Abstract
Background
Tracheobronchial colonization by Pseudomonas aeruginosa (PA) has been shown to negatively impact outcomes in cystic fibrosis and bronchiectasis. There is uncertainty whether the same association is prevalent in chronic obstructive pulmonary disease (COPD), especially in the outpatient setting. Our objective was to determine (1) whether PA isolation is associated with mortality and (2) changes in exacerbation and hospitalization rates within a longitudinal cohort of COPD outpatients.
Methods
Pseudomonas aeruginosa colonization was ascertained in monthly sputum cultures in a prospective cohort of COPD patients from 1994 to 2014. All-cause mortality was compared between patients who were colonized during their follow-up period (PA+) and those who remained free of colonization (PA−); Cox proportional hazards models were used. Exacerbation and hospitalization rates were evaluated by 2-rate χ 2 and segmented regression analysis for 12 months before and 24 months after PA isolation.
Results
Pseudomonas aeruginosa was isolated from sputum in 73 of 181 (40%) patients. Increased mortality was seen with PA isolation: 56 of 73 (77%) PA+ patients died compared with 73 of 108 (68%) PA− patients (P = .004). In adjusted models, PA+ patients had a 47% higher risk of mortality (adjusted hazard ratio = 1.47; 95% confidence interval, 1.03–2.11; P = .04). Exacerbation rates were higher for the PA+ group during preisolation (15.4 vs 9.0 per 100 person-months, P < .001) and postisolation periods (15.7 vs 7.5, P < .001). Hospitalization rates were higher during the postisolation period among PA+ patients (6.25 vs 2.44, P < .001).
Conclusions
Tracheobronchial colonization by PA in COPD outpatients was associated with higher morbidity and mortality. This suggests that PA likely contributes to adverse clinical outcomes rather than just a marker of worsening disease.
Keywords: chronic obstructive pulmonary disease, exacerbations, hospitalization, mortality, Pseudomonas aeruginosa
Pseudomonas aeruginosa (PA) is a significant pathogen in chronic airway diseases including cystic fibrosis (CF) and bronchiectasis [1, 2]. Chronic respiratory infection with PA is one of the main characteristics of CF and significantly contributes to morbidity and mortality [3, 4], with a reported 2.6-times higher risk of death in respiratory culture PA-positive patients [5]. Pseudomonas aeruginosa is isolated less frequently from patients with non-CF bronchiectasis, but once it becomes a chronic infection, it is rarely eradicated [6, 7]. A recent systematic review and meta-analysis showed that PA colonization in adult bronchiectasis is associated with a 3-fold increased risk of death along with increase hospital admissions, exacerbations, and worse quality of life [8].
Pseudomonas aeruginosa is isolated from the sputum of 4%–15% of adults with chronic obstructive pulmonary disease (COPD) in many cross-sectional studies and is more likely to be isolated from patients with severe disease [9–12]. Pseudomonas aeruginosa can cause acute exacerbations of COPD (AECOPD), and some studies have found that the presence of PA is associated with increased exacerbation and mortality in COPD [12–17]. However, these findings are based on patients hospitalized with an exacerbation or with multidrug-resistant organisms. In addition, previous studies have primarily been retrospective in nature and lacked systematic sampling of sputum cultures; therefore, the risk of bias may have been high. The relationship between PA isolation, clinical outcomes (eg, exacerbations and hospitalizations), and long-term mortality within COPD outpatients is less clear.
In this study, we evaluate the impact of initial PA isolation on clinical outcomes and mortality within a prospective outpatient COPD cohort. At this point, no definitive conclusion can be made as to whether PA serves as a marker of progressed disease or is independently associated with adverse clinical outcomes. A greater understanding of the influence of PA isolation on COPD mortality and morbidity in outpatients would help to guide specific therapies and help inform preventative and eradication strategies. Therefore, the goals of this study were (1) to determine the association between PA isolation and long-term mortality and (2) to evaluate the change in exacerbation and hospitalization rates after PA isolation.
METHODS
Study Design and Participants
The COPD study clinic is a prospective study that started in 1994 at the Buffalo VA Medical Center (VAMC) [18]. Study details are provided in the online Supplementary Material. In brief, participants were evaluated monthly and whenever they had symptoms suggestive of an exacerbation. At clinic visits, clinical information and sputum and serum samples were obtained. A clinical evaluation was performed at each visit to determine whether the patients had stable disease or an exacerbation as described earlier [13, 18].
Pseudomonas aeruginosa Strains
The processing of sputum samples is described in the online Supplement Material. In brief, spontaneously expectorated morning sputum samples was homogenized in 0.1% dithiothreitol, and serial dilutions were subjected to quantitative culture. Bacterial identification was performed using standard techniques. Pseudomonas aeruginosa isolation was identified by colony morphology, the absence of lactose fermentation, and the presence of oxidase.
We further identified PA sputum culture-positive patients utilizing the microbiology records within the Buffalo VAMC electronic medical record (EMR). For patients with repeated positive sputum cultures, the date of the first culture was recorded. For the purposes of this study, patients were divided into 2 groups: those in whom PA was isolated in sputum (PA+) and those in whom PA was not isolated in sputum over the study period (PA−).
