Abstract
Background
Dynamics of infection by Bartonella and Rickettsia species, which are epidemiologically associated in dogs, have not been explored in a controlled setting.
Objectives
Describe an outbreak investigation of occult Bartonella spp. infection among a group of dogs, discovered after experimentally induced Rickettsia rickettsii (Rr) infection.
Animals
Six apparently healthy purpose‐bred Beagles obtained from a commercial vendor.
Methods
Retrospective and prospective study. Dogs were serially tested for Bartonella spp. and Rr using serology, culture, and PCR, over 3 study phases: 3 months before inoculation with Rr (retrospective), 6 weeks after inoculation with Rr (retrospective), and 8 months of follow‐up (prospective).
Results
Before Rr infection, 1 dog was Bartonella henselae (Bh) immunofluorescent antibody assay (IFA) seroreactive and 1 was Rickettsia spp. IFA seroreactive. After inoculation with Rr, all dogs developed mild Rocky Mountain spotted fever compatible with low‐dose Rr infection, seroconverted to Rickettsia spp. within 4‐11 days, and recovered within 1 week. When 1 dog developed ear tip vasculitis with intra‐lesional Bh, an investigation of Bartonella spp. infection was undertaken. All dogs had seroconverted to 1‐3 Bartonella spp. between 7 and 18 days after Rr inoculation. Between 4 and 8 months after Rr inoculation, Bh DNA was amplified from multiple tissues from 2 dogs, and Bartonella vinsonii subsp. berkhoffii (Bvb) DNA was amplified from 4 of 5 dogs' oral swabs.
Conclusions and Clinical Importance
Vector‐borne disease exposure was demonstrated in research dogs from a commercial vendor. Despite limitations, our results support the possibilities of recrudescence of chronic subclinical Bartonella spp. infection after Rr infection and horizontal direct‐contact transmission between dogs.
Keywords: PCR, recrudescence, serology, transmission
Abbreviations
- BAPGM ePCR
Bartonella alpha‐proteobacteria growth medium enrichment PCR
- Bh SA2
Bartonella henselae San Antonio 2 strain
- Bh
Bartonella henselae
- Bk
Bartonella koehlerae
- Bvb I
Bartonella vinsonii subspecies berkhoffii genotype I
- Bvb
Bartonella vinsonii subspecies berkhoffii
- CVBD
canine vector‐borne diseases
- IFA
immunofluorescent antibody assay
- ITS
internal transcribed spacer
- LAR
Laboratory Animal Resources
- NCSU‐VBDDL
North Carolina State University College of Veterinary Medicine Vector Borne Diseases Diagnostic Laboratory
- RMSF
Rocky Mountain spotted fever
- Rr
Rickettsia rickettsii
- SFGR
spotted fever group Rickettsia spp
1. INTRODUCTION
The genus Bartonella consists of over 40 globally distributed species of alpha‐proteobacteria, infecting a wide range of mammalian hosts including dogs.1, 2 Studies on Bartonella exposure in dogs have described an epidemiologic association between spotted fever group Rickettsia spp. (SFGR) and Bartonella species.3, 4, 5, 6, 7 Based on infection of both fleas and ticks with Bartonella spp. and SFGR, it is assumed that the serologic association between these 2 pathogens represents exposure from coinfected vectors or sequential exposure to multiple infected vectors. As the dynamics of Bartonella spp. and SFGR seroreactivity in coexposed dogs have not previously been explored in a controlled setting, it is also possible that infection with Rickettsia rickettsii (Rr) could result in recrudescence of chronic subclinical Bartonella infection.
Vector transmission of different Bartonella species by sand flies, fleas, lice, ticks, and flies is reasonably well documented by laboratory and field studies8, 9, 10—and transmission by a variety of other vectors has been suspected—but defining a single natural vector for Bartonella transmission among dogs has proved difficult.1, 9, 10, 11 Nonvectorial routes of transmission of Bartonella spp. are also proposed. Being scratched by an infected, flea‐infested cat—allowing inoculation of flea feces under the skin—is a well‐known route of transmission for Bartonella henselae (Bh) to humans. Transmission of Bartonella spp. by needle stick and blood transfusion has been reported, demonstrating direct transmission via infected cells, blood, or interstitial fluid in the absence of passage through an arthropod vector.12, 13, 14, 15, 16 There are also reports implicating transmission by bites or suggesting the possibility of viable Bartonella spp. bacteria in the mouth or saliva.17, 18, 19 In Korea, Bh DNA was PCR‐amplified from over 15% of pet canine saliva samples and almost 30% of toenail samples,20 and in the United States 5 of 44 Golden Retrievers sampled had Bartonella spp. DNA on oral swabs.21 Bartonella henselae DNA was found in the saliva of a man with angioedema of the tongue and in his healthy dog,22 and in eastern China Bartonella exposure was associated with dog bites.23 However, the extent to which saliva might be infectious has not been established and direct transmission among dogs has not been reported.
Despite the evidence of nonvectorial routes of transmission, in the absence of concurrent flea infestation, the risk of Bartonella transmission is currently considered minimal.24, 25 However, if transmission can occur directly between dogs—or from dogs to humans in the absence of vectors—this could be of substantial importance. Establishment of an experimental model of Bartonella spp. infection in non‐reservoir hosts has thus far remained elusive,26 so investigation of the potential for direct transmission of Bartonella spp. has been confined to epidemiologic associations and case reports.
The original study objective was to evaluate sequentially timed serological response to low‐dose experimental Rr infection in laboratory‐raised dogs. However, after completion of the Rr study, Bh DNA was detected in ear‐tip vasculitis lesions in 1 dog. Subsequently, Bartonella spp. antibodies were documented in all dogs, either before or after the experimental Rr infection in a vector‐free biocontainment facility. This unexpected circumstance provided an opportunity to investigate both the serologic response to coinfection with these 2 previously associated pathogens, as well as to investigate the potential for reactivation and non‐vectorial transmission of Bartonella species. Therefore, the objective of this study was to describe an outbreak investigation of occult Bartonella spp. infection among a group of laboratory‐reared dogs subsequent to experimentally induced Rr infection.
2. METHODS
2.1. Animals
The animals included in this study were 6 healthy purpose‐bred laboratory‐reared female Beagles age 6‐12 months (to protect their identities, referred to here as Shok, Kat, Tan, Cher, Pam, and Sax). The dogs had received routine preventative care and vaccinations before arrival at the NCSU Laboratory Animal Resources (LAR) facility, including treatment with sulfamethoxazole (30 mg/kg PO daily) and fenbendazole (25 mg/kg PO daily) for 1 week for coccidiosis prior to transport. The dogs were reported by the vendor to be otherwise free from intestinal parasites; Dirofilaria immitis and Brucella canis testing were negative. The vendor's canine housing facility consists of indoor/outdoor concrete‐floor runs. The vendor practices routine pest control for the facility environment, but study dogs were not treated with flea/tick preventatives while housed at the vendor. The study was approved by the NCSU Institutional Animal Care and Use Committee (Protocol #16‐206).
2.2. Study timeline
The dogs were acquired from a commercial vendor and arrived at the NCSU LAR facility on December 19, 2016. The study timeline is divided into 3 phases: pre‐inoculation (PI phase, December 19, 2016‐March 19, 2017), before experimental intervention; Rr monitoring (RM phase, March 20, 2017‐April 28, 2017), the approximately 6‐week period after experimental inoculation with Rr, and extended follow up (EF phase, April 29, 2017‐December 12, 2017) when the remaining dogs were housed in the LAR facility and evaluated as needed and based on test results. All dogs were routinely vaccinated with DAPP and Rabies vaccines on 6 July, 2017. One dog (Tan) was adopted on August 24, 2017. Due to concerns for zoonotic disease transmission, 4 dogs were transferred to other investigators for studies that required euthanasia (October 24, 2017‐11 December, 2017). A gross necropsy was permitted for 1 of these 4 dogs (Shok, 11 December, 2017) but histopathology was not performed. One dog (Kat) remains a resident at NCSU LAR at the time of writing (approximately 2.5 years after arrival).
2.3. Study setting
From the date of arrival on December 19, 2017 through April 28, 2017 (the PI and RM phases), all dogs were housed in individual runs with access restricted to LAR personnel and study investigators. Figure 1 shows a housing schematic. The run enclosures had solid concrete 4‐ft walls. Metal chain‐link fence extended from the wall tops to the ceiling. The front and back of each run was enclosed with chain‐link fence, with front doors opening onto a common corridor run. Dogs were isolated from one another in these separated run enclosures, except during twice daily 5‐ to 10‐minute periods when runs were being cleaned. While runs were cleaned, each dog had access to the common corridor; the fencing between the corridor and each dog's individual run allowed for nose‐to‐nose contact between a dog in the corridor and any other dog.
Figure 1.
