Abstract
Background
Hepatic lipidosis is increasing in incidence in the Western world, with cats being particularly sensitive. When cats stop eating and start utilizing their fat reserves, free fatty acids (FFAs) increase in blood, causing an accumulation of triacylglycerol (TAG) in the liver.
Objective
Identifying potential new drugs that can be used to treat hepatic lipidosis in cats using a feline hepatic organoid system.
Animals
Liver organoids obtained from 6 cats.
Methods
Eight different drugs were tested, and the 2 most promising were further studied using a quantitative TAG assay, lipid droplet staining, and qPCR.
Results
Both T863 (a diacylglycerol O‐acyltransferase 1 [DGAT1] inhibitor) and 5‐aminoimidazole‐4‐carboxamide 1‐β‐D‐ribofuranoside (AICAR; an adenosine monophosphate kinase activator) decreased TAG accumulation by 55% (P < .0001) and 46% (P = .0003), respectively. Gene expression of perilipin 2 (PLIN2) increased upon the addition of FFAs to the medium and decreased upon treatment with AICAR but not significantly after treatment with T863.
Conclusions and Clinical Importance
Two potential drugs useful in the treatment of hepatic lipidosis in cats were identified. The drug T863 inhibits DGAT1, indicating that DGAT1 is the primary enzyme responsible for TAG synthesis from external fatty acids in cat organoids. The drug AICAR may act as a lipid‐lowering compound via decreasing PLIN2 mRNA. Liver organoids can be used as an in vitro tool for drug testing in a species‐specific system and provide the basis for further clinical testing of drugs to treat steatosis.
Keywords: AICAR, diacylglycerol O‐acyltransferase 1 inhibitor, hepatic organoids, perilipin 2, steatosis
Abbreviations
- AICAR
5‐aminoimidazole‐4‐carboxamide 1‐β‐D‐ribofuranoside
- AMPK
adenosine monophosphate‐activated protein kinase
- AMPKK
AMPK kinase
- CYP3A132
cytochrome 3A132
- DAG
diacylglycerol
- DGAT
diacylglycerol O‐acyltransferase
- FFA
free fatty acid
- HMBS
hydroxymethylbilane synthase
- HPRT‐1
hypoxanthine phosphoribosyltransferase 1
- LGR5
leucine‐rich repeat‐containing G protein‐coupled receptor 5
- NAFLD
non‐alcoholic fatty liver disease
- PLIN2
perilipin 2
- RPS5
ribosomal protein S5
- TAG
triacylglycerol
- TTR
transthyretin
- YWHAZ
tyrosine 3‐monooxygenase/tryptophan 5‐monooxygenase activation protein, zeta
- ZMP
5‐amino‐4‐imidazolecarboxamide ribose‐monophosphate
1. INTRODUCTION
Because of changes in diet and lifestyle, the incidence of obesity and non‐alcoholic fatty liver disease has increased worldwide, both in humans and their pets.1, 2, 3, 4 Cats are particularly sensitive to hepatic lipidosis, which occurs when, for example, obese cats stop eating and rapidly lose weight. Fat stores in adipose tissue are broken down to free fatty acids (FFAs) and transported through the blood to the liver, where they accumulate as triacylglycerol (TAG) in lipid droplets in hepatocytes. Cats with hepatic lipidosis accumulate approximately 34% more TAG in their livers compared to healthy cats.3, 5 They are often clinically presented with icterus, dehydration, lethargy, and ptyalism, with a history of anorexia and weight loss.6, 7 Treatment is mainly supportive and consists of correcting hypoperfusion using fluid therapy and reversing the negative energy balance by feeding the patient by means of force feeding or tube feeding.7 A mortality rate of 38% has been reported,6 and drugs to directly target lipidosis are not available yet. An in vitro model for hepatic lipidosis in cats has been described in which feline hepatic organoids were loaded with TAG.8 We aimed to use this model to test drugs for their potential to treat cats developing hepatic lipidosis. In cats fed a high‐fat diet that has induced early steatosis, both oleic and palmitic acid have been shown to increase in concentration in the serum.9 In feline liver organoids, adding additional oleic and palmitic acid to the medium resulted in the accumulation of TAG in lipid droplets, mimicking hepatic lipidosis.8 Furthermore, lipid accumulation also can be measured in organoids that are differentiated toward a hepatocyte‐like phenotype. This system allowed the effect of various drugs on TAG accumulation to be tested in a species‐specific system using primary feline liver cells, without the use of live animals.