Outcomes
Survival time was defined as the interval between study entry and the date of death or the date of final follow-up in the VAMC EMR (May 31, 2017). Exposure was defined as any PA+ sputum culture after the study participant’s entry date into the study until end of follow-up. Exposure date was defined as the date of the first PA+ sputum culture.
We compared PA+ and PA− groups with regard to changes in monthly exacerbation and hospitalization rates including COPD-related hospitalizations. Clinical outcomes data were collected from both COPD study records and the VAMC EMR. Exacerbations were counted as distinct events if there was a return to baseline symptoms between events.
Analysis of Antibody Response to Pseudomonas aeruginosa
Serum antibody responses were determined by enzyme-linked immunosorbent assay to a mixture of PA strains to assess immune response to PA in the time period spanning the isolation in PA+ patients, specifically, approximately 3 and 9 months before and after isolation. Sera samples spanning this time frame were available for 52 patients. For the PA− group, we assessed the immune response in the same time span around the control isolation time point, which was estimated by dividing the subjects into pre- and postisolation segments based on the mean time to PA isolation within the PA culture-positive group. We chose 16 patients from the PA− group who were matched for age and forced expiratory volume in 1 second (FEV1)% predicted with the 52 PA+ patients and had sera available in the appropriate time frame. The percentage change in optical density between sera was calculated.
To determine the level of a significant percentage antibody level change between sera, 10 paired samples of sera obtained 2 months apart from subjects in the study clinic with stable COPD and who had not been colonized by potential pathogens during that period were tested against the Pseudomonas antigen pool. The upper limit of the 99% confidence interval (CI) for the average change in these control samples was regarded as a significant change. We compared the frequency of significant increase in antibody levels in PA+ and PA− patients in postisolation compared with preisolation samples and in samples at −3 months compared with −9 months.
Statistical Analysis
Kaplan-Meier survival curves and the log-rank test were used to assess the association between PA isolation and mortality. Time-dependent Cox proportional hazard regression models were used to calculate hazard ratios (HRs) and 95% CIs associated with the risk of mortality. Pseudomonas aeruginosa included a time-dependent variable, taking the time period from study entry into account. Change in status from unexposed to exposed could only occur once, at the time of exposure date, and remained unchanged during the remaining follow-up. Based on a review of the literature, the following patient characteristics were investigated as confounders in the model: age, race, smoking status (current vs former), participant follow-up in primary study, Charlson comorbidity index, and FEV1% predicted [19]. All Cox models were checked to ensure that they fulfilled the proportional hazard assumption.
Morbidity outcomes were evaluated in 2 ways: (1) by comparing the exacerbation and hospitalization rates 12 months before the first positive PA culture (considered the preisolation segment) to the rates 24 months after PA isolation (considered the postisolation segment); and (2) using a segmented regression analysis of interrupted time series data to assess changes in level and slope of the regression lines in the pre- and postisolation segments. This analysis allowed estimation of changes in outcomes between pre- and postisolation phases, while accounting for both sudden changes and changes in trends for the outcomes of interest. Finally, we evaluated the difference in rates of each outcome between groups for every time period. Taking the difference allows us to collapse the 2 time series into 1 to estimate a difference-in-difference effect. Additional details are provided in the online Supplement.
Monthly exacerbation and hospitalization rates were modeled as incidence rates, including baseline age and FEV1% predicted as confounders. Only patients with complete data over the 36-month period were included in the exacerbation and hospitalization rate analyses (n = 133). There was no significant difference between groups in patients with complete data over the 36-month period (P = .18). A 2-tailed P value of .05 was deemed statistically significant, and all analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC).
RESULTS
Patient Characteristics
Over the study period, 181 COPD patients were enrolled, and PA was isolated in 73 (40%) patients (Supplementary Figure 1). Demographics and clinical characteristics of the PA culture-positive and culture-negative groups are presented in Table 1. The median age of the sample was 68 years (interquartile range [IQR], 61–74), and most patients were white (88%). The median baseline Charlson comorbidity index was 4 (IQR, 3–5), and the median pack-years of smoking was 75 (IQR, 50–100). The prevalence of bronchiectasis was 12% in the PA+ group compared with 14% in the PA− group (P = .71). There were no significant differences between the PA+ and PA− patients for any of these characteristics. However, PA+ patients did have a significantly lower baseline FEV1% predicted (44% vs 53%, P = .01) and were followed up for a longer duration (4.7 vs 2.4 years, P < .001). Pseudomonas aeruginosa was present in 719 (8.1%) study visits compared with 8124 (92%) visits in the culture-negative group, and the mean time to PA isolation was 2.6 ± 2.8 years.
Table 1.
