Abstract
Background
Glomerular filtration rate (GFR) estimation is the gold standard for assessment of renal function, although the clinical utility of this test is unclear.
Objectives
To describe the clinical utility of GFR estimation in dogs.
Animals
Medical records of 132 dogs that had serum iohexol clearance measured between 2012 and 2017.
Methods
Iohexol clearance and clinical records were reviewed and submitting practices contacted to obtain outcome data. Dogs were classified into 4 groups based on the reason for performing GFR estimation: A1 (screening for pre‐azotemic chronic kidney disease [CKD], n = 105), A2 (confirmation of azotemic CKD, n = 3), B (screening for pre‐azotemic acute kidney injury, n = 19), and C (miscellaneous causes, n = 5). Descriptive review of the clinical utility of GFR estimation is provided.
Results
For dogs in Group A1, renal disease was diagnosed in 9/9 dogs with a GFR ≥40% decreased below the mean GFR of their body weight category, in 5/6 dogs with a ≥30% but <40% reduction in GFR and in 7/9 dogs with a ≥20% but <30% reduction in GFR.
Conclusions and Clinical Importance
Glomerular filtration rate estimation is useful for the diagnosis of CKD before the onset of azotemia.
Keywords: canine, diagnosis, iohexol clearance, renal
Abbreviations
- AKI
acute kidney injury
- CKD
chronic kidney disease
- CRGV
cutaneous and renal glomerular vasculopathy
- GFR
glomerular filtration rate
- PLN
protein‐losing nephropathy
- RVC
Royal Veterinary College
- SDMA
symmetric dimethylarginine
- UCCR
urine cortisol‐to‐creatinine ratio
- USG
urine specific gravity
1. INTRODUCTION
Accurately assessing renal function can be useful in dogs with suspected kidney disease. Such situations include screening for renal dysfunction as a cause of polyuria and polydipsia in dogs that are non‐azotemic, have only a borderline increase in serum creatinine concentration, have isolated increases in novel markers such as symmetric dimethylarginine (SDMA), or have persistently reduced urine concentrating ability.1 Other indications include monitoring for progression of existing chronic kidney disease (CKD), screening for renal dysfunction in breeds predisposed to hereditary nephropathies,2 dosage adjustment of renally excreted drugs, and monitoring the effects of chronic administration of potentially nephrotoxic drugs.3
Glomerular filtration rate (GFR) estimation is the gold standard for assessment of renal function, as it is directly proportional to renal mass.4 Glomerular filtration rate is estimated by assessing the clearance of a marker of glomerular filtration.1 Urinary clearance of inulin is the reference method for estimating GFR in humans and dogs. A more practical alternative is to measure the plasma/serum clearance of 1 or more of various markers. Markers used to estimate GFR in animals include inulin, exogenous creatinine, radionucleotides, and iohexol.5, 6, 7, 8 Because of its ease of use, cost, and availability, plasma clearance of iohexol has become 1 of the more widely used markers of GFR in veterinary and human medicine.9, 10, 11, 12, 13, 14, 15, 16, 17, 18
Techniques using limited sampling have been used in human and veterinary medicine as a practical means to estimate GFR. When using such techniques, a correction formula must be applied to achieve more accurate approximations of GFR. The most widely used of these, the Brøchner‐Mortensen formula, has been extrapolated from human medicine for use in veterinary studies19, 20, 21; however, a systematic review of species differences has not been performed. More recently, Bexfield et al. described a correction formula for estimation of GFR in dogs using a 1‐compartmental clearance technique taking dog weight and age into account.21, 22
Serum creatinine, a surrogate marker of GFR, has largely replaced GFR estimation in clinical practice because of its availability, practicality of measurement, and widespread use in monitoring and staging kidney disease.23 However, serum creatinine is insensitive to early decline in GFR because of the exponential relationship between serum creatinine concentration and GFR. A further limitation is that reference intervals for creatinine vary with animal size, yet laboratories generally use 1 reference interval for all animals irrespective of their size. Additionally, reference intervals vary between laboratories depending on the method of measuring creatinine.24 Despite GFR estimation being the gold standard for assessing renal function, it remains infrequently used and data on the widespread clinical utility of GFR estimation in clinical practice are lacking. The aim of this study was to describe the clinical utility of GFR estimation determined by serum iohexol clearance in dogs.
2. MATERIALS AND METHODS
2.1. Data acquisition and analysis
The medical records of dogs which had samples submitted for GFR estimation by serum iohexol clearance to the Royal Veterinary College (RVC) GFR/therapeutic drug monitoring service from March 9, 2012, to November 4, 2017, were assessed. The project was reviewed and approved by the RVC clinical research and ethical review board, which allowed access to joint iohexol clearance test submission forms held by deltaDOT Ltd and the RVC. Additionally, contact with the veterinarians for access to the clinical records of the dogs under investigation and for completion of a short questionnaire regarding outcomes was approved. This contact was performed before the final implementation of the General Data Protection Regulation (EU) 2016/679.
2.2. Iohexol clearance protocol
A standard protocol was recommended to veterinarians collecting samples to be submitted for measurement of iohexol clearance. Dogs were well hydrated at the time of the test, had free access to water for 12 hours before testing, and had no clinical signs consistent with dehydration or hypovolemia. Food was withheld for 12 hours before testing. A single dose of iohexol (Omnipaque 300) was administered at 300 mg iodine/kg IV through a catheter. The IV catheter was then flushed with saline. Blood was collected into serum gel tubes precisely at 2, 3, and 4 hours after iohexol administration. Exact times of blood collection and iohexol administration were noted, and if there were discrepancies with the protocol, the actual time between dose administration and sampling was used in the calculation. Blood was centrifuged after clotting as per the centrifuge manufacturers' instructions for separation of serum. The serum samples were shipped at room temperature for next day delivery. Serum iohexol concentration for each serum sample was measured using deltaDOT Ltd's validated high‐performance capillary electrophoresis method.25 To correct for any variability in the amount of sample injected onto the column, 3 μL of iopromide was added to 57 μL of each sample (2‐, 3‐, and 4‐hour post‐iohexol serum samples) as an internal standard and this was used to correct the iohexol concentrations for variation in sample volume applied to the system. Data were analyzed using deltaDOT Ltd's generalized separation transform.
