Abstract
Background
Straight‐ and branched‐chain (BCFA) short‐chain fatty acids (SCFAs) are produced by colonic microbiota and have both beneficial and deleterious effects in humans with chronic kidney disease (CKD). Fecal SCFAs in cats with CKD have not been described.
Objective
To characterize fecal SCFA concentrations in cats with CKD as compared to healthy geriatric cats and correlate SCFA to serum indoxyl sulfate (IS) and p‐cresol sulfate (pCS) concentrations.
Animals
Twenty‐eight cats with CKD (International Renal Interest Society [IRIS] stages 2, 3, and 4) and 11 older (≥ 8 years) healthy geriatric cats.
Methods
Prospective, cross‐sectional study. Voided feces were analyzed using stable isotope dilution gas chromatography‐mass spectrometry to determine fecal concentrations of SCFAs. Serum concentrations of IS and pCS were measured using liquid chromatography tandem mass spectrometry.
Results
Fecal isovaleric acid concentrations were significantly higher in CKD cats(P = .02) Cats with IRIS CKD stage 3 and 4 had significantly higher fecal isovaleric acid concentrations compared to healthy geriatric cats (P = .03), but not compared to IRIS CKD stage 2 cats. Total fecal concentrations of BCFAs were found to correlate weakly with serum creatinine concentration (rho, 0.33; P = .05), blood urea nitrogen concentration (rho, 0.40; P = .01), and pCS concentration (rho, 0.35; P = .04).
Conclusions and Clinical Importance
Fecal isovaleric acid concentrations were higher in CKD cats, particularly in late stage disease, compared to healthy geriatric cats. Fecal BCFA concentrations correlated with pCS and were higher in cats with muscle wasting, providing evidence for malassimilation of protein in CKD cats.
Keywords: branched‐chain fatty acids, chronic renal failure, feline, isovaleric acid, short‐chain fatty acids, uremic toxins
Abbreviations
- BCS
body condition score
- BCFA
branched‐chain fatty acid
- CKD
chronic kidney disease
- DM
dry matter
- ESRD
end‐stage renal disease
- IS
indoxyl sulfate
- IRIS
International Renal Interest Society
- pCS
p‐cresol sulfate
- SCFA
straight‐chain short‐chain fatty acid
- SDMA
symmetric dimethylarginine
- USG
urine specific gravity
1. INTRODUCTION
The gut microbiome and its microbial metabolites have been implicated in the pathogenesis of chronic kidney disease (CKD). In humans with CKD, the number of beneficial colonic bacteria that produce short‐chain fatty acids (SCFAs) is decreased with a concurrent increase in bacteria that produce the major uremic toxins indoxyl sulfate (IS), p‐cresol sulfate (pCS), and trimethylamine‐N‐oxide.1, 2, 3
Although there is limited information regarding the microbiome and its link to kidney disease in veterinary medicine, cats with CKD have fecal dysbiosis characterized by decreased fecal microbial diversity and richness based on 16S ribosomal rRNA gene sequencing.4 Additionally, IS is increased in cats with CKD and is associated with disease progression,5, 6 and in some CKD cats, serum pCS concentrations are increased compared to healthy geriatric controls.4 However, to date, fecal SCFA concentrations have not been characterized in cats with CKD.
The SCFAs produced by the colonic microbiota consist of the straight‐chain SCFAs acetic acid, propionic acid, butyric acid, valeric acid, and the branched‐chain fatty acids (BCFA) isovaleric acid and isobutyric acid. The straight‐chain SCFAs are the most abundant SCFAs in the human intestinal tract, representing 90%‐95% of the SCFA present in the colon.7 Straight‐chain SCFAs are major end‐products of saccharolytic fermentation of complex polysaccharides (including non‐digestible dietary fiber) and epithelial‐derived mucus, and are essential nutrients vital for both intestinal and host health.8 They have several beneficial local and systemic effects including promotion of colonic motility, facilitation of lipid and glucose metabolism, blood pressure regulation, and anti‐inflammatory properties.9, 10, 11, 12, 13, 14 In contrast, BCFAs represent only a small portion (5%) of total SCFA production and are produced when protein passes through the small intestine unabsorbed and protein‐derived branched chain amino acids are fermented by microbiota in the colon.8, 15, 16 Branched‐chain SCFAs and other products of protein fermentation in the colon are considered deleterious to the gut and may promote inflammation as well as have negative effects on motility in a rodent model.15, 16, 17
Thus, because of the potential implications of SCFA for gut health, our primary aim was to characterize fecal SCFA concentrations in healthy geriatric cats and cats with CKD. A secondary aim was to evaluate the correlation between the fecal SCFA concentrations and serum concentrations of IS and pCS. We hypothesized that cats with CKD would have an altered SCFA profile when compared to healthy control cats, and fecal BCFAs would correlate positively to serum concentrations of IS and pCS.
2. MATERIALS AND METHODS
2.1. Study design and selection of cats
This prospective, cross‐sectional study was performed at Colorado State University Veterinary Teaching Hospital. The same population of CKD and geriatric control cats was used in a previous publication evaluating the fecal microbiome using 16S ribosomal RNA gene sequencing and serum IS and pCS concentrations.4 To be eligible for inclusion, CKD and control cats underwent a thorough evaluation that included a review of the past medical record, complete physical examination (including 9‐point body condition score [BCS; Nestle Purina, St. Louis, Missouri] and muscle condition score [MCS]), minimum database consisting of CBC (Advia 120 Hematology System, Siemens Healthineers, Erlangen, Germany), serum biochemistry panel (Cobas 6000, Roche Diagnostics, Indianapolis, Indiana), and urinalysis, serum total thyroxine (T4) concentration, blood pressure, fecal flotation, and urine protein:creatinine ratio (UPC; Cobas 6000, Roche Diagnostics; if urine dipstick testing detected ≥1+ protein). Evidence of azotemic CKD was defined as a serum creatinine concentration > 1.6 mg/dL on at least 2 time points (over at least 3 months) together with urine specific gravity (USG) <1.035 on at least 1 occasion or an increase in serum creatinine concentration on at least 2 time points together with an increase in symmetric dimethylarginine (SDMA) concentration (>14 μg/dL; IDEXX Laboratories, Westbrook, Maine) interpreted in combination with clinical history and physical examination findings consistent with CKD. The CKD cats were staged based on International Renal Interest Society (IRIS) guidelines.18 Geriatric cats (≥ 8 years) were recruited from employees, students, and staff of the veterinary teaching hospital. Geriatric cats were considered healthy based on unremarkable client history and past medical record review, physical examination, and normal laboratory test results including serum creatinine concentration <1.6 mg/dL and USG >1.035.
