Abstract
Infections are a common cause of hospitalization for patients with opioid use disorder (OUD), and hospital admissions are rising in the context of the worsening US opioid crisis. Infectious disease (ID) physicians are frequently the first point of medical contact for these patients. In this article, we discuss the integration of evidence-based management of OUD and patient-centered care of hospitalized persons with acute injection-related infections. We describe the following components of a comprehensive approach for OUD with inpatient ID consultations: (1) how to screen for OUD; (2) how to initiate the 3 US Food and Drug Administration-approved medications for OUD (buprenorphine, methadone, and extended-release naltrexone); (3) how to manage acute pain and opioid-related conditions; and (4) how to link and integrate ID and OUD treatment after hospital discharge. These strategies reduce unplanned discharges and increase completion of recommended antimicrobial regimens.
Keywords: addition-related infections, buprenorphine, opioids, outpatient parenteral antibiotic therapy (OPAT), substance use
With the opioid epidemic surging, the United States is facing a rising tide of infectious complications of drug use. In 2012, there were 530 000 opioid use disorder (OUD)-related hospitalizations nationally and, of these, $700 million in costs were due to OUD-related infections [1]. This is likely an underestimation in scale and cost. Perhaps the most feared bacterial complication of injection drug use (IDU), infective endocarditis, has increased in magnitude by as much as 12-fold from 2010 to 2015 [2].
Infectious disease (ID) physicians are in a unique position to diagnose comorbid OUD and deploy effective medications to treat OUD ([MOUD] eg, methadone, buprenorphine, extended-release naltrexone [XR-NTX]) for the persons with infections related to IDU that they see on a regular basis. Unfortunately, this is more often than not a missed opportunity; however, it is not due to a lack of efficacy of MOUD, which has demonstrated improved retention in treatment and a mortality benefit through a reduction in illicit opioid use and overdoses [3, 4]. Furthermore, MOUD has been found to reduce IDU risk behaviors, acquisition of hepatitis C virus (HCV), and human immunodeficiency virus (HIV), improve adherence to treatment for both viral infections, and maintain HIV viral suppression [5, 6].
The value of MOUD as an essential instrument in the ID toolkit is gaining recognition [7]. The Addiction Medicine specialty has expanded as an adjuvant to ID consultation to include MOUD treatment, which has shown impact across multiple ID outcomes [8]. However, there is a shortage of MOUD prescribers—as of 2012, there were 1 million patients without available providers for methadone or buprenorphine treatment, and this deficit has likely worsened [9]. Infectious disease physicians have the unique role of being the first and, at times, the last “touch point” for patients with substance use disorders (SUDs), especially for those with OUD and serious infections. There is a growing awareness of the need for integrated ID and addiction services [10]. Infectious disease physicians are one of many provider groups that can and should (1) screen for OUD, (2) initiate MOUD, (3) manage acute pain and opioid-related conditions, and (4) link and integrate ID and OUD care after hospital discharge. The following is a practical and stepwise approach for ID physicians to integrate ID and OUD treatment in the hospitalized patient to reduce morbidity and mortality from the opioid epidemic.
SCREENING FOR OPIOID USE DISORDER
Substance use disorders, including OUDs, are common in the inpatient hospitalized setting. Universal screening for OUD is recommended for all patients by the National Institute of Drug Abuse (NIDA) and the US Preventive Services Task Force; this can be quickly integrated into a standard ID consult on initial evaluation [11].
The NIDA Quick Screen (Table 1) is a single-question evaluation for substance use in the past year including nonopioid substances (alcohol, tobacco, other nonmedical prescribed or illicit drug use) [12]. An affirmative answer reflexes to a more detailed assessment of severity for all listed substances called the NIDA-modified Alcohol, Smoking and Substance Involvement Screening Test (NM-ASSIST) [12]. In particular, the NM-ASSIST can identify stimulant use that is increasingly comorbid with illicit opioid use [13]. The Rapid Opioid Dependence Screen ([RODS] created by author S.S., see Table 2) is an 8-question, brief assessment tool that is used for both screening and diagnosis of OUD and is validated to the Diagnostic Statistical Manual, Fourth Edition, for OUD diagnosis [14].
Table 1.
Screening Tools for Opioid Use Disorder
| NIDA Quick Screen (OUD) |
|---|
| In the past year, how often have you used the following? |
| Prescription drugs for non-medical reasons: |
| □ Once or twice □ monthly □ weekly □ daily or almost daily |
| Illegal drugs: |
| □ Once or twice □ monthly □ weekly □ daily or almost daily |
| Reflex positive to NM ASSIST |
Adapted from The National Institute on Drug Abuse. NIDA Drug Screening Tool, NIDA-Modified ASSIST (NM ASSIST). Available at: https://www.drugabuse.gov/nmassist/. Accessed 18 November 2019.
Table 2.
