Abstract
Background
Staphylococcus aureus causes serious health care– and community-associated disease, requiring improved preventive measures such as vaccines. The investigational S. aureus 4-antigen vaccine (SA4Ag), comprising capsular polysaccharide serotypes 5 and 8 (CP5 and CP8) conjugated to CRM197, recombinant mutant clumping factor A (rmClfA), and recombinant manganese transporter protein C (rP305A or rMntC), was well tolerated, inducing robust functional immune responses to all 4 antigens through 12 months postvaccination. This is a serological extension study through 36 months postvaccination.
Methods
In 2 previous studies, healthy adults received SA4Ag, SA3Ag (without rMntC), or placebo; serology was also assessed at ~24 and ~36 months postvaccination. Functional immune responses (antibody responses that facilitate killing of S. aureus or neutralize S. aureus virulence mechanisms) were assessed with opsonophagocytic activity killing assays (CP5 or CP8) and a fibrinogen-binding inhibition assay (ClfA). A competitive Luminex immunoassay assessed ClfA and rMntC responses. Adverse events within 48 hours of blood draw were recorded.
Results
Four hundred forty subjects (18–64 years old, 255; 65–85 years old, 185) were enrolled. At 24 and 36 months postvaccination, subjects receiving SA4Ag had substantially higher geometric mean titers (GMTs) for CP5, CP8, and ClfA vs baseline; geometric mean fold rises (GMFRs) from baseline to month 36 were 2.7–8.1. For rMntC, 36-month GMTs declined from peak levels but remained above baseline for all SA4Ag groups; GMFRs from baseline to month 36 were 1.8 and 1.5 in the younger and older cohorts, respectively.
Conclusions
Persistent functional immune responses to S. aureus antigens were observed through 36 months in healthy adults.
ClinicalTrials.gov
NCT01643941 and NCT01364571.
Keywords: orthopedic infections, SA4Ag, Staphylococcus aureus, vaccine, vaccine immunogenicity persistence
Staphylococcus aureus (S. aureus) is a major cause of health care–associated infections, including bacteremia, lower respiratory infection, skin and soft tissue infections, and surgical site infections [1–3]. Patients with S. aureus infections (either methicillin-sensitive or methicillin-resistant) experience worse outcomes than uninfected patients, as measured by length of hospital stay, cost of care/hospital charges, and hospital mortality [4].
Improved measures are urgently needed to prevent S. aureus infection, including vaccines [5], especially as antibiotic drug resistance has led to fewer treatment options and generally more complex treatment regimens [6, 7]. An investigational S. aureus vaccine has been under development for the prevention of invasive S. aureus disease in patients undergoing orthopedic surgery, including deep or organ/space surgical site infections and bloodstream infections [8]. The investigational vaccine targets highly conserved virulence factors expressed early during infection and has been evaluated in clinical studies, initially as a 3-antigen S. aureus vaccine (SA3Ag) [9, 10], and then as a 4-antigen S. aureus vaccine (SA4Ag) [11, 12]. SA3Ag contains capsular polysaccharide serotypes 5 and 8 (CP5 and CP8) conjugated to CRM197 and a recombinant surface protein clumping factor A (rmClfA) that is mutated to remove the ability to bind fibrinogen. In addition to the antigens in SA3Ag, SA4Ag includes the lipoprotein manganese transporter protein C (MntC), which is expressed as a recombinant nonlipidated protein (rMntC; also known as rP305A) [11, 12]. These antigens were selected based on several criteria described by Anderson and coworkers [13]. All 4 antigens are expressed during human infection [14] and were protective in preclinical models that mimic human disease [15]. Bacteria express CPs to help them evade innate immune responses. Although CPs are not naturally immunogenic, immunogenicity is improved when conjugated to a carrier protein, and vaccination with CP conjugates can induce antibodies capable of killing bacteria [16]. S. aureus uses ClfA to bind to human fibrinogen; this is an early step in the organism establishing persistent infection. Humans do not naturally express antibodies that prevent ClfA binding; however, the ClfA antigen was designed to induce antibodies that can block this virulence mechanism [17]. MntC was discovered during a proteomics screen of antigens that are expressed in vivo [18] and is not expressed in artificial media. MntC facilitates manganese acquisition and has a role in helping S. aureus survive phagocytosis [19, 20]. By targeting MntC, antibodies prevent S. aureus from acquiring this important element and evading the host defense [21].
Two studies of SA4Ag evaluated the immunogenicity and overall safety of a single vaccination up to 12 months postvaccination [11, 12]. Both studies found that SA4Ag was well tolerated, was not associated with vaccine-related serious adverse events, and elicited robust functional immune responses after a single vaccination, with rapidly induced, high levels of bacteria-killing antibodies and sustained immune responses observed at 12 months postvaccination. To evaluate the duration of immune responses, a serological extension study (Study B3451014) through ~36 months postvaccination was conducted. Although the SA4Ag vaccine development program is currently under evaluation due to meeting futility criteria at a prespecified interim efficacy assessment of a phase 2b clinical trial [22], antibody persistence data may inform future studies of S. aureus vaccines.
METHODS
Study Population
The study was conducted at 12 study sites in the United States from January 2014 to April 2016 and included subjects who were enrolled in previous studies (NCT01643941 and NCT01364571) evaluating SA4Ag [11, 12] and had blood collected at both the baseline (prevaccination) and month 12 postvaccination time points. Subjects in the current extension study were asked to donate additional blood samples at approximately 24 months (visit 1) and 36 months (visit 2) postvaccination. All subjects who received SA4Ag or placebo in the primary study were considered for participation; however, the reasons why subjects declined to participate in this extension study were not collected. Due to the timing of the initiation of the extension study, in some subjects, the timing of the first sample was as late as 30 months after vaccination, whereas others only had collection of the 36-month postvaccination specimen.