Exclusion criteria based on a review of available history, examination, and diagnostics tests included complications of CKD such as acute obstructive or neoplastic urinary disease, urinary tract infection, recent hospitalization (<2 months), medications known to alter the intestinal microbial composition received within 6 weeks before enrollment (antibiotics, antacids, probiotics), or diseases known to alter the intestinal microbial composition in cats including hyperthyroidism, diabetes mellitus, and known or suspected gastrointestinal disease including intestinal parasitism, food‐ or antibiotic‐responsive chronic enteropathy, and chronic diarrhea.4 A client questionnaire was provided to the owner and the following information was obtained: diet (brand name and amount offered of wet or dry food or both), current medications or supplements, medications or supplements administered in previous 3 months, appetite and fecal score (Nestle Purina) at the time of enrollment, clinical signs of constipation, and frequency of vomiting. A table with descriptions of MCS, appetite, fecal, and vomiting scores is provided in Table S1. The project was approved by the Clinical Review Board (#2016‐080) at Colorado State University, and all owners gave written informed consent before participation.
2.2. Fecal fatty acid analysis
At least 1 g of voided feces was collected by the owner within 12 hours of defecation and placed on ice until frozen within 24 hours of collection. The samples were stored at −80°C until analysis of fecal fatty acid concentrations. Fecal concentrations of straight‐chain SCFAs (i.e., acetic acid, propionic acid, butyric acid, valeric acid) and BCFAs (i.e., isobutyric acid, isovaleric acid) were measured using a stable isotope dilution gas chromatography‐mass spectrometryassay as previously described19 with some modifications. Briefly, the fecal samples were weighed, diluted 1:5 in extraction solution (2 N hydrochloric acid), and frozen at −80°C until analysis. After thawing, fecal samples were homogenized using a multitube vortexer for 30 minutes at room temperature, then fecal suspensions were centrifuged for 20 minutes at 2100g and 4°C. Supernatants then were collected using serum filters (Fisherbrand serum filter system; Fisher Scientific Inc, Pittsburgh, Pennsylvania). From each sample, 500 μL of supernatant was mixed with 25 μL of internal standard (200 mM heptadeuterated butyric acid) and extracted using diethyl ether on a C18 solid‐phase extraction column (Sep‐Pak C18 1 cc Vac Cartridge; Waters Corporation, Milford, Massachusetts). The organic phase of the samples was derivatized using N‐tert‐butyldimethylsilyl‐N‐methyltrifluoroacetamide (MTBSTFA) at room temperature for 60 minutes. A gas chromatograph (Agilent 7890A, Agilent Technologies Inc, Santa Clara, California) coupled with an electron ionization mass spectrometer (Agilent 5975C, Agilent Technologies Inc) was used for chromatographic separation and quantification of the derivatized samples. Separation was achieved using a DB‐1 ms capillary column (Agilent Technologies Inc). The gas chromatograph temperature program was as follows: 40°C held for 0.1 minute, increased to 70°C at 5°C/min, 70°C held for 3.5 minutes, increased to 160°C at 20°C/min, and finally increased to 280°C at 35°C/min, then held for 3 minutes. The total run time was 20.53 minutes. The mass spectrometer was operated in electron impact positive‐ion mode with selective ion monitoring at mass‐to‐charge ratios (M/Z) of 117 (acetate), 131 (propionate), 145 (isobutyrate and butyrate), 152 (heptadeuterated butyrate; internal standard), and 159 (isovalerate and valerate). Quantification was based on the ratio of the area under the curve of the internal standard and each of the fatty acids. The lower detection limits of fecal concentrations of acetate, propionate, butyrate, isobutyrate, isovalerate, and valerate were 1.33 μmol/g, 0.43 μmol/g, 0.12 μmol/g, 0.03 μmol/g, 0.02 μmol/g, and 0.05 μmol/g, respectively. To take into account differences in water content among fecal samples, final concentrations of fecal SCFAs were adjusted by fecal dry matter (DM) and expressed as μmol/g of fecal DM.
2.3. Assays for serum IS and p‐CS concentrations
Blood was collected in sterile non‐heparinized tubes and centrifuged at 5000 rpm for 5 minutes. Serum was harvested and samples were frozen and stored at −80°C until analysis by Colorado State University. Total IS and pCS serum concentrations were determined by liquid chromatography tandem mass spectrometry as previously described.4 The accuracy +/− coefficient of variation of quality assurance/quality control samples among the batches analyzed for this study was 93.7% ± 4.8% for IS and 92.5% ± 4.0% for pCS.
2.4. Statistical analysis
Because only 2 IRIS CKD stage 4 cats could be enrolled in the study, IRIS CKD stage 3 and 4 cats were combined for statistical analyses between the stages of CKD. When fecal fatty acid concentrations were compared to clinical scores and dietary consumption, the latter were grouped as follows for analysis: MCS (normal muscle mass [MCS 0] versus muscle atrophy [MCS 1‐3]), vomiting score (normal vomiting score [0‐1] versus abnormal vomiting score 2, 3), BCS categories (too thin [BCS 1‐4] versus ideal body weight [BCS 5] versus overweight [BCS 6‐9]), appetite (ate >75% of food offered by owner versus ≤75% of food offered by owner), and consumption of renal diet (exclusively consuming a renal diet versus consuming a maintenance diet). Data sets were assessed for normality using the Shapiro‐Wilk test and were analyzed using Prism (Version 8.0.2, Graph Pad Software Inc, La Jolla, California). In general, a Mann‐Whitney U test was used for comparison between 2 groups and Kruskal‐Wallis with Dunn's post hoc analysis used for comparison among ≥3 groups. Two‐tailed Spearman correlation coefficient (rho) was computed to evaluate the association between straight‐chain SCFAs and BCFAs and the following variables: hematocrit and serum creatinine, blood urea nitrogen (BUN), calcium, phosphorus, potassium, IS, and pCS concentrations. A value of 1.0 for analysis of total T4 results was used when the reported value was <1.0 (2/29 CKD cats). For all analyses, a value of P < .05 was considered significant, and data are represented as median and range.