Serum iohexol concentrations were used to calculate serum clearance of iohexol. Glomerular filtration rate was estimated from the serum clearance of iohexol by application of a compartmental model and canine‐specific correction formula,22 normalized to body weight in kilograms. For data analysis, dogs were divided into 4 weight quartiles; Category 1:1.8‐12.4 kg, Category 2:13.2‐25.5 kg, Category 3:25.7‐31.6 kg, and Category 4:32.0‐70.3 kg.22 In the event that a dog's body weight did not fall within the range of 1 of these body weight categories, the dog was included in the body weight category to which its body weight was closest. The estimated GFR of each dog was compared with the expected mean GFR of their respective body weight categories: 2.89 mL/kg/min for Category 1, 2.4 mL/kg/min for Category 2, 2.16 mL/kg/min for Category 3, and 2.25 mL/kg/min for Category 4.22
2.3. Clinical case data collection
Standardized submission forms were provided to be completed by a veterinarian with each GFR sample submission. Information requested was: signalment; weight; the exact volume of Omnipaque 300 administered; exact time of Omnipaque 300 administration and exact times of each serum sample collection; reason for GFR testing and case history (including current/previous medications and dose rates); estimated/measured water consumption; previous serum creatinine and SDMA concentrations; total/ionized blood calcium concentration; urinalysis; urine culture; urine protein:creatinine ratio; ACTH stimulation test results; low‐dose dexamethasone suppression test results; urine cortisol‐to‐creatinine ratio (UCCR); abdominal ultrasound findings; blood pressure; serum total T4, free T4, and TSH concentrations; and any other additional test results. When interpreting serum creatinine results, the reference intervals from the various laboratories to which each individual sample was submitted for serum creatinine measurement were used. When >1 serum creatinine concentration, urine‐specific gravity (USG), or UCCR result was provided for a dog, the median value was chosen for interpretation. Corrected GFR was then interpreted with regard to the dogs' medical history by a specialist panel consisting of a diplomate of the European College of Veterinary Pharmacology and Toxicology (ECVPT; J.E.), a joint diplomate of the European College of Veterinary Anaesthesia and Analgesia and the ECVPT (L.P.), and a diplomate of the American College of Veterinary Internal Medicine (small animal internal medicine; R.E.J.).
The veterinarian(s) who submitted each sample set for iohexol clearance to be measured was contacted via email and asked to complete a short questionnaire regarding case outcomes. Data collected included status (ie, alive/dead), date of euthanasia/death, reason for euthanasia/death if known, diagnosis reached for the clinical signs/routine laboratory findings that prompted GFR estimation, evidence for diagnosis of CKD (defined as persistently elevated creatinine or a single identified episode of elevated creatinine above the laboratory reference interval accompanied by inappropriately dilute urine [USG of <1.030]), and if a diagnosis of CKD was obtained, the time between GFR estimation and when evidence supporting a diagnosis of CKD was obtained. For dogs that were alive, follow‐up time was considered the days between GFR estimation and date of contacting the submitting veterinary surgeon. For dogs that were dead, follow‐up time was considered the days between GFR estimation and death/euthanasia. If no response to the questionnaire was received or the answers were insufficient to provide outcome information, the veterinarians were contacted directly by telephone to request the full clinical history and laboratory reports for the dogs in question from the time of iohexol clearance sample submission to the time of follow‐up.
2.4. Case classification
Dogs were classified into 1 of 4 groups depending on the reason for sample submission: Group A1: screening for non‐azotemic CKD; Group A2: confirmation of CKD with evidence of CKD already documented; Group B: suspicion for non‐azotemic acute kidney injury (AKI); and Group C: miscellaneous reasons. These classifications were based on review of data provided on the GFR submission forms. Group A1 cases included those with non‐azotemic polyuria and polydipsia, persistently inappropriately dilute urine concentrating ability and abnormalities identified on abdominal ultrasound examination compatible with either chronic nephropathy or renal dysplasia. Dogs with a borderline azotemia, defined as a creatinine value <0.3 mg/dL above the upper end of the reference interval from the corresponding laboratory, and azotemic dogs with a creatinine ≥0.3 mg/dL above the upper end of the reference interval from the corresponding laboratory but USG ≥1.030 were included in this group. Group A2 cases included those where there had been clinical suspicion for a diagnosis of CKD as per Group A1 but where in addition there was at least 1 measurement of creatinine from the submitting veterinarian that had been ≥0.3 mg/dL above their specific laboratory reference interval with concurrent USG <1.030, which was considered consistent with CKD. One dog with a serum creatinine concentration that was 0.54 mg/dL above the upper end of the reference interval with concurrent USG of 1.013 was included in Group A1 rather than in Group A2 as it was a Greyhound, a breed that has higher serum creatinine concentrations than other dog breeds.26 For classification into Groups A1 and A2, clinical changes were required to be present for >1 month indicating chronicity. Group B included those dogs in which there was suspicion for an AKI based on historical findings and duration of clinical signs being <1 month, and included screening for cutaneous and renal glomerular vasculopathy (CRGV) in cases that presented with unexplained skin lesions.27 Dogs not falling into Groups A1, A2, or B were considered miscellaneous (Group C).
Glomerular filtration rate estimation results from individual dogs in Groups A1, A2, and B were interpreted using categories of: GFR Group 1: GFR increased or <20% decreased from the mean GFR of the body weight category, kidney disease considered excluded or unlikely as a cause of presenting clinical signs; GFR Group 2: ≥20% but <30% decrease in GFR from the mean GFR of the body weight category, kidney disease considered possible but unconfirmed as an etiology for presenting clinical signs; GFR Group 3: ≥30% but <40% decrease in GFR from the mean GFR of the body weight category, kidney disease considered likely as an etiology for presenting clinical signs; and GFR Group 4: ≥40% decrease in GFR from the mean GFR of the body weight category, kidney disease considered almost certain as an etiology for presenting signs. The criterion for GFR Group 1 was based on the anticipated variability in iohexol clearance measurement where the within individual variability for GFR estimation via iohexol clearance has previously been reported as 19.9% for non‐azotemic cats.28 Criteria for Categories 3 and 4 were based on estimates of GFR reduction that have been associated with the point at which serum creatinine becomes elevated in previous studies.29
2.5. Statistical analysis
Iohexol clearance submission forms and follow‐up data from veterinarians were analyzed. Descriptive analysis is provided with clinicopathological data collected from submission forms and follow‐up questionnaire/telephone assessment presented as median (range) unless otherwise stated. Dogs were grouped according to reason for GFR assessment (Groups A1‐C) and the following parameters were evaluated: signalment, weight, presenting complaint, laboratory diagnostics performed, GFR (median and range), GFR analyzed by weight category, GFR % deviation from mean, final diagnosis, and status at the time of follow‐up.
3. RESULTS
3.1. Study population
A total of 132 dogs had samples submitted for GFR assessment between March 9, 2012, and November 4, 2017.
Forty‐six different breeds were represented in the study population (not including cross‐breeds). The most commonly represented were Labrador Retrievers (n = 20), West Highland White Terriers (n = 7), Border Collies (n = 7), Golden Retrievers (n = 6), Staffordshire Bull Terriers (n = 6), and Boxers (n = 6). A full list of the represented breeds is shown in Table 1.