2. MATERIALS AND METHODS
2.1. Cell culture
Organoids were derived from 6 different cats, 2 females and 4 males (sex distribution was a result of sample availability). The absence of liver pathology was assessed by liver histology, as reviewed by a board‐certified veterinary pathologist. Samples were obtained postmortem from surplus liver tissue; no animals were harmed or euthanized for the purpose of this study. Liver was sampled fresh within several hours after euthanasia or was obtained from a frozen (−70°C) tissue biobank. Feline liver organoid cultures were established according to a previously described method.8 In short, isolated biliary duct fragments were suspended in ice‐cold Matrigel (BD Biosciences, San Jose, California) and plated in drops on prewarmed 12‐well plates. The drops were allowed to solidify at 37°C before culture medium was added. Components for expansion and differentiation medium were similar to those previously described.8 Experiments were performed in expansion medium, unless stated otherwise. To differentiate into a more hepatocyte‐like phenotype, organoids from 3 different cats were plated in expansion medium and cultured for 2 days, after which time medium was changed toward differentiation medium for 10 days. At day 9 after switching to differentiation medium, the substrates and drugs were added. Medium was renewed every other day. Fatty acids used were oleic acid (C18:1) and palmitic acid (C16:0) at concentrations of 0.4 and 0.2 mM, respectively. Fatty acids were coupled to 12% (vol/vol) fatty acid‐free bovine serum albumin (Sigma‐Aldrich, St. Louis, Missouri). Bovine serum albumin without fatty acids was used as control. Before analyses, organoids were extracted from the Matrigel by washing with ice‐cold Hank's balanced salt solution and centrifugation at 360g for 5 minutes.
2.2. Drugs
Drugs (see Table S1 for targets and references) were dissolved in dimethyl sulfoxide and used at concentrations as described in the literature. Initial screening consisted of culturing undifferentiated feline liver organoids from 3 donors in the presence or absence of the drugs, as mentioned in Table S1, and in the presence of additional fatty acids to stimulate lipid accumulation. The TAG assay was used to determine TAG amounts. Drugs that inhibited TAG accumulation in organoids from 3 cats were selected for further testing. Selected drugs and concentrations used after initial screening were 5‐aminoimidazole‐4‐carboxamide 1‐β‐D‐ribofuranoside (AICAR; Sigma) at a concentration of 2 mM, T863 (Sigma) at a concentration of 20 μM, and PF 06424439 (Sigma) at a concentration of 50 μM. Organoids were cultured for 24 hours in the presence of additional fatty acids and drugs (or vehicle controls) before sampling.
2.3. Triacylglycerol assay
Samples were sonicated, and 10% of the sample was used to measure protein concentration for normalization purposes using a Pierce bicinchoninic acid Protein Assay Kit (ThermoFisher Scientific, Waltham, Massachusetts). The other part was used to extract lipids according to a previously described method.10 Samples were washed with methanol to prevent contamination with chloroform and dried under nitrogen gas. The TAG quantitation was performed using a TriglyceridesLiquiColor mono kit (HUMAN, Wiesbaden, Germany) with triolein as a standard. After incubation with the Triglycerides Liquicolor assay reagent for 90 minutes in a shaking water bath, TAG was measured using a spectrophotometer with a microplate reader at an extinction of 540 nm (Molecular Devices, VersaMax, Sunnyvale, California).
2.4. RNA isolation and quantitative PCR
Sample RNA was isolated using a RNeasy Micro Kit (Qiagen, Hilden, Germany) including an on‐column DNase‐I treatment to minimize gDNA contamination. Subsequently cDNA was synthesized using an iScript cDNA Synthesis Kit (Bio‐Rad, Hercules, California). The PCR amplifications were performed using a Bio‐Rad detection system with iQ SYBR Green Supermix (Bio‐Rad). Melt‐curve and sequence analysis confirmed the specificity of the amplicon, and the relative expression levels were normalized using the reference genes tyrosine 3‐monooxygenase/ tryptophan 5‐monooxygenase activation protein, zeta (YWHAZ), ribosomal protein S5(RPS5), hypoxanthine phosphoribosyltransferase (HPRT‐1), and hydroxymethylbilane synthase (HMBS). Primers used for PCR amplification of genes of interest and primers for the reference genes are shown in Table S2.
2.5. Fluorescent imaging of whole‐mount organoids
Organoids were carefully removed from Matrigel and fixed with 10% neutral buffered formalin. Nuclei were stained with 30 μg/mL Hoechst 33342 (Molecular Probes, Paisley, UK), and lipid droplets were stained with 0.1 μg/mL LD540 (lipophilic dye, kindly provided by Christoph Thiele). Fixed organoids were incubated with the dyes for 15 minutes in phosphate‐buffered saline, and after washing the organoids were mounted in FluorSave (Calbiochem, Billerica, Massachusetts). Images were taken with a Leica TCSSPE‐II confocal microscope at the Center of Cellular Imaging (Faculty of Veterinary Medicine, Utrecht University, The Netherlands).