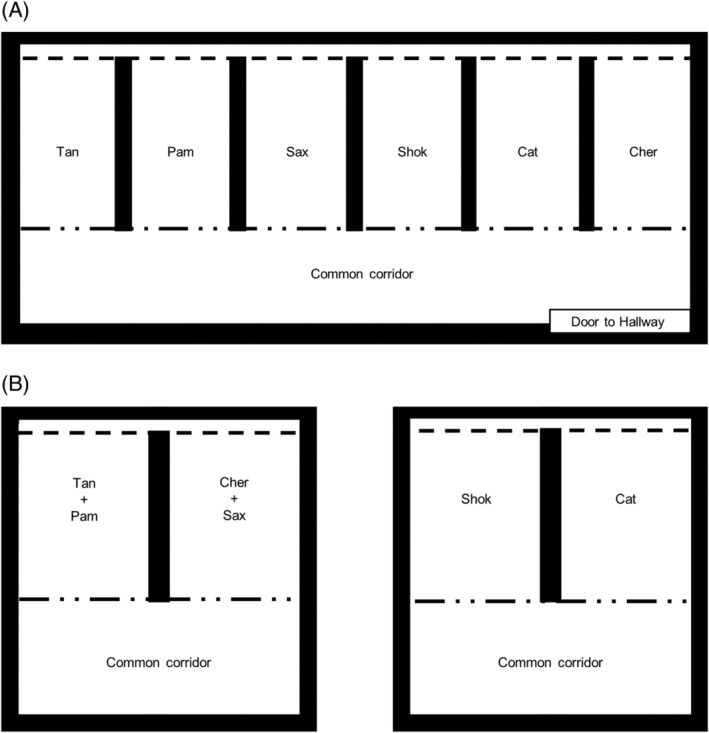
Schematic of dog housing in Laboratory Animal Resources. A, Housing during PI and RM phases. All dogs were housed in individual runs, with chain‐link fence doors (broken dotted line) opening onto a common corridor run in the front, and chain‐link fence (dashed line) in the rear. B, Housing during EF phase. Four dogs were housed as pairs, and 2 dogs were housed singly in the same room. Dashed line indicates chain‐link fence, broken dotted line indicates chain‐link door, thick black line indicates concrete walls and room borders. EF, extended follow up; PI, pre‐inoculation; RM, Rr monitoring
During the EF phase, dogs were moved to various locations within LAR (Figure 1B). Four dogs were housed as pairs (Tan and Pam together, and Cher and Sax together), and 2 dogs (Shok and Kat) were housed singly in the same room. During the EF phase only, all dogs were allowed short periods of outdoor access (5‐20 minutes) as part of their daily enrichment activities in compliance with the IACUC protocol and welfare standards. During this period, dogs were allowed to interact directly during twice daily exercise periods for approximately 5‐20 minutes, both in indoor common areas and an outdoor play area; detailed records of dog‐dog interactions were not available for review from the EF phase, but it is possible that any dog could come into contact with any other dog during this time. During all study phases, dogs were observed at least twice daily by LAR staff. At no time was any arthropod or insect vector found on dogs or within the LAR biocontainment facility. During the RM phase, all personnel wore personal protective equipment including a laboratory coat or disposable coveralls, shoe covers/booties, and gloves.
2.4. Rickettsia rickettsii experimental infection
According to the original objective of the study—to evaluate sequentially timed serological response to low‐dose experimental Rr infection in dogs—each dog was inoculated with 3 × 105 TCID50 (Median Tissue Culture Infectious Dose) of Rr via intradermal injection on March 20, 2017. Inoculum was prepared from frozen stocks of a canine Rr isolate derived from a clinical case of Rocky Mountain spotted fever (RMSF; NCSU‐2008‐CO4, “Murphy” strain).27 The inoculum was prepared in the NCSU College of Veterinary Medicine Biosafety Level III Laboratory and the dose determined from previous experiments.28, 29
To determine if the Rr cell culture inoculum used during the Rr experimental infection was contaminated with 1 or more Bartonella spp., DNA was extracted for PCR testing from the stored DH‐82 Rr cell culture inoculum (see details in “Diagnostic methods” section). The inoculum was not cultured in BAPGM due to biosafety concerns involved with handling of this BSL‐3 organism, as well as the presumed sensitivity of qPCR to amplify Bartonella spp. if concurrently growing in this cell line, since the DH82 cell line is also routinely used to grow Bartonella spp. intracellularly by the North Carolina State University College of Veterinary Medicine Vector Borne Diseases Diagnostic Laboratory (NCSU‐VBDDL).
2.5. Clinical monitoring
From arrival on December 19, 2017, through Rr inoculation on March 20, 2017 (PI phase), dogs were observed daily by LAR staff. After Rr inoculation and continuing for 35 days until April 28, 2017 (RM phase), dogs underwent daily observation as well as measurement of body temperature, pulse, and respiratory rate. Results were recorded by a veterinarian or veterinary staff member. After April 28, 2017 (EF phase), dogs were observed by LAR husbandry staff during the course of their daily care and examined by veterinary staff only if concerns were noted. A detailed timeline of diagnostic testing performed is included in the “Diagnostic sampling chronology” section. Complete blood counts were performed according to routine procedures by the NCSU Veterinary Hospital Clinical Pathology Laboratory.
2.6. Diagnostic methods
Serum samples were tested using previously described indirect immunofluorescent antibody (IFA) assays, with results considered seroreactive at titer of 1:64 or greater.30 Antibodies to 3 Bartonella species (Bartonella henselae San Antonio 2 strain [Bh SA2], Bartonella vinsonii subsp. berkhoffii genotype I [Bvb I], and Bartonella koehlerae [Bk]), as well as Rr, Ehrlichia canis, Babesia canis, Babesia gibsoni, and Leishmania infantum were assessed by IFA. Rickettsia rickettsii antibody titers were evaluated using 3 secondary antibodies: fluorescein isothiocyanate (FITC)‐labeled goat anti‐dog IgG (H + L), goat anti‐dog IgG (gamma), and goat anti‐dog IgM (mu) (KPL, Gaithersburg, Maryland).30 This Rr IFA cannot distinguish between SFGR species due to the strong cross‐reactivity within the group. Bartonella antibody titers were evaluated using 4 commercially available conjugates. The FITC‐labeled Goat anti‐Dog IgG (H + L) (Sigma‐Aldrich, St. Louis, Missouri) conjugate is used for commercially available diagnostic testing of Bartonella spp. through the NCSU‐VBDDL (referred to hereafter as “diagnostic IFA”). To elucidate serological response of different antibody isotypes over time, goat anti‐dog IgG (gamma), and goat anti‐dog IgM (mu) (KPL) were also used for IFA testing.12, 30 For manufacturer consistency with the isotype‐specific conjugates, FITC‐labeled goat anti‐dog IgG (H + L) (KPL) was also used to test a subset of samples. Positive and negative control sera were tested concurrently with each IFA run. If results were equivocal or difficult to interpret, IFA was repeated on the same sample and the more conservative (lower) titer was reported.
A commercially available ELISA (4 DX Plus SNAP test, IDEXX Laboratories, Westbrook, Maine) was used to test for Anaplasma phagocytophilum, Anaplasma platys, Borrelia burgdorferi, E canis, and Ehrlichia ewingii antibodies and Dirofilaria immitis antigen.
Bartonella spp. bacteremia was assessed using enrichment blood culture with the Bartonella alpha proteobacteria growth medium (BAPGM) as previously described.31 By using standard operating procedures, DNA was extracted from samples intended for Bartonella spp. PCR.30 Bartonella spp. and strain classification was performed using primers designed to amplify 2 consensus sequences in the Bartonella 16S‐23S internal transcribed spacer (ITS) region as described previously with minor modifications.30, 32, 33, 34 All amplicon products were commercially sequenced (Genewiz, Research Triangle Park, North Carolina) to determine the Bartonella sp. and strain type. DNA extracted from whole‐blood and tissue samples was also used for Rickettsia genus‐specific PCR as described previously.35
2.7. Diagnostic sampling chronology
The diagnostic testing timeline is shown in Figure 2. Briefly, blood and serum specimens from all dogs were obtained at prespecified intervals during the PI and RM phases: 4 time points during the PI phase and 3 times weekly for 2 weeks then twice weekly for the subsequent 4 weeks during the RM phase. During the EF phase, samples were obtained at various time points for the dogs remaining in the study, at the discretion of the investigators.
Figure 2.
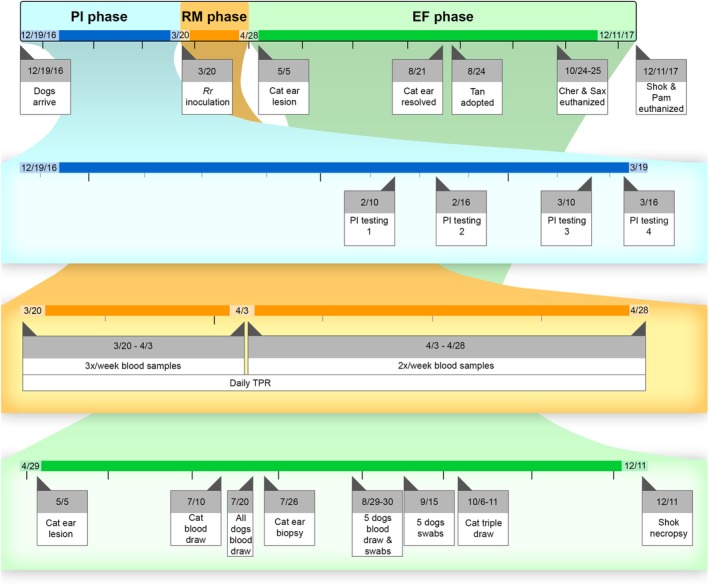
Study timeline. The overall timeline is shown on the top row, with the color indicating the study phase (blue = PI phase, orange = RM phase, green = EF phase). Black tick marks indicate 1 month, gray tick marks indicate 1 week. EF, extended follow up; PI, pre‐inoculation; RM, Rr monitoring
Rr IFA serology was performed on serum from each dog at every time point blood was drawn during the PI and RM phases, and at 1 time point during the EF phase (July 20, 2017). One dog (Kat) also had Rr IFA serology performed at multiple time points during the EF phase. Rickettsia spp. PCR was performed on whole blood from each dog at every time point blood was drawn during the PI and RM phases. One dog (Kat) also had Rickettsia spp. PCR performed once during the EF phase. Bartonella spp. IFA was performed on serum from each dog at every time point blood was drawn during all phases. Bartonella spp. PCR was performed on whole blood from all dogs at every time point blood was drawn during all phases. Bartonella alpha‐proteobacteria growth medium enrichment PCR (BAPGM ePCR) was performed on whole blood from all dogs at 1‐5 time points during the EF phase only.