Differences Between Pseudomonas aeruginosa Culture-Positive and Culture-Negative Groups Among Patients Enrolled in the COPD Study, Buffalo, New York, April 1994–June 2014
| Characteristic, N (%) or Median (IQR) | P aeruginosa Culture-Positive (N = 73) | P aeruginosa Culture-Negative (N = 108) | P |
|---|---|---|---|
| Age, years | 66 (61–73) | 68 (62–74) | .63 |
| Race | .32 | ||
| White | 62 (85) | 97 (90) | |
| Black | 11 (15) | 11 (10) | |
| Smoking Status | .95 | ||
| Current smoker | 24 (33) | 36 (33) | |
| Former smoker | 49 (67) | 72 (67) | |
| Pack-years of smoking | 75 (50–106) | 73 (50–96) | .28 |
| Charlson comorbidity index | 4 (3–5) | 4 (3–5) | .89 |
| Bronchiectasisa | 6 (12) | 11 (14) | .71 |
| FEV1, L | 1.42 (1.08–1.78) | 1.67 (1.25–2.19) | .01 |
| FEV1% predicted | 44 (33–54) | 53 (36–63) | .01 |
| Participant follow-up per study protocol, years (mean ± SD) | 5.7 ± 4.2 | 3.7 ± 3.7 | <.001 |
| Median (IQR) | 4.7 (2.8–7.6) | 2.4 (1.1–5.2) | <.001 |
| Total study visits | 719 (8.1)b | 8124 (92)b | |
| Exacerbation visits | 141 (20) | 1238 (15) | .003c |
| Stable visits | 578 (80) | 6886 (85) | |
| Time to P aeruginosa isolation | |||
| Mean ± SD, years | 2.6 ± 2.8 | ||
| Median (IQR), years | 1.6 (0.68–3.7) |
Abbreviations: COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 second; IQR, interquartile range; SD, standard deviation.
aNumber of patients with a high-resolution computed tomography image available for bronchiectasis assessment: P aeruginosa (PA) culture-positive, n = 50; PA culture-negative, n = 77.
bThe percentage indicates isolation from the total number of study visits (n = 8843).
c P value provides the difference in exacerbation and stable visits between PA culture-positive and -negative groups.
Relationship Between Pseudomonas aeruginosa Isolation and Long-Term Mortality
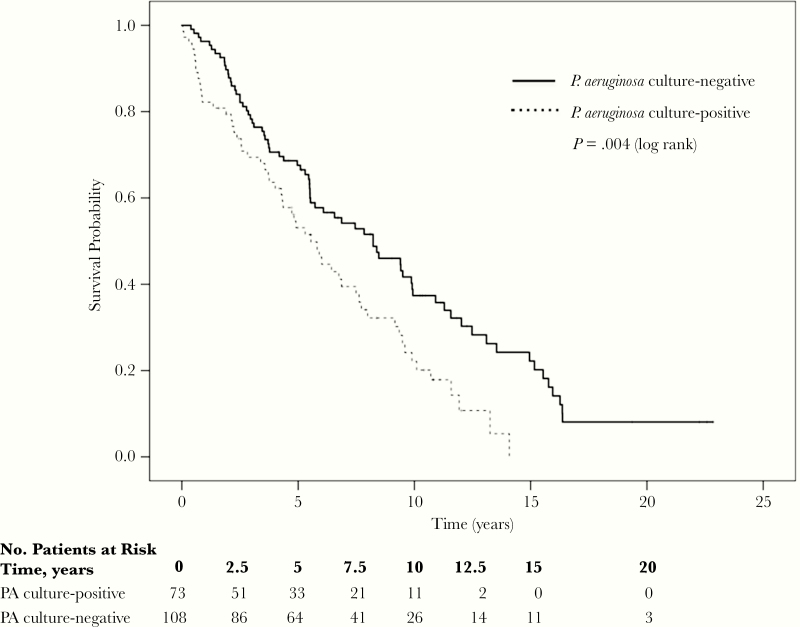
During the study period, 77% (56 of 73) of PA culture-positive patients died compared with 68% (73 of 108) of PA culture-negative patients, with a crude HR of 1.70 (95% CI, 1.18–2.44) (Table 2). The median survival for PA culture-positive patients was 5.5 (95% CI, 4.2–6.9) years compared with 8.2 (95% CI, 5.5–10.9) years in the PA culture-negative group (Figure 1).
Table 2.
Pseudomonas aeruginosa Isolation and Risk of Mortality in Adults With COPD, Buffalo Veterans Affairs Medical Center, Buffalo, New York, 1994–2014
| Group | Events/ Persons | Crudea,b HR (95% CI) | P | Adjusteda,c HR (95% CI) | P |
|---|---|---|---|---|---|
| P aeruginosa culture-negative | 73/108 | 1.0 (reference) | - | 1.0 (reference) | - |
| P aeruginosa culture-positive | 56/73 | 1.72 (1.21–2.43) | .002 | 1.78 (1.24–2.55) | .001 |
Abbreviations: CI, confidence interval; COPD, chronic obstructive pulmonary disease; HR, hazard ratio.
a Pseudomonas aeruginosa is modeled as a time-dependent variable.
bCox proportional hazards regression, unadjusted.
cCox proportional hazards model, covariates: age, forced expiratory volume in 1 second (FEV1)%, Charlson comorbidity index, baseline smoking status, participant follow-up, and race.