2.6. Statistics
For statistical analyses, a 2‐way analysis of variance using a Dunnett's multiple comparisons test was performed, with treatment and cat as the 2 levels.
3. RESULTS
Addition of palmitate and oleate to feline organoids resulted in the accumulation of TAG and lipid droplets, as observed by fluorescence microscopy and fluorescent‐activated cell sorting (FACS) analysis.8 Quantification of the TAG accumulation showed a 4‐fold (±1.3, P < .001) increase in TAG accumulation in feline organoids after 24 hours of incubation with additional fatty acids in the expansion medium (Figure 1). The quantification of TAG accumulation allowed us to screen systematically for drugs affecting hepatic lipidosis in cats. Several drugs have been described in the literature for their possible effect on hepatic lipidosis. In a pilot study, we screened 8 different drugs (Table S1) on feline organoids from 3 different donors in the presence of additional fatty acids to mimic the induction of hepatic lipidosis. From these 8 different drugs at the described concentrations, 2 drugs induced lower amounts of TAG in all 3 donors as compared to fatty acid treatment without drugs and were subjected to further investigation. These 2 drugs were AICAR, an adenosine monophosphate (AMP) analog acting as an AMP kinase activator and T863, a diacylglycerol O‐acyltransferase 1 (DGAT1) inhibitor. Treatment of undifferentiated feline organoids from 6 donors with AICAR significantly decreased TAG accumulation by 46% (P = .003); treatment with T863 resulted in a decrease of 55% (P < .001; Figure 1). The DGAT2 inhibitor PF 06424439 did not induce a clear decrease in TAG accumulation on its own, but, when combined with the DGAT1 inhibitor, TAG synthesis was completely prevented, resulting in TAG amounts that were similar to those present in organoids cultured without added fatty acids. Analysis of organoids by fluorescence microscopy using LD540, a marker for lipid droplets, showed an increase in lipid droplets in FFA‐treated compared to control‐treated organoids. A clear decrease in the amount of fluorescent signal was observed when the selected drugs were present (Figure 2), a finding consistent with the quantitative TAG measurements. Drug treatment did not cause visible morphological changes to the organoids. Organoids had a darker appearance after the addition of fatty acids, as previously described (see Kruitwagen et al8 and Figure S1).
Figure 1.
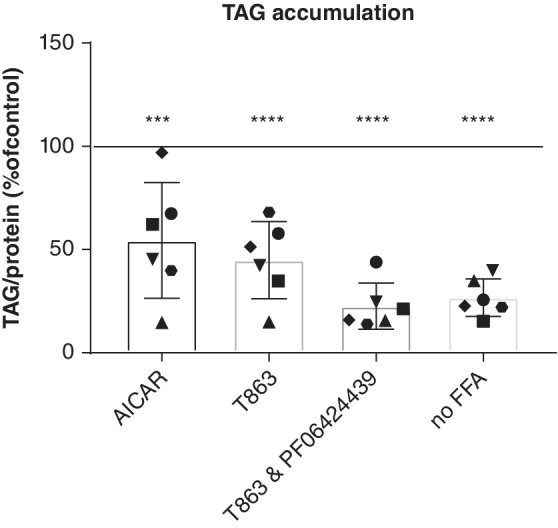
Triacylglycerol accumulation in undifferentiated feline liver organoids. Organoids from 6 different cats were incubated in the absence (no‐free fatty acids [FFA]) or presence of additional fatty acids and in the absence or presence of 5‐aminoimidazole‐4‐carboxamide 1‐β‐D‐ribofuranoside (AICAR, an adenosine monophosphate kinase activator, 2mM), T863 (diacylglycerol O‐acyltransferase 1 [DGAT1] inhibitor, 20 μM) or a combination of a T863 (DGAT1 inhibitor, 20 μM) and PF 06424439 (DGAT2 inhibitor, 50 μM). *** P = .003, **** P < .001. 100% represents TAG accumulation in the presence of additional fatty acids and dimethyl sulfoxide as vehicle control. Each dot represents the average of a culture duplicate from 1 cat
Figure 2.
