Abstract
Superior vena cava (SVC) and inferior vena cava obstructions were once well-documented complications from the Mustard repair for D-transposition of the great arteries (TGA), occurring in 10%–40% patients; however, they are rarely documented with the current, more common arterial switch operation (ASO). Similarly, SVC thrombosis is an uncommon, severe complication following neonatal cardiac surgery. We report a case of persistent SVC thrombosis, SVC syndrome, and chylothorax arising after ASO, refractory to thrombolysis and stent placement. A 6-day-old neonate with prenatally known TGA underwent an arterial switch procedure. Despite an initially unremarkable postoperative course, he developed respiratory difficulty after starting enteral feeding. Soft-tissue swelling was noted in the neck, chest, and upper face. An SVC thrombus was identified with cardiac catheterization. Multiple thrombolytic modalities were attempted. His postoperative course was further complicated by recurrent chylothoraces, respiratory failure, sepsis, anasarca, and renal failure. He was eventually transferred to a larger center for a special lymphatics evaluation, where two lymphovenous anastomoses were unsuccessful. He was sent to his home hospital, where he died from extended-spectrum beta-lactamase Klebsiella sepsis. Early diagnosis of SVC syndrome and prompt thrombolysis may prevent the complications encountered in this patient. More research is needed in neonatal thrombolysis and anticoagulation.
Keywords: Anticoagulation, arterial switch operation, superior vena cava syndrome, thrombus
INTRODUCTION
D-Transposition of the great arteries (D-TGA) accounts for 5%–7% of all congenital heart defects, with a prevalence of 0.2/1000 live births.[1] The arterial switch operation (ASO) is the standard of care for neonates with TGA. Currently, the early mortality rate is low (<5%).[2] Long-term survival and functional outcomes are excellent, with most surgically treated patients living to adulthood and a 20-year survival of nearly 90%.[1,3] Superior vena cava (SVC) and inferior vena cava obstruction were well-documented complications from the Mustard repair, occurring in 10%–40% patients;[4] however, they are rarely documented after ASO. SVC thrombosis, however, is an uncommon, severe complication following neonatal cardiac surgery. SVC thrombosis can cause chylothorax, hydrocephalus, and respiratory complications with high morbidity and mortality.[5] We report a case of persistent SVC thrombosis, SVC syndrome, and chylothorax arising after ASO, refractory to thrombolysis and stent placement.
CASE REPORT
A male infant was born at 36 weeks through C section. He was a prenatally diagnosed D-TGA with an intact ventricular septum. Postnatal echo confirmed the anatomy, with the presence of adequate interatrial communication, and prostaglandin was initiated. On the day before surgery, an umbilical venous catheter (UVC) was removed, and a peripherally inserted central catheter (PICC) was inserted in his right upper arm, as it is our institutional policy to replace a UVC with a PICC if more long-term central access is anticipated.
On day of life (DOL)#6, the patient underwent ASO. His coronary pattern was normal. No thrombi were identified [Figure 1]. During the operation, the SVC was found to be a small vessel; so, after a small (8 French) SVC cannula was removed, the site was repaired carefully with multiple interrupted sutures of prolene to prevent further narrowing. Bicaval cannulation was thought to be safer than single right atrial cannulation, due to the following reasons: with a single venous cannula in right atrium, a period of circulatory arrest is needed to close the atrial septal defect, as it will prevent opening the atria under continuous bypass. Furthermore, it would complicate the use of retrograde cardioplegia, which is an important adjunct to achieve myocardial protection during the arterial switch. His immediate postoperative course was unremarkable, and by postoperative day (POD)#4, he was extubated to high flow through the nasal cannula.
Figure 1.