Subjects were excluded from taking part in the extension study after participation in the primary SA4Ag vaccine trials if they had (1) participated in clinical studies investigating the use of immune modulators and/or non-Pfizer S. aureus/Candida vaccine studies; (2) developed a known or suspected defect of the immune system, including those receiving chronic, systemic corticosteroid therapies or those receiving immunosuppressive therapy; (3) received blood products or immunoglobulins (including monoclonal antibodies) within 6 months before a study visit; (4) developed a bleeding diathesis or condition associated with prolonged bleeding time that could affect routine blood draw; or (5) developed a severe physical or mental condition or laboratory abnormality that the investigator deemed would adversely affect the study participation, making the subject inappropriate for study entry.
The primary immunogenicity population was the evaluable immunogenicity population and included all subjects who were eligible, had blood drawn within the protocol-specified time frame, had valid and determinate assay results for the proposed analysis, and had no major protocol deviations. Subjects who had a confirmed S. aureus infection since completion of the primary study were excluded from the evaluable immunogenicity population.
The study protocol was approved by the institutional review board or the independent ethics committee for each of the study sites. The study was conducted in compliance with original or derivative ethical principles from the Declaration of Helsinki and in compliance with all guidelines of the International Council on Harmonization (ICH) Good Clinical Practice (GCP). In addition, all local regulatory requirements were followed. Written informed consent was obtained from each subject before enrollment in the study and before performance of any study-related procedures.
Study Vaccine
No vaccines were administered in this extension study. Subjects were vaccinated in the 2 primary vaccination studies [11, 12] with a single intramuscular dose of 1 of the 3 formulations of SA4Ag, with placebo, or with SA3Ag—a formulation without rMntC used only in the study for subjects 65–85 years of age [12]. The 3 formulations of SA4Ag and the single formulation of SA3Ag included fixed-dose CP5-CRM197, CP8-CRM197, and rmClfA (30 μg, 30 μg, and 60 μg, respectively). SA4Ag also included rMntC at 20 μg (low-dose SA4Ag), 60 μg (mid-dose SA4Ag), or 200 μg (high-dose SA4Ag).
Study Assessments
Immunogenicity
The primary studies were unblinded as planned on completion; thus, in this extension study, the subjects, investigators, and study site staff were unblinded. However, immunogenicity assays were performed by laboratory staff unaware of the study vaccine received by the subject.
Immunogenicity was assessed at baseline (prevaccination), day 29, month 12, month 24/30, and month 36 postvaccination. Serum samples of baseline, day 29, and month 12 time points were collected from the primary studies but were reassessed using the current validated assays to allow evaluation of the immune response kinetics from vaccination through month 36.
Five assays were performed at each blood sampling time point to assess the immune response to the vaccine [11, 12]. Functional immune responses (antibody responses that facilitate the killing of S. aureus or neutralize S. aureus virulence mechanisms) were assessed using validated opsonophagocytic activity (OPA) killing assays using CP5- or CP8-expressing S. aureus strains [16] and using the qualified fibrinogen-binding inhibition (FBI) assay [17] for ClfA. Responses to ClfA and rMntC were measured using a validated multiplex competitive Luminex immunoassay (cLIA) [14].
Qualified OPAs were used to measure functional antibodies specific to CP5- or CP8-expressing strains of S. aureus [16] in human sera. Human test serum was serially diluted 2-fold and added to microtiter assay plates. Live target bacterial strains (CP5 or CP8) were added to the wells, and the plates were shaken at 4°C for 30 minutes. Differentiated HL-60 cells (phagocytes) and baby rabbit serum (complement source) were added to the wells, and the plates were shaken at 37°C for 60 minutes. After these incubations, an aliquot of assay reaction from each well was transferred to filter plates to enumerate surviving bacteria. The filter plates were incubated overnight at 37°C to allow bacterial colonies to form. After incubation, the plates were fixed, and the colonies were stained with Coomassie blue stain. Colonies were imaged and counted using CTL ImmunoSpot readers. Raw colony counts were used to plot kill curves and calculate OPA titers. The OPA titer is defined as the reciprocal dilution that results in a 50% reduction in bacterial count compared with control wells without test serum. The OPA titer is interpolated from the 2 dilutions that encompass this 50% killing cutoff. The reported OPA titer is the geometric mean of 2 replicate OPA titers.
A qualified FBI assay was used to measure functional clumping factor A (ClfA)–specific immunoglobulin in human sera [17]. The FBI assay measures the ability of anti-ClfA antibodies to prevent the binding of live ClfA-expressing S. aureus isolates to plate-immobilized human fibrinogen. Briefly, 2-fold serial dilutions of human test serum were incubated with a fixed amount of live bacteria with shaking at 37ºC for 30–60 minutes. The opsonized bacteria were then added to plates precoated with human fibrinogen and incubated at 37ºC for 60 minutes. After subsequent washing steps, the amount of live bacteria bound to the immobilized fibrinogen was detected by incubation with BacTiter-Glo detection reagent at 37ºC for 15 minutes. After incubation, assay plates were read using a luminometer. The FBI assay titer is defined as the reciprocal dilution that results in 75% inhibition of bacterial binding, compared with the average binding observed in control wells without test serum.