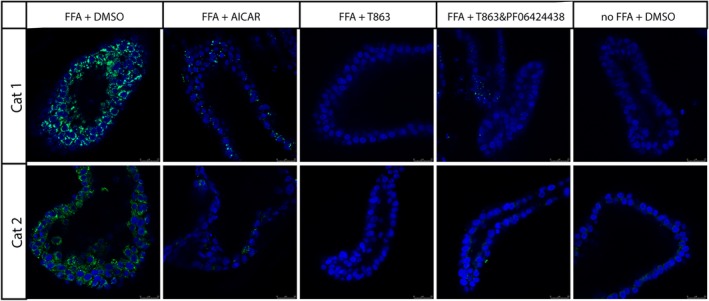
Presence of lipid droplets in feline liver organoids after inducing lipid accumulation. Organoids were incubated in the absence (no‐free fatty acids [FFA]) or presence (FFA) of additional fatty acids and in the absence (dimethyl sulfoxide) or presence of 5‐aminoimidazole‐4‐carboxamide 1‐β‐D‐ribofuranoside (AICAR, an adenosine monophosphate kinase activator, 2mM), T863 (diacylglycerol O‐acyltransferase 1 [DGAT1] inhibitor, 20 μM), or a combination of a T863 (DGAT1 inhibitor, 20 μM) and PF 06424439 (DGAT2 inhibitor, 50 μM). Fluorescence microscopic images are from 2 different cats (cat 1 and cat 2). Blue = Hoechst, green = LD540
Because differentiated organoids might even more closely mimic in vivo liver, organoids from 3 different donors were differentiated toward the hepatocyte lineage. Differentiation was confirmed using qPCR (Figure 3A). Expression of the stem cell marker leucine‐rich repeat‐containing G protein‐coupled receptor 5 decreased and expression of hepatic markers transthyretin and CYP3a132 increased. Basic TAG concentrations (without addition of FFAs and drugs) increased in organoids after differentiation compared to organoids before differentiation (Figure 3B). When differentiated organoids were treated with AICAR and T863, the results were similar to those of the undifferentiated organoids. Treatment with AICAR decreased TAG accumulation by 45% (P = .006), and treatment with T863 decreased TAG accumulation by 52% (P = .002; Figure 3C).
Figure 3.
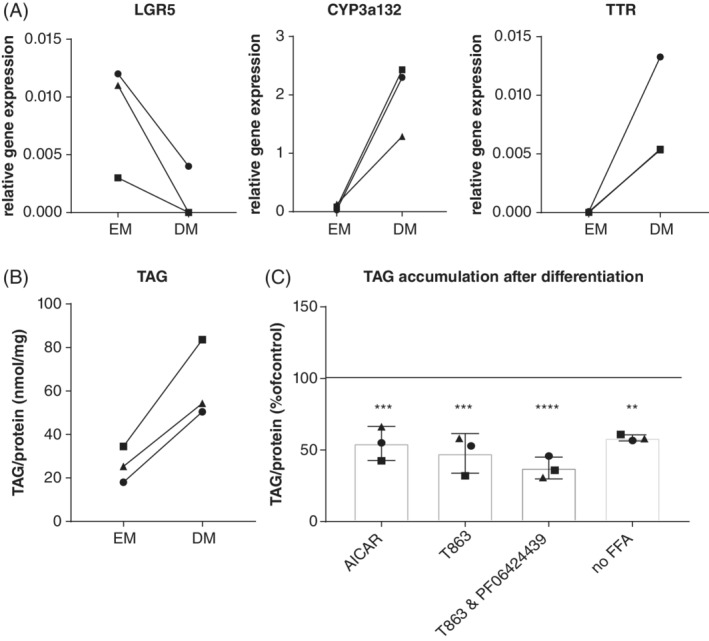
Triacylglycerol accumulation in differentiated feline liver organoids. A and B, Organoids were cultured in expansion medium (EM) or differentiation medium (DM), and relative gene expression of stem cell (leucine‐rich repeat‐containing G protein‐coupled receptor 5) and hepatocyte (TTR and CYP3a132) marker genes (A) and intracellular TAG concentrations (B) was measured. C, TAG accumulation in differentiated feline liver organoids after administration of drugs. Organoids were incubated in the absence (no‐free fatty acids [FFA]) or presence of additional fatty acids and in the absence or presence of 5‐aminoimidazole‐4‐carboxamide 1‐β‐D‐ribofuranoside (AICAR, an adenosine monophosphate kinase activator, 2mM), T863 (diacylglycerol O‐acyltransferase 1 [DGAT1] inhibitor, 20 μM), or a combination of a T863 (DGAT1 inhibitor, 20 μM) and PF 06424439 (DGAT2 inhibitor, 50 μM). 100% represents TAG accumulation in the presence of additional fatty acids, without drugs. **P = .01 ***P = .006, ****P < .001
The mechanism by which T863 decreased the accumulation of TAG most likely is by inhibiting its target, the TAG‐synthesizing enzyme DGAT1. Less obvious is the mechanism by which the AMP kinase activator AICAR decreases TAG. Perilipin 2 (PLIN2, also known as adipose differentiation‐related protein) is a protein present on lipid droplets and is known to stabilize lipid droplets. Gene expression of PLIN2 increases with the addition of fatty acids to the medium.8 We therefore measured the PLIN2 mRNA concentrations using qPCR and found that treatment with AICAR markedly decreased the expression of PLIN2 in lipid‐loaded organoids (Figure 4). Under these conditions, PLIN2 expression was not statistically different from incubation in the absence of additional fatty acids. This effect was specific for AICAR, as the other TAG‐lowering drug T863 did not significantly affect PLIN2 mRNA concentrations. Both differentiated and undifferentiated organoids behaved similarly (P = .57; Figure 4).