Rapid Opioid Dependence Screen (RODS)
| 1. Have you ever taken any of the following drugs: | |
| Heroin | □ Yes □ No |
| Methadone | □ Yes □ No |
| Buprenorphine | □ Yes □ No |
| Morphine MS Contin | □ Yes □ No |
| Oxycontin | □ Yes □ No |
| Oxycodone | □ Yes □ No |
| Other opioid analgesics (eg, Vicodin, Darvocet, Fentanyl, etc) | □ Yes □ No |
| If no, skip to “Scoring Instructions” | |
| 2. Did you ever need to use more opioids to get the same high as when you first started using opioids? | □ Yes □ No |
| 3. Did the idea of missing a fix (or dose) ever make you anxious or worried? | □ Yes □ No |
| 4. In the morning, did you ever use opioids to keep from feeing “dope sick” or did you ever feel “dope sick?” | □ Yes □ No |
| 5. Did you ever worry about your use of opioids? | □ Yes □ No |
| 6. Did you ever find it difficult to stop or not use opioids? | □ Yes □ No |
| 7. Did you ever need to spend a lot of time/energy on finding opioids or recover from feeling high? | □ Yes □ No |
| 8. Did you ever miss important things like doctor’s appointments, family/friend activities, or other things because of opioids? | □ Yes □ No |
| Scoring Instructions: Add the number of “yes” responses for Questions 2 to 8. If total answer is ≥3, RODS screen is positive |
Created by author Springer SA. Adapted from Wickersham JA, Azar MM, Cannon CM, Altice FL, Springer SA. Validation of a brief measure of opioid dependence: the Rapid Opioid Dependence Screen (RODS). JCHC. 2015;21:12–26.
Diagnosing Opioid Use Disorder
Establishment of a diagnosis of OUD and a brief assessment of severity is warranted for those that screen positive. Per the Diagnostic and Statistical Manual, Fifth Edition, DSM-5 criteria, an OUD constitutes a problematic pattern of opioid use resulting in clinically significant impairment [15]. The 3 broad criteria categories are (1) loss of control, (2) adverse consequences, including health, legal, etc, and (3) presence of strong cravings or desires. These are known in shorthand as the “three Cs” (craving, consequences, loss of control). Presence of 2 or more criteria is consistent with a diagnosis of OUD, and severity is graded from mild to moderate to severe as determined by total number of criteria. As noted above, the RODS can also diagnose moderate to severe OUD as well [16].
Harm Reduction and Additional Opioid Use Disorder Assessment for the Infectious Disease Provider
After diagnosis of OUD, providers should assess additional drug use-related history and risk practices before initiation of MOUD, which can be woven into an ID consult history and physical examination. The infectious risk during the act of opioid consumption can be broadly grouped into 2 categories: (1) risks related to route of entry and (2) risk from drug preparation/paraphernalia. Understanding the drug preparation and injection process will help to provide harm reduction for infectious and noninfectious comorbidities.
The infectious risk from opioids and other illicit substance use is largely defined by route of drug entry—infections from skin flora during needle puncture, such as Staphylococcus aureus and Streptococcal species, are most commonly encountered [17]. Gram-negative and anaerobic bacterial infections occur when persons utilize femoral or lower extremity veins for injection. Very high-risk injection into arteries can lead to aneurysmal infections. Injecting into subcutaneous or muscle tissue, known as skin-popping or muscle-popping, respectively, predisposes to abscess formation or growth of spore-forming bacteria such as Clostridial and Bacillus species. These can cause a wide variety of clinical manifestations such as necrotizing soft tissue infections (Clostridium novyi), tetanus (Clostridium tetani), wound botulism (Clostridium botulinum), and anthrax (Bacillus anthracis) [18].
The drug preparation process for injecting opioids such as heroin and fentanyl involves a series of steps with which ID providers should be familiar. In general, these are (1) dissolving powdered drug with water in a cooker, (2) heating the cooker to complete the solubilization process, (3) drawing up the drug into a needle with a syringe, with or without a filter to strain out impurities, and (4) evacuation of air bubbles from the syringe. At times, a mild acid agent is added to assist with the solubility process. Ideally, all of these steps are performed with sterile equipment, but in reality each step provides opportunity for microbial contamination. Nonsterile water can introduce environmental bacteria, particularly if sourced from overtly contaminated sources. Acidification of drug with citrus juices increases the risk for Candidal species [19]. Licking of the needle to recover residual heroin after evacuation of air bubbles poses the clear risk of oral flora exposure. Drug paraphernalia are often shared or reused, although the possibility of contamination with body fluids depends on the mechanism of sharing. During the injection process, blood is drawn via the needle tip into the syringe to confirm entry into the vessel before injection of drug, resulting in potential passage of HIV, hepatitis B virus, and HCV throughout the drug and drug works.
There are a variety of real-world practices that people who inject drugs use in attempts to reduce harm. We recommend decontamination with undiluted household bleach, retained for at least 2 minutes [20]. Patients should be counseled on alcohol swabbing and general hygiene practices before injection, which likely reduce skin and soft tissue infections [21]. All drugs should be separated and individually injected with new syringes if it is to be divided amongst users. Local availability of other harm reduction strategies such as needle and syringe exchange programs and overdose education and naloxone distribution will guide subsequent counseling. Risk of sexual behaviors such as exchanging sex for drugs and/or money are more common in substance using populations, and thus barrier protection use should be recommended, and pre-exposure prophylaxis should be offered to those who test negative for HIV.