As noted above, a qualified cLIA was used to measure antibody responses to ClfA [17] and rMntC [21] in human serum. Briefly, antigen-coated microspheres were incubated overnight with appropriately diluted serum samples, controls, and reference standard serum. A mixture of phycoerythrin-labeled ClfA and r305-specific mouse monoclonal antibodies were then added to the microsphere/serum mixture, and after a 2-hour incubation, the unbound components were washed off using a magnetic bead washer. The magnitude of the fluorescence signal allowed for measurement of the antibody bound to the antigen-coated microspheres by the Bio-Plex reader. Raw data were expressed as median fluorescence intensities and read against a reference standard.
Safety
Adverse events (AEs) occurring within 48 hours of the immunogenicity blood draw were recorded. Serious AEs (SAEs) considered by the investigator to be possibly vaccine-related were reported as a part of follow-up from the primary studies.
Immunogenicity End Points
At each time point, the following analyses were performed: (1) proportions of subjects achieving titers greater than or equal to predefined threshold criteria that were considered potentially biologically relevant, based on prior clinical studies and animal models, as previously described [11, 12] (for the OPA assays, the thresholds were ≥1000 for CP5-CRM197 and ≥2000 for CP8-CRM197; for rmClfA, the threshold was greater than or equal to the lower limit of quantitation [LLOQ; 121] based on the FBI assay; for rMntC, it was greater than or equal to the LLOQ [128.00] based on the cLIA); (2) geometric mean titers (GMTs) for functional antibody assays (including OPA for CP5/CP8 and FBI for ClfA) and antigen-specific cLIA titers (for ClfA and rMntC); and (3) geometric mean fold rises (GMFRs) from baseline (prevaccination) for each antigen (measured by OPA, FBI, or cLIA).
Statistical Analysis
This study did not test a hypothesis; instead, descriptive statistics were compiled and analyzed. For each time point in each assay, GMTs were calculated by log-transforming assay values, calculating means, then exponentially converting the means to return them to the original scale. For values below the LLOQ, the value used was half the LLOQ of the specific assay. The 95% confidence intervals (CIs) were determined through back-transformations of the confidence limits computed for the mean of the logarithmically transformed assay data based on the Student t distribution.
RESULTS
Participants
Four hundred forty (440) of the 737 subjects vaccinated in the primary studies were enrolled (Table 1). Of the 454 subjects in the initial study of 18–64-year-olds [11], 255 (56%) participated in the extension study, and 185 of the 283 (65%) subjects aged 65–85 years also participated [12].
Table 1.
Disposition of Enrolled Subjects
| Vaccine Groupsa | ||||||
|---|---|---|---|---|---|---|
| Placebo | Low-Dose SA4Ag | Mid-Dose SA4Ag | High-Dose SA4Ag | SA3Agb | Total | |
| No. | No. | No. | No. | No. | No. | |
| Younger cohort: subjects 18–64 years of age | 66 | 63 | 69 | 57 | N/A | 255 |
| Enrolled at visit 1 (month 24/30)c | 61 | 59 | 65 | 52 | – | 237 |
| Withdrawn before visit 2 (month 36) | 12 | 6 | 8 | 4 | – | 30 |
| Lost to follow-up | 6 | 5 | 5 | 1 | – | 17 |
| No longer meets entrance criteria | 0 | 0 | 1 | 2 | – | 3 |
| No longer willing to participate in study | 5 | 0 | 1 | 1 | – | 7 |
| Other | 1 | 1 | 1 | 0 | – | 3 |
| Enrolled at visit 2 (month 36) | 5 | 4 | 4 | 5 | – | 18 |
| Completed visit 2 (month 36) | 54 | 57 | 61 | 53 | – | 225 |
| Older cohort: subjects 65–85 years of age | 38 | 43 | 33 | 37 | 34 | 185 |
| Enrolled at visit 1 (month 24) | 37 | 43 | 32 | 37 | 34 | 183 |
| Withdrawn before visit 2 (month 36) | 2 | 1 | 1 | 0 | 2 | 6 |
| Lost to follow-up | 1 | 0 | 0 | 0 | 1 | 2 |
| No longer meets entrance criteria | 0 | 1 | 0 | 0 | 0 | 1 |
| Other | 1 | 0 | 1 | 0 | 1 | 3 |
| Enrolled at visit 2 (month 36) | 1 | 0 | 1 | 0 | 0 | 2 |
| Completed visit 2 (month 36) | 36 | 42 | 32 | 37 | 32 | 179 |
Abbreviations: CP5, capsular polysaccharide serotype 5; CP8, capsular polysaccharide serotype 8; N/A, not applicable; rmClfA, recombinant mutant clumping factor A; rMntC, recombinant manganese transporter protein C; SA3Ag, Staphylococcus aureus 3-antigen vaccine; SA4Ag, Staphylococcus aureus 4-antigen vaccine.
aVaccine groups labeled low-dose, mid-dose, and high-dose SA4Ag refer only to differences in rMntC dose levels (20 μg, 60 μg, and 200 μg, respectively). CP5-CRM197, CP8-CRM197, and rmClfA dose levels (30 μg, 30 μg, and 60 μg, respectively) are the same in each SA4Ag dose level and in SA3Ag.
bFor subjects 65–85 years of age only.
cForty-three subjects were enrolled at month 30.
Thirty-six of the 420 subjects who enrolled at visit 1 withdrew from the study before visit 2, and 20 subjects who missed visit 1 were enrolled at visit 2. None of the subjects withdrew for safety-related reasons.