Figure 4.
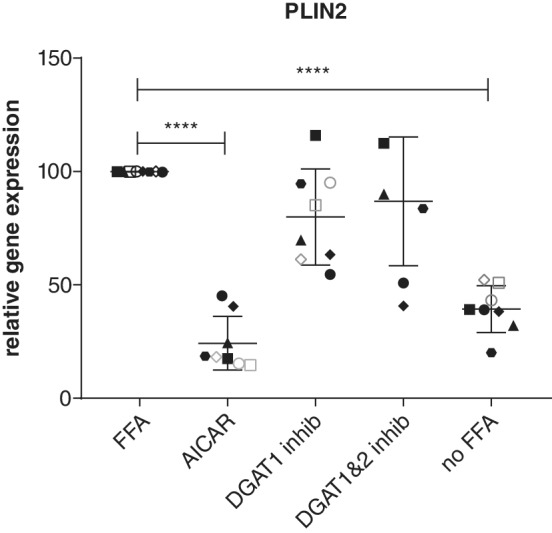
Relative gene expression of perilipin 2. Organoids were incubated in the absence (no‐free fatty acids [FFA]) or presence of additional fatty acids and in the absence or presence of 5‐aminoimidazole‐4‐carboxamide 1‐β‐D‐ribofuranoside (AICAR, an adenosine monophosphate kinase activator, 2mM), T863 (diacylglycerol O‐acyltransferase 1 [DGAT1] inhibitor, 20 μM), or a combination of a T863 (DGAT1 inhibitor, 20 μM) and PF 06424439 (DGAT2 inhibitor, 50 μM). Closed dots represent undifferentiated organoids, each dot one donor. Open dots represent differentiated organoids, each dot one donor. **** P < .001. PLIN2 gene expression in the presence of additional fatty acids is set at 100%
4. DISCUSSION
The feline hepatic organoid system was successfully used to identify drugs potentially useful in treating cats developing hepatic lipidosis. Both T863 (a DGAT1 inhibitor) and AICAR (an AMP kinase activator) decreased TAG accumulation, both before and after differentiation of the adult hepatic stem cells toward the hepatocyte lineage. The fact that undifferentiated liver organoids gave similar results as did differentiated organoids strengthens the use of undifferentiated liver organoids for future drug screening and more mechanistic studies, thereby decreasing the amount of time and resources for these experiments as well as our conclusion that these agents are promising candidate drugs for clinical application.
Two enzymes are known to catalyze the final step in the formation of TAG from diacylglycerol (DAG) and fatty acyl‐CoA: DGAT 1 and 2. Diacylglycerol O‐acyltransferase 1 knock‐out mice are viable, in contrast to DGAT2 knock‐out mice. Moreover, DGAT1‐deficient mice are resistant to obesity induced by a high‐fat diet and have increased sensitivity to insulin.11, 12 They are protected from hepatic lipidosis induced by exogenous fatty acids, either from a high‐fat diet or from fasting. Both a liver‐specific and a general knock‐down resulted in the protection against hepatic lipidosis.13 Several DGAT1 inhibitors have been developed, T863 is one among them. Administration of DGAT1 inhibitors to mice led to a decrease in serum and liver TAGs, caused weight loss in obese mice, and increased insulin sensitivity.14, 15 Whereas mice tolerate treatment with DGAT1 inhibitors very well, clinical trials in humans all have been associated with adverse gastrointestinal effects, mainly severe diarrhea.15 This species difference might be caused by the fact that humans lack DGAT2 expression in the small intestine.16 Whether DGAT2 is expressed in the feline intestine is unknown.
In our feline model, a DGAT1 inhibitor decreased the accumulation of TAG, whereas a DGAT2 inhibitor did not. This observation suggests that DGAT1 mainly is important in synthesizing TAG from external fatty acids. This conclusion is in agreement with a previously described model system,13 showing a role for DGAT1 in the response to exogenous fatty acids. Administration of a combination of DGAT1 and DGAT2 inhibitors resulted in stronger inhibition of TAG accumulation than did the DGAT1 inhibitor alone. Potentially, DGAT2 might be involved in TAG synthesis in our system in which DGAT1 can compensate for DGAT2 inhibition but not conversely. It is also possible that DGAT2 only becomes active if no DGAT1 activity is present.