Gathering information on a patient’s substance use history is informative in anticipation of initiating MOUD treatment. A brief history will include other forms of substance use such as alcohol and stimulant use, past use of MOUD, response, and information on relapse. History of overdose, hospitalization, and rehabilitation is also relevant to the overall treatment plan. Table 3 contains language that can be used for open-ended and nonjudgmental communication with patients about substance use.
Table 3.
Question Stems for Taking a History of Substance Use
| Assure the patient of confidentiality and a non-punitive environment | ||
|---|---|---|
| “I’d like to ask some questions about your drug use history that we ask all patients- this is completely confidential and this is to help me provide better treatment for you” | ||
| Aim for patient-centeredness in management decisions | ||
| “We have great medication treatments for opioid use disorder these days, and there are also lots of ways to keep yourself as safe as possible if you continue to use” | ||
| Ask about the source and potential sharing of all components of drug paraphernalia (“drug works”) for Infectious Disease related harm reduction | ||
| “Can you take me through the process of how you normally inject your drugs?” | ||
| Guide the open ended evaluation through the various components of the patient’s drug works | ||
| ○ water source | ○ acid agent (if used) | ○ needles |
| ○ filters | ○ syringes |
INITIATING MEDICATION TREATMENT FOR OPIOID USE DISORDER
After making a diagnosis of OUD, providers should discuss initiation of MOUD with the patient. Initiation of MOUD in the hospital setting prevents opioid withdrawal to relieve the discomfort patients experience (eg, pain, anxiety, diaphoresis, diarrhea, nausea/vomiting) and promote retention in medical care. This will ideally be followed by sustained treatment of OUD when patients are discharged into the community.
Forms of medications to treat opioid use disorder
There are 3 forms of US Food and Drug Administration (FDA)-approved MOUD that can be chosen, as discussed in Table 4. Although agonist-based treatment (buprenorphine, methadone) is recommended for alleviating acute opioid withdrawal symptoms, there is a preponderance of evidence for both agonist and nonagonist (XR-NTX) based therapy for maintenance treatment of OUD. There is no consensus guideline on the preferred form of MOUD for maintenance treatment of OUD.
Table 4.
FDA-Approved Medications for Opioid Use Disorder
| Medications | Mechanism of Action | Adverse Effects | Formulations | Relevant ART Interactions in Persons With HIV | Other Relevant Interactions |
|---|---|---|---|---|---|
| Buprenorphine | Partial μ-receptor agonist | Opioid agonist effects (nausea, constipation), potential precipitated withdrawal | (1) Daily SL (tablet or buccal film), can be coformulated with naloxone or alone (2) Every 6 months subdermal implant (Probuphine), (3) Monthly subcutaneous injection (Sublocade) | Atazanavir: ↑ buprenorphine levels | Metabolized by CYP3A4 (caution with rifamycins, macrolides, azoles, etc) |
| Methadone | Full μ-receptor agonist | Opioid agonist effects (nausea, constipation), tolerance usually develops | Given PO daily (tablet or liquid form) | ABC:↓methadone levels AZT: ↑ AZT levels EFV:↓methadone levels RPV: ↓methadone levels DRV/r: ↓methadone levels | QTc prolongation (caution with macrolides, FQLs) Extensively metabolized by CYP450 system (caution with rifamycins, macrolides, azoles, etc) |
| Extended-release naltrexone | Full μ-receptor antagonist | Injection site reactions, potential precipitated withdrawal | Intramuscular every 28 days | n/a | n/a |
Abbreviations: ABC, abacavir; ART, antiretroviral therapy; AZT, zidovudine; CYP450, cytochrome P450; DRV/r, darunavir/ritonavir; EFV, efavirenz; FDA, US Food and Drug Administration; FQL, fluoroquinolones; HIV, human immunodeficiency virus; n/a, not applicable; PO, oral; RPV, rilpivirine; SL, sublingual.
The choice between MOUD therapies is based on a variety of factors including comorbidities, availability, site of treatment, medication profile, and patient preference. However, buprenorphine is considered the most efficacious and convenient form of MOUD because it has the advantages of both strong evidence for mortality reduction and ease of prescription for providers who obtain a Drug Enforcement Administration (DEA) exemption waiver.
Buprenorphine
Buprenorphine is a semisynthetic opioid with partial agonist effect at the mu-opioid receptor. The partial agonist properties confer a “ceiling effect” on sedative symptoms at higher doses, reducing the risk for respiratory depression. Because it exhibits high mu receptor affinity, buprenorphine can displace other mu agonists and precipitate withdrawal if dosed in conjunction with other opioids. Naloxone is often added for coformulation with the intent to dissuade from intravenous illicit use; it is negligibly absorbed via sublingual or oral form and hence plays no additional role for prescribed use.