Table 1.
Dog breeds represented in the study population
| Number of dogs | Breed(s) |
|---|---|
| 20 | Labrador Retriever |
| 7 | West Highland White Terrier, Border Collie |
| 6 | Golden Retriever, Staffordshire Bull Terrier, Boxer |
| 4 | Greyhound, Lurcher |
| 3 | Springer Spaniel, Cocker Spaniel, Hungarian Vizsla, Beagle |
| 2 | German Shorthaired Pointer, German Shepard, Jack Russell Terrier, Doberman Pinscher, Yorkshire Terrier, Border Terrier, Pug |
| 1 | Japanese Akita, Australian Shepard, Australian Cattle Dog, Bearded Collie, Havanese, Dalmatian, Dachshund, English Bull Terrier, English Setter, Flat‐coated Retriever, French Bulldog, Giant Schnauzer, Great Dane, Irish Setter, Kerry Blue Terrier, Alaskan Malamute, Papillon, Pomeranian, Poodle, Rottweiler, Saluki, Shar Pei, Shetland Sheepdog, Shih Tzu, Swiss Mountain Dog, Weimaraner, Welsh Springer Spaniel |
| 19 | Cross‐breeds |
There were 70 females (7 intact, 50 neutered, and 13 of unspecified neuter status) and 62 males (15 entire, 29 neutered, and 18 of unspecified neuter status) in the study population. Ages ranged from 0.5 to 15.5 years with a median age of 6.2 years. Weight ranged from 2.45 to 59.3 kg with a median weight of 20.7 kg. Dogs were assigned to 1 of 4 weight categories22 (Category 1:1.8‐12.4 kg, n = 30; Category 2:13.2‐25.5 kg, n = 55; Category 3:25.7‐31.6 kg, n = 21; and Category 4:32.0‐70.3 kg, n = 26). One dog's body weight (25.55 kg) did not fall within the previously described ranges of these body weight categories; this dog was ultimately included in body weight Category 1 given that its body weight was nearer to the upper limit of category 1 (25.5 kg) than the lower limit of Category 2 (25.7 kg).
Samples were submitted from 3 countries: the United Kingdom (n = 127), Norway (n = 3), and Denmark (n = 2). Seventy‐seven samples were submitted from diplomates of the European or American College of Veterinary Internal Medicine, Royal College of Veterinary Surgeons (RCVS)‐recognized specialists in small animal internal medicine or by residents working under the supervision of the aforementioned diplomates. Fifty‐four samples were submitted from general practitioner veterinarians. It was unknown whether 1 sample was submitted from a specialist or general practitioner because of lack of information on the submission form. Submission form information was available for all 132 dogs. Follow‐up data were available for 81% (107/132) of dogs: questionnaire evaluation in 47/132 cases and telephone follow‐up was required in 60/132 cases. The time between GFR estimation and when the submitting veterinarians was contacted (n = 132) ranged from 2 to 1350 days with a median of 402 days. Survival time for those dogs that had died or been euthanized was a median of 325 days (range, 2‐849). In all dogs for which follow‐up data were available, a final diagnosis was reached in 75.7% (81/107) of cases.
3.2. Glomerular filtration rate estimation
Median GFR was 2.15 mL/kg/min across all body weight categories with a range of 1.03‐4.04 mL/kg/min. Percentage deviation from the mean of the body weight category ranged from −78.6 to +68.0% with a median deviation of −12% from the mean. When GFR estimation results from all dogs were analyzed together by body weight categories, the results were:
Body weight Category 1 (1.8‐12.4 kg): median GFR 2.66 mL/kg/min (range, 1.3‐4.04 mL/kg/min); median % deviation in GFR from bodyweight category mean −9.45% (range, −55 to +42.9%).
Body weight Category 2 (13.2‐25.5 kg): median GFR 2.22 mL/kg/min (range, 1.2‐4.03 mL/kg/min); median % deviation in GFR from bodyweight category mean −7.05% (range, −50 to +68%).
Body weight Category 3 (25.7‐31.6 kg): median GFR 1.83 mL/kg/min (range 1.09‐3.23 mL/kg/min); median % deviation in GFR from bodyweight category mean −15.55% (range, −49.5 to +49.5%).
Body weight Category 4 (32.0‐70.3 kg): median GFR 1.83 mL/kg/min (range, 1.03‐3.1 mL/kg/min); median % deviation in GFR from bodyweight category mean −14.49% (range, −54.5 to +37.6%).
Glomerular filtration rate estimation results per body weight category are represented graphically in Figure 1.
Figure 1.
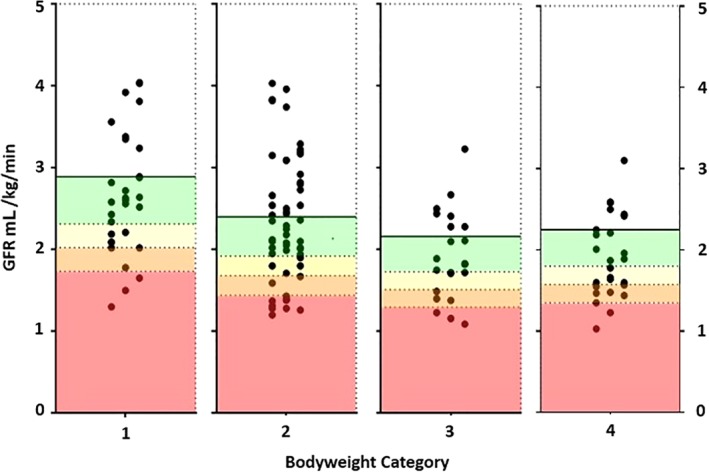
Glomerular filtration rate (GFR) estimation results (mL/kg/min) represented graphically, separated by patient body weight Categories 1‐4. Each dot represents the GFR result from a patient. The area on each chart with a green background represents a GFR decrease of <20% from the mean GFR of the body weight category, the yellow background represents a GFR decrease of ≥20% but <30% from mean GFR, the orange background represents a ≥30% but <40% decrease in GFR from mean GFR, and the red background represents a ≥40% decrease in GFR from the mean GFR
3.3. Clinical case evaluation
Dogs were categorized based on presenting reason for GFR assessment before further analysis; Group A1, n = 105; Group A2, n = 3; Group B, n = 19; and Group C, n = 5. Clinical signs, signalment, and laboratory findings for dogs based on reason for presentation groups are presented in Tables 2 and 3. Of the dogs in Group A1, 4 had suspected renal dysplasia based on imaging findings and on their age being <1 year. Of dogs in Group B, 17 were being screened for pre‐azotemic AKI because of suspected CRGV, 1 had suspected AKI because of recent raisin ingestion, and 1 had suspected AKI because of recent jerky treat ingestion. Of the 5 dogs in Group C (miscellaneous causes), 3 were undergoing chemotherapy and had GFR estimation performed to assess the need for carboplatin dose adjustment and in the case of the remaining 2 dogs insufficient information was available on the submission forms to establish the reason for GFR estimation and follow‐up information could not be obtained. Data relating to GFR estimation and percentage change in GFR for all dogs, and divided according to body weight category per reason for presentation group, are presented in Table 3. A full list of the tests performed before GFR estimation in dogs in Groups A1, A2, B, and C is provided in Supplemental Table 1.