As with all drugs interfering with TAG synthesis and accumulation, lipotoxicity caused by FFAs might be a reason for caution in treating cats with hepatic lipidosis using DGAT1 inhibitors. Because in cats with hepatic lipidosis FFA concentration and flux in the blood are high, preventing TAG formation might cause relocation of fatty acids to other tissues or might increase fatty acid breakdown. Only when there is insufficient capacity to oxidize fatty acids might an accumulation of FFAs cause cytotoxicity. Moreover, DGAT1 has been implicated in the protection of mitochondrial function by preventing lipotoxicity during autophagy induced by starvation.17 Likewise, DGAT1 has been reported to protect the endoplasmic reticulum of adipocytes from lipotoxicity during lipolysis by repacking part of the released FFAs back into TAG.18
Although mice tolerate DGAT1 inhibition well, the adverse gastrointestinal effects observed in humans and the potential of increased lipotoxicity suggests caution in using them to treat cats with hepatic lipidosis. To our knowledge, DGAT1 inhibitors have not been used in cats before and potential adverse in vivo effects in this species therefore are unknown.
The drug AICAR is mainly known to the public for its potential use in doping, and it has been placed on the list of illegal substances by the World Anti‐Doping Agency. The reason it can be used for doping is that it is an exercise mimetic.19 After phosphorylation within the cell, AICAR becomes 5‐amino‐4‐imidazolecarboxamide ribose‐monophosphate (ZMP), an analog of AMP. Adenosine monophosphate (and ZMP) can activate AMP‐activated protein kinase (AMPK) both by direct allosteric activation and by promoting the reactivation of dephosphorylated AMPK by AMPK kinase (AMPKK).20 Activation of AMPK has various effects in different tissues. For example, it increases food intake by stimulating the hypothalamus. In muscles it increases fatty acid uptake and oxidation, in adipose tissue it decreases fatty acid synthesis and lipolysis, and in the liver it decreases gluconeogenesis and fatty acid synthesis and increases fatty acid oxidation. In short, it switches on catabolic pathways and switches off anabolic pathways (reviewed by Kahn et al21 and Viollet et al22). Activation of AMPK in mice with a fatty liver decreased the lipid content back to normal.23 Whereas these effects of AICAR on lipid metabolism would be very beneficial in cats with hepatic lipidosis, its inhibitory effect on gluconeogenesis might pose a risk for anorectic cats. A marked decrease in plasma glucose concentration in fasting mice after intraperitoneal injection of AICAR has been reported.24 On the other hand, cats with hepatic lipidosis have been reported to quickly become hyperglycemic after refeeding or after glucose infusion,25 in which case a decrease in plasma glucose concentration might also be beneficial.
Our results show a decrease in the expression of PLIN2 after treatment with AICAR. Perilipin 2 is a protein present on lipid droplets that is known to influence lipid storage within lipid droplets. Knockdown of PLIN2 in mice decreases the size and number of lipid droplets and decreases total TAG amounts within livers of mice. Knockdown protects against hepatic lipidosis induced by feeding mice a high‐fat diet.26, 27 The presence of PLIN2 on lipid droplets has been suggested to protect lipid droplets from degradation by autophagy.26 The PLIN2 protein can be phosphorylated by AMPK, after which it is targeted for degradation in lysosomes by chaperone‐mediated autophagy.28 After removal of PLIN2, the lipid droplets become susceptible to degradation either by cytosolic lipases or by macroautophagy.29 Our results show that mRNA expression of PLIN2 increases upon the addition of fatty acids to the medium, and this increase is prevented by treatment with AICAR but not significantly after treatment with T863. It remains to be determined whether AICAR acts directly or indirectly on PLIN2 expression.
The feline liver organoid system previously was introduced as a means to model hepatic lipidosis.8 To measure changes in lipid content, these investigators used an FACS‐based method. This approach is a time‐consuming and indirect method because it measures lipid content using LD540 as a fluorescent dye. It is not clear whether measuring LD540 by FACS is linear, and by generating single cells from organoids, a potential bias might be introduced, for example, if cells with large amounts of TAG are more vulnerable to cell death during these procedures. The method we used works faster and measures TAG directly by an established method that is linear to many orders of magnitude. Results from samples in the absence or presence of additional FFAs showed that this new method is sensitive enough to detect basal concentrations of intracellular TAGs and the increase induced by additional fatty acids. Preventing synthesis of TAG by addition of both a DGAT1‐ and a DGAT2‐ inhibitor resulted in no increase in TAG. Although this hepatic model is the only species‐specific in vitro model available for cats, drugs identified in our study require further testing before application in the clinic. For the DGAT1 inhibitor, a study to evaluate potential gastrointestinal adverse effects in healthy cats is advisable. In the case of AICAR, plasma glucose concentrations must be closely monitored in cats with hepatic lipidosis.