3. RESULTS
3.1. Cats
Fecal straight‐chain SCFA and BCFA concentrations were measured in 28 cats with CKD (16 cats had IRIS CKD stage 2, 10 cats had IRIS CKD stage 3, 2 cats had IRIS CKD stage 4) and 11 healthy geriatric control cats. Serum IS and pCS concentrations were available in a subset of healthy control cats (n = 10) and CKD cats (n = 26) from a previous analysis on the study population.4
Descriptions of age, BCS, MCS, clinical scores, and diagnostic laboratory findings from cats in each group (healthy geriatric cats, IRIS CKD stage 2 cats, IRIS CKD stage 3 and 4 cats) are summarized in Table 1. Twenty‐one of 28 (75%) cats with CKD had a urinary ultrasound examination with 18 of 28 cats having changes consistent with chronic renal degenerative disease, 2 of 29 cats with apparently normal renal architecture, and 1 of 29 cats with chronic unilateral obstructive ureteroliths and concurrent renal degenerative disease. No sonographic evidence of neoplasia or pyelonephritis was identified in the CKD cats. Abdominal imaging was not performed on healthy geriatric cats. Healthy geriatric cats were significantly younger compared to the IRIS CKD stage 2 cats (P = .006). No significant difference was found in the age of IRIS CKD stage 3 and 4 cats compared to healthy geriatric cats and IRIS CKD stage 2 cats. Serum total T4 concentration at time of enrollment excluded hyperthyroidism. Two CKD cats had serum total T4 concentration <1.0 which was suspected to be associated with non‐thyroidal illness. All healthy geriatric controls and most CKD cats (22/28) were negative for proteinuria on urine dipstick testing. Of the 6 of 28 CKD cats that had ≥1+ protein on urine dipstick testing, only 3 had borderline proteinuria (UPC, 0.22, 0.23, 0.24), and the remaining cats were non‐proteinuric (UPC < 0.2).
Table 1.
Characteristics of study groups including healthy control cats, IRIS CKD stage 2 cats, and IRIS CKD stage 3 and 4 cats
| Healthy control cats (n = 11) | CKD stage 2 (n = 16) | CKD stages 3 and 4 (n = 12) | |
|---|---|---|---|
| Variable (reference interval) | Median (range) | Median (range) | Median (range) |
| Age (y) | 11 (8‐12)a | 15 (7‐22)b | 14.5 (7‐17.5) |
| BCS (1‐9) | 5 (4‐8) | 5 (3‐7) | 5 (2‐8) |
| MCS (0‐3) | 0 (0‐1)a | 1 (0‐2)b | 1 (0‐3)b |
| Fecal score (1‐7) | 2 (2) | 2 (1‐3) | 2 (2‐3) |
| Appetite score (0‐4) | 0 (0‐1)a | 1 (0‐3)b | 1 (0‐3)b |
| Vomiting score (0‐3) | 0 (0‐2)a | 2 (0‐3)b | 2 (0‐3) |
| Creatinine (0.8‐2.4 mg/dL) | 1.2 (0.7‐1.6)a | 2 (1.6‐2.6)b | 3.1 (2.9‐6.9)c |
| BUN (18‐35 mg/dL) | 24 (20‐38)a | 44 (20‐60)b | 54 (33‐98)b |
| Total calcium (9.2‐11.1 mg/dL) | 9.8 (9.1‐11.3)a | 10.1 (9.1‐10.7) | 10.5 (10‐11.4)b |
| Phosphorus (3.0‐6.0 mg/dL) | 4.3 (2.9‐5.0) | 3.7 (2.6‐5.6) | 4.4 (3.3‐8.1) |
| Potassium (3.7‐5.4 mEq/L) | 4.6 (4.2‐5.5) | 4.5 (3.9‐5.3) | 4.4 (4.1‐5.2) |
| Total T4 (1.2‐4.8 μg/dL) | 3.1 (1.9‐3.6)a | 2.0 (1.0‐3.7)b | 1.7 (1.0‐2.9)b |
Note: Rows with different superscript letters are significantly different from one another.
Abbreviations: BCS, body condition score; BUN, blood urea nitrogen; CKD, chronic kidney disease; IRIS, International Renal Interest Society; MCS, muscle condition score.
When clinical scores (BCS 1‐9, MCS 0‐3, fecal score 1‐7, vomiting score 0‐3, appetite score 0‐4) were compared between healthy geriatric cats and CKD cats, BCS was not significantly different between healthy control cats and CKD cats. Despite a similar BCS, MCS and thus severity of muscle atrophy were significantly more prominent in CKD cats (median score, 1; range, 0‐3) compared to healthy geriatric cats (median score, 0; range, 0‐1; P = .0002). When MCS was compared between stages of CKD, IRIS stage 2 (P = .048) and IRIS stage 3 and 4 (P = .0009) CKD cats had significantly more muscle atrophy when compared to healthy geriatric cats, but no difference was found between IRIS CKD stage 2 and stage 3 and 4 cats (Figure 1). Based on the owner questionnaire, cats with CKD (median score, 2; range, 0‐3) vomited more frequently compared to the healthy control cats (median score, 0; range, 0‐2; P = .004). Cats with CKD had poorer appetite (median score, 1; range, 0‐3) compared to healthy control cats (median score, 0; range, 0‐1; P = .001). Fecal score was not significantly different between healthy control cats and CKD cats.
Figure 1.
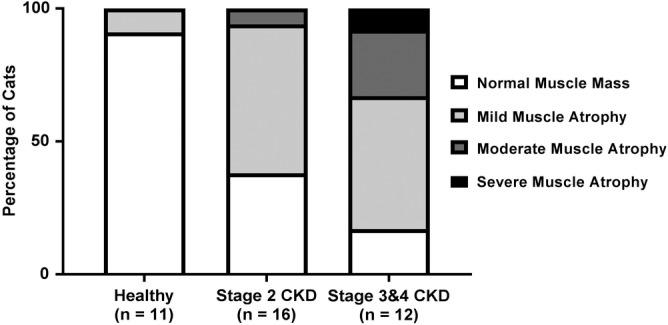
Percentage of cats affected by each muscle condition score for healthy control cats, IRIS CKD stage 2 cats, and IRIS CKD stage 3 and 4 cats. Muscle condition score was significantly higher in IRIS CKD stage 2 (P = .05) and IRIS CKD stage 3 and 4 cats (P = .0009) in comparison to healthy geriatric cats. CKD, chronic kidney disease; IRIS, International Renal Interest Society
For IRIS CKD stage 2 cats, 3 of 16 (19%) cats ate exclusively a commercial renal diet, 3 of 16 (19%) cats ate a combination of commercial renal diet and maintenance diet, and 10 of 16 (63%) cats ate variable commercial maintenance diets. For IRIS CKD stage 3 and 4 cats, 5 of 12 (42%) cats ate exclusively a commercial renal diet, 3 of 12 (25%) cats ate a combination of commercial renal diet and maintenance diet, and 4 of 12 (33%) cats ate variable commercial maintenance diets. All healthy geriatric cats were being fed variable commercial maintenance diets. Current medications at the time of enrollment for the CKD cats included transdermal mirtazapine gel (4/28 cats), amlodipine (4/28 cats), PO potassium supplementation (3/28 cats), and alendronate, maropitant, psyllium powder, aluminum hydroxide, glucosamine/chondroitin joint supplement, PO buprenorphine, and polyethylene glycol (1 cat each). None of the healthy geriatric cats were on medications at the time of enrollment.