Figure 1.
Kaplan-Meier curves comparing overall survival for the Pseudomonas aeruginosa (PA) culture-positive and culture-negative groups.
Kaplan-Meier survival curves with log-rank analysis indicated that PA isolation was associated with mortality (P = .004). Age and FEV1% were the only variables considered a confounder based on a change in the HR of interest by 10% of more and were included in the primary model. After adjustment, the Cox proportional hazards model indicated a 47% higher risk of mortality in the PA culture-positive group versus the culture-negative group (adjusted HR [aHR] = 1.47; 95% CI, 1.03–2.11; P = .04) (Table 2). As a sensitivity analysis, we performed an additional multivariable model adjusting for variables believed to be clinically important including age, FEV1%, Charlson comorbidity index, baseline smoking status, and race. The results were similar between models (aHR = 1.49; 95% CI, 1.04–2.13; P = .03). We further analyzed whether persistent PA sputum cultures, which we defined as >1 repeat positive PA culture, was associated with mortality. Forty-eight of the 73 (66%) subjects had persistent PA+ sputum cultures, and we found no association between persistence and mortality (P = .49). As an additional sensitivity analysis, we included the presence of bronchiectasis within our adjusted models and found that our estimates were robust (aHR = 1.89; 95% CI, 1.22–2.94; P = .005) (Supplementary Table 1). The proportionality hazard assumption was tested using the Schoenfeld residuals and was nonsignificant in all models, indicating that the assumption was not violated.
Relationship Between Pseudomonas aeruginosa Isolation and Clinical Outcomes
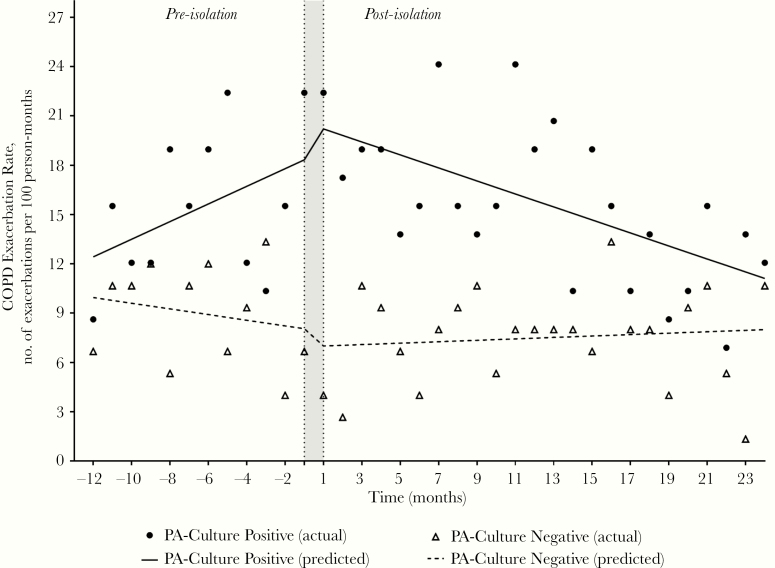
For the PA culture-positive group, the exacerbation rate was 15.4 per 100 person-months in the preisolation segment and 15.7 per 100 person-months in the postisolation segment (Table 3). The exacerbation rate was higher in the PA culture-positive group versus the culture-negative group in both the preisolation (15.4 vs 9.0, P < .001) and postisolation (15.7 vs 7.5, P < .001) segments. The results of the segmented regression analysis for exacerbation rates are summarized in Table 4 and Figure 2. For the PA culture-positive group, a significant decrease in the slope (coefficient −1.19, P = .02) in the postisolation segment was observed, but no significant changes in slope or level were observed in the PA culture-negative group.
Table 3.
Exacerbation and Hospitalization Rates in Pseudomonas aeruginosa Culture-Positive and Culture-Negative Groups During the Pre- and Postisolation Segments, Buffalo, New York, April 1994–June 2014
| Outcome | Groupa | Preisolation Segmentb (95% CI) | Postisolation Segmentb (95% CI) | P Value (pre vs post) |
|---|---|---|---|---|
| Exacerbation Rate | PA culture-positive | 15.4 (12.6–18.6) | 15.7 (13.7–17.9) | .88 |
| PA culture-negative | 9 (7.1–11.2) | 7.5 (6.3–8.9) | .20 | |
| P value (positive vs negative groups) | <.001 | <.001 | ||
| Overall Hospitalization Rate | PA culture-positive | 4.74 (3.26–6.65) | 6.25 (5.01–7.71) | .17 |
| PA culture-negative | 4.33 (3.08–5.92) | 2.44 (1.78–3.29) | .01 | |
| P value (positive vs negative groups) | .71 | <.001 | ||
| COPD-Related Hospitalization Rate | PA culture-positive | 2.44 (1.42–3.91) | 1.87 (1.22–2.74) | 0.39 |
| PA culture-negative | 1.44 (0.77–2.47) | 0.89 (0.51–1.44) | 0.20 | |
| P value (positive vs negative groups) | .15 | .02 |
Abbreviations: CI, confidence interval; COPD, chronic obstructive pulmonary disease; PA, P aeruginosa.
aIncludes only patients with nonmissing data over the 36-month period: PA culture-positive (n = 58); PA culture-negative (n = 75).
bRates are expressed as per 100 person-months.
