pressure among the 3 groups at the start of perfusion preservation, but with pressure decreasing after 60 min. An intermediate pressure was maintained in Group 3 (SDP).
AST and LDH in the perfusion solution during perfusion preservation (Figures 5, 6)
Figure 5.
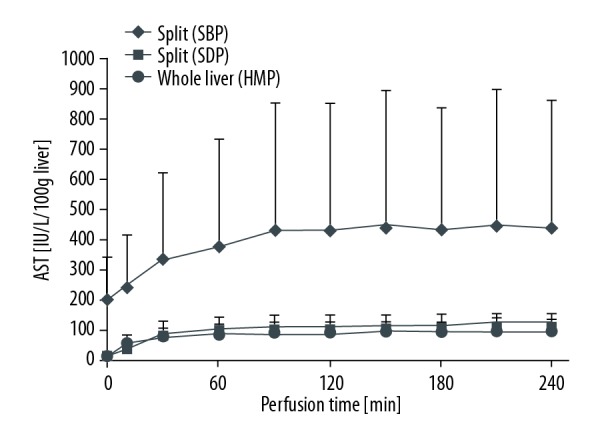
AST during perfusion. AST – aspartate aminotransferase.
Figure 6.

LDH during perfusion. LDH – lactate dehydrogenase.
In Group 2 (SBP), the AST was 433.66±420.33 IU/L/100 g liver at 120 min of perfusion preservation and 436.43±422.78 IU/L/100 g liver at 240 min, which was significantly higher than in Group 3 (SDP) and 4 (Whole Liver). In Group 3 (SDP), the AST was 112.48±33.59 IU/L/100 g liver at 120 min of perfusion preservation and 120.80±33.94 IU/L/100 g liver at 240 min. In Group 4 (Whole Liver), the AST was 87.52±39.66 IU/L/100 g liver at 120 min of perfusion and 96.03±39.29 IU/L/100 g liver at 240 min, which was the lowest among the 3 groups.
In Group 2 (SBP), the LDH was 601.04±507.86 IU/L/100 g liver at 120 min of perfusion preservation and 622.97±518.84 IU/L/100 g liver at 240 min, which was significantly higher than in Group 3 (SDP) and 4 (Whole Liver). In Group 3 (SDP), the LDH was 172.93±84.74 IU/L/100 g liver at 120 min of perfusion preservation and 180.23±86.94 IU/L/100 g liver at 240 min. In Group 4 (Whole Liver), the LDH was 107.28±30.88 IU/L/100 g liver at 120 min of perfusion preservation and 110.31±25.74 IU/L/100 g liver at 240 min, which was the lowest among the 3 groups.
PV pressure (PVP) and HA pressure (HAP) resistance after reperfusion (Figures 7, 8)
Figure 7.

PVP resistance during reperfusion. PVP – portal vein pressure.
Figure 8.

HAP resistance during reperfusion. HAP – hepatic artery pressure.
In Group 2 (SBP), the PVP resistance was 0.065±0.012 mmHg/ml/min at 60 min of reperfusion and 0.059±0.012 mmHg/ml/min at 120 min, which was the highest among the 4 groups. In Group 3 (SDP), the PVP resistance was 0.033±0.009 mmHg/ml/min at 60 min of reperfusion and 0.030±0.010 mmHg/ml/min at 120 min, which was significantly lower than in Group 2 (SBP). In Group 1, static cold storage (CS), the PVP resistance was 0.053±0.017 mmHg/ml/min at 60 min of reperfusion and 0.041±0.017 mmHg/ml/min at 120 min. In Group 4 (Whole Liver), the PVP resistance was 0.035±0.025 mmHg/ml/min in 60 min of reperfusion and 0.027±0.011 mmHg/ml/min at 120 min. These results showed that the PVP resistance was lowest in whole liver preserved with CS and was highest in Group 1 (CS).
In Group 1 (CS), the HAP resistance was 3.84±1.08 mmHg/ml/min at 60 min of reperfusion and 3.74±0.90 mmHg/ml/min at 120 min, which was the highest among the 4 groups. In Group 2 (SBP), the HAP resistance was 2.14±1.37 mmHg/ml/min at 60 min of reperfusion and 2.14±1.02 mmHg/ml/min at 120 min. In Group 3 (SDP), the HAP resistance was 1.51±0.74 mmHg/ml/min at 60 min of reperfusion and 1.33±0.70 mmHg/ml/min at 120 min. In Group 4 (Whole Liver), the HAP resistance was 0.72±0.56 mmHg/ml/min at 60 min of reperfusion and 0.72±0.54 mmHg/ml/min at 120 min.