Table 2.
Clinical presenting complaints and reasons for dogs presenting for glomerular filtration rate (GFR) estimation
| Clinical sign/laboratory finding | Group A1 | Group A2 | Group B | Group C |
|---|---|---|---|---|
| Polyuria‐polydipsia | 71 | 1 | 3 | ‐ |
| Azotemia | 26 | 3 | 1 | |
| Urinary incontinence | 18 | 1 | 2 | ‐ |
| Proteinuria | 13 | ‐ | ‐ | ‐ |
| Isosthenuria | 12 | ‐ | ‐ | ‐ |
| Increased SDMA | 6 | ‐ | 1 | ‐ |
| Abnormal kidneys on imaging | 5 | ‐ | ‐ | ‐ |
| Lethargy | 4 | ‐ | 1 | ‐ |
| Hematuria | 3 | 1 | 1 | ‐ |
| Nocturia | 3 | ‐ | ‐ | ‐ |
| Inappropriate urination | 3 | ‐ | ‐ | ‐ |
| Weight loss | 3 | ‐ | ‐ | ‐ |
| Hypertension | 3 | ‐ | ‐ | ‐ |
| Therapeutic drug monitoring | ‐ | ‐ | ‐ | 3 |
| Hyposthenuria | 1 | 1 | ‐ | ‐ |
| Inappropriately concentrated urine | 2 | ‐ | ‐ | ‐ |
| Polydipsia | 2 | ‐ | ‐ | ‐ |
| Polyuria | 2 | ‐ | ‐ | ‐ |
| Increased urea | 2 | ‐ | ‐ | ‐ |
| Vomiting | 2 | ‐ | ‐ | ‐ |
| Inappetence | 1 | ‐ | ‐ | ‐ |
| Renal mass on imaging | 1 | ‐ | ‐ | ‐ |
| Recent jerky treat ingestion | ‐ | ‐ | 1 | ‐ |
| Research purposes (details unspecified) | ‐ | ‐ | ‐ | 1 |
| Skin lesions | ‐ | ‐ | 17 | ‐ |
| Recent raisin ingestion | ‐ | ‐ | 1 | ‐ |
Abbreviation: SDMA, symmetric dimethylarginine.
Table 3.
Signalment and clinicopathological variables for dogs presenting for glomerular filtration rate (GFR) estimation
| Group A1 (n = 105) | Group A2 (n = 3) | Group B (n = 19) | Group C (n = 5) | |||||
|---|---|---|---|---|---|---|---|---|
| Median (range) | N | Median (range) | N | Median (range) | N | Median (range) | N | |
| Age (y) | 6.25 (0.5‐14) | 7.8 (5.4‐10) | 6 (1‐11.2) | 4.1 (3‐15.6) | ||||
| Body weight (kg) | 20.88 (2.45‐59.3) | 30.5 (19.1‐41.7) | 19.6 (6.7‐41.5) | 18 (4.5‐32.6) | ||||
| Weight category | ||||||||
| 1 | 9.3 (2.5‐12.4) | 65 | ‐ | ‐ | 9.1 (3.42‐12.4) | 4 | 6.3 (4.5‐8) | 2 |
| 2 | 20.38 (13.2‐25.55) | 12 | 19.1 | 1 | 18.1 (13.5‐21.3) | 10 | 21.5 (18‐24.9) | 2 |
| 3 | 28.8 (25.8‐30.3) | 12 | 28.6 | 1 | 29.3 (27.9‐31.5) | 3 | ‐ | ‐ |
| 4 | 38 (32‐59.3) | 16 | 41.7 | 1 | 38.6 (35.6‐41.5) | 2 | 32.6 | 1 |
| Sex | ||||||||
| FN | 43 | ‐ | 7 | ‐ | ||||
| FE | 5 | ‐ | 2 | ‐ | ||||
| F (unknown neuter status) | 11 | ‐ | 2 | ‐ | ||||
| MN | 22 | 3 | 3 | 1 | ||||
| ME | 12 | ‐ | 2 | 1 | ||||
| M (unknown neuter status) | 12 | ‐ | 3 | 3 | ||||
| Creatinine (mg/dL) | 97 (26‐233) | 103 | 157 (156‐183) | 3 | 96 (70‐165) | 19 | 136 (128‐149) | 4 |
| Symmetric dimethylarginine (μg/dL) | 15 (8‐25) | 6 | ‐ | ‐ | 8 | 1 | ‐ | ‐ |
| USG | 1.015 (1.005‐1.042) | 98 | 1.013 (1.008‐1.025) | 3 | 1.042 (1.015‐1.055) | 14 | 1.030 (1.020‐1.040) | 4 |
| UPC | 0.16 (0‐23.4) | 87 | 0.15 (0.05‐1.45) | 3 | 0.12 (0‐0.2) | 5 | 0.07 (0.03‐0.36) | 3 |
| Urine culture | ||||||||
| Total in which performed | 87 | 3 | 3 | 1 | ||||
| Positive | 7 | 0 | 1 | ‐ | ||||
| Negative | 79 | 3 | 2 | 1 | ||||
| Result unknown | 1 | ‐ | ‐ | ‐ | ||||
| GFR (mL/kg/min) | 2.12 (1.09‐4.04) | 1.37 (1.03‐1.4) | 2.58 (1.72‐3.96) | 1.75 (1.3‐3.92) | ||||
| GFR (mL/kg/min) by weight category | ||||||||
| 1 | 2.58 (1.5‐4.04) | ‐ | 2.74 (2.52‐3.56) | 2.62 (1.3‐3.92) | ||||
| 2 | 2.18 (1.2‐4.03) | 1.38 | 2.77 (2.05‐3.96) | 1.94 | ||||
| 3 | 1.83 (1.09‐2.67) | 1.4 | 2.5 (1.72‐3.23) | 1.75 | ||||
| 4 | 1.87 (1.23‐3.1) | 1.03 | 2.11 (2.01‐2.21) | 1.48 | ||||
| Percentage change in GFR compared to body weight GFR category | −12.6 (−50 to +68) | −42.4 (−54.4 to −35.2) | 0 (−20.4 to +65) | −27.3 (−55 to +35.6) | ||||
| Percentage change in GFR compared to body weight GFR category by weight category | ||||||||
| 1 | −10.8 (−48 to +40.8) | ‐ | −5.4 (−12.8 to +23.7) | −9.7 (−55 to +35.6) | ||||
| 2 | −7.8 (−46.7 to +68) | −42.4 | 15.2 (−14.6 to +65) | −19 | ||||
| 3 | −15.6 (−49.5 to +23.5) | −35.2 | 15.6 (−20.4 to +49.5) | 35.6 | ||||
| 4 | −16.9 (−43.5 to +37.6) | −54.4 | −6.2 (−10.7 to −1.8) | −27.3 | ||||
Abbreviations: UPC, urine protein‐to‐creatinine ratio; USG, urine specific gravity.