3.2. Fecal SCFA and BCFA analysis
For healthy geriatric controls, the mean percentages of the total SCFA fecal concentration for acetic acid, propionic acid, butyric acid, valeric acid, isovaleric acid, and isobutyric acid were 60%, 22%, 12%, 2%, 3%, and 2%, respectively. For cats with CKD, the mean percentages of the total SCFA fecal concentration for acetic acid, propionic acid, butyric acid, valeric acid, isovaleric acid, and isobutyric acid were 58%, 21%, 11%, 4%, 5%, and 3%, respectively. Fecal fatty acid concentrations and serum IS and pCS concentrations for healthy geriatric control cats, IRIS CKD stage 2 cats, and IRIS CKD stage 3 and 4 cats are presented in Table 2. No significant differences among all CKD cats, IRIS CKD stages (stage 2, 3, and 4), and healthy geriatric cats were found in total fecal straight‐chain SCFA, total BCFA concentrations, or individual fecal concentrations of acetic acid, propionic acid, butyric acid, valeric acid, and isobutyric acid. Fecal concentrations of isovaleric acid were significantly increased in cats with CKD (median, 7.7 μmol/g; range, 2.5‐65.1) compared to healthy geriatric cats (median, 4.3 μmol/g; range, 2.9‐7.4; P = .02; Figure 2). When compared between the stages of CKD, IRIS CKD stage 3 and 4 cats had significantly higher (P = .03) fecal concentrations of isovaleric acid compared to healthy geriatric cats, but no significant difference was found between IRIS CKD stage 2 cats and IRIS CKD stage 3 and 4 cats, or between IRIS CKD stage 2 cats and healthy geriatric cats (Table 2).
Table 2.
Fecal fatty acid concentrations and serum IS and pCS concentrations in healthy geriatric control cats, IRIS CKD stage 2 cats, and IRIS CKD stage 3 and 4 cats
| Healthy controls cats (n = 11) | CKD stage 2 (n = 16) | CKD stages 3 and 4 (n = 12) | |
|---|---|---|---|
| Variable | Median (range) | Median (range) | Median (range) |
| Fecal straight‐chain SCFA | |||
| Acetic (μmol/g) | 130 (28‐371) | 144 (31‐347) | 200 (43‐321) |
| Propionic (μmol/g) | 42 (11‐154) | 53 (8.1‐270) | 45 (11‐125) |
| Butyric (μmol/g) | 24 (7.0‐238) | 22 (4.9‐122) | 21 (2.3‐88) |
| Valeric (μmol/g) | 3.0 (0.4‐23) | 4.1 (0.2‐47) | 2.9 (0.4‐48) |
| Total SCFA (μmol/g) | 203 (48‐635) | 263 (50‐533) | 299 (59‐488) |
| Fecal BCFA | |||
| Isovaleric (μmol/g) | 4.3 (2.9‐7.4)a | 5.7 (3.1‐24) | 9.6 (2.5‐65)b |
| Isobutyric (μmol/g) | 3.4 (2.4‐6.6) | 4.4 (1.9‐17) | 6.4 (1.2‐26) |
| Total BCFA (μmol/g) | 7.8 (5.3‐14) | 10 (4.9‐40) | 15 (3.7‐91) |
| Serum gut‐derived uremic toxins | |||
| IS (ng/mL) | 1201 (202‐2860)a | 3200 (746‐10300)b | 5070 (1020‐27 600)b |
| pCS (ng/mL) | 2905 (901‐7220) | 7290 (34‐30 600) | 5890 (189‐35 300) |
Note: Rows with a different superscript letters are significantly different from one another.
Abbreviations: BCFA, branched‐chain fatty acid; IS, indoxyl sulfate; pCS, p‐cresol sulfate; SCFA, short‐chain fatty acid.
Figure 2.
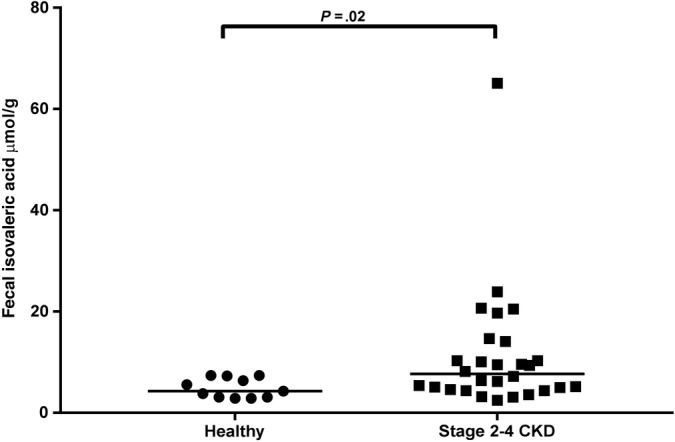
Fecal isovaleric acid concentrations in healthy geriatric cats and cats with IRIS stage 2‐4 CKD. Significantly higher isovaleric acid concentrations were seen in cats with CKD in comparison to healthy geriatric cats (P = .02). Significance remained when data were analyzed without outlier (P = .02). Dot plot illustrates the median values. CKD, chronic kidney disease; IRIS, International Renal Interest Society
When total fecal straight‐chain SCFA, total fecal BCFA, and individual fecal fatty acid concentrations (i.e. acetic acid, propionic acid, butyric acid, isovaleric acid, isobutyric acid, valeric acid) were compared to clinical scores (BCS, MCS, vomiting score, and appetite score categories) for all enrolled cats, no significant difference was found for BCS, vomiting score, or appetite score categories. For MCS, fecal total BCFA concentrations (P = .01; Figure 3), isovaleric acid concentrations (P = .01), and isobutyric acid concentrations (P = .03) were significantly higher in cats with muscle atrophy (MCS 1‐3) compared to cats with a normal muscle mass (MCS 0; Table 3). Serum IS concentrations were significantly higher in cats with muscle atrophy compared to cats without muscle atrophy (P = .01). No significant difference was found in serum pCS concentrations between cats with and without muscle atrophy.
Figure 3.
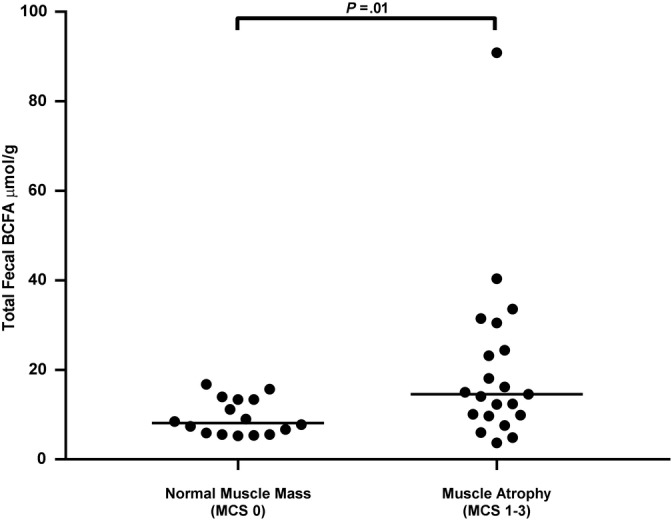
Fecal total branched‐chain fatty acid (BCFA) concentrations in cats with normal muscle mass (muscle condition score [MCS] 0) and cats with muscle atrophy (MCS 1‐3). Cats with muscle atrophy had significantly higher fecal total BCFA concentration compared to cats with normal muscle mass (P = .01). Significance remained when data were analyzed without outlier (P = .02). Dot plot illustrates the median values
Table 3.