Buprenorphine has few true interactions or barriers to initiation. Overdose is rare; co-occurring stimulant use disorder and benzodiazepine use are not absolute contraindications for prescribing. Buprenorphine is metabolized by the CYP3A4 mechanism, and, although there have been some reported interactions with protease inhibitors as referenced in Table 4, “there are no clinically significant interactions with US Department of Health and Human Service (DHHS) guideline recommended first line antiretroviral therapies including integrase inhibitor based regimens” [22]. In general, patients should be in at least mild to moderate opioid withdrawal before initiation to avoid precipitated withdrawal if actively using. Those persons with a history of OUD who are in a supervised setting such as a prolonged hospitalization or incarceration may not be actively using and thus might be opioid naive. Although they lack withdrawal symptoms, they will require induction on MOUD before discharge to prevent relapse upon release. Patients should be counseled against swallowing the sublingual or buccal forms of buprenorphine and to let them dissolve fully to be absorbed.
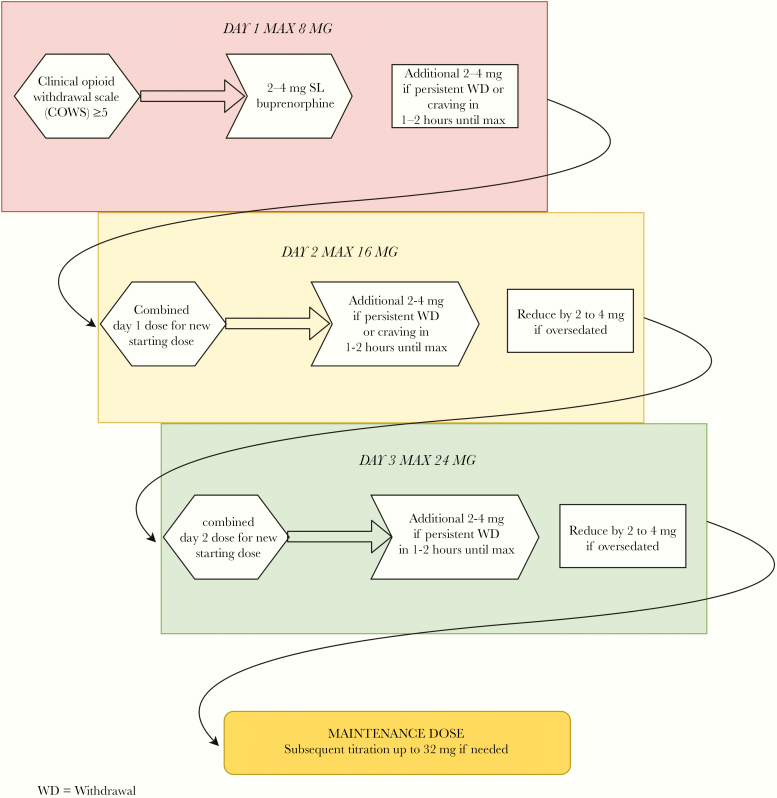
A preceding abstinence period, dependent on the half-life of the opioid in question, is needed before starting buprenorphine to avoid precipitated withdrawal. The Clinical Opioid Withdrawal Scale ([COWS] see Table 5 Appendix) allows for quantification of withdrawal symptoms in the clinical setting. For those with moderate to severe OUD, symptoms consistent with “at least” mild withdrawal (COWS ≥5) are sufficient for initiation of buprenorphine. Figure 1 describes the stepwise process for buprenorphine induction as described in SAMHSA TIP 63 [23]. In general, 2 to 4 mg of transmucosal buprenorphine is initially administered with additional dosing of up to 8 mg on the first day, based on COWS scoring and satiety of craving. On day 2, the cumulative dose from the day prior is given to start, and additional dosing of up to 16 mg is allowed, once again titrated to reduce withdrawal and craving response. This process is repeated on day 3—the majority of patients should ultimately stabilize in the 8- to 16-mg dose range; however, doses up to 32 mg of transmucosal buprenorphine may be used. Although alpha-2 adrenergic agonists (ie, clonidine, lofexidine) are superior to placebo in treating opioid withdrawal, they are inferior to agonist therapy and should only be used for adjunctive support during the induction period [24].
Figure 1.
Flow diagram for sublingual buprenorphine induction in persons with active opioid addiction.
Two additional formulations of buprenorphine, Probuphine and Sublocade, offer the benefit of extended-release dosing for maintenance treatment of OUD. Probuphine is a subdermally implantable rod that delivers medication at a steady state over 6 months. Sublocade is a subcutaneous depot formulation of buprenorphine administered monthly with 2 dose options, 100 mg and 300 mg. It is recommended that patients be on a stable sublingual dose of at least 8 mg of buprenorphine for 7 days before initiation for both medications.
In the United States, the Drug Addiction Treatment Act (DATA) of 2000 was created as an exception to the Controlled Substances Act to permit buprenorphine to be prescribed outside opioid treatment programs (OTPs) if specific training is completed and an exemption waiver is obtained. “Federal law, however, states that inpatient providers are exempt from waiver requirements for maintenance or withdrawal treatment if the patient is admitted for reasons that are not directly related to withdrawal” [25]. Therefore, ID physicians are allowed by law to prescribe buprenorphine in this scenario, including the ability to prescribe up to 3 days of medication on discharge, to be dispensed by one’s institutional emergency department or clinic as well [23]. Outpatient prescribing does require a DEA-X waiver for buprenorphine—this is obtained via an 8-hour physician training (24 hours for nurse practitioners/physician assistants) such as the training offered in conjunction with IDweek. Information about local sessions or free internet-based waiver training can be found at https://pcssnow.org/medication-assisted-treatment.