A total of 47 (35.6%) dogs across Groups A1‐C were receiving medications at the time of GFR estimation. Of these, 18 were receiving medications that could influence GFR (angiotensin‐converting enzyme‐inhibitors n = 9, glucosamine n = 3, levothyroxine n = 3, angiotensin‐receptor antagonist n = 1, prednisolone n = 1, and topical prednisolone n = 1). Details on drugs being administered at the time of GFR estimation in each group are presented in Table S2.
3.4. Evaluation of outcome data: Group A1
3.4.1. Diagnosis
In Group A1, follow‐up data were available for 79 cases and on review of this information a final diagnosis was reached in 77% (61/79). Overall, the most common diagnoses reached in Group A1 were psychogenic polydipsia (n = 15), progressive CKD (n = 15), protein‐losing nephropathy (PLN, n = 4), and central diabetes insipidus (n = 4). Diagnoses were further analyzed based on dog GFR categorization as described in Figure 2. In 9/9 (100%) dogs for which a follow‐up diagnosis was available and that had a GFR decrease of ≥40% below the mean GFR for their bodyweight category (GFR Group 4), a renal etiology was ultimately diagnosed for their clinical signs or laboratory findings that prompted GFR estimation. Five of 6 dogs (83.3%) with an available final diagnosis in GFR Group 3 (≥30% but <40% reduction in GFR) and 7/9 (77.8%) dogs with an available final diagnosis in GFR Group 2 (≥20% but <30% reduction in GFR) were ultimately diagnosed with a renal etiology of their presenting clinical signs. Five of 37 dogs (13.5%) with an available diagnosis in GFR Group 1 (GFR increased or <20% reduction in GFR) were ultimately diagnosed with a renal etiology of their presenting clinical signs. Of the remaining 32 dogs in GFR Group 1 that were diagnosed with a nonrenal etiology of their presenting clinical signs, none developed progressive CKD during the follow‐up period.
Figure 2.
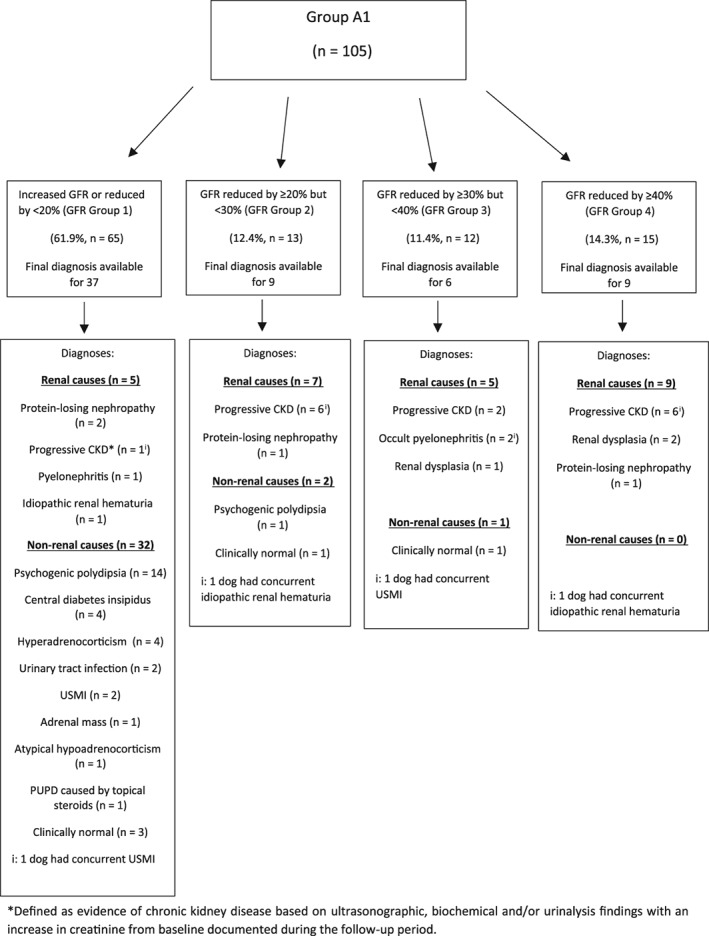
Distribution of dogs in Group A1 (glomerular filtration rate [GFR] estimation performed to screen for pre‐azotemic chronic kidney disease) and documented outcome
3.4.2. Development of azotemia
Of the 103 dogs in Group A1 for which serum creatinine was available at the time of GFR estimation, 23 (22%) dogs had azotemia documented before GFR estimation, whereas 80 (78%) dogs were not azotemic. In 18 of the 23 azotemic cases, the azotemia was considered borderline, that is, ≤0.3 mg/dL above the upper end of the laboratory reference interval for creatinine. In the remaining 5 cases, the creatinine was >0.3 mg/dL above the upper end of the laboratory reference interval but 3 dogs were included in Group A1 because of their USG, which was well concentrated, suggesting a potential prerenal component contributing to azotemia (USG 1.030, 1.034, 1.042) and in 2 cases because of being a Greyhound.26 Six dogs in Group A1 that were not azotemic at the time of GFR estimation and were ultimately diagnosed with progressive CKD developed azotemia during the follow‐up period, with median time between GFR estimation and documentation of azotemia of 335 days (range, 76‐827 days).
3.4.3. Status
Data pertaining to status was available for 83 (79%) dogs in Group A1. Sixty‐seven dogs were alive at the time of follow‐up, while 16 dogs had died. For those dogs that had died, median time between GFR estimation and death/euthanasia was 326 days (range, 2‐914 days). A reason for death/euthanasia was available for 16/18 dogs. Causes of death/euthanasia included neoplasia (n = 3), spinal cord disease (n = 2), age‐related poor quality of life (n = 2), acute gastroenteritis (n = 2), uroabdomen (n = 1), pancreatitis (n = 1), meningoencephalitis of unknown etiology (n = 1), cluster seizures (n = 1), and progression of renal dysplasia (n = 1).
