Abstract
Background
Steroids administered PO and intramuscularly are associated with development of congestive heart failure in cats without prior signs of heart disease, but criteria to identify cats at increased risk for steroid‐induced heart failure are not established.
Hypothesis
Cats administered steroids PO for 5 to 7 days will develop increased N terminal pro‐B‐type natriuretic peptide (NT‐proBNP) concentration and heart size.
Animals
Ten client‐owned cats.
Methods
Observational cohort study. Cats intended to initiate at least a 5‐day course of steroids administered PO were consecutively recruited.
Results
Steroids administered PO to cats are not associated with differences in absolute concentration of NT‐proBNP (baseline: 49 pmol/L [range, 24‐1013]; after steroids: 85 pmol/L [range, 46‐1367]; P = .23), blood pressure (baseline: 145 mm Hg [range, 116‐163]; after steroids: 145 mm Hg [range, 115‐230]; P = .94), nor blood glucose concentration (baseline: 125 mg/dL [range, 92‐254]; after steroids: 144 mg/dL [range, 114‐307]; P = .43), but are associated with increased median left atrial dimension (baseline: 1.26 cm [range, 0.96‐2.03; after steroids: 1.38 [range, 1.03‐2.20]; P = .02) and mean left ventricular internal diameter (baseline: 1.55 cm [standard deviation—SD, 0.28; after steroids: 1.72 cm [SD, 0.28]; P = .03). Six of 10 (60%) cats had a percentile change in NT‐proBNP >60% after steroids, and 3 of 8 (38%) cats with baseline BNP <100 pmol/L had an NT‐proBNP >100 pmol/L after steroids.
Conclusions and Clinical Importance
Increased heart size and percentage change in individual NT‐proBNP concentration suggests plasma volume expansion secondary to steroids administered PO in cats. A serial assessment of an individual cat's change in NT‐proBNP might be clinically useful for judging risk for volume expansion.
Keywords: budesonide, heart failure, N‐terminal pro‐B‐type natriuretic peptide, prednisolone
Abbreviations
- AoVmax
maximal aortic outflow velocity
- BG
blood glucose
- CHF
congestive heart failure
- LVIDd
left ventricular internal diameter in diastole
- LVIDs
left ventricular internal diameter in systole
- NT‐proBNP
N terminal pro‐B‐type natriuretic peptide
- TS
total solids
1. INTRODUCTION
Steroids are implicated in structural and hemodynamic cardiovascular changes in both human and veterinary patients. Steroids administered intramuscularly in cats increase plasma volume up to 49.7% without a change in body weight or total body water.1 Postulated mechanisms include mineralocorticoid‐induced sodium and water retention, and insulin insensitivity with associated hyperglycemia and fluid shifts from intracellular to extracellular spaces, with the latter proposed to be most important.1 Exogenous and endogenous steroids are associated with increased left ventricular mass and diastolic dysfunction in people,2, 3 and increased left ventricular wall thickness and evidence of diastolic dysfunction occurs in normotensive dogs with naturally occurring hyperadrenocorticism.4 Additionally, glucocorticoid and mineralocorticoid receptors on vascular smooth muscle mediate vasoconstriction by affecting transmembrane sodium and calcium flux in canine and lapine arteries, and therefore steroid therapy might increase vascular resistance.5 Increased systemic blood pressure is associated with a short course of steroids administered PO in dogs.6 Thus, increased plasma volume, hypertrophy‐associated diastolic dysfunction, and increased afterload due to enhanced systemic vascular resistance are all mechanisms by which steroids might predispose a cat to develop congestive heart failure (CHF). Steroids administered PO and intramuscularly are associated with development of CHF in cats without prior signs of heart disease,7 but criteria to identify cats at increased risk for steroid‐induced heart failure are not established. Plasma N terminal pro‐B‐type natriuretic peptide (NT‐proBNP) increases with myocardial stretch and increases with atrial dilation and left ventricular hypertrophy in people and cats8, 9, 10, 11; however, there is limited data regarding changes in NT‐pro BNP and echocardiographic indices of myocardial remodeling and hemodynamics associated with steroid use in cats.12 Short‐term glucocorticoids increase circulating natriuretic peptides in human patients.13 The primary objective of this study was to measure NT‐proBNP and echocardiographic variables in cats administered steroid treatment PO with the hypothesis that heart size and NT‐proBNP would increase, supporting increased plasma volume.