The demographics of the older cohort (65–85-year-olds) were comparable to the primary study [12]; however, subjects from the younger cohort (18–64-year-olds) were slightly older, with more female and more white subjects compared with the primary study population [11]. In this follow-up study, more female than male subjects were enrolled (61.6% and 51.9% in the younger and older cohorts, respectively), and the majority of subjects were white (83.5% and 91.9% in the younger and older cohorts, respectively). The mean age at vaccination was 46.3 years in the total younger cohort and 70.7 years in the total older cohort; the mean age in each cohort was similar across active vaccine and placebo groups. The mean age at enrollment was 48.5 years in the total younger cohort and 72.7 years in the total older cohort; the mean age in each cohort was similar across the active vaccine and placebo groups (Supplementary Table 1).
Immunogenicity
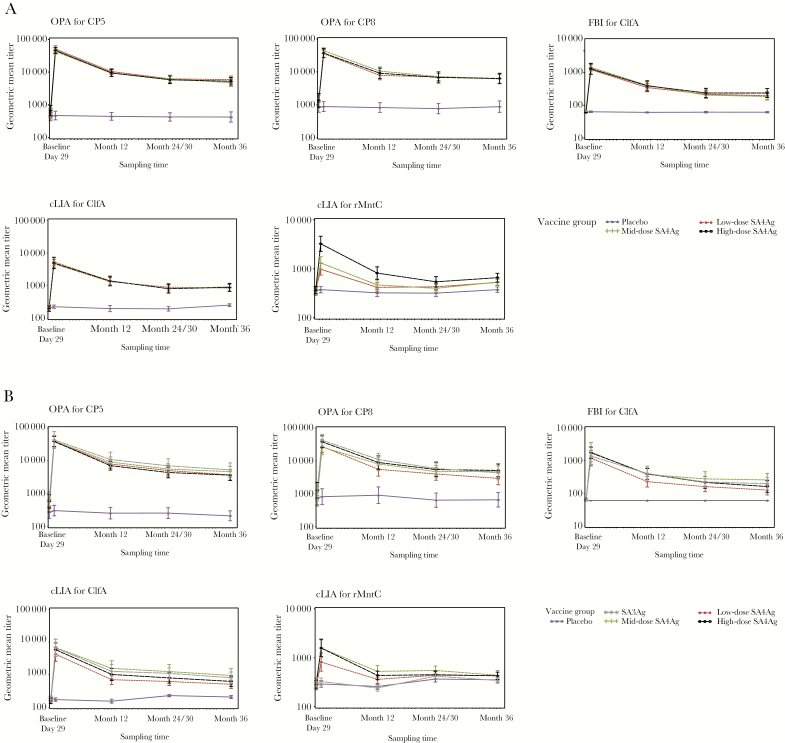
Overall, 94.9% (242/255) and 96.2% (178/185) of subjects were included in the evaluable population in the younger and older cohorts, respectively. In all SA4Ag groups, rapid and substantial increases in OPA, FBI, and cLIA GMTs for all 4 antigens were observed at day 29 postvaccination, with dose-dependent cLIA GMTs observed for rMntC [11, 12]. In comparison to the gradual declines of OPA, FBI, and cLIA GMTs for all 4 antigens observed through month 12 postvaccination [11, 12], a lower rate of decline was observed for all 4 antigens from month 12 to month 36 (Figure 1), indicating sustained immune responses. Although GMTs for rMntC declined from month 12 to near baseline and placebo levels at month 36, they remained higher at month 36 than at baseline in both age cohorts in all SA4Ag groups (Figure 1), as the lower bounds of the GMFRs were above 1 for all subjects: 1.2–1.5 in the younger cohort and 1.1–1.4 in the older cohort (data not shown).
Figure 1.
Immunogenicity results in the evaluable immunogenicity population.a A, The younger cohort (18–64 years of age). B, The older cohort (65–85 years of age). aThe evaluable immunogenicity population included all subjects who were eligible, had no confirmed Staphylococcus aureus infection since completion of the primary study, had blood drawn within the protocol-specified time frame, had valid and determinate assay results for the proposed analysis, and had no major protocol deviations. bVaccine groups labeled low-dose, mid-dose, and high-dose SA4Ag refer only to differences in rMntC levels (20 μg, 60 μg, and 200 μg, respectively). CP5-CRM197, CP8-CRM197, and rmClfA levels (30 μg, 30 μg, and 60 μg, respectively) are the same in each SA4Ag dose level and in SA3Ag. Abbreviations: ClfA, surface protein clumping factor A; cLIA, competitive Luminex immunoassay; CP5, capsular polysaccharide serotype 5; CP8, capsular polysaccharide serotype 8; FBI, fibrinogen-binding inhibition; OPA, opsonophagocytic activity; rMntC, recombinant manganese transporter protein C; SA3Ag, Staphylococcus aureus 3-antigen vaccine; SA4Ag, S. aureus 4-antigen vaccine.
In the high-dose SA4Ag group, the CP5, CP8, and ClfA GMTs at 36 months postvaccination were well above baseline (Table 2), with GMFRs from baseline to month 36 ranging from 2.7 to 8.1 (Table 3). GMFRs for CP5 (OPA), CP8 (OPA), ClfA (FBI), and ClfA (cLIA) were 8.1, 4.5, 3.9, and 4.4 in the younger cohort, respectively; these were 6.3, 4.3, 2.7, and 4.0 in the older cohort, respectively (Table 3). For rMntC, the GMTs at 36 months postvaccination remained higher than at baseline in both age cohorts (Table 2), with GMFRs from baseline to month 36 of 1.8 in the younger cohort (95% CI, 1.5–2.1) and 1.5 in the older cohort (95% CI, 1.2–1.8) (Table 3).
Table 2.