Table 4.
Changes in Exacerbation and Hospitalization Rates Between Pseudomonas aeruginosa Culture-Positive and Culture-Negative Groups Using Segmented Regression Analysis, Buffalo, New York, April 1994–June 2014a,b
| Outcome | Group | Baseline Trend (per 100 person-months) | Change in Level | Trend Change After Month 12 (per 100 person-months) | |||
|---|---|---|---|---|---|---|---|
| Coefficient (SE) | P | Coefficient (SE) | P | Coefficient (SE) | P | ||
| Exacerbation Rate | PA culture-positive | 0.537 (0.339) | .12 | 1.34 (3.27) | .69 | −1.19 (0.472) | .02 |
| PA culture-negative | −0.356 (0.458) | .44 | −1.99 (3.02) | .52 | 0.225 (0.405) | .58 | |
| Difference | 0.709 (0.434) | .11 | 2.70 (4.18) | .52 | −1.18 (0.603) | .06 | |
| Hospitalization Rate | PA culture-positive | 0.247 (0.281) | .39 | 1.83 (2.71) | .50 | −0.312 (0.391) | .43 |
| PA culture-negative | −0.533 (0.289) | .07 | −2.84 (1.90) | .15 | 0.343 (0.255) | .19 | |
| Difference | 0.522 (0.263) | .06 | 3.33 (2.54) | .20 | −0.561 (0.366) | .14 |
Abbreviations: PA, P aeruginosa; SE, standard error.
aEach model is adjusted for age and forced expiratory volume in 1 second (FEV1)%.
bSignificant results are shown in boldface.
Figure 2.
Chronic obstructive pulmonary disease (COPD) exacerbation rates comparing Pseudomonas aeruginosa (PA) culture-positive and culture-negative groups in the pre- and postisolation segments. The shaded area indicates time of PA isolation.
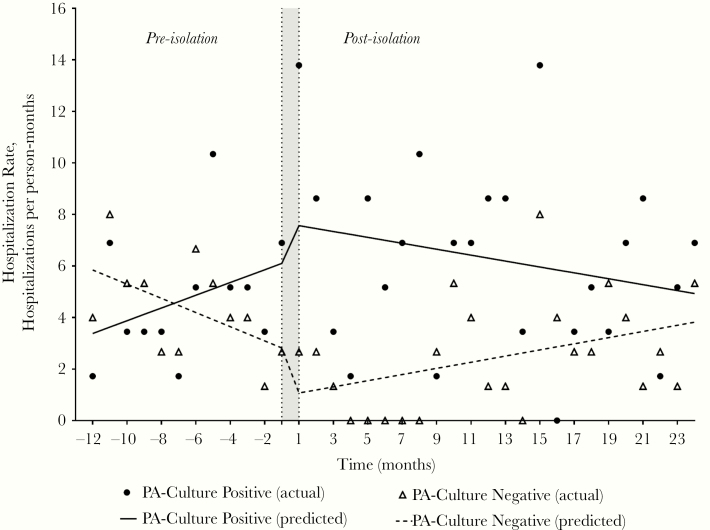
The overall hospitalization rate in the PA+ group increased from 4.74 per 100 person-months in the preisolation segment to 6.25 per 100 person-months in the postisolation segment, but the change was not statistically significant (P = .17) (Table 3). Hospitalization rates were similar between groups in the preisolation segment (P = .71) and significantly higher within the culture-positive group in the postisolation segment (6.25 vs 2.44 hospitalizations per 100 person-months, P = .01). A decreasing trend in overall hospitalization rate within the PA− group was seen preisolation (P = .07) with an almost significant difference from the PA+ group (P = .06); however, no significant changes in slope or level within either group were seen within the pre- and postisolation segments (Figure 3, Table 4). The PA+ group had higher rates for COPD-related hospitalizations than the PA− group, and the difference was statistically significant in the postisolation segment (Table 3).
Figure 3.
Hospitalization rates comparing Pseudomonas aeruginosa (PA) culture-positive and culture-negative groups in the pre- and postisolation segments. The shaded area indicates time of PA isolation.
Antibiotic and Steroid Use Between Groups
Antibiotic and oral steroid treatments for acute exacerbations were evaluated during the 36-month period among patients who had data for this time frame (n = 133). We evaluated antibiotic use as both the number of treatment regimens for an AECOPD and the total number of antibiotic administered to the patient. The PA+ group received a higher rate of antibiotic and oral steroid treatments within both the pre- and postisolation segments (P < .01) (Table 5). Total antibiotic exposure was also significantly higher in the PA+ group compared with the PA− group in both segments (P < .001). We saw an increase in ciprofloxacin (antibiotic with potential activity against PA) exposure in the postisolation segment within the PA+ group (n = 23, 7.8%) compared with the PA− group (n = 6, 3.7%; P = .08).