Methadone
Methadone is a full mu-opioid agonist that has been in clinical use for both withdrawal and maintenance treatment of OUD since the 1960s in the United States. It is dosed once a day but has a uniquely long half-life resulting in accumulation of dose effects, requiring slow titration. Hence, patients with OUD will often not feel the full agonist effects until approximately day 4 or more of induction. An initial dose of 10 to 30 mg is recommended, with the option to give additional doses up to 40 mg on first day based on need [23]. For those that are opioid abstinent (ie, incarcerated, legally mandated abstinence), a starting dose of 5 to 10 mg is adequate with slow titration afterwards (5 mg per week). It can be initiated in the hospital for withdrawal management for up to 72 hours and continued for maintenance treatment by inpatient addiction specialists, but it is limited in that it can only be dispensed in the outpatient setting by federally designated OTPs. Medication interactions should be evaluated before initiation given both its extensive metabolism by cytochrome P450 and its QTc prolonging effects (see Table 4).
Extended-Release Naltrexone
Naltrexone is a competitive mu-opioid antagonist that blocks the euphoric effects of opioids. Antagonist therapy with naltrexone was first approved in 1984 as an oral formulation but was not found to be effective in reducing opioid use secondary to poor adherence [26]. The long-acting injectable formulation, XR-NTX, was FDA approved in 2010 in the United States for treatment of OUD and is associated with reduction in relapse to illicit opioid use, overdose, and improved treatment retention [27, 28]. In addition, XR-NTX improves HIV viral suppression in persons with HIV (PWH) released from prison or jail with comorbid OUD [29]. Extended-release naltrexone is an intramuscular injection given as a fixed dose of 380 mg administered every 4 weeks and does not require a regulatory waiver to prescribe. Due to its opioid antagonistic properties, 7 days of opioid abstinence is required before XR-NTX initiation to prevent precipitated withdrawal. Thus, this is not an appropriate medication to alleviate opioid withdrawal symptoms, but it is a good candidate for maintenance treatment if a patient is interested in a nonagonist-based therapy and does not have any underlying pain conditions requiring an opioid-based treatment.
MANAGING ACUTE PAIN AND OPIOID-RELATED CONDITIONS
Those with OUD face significant barriers to healthcare including criminalization of their substance use, stigma, and health disparities due to socioeconomic or rural status [30]. Yet, many of the issues that arise during a hospitalization are treatable, including the patient’s underlying OUD, complex pain symptoms, cravings, and withdrawal. Undertreatment of these conditions can lead to illicit inpatient opioid use and elopement. There is a growing body of literature that patient-centered care and MOUD can improve overall care including reduction in unplanned discharges and readmissions [31].
Opioid use disorder is independently associated with conditions such as trauma and pre-existing chronic pain [32]. In addition, chronic opioid use predisposes to opioid tolerance and sensitization of pain receptors leading to hyperalgesia. When a patient with OUD is hospitalized, these factors are compounded by acute infection-related pain. Uncontrolled pain is a trigger for relapse when patients attempt to self-medicate [33]. Hence, acute pain should be managed aggressively, targeting both opioid and nonopioid pain pathways. Agonist MOUD (ie, buprenorphine) provides potent analgesia that is not as long lasting as its effects on drug craving. Thus, dividing dosing to 3 times a day can improve effects. When possible, providers should add nonsteroidal anti-inflammatory drugs and acetaminophen. Consultation with anesthesia or pain management can be helpful. Providers should NOT withhold additional opioid pain medications if the patient has been stabilized on MOUD and it is considered to be medically warranted.
The prevalence of unplanned discharge also known as discharge “against medical advice” (AMA) is understandably higher in substance using populations compared with the general population. The term AMA itself carries stigma and belies the underlying chronic relapsing brain pathology of addiction; other more patient-centered terms such as “incomplete discharge” have been suggested. As many as 30% of hospitalizations in patients with OUD lead to incomplete discharge [34]. Because incomplete discharges are unplanned, they interrupt services, curtail appropriate infection treatment, and can preclude adequate linkage to care for MOUD treatment after discharge. A Canadian study showed a 3-fold elevation in 1-year mortality risk after incomplete discharge [31]. However, the powerful effects of MOUD to treat craving and withdrawal symptoms can improve inpatient retention. In-hospital methadone use has been associated with reduction in incomplete discharge amongst PWH and a history of IDU [35]. A retrospective evaluation found that addiction medicine consultation decreased incomplete discharge and increased antimicrobial treatment completion—this was likely mainly powered by group differences in MOUD receipt, which was 86% versus 17% in the group without addiction consultation [8].