Immunofluorescent antibody assay serology for other canine vector‐borne diseases (CVBDs) as described above was performed on serum from each dog at every time point blood was drawn during the PI phase only. The SNAP 4DX Plus was performed on serum from each dog at the first PI time point (10 February, 2017) and 1 EF time point (July 20, 2017). One dog (Kat) also had IFA serology for other CVBDs and SNAP 4DX Plus test performed multiple times during the EF phase. Complete blood counts were performed on whole blood from each dog at 1 time point during PI phase (March 16, 2017) and at each time point blood was drawn during RM phase.
During the EF phase, tissue samples were obtained from 2 dogs. One dog (Kat) had biopsies taken from skin lesions on the pinnae, as well as from normal skin on the abdomen. At the time of euthanasia, approximately 1 year after arrival (11 December, 2017), another dog (Shok) had postmortem samples taken from bone marrow, spleen, lung, and submandibular and mesenteric lymph nodes. To determine if body fluids could be a source of Bartonella spp. transmission in this setting, sterile swabs were used to collect samples from the periodontal surface, buccal mucus membranes, and vaginal vault from 5 dogs; oral swab samples were collected twice (August 30, 2017 and September 15, 2017), while vaginal swab samples were only collected on September 15, 2017. These tissues and swabs were all tested for Rickettsia and Bartonella spp. by PCR.
3. RESULTS
All dogs were clinically healthy during the PI phase, with no reported problems from the LAR husbandry staff. All dogs had normal physical examinations and vital signs (body temperature, heart rate, respiration rate) on the day of Rr inoculation (March 20, 2017). After Rr inoculation, dogs exhibited a mild, short‐duration systemic illness. All dogs had a mildly increased body temperature at various points between 2 and 7 days after inoculation (Figure 3A,B). One dog (Pam) also had an increased body temperature on April 10, 2017, and April 24, 2017 (3 and 5 weeks after inoculation). All dogs were clinically recovered based on physical examination and behavioral observation by 1 week after inoculation, without antibiotic administration.
Figure 3.
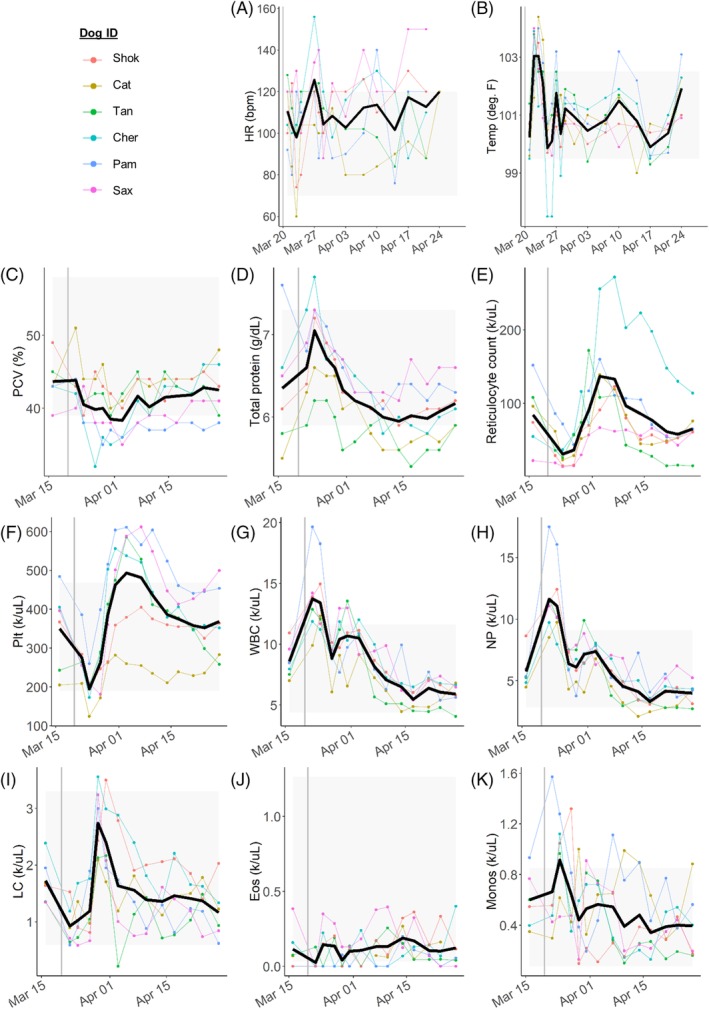
Spaghetti plots showing clinical and CBC results for all dogs. For all plots, the x‐axis shows the testing date and the y‐axis shows the value of each parameter. Vertical gray line represents the date of Rr inoculation. Gray boxes show reference ranges for each parameter. Black line represents the mean for all dogs. Colors correspond to each individual dog, shown in the top left. A, Heart rate. B, Body temperature. C‐K, CBC parameters. Eos, absolute eosinophil count; HR, heart rate; LC, absolute lymphocyte count; Monos, absolute monocyte count; NP, absolute neutrophil count; PCV, packed cell volume; Plt, platelet count; Rr, Rickettsia rickettsii; Temp, body temperature; WBC, absolute white blood cell count
During the EF phase, 5 of the 6 dogs remained clinically healthy. As reported,36 about 1 week into the EF phase (May 5, 2017, 46 days after Rr inoculation), 1 dog (Kat) developed superficial skin lesions on the tip of the right pinna that resolved after about 1 week. Similar but more severe lesions recurred 3 times, and when the lesions persisted, biopsies were performed (July 20, 2017) and the dog was diagnosed with small vessel vasculitis and dermatitis. Photographs of these lesions and other pertinent details of the clinical case have been previously published.36 Bartonella henselae DNA was amplified and sequenced, and Bartonella organisms were visualized by laser scanning confocal immunohistochemistry, from the aural margin biopsies.36 After this diagnosis, the dog was treated with doxycycline 10 mg/kg PO every 12 hours and enrofloxacin 10 mg/kg PO every 24 hours for 6 weeks (August 22, 2017‐October 3, 2017). The lesions improved and did not recur after this treatment, and the dog remains clinically healthy at the time of writing (approximately 2 years after onset of ear‐tip vasculitis).
3.1. Rickettsia infection
Rickettsia titers for each antibody isotype (IgG H + L, IgM, and IgG gamma) throughout the study (all phases) are shown in Figure 4. One dog was Rr seroreactive during the PI phase (Kat, February 10, 2017), with an IFA titer of 1:128; however, this dog was not Rr seroreactive at any of the 3 other PI phase time points or by IFA using IgM or IgG isotype‐specific conjugates. The remaining 5 dogs were not Rr seroreactive during the PI phase using IgG H + L, IgM, or IgG gamma isotype‐specific conjugates.
Figure 4.