Table 5.
Antibiotic and Steroid Utilization Comparing Pseudomonas aeruginosa Culture-Positive and Culture-Negative Groupsa,b
| Antibiotic and Steroid Utilization | Preisolation Segment (12 months) | P | Postisolation Segment (24 months) | P | ||
|---|---|---|---|---|---|---|
| (+) | (−) | (+) | (−) | |||
| No. of antibiotic treatments for an acute exacerbation | 118 | 86 | 249 | 142 | ||
| Antibiotic treatment rate (95% CI)c | 16.9 (14–20.3) | 9.6 (7.6–11.8) | <.001 | 17.9 (15.7–20.2) | 7.9 (6.6–9.3) | <.001 |
| No. of oral steroid treatments for an acute exacerbation | 54 | 40 | 120 | 85 | ||
| Steroid treatment rate (95% CI)c | 7.8 (5.8–10.1) | 4.4 (3.2–6.1) | .007 | 8.6 (7.1–10.3) | 4.7 (3.7–5.8) | <.001 |
| Total antibiotic exposure, no. | 146 | 96 | 293 | 162 | ||
| Antibiotic exposure rate (95% CI)c | 20.9 (17.7–24.7) | 10.7 (8.6–13) | <.001 | 21 (18.7–23.6) | 9 (7.7–10.5) | <.001 |
| Antibiotic classes, no. (%) | ||||||
| β-lactam | 43 (29) | 24 (25) | .45 | 92 (31) | 43 (27) | .28 |
| Macrolide | 30 (21) | 28 (29) | .13 | 62 (21) | 44 (27) | .15 |
| Fluoroquinoloned | 31 (21) | 23 (24) | .62 | 61 (21) | 45 (28) | .10 |
| Ciprofloxacin | 7 (4.8) | 4 (4.2) | .84 | 23 (7.8) | 6 (3.7) | .08 |
| Folate antagonists | 24 (16) | 13 (14) | .55 | 35 (12) | 19 (12) | .95 |
| Other | 11 (7.5) | 4 (4.2) | .30 | 6 (2) | 5 (3) | .50 |
Abbreviations: CI, confidence interval.
a(+) and (−) indicate the P aeruginosa culture-positive and culture–negative groups, respectively.
bIncludes only patients with nonmissing data over the 36-month period: P aeruginosa (PA) culture-positive (n = 58); PA culture-negative (n = 75).
cRates are expressed per 100 person-months.
dCiprofloxacin is presented separately and not counted within the fluoroquinolone group.
Immune Response to Pseudomonas aeruginosa
The 10 paired control samples from patients without colonization demonstrated 4.3 ± 6.4 (mean ± 1 standard deviation) change in optical density (OD), with a 19.2% change representing the upper limit of the 99% CI. Therefore, an increase in OD450 of more than 19.2% between sera was regarded as a significant serum immune response to the PA strain. Enzyme-linked immunosorbent assays detected a significant antibody response before the first positive culture for PA in 6 of 52 (12%) PA+ patients and in 1 of 16 (6%) PA− patients (P = .61).
Discussion
In this study, we show that even in the outpatient setting, PA tracheobronchial colonization is associated with long-term mortality in COPD. Pseudomonas aeruginosa colonization is also associated with greater morbidity, including a higher exacerbation frequency observed in the year before and 2 years after isolation. In addition, PA culture-positive patients had higher, sustained hospitalization rates including COPD-related hospitalizations over the observation period.
Our data indicate that the isolation of PA from an outpatient-based COPD population is associated with worse survival. Previous studies evaluating this relationship were based on patients hospitalized for COPD exacerbations. Almagro et al [14] prospectively studied patients hospitalized with a COPD exacerbation and reported that PA isolation in sputum was associated with a higher mortality at 3 years. Likewise, Lin et al [20] described 494 COPD exacerbations in hospitalized patients in Taiwan and found that PA was a common pathogen and independent risk factor for inpatient mortality. Multidrug-resistant PA isolation in hospitalized patients has also been shown to be associated with mortality in COPD [16, 21]. In contrast to our observations, Boutou et al [15] found no relationship between PA isolation and long-term mortality using hospital-based microbiology records to identify isolation. The retrospective nature of their study and lack of systematic sampling may explain why their results are different from ours.
Chronic obstructive pulmonary disease exacerbations incur a substantial burden on healthcare systems and are a major cause of mortality, morbidity, and poor health status [22, 23]. Our results suggest that patients with PA-positive sputum cultures have higher exacerbation rates. We have previously shown in the same cohort of patients that isolation of a new PA strain was associated with exacerbation [13]. Gallego et al [24] recently reported that PA is associated with a higher exacerbation frequency (4.4 ± 4 vs 2.5 ± 2, P = .008) in a prospective study of 118 COPD patients followed for up to 1 year after hospital discharge. Boutou et al [15] also reported a significantly higher exacerbation rate per year in PA isolation-positive patients. Increased exacerbation frequency and corresponding antibiotic and systemic corticosteroid use in the PA+ patients predated the acquisition of PA. Potential explanations include that host exacerbation susceptibility factors (eg, impaired macrophage function) overlap with those that lead to PA acquisition [25]. Antibiotic selection pressure and repeated exposures to systemic steroids could also account for subsequent PA acquisition. Subclinical PA acquisition, as discussed below, could also have contributed. Long-term, low-dose macrolide therapy has been shown to be effective in the reduction of exacerbations, and in vitro studies have shown macrolides to interfere with PA quorum sensing [26–28]. This has the potential to reduce exacerbations in Pseudomonas infections and influence mortality; however, none of the subjects within this study were on long-term macrolide therapy. Further studies are required to dissect these potential mechanisms.