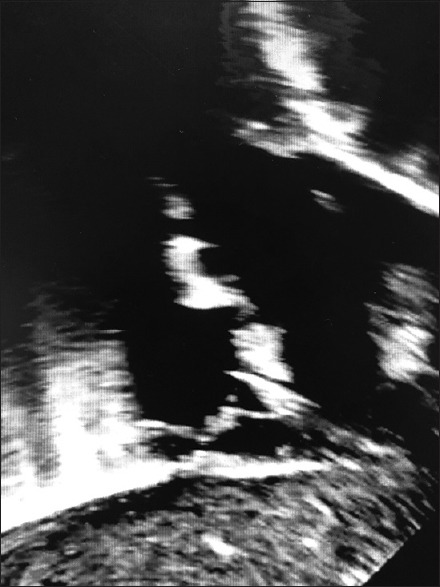
Preoperative subcostal short-axis echocardiogram. The right atrium can be seen on the left of the image with a patent superior vena cava seen superiorly
Despite initially doing well after extubation, he developed respiratory distress when starting feeds on POD#5. Swelling of the soft tissues in the neck, upper chest, and face developed concurrently, suggesting SVC syndrome. Chest computed tomography (CT) angiography demonstrated large, bilateral pleural effusions and a very small caliber SVC, with no obvious occlusion. The placement of bilateral chest tubes yielded chylous fluid. An echocardiogram was suspicious for a thrombus in the SVC–right atrial junction [Figure 2], with no flow by color Doppler, but a small amount of flow through the SVC by bubble study. Enoxaparin was started immediately and then switched to heparin for better monitoring control. Cardiac catheterization showed irregular occlusive clot formation in the SVC and innominate veins. Thrombolysis was attempted by Pronto catheter after angioplasty and ballooning, which improved flow temporarily. He was maintained on a tissue plasminogen activator (TPA) infusion and heparin.
Figure 2.
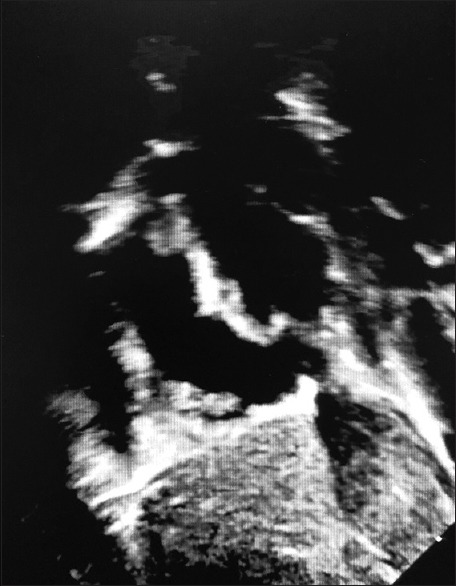
Postoperative subcostal sagittal echocardiogram. The superior vena cava can be seen to the right and superiorly to the atrial septum, and a thrombus can be seen at the superior vena cava– right atrium junction
Since repeat echocardiograms still did not show any spontaneous flow through the SVC, vascular interventional radiology performed clot-directed TPA. He then had a stent placed in the caval–atrial junction and thromboplasty. No flow was noted through the SVC on subsequent echocardiograms, even after multiple treatments of thrombolysis, stent placement, and different anticoagulation regimens such as heparin, bivalirudin, and enoxaparin. No specific thrombophilic disorder was found, but the investigation was limited by anticoagulation.
Chylothorax was a major complication of this patient's SVC thrombosis. Octreotide was not beneficial. Pleurodesis helped temporarily. Enteral feeds with Enfaport were titrated with parental nutrition. He required a peritoneal drain initially for ascites and eventually required peritoneal dialysis for anasarca, oliguria, and renal failure. A tracheostomy was performed for chronic respiratory failure. Multiple product replacements were needed for ongoing losses, including intravenous immunoglobulin (IVIG), albumin, red blood cells, and antithrombin III. Bactrim and fluconazole prophylaxis were continued for lymphopenia. He had recurrent fever and hypotension triggering multiple sepsis investigations, with multiple courses of empiric antibiotics.
Due to his persistent high-volume chylothorax, chronic respiratory failure, and renal insufficiency, he was transferred to a larger center for special lymphatics evaluation, where he had two lymphovenous anastomoses, which were ultimately unsuccessful. He continued to have high-volume chylothoraces, ascites, renal insufficiency, and multiple electrolyte abnormalities requiring constant replacements. He was transferred back to his home hospital and died later from extended-spectrum beta-lactamase Klebsiella sepsis.