2. MATERIALS AND METHODS
2.1. Case selection
Cats were recruited from a single private specialty hospital, Friendship Hospital for Animals, for this prospective observational study. The study was reviewed and approved by the hospital administration, and informed owner consent to participate was obtained for all cases. Cats for which the primary clinician intended to initiate at least a 5‐day course of steroids administered PO at a consistent dosage for any clinical reason were consecutively recruited. The primary clinician determined the dose and frequency of steroid administration. Cats were excluded from the study if they had concurrent disease that might affect NT‐proBNP, including uncontrolled hyperthyroidism (total thyroxine ≥4.0 μg/dL), systemic hypertension (systolic blood pressure ≥160 mm Hg), or moderate to severe chronic renal disease (IRIS stage III or higher). Cats receiving diuretics, angiotensin‐converting enzyme inhibitors, or fluids administered SC or IV for any reason were excluded. Cats that received steroid treatment for any reason in the 2 weeks before enrollment were excluded. Owners were instructed to continue feeding their cat's usual diet for the duration of the study.
At the initial visit, baseline population characteristics were documented including age, sex, breed, and the reason for planned initiation of steroids. Baseline values were established for body weight and heart rate, and the presence or absence of a murmur or gallop sound was documented. Systolic blood pressure was obtained via Doppler using a cuff measured to 40% of the limb circumference, and the cat position, cuff size, and limb used were recorded. The mean of 3 consecutive measurements was recorded. Venipuncture was performed for evaluation of PCV, total solids (TS) concentration, and blood glucose (BG) concentration. Additionally, if the cat's medical records did not show results of blood urea nitrogen, creatinine, or total thyroxine (T4) within the past 6 months, these tests were performed. Venipuncture was performed and EDTA plasma was stored up to 12 hours and transported on ice packs by courier to a central laboratory, and plasma NT‐proBNP concentration was determined using a commercially available quantitative assay with an upper reference value of 100 pmol/L (Cardiopet; proBNP, IDEXX Laboratories, Westbrook, Maine). The time of day at which the blood sample for NT‐proBNP assay was collected was recorded.
Echocardiography with simultaneous ECG using a 12 MHz transducer (GE Vivid E9 Cardiac Ultrasound, General Electric Company, Boston, Massachusetts) was performed by 1 board‐certified cardiologist (C.L.B.) according to accepted techniques.14 Cats were not sedated and were lightly restrained in lateral recumbency. Cine loops were saved and upon completion of enrollment of all cats were batch analyzed for purposes of the study in blinded fashion. A technician used an online random number generator to assign a number to each study and sequentially loaded studies into an offline station (EchoPACs, General Electric Company) for measurement by a single individual (C.L.B.). Opaque tape was placed across the computer screen to blind the cardiologist from identifying patient information and date of the examination. Echocardiographic data collected were left atrial dimension, left atrial to aortic root ratio (LA:Ao), maximal left atrial area, left atrial plus auricular area, right atrial maximal area, left ventricular internal diameter in diastole (LVIDd) and in systole (LVIDs), interventricular septal wall thickness in diastole, left ventricular free wall thickness in diastole, maximal aortic outflow velocity, and velocity time integral of aortic outflow. Left atrial and aortic root dimensions were measured from right parasternal short‐axis base views by directing the cursor through the left atrium in a line along the commissure of the left and noncoronary aortic valve cusps.15 Maximal left atrial area was calculated by tracing the endocardial surface in a single plane (right parasternal long axis view) just before mitral valve opening in early diastole as previously described.16 Maximal right atrial area was calculated in a similar fashion. Left atrial plus auricular area was calculated by tracing the endocardial surface in a single plane (right parasternal short‐axis view) at aortic valve closure as previously described.17 For each variable, the mean of measurements from 3 consecutive cardiac cycles was used.