We observed no significant differences in exacerbation or hospitalization rates in the 24 months after PA isolation. This may be explained by a combination of pathogenic and host factors that determine outcome of acquisition of a bacterial strain. Not all isolations of pathogenic bacteria are followed by exacerbations. In addition, we observed an increase in ciprofloxacin use after Pseudomonas isolation. However, increased COPD-related hospitalization rates in the PA+ patients demonstrate that the severe exacerbations are enhanced by PA isolation, and these are the ones that are associated with the most morbidity and mortality in COPD.
Pseudomonas aeruginosa airway infection is associated with the development of systemic antibody response, and serum antibody levels to PA have been used to examine temporal relationships of PA isolation in CF [13]. West et al [29] showed that titers of antibodies directed against PA were elevated up to 11.9 months before PA isolation in a cohort of children with CF. We have observed that 12% of our PA+ patients exhibited antibody responses to PA before their first positive culture. The most likely explanation for this is that sputum cultures remained negative despite the patient having acquired PA. This could also underlie the increased exacerbation rate before PA isolation.
Does PA colonization directly increase COPD morbidity and mortality, or is it a marker of another cause of worse clinical outcomes? The accumulating evidence for the vicious cycle hypothesis in COPD—that bacterial presence in the tracheobronchial tree, even in clinically stable COPD, is proinflammatory and associated with pathological and structural evidence of chronic infection—would suggest that PA is a direct driver of adverse outcomes rather than a marker. The clear-cut adverse consequences of PA colonization in bronchiectasis and CF have been described in several studies, and, although differences exist, a final common pathway of infection-associated inflammatory damage to the airways is likely too prevalent in these airway diseases.
A limitation of our study was the relatively small number of patients included; however, most published studies in this field have used similar sample sizes [14, 15, 30]. In addition, a limitation, as with all observational studies, is the risk of residual confounding factors. We found no relationship between PA isolation and bronchiectasis; however, high-resolution computed tomography was not systematically performed to diagnose bronchiectasis, so we only estimated prevalence based on a subset of patients. Because the patient sample included predominantly moderately severe and severe COPD with chronic bronchitis, we would not expect these results to be different within the entire patient cohort. Previous studies have found no relationship between bronchiectasis score and spriometric measurements or the presence of PA in sputum [30, 31]. This study was conducted at a single center, which may limit its generalizability; however, the findings of this study are enhanced by its internal validity with a rigorous approach to monthly sputum collections and diagnoses of exacerbations. Our sample of COPD patients were older and predominantly male with moderate to severe COPD at baseline. Further study in mild disease in young patients with female gender representation is required to determine whether the identified relationships and outcomes are applicable across the spectrum of COPD. Future clinical trials are needed to confirm our results and determine stratification criteria for PA eradication in COPD.
Conclusions
Isolation of PA in sputum was associated with long-term mortality in COPD outpatients. Pseudomonas aeruginosa isolation was also associated with adverse clinical outcomes including higher exacerbation, overall hospitalization, and COPD-related hospitalization rates. These findings suggest that PA likely contributes to adverse clinical outcomes rather than just a marker of worsening disease. Because early detection and prompt treatment of exacerbations are essential to reduce COPD burden, a prompt diagnosis of PA-related infection should be a priority to limit long-term sequelae. Because chronic colonization by PA also has adverse consequences, whether early eradication, as is practiced in CF with success, is applicable to COPD could be considered. Current clinical practice in CF includes screening for PA isolation, early eradication of recent acquisition, and aggressive treatment of PA-related exacerbations. The potential importance of such approaches in COPD is supported by this study, and these methods should be tested in prospective trials.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Figure 1. Flow diagram. This figure shows the flow of patients included in the analyses. (aNo significant difference between groups in the proportion of missing data excluded from these analyses.)
Acknowledgments
Author contributions. D. M. J., H. M. O.-B., K. N., J. Z., T. F. M., and S. S. contributed to study concept and design. All authors contributed to acquisition, analysis, or interpretation of the data. D. M. J., J. Z., H. M. O.-B., and S. S. contributed to statistical analysis. D. M. J., H. M. O.-B., and S. S. generated the initial draft of the manuscript. All authors contributed to critical revisions of the manuscript. All authors provided final approval of the manuscript. D. M. J. and S. S. supervised the study.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official view of the National Institutes of Health.