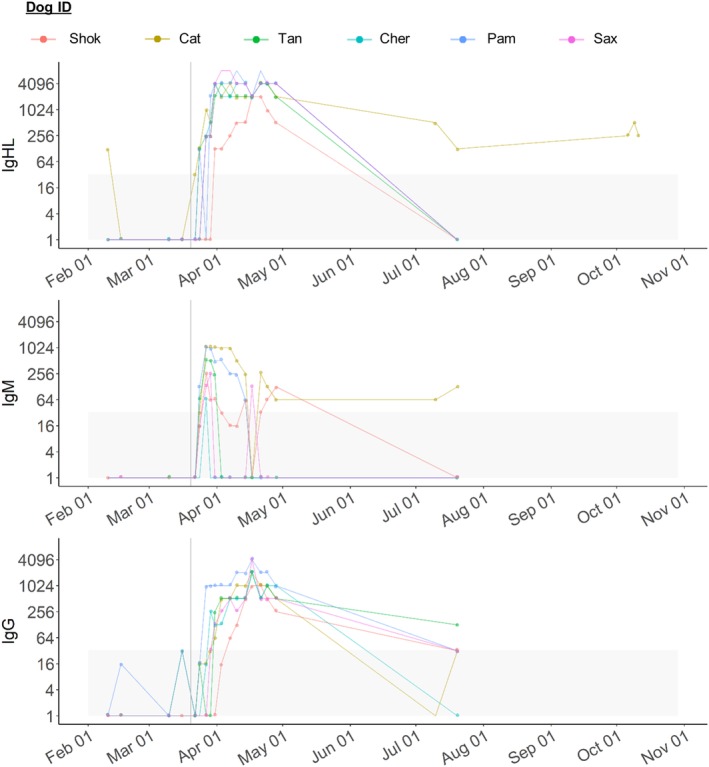
Spaghetti plots showing Rickettsia spp. Immunofluorescent antibody (IFA) titers before and after experimental Rickettsia rickettsii (Rr) inoculation. For all plots, the x‐axis shows the testing date and the y‐axis shows IFA titer of 1:value. Titers for Rickettsia spp. are shown for each specific antibody (IgHL top, IgM middle, IgG bottom). Vertical gray line represents date of Rr inoculation. Gray boxes show non‐seroreactive titers. Colors correspond to each individual dog, shown in at the top of the figure. IgG, IgG gamma; IgHL, IgG heavy and light chains; IgM, IgM mu
By using the diagnostic IgG H + L IFA, we found that all dogs seroconverted to Rr by post‐inoculation day 11 (March 31, 2017, Table 1) on the IgG H + L IFA assay. Three dogs (Kat, Tan, Pam) became seroreactive by post‐inoculation day 4 (March 24, 2017). All dogs remained seroreactive (IgG H + L) until the end of the RM phase (April 28, 2017, 39 days after inoculation). When next tested 4 months after Rr inoculation, only 1 of the 6 dogs (Kat) was Rr seroreactive (Figure 4). This dog remained Rr‐seroreactive when tested again nearly 7 months after Rr inoculation and was the same dog that was Rr seroreactive when first tested during the PI phase.
Table 1.
Rickettsia spp. seroconversion following Rickettsia rickettsii inoculation
| IgHL | IgM | IgG | |
|---|---|---|---|
| Shok | 11 | 7 | 18 |
| Cat | 4 | 7 | 11 |
| Tan | 4 | 4 | 11 |
| Cher | 7 | 7 | 9 |
| Pam | 4 | 4 | 7 |
| Sax | 7 | 7 | 11 |
The number of days post‐inoculation that each dog seroconverted based on each immunofluorescent antibody assay antibody is shown.
Abbreviations: IgG, IgG gamma; IgHL, IgG heavy and light chains; IgM, IgM mu.
Two dogs became Rr IgM seroreactive by 4 days after inoculation, and all dogs were IgM seroreactive at 7 days after inoculation. IgM seroreactivity persisted for a variable period of time; 4 of the 6 dogs were IgM non‐seroreactive by 32 days after inoculation. However, IgM seroreactivity was detected in 1 dog (Shok) erratically, with low titers persisting until 39 days after inoculation. One dog was IgM seroreactive during the EF phase (Kat, July 20, 2017, 122 days after inoculation).
Two dogs became Rr IgG gamma seroreactive by 7 days after inoculation, and all dogs were IgG seroreactive by 18 days after inoculation. All dogs were IgG gamma seroreactive until the end of the RM phase (April 28, 2017, 39 days after inoculation). Only 1 dog was IgG gamma seroreactive during the EF phase (Tan, July 20, 2017, 122 days after inoculation).
Dogs were tested for rickettsemia by PCR of whole‐blood DNA extractions. Rickettsia rickettsii DNA was not amplified from any dog's blood at any time point either before or after Rr experimental inoculation.
3.2. Bartonella serology
Bartonella spp. titers for diagnostic IgG H + L throughout the study (all phases) are shown in Figure 5. Based on the combined results using all 4 IFA isotype‐specific conjugates and all 3 Bartonella sp. antigens, during the PI phase 3 dogs were Bartonella spp. seroreactive. One dog (Shok) was Bh seroreactive on February 10, 2017, and February 16, 2017, using the diagnostic IFA assay. Another dog (Kat) was Bvb seroreactive on February 10, 2017, and March 16, 2017, using the alternative IgG H + L isotype‐specific conjugate but non‐seroreactive using the diagnostic Bvb assay at all 4 PI phase time points. A third dog (Tan) was Bvb seroreactive using the IgM isotype‐specific conjugate only, on February 10, 2017. The remaining 3 dogs (Cher, Pam, and Sax) were Bh, Bvb, and Bk non‐seroreactive using any of the 4 isotype conjugates during the PI phase. All 6 dogs were E canis, B canis, B gibsoni, and L infantum IFA non‐seroreactive, and A phagocytophilum, A platys, B burgdorferi, E canis, and E ewingii and D immitis ELISA negative during the PI phase.
Figure 5.
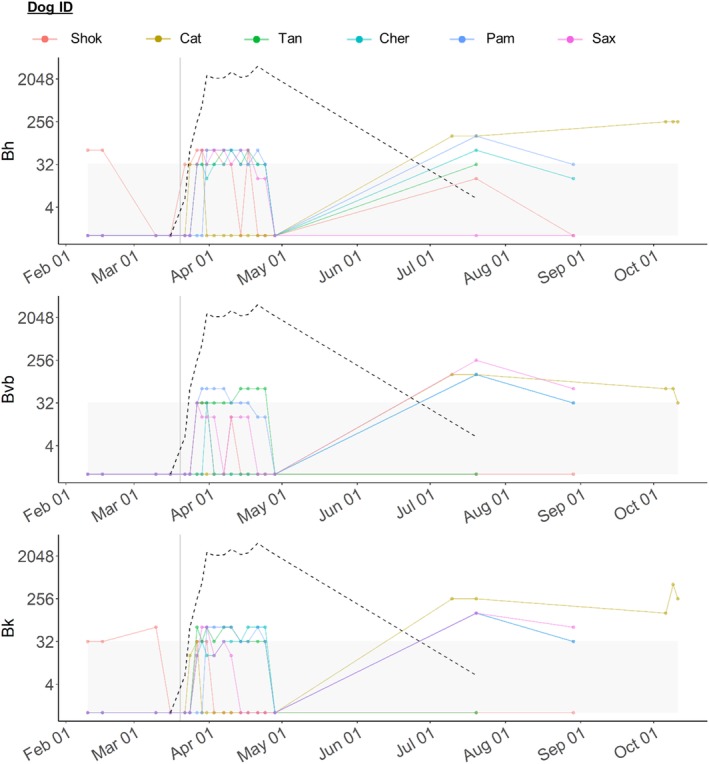
Spaghetti plots showing Bartonella spp. Immunofluorescent antibody (IFA) titers before and after experimental Rickettsia rickettsii (Rr) inoculation. For all plots, the x‐axis shows testing date and the y‐axis shows IFA titer of 1:value. Titers for diagnostic IFA (IgG H + L) are shown for each Bartonella species (Bh top, Bvb middle, Bk bottom). Vertical gray line represents date of Rr inoculation. Gray boxes show non‐seroreactive titers. Black dotted line shows the mean Rickettsia spp. Immunofluorescent antibody (IFA) titer for all dogs to indicate the timeline of Rickettsia seroconversion. Colors correspond to each individual dog, shown at the top of the figure
Based on the combined results using all 4 IFA isotype‐specific conjugates and all 3 Bartonella sp. antigens, during the RM phase all 6 dogs became Bartonella spp. seroreactive: all 6 dogs became Bh seroreactive, 2 dogs (Tan and Pam) became Bvb seroreactive, and 4 dogs (Tan, Cher, Pam, and Sax) became Bk seroreactive (Figure 5). When using the alternative IgG H + L isotype‐specific conjugate, it was found that only 3 dogs were Bartonella spp. seroreactive during the RM phase: 2 dogs (Tan and Cher) were Bh seroreactive at a single time point each, 1 dog (Tan) was Bvb seroreactive at a single time point and 1 dog (Pam) was Bk seroreactive at multiple time points. Each dog that was seroreactive using the alternative IgG H+L conjugate was also seroreactive to the same Bartonella spp. antigen on the diagnostic IFA. When using the IgM and IgG gamma isotype‐specific conjugates, it was found that only 1 dog (Cher) was Bartonella spp. seroreactive during the RM phase. This dog was Bartonella spp. seroreactive at 2 time points: On April 7, 2017, she was Bk IgM and Bh and Bk IgG gamma seroreactive, and on April 14, 2017, she was Bh IgG gamma seroreactive. She was Bvb IgM and IgG gamma non‐seroreactive at all time points during the RM phase. The remaining 5 dogs were Bh, Bvb, and Bk IgM and IgG gamma non‐seroreactive during the RM phase.
Based on the combined results using all 4 IFA isotype‐specific conjugates and all 3 Bartonella sp. antigens, during the EF phase all 6 dogs were Bartonella spp. seroreactive. With the use of the diagnostic assay, 3 dogs (Kat, Cher, Pam) were seroreactive to all 3 Bartonella spp. and 1 dog (Sax) was seroreactive to Bvb and Bk (Figure 5). When using the alternative IgG H + L isotype‐specific conjugate, it was found that only 3 dogs were seroreactive, each to a single species at a single time point (Tan to Bvb on July 20, 2017, Shok to Bvb on August 29, 2017, and Cher to Bk on August 29, 2017). One dog (Cher) was IgM seroreactive during the EF phase, with a titer of 1:128 to Bvb on August 29, 2017. The remaining 5 dogs were Bh, Bvb, and Bk IgM non‐seroreactive during the EF phase. No dog was IgG gamma seroreactive to any Bartonella spp. during the EF phase.