AST, LDH and hyaluronic acid in the perfusion solution after reperfusion (Figures 9–11)
Figure 9.
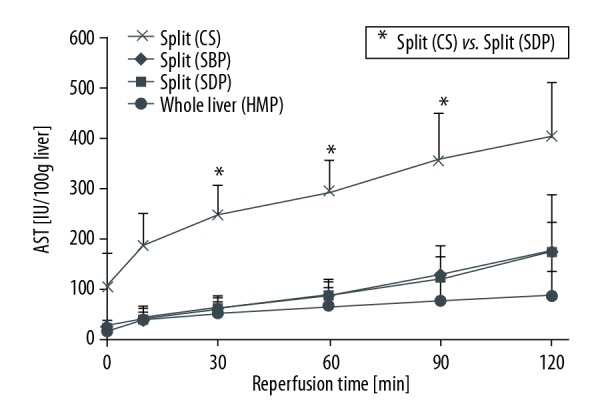
AST during reperfusion.
Figure 10.

LDH during reperfusion
Figure 11.
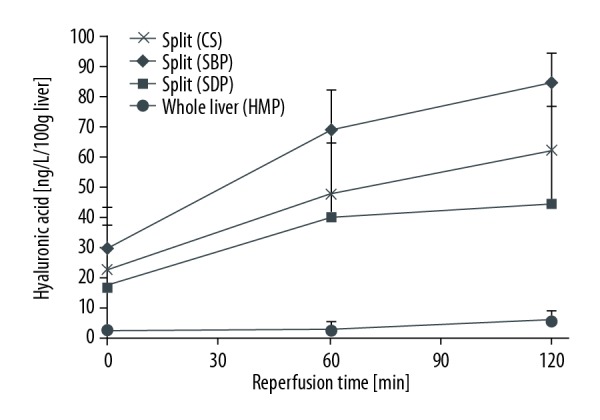
Hyaluronic acid during reperfusion.
In Group 1 (CS), the AST was 295.00±64.11 IU/L/100 g liver at 60 min of reperfusion and 404.30±107.70 IU/L/100 g liver at 120 min, which was the highest AST among the 4 groups. In Group 3 (SDP), the AST was 88.15±33.16 IU/L/100 g liver at 60 min of reperfusion and 176.61±113.45 IU/L/100 g liver at 120 min, which was significantly lower than in Group 1 (CS). In Group 3 (SDP), the AST was 91.13±26.78 IU/L/100 g liver at 60 min of reperfusion and 176.60±58.68 IU/L/100 g liver at 120 min. In Group 4 (Whole Liver), the AST was 67.28±39.52 IU/L/100 giver at 60 min of reperfusion and 89.54±46.60 IU/L/100 g liver at 120 min. In Group 1 (CS), the LDH was 428.12±158.89 IU/L/100 g liver at 60 min of reperfusion and 616.13±314.32 IU/L/100 g liver at 120 min, which was the highest among the 4 groups. In Group 3 (SDP), the LDH was 141.55±13.08 IU/L/100 g liver at 60 min of reperfusion and 232.64±54.28 IU/L/100 g liver at 120 min. In Group 2 (SBP), the LDH was 181.76±53.47 IU/L/100 g liver at 60 min of reperfusion and 272.68±41.02 IU/L/100 g liver at 120 min. In Group 4 (Whole Liver), the LDH was 119.38±38.3 IU/L/100 g liver at 60 min of reperfusion and 151.07 ± 53.66 IU/L/100 g liver at 120 min.
Histological findings during reperfusion (Figures 12, 13)
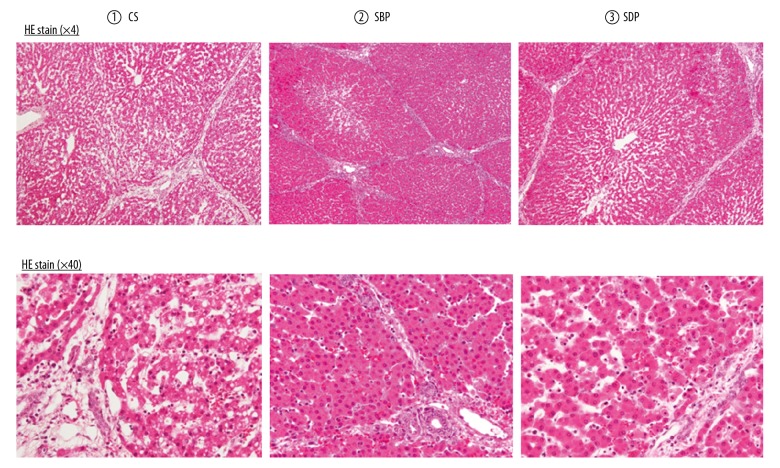
Figure 12.