DISCUSSION
SVC thrombosis is a rare complication of cardiac surgeries. A retrospective study by Sharoni et al.[6] showed that 9 of 1853 children who underwent any cardiac surgical procedure developed SVC syndrome (an incidence of 0.5%). Interestingly, 5 of the 9 patients were also neonates, suggesting that neonates may have a higher risk of SVC syndrome, likely from an anatomically smaller SVC. The development of SVC thrombosis in this patient's case was likely multifactorial, from endothelial cell lining alterations during cardiopulmonary bypass, a PICC, small caliber SVC, and surgical manipulations of the vessel. He may have had an unknown underlying thrombophilia, which was challenging to diagnose on chronic anticoagulation.
Several techniques for treating SVC thrombosis have been suggested, including thrombolytic therapy with anticoagulation, balloon dilation, stent implantation, thrombectomy, and atriojugular bypass.[7] This patient developed collaterals, so thrombectomy and bypass were not performed. Sharoni et al. suggest that early treatment with thrombolytic agents may prevent the sequelae of SVC obstruction.[6,7] Even though SVC thrombosis was diagnosed early, thrombolytic therapy was delayed with concern for postoperative bleeding risk from suture lines. Further studies are needed in thrombolysis in postoperative cardiac patients and anticoagulation of neonates. Furthermore, chylothoraces complicate anticoagulation because of chronic antithrombin III losses in the chylous fluid. Unfortunately, there is a paucity of guidelines for thrombin inhibitors in pediatrics. Similarly, further studies are needed in children who suffer this rare complication, and a multicenter review of these cases, identifying possible risk factors and treatment modalities, would be beneficial.
It was challenging managing this patient's intravascular volume status in the setting of capillary leak syndrome, fluid overload, and acute kidney injury requiring peritoneal dialysis. His chylothorax also resulted in hypogammaglobulinemia, hypoproteinemia, and lymphopenia requiring frequent IVIG, albumin, and multiple courses of antibiotics for clinical sepsis, which ultimately resulted in death from Klebsiella bacteremia.
Implications to clinical practice
Infants with SVC syndrome from cardiac surgery have prolonged hospitalizations, including a lengthy stay in the intensive care unit, chronic respiratory failure from chylous effusions, difficult intravenous access, and immunocompromise from chronic lymphopenia. Early diagnosis of SVC syndrome, thrombolytic therapy, and possibly thrombectomy may prevent further complications. Direct cannulation of the SVC should be avoided, if possible, if the SVC is noted to be small at the time of surgery.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Fraser CD., Jr The neonatal arterial switch operation: Technical pearls. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2017;20:38–42. doi: 10.1053/j.pcsu.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 2.Stoica S, Carpenter E, Campbell D, Mitchell M, da Cruz E, Ivy D, et al. Morbidity of the arterial switch operation. Ann Thorac Surg. 2012;93:1977–83. doi: 10.1016/j.athoracsur.2011.11.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Villafañe J, Lantin-Hermoso MR, Bhatt AB, Tweddell JS, Geva T, Nathan M, et al. D-transposition of the great arteries: The current era of the arterial switch operation. J Am Coll Cardiol. 2014;64:498–511. doi: 10.1016/j.jacc.2014.06.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arciniegas E, Farooki ZQ, Hakimi M, Perry BL, Green EW. Results of the mustard operation for dextro-transposition of the great arteries. J Thorac Cardiovasc Surg. 1981;81:580–7. [PubMed] [Google Scholar]
- 5.Bauman ME, Moher C, Bruce AK, Kuhle S, Kaur S, Massicotte MP. Chylothorax in children with congenital heart disease: Incidence of thrombosis. Thromb Res. 2013;132:e83–5. doi: 10.1016/j.thromres.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 6.Sharoni E, Erez E, Birk E, Katz J, Dagan O. Superior vena cava syndrome following neonatal cardiac surgery. Pediatr Crit Care Med. 2001;2:40–3. doi: 10.1097/00130478-200101000-00009. [DOI] [PubMed] [Google Scholar]
- 7.Kumar TK, Subramanian S, Sathanandam S, Alexander J, Ali M, Knott-Craig CJ. Superior vena cava reconstruction for treatment of chylothorax resulting from thrombosis of superior vena cava in young infants. Ann Thorac Surg. 2015;100:1432–6. doi: 10.1016/j.athoracsur.2015.06.021. [DOI] [PubMed] [Google Scholar]