Cats were presented for repeat evaluation 5‐7 days after initiation of steroid treatment. Owners were encouraged to return for the repeat examination at approximately the same time of day as the initial visit to avoid any confounding effect of circadian rhythm on NT‐proBNP. The number of days of steroid treatment and the time elapsed since most recent dose were recorded. Body weight and heart rate were again measured, and the presence or absence of a murmur or gallop was noted. Measurement of systolic blood pressure was repeated using the same cuff, limb, and body position. Venipuncture was performed and PCV, TS, BG, and NT‐proBNP were repeated. An echocardiogram was repeated, and cine loops were saved for later evaluation in blinded fashion.
2.2. Statistical analyses
Healthy cats without signs of heart failure typically have NT‐proBNP <100 pmol/L.10, 18 This study was powered based on an alternate hypothesis that mean NT‐proBNP in cats would rise from a value of approximately 35 to 100 pmol/L post‐steroids. Ten cats were required to detect a significant difference versus the null hypothesis with a power of 80%. Data were examined with a D'Agostino & Pearson normality test to determine distribution. Normal data are presented as mean ± standard deviation, and non‐normal data are presented as median (range [ie, min‐max]). A paired t test or Wilcoxon signed rank test was performed to compare baseline and recheck measurements. A percentage change in NT‐proBNP >60% in individual cats is superior to the use of absolute values compared to a reference range due to high individual variability of NT‐proBNP in cats.19 Thus, the study also considered the percentage change in NT‐proBNP between the baseline values and values after steroid treatment in individual cats. Specifically, the number of cats whose change in NT‐proBNP concentration exceeded 60% of the baseline or whose NT‐proBNP concentration was >100 pmol/L after steroid treatment were tabulated. Statistical analyses were performed with commercially available software (Prism 7.0, GraphPad Software, La Jolla, California; STATA 14.2, Stata Corp., College Station, Texas). Statistical significance was set at <0.05. Corrections were not made for multiple comparisons.
3. RESULTS
Ten cats were recruited and all completed the study. Breeds represented included Domestic Shorthair (5), Domestic Medium Hair (2), Domestic Long Hair (1), Russian Blue (1), and Maine Coon (1). Ages ranged from 2 to 13.5 years (median 9.5 years). Clinical reasons for steroid use included asthma (2), dermatitis (2), pemphigus (1), chronic rhinitis (1), intestinal small cell lymphoma (1), neoplastic pleural effusion (1), oral ulcers (1), and immune‐mediated hemolytic anemia (1). No cat appeared clinically dehydrated at initial examination. Eight cats had no echocardiographic evidence of cardiac disease. Baseline NT‐proBNP values for these cats was normal in all cases with a range of 24‐94 pmol/L (median 38.5 pmol/L). One cat was newly diagnosed with complete AV block and 4‐chamber cardiac dilation and had increased baseline NT‐proBNP. Another cat was newly diagnosed with hypertrophic obstructive cardiomyopathy with normal left atrial size and had markedly increased baseline BNP. One owner began the first dose of the steroid taper (administered 50% of initial dose) the day before recheck. All other cats received steroids administered PO as prescribed, and all cats returned for follow‐up within 5‐7 days as specified by the study protocol (median 7 days). One cat was treated with budesonide administered PO (0.2 mg/kg once daily), and the remainder were treated with prednisolone administered PO (dose range 1‐3.3 mg/kg/day; median 2 mg/kg/day). Results are presented in Table 1. There was no statistically significant difference in NT‐proBNP concentrations at baseline as compared to NT‐proBNP after steroid treatment (Figure 1). Six of 10 (60%) cats had a change in NT‐proBNP >60% after steroid treatment, and 3/8 (38%) cats with a baseline BNP <100 pmol/L had NT‐proBNP >100 pmol/L after steroid treatment. There was a statistically significant increase in TS concentration. There was no difference in heart rate, blood pressure, glucose concentration, or body weight. There was a statistically significant increase in LVIDd, LVIDs, LA dimension, LA/Ao ratio, and left atrial and auricular area (Table 1; Figure 2). There was no significant difference in any other echocardiographic variable assessed. One cat had a particularly large increase in NT‐proBNP concentration (30‐414 pmol/L) with a concentration after steroid treatment that is associated with heart failure in cats.18 This cat also developed a new murmur and gallop, had a dramatic drop in PCV without clinical signs of hemorrhage, and developed a dilated left ventricle and pseudonormalization of the mitral E and A wave pattern suggesting volume expansion.