3.5. Evaluation of outcome data: Group A2
In Group A2, follow‐up data were available for 2 of 3 cases. All 3 dogs in Group A2 were assessed to have been diagnosed with CKD before GFR estimation on the basis of overt azotemia (creatinine 2.0, 1.76, and 1.78 mg/dL), and follow‐up data confirmed the diagnosis of CKD in both dogs for which these data were available. Glomerular filtration rate categorization for Group A2 showed that in 1 dog, GFR was reduced by ≥30% but <40%, and in 2 dogs ≥40% commensurate with their documented azotemia. Both dogs for which follow‐up data were available were alive at the time of follow‐up.
3.6. Evaluation of outcome data: Group B
3.6.1. Diagnosis
In Group B, follow‐up data were available for 15/19 cases and on review of this information a final diagnosis was reached in 100% (15/15). The most common diagnosis was a nonspecified dermatopathy (n = 13), whereas the remaining 2 dogs were considered clinically normal. Diagnoses were further analyzed based on GFR categorization as described in Figure 3. No dogs (0/15) in GFR Group 1 were ultimately diagnosed with a renal etiology of their clinical signs. Similarly, no dogs (0/1) in GFR Group 2 were ultimately diagnosed with a renal etiology of their clinical signs.
Figure 3.
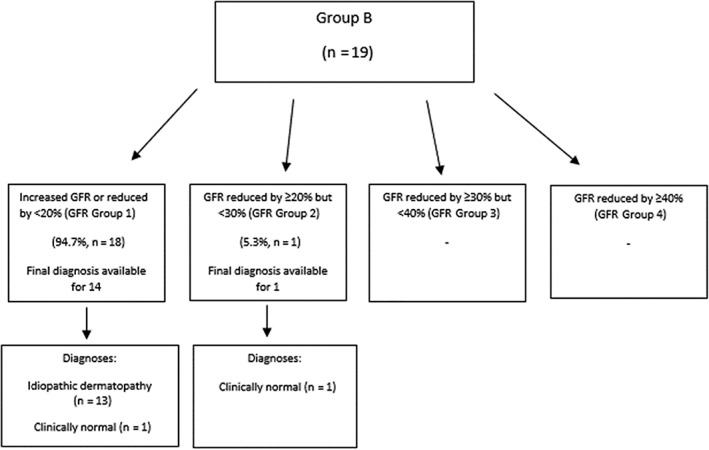
Distribution of dogs in Group B (glomerular filtration rate [GFR] estimation performed to screen for pre‐azotemic acute kidney injury) and documented outcome
3.6.2. Development of azotemia
Three out of 19 dogs in Group B had azotemia documented before GFR estimation, whereas the remaining 16 dogs in Group B were not azotemic. In all 3 azotemic cases, the azotemia was considered borderline, that is, <0.3 mg/dL above the upper end of the laboratory reference interval for creatinine. No dogs in Group B developed azotemia during the follow‐up period.
3.6.3. Status
All 15 dogs for which follow‐up data were available were alive at the time of follow‐up and none had gone on to develop progressive CKD.
3.7. Evaluation of outcome data: Group C
Glomerular filtration rate categorization for Group C showed that in 1 dog GFR was reduced by <20%, in 1 dog GFR was reduced by ≥30% but <40%, and in 1 dog ≥40%. Follow‐up data pertaining to whether or not carboplatin dose was adjusted based on GFR estimation was only available for 1 of 3 dogs. This dog had a 19% reduction in GFR from the mean of its body weight category and did not have carboplatin dose adjusted. Follow‐up data pertaining to status were available for all 3 dogs. Two dogs were dead and 1 was alive at the time of follow‐up. Of the 2 dead dogs, 1 was euthanized because of metastatic colonic adenocarcinoma 288 days after GFR estimation. The date and cause of death of the other dog was unknown.
4. DISCUSSION
This study demonstrates the clinical utility of GFR estimation in dogs. In Group A1 for which GFR estimation was performed for suspicion of pre‐azotemic CKD, using our preliminary categorization criteria, 100% of dogs in GFR Group 4, 83.3% of dogs in GFR Group 3, and 77.8% of dogs in GFR Group 2 (for which final diagnoses were available) were ultimately diagnosed with a renal etiology of their clinical signs or laboratory findings that prompted GFR estimation. These results suggest that the decline in GFR is detectable before the onset of azotemia in this subgroup of dogs, with GFR estimation therefore contributing to the diagnostic investigation. Relatively few (n = 6) dogs had SDMA measurements performed by their local veterinarian; therefore, based on the data provided, it was impossible to determine whether SDMA would have indicated reduced renal function in these dogs where creatinine concentration had not done so.
One dog in each of GFR Groups 2 and 3 (GFR reduction of ≥20% but <30% and ≥30% but <40%, respectively) was considered clinically normal by the submitting veterinarians at the time of follow‐up. Unfortunately, this assessment was based on spontaneous resolution of the dogs' clinical signs (polyuria‐polydipsia in both cases) rather than on longitudinal monitoring of their kidney function. Therefore, the authors cannot exclude the possibility that these dogs had undetected kidney disease at the time of follow‐up, and that progression of kidney disease could have been documented either through serial assessment of serum creatinine concentration or repeat GFR estimation. The ultimate classification of these dogs as normal can therefore be questioned. Other possible explanations were that the GFR estimation results in these 2 dogs were erroneous, or that the dogs did indeed have CKD but had a transient GFR reduction that later returned to normal; after the loss of nephrons, the kidney adapts via hyperfiltration of the remaining nephrons.30, 31 This compensatory response could have led to a return of GFR back forwards normal and a subsequent resolution in clinical signs.
A range of diagnoses were obtained in dogs in Group A1 that demonstrated an increase or up to 20% reduction in their GFR value (GFR Group 1). However, in 5 of the 37 (14%) dogs in this GFR category for which a final diagnosis was available, a renal etiology of the dog's clinical signs was ultimately obtained (PLN, n = 2; idiopathic renal hematuria, n = 1; progressive CKD, n = 1; and pyelonephritis, n = 1).
The 3 dogs classified into Group A2 were assessed to already have a diagnosis of CKD based on the data provided by submitting veterinarians. In these dogs, the authors believe that performing GFR estimation was not indicated, given these dogs already had definitive evidence of CKD based on their historical creatinine, USG +/− renal ultrasonographic findings. If dogs already had a diagnosis of azotemic CKD, GFR estimation provided no additional diagnostic benefit unless the authors were unaware, for example, of the requirement for drug dosage adjustment. However, it is possible that GFR estimation could help in longitudinal monitoring of dogs with azotemic CKD in which creatinine might not change as GFR drops because of muscle atrophy; having a baseline GFR at the time of diagnosis of CKD with which to compare subsequent GFR estimations could be helpful in this group of dogs.