3.3. Bartonella PCR and DNA sequencing
Bartonella PCR on whole blood from all dogs was negative at every time point blood was drawn during all phases (Figure 2). Bartonella alpha‐proteobacteria growth medium enrichment PCR on whole blood from all dogs was negative at every point tested (Figure 2, Table 2).
Table 2.
Bartonella testing of body fluids during EF phase
| 7/20 Bartonella IFA | 7/20 PCR & BAPGM blood | 7/26 Pinnae & skin biopsies | 8/29 Bartonella IFA | 8/29 PCR & BAPGM blood | 8/30 cheek | 8/30 periodontal | 9/15 cheek | 9/15 periodontal | 9/15 vaginal | 10/6‐25 PCR & BAPGM blood | 12/11 Tissue biopsiesa | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Shok | − | − | NA | Bvbb | − | Bvb | − | − | − | − | NA | Bhc |
| Cat | Bh + Bvb + Bk | − | Bhc | NA | NA | Bvb | − | − | − | − | −×3d | NA |
| Tan | Bvbb | − | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Chere | Bh + Bvb + Bk | − | NA | Bvbf + Bkb | − | − | − | − | − | − | − | NA |
| Pam | Bh + Bvb + Bk | − | NA | − | − | Bvb | Bvb | − | Bartonella spp.g | − | NA | NA |
| Saxe | Bvb + Bk | − | NA | Bvb + Bk | − | Bvb | Bvb | − | − | − | − | NA |
Blood samples and swabs from the oral cavity and vaginal vault were taken on each date shown. Negative test results are indicated by the minus sign (“−”). Samples that were not available for testing are indicated by “NA.”
Abbreviations: BAPGM, Bartonella alpha‐proteobacteria growth medium; Bh, Bartonella henselae; Bk, Bartonella koehlerae; Bvb, Bartonella vinsonii subsp. berkhoffii; IFA, immunofluorescent antibody.
Tissues included lung, bone marrow, lymph nodes.
Detected with the alternative KPL secondary antibody only.
Same Bartonella species and strain (Bh SA2).
Three blood samples tested.
Housed together in a single run.
Detected with the IgM (mu) antibody only.
Detected on real‐time PCR using TaqMan probe, species unable to be determined.
Bartonella PCR results during the EF phase are summarized in Table 2.On July 26, 2017, 1 dog (Kat) had biopsies obtained from skin lesions on the pinnae and from normal appearing skin on the abdomen. Bartonella henselae San Antonio 2 strain DNA was amplified and sequenced from the skin lesions on both pinnae and from the normal abdominal skin as previously reported.36 Rr DNA was not PCR‐amplified from any of the biopsies. Just before the biopsies (July 10, 2017, and July 20, 2017), this dog was Rr seroreactive (1:64 and 1:128), seroreactive to all 3 Bartonella spp. (Bh 1:128, Bvb 1:128, Bk 1:256), and Babesia canis seroreactive (1:64). Blood BAPGM ePCR was negative. She was not seroreactive to B gibsoni, E canis, or L infantum, and was negative on the SNAP 4DX Plus test. Additionally, this dog had blood samples taken on October 6, 2017, October 9, 2017, and October 11, 2017; she remained Rr and Bartonella spp. seroreactive (Figures 4 and 5) but was no longer B canis seroreactive. At each of those 3 time points, blood BAPGM ePCR was negative.
To determine if body fluids could be serving as a source of transmission among dogs in this confined vector‐free setting, PCR was performed on oral and vaginal swabs on the 5 remaining dogs on August 30, 2017, and September 15, 2017. PCR results from these swabs are shown in Table 2. Four of the 5 dogs had Bvb I DNA amplified from 1 or more oral swabs on August 30, 2017 with 2 primer sets (100% DNA sequence identity, 399/399 and 563/563 bp, GenBank accession #AF167988.1). Vaginal swabs were not collected on this date. When sampling was repeated on September 15, 2017, only 1 dog (Pam) had Bartonella spp. DNA amplified from an oral swab (99.3% DNA identity, 138/139 bp Bh SA2, GenBank accession #AF369529). Due to the short DNA sequence obtained with the real‐time PCR TaqMan probe, the Bartonella sp. could not be definitively determined; however, the sequence was not consistent with any of the 4 Bvb genotypes. DNA concentration of the swabs from September 15, 2017, were low (<2.5 ng/μL) compared to that of swabs obtained on August 30, 2017 (10‐63 ng/μL). Also, during the EF phase, all dogs were tested 1‐2 times for Bartonella bacteremia with BAPGM ePCR (all dogs on July 20, 2017, Kat on October 6, 2017, October 9, 2017, and October 11, 2017, Sax on October 24, 2017, and Cher on October 25, 2017): all were PCR‐negative.
On December 11, 2017, at the time of euthanasia, tissues were collected from 1 dog (Shok). Gross postmortem examination findings were normal, and histopathology was not performed. Based on PCR and sequencing, Bh DNA was amplified from multiple tissues (lung, bone marrow, and lymph nodes). When multiple 16S‐23S ITS primer sets were used, all amplified 16‐23S rDNA sequences shared 100% identity with Bh SA2 (GenBank accession #AF369529): lung (363/363 bp), bone marrow (551/551 bp), and submandibular lymph node (527/527 bp). Mesenteric lymph node was also Bartonella genus positive by real‐time PCR using a TaqMan probe, but the amplified sequence did not allow for discrimination among several Bartonella species. Bartonella spp. DNA was not amplified from the spleen. No Bvb, Bk or Rr DNA was amplified from this dog's tissues. This dog was Bh seroreactive before Rr inoculation, but Bh non‐seroreactive during the EF phase. The Bartonella species and strain (Bh SA2) amplified from this dog's tissues were the same as that amplified from another dog's (Kat) tissues 5 months earlier.
The stored Rr inoculum was tested for Bartonella spp. by PCR: Bartonella spp. DNA was not amplified from stored inoculum.
3.4. Complete blood count results
Complete blood count results for each dog during the PI and RM phases are shown in Figure 3C‐K. Before Rr inoculation, most dogs had normal CBC findings, but 2 dogs (Kat and Tan) had mild hypoproteinemia and 1 dog (Pam) had multiple mild abnormalities including thrombocytosis, hyperproteinemia, monocytosis, and reticulocytosis. Kat was also the 1 dog Rr seroreactive during the PI phase, and Kat and Tan were 2 of the 3 dogs Bartonella spp. seroreactive during the PI phase. Pam was Bartonella spp. and Rr non‐seroreactive during the PI phase.
After infection with Rr, there were multiple CBC changes. The PCV, hematocrit (HCT), or both decreased in all dogs; 2 dogs (Cher and Pam) developed anemia while the remaining dogs maintained PCV within reference range. All dogs developed reticulocytosis, but MCV and MCHC remained within reference range for all dogs. Platelet count initially decreased in all dogs and then increased above pre‐Rr inoculation baseline; 2 dogs (Kat and Tan) developed thrombocytopenia and 4 dogs (Cher, Tan, Pam, Sax) developed rebound thrombocytosis. White blood cell count increased in all dogs, and all dogs developed mild neutrophilia and monocytosis. Two dogs (Kat and Tan) developed neutropenia 3‐4 weeks after Rr infection.
4. DISCUSSION
There were 2 unexpected findings during this investigation: after experimental Rr infection at a vector‐free biocontainment facility, we found that all dogs developed Bartonella spp. seroreactivity and multiple dogs had DNA evidence of Bartonella spp. infection. Based on PCR from tissues, at least 2 of these 6 seroreactive dogs were actively infected with 1 or more Bartonella species at the time of testing: Kat had Bh amplified from skin biopsy specimens taken from lesions in July 2017, and Shok had Bh amplified from postmortem tissues in December 2017. In addition, 4 of 5 dogs had Bvb I amplified from oral swabs in August 2017, and 1 of those 4 dogs had another Bartonella sp. (most compatible with Bh SA2) amplified from an oral swab 2 weeks later. It is possible that these swab results represent active infection with salivary shedding of Bvb and Bh, occurring in 4 of 5 dogs. Despite detection of Bartonella spp. antibodies in all dogs at multiple time points, and amplification of Bartonella spp. DNA from tissues and oral swabs, Bartonella spp. DNA was not amplified from blood during the course of this investigation. While 5 of 6 dogs had Bk antibodies, Bk DNA was not amplified from either tissues or swabs.
We are only able to speculate on the origin of the Bh and putative Bvb infections. These dogs were either infected before arrival at NCSU LAR or newly infected while residing in the presumed vector‐free housing at the NCSU LAR. If the dogs were infected before arrival at the NCSU, it is possible that the physiologic stress of experimental Rr infection caused reactivation of latent Bartonella infections. At the vendor's facility, the dogs were housed in indoor/outdoor runs and not treated with flea or tick preventatives, so it is possible that they were exposed during that time. The 3 dogs that were Bartonella spp. seroreactive at the first testing time point (February 10, 2017), and before Rr inoculation, support the possibility of preexisting chronic subclinical infection. The rapid development of Bartonella antibodies (by 9 days after Rr inoculation in 5 of 6 dogs), particularly those dogs that seroconverted to multiple Bartonella spp., also supports reactivation of latent infection. As these dogs had resided at the NCSU LAR for over 7 weeks at the time of this first blood collection, however, it is not possible to determine whether transmission occurred before or after shipment from the vendor. All dogs did receive 1 week of sulfamethoxazole for coccidiosis before shipping, but it is unlikely that would clear Bartonella spp. infections.37 If the dogs were exposed at the vendor's facility, this highlights the need for researchers to specifically request dogs be treated with flea/tick preventatives and tested for infection prior to research studies, so that coinfection with these vector‐borne diseases does not bias the results of their studies.