Table 1.
Physical examination, clinicopathological, and select echocardiographic data at baseline at after 5‐7 days of corticosteroids administered PO in 10 cats
| Visit 1 | Visit 2 | P value | |
|---|---|---|---|
| Body weight (kg) | 5.2 ± 1.4 | 5.1 ± 1.4 | .06 |
| Heart rate (bpm) | 199 ± 45 | 191 ± 39 | .50 |
| Blood pressure (mmHg systolic) | 145 (116‐163) | 145(115‐230) | .94 |
| NT‐proBNP (pmol/L) | 49 (24‐1013) | 85 (46‐1367) | .23 |
| Total solids (g/dL) | 7.35 ± 0.94 | 8.00 ± 0.96 | .003* |
| PCV (%) | 37.5 (15‐43) | 32.7 ± 7.558 | .06 |
| Glucose (mg/dL) | 125 (92‐254) | 144 (114‐307) | .43 |
| LVIDd (cm) | 1.55 ± 0.28 | 1.72 ± 0.28 | .03* |
| LVIDs (cm) | 0.67 ± 0.26 | 0.81 ± 0.25 | .002* |
| LAD (cm) | 1.26 (0.96‐2.03) | 1.38 (1.03‐2.20) | .02* |
| LA:Ao | 1.25 (1.16‐1.91) | 1.50 ± 0.30 | .008* |
| LAAuricle (cm2) | 1.72 (1.03‐6.46) | 1.93 (1.3‐7.3) | .04* |
| LAarea (cm2) | 2.00 (1.20‐6.10) | 2.12 (1.23‐6.03) | .70 |
| RAarea (cm2) | 1.43 ± 0.462 | 1.69 ± 0.83 | .37 |
| IVSd (cm) | 0.38 (0.26‐0.66) | 0.33 (0.23‐0.50) | .28 |
| LVFWd (cm) | 0.50 ± 0.10 | 0.48 ± 0.08 | .51 |
| AoVmax (m/s) | 0.81 (0.59‐3.50) | 0.87 (0.72‐2.53) | .70 |
| AoVTI (cm) | 6.61 ± 1.75 | 7.70 ± 1.33 | .08 |
Data are presented as mean ± standard deviation and or median (range).* denotes a statistically significant difference between groups.
Abbreviations: Ao Vmax, maximal aortic outflow velocity; AoVTI, aortic velocity time integral; IVSD, interventricular septal wall thickness in diastole; LA:Ao, left atrial to aortic root ratio; LAarea, left atrial area; LAAuricle, left atrial and auricular area; LAD, left atrial dimension; LVFWd, left ventricular free wall thickness in diastole; LVIDd, left ventricular internal diameter in diastole; LVIDs, left ventricular internal diameter in systole; NT‐proBNP, N terminal pro‐B‐type natriuretic peptide; RAarea, right atrial area.
Figure 1.