Fecal branched‐chain fatty acid (BCFA) and serum indoxyl sulfate and p‐cresol sulfate concentrations in cats with normal muscle mass and cats with muscle atrophy
| Normal muscle mass (MCS 0) (n = 16) | Muscle atrophy (MCS 1‐3) (n = 21) | P‐value | |
|---|---|---|---|
| Fecal BCFA | |||
| Isovaleric (μmol/g) | 4.5 (2.9‐10.3) | 9.4 (2.5‐65.1) | .01 |
| Isobutyric (μmol/g) | 3.5 (2.2‐7.2) | 5.9 (1.2‐25.9) | .03 |
| Total BCFA (μmol/g) | 8.2 (5.3‐16.8) | 14.6 (3.7‐90.9) | .01 |
| Serum gut‐derived uremic toxins | |||
| Indoxyl sulfate (ng/mL) | 2170 (165‐4960) | 5070 (746‐27 600) | .01 |
| p‐Cresol sulfate (ng/mL) | 3620 (901‐13 100) | 4890 (34.3‐35 300) | .44 |
No significant differences in total or individual fecal SCFA concentrations were found when all CKD cats consuming exclusively a prescription renal diet were compared to those not exclusively consuming a renal diet. No significant differences in fecal SCFA concentrations were found between cats of the same CKD stage exclusively consuming a renal diet in comparison to those not exclusively consuming a renal diet, However, the highest concentrations of isovaleric acid in IRIS CKD stage 3 and 4 cats were observed in those cats not consuming a renal diet (n = 6; median, 14.7 μmol/g; range, 3.2‐65.1) versus IRIS CKD stage 3 and 4 cats that were consuming a renal diet (n = 5; median, 9.4 μmol/g; range, 2.5‐10.1).
Spearman correlation coefficients and P‐values for significant correlations between fecal fatty acid concentrations, serum IS and pCS concentrations, and renal laboratory variables are presented in Table 4. Neither total nor individual concentrations of fecal straight‐chain SCFAs correlated with serum IS or pCS concentrations or renal laboratory variables, with the exception of fecal concentration of valeric acid which was positively correlated with pCS serum concentration (rho, 0.48; P = .003). Total fecal concentrations of BCFAs correlated with serum creatinine (rho, 0.37; P = .02), BUN (rho, 0.43; P = .006), and pCS (rho, 0.37; P = .02) concentrations. When individual concentrations of BCFA were assessed, fecal isovaleric acid concentrations were correlated with serum creatinine (rho, 0.39; P = .01), BUN (rho, 0.46; P = .003), and pCS (rho, 0.36; P = .03) concentrations, but not with IS concentrations (P = .07).
Table 4.
Spearman correlation coefficients and P‐value for significant correlations found between fecal fatty acid concentrations, serum IS and pCS concentrations, and renal laboratory variables
| Spearman correlation coefficient | P‐value | |
|---|---|---|
| Valeric acid | ||
| pCS | 0.48 | .003 |
| Fecal total BCFA | ||
| Creatinine | 0.37 | .02 |
| BUN | 0.43 | .006 |
| pCS | 0.37 | .03 |
| Fecal isovaleric acid | ||
| Creatinine | 0.39 | .01 |
| BUN | 0.46 | .003 |
| pCS | 0.36 | .03 |
Abbreviations: BCFA, branched‐chain fatty acid; BUN, blood urea nitrogen; IS, indoxyl sulfate; pCS, p‐cresol sulfate.
When analysis was performed excluding an outlier stage 3 CKD cat that had extremely high fecal isovaleric acid concentration, CKD cats (all cats IRIS stage 2‐4) still had significantly higher fecal concentrations of isovaleric acid compared to healthy geriatric cats (P = .02). When individual stages of CKD were compared to healthy geriatric cats, no significant difference was found between healthy geriatric cats and IRIS stage 3 and 4 CKD cats (P = .05). Fecal total BCFA (P = .02), fecal isovaleric acid (P = .02), and fecal isobutyric acid (P = .05) concentrations continued to be significantly increased in cats with muscle atrophy. For the correlation analysis, total fecal concentrations of BCFAs still correlated with serum creatinine (rho, 0.33; P = .05), BUN (rho, 0.40; P = .01), and pCS (rho, 0.35; P = .03) concentrations. Fecal isovaleric acid concentrations continued to correlate with serum creatinine (rho, 0.35; P = .04) and BUN (rho, 0.43; P = .008) concentrations but no longer correlated with serum pCS concentrations (rho, 0.33; P = .05).
4. DISCUSSION
Our goal was to characterize fecal straight‐chain and branched‐chain SCFA concentrations in cats with CKD and compare these findings to a population of healthy geriatric cats. A prominent finding was significantly higher fecal concentrations of the BCFA isovaleric acid in CKD cats compared to control cats and its positive correlation with serum creatinine, BUN, and pCS concentrations. Both BCFAs and the uremic toxins IS and pCS are produced when amino acids escape absorption in the small intestine and are metabolized by the colonic microbiota.15 Because cats with CKD have higher serum IS and pCS concentrations,4, 5 these findings taken together suggest that cats with CKD may have protein malassimilation in the upper small intestinal tract. Similar findings are seen in both rat models and in humans with end‐stage renal disease (ESRD) where increased abundance of proteolytic bacteria and increased amounts of undigested amino acids in the colon have been described.20, 21 Protein malassimilation also is documented in humans with CKD.22 In 1 study, the percentage of dietary protein‐derived leucine that appeared in plasma after consumption was significantly decreased (41% ± 5%) compared to healthy controls (61% ± 4%).23 This finding is of particular interest because leucine is an amino acid that results in formation of BCFA in the colon.