Glomerular filtration rate estimation was also useful in ruling out AKI in dogs classified into Group B. No dogs (0/15) in GFR Group 1 or GFR Group 2 (0/1) were ultimately diagnosed with a renal etiology of their clinical signs. A GFR result that was increased or <20% decreased below the mean GFR of the dog's bodyweight category reliably excluded pre‐azotemic AKI. Obtaining a GFR result ≥20% but <30% decreased below mean GFR could also be reliable for excluding pre‐azotemic AKI; however, the fact that a final diagnosis was only available for 1 dog in this GFR category means it is impossible to accurately determine this in our study. The unusual demographic of idiopathic dermatopathy given as a final diagnosis in many of these cases reflects the recent emergence of cutaneous renal glomerular vasculopathy in the United Kingdom.27
This study has multiple limitations. First, the study was retrospective in nature and the study population was relatively small. Submissions came from many different practices, including referral centers and general practices. There is therefore inherent variation in the extent and quality of the diagnostic investigation that was performed in dogs before and after GFR estimation. This led to difficulties in terms of reaching final diagnoses for some dogs. The clinical histories of dogs for which a confirmed final diagnosis was reported by submitting veterinarians were reviewed by the authors; if there was a high level of confidence that the diagnosis reported accounted for the dogs' presenting reason for GFR estimation, the dogs were assessed to have a confirmed final diagnosis. If the diagnosis was assessed to be presumptive, or incorrect, then the dogs were recorded as not having a confirmed final diagnosis. Given that we did not directly examine dogs or perform the diagnostic workup in the study population, it is possible that our interpretation of the final diagnoses could be inaccurate for some dogs. This study relied on submitting veterinarians following a standard protocol to measure iohexol clearance. To the best of our knowledge, the recommended protocol was followed by submitting veterinarians, but we acknowledge the possibility that in some cases deviation from the protocol could have occurred with subsequent effects on the results. The authors also acknowledge that interindividual and intraindividual variability in GFR exists, which could lead to some dogs with normal kidney function ultimately being classified into GFR Groups 2 or, less likely GFR Group 3, where kidney disease is considered possible or likely respectively. The different veterinary practices submitting for GFR estimation used a variety of laboratories for measuring serum creatinine, such that different reference intervals were used for each individual dog in this study and to determine whether the dogs were initially azotemic or not at the time of GFR submission. A further limitation is that clinical history and important clinicopathologic data such as serum creatinine and USG were missing for some dogs at the time of GFR estimation, making interpretation of their GFR result more challenging. Finally, follow‐up data were not available for all dogs that had GFR estimation performed, limiting the amount of longitudinal information available for assessment of the clinical utility of GFR estimation and resulting in the ultimate number of dogs, where progression of renal disease could be assessed, being relatively small.
The authors acknowledge that using breed‐specific reference ranges for creatinine or serial monitoring of creatinine concentrations could increase the sensitivity of creatinine for detection of CKD or AKI. Given that creatinine values for this study came from many different laboratories using different reference intervals, comparing the sensitivity of GFR estimation for the detection of pre‐azotemic CKD or AKI to the use of breed‐specific creatinine reference intervals or serial creatinine measurements was not possible. Such a comparison could be a focus for future study. Future studies are also required to compare the clinical utility of GFR estimation for detecting pre‐azotemic CKD and AKI to other biomarkers, for example, SDMA which was infrequently available in this population of dogs. Longer term studies would be required to better evaluate the predictive capacity of GFR estimation for the future development of azotemic CKD.
In conclusion, in our population of dogs, GFR estimation via iohexol clearance was useful for the diagnosis of CKD before the onset of azotemia and for ruling out pre‐azotemic AKI. In dogs that already had a diagnosis of azotemic CKD, GFR estimation provided no additional diagnostic benefit. Based on data from previous publications,32 GFR estimation is also useful to screen for the need for carboplatin dose adjustment in dogs undergoing chemotherapy, although it was not possible to determine the clinical utility of GFR estimation for this purpose in our study because of lack of follow‐up data from this subset of dogs. The clinical utility of GFR estimation must be balanced against the theoretical risk for iohexol administration to contribute to an AKI.33
CONFLICT OF INTEREST DECLARATION
Ludovic Pelligand has affiliation with deltaDOT through a Concept Development Partnership (shared company/RVC investment) which resulted in employment of a postdoctoral researcher for 4 years for the development of the GFR service.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
This project was reviewed and approved by the Royal Veterinary College (RVC) Social Sciences Research Ethical Review Board (reference URN SR2017‐1223) which granted approval for access to joint iohexol clearance test submission forms held by deltaDOT Ltd and the RVC and for contact with the veterinarians for access to the clinical records of the dogs under investigation for completion of a short questionnaire regarding outcomes.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
Supporting information
Supplemental Table 1 Tests performed prior to GFR estimation in patients in groups A, B and C
Supplemental Table 2 Number of patients receiving medications at the time of GFR estimation
ACKNOWLEDGMENT
An abstract of this paper was presented at the 28th ECVIM Congress in Rotterdam, the Netherlands, in September 2018.