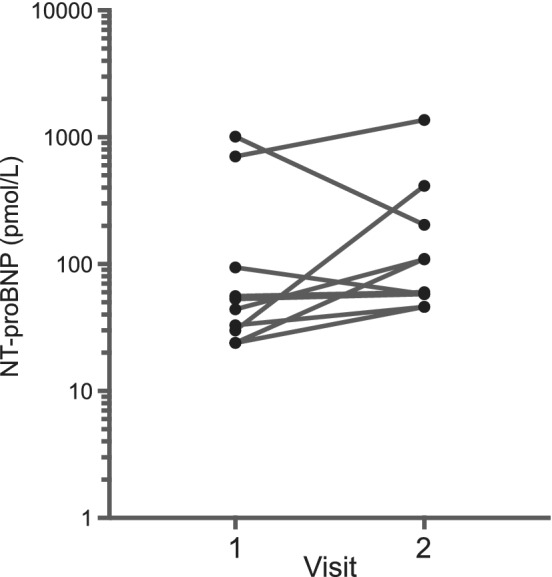
N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP) concentration at baseline (v1) and after 5 to 7 days (v2) of PO administered corticosteroids in 10 cats
Figure 2.
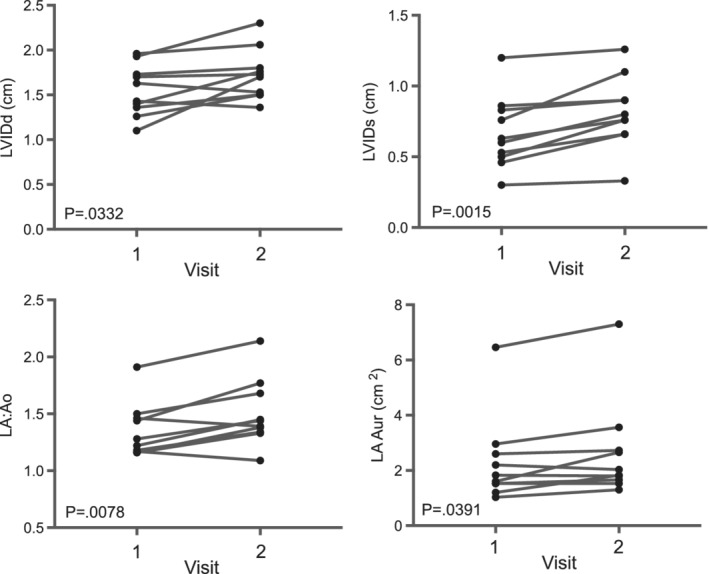
Select echocardiographic data at baseline (v1) and after 5 to 7 days (v2) of PO administered corticosteroids in 10 cats
One cat was prescribed budesonide administered PO due to the presence of complete heart block with 4‐chamber dilation and concerns for provoking heart failure; the clinician cited anecdotal evidence that budesonide might cause less plasma volume expansion than prednisolone. After steroid administration, NT‐proBNP in this cat increased by 93% (707‐1367 pmol/L). Four months after the study ended and while still receiving budesonide, the cat developed a large volume of abdominal effusion. The cause of effusion was not determined. At the time of writing, no other cats had developed signs consistent with CHF.
4. DISCUSSION
In this study, the short‐term effect of steroids administered PO was not associated with a statistically significant increase in absolute concentration of NT‐proBNP; however, the significant change in left atrial and ventricular size suggests that steroid administration increased circulating plasma volume. Glucocorticoid‐induced hyperglycemia has been proposed as the primary factor in volume expansion and precipitation of heart failure after steroids administered intramuscularly in cats,1 but this reasoning is controversial.12 In the current study, the increase in heart size was likely due to volume expansion secondary to mineralocorticoid effects since there was no significant change in BG concentration with steroid treatment. Blood pressure was not significantly different after steroid treatment and systemic hypertension does not seem to be a clinically relevant effect of steroids administered PO in cats12 as in dogs.6 Total solids concentration significantly increased after steroid treatment and might be due to increased hepatic synthesis of albumin or increased circulating lipids.20 Despite an overall lack of significant difference in absolute values of NT‐proBNP, 60% of the cats had a serial change in NT‐proBNP, which is greater than expected weekly intra‐individual variation,19 and over a third of cats with normal NT‐proBNP had increases of NT‐proBNP after steroid treatment that exceeded the upper normal reference limit. Due to weekly variability, an individual's serial change in NT‐proBNP concentration >60% of the baseline value might be a better indicator of clinically relevant change versus the use of a population‐based reference value.19 A serial assessment of NT‐proBNP in an individual cat undergoing steroid treatment might be useful in assessing risk of causing volume overload.