Specialty services and novel models of care are emerging to better integrate OUD and ID treatment. The addiction medicine service is an invaluable resource and can be used as comanagement with ID providers. Multidisciplinary models incorporate a range of services throughout the duration of the hospitalization such as “Patient Safety Care Plans” for patients with possibility of inpatient illicit use and “PICC Community Safety Assessments” [36].
LINKING AND INTEGRATING INFECTIOUS DISEASE AND OPIOID USE DISORDER TREATMENT AFTER HOSPITAL DISCHARGE
Persons with OUD and related infections are best served by an effective transition to outpatient care to improve outcomes for both their addiction and infection. Infectious disease providers often manage outpatient parenteral antibiotic therapy (OPAT) in the vulnerable posthospitalization period when patients might fail to link effectively and thus can also provide continued MOUD. The peripherally inserted central catheter is a mainstay of long-term antimicrobial treatment, but previous guideline statements have argued against its use in persons who inject drugs [37]. However, data from the past decade has shown successful implementation of OPAT programs for those with SUDs [38]. Readmissions do appear to be higher, ranging from 20% to 41% in people who use drugs compared with 3% to 12% in the general population [39]. These data are derived from nonrandomized studies and, therefore, confounded by issues of OPAT patient selection. One internally validated risk assessment tool identified low-risk persons with IDU history using a 9-point questionnaire and allowed them to receive OPAT; all others received prolonged hospital care. This tool significantly reduced length of stay and costs [40].
Continued addiction management by ID providers in the outpatient setting can take the form of chronic management akin to an HIV primary care model or bridge-prescribing until linkage to durable community addiction services are facilitated. The latter is an underutilized opportunity given the postacute care that is delivered by ID providers for addiction-related infections that is outside the scope of inpatient hospitalist care. Substance use treatment can be billed for via multiple mechanisms, including elevation of level of service and procedural reimbursement of clinic-administered medications such as XR-NTX or injectable formulation of buprenorphine.
CONCLUSIONS
Ultimately, if the opioid epidemic is to be overcome, every medical specialty will need to find its role in mitigating its effects. Infectious disease has always been an exceptionally dynamic specialty that has recognized the social determinants or associated conditions in our field and evolved to address them. Some might see the integration of addiction management into the ID consult as an expansion of an already burdened workload. Although this adoption of change might not be for all, comprehensive and integrated services add value to our professional skillset and, certainly, to the lives of patients. For other ID providers, especially those practicing in areas on the frontlines of the opioid epidemic with no adjunctive specialty services for assistance, they will find that having the tools to treat infection-related addiction will be a vital, sorely needed compendium.
Acknowledgments
Disclaimer. The funders had no involvement in the development of this manuscript nor the recommendations as provided within the manuscript.
Financial support. Funding for career development was received from the National Institutes on Drug Abuse (K02 DA032322: to S. A. S.).
Potential conflicts of interest. E. E. reports grants from the Agency of Health Research and Quality, the Center for AIDS Research, the Gilead HIV Research Scholarship, the Omenn Fellowship in Public Health and Policy, and Viiv. S. A. S. reports grants from National Institute of Health. Veterans Affairs and consulting from Alkermes Inc and Gilead. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
APPENDIX
Table 5. Clinical Opiate Withdrawal Scale (COWS)
| Resting Pulse Rate (Beats per minute) ◯ 0 = pulse rate <80 ◯ 1 = pulse rate 81–100 ◯ 2 = pulse rate 101–120 ◯ 4 = pulse rate greater than 120 | GI Upset (in past ½ hour) ◯ 0 = no GI symptoms ◯ 1 = stomach cramping ◯ 2 = nausea/loose stools ◯ 3 = vomiting/diarrhea ◯ 5 = multiple episodes of diarrhea or vomiting |
| Sweating (in past ½ hour) ◯ 0 = No report of chills or flushing ◯ 1 = Subjective report of chills or flushing ◯ 2 = Flushed or observable moistness on face ◯ 3 = Beads of sweat on brow or face ◯ 4 = Sweat streaming off face | Tremor ◯ 0 = no tremor ◯ 1 = tremor can be felt, but not observed ◯ 2 = slight tremor observable ◯ 4 = gross tremor/muscle twitching |
| Restlessness ◯ 0 = able to sit still ◯ 1 = subjective difficulty sitting still but able to do so ◯ 3 = frequent shifting/movement of hands/arms ◯ 5 = unable to sit still for more than a few seconds | Yawning ◯ 0 = no yawning ◯ 1 = yawning once or twice during assessment ◯ 2 = yawning 3 or more times during assessment ◯ 4 = yawning several times a minute |
| Pupil Size ◯ 0 = pupils pinned or normal size for room light ◯ 1 = pupils possibly larger than normal for room light ◯ 2 = pupils moderately dilated ◯ 5 = pupils dilated, only rim of iris visible | Irritability/Anxiety ◯ 0 = none ◯ 1 = subjective increased irritability/anxiousness ◯ 2 = patient obviously irritable/anxious ◯ 4 = irritability/anxiousness makes assessment difficult |
| Muscle/Bone/Joint Aches ◯ 0 = not present ◯ 1 = mild diffuse discomfort ◯ 2 = patient reports severe diffuse aching of joints/muscles ◯ 4 = patient rubbing joints/muscles and unable to sit still due to discomfort | Piloerection ◯ 0 = skin is smooth ◯ 3 = piloerection of skin can be felt, arm hair standing up ◯ 5 = prominent piloerection |
| Rhinorrhea/Lacrimation ◯ 0 = not present ◯ 1 = nasal stuffiness/unusually moist eyes ◯ 2 = nose running or tearing ◯ 4 = nose constantly running or tears streaming down cheeks | Total: Score: 5–12 = mild; 13–24 = moderate; 25–36 = moderately severe; greater than 36 = severe |
Adapted from Wesson DR and Ling W. The Clinical Opiate Withdrawal Scale (COWS). J Psychoactive Drugs. 2003;35:253–9.