Staphylococcus aureus Antigen-Specific GMTs in the Evaluable Immunogenicity Populationa
| Antigen and Age Groupb | Placebo | High-Dose SA4Age | ||||||
|---|---|---|---|---|---|---|---|---|
| Baselinec (95% CI) | Day 29d (95% CI) | Month 12d (95% CI) | Month 36d (95% CI) | Baselinec (95% CI) | Day 29d (95% CI) | Month 12d (95% CI) | Month 36d (95% CI) | |
| CP5f | ||||||||
| 18–64 | 440.0 (324.3–597.0) | 469.4 (346.8–635.2) | 443.5 (337.5–582.8) | 423.7 (295.6–607.3) | 671.8 (466.4–967.6) | 45 715.6 (37 231.4–56 133.1) | 9408.1 (7418.7–11 931.0) | 5242.9 (4033.3–6815.4) |
| 65–85 | 266.7 (175.9–404.3) | 303.4 (210.0–438.4) | 253.6 (168.7–381.3) | 210.1 (148.0–298.2) | 581.7 (366.7–922.8) | 38 912.6 (27 278.4–55 508.9) | 7144.5 (5227.0–9765.6) | 3661.5 (2514.5–5331.8) |
| CP8f | ||||||||
| 18–64 | 837.2 (592.5–1182.9) | 880.9 (622.5–1246.5) | 823.3 (586.7–1155.1) | 878.2 (588.6–1310.1) | 1367.2 (844.7–2213.1) | 37 306.1 (27 170.8–51 222.0) | 9127.7 (6375.6–13 067.7) | 6313.5 (4376.5–9107.6) |
| 65–85 | 769.1 (433.3–1365.3) | 825.7 (474.1–1438.2) | 914.9 (512.5–1633.3) | 663.6 (399.7–1101.7) | 1255.5 (711.1–2216.7) | 38 609.3 (25 389.7–58 712.0) | 9064.3 (5690.3–14 438.8) | 5195.9 (3296.7–8189.1) |
| ClfAg | ||||||||
| 18–64 | 62.8 (60.2–65.5) | 63.7 (60.5–67.0) | 61.2 (59.8–62.6) | 61.9 (59.1–64.8) | 60.5 (NE–NE) | 1322.7 (899.7–1944.5) | 396.9 (278.0–566.7) | 238.8 (174.8–326.3) |
| 65–85 | 61.7 (59.3–64.2) | 60.5 (NE–NE) | 60.5 (NE–NE) | 60.5 (NE–NE) | 60.5 (NE–NE) | 1756.3 (1172.4–2630.8) | 395.7 (271.7–576.5) | 162.3 (108.0–243.9) |
| ClfAh | ||||||||
| 18–64 | 199.0 (165.2–239.7) | 221.8 (196.9–249.8) | 194.7 (156.6–242.1) | 248.2 (224.7–274.2) | 193.4 (159.7–234.2) | 4841.0 (3228.8–7258.3) | 1359.9 (963.7–1918.9) | 867.3 (653.7–1150.7) |
| 65–85 | 187.3 (160.7–218.2) | 171.9 (151.8–194.6) | 153.9 (133.9–176.9) | 206.8 (184.9–231.4) | 156.7 (129.2–190.0) | 5811.0 (3596.7–9388.6) | 1012.2 (660.1–1552.2) | 621.6 (432.1–894.2) |
| rMntCh | ||||||||
| 18–64 | 358.6 (303.2–424.3) | 375.5 (322.8–436.8) | 322.8 (270.2–385.7) | 374.6 (330.4–424.6) | 367.6 (308.2–438.4) | 3244.0 (2272.6–4630.6) | 817.4 (609.9–1095.5) | 658.9 (535.9–810.2) |
| 65–85 | 286.8 (240.7–341.7) | 292.2 (249.8–341.9) | 259.2 (226.0–297.2) | 354.8 (317.8–396.2) | 288.0 (239.1–346.8) | 1581.9 (1068.5–2342.1) | 435.2 (354.5–534.2) | 427.7 (359.5–508.7) |
Abbreviations: CI, confidence interval; ClfA, surface protein clumping factor A; cLIA, competitive Luminex immunoassay; CP5, capsular polysaccharide serotype 5; CP8, capsular polysaccharide serotype 8; FBI, fibrinogen-binding inhibition; GMT, geometric mean titer; NE, not estimable; OPA, opsonophagocytic activity; rMntC, recombinant manganese transporter protein C; SA4Ag, Staphylococcus aureus 4-antigen vaccine.
aThe evaluable immunogenicity population included all subjects who were eligible, had no confirmed S. aureus infection since completion of the primary study, had blood drawn within the protocol-specified time frame, had valid and determinate assay results for the proposed analysis, and had no major protocol deviations.
bAge group in years.
cPrevaccination.
dPostvaccination.
eHigh-dose SA4Ag included CP5-CRM197 30 μg, CP8-CRM197 30 μg, rmClfA 60 μg, and rMntC 200 μg.
fDetermined via OPA assay.
gDetermined via FBI assay.
hDetermined via cLIA.
Table 3.