Histopathological findings of the isolated ex vivo reperfusion model. The histological findings show that the Glisson sheath was more edematous in Group 1 (CS) than in Groups 2 (SBP) and 3 (SDP). Furthermore, the liver cell structure was irregular in Group 1 (CS) but remained regular in Groups 2 (SBP) and 3 (SDP). Under high-power magnification, the liver cells showed more lipid drops in Group 1 (CS) than in Groups 2 (SBP) and 3 (SDP). CS – cold storage; SBP – split before perfusion; SDP – split during perfusion.
Figure 13.

Histological scores (Suzuki scores).
The histological findings show that the Glisson sheath was more edematous in Group 1 (CS) than in Groups 2 (SBP) and 3 (SDP). Furthermore, the liver cell structure was irregular in Group 1 (CS) but remained regular in Groups 2 (SBP) and 3 (SDP). Under high-power magnification, the liver cells showed more lipid drops in Group 1 (CS) than in Groups 2 (SBP) and 3 (SDP).
In addition, H&E staining revealed significant morphological changes in total Suzuki scores (12.0±0.0 vs. 6.7±0.7 vs. 4.6±0.7) in Groups 2 (SBP) and 3 (SDP) compared with Group 1 (CS).
Discussion
The current severe donor shortage is a serious global problem. However, while split-liver transplantation can be performed under limited donor conditions, this transplantation can create 2 extended-criteria grafts and increase the risk of transplant failure [1,2], Split-liver transplantation is thought to be a type of “marginal” donor liver transplantation because of the small size of the graft [3]. Split-liver transplantation can produce insufficient grafts, and the transplantation of such grafts often causes excessive PV blood flow due to HA spasm via HA buffer response [13]. These conditions can increase the risk of HA thrombosis [13]. This HA spasm can reduce blood supply to the biliary system, which can increase the risk of biliary tract complications [14]. When small-for-size grafts are transplanted to recipients with severe portal hypertension, they have a risk of primary graft dysfunction resulting from the inconsistency of GRWR [15]. Thus, both small split-liver grafts or marginal-size livers are generally exposed to severe ischemic reperfusion injury. The shear stress to the sinusoidal and parenchymal cells after reperfusion can result in ischemic reperfusion injury.
The current standard method of organ preservation is CS. However, it has been recently suggested that the MP technique for ex vivo organ preservation could replace CS. The MP technique is considered effective, particularly for organ preservation and resuscitation in marginal donors, including those obtained after cardiac death or from extended-criteria donor grafts [6]. HMP allows for successive circulation of preservative solution and metabolic substances. Simultaneously, the associated washout effect removes waste products from endothelial and parenchymal cells and dilutes the products to stabilize microcirculation. HMP has been reported to improve early graft function [4] and ATP levels in tissues during reperfusion [8]. In addition, HMP allows for pharmacological manipulation and the pre-transplant evaluation of grafts [4] and has enhanced the use of grafts obtained from extended-criteria donors [7]. Recent reports have also suggested that HMP has a benefit in long-term survival of grafts [16].
On comparing whole liver with split liver of any small size in the present study, it was clear that the split graft was a marginal graft based on an evaluation using the MP system. Our findings showed that MP is useful for small split-liver grafts as an effective preservation procedure. The AST, LDH, and HA levels were lower in Groups 2 (SBP) and 3 (SDP) than in Group 1 (CS). An evaluation by ex vivo isolated liver reperfusion indicated that the early graft function was significantly different between Group 1 (CS) and Groups 3 (SDP) and 2 (SBP).
Preserving small split-liver grafts using oxygenated HMP may be much more effective than other techniques and positively affect the prognosis of split-liver transplantation, potentially leading to liver regeneration. The tissue-protective effect induced by oxygenated HMP is achieved by the reactivation of mitochondrial respiration, which occurs prior to reperfusion, when HMP oxidizes mitochondrial electron complexes. Mitochondrial electron overload upon reperfusion is prevented by the decreased production of reactive oxygen species through a reduction in lipid peroxidation and the storage of intracellular glutathione, as observed in rat livers [8,17]. Other studies have suggested that preservation without oxygenation also protects the endoplasmic reticulum and the vascular endothelium [18].