Cats with muscle atrophy (MCS 1‐3) had significantly higher fecal isovaleric acid, isobutyric acid, and total BCFA concentrations as compared to cats with normal muscle mass (MCS 0). Previous literature suggests that weight loss and muscle wasting in human ESRD patients is caused, at least in part, by impaired small intestinal protein digestion and absorption as well as by alterations in protein metabolism and inadequate protein intake.24, 25 This scenario may be further complicated by age‐related changes in cats, with decreased protein digestibility described as an aspect of impaired digestive function in geriatric cats.26 In our study, 1 IRIS CKD stage 3 cat was an outlier compared to the other enrolled cats and had an extremely high fecal isovaleric acid concentration (Figure 2). When investigated, it was discovered that this patient was being fed a partially raw, high protein diet (Instinct Dry, Nature's Variety, St. Louis, Missouri) and was the only cat in the study to be receiving a high protein diet. When this cat was excluded from fecal fatty acid analysis, results were similar to those obtained when all cats were included. The exception was that a significant difference between healthy geriatric cats and IRIS CKD stage 3 and 4 cats no longer existed (P = .05). Taken overall, increased fecal BCFA concentrations may represent an indirect indicator of the efficiency of protein malassimilation in cats with CKD.27
Increased concentrations of fecal isovaleric acid and serum IS and pCS in cats with CKD may have implications for gastrointestinal health and motility. Although straight‐chain SCFAs, primarily butyrate and propionate, have been shown to have a prokinetic effect by promoting contraction of smooth muscle in the colon in ex vivo studies evaluated in colonic tissue from dogs,28 cats,29 and guinea pigs,30 BCFAs and other products of protein fermentation in the colon are considered deleterious to the gut. For example, isovaleric acid has been shown to cause colonic smooth muscle relaxation in an ex vivo rodent model.17 In addition, decreased gastrointestinal motility has been linked directly to uremia by causing dysbiosis and colonic inflammation secondary to increased uremic toxin exposure in rodent models.31, 32, 33 In an ex vivo rodent model, incubation of colons with the gut‐derived uremic toxins IS and pCS, but not urea, was shown to decrease force of contraction by 66% and 55%, respectively.33 This finding supports the idea that uremia directly causes impaired colonic motility, although other factors such as dehydration and hypokalemia also may be factors. Constipation is a common problem in humans on hemodialysis and has been shown to negatively impact health‐related quality of life.34, 35 Although the prevalence of constipation in cats with CKD is unknown, CKD in cats is associated with an increased risk of constipation.36 It is possible that higher fecal isovaleric acid and serum IS concentrations in CKD cats might contribute to constipation. In our study population, only 2 of 30 CKD cats had a fecal score of 1 (small hard feces) that may be associated with constipation, and no owners reported concern for overt signs of constipation in their CKD cats. However, subtle signs of constipation are difficult to detect for cat owners, and normal feces does not exclude the possibility of decreased frequency of defecation. Additional studies assessing the relationships among fecal isovaleric acid, defecation frequency, and stool quality are warranted.
Cats with CKD did not have significantly different fecal straight‐chain SCFA concentrations as compared to healthy geriatric cats. Factors that affect straight‐chain SCFA concentrations include the amount and type of fermentable carbohydrate consumption, composition and diversity of the intestinal microbiota, colonic transit time, and interactions between microbes and the host.37, 38, 39 In humans with CKD and ESRD, it is commonly recommended to decrease the dietary intake of fruits and vegetables to limit potassium intake and prevent hyperkalemia.40 This decrease in dietary fiber leads to a decrease in bacterial groups known to produce straight‐chain SCFAs in the colon, in particular butyric acid.1, 41 Dietary fiber restriction to prevent hyperkalemia is not recommended in cats with CKD because hypokalemia is a more common sequela of the disease. Therefore, microbial SCFA production may not be affected in cats to the same extent as in humans with CKD. Although cats are obligate carnivores, and comparison to humans may not be appropriate, it has been documented that dietary protein and fiber intake and nutrient sources affect both the microbiome composition of cats and microbial metabolite production in healthy cats, similar to what is observed in humans.42, 43, 44, 45 Specifically, the fiber source has been shown to affect fecal SCFA concentrations in healthy cats.42 A previous study evaluated the effect that 3 fiber sources with differences in fermentability, solubility, and prebiotic potential had on fecal microbial metabolites in healthy cats. Fecal acetate and propionate concentrations increased in cats that received a highly soluble, fermentable fiber (pectin), whereas fecal butyrate and BCFAs increased after supplementation with rapidly fermented, prebiotic fiber (fructooligosaccharide), and pectin.42 In our study, the diets of the enrolled cats were variable, and unfortunately the fiber source and intake for the cats could not be accurately determined. In addition, the specific microbiota responsible for SCFA production has yet to be determined in veterinary medicine. Although fecal bacterial composition determined by 16S rRNA gene sequencing previously was published in the same group of cats and the study confirmed dysbiosis in cats with CKD, no specific bacterial taxa were associated with the CKD cats compared to healthy geriatric cats.4 To further define the link between gut microbial composition and SCFA production in cats, shotgun metagenomic analysis on fecal samples in conjunction with targeted analysis of fecal SCFAs in cats on a well‐defined diet is needed to determine the bacterial groups responsible for fecal acid production in the colon of cats.
A few observations are important to note when evaluating differences in fecal SCFAs in CKD cats exclusively consuming a prescription renal diet versus those CKD cats consuming a maintenance diet in our study. Although most commercial renal diets are highly digestible and therefore moderately restricted in fiber content, in our study, CKD cats consuming a renal diet did not have statistically different fecal straight‐chain SCFA concentrations compared to cats fed a commercial maintenance diet. Additionally, renal diets are often lower in protein and highly digestible, which may explain the observed lower fecal isovaleric acid concentrations in CKD cats consuming a renal diet as compared to those that were not consuming a renal diet, most notably in the advanced stage IRIS CKD 3 and 4 cats. This difference, however, was not statistically significant, which may be a consequence of small sample size. Further evaluation of the link between dietary intake and fecal microbial metabolites could help advance our understanding of the potential benefits provided by nutritional modifications on cats with CKD.
Our study had several limitations. As previously mentioned, accurate determination of dietary protein and fiber intake was not possible because of the common practice of feeding a mixture or rotation of diets. Thus, analysis of a relationship between dietary protein and fiber source and fecal fatty acid concentrations was not possible. Although observations in people largely rely on the measurement of fecal SCFA concentrations, it is unclear whether fecal SCFA concentrations are a reliable proxy for luminal SCFA production.46 With regard to the cat populations, the control group was age‐group‐matched (≥ 8 years) rather than age‐matched to the CKD group. Because of the high prevalence of CKD in geriatric cats and the presence of concurrent disease (e.g., hyperthyroidism, chronic enteropathy), an age‐matched healthy control population could not be identified in the referral hospital population of cats. Additionally, although cats receiving medications documented to affect the microbiome in veterinary species were excluded from the study, several enrolled cats with CKD were receiving medications that might have affected the microbiome to an unknown extent. Because CKD patients typically are receiving multiple medications to manage their disease, it was not feasible to only enroll cats not receiving medication. Because of the exclusion criteria, only 2 IRIS stage 4 CKD cats were enrolled in our study; therefore, IRIS stage 3 and 4 CKD cats were combined into a single group for statistical analysis. Moreover, 2 cats with CKD maintained normal USG >1.035 and were diagnosed in accordance with IRIS recommendations18 based on persistently increased serum creatinine concentration >1.6 mg/dL over a 3‐month period and increased serum SDMA concentration. An additional limitation was that muscle mass score was based on physical examination, which previously has been shown to be somewhat imprecise.47 Lastly, a few geriatric cats in the healthy control group had laboratory results on the serum biochemistry panel that were outside the reference range (Table 1). Although the abnormal results were minimal, and not considered clinically relevant, we cannot exclude the possibility that the healthy control cat had subclinical disease that could have explained these abnormalities.