Staphylococcus aureus Antigen-Specific Responses in the Evaluable Immunogenicity Population: GMFR From Baseline at Month 36
| Antigen | Age Group, y | Placebo | High-Dose SA4Aga |
|---|---|---|---|
| GMFR (95% CI) | GMFR (95% CI) | ||
| CP5b | 18–64 | 1.0 (0.8–1.3) | 8.1 (6.0–10.8) |
| 65–85 | 0.8 (0.6–0.9) | 6.3 (4.0–9.8) | |
| CP8b | 18–64 | 1.0 (0.8–1.2) | 4.5 (3.2–6.4) |
| 65–85 | 0.8 (0.6–1.0) | 4.3 (2.5–7.3) | |
| ClfAc | 18–64 | 1.0 (1.0–1.1) | 3.9 (2.8–5.3) |
| 65–85 | 1.0 (0.9–1.0) | 2.7 (1.8–4.0) | |
| ClfAd | 18–64 | 1.3 (1.0–1.6) | 4.4 (3.1–6.3) |
| 65–85 | 1.1 (1.0–1.3) | 4.0 (2.9–5.4) | |
| rMntCd | 18–64 | 1.0 (0.9–1.3) | 1.8 (1.5–2.1) |
| 65–85 | 1.3 (1.1–1.5) | 1.5 (1.2–1.8) |
Abbreviations: CI, confidence interval; ClfA, surface protein clumping factor A; cLIA, competitive Luminex immunoassay; CP5, capsular polysaccharide serotype 5; CP8, capsular polysaccharide serotype 8; FBI, fibrinogen-binding inhibition; GMFR, geometric mean fold rise; OPA, opsonophagocytic activity; rMntC, recombinant manganese transporter protein C; SA4Ag, Staphylococcus aureus 4-antigen vaccine.
aHigh-dose SA4Ag included CP5-CRM197 30 μg, CP8-CRM197 30 μg, rmClfA 60 μg, and rMntC 200 μg.
bDetermined via OPA assay.
cDetermined via FBI assay.
dDetermined via cLIA.
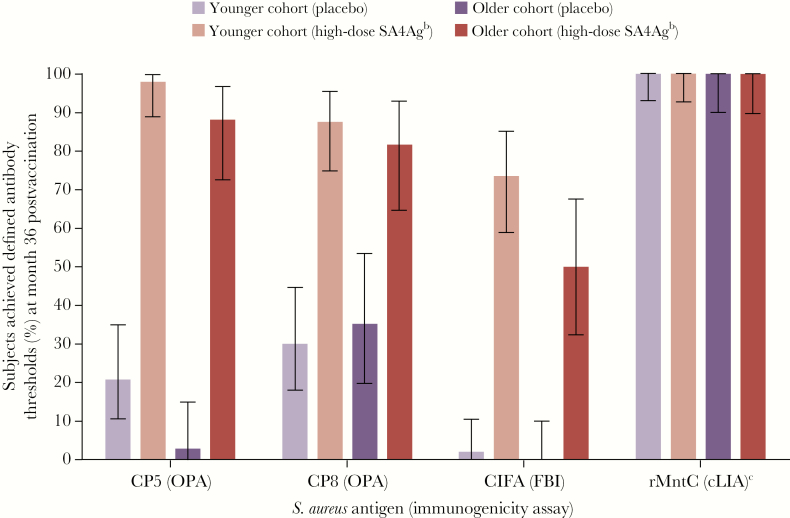
In the high-dose SA4Ag group, the proportions of subjects achieving predefined OPA threshold functional response criteria for CP5 and CP8 at month 36 ranged from 81.8% to 97.9% in both age cohorts (compared with 2.9%–35.3% of placebo recipients). In the younger cohort, the proportions were 97.9% and 87.5%, respectively; in the older cohort, the proportions were 88.2% and 81.8%, respectively (Figure 2). The proportion of subjects achieving predefined FBI threshold functional response criteria for ClfA at month 36 was 73.5% in the younger cohort, and it was 50.0% in the older cohort (compared with 2.0% and 0.0% of placebo recipients in those cohorts, respectively) (Figure 2). Nearly all subjects (>90%) achieved the predefined cLIA threshold for rMntC at all time points from baseline to month 36 in both cohorts, including placebo recipients (Figure 2). The threshold for cLIA results was set at the LLOQ of the assay, which was determined based on assay performance (assay linearity and precision); this threshold is relatively low, and thus the immune responses elicited by rMntC, as shown by substantial rises in GMTs and GMFRs (Tables 2 and 3), were not able to be demonstrated by the threshold analysis, due to the high proportions of participants with assay results above threshold at baseline.
Figure 2.
Percentage of subjects achieving predefined antibody thresholds at month 36 postvaccination in the evaluable immunogenicity population.a Error bars indicate 95% confidence intervals for each value. Younger cohort: 18–64 years of age; older cohort: 65–85 years of age. aThe evaluable immunogenicity population included all subjects who were eligible, had no confirmed Staphylococcus aureus infection since completion of the primary study, had blood drawn within the protocol-specified time frame, had valid and determinate assay results for the proposed analysis, and had no major protocol deviations. bVaccine groups labeled low-dose, mid-dose, and high-dose SA4Ag refer only to differences in rMntC levels (20 μg, 60 μg, and 200 μg, respectively). CP5-CRM197, CP8-CRM197, and rmClfA levels (30 μg, 30 μg, and 60 μg, respectively) are the same in each SA4Ag dose level and in SA3Ag. cFor rMntC, the threshold for cLIA results was set at the lower limit of quantitation of the assay, which was determined based on assay linearity and precision; this threshold is relatively low, and thus the immune responses elicited by rMntC were not able to be detected due to the high proportions of participants with assay results above the threshold. Abbreviations: ClfA, surface protein clumping factor A; cLIA, competitive Luminex immunoassay; CP5, capsular polysaccharide serotype 5; CP8, capsular polysaccharide serotype 8; FBI, fibrinogen-binding inhibition; OPA, opsonophagocytic activity; rMntC, recombinant manganese transporter protein C; SA3Ag, Staphylococcus aureus 3-antigen vaccine; SA4Ag, S. aureus 4-antigen vaccine.
Safety
No AEs were reported in the 48 hours after each blood draw. No vaccine-related SAEs were reported up to month 36 postvaccination.