References
- 1. Ronan MV, Herzig SJ. Hospitalizations related to opioid abuse/dependence and associated serious infections increased sharply, 2002-12. Health Aff (Millwood) 2016; 35:832–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fleischauer AT, Ruhl L, Rhea S, Barnes E. Hospitalizations for endocarditis and associated health care costs among persons with diagnosed drug dependence - North Carolina, 2010-2015. MMWR Morb Mortal Wkly Rep 2017; 66:569–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sordo L, Barrio G, Bravo MJ, et al. . Mortality risk during and after opioid substitution treatment: systematic review and meta-analysis of cohort studies. BMJ 2017; 357:j1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mattick RP, Breen C, Kimber J, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev 2014; Cd002207. [DOI] [PubMed] [Google Scholar]
- 5. Altice FL, Bruce RD, Lucas GM, et al. . HIV treatment outcomes among HIV-infected, opioid-dependent patients receiving buprenorphine/naloxone treatment within HIV clinical care settings: results from a multisite study. J Acquir Immune Defic Syndr 2011; 56Suppl 1:S22–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Platt L, Minozzi S, Reed J, et al. . Needle and syringe programmes and opioid substitution therapy for preventing HCV transmission among people who inject drugs: findings from a Cochrane Review and meta-analysis. Addiction 2018; 113:545–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Westlake AA, Eisenberg MP. Infectious disease (ID) learning unit: what the ID clinician needs to know about buprenorphine treatment for opioid use disorder. Open Forum Infect Dis 2017; 4:ofw251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Marks LR, Munigala S, Warren DK, et al. . Addiction medicine consultations reduce readmission rates for patients with serious infections from opioid use disorder. Clin Infect Dis 2019; 68:1935–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jones CM, Campopiano M, Baldwin G, McCance-Katz E. National and state treatment need and capacity for opioid agonist medication-assisted treatment. Am J Public Health 2015; 105:e55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Serota DP, Barocas JA, Springer SA. Infectious complications of addiction: a call for a new subspecialty within infectious diseases. Clin Infect Dis 2019. doi:10.1093/cid/ciz804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Force USPST. Draft Recommendation Statement: Illicit Drug Use, Including Nonmedical Use of Prescription Drugs: Screening. US Preventive Services Task Force; 2019. https://www.uspreventiveservicestaskforce.org/Page/Document/draft-recommendation-statement/drug-use-in-adolescents-and-adults- including-pregnant-women-screening [Google Scholar]
- 12. NIDA. Resource Guide: Screening for Drug Use in General Medical Settings. National Institute on Drug Abuse website; 2012. https://www.drugabuse.gov/publications/resource-guide-screening-drug-use-in-general-medical-settings. Accessed 24 November 2019. [Google Scholar]
- 13. Kariisa M, Scholl L, Wilson N, et al. . Drug overdose deaths involving cocaine and psychostimulants with abuse potential - United States, 2003-2017. MMWR Morb Mortal Wkly Rep 2019; 68:388–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wickersham JA, Azar MM, Cannon CM, et al. . Validation of a brief measure of opioid dependence: the rapid opioid dependence screen (RODS). J Correct Health Care 2015; 21:12–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.American Psychiatric Association. Diagnostic and Statistical Manual for Mental Disorders. 5th Edition. Washington, DC: American Psychiatric Association; 2013. [Google Scholar]
- 16. Babu KM, Brent J, Juurlink DN. Prevention of opioid overdose. N Engl J Med 2019; 380:2246–55. [DOI] [PubMed] [Google Scholar]
- 17. Gordon RJ, Lowy FD. Bacterial infections in drug users. N Engl J Med 2005; 353:1945–54. [DOI] [PubMed] [Google Scholar]
- 18. Palmateer NE, Hope VD, Roy K, et al. . Infections with spore-forming bacteria in persons who inject drugs, 2000-2009. Emerg Infect Dis 2013; 19:29–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Scheidegger C, Pietrzak J, Frei R. Methadone diluted with contaminated orange juice or raspberry syrup as a potential source of disseminated candidiasis in drug abusers. Eur J Clin Microbiol Infect Dis 1993; 12:229–31. [DOI] [PubMed] [Google Scholar]
- 20. Binka M, Paintsil E, Patel A, et al. . Disinfection of syringes contaminated with hepatitis C virus by rinsing with household products. Open Forum Infect Dis 2015; 2:ofv017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vlahov D, Sullivan M, Astemborski J, Nelson KE. Bacterial infections and skin cleaning prior to injection among intravenous drug users. Public Health Rep 1992; 107:595–8. [PMC free article] [PubMed] [Google Scholar]
- 22. What to Start: Initial Combination Regimens for the Antiretroviral-Naive Patient Available at: https://aidsinfo.nih.gov/guidelines/html/1/adult-and-adolescent-arv/11/what-to-start. Accessed 21 November 2019.