In conclusion, CKD in cats is associated with increased fecal isovaleric acid, particularly in IRIS CKD stage 3 and 4 cats. Fecal BCFA concentrations were positively correlated with serum pCS concentrations and severity of azotemia. Cats with muscle atrophy had higher fecal BCFA concentrations compared to cats without muscle atrophy. These findings support malassimilation of protein in cats with CKD. Future studies are needed to fully elucidate links among the gut microbiome, protein malassimilation, microbial fatty acid production, and clinical consequences in cats with CKD.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Approved by the Clinical Review Board (#2016‐080) at Colorado State University and all owners gave written informed consent before participation.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
Supporting information
Table S1 Clinical scoring system used to determine muscle condition, appetite, consistency of feces, and frequency of vomiting.
ACKNOWLEDGMENT
This study was presented in part in abstract form at the 2019 ACVIM Forum, Phoenix, AZ.
Summers S, Quimby JM, Phillips RK, et al. Preliminary evaluation of fecal fatty acid concentrations in cats with chronic kidney disease and correlation with indoxyl sulfate and p‐cresol sulfate. J Vet Intern Med. 2020;34:206–215. 10.1111/jvim.15634
Funding information Buttons Fund For Feline Chronic Kidney Disease Research; University of Colorado Cancer Center Shared Resource Support Grant (P30CA046934) supporting the Pharmacology Shared Resource
REFERENCES
- 1. Wong J, Piceno YM, DeSantis TZ, et al. Expansion of urease‐ and uricase‐containing, indole‐ and p‐cresol‐forming and contraction of short‐chain fatty acid‐producing intestinal microbiota in ESRD. Am J Nephrol. 2014;39:230‐237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bain MA, Faull R, Fornasini G, Milne RW, Evans AM. Accumulation of trimethylamine and trimethylamine‐N‐oxide in end‐stage renal disease patients undergoing haemodialysis. Nephrol Dial Transplant. 2006;21:1300‐1304. [DOI] [PubMed] [Google Scholar]
- 3. Evenepoel P, Meijers BK, Bammens BR, Verbeke K. Uremic toxins originating from colonic microbial metabolism. Kidney Int Suppl. 2009;114:S12‐S19. [DOI] [PubMed] [Google Scholar]
- 4. Summers SC, Quimby JM, Isaiah A, et al. The fecal microbiome and serum concentrations of indoxyl sulfate and p‐cresol sulfate in cats with chronic kidney disease. J Vet Intern Med. 2019;33:662‐669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cheng FP, Hsieh MJ, Chou CC, Hsu WL, Lee YJ. Detection of indoxyl sulfate levels in dogs and cats suffering from naturally occurring kidney diseases. Vet J. 2015;205:399‐403. [DOI] [PubMed] [Google Scholar]
- 6. Chen CN, Chou CC, Tsai PSJ, Lee YJ. Plasma indoxyl sulfate concentration predicts progression of chronic kidney disease in dogs and cats. Vet J. 2018;232:33‐39. [DOI] [PubMed] [Google Scholar]
- 7. Rios‐Covian D, Ruas‐Madiedo P, Margolles A, et al. Intestinal short chain fatty acids and their link with diet and human health. Front Microbiol. 2016;7:185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Morrison DJ, Preston T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes. 2016;7:189‐200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Donohoe DR, Garge N, Zhang X, et al. The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell Metab. 2011;13:517‐526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Park J, Kim M, Kang SG, et al. Short‐chain fatty acids induce both effector and regulatory T cells by suppression of histone deacetylases and regulation of the mTOR‐S6K pathway. Mucosal Immunol. 2015;8:80‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Topping DL, Clifton PM. Short‐chain fatty acids and human colonic function: roles of resistant starch and nonstarch polysaccharides. Physiol Rev. 2001;81:1031‐1064. [DOI] [PubMed] [Google Scholar]
- 12. Smith PM, Howitt MR, Panikov N, et al. The microbial metabolites, short‐chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341:569‐573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fukumoto S, Tatewaki M, Yamada T, et al. Short‐chain fatty acids stimulate colonic transit via intraluminal 5‐HT release in rats. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1269‐R1276. [DOI] [PubMed] [Google Scholar]
- 14. Miyamoto J, Kasubuchi M, Nakajima A, et al. The role of short‐chain fatty acid on blood pressure regulation. Curr Opin Nephrol Hypertens. 2016;25:379‐383. [DOI] [PubMed] [Google Scholar]
- 15. Scott KP, Gratz SW, Sheridan PO, Flint HJ, Duncan SH. The influence of diet on the gut microbiota. Pharmacol Res. 2013;69:52‐60. [DOI] [PubMed] [Google Scholar]
- 16. Russell WR, Gratz SW, Duncan SH, et al. High‐protein, reduced‐carbohydrate weight‐loss diets promote metabolite profiles likely to be detrimental to colonic health. Am J Clin Nutr. 2011;93:1062‐1072. [DOI] [PubMed] [Google Scholar]
- 17. Blakeney BA, Crowe MS, Mahavadi S, Murthy KS, Grider JR. Branched short‐chain fatty acid isovaleric acid causes colonic smooth muscle relaxation via cAMP/PKA pathway. Dig Dis Sci. 2018;64:1171‐1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kamstock D, Guth A, Elmslie R, et al. Liposome‐DNA complexes infused intravenously inhibit tumor angiogenesis and elicit antitumor activity in dogs with soft tissue sarcoma. Cancer Gene Ther. 2006;13:306‐317. [DOI] [PubMed] [Google Scholar]
- 19. Zimmermann J, Herrlinger S, Pruy A, Metzger T, Wanner C. Inflammation enhances cardiovascular risk and mortality in hemodialysis patients. Kidney Int. 1999;55:648‐658. [DOI] [PubMed] [Google Scholar]
- 20. Liu Y, Li J, Yu J, et al. Disorder of gut amino acids metabolism during CKD progression is related with gut microbiota dysbiosis and metagenome change. J Pharm Biomed Anal. 2018;149:425‐435. [DOI] [PubMed] [Google Scholar]
- 21. Liu S, Liang S, Liu H, et al. Metabolite profiling of feces and serum in hemodialysis patients and the effect of medicinal charcoal tablets. Kidney Blood Press Res. 2018;43:755‐767. [DOI] [PubMed] [Google Scholar]