However, extended periods of preservation without oxygenation are also associated with certain drawbacks and limitations, particularly with regard to endoplasmic stress and changes in the sinusoidal endothelial cells [19]. Shear stress occurs due to the reaction of oxygen with increased concentrations of intracellular redox-active iron, which triggers free-radical-mediated cell injury [20]. The present findings suggest that oxidative stress and endothelial shear stress can be reduced by regulating the perfusion temperature and oxygenation. The tissue-protective effect induced by oxygenated HMP is achieved by the reactivation of mitochondrial respiration, which occurs prior to reperfusion, when HMP oxidizes mitochondrial electron complexes. Mitochondrial electron overload upon reperfusion is prevented by the decreased production of reactive oxygen species [21]. Even short-term (2 h) oxygenated HMP before transplantation ameliorates the mitochondrial and energetic disturbances and injuries associated with cold ischemia. The results of the present study thus suggest that oxidative stress and endothelial shear stress can be decreased by regulating the perfusion and oxygenation for a short time.
Zhang et al. reported the effectiveness and stability of normothermic machine perfusion (NMP) by splitting pig livers in perfusion preservation. Their results showed that NMP can be used for split-liver preservation without causing significant liver damage. The effects of MP include the washout effect and the successive supply of metabolic substrates and antioxidant agents to the endothelium and the parenchyma. [22]. However, they did not demonstrate any marked difference between CS, HMP, and NMP. The theory of NMP is that normal temperature is maintained and the substrates essential for cell metabolism are provided so that physiological circumstances can be reproduced [23].
However, due to the very high demand for oxygen and energy under conditions of normal temperature, NMP requires oxygen carriers and dialysis. Furthermore, in clinical settings, organ transportation between facilities may require cold preservation [12]. Currently, no conclusion has been reached on the ideal temperature for MP, as few studies have directly compared the results of HMP with those of NMP under the same conditions.
Conclusions
Splitting under conditions of MP can aid in recovery of function and help reduce ischemia-reperfusion injury. HMP is safe and can improve graft function while attenuating liver preservation injury.
Abbreviations
- AST
aspartate aminotransferase
- ATP
adenosine triphosphate
- CS
cold storage
- HA
hepatic artery
- HAP
hepatic artery pressure
- HMP
hypothermic machine perfusion preservation
- LDH
lactate dehydrogenase
- MP
machine perfusion
- NMP
normothermic machine perfusion
- PV
portal vein
- PVP
portal vein pressure
- SBP
split before perfusion
- SDP
split during perfusion
Footnotes
Source of support: This work was supported in part by a grant-in-aid for (B) (#15H03922 to H.O.) from the Japan Society for the Promotion of Science (JSPS) and a grant-in-aid for Innovative Research in Life Science from Asahikawa Medical University to N.M
Conflicts of interest
None.
References
- 1.Pichlmayr R, Ringe B, Gubernatis G, et al. Transplantation of a donor liver to 2 recipients (splitting transplantation) – a new method in the further development of segmental liver transplantation. Langenbecks Arch Chir. 1988;373:127–30. [PubMed] [Google Scholar]
- 2.Dalal AR. Split liver transplantation: What’s unique? World J Transplant. 2015;5:89–94. doi: 10.5500/wjt.v5.i3.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hashimoto K, Fujiki M, Quintini C, et al. Split liver transplantation in adults. World J Gastroenterol. 2016;22:7500–6. doi: 10.3748/wjg.v22.i33.7500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guarrera JV, Henry SD, Samstein B, et al. Hypothermic machine preservation in human liver transplantation: The first clinical series. Am J Transplant. 2010;10:372–81. doi: 10.1111/j.1600-6143.2009.02932.x. [DOI] [PubMed] [Google Scholar]
- 5.de Vera ME, Lopez-Solis R, Dvorchik I, et al. Liver transplantation using donation after cardiac death donors: Long-term follow-up from a single center. Am J Transplant. 2009;9:773–81. doi: 10.1111/j.1600-6143.2009.02560.x. [DOI] [PubMed] [Google Scholar]