DISCUSSION
The current study demonstrated that antibody levels at 3 years after single-dose vaccination with SA4Ag remained above prevaccination levels in adults 18 to 85 years of age. The robust functional immune responses elicited against key S. aureus virulence mechanisms were demonstrated by OPA killing assays (>80% of subjects achieved predefined OPA titer thresholds at 36 months) and functional antibody responses to ClfA above the FBI predefined threshold in most subjects. Responses to the fourth antigen (rP305A or rMntC) had substantially declined at 36 months; however, the GMTs remained above baseline level in both age groups.
Increased age may impact vaccine immunogenicity and efficacy; for influenza vaccines, this has led to the development of high-dose influenza vaccine formulations to increase vaccine efficacy in the elderly [23]. However, immune responses to vaccines are not necessarily impaired in older age, as demonstrated by durable immune responses in the elderly in this study and in the primary SA4Ag study by Creech et al. [12]. Similarly, protective responses of licensed vaccines in older age have been reported. For example, large phase 3 studies of a herpes zoster vaccine have shown comparable efficacy against herpes zoster across 3 age groups (50–59, 60–69, and ≥70 years of age) [24] and no difference in vaccine efficacy in participants 70–79 (90.0% efficacy) and ≥80 years of age (89.1% efficacy) [25].
There are many potential indications for an S. aureus vaccine, given the spectrum of disease and various populations at risk [26]. However, elective surgery represents a priority population due to the relatively high incidence of disease [27], a well-defined risk period (the time of surgery) [5, 7], and the opportunity to vaccinate before the period of risk. SA4Ag efficacy has been undergoing efficacy assessment in a randomized placebo-controlled study among patients undergoing elective instrumented spinal fusion surgery (ClinicalTrials.gov identifier NCT02388165). Recently, this clinical trial ceased enrollment due to meeting its prespecified futility criteria at an interim efficacy analysis in December 2018 [22]; however, there were no safety concerns. Investigation is currently underway to understand these results in the elective spinal surgical population evaluated. Other investigational S. aureus vaccines are under development, including other candidates that share antigens with SA4Ag (eg, CP5, CP8, and ClfA) [28]. The antibody persistence results we present in this healthy population are therefore relevant to the S. aureus vaccine development field. Given the substantial burden of health care– and community-associated disease, there remains an unmet medical need to develop a vaccine to prevent S. aureus disease. Further research into humoral and cell-mediated immune responses to S. aureus antigens is critical to support ongoing vaccine development.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
Editorial support was provided by Thomas Gegeny, PhD, and Chu Kong Liew, PhD, of Engage Scientific Solutions and was funded by Pfizer Inc. We thank Pfizer colleagues from Vaccine Clinical Research & Development, High-Throughput Clinical Testing, Clinical Development Operations, Pharmaceutical Sciences, Vaccine Regulatory and Project Management, clinical center collaborators, and clinical trial participants.
Financial support. This study was sponsored by Pfizer Inc.
Potential conflicts of interest. C.B.C., R.W.F., R.F., and M.K.K. received grant support from Pfizer to conduct the study. All other authors were employees of Pfizer. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Author contributions. All authors participated in the interpretation of study results and in the drafting, critical revision, and approval of the final version of the manuscript. D.C., J.B., J.E., W.C.G., S.P., A.S.A., and K.U.J. were involved in the study design. C.B.C., R.W.F., S.P., and D.C. collected the data. C.B.C., A.F., R.W.F., M.K.K., S.P., J.B., D.R., D.C., J.E., W.C.G., A.S.A., and K.U.J. interpreted the results. C.B.C., R.W.F., and M.K.K. were investigators in the study. A.F. and D.R. conducted the statistical analysis.
Data availability. Upon request, and subject to certain criteria, conditions, and exceptions (see https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information), Pfizer will provide access to individual de-identified participant data from Pfizer-sponsored global interventional clinical studies conducted for medicines, vaccines, and medical devices (1) for indications that have been approved in the United States and/or European Union or (2) in programs that have been terminated (ie, development for all indications has been discontinued). Pfizer will also consider requests for the protocol, data dictionary, and statistical analysis plan. Data may be requested from Pfizer trials 24 months after study completion. The de-identified participant data will be made available to researchers whose proposals meet the research criteria and other conditions, and for which an exception does not apply, via a secure portal. To gain access, data requestors must enter into a data access agreement with Pfizer.