One cat in this study received budesonide administered PO and ultimately developed abdominal effusion. Budesonide has been anecdotally suggested to be safer than prednisolone with reduced mineralocorticoid effects; in humans it is less bioavailable and in dogs fewer side‐effects have been noted (though adrenal suppression does occur), but to the authors' knowledge it has not been extensively evaluated in cats.21
There are several potential limitations to the present study. Hemodynamic changes were indirectly measured using NT‐proBNP and echocardiography; however, assessment of plasma volume or total body water using isotope or dye dilution or densitometry is cumbersome and poorly suited to routine clinical practice.22 Differences in hydration status could have affected echocardiographic measurements in this study17; however, cats were examined at roughly the same time of day for each visit to minimize circadian effects on NT‐proBNP or hydration. Despite these efforts, the duration of hospital visits were not standardized and cats were not acclimatized to the hospital before recording heart rate and blood pressure measurements. Additionally, treatment of the cats' underlying disorders with steroids might have affected water intake and thus hydration status at follow‐up examination, though no cat appeared clinically dehydrated at initial examination. Variability in gastrointestinal absorption might affect plasma steroid concentrations, which were not measured. This study was not designed to assess the effects of more protracted courses of steroids, thus conclusions regarding long‐term effects of steroids administered PO require additional studies.
CONFLICT OF INTEREST DECLARATION
Mark A. Oyama has received research funding from IDEXX Laboratories, Westbrook, ME within the past 3 years.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
The study was reviewed and approved by the hospital administration and informed owner consent to participate was obtained for all cases.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
ACKNOWLEDGMENTS
The authors thank Jessica Mansour and Caitlin Liscinski for help with data collection.
Block CL, Oyama MA. Echocardiographic and biomarker evidence of plasma volume expansion after short‐term steroids administered orally in cats. J Vet Intern Med. 2020;34:29–34. 10.1111/jvim.15678
REFERENCES
- 1. Ployngam T, Tobias AH, Smith SA, Torres SMF, Ross SJ. Hemodynamic effects of methylprednisolone acetate administration in cats. Am J Vet Res. 2006;67(4):583‐587. [DOI] [PubMed] [Google Scholar]
- 2. Pearson AC, Schiff M, Mrosek D, Labovitz AJ, Williams GA. Left ventricular diastolic function in weight lifters. Am J Cardiol. 1986;58:1254‐1259. [DOI] [PubMed] [Google Scholar]
- 3. Muiesan ML, Lupia M, Salvetti M, et al. Left ventricular structural and functional characteristics in Cushing's syndrome. J Am Coll Cardiol. 2003;41:2275‐2279. [DOI] [PubMed] [Google Scholar]
- 4. Takano H, Kokubu A, Sugimoto K, Sunahara H, Aoki T, Fujii Y. Left ventricular structural and functional abnormalities in dogs with hyperadrenocorticism. J Vet Cardiol. 2015;17:173‐181. [DOI] [PubMed] [Google Scholar]
- 5. Kornel L. The role of vascular steroid receptors in the control of vascular contractility and peripheral vascular resistance. J Steroid Biochem Mol Biol. 1993;45:195‐203. [DOI] [PubMed] [Google Scholar]
- 6. Masters AK, Berger DJ, Ware WA, et al. Effects of short‐term anti‐inflammatory glucocorticoid treatment on clinicopathologic, echocardiographic, and hemodynamic variables in systemically healthy dogs. Am J Vet Res. 2018;79(4):411‐423. [DOI] [PubMed] [Google Scholar]