- 6.Dutkowski P, Polak WG, Muiesan P, et al. First comparison of hypothermic oxygenated perfusion versus static cold storage of human donation after cardiac death liver transplants: An international-matched case analysis. Ann Surg. 2015;262:764–70. doi: 10.1097/SLA.0000000000001473. discussion 770–71. [DOI] [PubMed] [Google Scholar]
- 7.Guarrera JV, Henry SD, Samstein B, et al. Hypothermic machine preservation facilitates successful transplantation of “orphan” extended criteria donor livers. Am J Transplant. 2015;15:161–69. doi: 10.1111/ajt.12958. [DOI] [PubMed] [Google Scholar]
- 8.Dutkowski P, Furrer K, Tian Y, et al. Novel short-term hypothermic oxygenated perfusion (HOPE) system prevents injury in rat liver graft from non-heart beating donor. Ann Surg. 2006;244:968–76. doi: 10.1097/01.sla.0000247056.85590.6b. discussion 976–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matsuno N, Obara H, Watanabe R, et al. Rewarming preservation by organ perfusion system for donation after cardiac death liver grafts in pigs. Transplant Proc. 2014;46:1095–98. doi: 10.1016/j.transproceed.2013.12.035. [DOI] [PubMed] [Google Scholar]
- 10.Morito N, Obara H, Matsuno N, et al. Oxygen consumption during hypothermic and subnormothermic machine perfusions of porcine liver grafts after cardiac death. J Artif Organs. 2018;21(4):450–57. doi: 10.1007/s10047-018-1063-0. [DOI] [PubMed] [Google Scholar]
- 11.Yoshikawa R, Obara H, Matsuno N, et al. Ex-vivo reperfusion model to evaluate the utility of machine preservation for porcine liver donated after cardiac death. Transplant Proc. 2018;50:2826–29. doi: 10.1016/j.transproceed.2018.04.020. [DOI] [PubMed] [Google Scholar]
- 12.Suzuki S, Nakamura S, Koizumi T, et al. The beneficial effect of a prostaglandin 12 analog on ischemic rat liver. Tansplantation. 1991;52:979–83. doi: 10.1097/00007890-199112000-00008. [DOI] [PubMed] [Google Scholar]
- 13.Nesher E, Island E, Tryphonopoulos P, et al. Split liver transplantation. Transplant Proc. 2011;43:1736–41. doi: 10.1016/j.transproceed.2010.11.031. [DOI] [PubMed] [Google Scholar]
- 14.Hashimoto K, Miller CM, Quintini C, et al. Is impaired hepatic arterial buffer response a risk factor for biliary anastomotic stricture in liver transplant recipients? Surgery. 2010;148:582–88. doi: 10.1016/j.surg.2010.01.019. [DOI] [PubMed] [Google Scholar]
- 15.Boillot O, Delafosse B, Méchet I, et al. Small-for-size partial liver graft in an adult recipient; a new transplant technique. Lancet. 2002;359:406–7. doi: 10.1016/S0140-6736(02)07593-1. [DOI] [PubMed] [Google Scholar]
- 16.Schlegel A, de Rougemont O, Graf R, et al. Protective mechanisms of end-ischemic cold machine perfusion in DCD liver grafts. J Hepatol. 2013;58:278–86. doi: 10.1016/j.jhep.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 17.Kim JS, Boudjema K, D’Alessandro A, Southard JH. Machine perfusion of the liver: Maintenance of mitochondrial function after 48-hour preservation. Transplant Proc. 1997;29:3452–54. doi: 10.1016/s0041-1345(97)00975-5. [DOI] [PubMed] [Google Scholar]
- 18.Minor T, Manekeller S, Sioutis M, Dombrowski F. Endoplasmic and vascular surface activation during organ preservation: Refining upon the benefits of machine perfusion. Am J Transplant. 2006;6:1355–66. doi: 10.1111/j.1600-6143.2006.01338.x. [DOI] [PubMed] [Google Scholar]
- 19.Reddy SP, Brockmann J, Friend PJ. Normothermic perfusion: a mini-review. Transplantation. 2009;87:631–32. doi: 10.1097/TP.0b013e3181995e83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rauen U, Petrat F, Li T, De Groot H. Hypothermia injury/cold-induced apoptosis – evidence of an increase in chelatable iron causing oxidative injury in spite of low O2-/H2O2 formation. FASEB J. 2000;14:1953–64. doi: 10.1096/fj.00-0071com. [DOI] [PubMed] [Google Scholar]
- 21.Du C, Fang M, Li Y, et al. Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell. 2000;102:33–42. doi: 10.1016/s0092-8674(00)00008-8. [DOI] [PubMed] [Google Scholar]
- 22.Zhang ZB, Gao W, Liu L, et al. Development and assessment of normothermic machine perfusion preservation for extracorporeal splitting of pig liver. Ann Transplant. 2017;22:507–17. doi: 10.12659/AOT.904483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.op den Dries S, Karimian N, Sutton ME, et al. Ex vivo normothermic machine perfusion and viability testing of discarded human donor livers. Am J Transplant. 2013;13:1327–35. doi: 10.1111/ajt.12187. [DOI] [PubMed] [Google Scholar]