McKenna M, Pelligand L, Elliott J, Walker D, Jepson R. Clinical utility of estimation of glomerular filtration rate in dogs. J Vet Intern Med. 2020;34:195–205. 10.1111/jvim.15561
REFERENCES
- 1. Heiene R, Moe L. Pharmacokinetic aspects of measurement of glomerular filtration rate in the dog: a review. J Vet Intern Med. 1998;12(6):401‐414. [DOI] [PubMed] [Google Scholar]
- 2. Bailey D, Rassnick K, Erb H, et al. Effect of glomerular filtration rate on clearance and myelotoxicity of carboplatin in cats with tumors. Am J Vet Res. 2004;65(11):1502‐1507. [DOI] [PubMed] [Google Scholar]
- 3. Nabity M, Lees G, Cianciolo R, et al. Urinary biomarkers of renal disease in dogs with X‐linked hereditary nephropathy. J Vet Intern Med. 2012;26(2):282‐293. [DOI] [PubMed] [Google Scholar]
- 4. Kerl M, Cook C. Glomerular filtration rate and renal scintigraphy. Clin Tech Small Anim Pract. 2005;20(1):31‐38. [DOI] [PubMed] [Google Scholar]
- 5. Finco D. Measurement of glomerular filtration rate via urinary clearance of inulin and plasma clearance of technetium Tc 99m pentetate and exogenous creatinine in dogs. Am J Vet Res. 2005;66(6):1046‐1055. [DOI] [PubMed] [Google Scholar]
- 6. Kampa N, Bostrom I, Lord P, Wennstrom U, Ohagen P, Maripuu E. Day‐to‐day variability in glomerular filtration rate in normal dogs by scintigraphic technique. J Vet Med. 2003;50(1):37‐41. [DOI] [PubMed] [Google Scholar]
- 7. Moe L, Heiene R. Estimation of glomerular filtration rate in dogs with 99mTc‐DTPA and iohexol. Res Vet Sci. 1995;58(2):138‐143. [DOI] [PubMed] [Google Scholar]
- 8. Brown S, O'Reilly P. Iohexol clearance for the determination of glomerular filtration rate in clinical practice: evidence for a new gold standard. J Urol. 1991;146(3):675‐679. [DOI] [PubMed] [Google Scholar]
- 9. Frennby B, Sterner G. Contrast media as markers of GFR. Eur Radiol. 2002;12(2):475‐484. [DOI] [PubMed] [Google Scholar]
- 10. Schwartz G, Furth S, Cole S, et al. Glomerular filtration rate via plasma iohexol disappearance: pilot study for chronic kidney disease in children. Kidney Int. 2006;69(11):2070‐2077. [DOI] [PubMed] [Google Scholar]
- 11. Braselton W, Stuart K, Kruger J. Measurement of serum iohexol by determination of iodine with inductively coupled plasma‐atomic emission spectroscopy. Clin Chem. 1997;43(8):1429‐1435. [PubMed] [Google Scholar]
- 12. Brown S, Finco D, Boudinot F, Wright J, Taver SL, Cooper T. Evaluation of a single injection method, using iohexol, for estimating glomerular filtration rate in cats and dogs. Am J Vet Res. 1996;57(1):105‐110. [PubMed] [Google Scholar]
- 13. Finco D, Braselton W, Cooper T. Relationship between plasma iohexol clearance and urinary exogenous creatinine clearance in dogs. J Vet Intern Med. 2001;15(4):368‐373. [PubMed] [Google Scholar]
- 14. Passos M, Nishida S, Camara N, et al. Iohexol clearance for determination of glomerular filtration rate in rats induced to acute renal failure. PLoS One. 2015;10(4):e0123753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Goy‐Thollot I, Chafotte C, Besse S, et al. Iohexol plasma clearance in healthy dogs and cats. Vet Radiol Ultrasound. 2006;47(2):168‐173. [DOI] [PubMed] [Google Scholar]
- 16. Laroute V, Lefebvre HP, Costes G, Toutain PL. Measurement of glomerular filtration rate and effective renal plasma flow in the conscious beagle dog by single intravenous bolus of iohexol and p‐aminohippuric acid. J Pharmacol Toxicol Methods. 1999;41(1):17‐25. [DOI] [PubMed] [Google Scholar]
- 17. Laroute V, Chetboul V, Roche L, et al. Quantitative evaluation of renal function in healthy Beagle puppies and mature dogs. Res Vet Sci. 2005;79(2):161‐167. [DOI] [PubMed] [Google Scholar]
- 18. Westhoff A, Meyerlindenberg A, Wohlsein P, et al. Determination of the glomerular filtration rate (GFR) in dog by an iodine contrast medium with the Renalyzer‐PRX‐90. Monatsh Veterinarmed. 1993;48(11):573‐582. [Google Scholar]
- 19. Brøchner‐Mortensen J. A simple method for the determination of glomerular filtration rate. Scand J Clin Lab Invest. 1972;30(3):271‐274. [DOI] [PubMed] [Google Scholar]
- 20. Gleadhill A, Michell A. Evaluation of iohexol as a marker for the clinical measurement of glomerular filtration rate in dogs. Res Vet Sci. 1996;60(2):117‐121. [DOI] [PubMed] [Google Scholar]
- 21. Heiene R, Moe L. The relationship between some plasma clearance methods for estimation of glomerular filtration rate in dogs with pyometra. J Vet Intern Med. 1999;13(6):587‐596. [DOI] [PubMed] [Google Scholar]
- 22. Bexfield N, Heiene R, Gerritsen R, et al. Glomerular filtration rate estimated by 3‐sample plasma clearance of Iohexol in 118 healthy dogs. J Vet Intern Med. 2008;22(1):66‐73. [DOI] [PubMed] [Google Scholar]
- 23. IRIS Kidney – Guidelines – IRIS Staging of CKD [Internet]. http://iris-kidney.com. http://www.iris-kidney.com/guidelines/staging.html. Accessed July 23, 2018.
- 24. Ulleberg T, Robben J, Nordahl KM, Ulleberg T, Heiene R. Plasma creatinine in dogs: intra‐ and inter‐laboratory variation in 10 European veterinary laboratories. Acta Vet Scand. 2011;53(1):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Willams S, Pelligand L, Devanur D, et al. Validation of a high performance capillary electrophoresis method of glomerular filtration rate by serum iohexol clearance in dogs. Paper presented at: Proceedings of the European College of Veterinary Medicine Congress; September 4‐6, 2014; Mainz, Germany.
- 26. Zaldívar‐López S, Marín L, Iazbik M, et al. Clinical pathology of Greyhounds and other sighthounds. Vet Clin Pathol. 2011;40(2):414‐425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Holm LP, Hawkins I, Robin C, et al. Cutaneous and renal glomerular vasculopathy as a cause of acute kidney injury in dogs in the UK. Vet Rec. 2015;176(15):384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Finch NC, Syme H, Elliott J. Repeated measurements of renal function in evaluating its decline in cats. J Feline Med Surg. 2018;20(12):1144‐1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nabity NB, Lees GE, Boggess M, et al. Symmetric dimethylarginine assay validation, stability, and evaluation as a marker for the early detection of chronic kidney disease in dogs. J Vet Intern Med. 2015;29:1036‐1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hostetter T, Olson J, Rennke H, et al. Hyperfiltration in remnant nephrons: a potentially adverse response to renal ablation. Am J Physiol. 1981;241(1):85‐93. [DOI] [PubMed] [Google Scholar]
- 31. Brown S, Brown C. Single‐nephron adaptions to partial renal ablation in cats. Am J Physiol. 1995;269(5):1002‐1008. [DOI] [PubMed] [Google Scholar]
- 32. Bailey DB, Rassnick KM, Prey JD, Dykes NL. Evaluation for serum iohexol clearance use in predicting carboplatin clearance in cats. Am J Vet Res. 2009;70(9):1135‐1140. [DOI] [PubMed] [Google Scholar]
- 33. Goic JB, Koenigshof AM, McGuire LD, et al. A retrospective evaluation of contrast‐induced kidney injury in dogs (2006‐2012). J Vet Emerg Crit Care. 2016;26(5):713‐719. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1 Tests performed prior to GFR estimation in patients in groups A, B and C
Supplemental Table 2 Number of patients receiving medications at the time of GFR estimation