- 7. Smith SA, Tobias AH, Fine DM, et al. Corticosteroid‐associated congestive heart failure in 12 cats. Int J Appl Res Vet Med. 2004;2(3):159‐170. [Google Scholar]
- 8. Connolly DJ, Soares Magalhaes RJ, Syme HM, et al. Circulating natriuretic peptides in cats with heart disease. J Vet Intern Med. 2008;22:96‐105. [DOI] [PubMed] [Google Scholar]
- 9. Fox PR, Rush JE, Reynolds CA, et al. Multicenter evaluation of plasma N‐terminal probrain natriuretic peptide (NT‐pro BNP) as a biochemical screening test for asymptomatic (occult) cardiomyopathy in cats. J Vet Intern Med. 2001;25:1010‐1016. [DOI] [PubMed] [Google Scholar]
- 10. Wess G, Daisenberger P, Mahling M, Hirschberger J, Hartmann K. Utility of measuring plasma N‐terminal pro‐brain natriuretic peptide in detecting hypertrophic cardiomyopathy and differentiating grades of severity in cats. Vet Clin Pathol. 2011;40(2):237‐244. [DOI] [PubMed] [Google Scholar]
- 11. Kim SW, Park SW, Lim SH, et al. Amount of left ventricular hypertrophy determines the plasma N‐terminal pro‐brain natriuretic peptide level in patients with hypertrophic cardiomyopathy and normal left ventricular ejection fraction. Clin Cardiol. 2006;29:155‐160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Imal AK, Berger DJ, Mochel JP, et al. Clinicopathologic, hemodynamic, and echocardiographic effects of anti‐inflammatory glucocorticoids in systemically healthy cats. 2018 American College of Veterinary Internal Medicine (ACVIM) forum research abstract program. J Vet Intern Med. 2018;32:C40‐C2309. 10.1111/jvim.15319. [DOI] [Google Scholar]
- 13. Brotman DJ, Girod JP, Garcia MJ, et al. Effects of short‐term glucocorticoids on cardiovascular biomarkers. J Clin Endocrinol Metab. 2005;90(6):3202‐3208. [DOI] [PubMed] [Google Scholar]
- 14. Thomas WP, Gaber CE, Jacobs GJ, et al. Recommendations for standards in transthoracic two‐dimensional echocardiography in the dog and cat. Echocardiography Committee of the Specialty of Cardiology, American College of Veterinary Internal Medicine. J Vet Intern Med. 1993;7:247‐252. [DOI] [PubMed] [Google Scholar]
- 15. Rishniw M, Erb HN. Evaluation of four 2‐dimensional echocardiographic methods of assessing left atrial size in dogs. J Vet Intern Med. 2000;14:429‐435. [DOI] [PubMed] [Google Scholar]
- 16. Schober KE, Maerz I, Ludewig E, Stern JA. Diagnostic accuracy of electrocardiothoracic and thoracic radiography in the assessment of left atrial size in cats: comparison with transthoracic 2‐dimensional echocardiography. J Vet Intern Med. 2007;21:709‐718. [DOI] [PubMed] [Google Scholar]
- 17. Campbell FE, Kittleson MD. The effect of hydration status on the echocardiographic measurements of normal cats. J Vet Intern Med. 2007;21:1008‐1015. [DOI] [PubMed] [Google Scholar]
- 18. Fox PR, Oyama MA, Reynolds C, et al. Utility of plasma N‐terminal pro‐brain natriuretic peptide (NT‐proBNP) to distinguish between congestive heart failure and non‐cardiac causes of acute dyspnea in cats. J Vet Cardiol. 2009;11:S51‐S61. [DOI] [PubMed] [Google Scholar]
- 19. Harris AN, Estrada AH, Gallagher AE, et al. Biologic variability of N‐terminal pro‐brain natriuretic peptide in adult healthy cats. J Feline Med Surg. 2017;19(2):216‐223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lowe AD, Campbell KL, Barger A, Schaeffer DJ, Borst L. Clinical, clinicopathological and histological changes observed in 14 cats treated with glucocorticoids. Vet Rec. 2008;162(24):777‐783. [DOI] [PubMed] [Google Scholar]
- 21. Trepanier L. Idiopathic inflammatory bowel disease in cats. J Feline Med Surg. 2009;11:32‐38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Munday HS. Assessment of body composition in cats and dogs. Int J Obes Relat Metab Disord. 1994;18(Suppl 1):S14‐S21. [PubMed] [Google Scholar]