- 22. Bammens B, Verbeke K, Vanrenterghem Y, Evenepoel P. Evidence for impaired assimilation of protein in chronic renal failure. Kidney Int. 2003;64:2196‐2203. [DOI] [PubMed] [Google Scholar]
- 23. van Vliet S, Skinner SK, Beals JW, et al. Dysregulated handling of dietary protein and muscle protein synthesis after mixed‐meal ingestion in maintenance Hemodialysis patients. Kidney Int Rep. 2018;3:1403‐1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Price SR, Mitch WE. Metabolic acidosis and uremic toxicity: protein and amino acid metabolism. Semin Nephrol. 1994;14:232‐237. [PubMed] [Google Scholar]
- 25. Mitch WE, Price SR, May RC, Jurkovitz C, England BK. Metabolic consequences of uremia: extending the concept of adaptive responses to protein metabolism. Am J Kidney Dis. 1994;23:224‐228. [DOI] [PubMed] [Google Scholar]
- 26. Laflamme D, Gunn‐Moore D. Nutrition of aging cats. Vet Clin North Am Small Anim Pract. 2014;44:761‐774. vi. [DOI] [PubMed] [Google Scholar]
- 27. Reichardt N, Duncan SH, Young P, et al. Phylogenetic distribution of three pathways for propionate production within the human gut microbiota. ISME J. 2014;8:1323‐1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. McManus CM, Michel KE, Simon DM, Washabau RJ. Effect of short‐chain fatty acids on contraction of smooth muscle in the canine colon. Am J Vet Res. 2002;63:295‐300. [DOI] [PubMed] [Google Scholar]
- 29. Rondeau MP, Meltzer K, Michel KE, McManus CM, Washabau RJ. Short chain fatty acids stimulate feline colonic smooth muscle contraction. J Feline Med Surg. 2003;5:167‐173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hurst NR, Kendig DM, Murthy KS, Grider JR. The short chain fatty acids, butyrate and propionate, have differential effects on the motility of the Guinea pig colon. Neurogastroenterol Motil. 2014;26:1586‐1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fu RG, Wang Y, Yuan HZ, et al. Effects of chronic renal failure on gastrointestinal motility: a study on the changes of gastric emptying, small intestinal transit, interdigestive myoelectric complex, and fecal water content. Ren Fail. 2011;33:615‐621. [DOI] [PubMed] [Google Scholar]
- 32. Nishiyama K, Aono K, Fujimoto Y, et al. Chronic kidney disease after 5/6 nephrectomy disturbs the intestinal microbiota and alters intestinal motility. J Cell Physiol. 2019;234:6667‐6678. [DOI] [PubMed] [Google Scholar]
- 33. Hoibian E, Florens N, Koppe L, Vidal H, Soulage CO. Distal colon motor dysfunction in mice with chronic kidney disease: putative role of uremic toxins. Toxins (Basel). 2018;10:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ikee R, Toyoyama T, Endo T, Tsunoda M, Hashimoto N. Clinical factors associated with constipation in hemodialysis patients. Int Urol Nephrol. 2016;48:1741‐1742. [DOI] [PubMed] [Google Scholar]
- 35. Zhang J, Huang C, Li Y, et al. Health‐related quality of life in dialysis patients with constipation: a cross‐sectional study. Patient Prefer Adherence. 2013;7:589‐594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Benjamin SE, Drobatz KJ. Retrospective evaluation of risk factors and treatment outcome predictors in cats presenting to the emergency room for constipation. J Feline Med Surg. 2019; 1098612X19832663. 10.1177/1098612X19832663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Li L, Ma L, Fu P. Gut microbiota‐derived short‐chain fatty acids and kidney diseases. Drug des Devel Ther. 2017;11:3531‐3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Aw W, Fukuda S. Toward the comprehensive understanding of the gut ecosystem via metabolomics‐based integrated omics approach. Semin Immunopathol. 2015;37:5‐16. [DOI] [PubMed] [Google Scholar]
- 39. Wong JM, de Souza R, Kendall CW, Emam A, Jenkins DJ. Colonic health: fermentation and short chain fatty acids. J Clin Gastroenterol. 2006;40:235‐243. [DOI] [PubMed] [Google Scholar]
- 40. Cupisti A, Kovesdy CP, D'Alessandro C, Kalantar‐Zadeh K. Dietary approach to recurrent or chronic hyperkalaemia in patients with decreased kidney function. Nutrients. 2018;10:261. 10.3390/nu10030261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jiang S, Xie S, Lv D, et al. A reduction in the butyrate producing species Roseburia spp. and Faecalibacterium prausnitzii is associated with chronic kidney disease progression. Antonie Van Leeuwenhoek. 2016;109:1389‐1396. [DOI] [PubMed] [Google Scholar]
- 42. Barry KA, Wojcicki BJ, Middelbos IS, et al. Dietary cellulose, fructooligosaccharides, and pectin modify fecal protein catabolites and microbial populations in adult cats. J Anim Sci. 2010;88:2978‐2987. [DOI] [PubMed] [Google Scholar]
- 43. Bermingham EN, Young W, Butowski CF, et al. The fecal microbiota in the domestic cat (Felis catus) is influenced by interactions between age and diet; a five year longitudinal study. Front Microbiol. 2018;9:1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Barry KA, Middelbos IS, Vester Boler BM, et al. Effects of dietary fiber on the feline gastrointestinal metagenome. J Proteome Res. 2012;11:5924‐5933. [DOI] [PubMed] [Google Scholar]
- 45. Lubbs DC, Vester BM, Fastinger ND, Swanson KS. Dietary protein concentration affects intestinal microbiota of adult cats: a study using DGGE and qPCR to evaluate differences in microbial populations in the feline gastrointestinal tract. J Anim Physiol Anim Nutr. 2009;93:113‐121. [DOI] [PubMed] [Google Scholar]
- 46. Vogt JA, Wolever TM. Fecal acetate is inversely related to acetate absorption from the human rectum and distal colon. J Nutr. 2003;133:3145‐3148. [DOI] [PubMed] [Google Scholar]
- 47. Michel KE, Anderson W, Cupp C, Laflamme DP. Correlation of a feline muscle mass score with body composition determined by dual‐energy X‐ray absorptiometry. Br J Nutr. 2011;106(Suppl 1):S57‐S59. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Clinical scoring system used to determine muscle condition, appetite, consistency of feces, and frequency of vomiting.