- 23.Substance Abuse and Mental Health Services Administration. Medications for Opioid Use Disorder. Treatment Improvement Protocol (TIP) Series 63, Full Document. HHS Publication No. (SMA) 18-5063 FULLDOC. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2018. [Google Scholar]
- 24. Meader N. A comparison of methadone, buprenorphine and alpha(2) adrenergic agonists for opioid detoxification: a mixed treatment comparison meta-analysis. Drug Alcohol Depend 2010; 108:110–4. [DOI] [PubMed] [Google Scholar]
- 25. Title 21 Code of Federal Regulations. §1306.07 Administering or dispensing of narcotic drugs. US Drug Enforcement Administration Office of Diversion Control; https://www.deadiversion.usdoj.gov/21cfr/cfr/1306/1306_07.htm. Accessed 26 November 2019. [Google Scholar]
- 26. Minozzi S, Amato L, Vecchi S, Davoli M, Kirchmayer U, Verster A. Oral naltrexone maintenance treatment for opioid dependence. Cochrane Database Syst Rev 2011; Cd001333. [DOI] [PubMed] [Google Scholar]
- 27. Tanum L, Solli KK, Latif ZE, et al. . Effectiveness of injectable extended-release naltrexone vs daily buprenorphine-naloxone for opioid dependence: a randomized clinical noninferiority trial. JAMA Psychiatry 2017; 74:1197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Krupitsky E, Nunes EV, Ling W, et al. . Injectable extended-release naltrexone for opioid dependence: a double-blind, placebo-controlled, multicentre randomised trial. Lancet 2011; 377:1506–13. [DOI] [PubMed] [Google Scholar]
- 29. Springer SA, Di Paola A, Azar MM, et al. . Extended-release naltrexone improves viral suppression among incarcerated persons living with HIV with opioid use disorders transitioning to the community: results of a double-blind, placebo-controlled randomized trial. J Acquir Immune Defic Syndr 2018; 78:43–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bearnot B, Mitton JA, Hayden M, Park ER. Experiences of care among individuals with opioid use disorder-associated endocarditis and their healthcare providers: Results from a qualitative study. J Subst Abuse Treat 2019; 102:16–22. [DOI] [PubMed] [Google Scholar]
- 31. Choi M, Kim H, Qian H, Palepu A. Readmission rates of patients discharged against medical advice: a matched cohort study. PLoS One 2011; 6:e24459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Seal KH, Shi Y, Cohen G, et al. . Association of mental health disorders with prescription opioids and high-risk opioid use in US veterans of Iraq and Afghanistan. JAMA 2012; 307:940–7. [DOI] [PubMed] [Google Scholar]
- 33. Voon P, Greer AM, Amlani A, et al. . Pain as a risk factor for substance use: a qualitative study of people who use drugs in British Columbia, Canada. Harm Reduct J 2018; 15:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ti L, Ti L. Leaving the hospital against medical advice among people who use illicit drugs: a systematic review. Am J Public Health 2015; 105:e53–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chan AC, Palepu A, Guh DP, et al. . HIV-positive injection drug users who leave the hospital against medical advice: the mitigating role of methadone and social support. J Acquir Immune Defic Syndr 2004; 35:56–9. [DOI] [PubMed] [Google Scholar]
- 36. Englander H, Mahoney S, Brandt K, et al. . Tools to support hospital-based addiction care: core components, values, and activities of the improving addiction care team. J Addict Med 2019; 13:85–9. [DOI] [PubMed] [Google Scholar]
- 37. Tice AD, Rehm SJ, Dalovisio JR, et al. ; IDSA Practice guidelines for outpatient parenteral antimicrobial therapy. IDSA guidelines. Clin Infect Dis 2004; 38:1651–72. [DOI] [PubMed] [Google Scholar]
- 38. Suzuki J, Johnson J, Montgomery M, et al. . Outpatient parenteral antimicrobial therapy among people who inject drugs: a review of the literature. Open Forum Infect Dis 2018; 5:ofy194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. MacKenzie M, Rae N, Nathwani D. Outcomes from global adult outpatient parenteral antimicrobial therapy programmes: a review of the last decade. Int J Antimicrob Agents 2014; 43:7–16. [DOI] [PubMed] [Google Scholar]
- 40. Eaton EF, Mathews RE, Lane PS, et al. . A 9-point risk assessment for patients who inject drugs and require intravenous antibiotics: focusing inpatient resources on patients at greatest risk of ongoing drug use. Clin Infect Dis 2019; 68:1041–3. [DOI] [PubMed] [Google Scholar]