The possibility of reactivation of latent Bartonella infection in both humans and dogs has been raised in case reports previously. Bartonella henselae and Epstein‐Barr virus were found simultaneously in a man with fever and lymphadenopathy after acute mononucleosis.38 A dog presumed to have immune‐mediated ineffective erythropoiesis was pharmacologically immunosuppressed; his HCT was improving or stable until he had an episode of presumptive kennel cough, at which time his HCT and regenerative response worsened with no change to his immunosuppressive medications.39 Bartonella henselae was amplified from this dog's blood soon after, and once immunosuppression was discontinued and appropriate antibiotics administered, his ineffective erythropoiesis resolved. Therefore, it is possible that chronic Bh infection reactivated after infectious tracheobronchitis. In a second case,4 an acutely ill Rr seroreactive dog failed to respond to antibiotic treatment directed against RMSF (doxycycline). When tested 10 days later, the dog had seroconverted to Bh and Bk. Unlike the dogs in this study, this dog developed very high Bh, Bk, and Rr titers simultaneously, supporting the theory that the dog was exposed to all 3 pathogens on or around the same time. However, it is also possible that that dog was chronically infected with 1 or more Bartonella spp. and experienced reactivation when infected with an SFGR species. Based on these previous reports and the results in this case series, it is likely that dogs can harbor chronic subclinical Bartonella infections that become recrudescent when exposed to the physiologic stress induced by coinfection.
However, it is instead possible that the dogs reported here were newly infected while residing at the NCSU LAR, which begs the question of transmission route. Because Bartonella species are considered to be primarily vector‐borne, the animal housing areas at the NCSU LAR are maintained with strict vector‐prevention methods. These dogs were checked daily for ectoparasites: none were reported throughout the duration of the study. Dogs were only allowed outdoor access during the EF phase (by which point all dogs had already seroconverted to Bartonella spp.), and all dogs that were given access to outdoor areas were treated monthly with a topical flea and tick preventative (Frontline Plus for Dogs, Merial Inc, Duluth, Georgia). There remains the unlikely possibility that a previously unknown vector remained undetected in the LAR facility and was able to facilitate transmission between dogs.
If we remove the possibility for vector transmission, then we must consider the possibility of direct transmission, via the contact allowed by the chain‐link fence between individual runs during the PI and RM phase, comingling during the EF phase, or both. The amplification of Bvb from 4 of 5 dogs' oral swabs during the EF phase is supportive of the possibility for direct transmission via body fluids or oral mucus membranes. At that time the only 2 dogs remaining housed together (who had shared a run for approximately 4 months) had disparate results: Sax had Bvb DNA amplified from both cheek and periodontal swabs, but her kennel‐mate Cher had no Bartonella spp. DNA amplified from any similar samples. However, at that time, Cher was seroreactive to Bvb IgM, which could be indicative of exposure due to direct transmission from Sax. While it is likely that the Bvb and other Bartonella spp. DNA found on oral/dental swabs in the dogs reported here were associated with active infection and shedding into the oral cavity, it is possible that this DNA represents environmental contamination from another source within LAR. If associated with active infection and shedding, the host and pathogen dynamics governing potential direct transmission should be urgently explored.
Regardless of the origin of Bartonella spp. exposure, all 6 dogs developed specific Bh, Bvb, and Bk antibody responses after Rr inoculation. Previous studies have documented that there is no cross‐reactivity between Rr and Bh or Bvb using the 3 respective IFA assays.6, 40 In the dogs reported here, the Bartonella spp. antibody response lagged behind the Rr seroconversion, and multiple dogs were Bartonella spp. seroreactive during the EF phase when Rr titers had waned. This further supports the conclusion that the Bartonella spp. seroreactivity was not cross‐reactive with Rr cell culture grown antigens.
Our results also highlight the previously reported poor sensitivity of the diagnostic Bartonella spp. IFA,26, 41, 42 as evidenced by the lack of Bartonella seroreactivity at various time points in dogs with positive tissue PCR (Shok and Kat) or oral swab PCR (Pam). Sensitivity and specificity for the IFAs performed in this study have not been previously explicitly evaluated; however, previous studies using similar IFA protocols have been done.26, 43, 44 When 20 naturally infected Bh PCR‐positive dogs had serology performed (on serum sampled concurrently with the PCR‐positive blood samples), only 1 dog was Bh seroreactive for the antigen used in this study (Bh SA2).43 In 3 dogs experimentally infected with Bh H1 and proven bacteremic, none were seroreactive against Bh SA2 (though all seroconverted to Bh H1).44 Conversely, a single dog experimentally infected with Bh SA2 seroconverted to Bh SA2 2 weeks after infection and maintained seroreactivity for approximately 6 weeks, after which titers waxed and waned until finally remaining below 1:16 after 12 weeks.26 The lack of sensitivity of Bartonella IFA overall could be due to immune‐complexing of antibodies, antibodies below the level of detection, relapsing infection, genotype‐specific antibody responses, or other as‐yet undetermined mechanisms.41, 43 The specificity of IFA is higher than the sensitivity and was recently estimated to be at least 85% for an expanded panel of antigens but could be significantly higher given the previously described level of seroreactivity in the healthy/blood donor dog population.43, 45
In addition to the poor sensitivity of IFA, there are other limitations to using IFA as a diagnostic test that impeded our ability to determine the origin or course of Bartonella spp. infection in this study. When evaluating IFA slides, interobserver differences in interpretation may include 1 dilution step (ie, 1:32 to 1:64, or 1:256 to 1:512), which is why as a general rule seroconversion is considered to occur only when antibody titers rise by 2 or more dilution steps (4‐fold). A previous study of Bartonella IFA showed that even when evaluated by blinded, experienced scientists, disagreements on dilution can occur, highlighting the somewhat subjective nature of this diagnostic test.46 Along with the low sensitivity, these inherent limitations of IFA may help to explain the differences in seroreactivity to each Bartonella spp. over time in the dogs reported here. However, studies of serological response to Bh infection in humans with classical Cat Scratch Disease have shown that even in that most straightforward, acute presentation, there is no “standard course” of anti‐Bh IgG or IgM production: some patients produce high levels of both isotypes, some produce only high levels of IgM, and some produce only low levels of either isotype.46, 47, 48 Though IgM and IgG isotype‐specific serology is routinely used clinically in humans to attempt to distinguish acute infection from previous exposure, evaluation of Bartonella IgG and IgM isotype‐specific antibody responses has not, to the authors knowledge, been previously reported in dogs (nor are these assays commercially available). Based on our results, the poor sensitivity of the IgG and IgM isotype‐specific IFA precludes their routine use in clinical diagnosis of Bartonella spp. exposure or infection in dogs. Overall, since seroreactivity was detected only sporadically in all 6 dogs throughout the study and did not always match the species ultimately identified on PCR, failure to detect antibodies to any of 3 Bartonella spp. does not rule out the possibility of concurrent occult infection.
While previous studies have supported the diagnostic utility of BAPGM ePCR blood culture for confirmation of bloodstream infection in both healthy and sick dogs,31, 45, 49, 50 our efforts to PCR amplify or culture Bartonella spp. DNA from blood were not successful, even in dogs with PCR‐positive saliva or tissue samples. Whether this is due to extremely low bloodstream levels, a relapsing time course of Bartonella spp. bacteremia, or sequestration of Bartonella organisms in cells outside the bloodstream (such as endothelial cells) is unknown. Additionally, we were unable to amplify Bk from any sample despite all but 1 dog seroconverting to Bk during either RM or EF phases. In a previous study,26 when dogs naturally infected with Bk were challenged by intradermal inoculation with either Bh or Bvb, dogs seroconverted only to the inoculated species.
With regard to the Rr infection, we showed that the serologic response was consistent with previous experimental studies of RMSF in dogs.28, 29 Because Rr is an endotheliotropic pathogen infecting predominantly endothelial cells of small‐ and medium‐size blood vessels, low numbers of Rr circulate in peripheral blood of most infected patients.51, 52 As such, Rickettsia genus‐specific PCR using the 23S‐5S intergenic region from blood specimens (as was performed in this study) was not expected to be positive in these dogs inoculated with low‐dose Rr (although the use of more specific primers or nested PCR can enhance detection).52, 53, 54 As expected, therefore, Rr DNA was not amplified at any time from these dogs, and IFA seroconversion was used to document infection. All dogs seroconverted to IgM by day 7, and all but 1 dog seroconverted to IgG gamma on days 7 through 11. This 1 dog (Shok) had an IgM response comparable to the other 5 dogs, but low IgG gamma titers and a slightly prolonged IgG gamma seroconversion (18 days after inoculation). While clinically healthy both before and after the mild RMSF, this dog was Bh seroreactive both pre‐ and post‐Rr inoculation and had Bh DNA amplified from multiple tissues at the time of euthanasia approximately 8 months later. These findings may indicate delayed class shift in this dog associated with chronic subclinical Bh infection. We are not aware of other documented examples of a delayed class shift in association with occult infections in dogs that were challenged with a highly virulent organism such as Rr. Whether the delay in IgG gamma seroconversion in this dog was a cause or consequence of Bh coinfection is unknown.