References
- 1. Diekema DJ, Pfaller MA, Schmitz FJ, et al. . Survey of infections due to Staphylococcus species: frequency of occurrence and antimicrobial susceptibility of isolates collected in the United States, Canada, Latin America, Europe, and the Western Pacific region for the SENTRY Antimicrobial Surveillance Program, 1997–1999. Clin Infect Dis 2001; 32(Suppl 2):S114–32. [DOI] [PubMed] [Google Scholar]
- 2. Owens CD, Stoessel K. Surgical site infections: epidemiology, microbiology and prevention. J Hosp Infect 2008; 70(Suppl 2):3–10. [DOI] [PubMed] [Google Scholar]
- 3. Baker AW, Dicks KV, Durkin MJ, et al. . Epidemiology of surgical site infection in a community hospital network. Infect Control Hosp Epidemiol 2016; 37:519–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Campbell RS, Emons MF, Mardekian J, et al. . Adverse clinical outcomes and resource utilization associated with methicillin-resistant and methicillin-sensitive Staphylococcus aureus infections after elective surgery. Surg Infect (Larchmt) 2015; 16:543–52. [DOI] [PubMed] [Google Scholar]
- 5. Mohamed N, Wang MY, Le Huec JC, et al. . Vaccine development to prevent Staphylococcus aureus surgical-site infections. Br J Surg 2017; 104:e41–54. [DOI] [PubMed] [Google Scholar]
- 6. Lowy FD. Staphylococcus aureus infections. N Engl J Med 1998; 339:520–32. [DOI] [PubMed] [Google Scholar]
- 7. Anderson DJ, Kaye KS. Staphylococcal surgical site infections. Infect Dis Clin North Am 2009; 23:53–72. [DOI] [PubMed] [Google Scholar]
- 8. Gurtman A, Begier E, Mohamed N, et al. . The development of a Staphylococcus aureus four antigen vaccine for use prior to elective orthopedic surgery. Hum Vaccin Immunother 2019; 15:358–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nissen M, Marshall H, Richmond P, et al. . A randomized phase I study of the safety and immunogenicity of three ascending dose levels of a 3-antigen Staphylococcus aureus vaccine (SA3Ag) in healthy adults. Vaccine 2015; 33:1846–54. [DOI] [PubMed] [Google Scholar]
- 10. Marshall H, Nissen M, Richmond P, et al. . Safety and immunogenicity of a booster dose of a 3-antigen Staphylococcus aureus vaccine (SA3Ag) in healthy adults: a randomized phase 1 study. J Infect 2016; 73:437–54. [DOI] [PubMed] [Google Scholar]
- 11. Frenck RW Jr, Creech CB, Sheldon EA, et al. . Safety, tolerability, and immunogenicity of a 4-antigen Staphylococcus aureus vaccine (SA4Ag): results from a first-in-human randomised, placebo-controlled phase 1/2 study. Vaccine 2017; 35:375–84. [DOI] [PubMed] [Google Scholar]
- 12. Creech CB, Frenck RW Jr, Sheldon EA, et al. . Safety, tolerability, and immunogenicity of a single dose 4-antigen or 3-antigen Staphylococcus aureus vaccine in healthy older adults: results of a randomised trial. Vaccine 2017; 35:385–94. [DOI] [PubMed] [Google Scholar]
- 13. Anderson AS, Miller AA, Donald RG, et al. . Development of a multicomponent Staphylococcus aureus vaccine designed to counter multiple bacterial virulence factors. Hum Vaccin Immunother 2012; 8:1585–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rozemeijer W, Fink P, Rojas E, et al. . Evaluation of approaches to monitor Staphylococcus aureus virulence factor expression during human disease. PLoS One 2015; 10:e0116945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Scully IL, Liberator PA, Jansen KU, Anderson AS. Covering all the bases: preclinical development of an effective Staphylococcus aureus vaccine. Front Immunol 2014; 5:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nanra JS, Buitrago SM, Crawford S, et al. . Capsular polysaccharides are an important immune evasion mechanism for Staphylococcus aureus. Hum Vaccin Immunother 2013; 9:480–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hawkins J, Kodali S, Matsuka YV, et al. . A recombinant clumping factor A-containing vaccine induces functional antibodies to Staphylococcus aureus that are not observed after natural exposure. Clin Vaccine Immunol 2012; 19:1641–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Anderson AS, Scully IL, Timofeyeva Y, et al. . Staphylococcus aureus manganese transport protein C is a highly conserved cell surface protein that elicits protective immunity against S. aureus and Staphylococcus epidermidis. J Infect Dis 2012; 205:1688–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Handke LD, Gribenko AV, Timofeyeva Y, et al. . MntC-dependent manganese transport is essential for Staphylococcus aureus oxidative stress resistance and virulence. mSphere 2018; 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Handke LD, Hawkins JC, Miller AA, et al. . Regulation of Staphylococcus aureus MntC expression and its role in response to oxidative stress. PLoS One 2013; 8:e77874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gribenko AV, Parris K, Mosyak L, et al. . High resolution mapping of bactericidal monoclonal antibody binding epitopes on Staphylococcus aureus antigen MntC. PLoS Pathog 2016; 12:e1005908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pfizer Inc. Independent data monitoring committee recommends discontinuation of the phase 2B STRIVE clinical trial of Staphylococcus aureus vaccine following planned interim analysis 2018. Available at: https://investors.pfizer.com/investor-news/press-release-details/2018/Independent-Data-Monitoring-Committee-Recommends-Discontinuation-of-the-Phase-2b-STRIVE-Clinical-Trial-of-Staphylococcus-aureus-Vaccine-Following-Planned-Interim-Analysis/default.aspx. Accessed 7 February 2019.
- 23. Izurieta HS, Thadani N, Shay DK, et al. . Comparative effectiveness of high-dose versus standard-dose influenza vaccines in US residents aged 65 years and older from 2012 to 2013 using Medicare data: a retrospective cohort analysis. Lancet Infect Dis 2015; 15:293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lal H, Cunningham AL, Godeaux O, et al. ; ZOE-50 Study Group Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N Engl J Med 2015; 372:2087–96. [DOI] [PubMed] [Google Scholar]
- 25. Cunningham AL, Lal H, Kovac M, et al. ; ZOE-70 Study Group Efficacy of the herpes zoster subunit vaccine in adults 70 years of age or older. N Engl J Med 2016; 375:1019–32. [DOI] [PubMed] [Google Scholar]
- 26. Tong SY, Davis JS, Eichenberger E, et al. . Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin Microbiol Rev 2015; 28:603–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. World Health Organization. Global Guidelines for the Prevention of Surgical Site Infection. Geneva: World Health Organization; 2016. [PubMed] [Google Scholar]
- 28. Levy J, Licini L, Haelterman E, et al. . Safety and immunogenicity of an investigational 4-component Staphylococcus aureus vaccine with or without AS03B adjuvant: results of a randomized phase I trial. Hum Vaccin Immunother 2015; 11:620–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.