To conclude, 2 potential drugs useful in the treatment of cats with hepatic lipidosis were identified. The drug T863 inhibits DGAT1, indicating that DGAT1 is the primary enzyme responsible for TAG synthesis from external fatty acids in cat liver organoids. The drug AICAR may act as a lipid‐lowering compound by decreasing PLIN2 mRNA expression. Our study shows the potential for the use of feline adult stem cells as an in vitro tool for drug testing in a species‐specific system and provides the basis for further clinical testing of these steatosis‐lowering drugs.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Authors declare no IACUC or other approval was needed.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
Supporting information
Figure S1 Morphology of feline liver organoids after inducing lipid accumulation in the presence or absence of drugs. Organoids were incubated in the absence (noFFA) or presence (FFA) of additional fatty acids and in the absence (DMSO) or presence of AICAR (AMP kinase activator, 2mM), T863 (DGAT1 inhibitor, 20 μM) or a combination of a T863 (DGAT1 inhibitor, 20 μM) and PF 06424439 (DGAT2 inhibitor, 50 μM).
Table S1 Supporting information
Table S2: Supporting information
ACKNOWLEDGMENTS
This study was sponsored by the Winn Feline Foundation (grant no. W17‐015). We thank Ingrid Vernooij for performing pilot experiments for the project.
Haaker MW, Kruitwagen HS, Vaandrager AB, et al. Identification of potential drugs for treatment of hepatic lipidosis in cats using an in vitro feline liver organoid system. J Vet Intern Med. 2020;34:132–138. 10.1111/jvim.15670
Maya W. Haaker and Hedwig S. Kruitwagen contributed equally to this work.
Funding information Winn Feline Foundation, Grant/Award Number: W17‐015
REFERENCES
- 1. Seidell JC. Obesity, insulin resistance and diabetes—a worldwide epidemic. Br J Nutr. 2000;83(S1):8‐11. 10.1017/S000711450000088X. [DOI] [PubMed] [Google Scholar]
- 2. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease—meta‐analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73‐84. 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 3. Armstrong PJ, Blanchard G. Hepatic Lipidosis in cats. Vet Clin North Am Small Anim Pract. 2009;39(3):599‐616. 10.1016/j.cvsm.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 4. Chandler M, Cunningham S, Lund EM, et al. Obesity and associated comorbidities in people and companion animals: a one health perspective. J Comp Pathol. 2017;156(4):296‐309. 10.1016/j.jcpa.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 5. Hall JA. Lipid composition of hepatic and adipose tissues from normal cats and from cats with idiopathic hepatic lipidosis. J Vet Intern Med. 1997;11(4):238‐242. [DOI] [PubMed] [Google Scholar]
- 6. Kuzi S, Segev G, Kedar S, Yas E, Aroch I. Prognostic markers in feline hepatic lipidosis: a retrospective study of 71 cats. Vet Rec. 2017;181(19):512 10.1136/vr.104252. [DOI] [PubMed] [Google Scholar]
- 7. Valtolina C, Favier RP. Feline Hepatic Lipidosis. Vet Clin North Am Small Anim Pract. 2017;47(3):683‐702. 10.1016/j.cvsm.2016.11.014. [DOI] [PubMed] [Google Scholar]
- 8. Kruitwagen HS, Oosterhoff LA, Vernooij IGWH, et al. Long‐term adult feline liver organoid cultures for disease modeling of hepatic Steatosis. Stem Cell Rep. 2017;8(4):822‐830. 10.1007/s11015-011-9426-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fujiwara M, Mori N, Sato T, et al. Changes in fatty acid composition in tissue and serum of obese cats fed a high fat diet. BMC Vet Res. 2015;11(1):1‐8. 10.1186/s12917-015-0519-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911‐917. [DOI] [PubMed] [Google Scholar]
- 11. Chen HC, Smith SJ, Ladha Z, et al. Increased insulin and leptin sensitivity in mice lacking acyl CoA:diacylglycerol acyltransferase 1. J Clin Invest. 2002;109(8):1049‐1055. 10.1172/JCI0214672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Smith SJ, Cases S, Jensen DR, et al. Obesity resistance and multiple mechanisms of triglyceride synthesis in mice lacking Dgat. Nat Genet. 2000;25(1):87‐90. 10.1007/BF02897157. [DOI] [PubMed] [Google Scholar]