Previous studies have shown that after experimental infection, repeat inoculation with Rr elicits no clinical illness, hematological changes, or recall IgM response.28, 29 Additionally, recrudescent infection with Rr is not thought to occur in dogs or humans.55 When inoculated with Rr, the 1 dog in this study (Kat) that was Rr seroreactive became clinically ill and had a robust IgM response, making it unlikely that she was previously infected with Rr. Because Rr IFA cross‐reacts with multiple SFGR species, this dog likely had exposure to another SFG rickettsia (or a cross‐reactive non‐Rickettsia species) that did not provide cross‐protection to Rr infection.
The major limitation of this study is the lack of sequential, prospective sampling of all dogs, particularly during the EF phase. For several reasons, samples were not obtained at predetermined time intervals after the RM phase. Prospective sampling to assess Bartonella spp. reactivation, transmission, or both possibilities was pursued only after documentation of intra‐lesional Bh in the dog with ear‐tip vasculitis.36 Lack of sequential sample collection limits our ability to draw conclusions about when, where, and how the dogs in this study became infected. It is unfortunate that we did not obtain blood and tissue samples from the dogs immediately upon arrival at LAR, allowing us to determine more definitively the timing of infection. Additionally, there are inherent limitations in using IFA and PCR as previously discussed. Interpretation of IFA is subjective, and determining the end point titer is usually known to have a margin of error of 1‐fold dilution above or below. This can result in misclassification bias, particularly when the IFA is performed unblinded (as ours were in this study). In few cases where IFA results differed upon repeat testing, we reported the more conservative (lower) titer, to limit the likelihood of false‐positives.
Despite limitations, this study supports the possibility of chronic subclinical Bh infection with recrudescence after infection with Rr. Additionally, the possibility for nonvectorial direct transmission via saliva or other body fluids should be further investigated with controlled experimental studies. Occult infections in research animals could cause spurious conclusions in studies utilizing these animals for infectious disease or other biomedical research. Also, if direct transmission is able to occur for this pathogen that has been considered primarily vector‐borne, there are wide‐ranging biosafety and zoonotic disease implications of substantial veterinary and human medical importance.
CONFLICT OF INTEREST DECLARATION
In conjunction with Dr. S. Sontakke and North Carolina State University, E. B. Breitschwerdt holds US Patent No. 7,115,385; Media and Methods for Cultivation of Microorganisms, which was issued on October 3, 2006. He is a co‐founder, shareholder and Chief Scientific Officer for Galaxy Diagnostics, a company that provides advanced diagnostic testing for the detection of Bartonella spp. infections. Ramaswamy Chandrashekar is employed by IDEXX Laboratories. The remaining authors declare no conflicts of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
The study was approved by the North Carolina State University IACUC (Protocol #16‐206).
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
ACKNOWLEDGMENTS
We thank Barbara Hegarty for preparation of the Bartonella and R. rickettsii antigen slides used for indirect fluorescent antibody testing and for generating the R. rickettsii inoculum used in this study. We also thank Dr. Barbara Qurollo for performing Rickettssia spp. PCR.
Lashnits E, Neupane P, Maggi RG, et al. Detection of Bartonella spp. in dogs after infection with Rickettsia rickettsii . J Vet Intern Med. 2020;34:145–159. 10.1111/jvim.15675
Present Address Nandhakumar Balakrishnan, Department of Health and Human Services, Bureau of Laboratories, Division of Infectious Diseases, 3350 N Martin Luther King Jr Blvd, Lansing, MI 48906.
Funding information Bartonella/Vector Borne Disease Research Fund; IDEXX Laboratories Inc.; NIH Office of the Director, Grant/Award Number: T32OD011130; North Carolina State University Veterinary Medical Foundation
REFERENCES
- 1. Breitschwerdt EB. Bartonellosis, one health and all creatures great and small. Vet Dermatol. 2017;28(1):96‐e21. [DOI] [PubMed] [Google Scholar]
- 2. Breitschwerdt E. Bartonellosis: one health perspectives for an emerging infectious disease. ILAR J. 2014;55(1):46‐58. [DOI] [PubMed] [Google Scholar]
- 3. Lashnits E, Correa M, Hegarty BC, Birkenheuer A, Breitschwerdt EB. Bartonella seroepidemiology in dogs from North America, 2008‐2014. J Vet Intern Med. 2018;32(1):222‐231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Golly E, Breitschwerdt EB, Balakrishnan N, et al. Bartonella henselae, Bartonella koehlerae, and Rickettsia rickettsii seroconversion and seroreversion in a dog with acute‐onset fever, lameness, and lymphadenopathy followed by a protracted disease course. Vet Parasitol Reg Stud Report. 2016;7:19‐24. [DOI] [PubMed] [Google Scholar]
- 5. Breitschwerdt EB, Blann KR, Stebbins ME, et al. Clinicopathological abnormalities and treatment response in 24 dogs seroreactive to Bartonella vinsonii (berkhoffii) antigens. J Am Anim Hosp Assoc. 2004;40(2):92‐101. [DOI] [PubMed] [Google Scholar]
- 6. Solano‐Gallego L, Bradley J, Hegarty B, et al. Bartonella henselae IgG antibodies are prevalent in dogs from southeastern USA. Vet Res. 2004;34(1):585‐595. [DOI] [PubMed] [Google Scholar]
- 7. Kordick SK, Breitschwerdt EB, Hegarty BC, et al. Coinfection with multiple tick‐borne pathogens in a Walker hound kennel in North Carolina. J Clin Microbiol. 1999;37(8):2631‐2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Regier Y, O'Rourke F, VAJ K. Bartonella spp. ‐ a chance to establish One Health concepts in veterinary and human medicine. Parasit Vectors. 2016;9(1):261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Angelakis E, Billeter SA, Breitschwerdt EB, Chomel BB, Raoult D. Potential for tick‐borne bartonelloses. Emerg Infect Dis. 2010;16(3):385‐391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Billeter SA, Levy MG, Chomel BB, Breitschwerdt EB. Vector transmission of Bartonella species with emphasis on the potential for tick transmission. Med Vet Entomol. 2008;22(1):1‐15. [DOI] [PubMed] [Google Scholar]
- 11. Cherry NA, Maggi RG, Rossmeisl JH, Hegarty BC, Breitschwerdt EB. Ecological diversity of Bartonella species infection among dogs and their owner in Virginia. Vector‐Borne Zoonotic Dis. 2011;11(11):1425‐1432. [DOI] [PubMed] [Google Scholar]
- 12. Oliveira AM, Maggi RG, Woods CW, Breitschwerdt EB. Suspected needle stick transmission of Bartonella vinsonii subspecies berkhoffii to a veterinarian. J Vet Intern Med. 2010;24(5):1229‐1232. [DOI] [PubMed] [Google Scholar]
- 13. Lin J‐W, Chen C‐M, Chang C‐C. Unknown fever and Back pain caused by Bartonella henselae in a veterinarian after a needle puncture: a case report and literature review. Vector‐Borne Zoonotic Dis. 2011;11(5):589‐591. [DOI] [PubMed] [Google Scholar]
- 14. Silva MN, Vieira‐Damiani G, Ericson ME, et al. Bartonella henselae transmission by blood transfusion in mice. Transfusion. 2016;56(6 pt 2):1556–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pitassi LHU, de Paiva Diniz PPV, Scorpio DG, et al. Bartonella spp. bacteremia in blood donors from Campinas, Brazil. PLoS Negl Trop Dis. 2015;9(1): e0003467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Abbott RC, Chomel BBB, Kasten RWRW, et al. Experimental and natural infection with Bartonella henselae in domestic cats. Comp Immunol Microbiol Infect Dis. 1997;20(1):41‐51. [DOI] [PubMed] [Google Scholar]
- 17. Breitschwerdt EB, Maggi RG, Sigmon B, Nicholson WL. Isolation of Bartonella quintana from a woman and a cat following putative bite transmission. J Clin Microbiol. 2007;45(1):270‐272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rolain JM, Boureau‐Voultoury A, Raoult D. Serological evidence of Bartonella vinsonii lymphadenopathies in a child bitten by a dog. Clin Microbiol Infect. 2009;15(Suppl 2):122‐123. [DOI] [PubMed] [Google Scholar]