- 13. Villanueva CJ, Monetti M, Shih M, et al. Specific role for acyl CoA:diacylglycerol acyltransferase 1 (Dgat1) in hepatic steatosis due to exogenous fatty acids. Hepatology. 2009;50(2):434‐442. 10.1002/hep.22980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cao J, Zhou Y, Peng H, et al. Targeting acyl‐CoA:Diacylglycerol Acyltransferase 1 (DGAT1) with small molecule inhibitors for the treatment of metabolic diseases. J Biol Chem. 2011;286(48):41838‐41851. 10.1074/jbc.M111.245456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Devita RJ, Pinto S. Current status of the research and development of diacylglycerol o ‐acyltransferase 1 (DGAT1) inhibitors. J Med Chem. 2013;56(24):9820‐9825. 10.1021/jm4007033. [DOI] [PubMed] [Google Scholar]
- 16. Haas JT, Winter HS, Lim E, et al. DGAT1 mutation is linked to a congenital diarrheal disorder. J Clin Invest. 2012;122(12):4680‐4684. 10.1172/JCI64873DS1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nguyen TB, Louie SM, Daniele JR, et al. DGAT1‐dependent lipid droplet biogenesis protects mitochondrial function during starvation‐induced autophagy. Dev Cell. 2017;42(1):9‐21.e5. 10.1016/j.devcel.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chitraju C, Mejhert N, Haas JT, et al. Triglyceride synthesis by DGAT1 protects adipocytes from lipid‐induced ER stress during lipolysis. Cell Metab. 2017;26(2):407‐418. 10.1016/j.cmet.2017.07.012.Triglyceride. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Guerrieri D, Moon HY, van Praag H. Exercise in a pill: the latest on exercise‐Mimetics. Brain Plast. 2017;2(2):153‐169. 10.3233/BPL-160043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Corton JM, Gillespie JG, Hawley SA, Hardie DG. 5‐Aminoimidazole‐4‐Carboxamide Ribonucleoside: a specific method for activating AMP‐activated protein kinase in intact cells? Eur J Biochem. 1995;565:1‐8. [DOI] [PubMed] [Google Scholar]
- 21. Kahn BB, Alquier T, Carling D, Hardie DG. AMP‐activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 2005;1(1):15‐25. 10.1016/j.cmet.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 22. Viollet B, Foretz M, Guigas B, et al. Activation of AMP‐activated protein kinase in the liver: a new strategy for the management of metabolic hepatic disorders. J Physiol. 2006;574(1):41‐53. 10.1113/jphysiol.2006.108506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Boudaba N, Marion A, Huet C, Pierre R, Viollet B, Foretz M. AMPK re‐activation suppresses hepatic Steatosis but its Downregulation does not promote fatty liver development. EBioMedicine. 2018;28:194‐209. 10.1016/j.ebiom.2018.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vincent MF, Erion MD, Gruber HE, Van Den Berghe G. Hypoglycaemic effect of AICAriboside in mice. Diabetologia. 1996;39(10):1148‐1155. 10.1007/BF02658500. [DOI] [PubMed] [Google Scholar]
- 25. Biourge V, Nelson RW, Feldman EC, Willits NH, Morris JG, Rogers QR. Effect of weight gain and subsequent weight loss on glucose tolerance and insulin response in healthy cats. J Vet Intern Med. 1997;11(2):86‐91. 10.1111/j.1939-1676.1997.tb00078.x. [DOI] [PubMed] [Google Scholar]
- 26. Tsai TH, Chen E, Li L, et al. The constitutive lipid droplet protein PLIN2 regulates autophagy in liver. Autophagy. 2017;13(7):1130‐1144. 10.1080/15548627.2017.1319544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chang BH‐J, Li L, Paul A, et al. Protection against fatty liver but Normal Adipogenesis in mice lacking adipose differentiation‐related protein. Mol Cell Biol. 2006;26(3):1063‐1076. 10.1128/MCB.26.3.1063-1076.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kaushik S, Cuervo AM. AMPK‐dependent phosphorylation of lipid droplet protein PLIN2 triggers its degradation by CMA. Autophagy. 2016;12(2):432‐438. 10.1080/15548627.2015.1124226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kaushik S, Cuervo AM. Degradation of lipid droplet‐associated proteins by chaperone‐ mediated autophagy facilitates lipolysis. Nat Cell Biol. 2015;17(6):759‐770. 10.1586/14737175.2015.1028369.Focused. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Morphology of feline liver organoids after inducing lipid accumulation in the presence or absence of drugs. Organoids were incubated in the absence (noFFA) or presence (FFA) of additional fatty acids and in the absence (DMSO) or presence of AICAR (AMP kinase activator, 2mM), T863 (DGAT1 inhibitor, 20 μM) or a combination of a T863 (DGAT1 inhibitor, 20 μM) and PF 06424439 (DGAT2 inhibitor, 50 μM).
Table S1 Supporting information
Table S2: Supporting information