- 19. Breitschwerdt EB, Maggi RG, Lantos PM, et al. Bartonella vinsonii subsp. berkhoffii and Bartonella henselae bacteremia in a father and daughter with neurological disease. Parasites and Vectors. 2010;3:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kim Y, Seo K, Lee J, et al. Prevalence of Bartonella henselae and Bartonella clarridgeiae in cats and dogs in Korea. J Vet Sci. 2009;10(1):85‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Duncan AW, Maggi RG, Breitschwerdt EB. Bartonella DNA in dog saliva. Emerg Infect Dis. 2007;13(12):1948‐1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lösch B, Wank R. Life‐threatening angioedema of the tongue: the detection of the RNA of B henselae in the saliva of a male patient and his dog as well as of the DNA of three Bartonella species in the blood of the patient. BMJ Case Rep. 2014;20:2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sun J, Fu G, Lin J, Song X, Lu L, Liu Q. Seroprevalence of Bartonella in eastern China and analysis of risk factors. BMC Infect Dis. 2010;10:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bradbury CA, Lappin MR. Evaluation of topical application of 10% imidacloprid–1% moxidectin to prevent Bartonella henselae transmission from cat fleas. J Am Vet Med Assoc. 2010;236(8):869‐873. [DOI] [PubMed] [Google Scholar]
- 25. Lappin MR, Davis WL, Hawley JR, Brewer M, Morris A, Stanneck D. A flea and tick collar containing 10% imidacloprid and 4.5% flumethrin prevents flea transmission of Bartonella henselae in cats. Parasit Vectors. 2013;6:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Balakrishnan N, Cherry NA, Linder KE, et al. Experimental infection of dogs with Bartonella henselae and Bartonella vinsonii subsp. berkhoffii. Vet Immunol Immunopathol. 2013;156(1–2):153‐158. [DOI] [PubMed] [Google Scholar]
- 27. Kidd L, Hegarty B, Sexton D, Breitschwerdt E. Molecular characterization of Rickettsia rickettsii infecting dogs and people in North Carolina. Ann N Y Acad Sci. 2006;1078:400‐409. [DOI] [PubMed] [Google Scholar]
- 28. Breitschwerdt EB, Levy MG, Davidson MG, et al. Kinetics of IgM and IgG responses to experimental and naturally acquired Rickettsia rickettsii infection in dogs. Am J Vet Res. 1990;51(8):1312‐1316. [PubMed] [Google Scholar]
- 29. Breitschwerdt EB, Walker DH, Levy MG, et al. Clinical, hematologic, and humoral immune response in female dogs inoculated with Rickettsia rickettsii and Rickettsia montana. Am J Vet Res. 1988;49(1):70‐76. [PubMed] [Google Scholar]
- 30. Kidd L, Qurollo B, Lappin M, et al. Prevalence of vector‐borne pathogens in Southern California dogs with clinical and laboratory abnormalities consistent with immune‐mediated disease. J Vet Intern Med. 2017;31(4):1081‐1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Duncan AW, Maggi RG, Breitschwerdt EB. A combined approach for the enhanced detection and isolation of Bartonella species in dog blood samples: pre‐enrichment liquid culture followed by PCR and subculture onto agar plates. J Microbiol Methods. 2007;69(2):273‐281. [DOI] [PubMed] [Google Scholar]
- 32. Lappin MR, Hawley J. Presence of Bartonella species and Rickettsia species DNA in the blood, oral cavity, skin and claw beds of cats in the United States. Vet Dermatol. 2009;20(5–6):509‐514. [DOI] [PubMed] [Google Scholar]
- 33. Namekata DY, Kasten RW, Boman DA, et al. Oral shedding of Bartonella in cats: correlation with bacteremia and seropositivity. Vet Microbiol. 2010;146(3–4):371‐375. [DOI] [PubMed] [Google Scholar]
- 34. Breitschwerdt EB, Mascarelli PE, Schweickert LA, et al. Hallucinations, sensory neuropathy, and peripheral visual deficits in a young woman infected with Bartonella koehlerae. J Clin Microbiol. 2011;49(9):3415‐3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lado P, Qurollo B, Williams C, Junge R, Klompen H. The microbiome of Haemaphysalis lemuris (Acari: Ixodidae), a possible vector of pathogens of endangered lemur species in Madagascar. Ticks Tick Borne Dis. 2018;9(5):1252‐1260. [DOI] [PubMed] [Google Scholar]
- 36. Southern BL, Neupane P, Ericson ME, et al. Bartonella henselae in a dog with ear tip vasculitis. Vet Dermatol. 2018;29(6):537‐e180. [DOI] [PubMed] [Google Scholar]
- 37. Rolain JM, Brouqui P, Koehler JE, Maguina C, Dolan MJ, Raoult D. Recommendations for treatment of human infections caused by Bartonella species. Antimicrob Agents Chemother. 2004;48(6):1921‐1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zbinden R, Kurer SB, Altwegg M, Weber R. Generalized infection with Bartonella henselae following infection due to Epstein‐Barr virus. Clin Infect Dis. 1996;23(5):1184‐1185. [DOI] [PubMed] [Google Scholar]
- 39. Randell MG, Balakrishnan N, Gunn‐Christie R, Mackin A, Breitschwerdt EB. Bartonella henselae infection in a dog with recalcitrant ineffective erythropoiesis. Vet Clin Pathol. 2018;47(1):45‐50. [DOI] [PubMed] [Google Scholar]
- 40. Pappalardo BL, Correa MT, York CC, Peat CY, Breitschwerdt EB. Epidemiologic evaluation of the risk factors associated with exposure and seroreactivity to Bartonella vinsonii in dogs. Am J Vet Res. 1997;58(5):467‐471. [PubMed] [Google Scholar]
- 41. Kordick DL, Breitschwerdt EB. Relapsing bacteremia after blood transmission of Bartonella henselae to cats. Am J Vet Res. 1997;58(5):492‐497. [PubMed] [Google Scholar]
- 42. Breitschwerdt EB, Greenberg R, Maggi RG, Mozayeni BR, Lewis A, Bradley JM. Bartonella henselae bloodstream infection in a boy with pediatric acute‐onset neuropsychiatric syndrome. J Cent Nerv Syst Dis. 2019;11: 117957351983201. https://www.ncbi.nlm.nih.gov/pubmed/30911227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Neupane P, Hegarty BC, Marr HS, Maggi RG, Birkenheuer AJ, Breitschwerdt EB. Evaluation of cell culture‐grown Bartonella antigens in immunofluorescent antibody assays for the serological diagnosis of bartonellosis in dogs. J Vet Intern Med. 2018;32(6):1958‐1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hegarty BC, Bradley JM, Lappin MR, Balakrishnan N, Mascarelli PE, Breitschwerdt EB. Analysis of seroreactivity against cell culture‐derived bartonella spp. antigens in dogs. J Vet Intern Med. 2014;28(1):38‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Balakrishnan N, Musulin S, Varanat M, et al. Serological and molecular prevalence of selected canine vector borne pathogens in blood donor candidates, clinically healthy volunteers, and stray dogs in North Carolina. Parasit Vectors. 2014;7:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Vermeulen MJ, Herremans M, Verbakel H, et al. Serological testing for Bartonella henselae infections in the Netherlands: clinical evaluation of immunofluorescence assay and ELISA. Clin Microbiol Infect. 2007;13(6):627‐634. [DOI] [PubMed] [Google Scholar]
- 47. Metzkor‐Cotter E, Kletter Y, Avidor B, et al. Long‐term serological analysis and clinical follow‐up of patients with cat scratch disease. Clin Infect Dis. 2003;37(9):1149‐1154. [DOI] [PubMed] [Google Scholar]
- 48. Bergmans AMC, Peeters MF, Schellekens JFP, et al. Pitfalls and fallacies of cat scratch disease serology: evaluation of Bartonella henselae‐based indirect fluorescence assay and enzyme‐linked immunoassay. J Clin Microbiol. 1997;35:1931‐1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Perez C, Maggi RG, Diniz PPVP, Breitschwerdt EB. Molecular and serological diagnosis of Bartonella infection in 61 dogs from the United States. J Vet Intern Med. 2011;25(4):805‐810. [DOI] [PubMed] [Google Scholar]
- 50. Kordick DL, Breitschwerdt EB. Persistent infection of pets within a household with three Bartonella species. Emerg Infect Dis. 1998;4(2):325‐328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kato C, Chung I, Paddock C. Estimation of Rickettsia rickettsii copy number in the blood of patients with Rocky Mountain spotted fever suggests cyclic diurnal trends in bacteraemia. Clin Microbiol Infect. 2016;22(4):394‐396. [DOI] [PubMed] [Google Scholar]
- 52. Kidd L, Maggi R, Diniz PPVP, Hegarty B, Tucker M, Breitschwerdt E. Evaluation of conventional and real‐time PCR assays for detection and differentiation of spotted fever group rickettsia in dog blood. Vet Microbiol. 2008;129(3–4):294‐303. [DOI] [PubMed] [Google Scholar]
- 53. Levin ML, Killmaster LF, Zemtsova GE, et al. Clinical presentation, convalescence, and relapse of Rocky Mountain Spotted Fever in dogs experimentally infected via tick bite. PLoS One. 2014;9(12): e115105 https://www.ncbi.nlm.nih.gov/pubmed/25542001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Breitschwerdt EB, Papich MG, Hegarty BC, Gilger B, Hancock SI, Davidson MG. Efficacy of doxycycline, azithromycin, or trovafloxacin for treatment of experimental Rocky Mountain spotted fever in dogs. Antimicrob Agents Chemother. 1999;43(4):813‐821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. McQuiston JH, Wiedeman C, Singleton J, Carpenter LR, et al. Inadequacy of IgM antibody tests for diagnosis of Rocky Mountain spotted fever. Am J Trop Med Hyg. 2014;91(4):767‐770. [DOI] [PMC free article] [PubMed] [Google Scholar]