Financial support. D. M. J. was funded by the National Institutes of Health, National Heart, Lung, and Blood Institutes Loan Repayment Program (1 L30 HL138791-01). The COPD study was supported by a Merit Review grant from the Department of Veterans Affairs (to T.F.M. and S.S.). Research reported in this publication was funded by the National Center for Advancing Translational Sciences of the National Institutes of Health under award number ULTR001412 to the University at Buffalo.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Razvi S, Quittell L, Sewall A, et al. Respiratory microbiology of patients with cystic fibrosis in the United States, 1995 to 2005. Chest 2009; 136:1554–60. [DOI] [PubMed] [Google Scholar]
- 2. Folkesson A, Jelsbak L, Yang L, et al. Adaptation of Pseudomonas aeruginosa to the cystic fibrosis airway: an evolutionary perspective. Nat Rev Microbiol 2012; 10:841–51. [DOI] [PubMed] [Google Scholar]
- 3. Douglas TA, Brennan S, Gard S, et al. Acquisition and eradication of P. aeruginosa in young children with cystic fibrosis. Eur Respir J 2009; 33:305–11. [DOI] [PubMed] [Google Scholar]
- 4. Rosenfeld M, Emerson J, Accurso F, et al. Diagnostic accuracy of oropharyngeal cultures in infants and young children with cystic fibrosis. Pediatr Pulmonol 1999; 28:321–8. [DOI] [PubMed] [Google Scholar]
- 5. Emerson J, Rosenfeld M, McNamara S, et al. Pseudomonas aeruginosa and other predictors of mortality and morbidity in young children with cystic fibrosis. Pediatr Pulmonol 2002; 34:91–100. [DOI] [PubMed] [Google Scholar]
- 6. Rayner CF, Tillotson G, Cole PJ, Wilson R. Efficacy and safety of long-term ciprofloxacin in the management of severe bronchiectasis. J Antimicrob Chemother 1994; 34:149–56. [DOI] [PubMed] [Google Scholar]
- 7. Davies G, Wells AU, Doffman S, et al. The effect of Pseudomonas aeruginosa on pulmonary function in patients with bronchiectasis. Eur Respir J 2006; 28:974–9. [DOI] [PubMed] [Google Scholar]
- 8. Finch S, McDonnell MJ, Abo-Leyah H, et al. A comprehensive analysis of the impact of Pseudomonas aeruginosa colonization on prognosis in adult bronchiectasis. Ann Am Thorac Soc 2015; 12:1602–11. [DOI] [PubMed] [Google Scholar]
- 9. Murphy TF. Pseudomonas aeruginosa in adults with chronic obstructive pulmonary disease. Curr Opin Pulm Med 2009; 15:138–42. [DOI] [PubMed] [Google Scholar]
- 10. Miravitlles M, Espinosa C, Fernández-Laso E, et al. Relationship between bacterial flora in sputum and functional impairment in patients with acute exacerbations of COPD. Study group of bacterial infection in COPD. Chest 1999; 116:40–6. [DOI] [PubMed] [Google Scholar]
- 11. Eller J, Ede A, Schaberg T, et al. Infective exacerbations of chronic bronchitis: relation between bacteriologic etiology and lung function. Chest 1998; 113:1542–8. [DOI] [PubMed] [Google Scholar]
- 12. Lode H, Allewelt M, Balk S, et al. A prediction model for bacterial etiology in acute exacerbations of COPD. Infection 2007; 35:143–9. [DOI] [PubMed] [Google Scholar]
- 13. Murphy TF, Brauer AL, Eschberger K, et al. Pseudomonas aeruginosa in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2008; 177:853–60. [DOI] [PubMed] [Google Scholar]
- 14. Almagro P, Salvadó M, Garcia-Vidal C, et al. Pseudomonas aeruginosa and mortality after hospital admission for chronic obstructive pulmonary disease. Respiration 2012; 84:36–43. [DOI] [PubMed] [Google Scholar]
- 15. Boutou AK, Raste Y, Reid J, et al. Does a single Pseudomonas aeruginosa isolation predict COPD mortality? Eur Respir J 2014; 44:794–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Montero M, Domínguez M, Orozco-Levi M, et al. Mortality of COPD patients infected with multi-resistant Pseudomonas aeruginosa: a case and control study. Infection 2009; 37:16–9. [DOI] [PubMed] [Google Scholar]
- 17. Sethi S, Murphy TF. Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N Engl J Med 2008; 359:2355–65. [DOI] [PubMed] [Google Scholar]
- 18. Sethi S, Evans N, Grant BJ, Murphy TF. New strains of bacteria and exacerbations of chronic obstructive pulmonary disease. N Engl J Med 2002; 347:465–71. [DOI] [PubMed] [Google Scholar]
- 19. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40:373–83. [DOI] [PubMed] [Google Scholar]
- 20. Lin SH, Kuo PH, Hsueh PR, et al. Sputum bacteriology in hospitalized patients with acute exacerbation of chronic obstructive pulmonary disease in Taiwan with an emphasis on Klebsiella pneumoniae and Pseudomonas aeruginosa. Respirology 2007; 12:81–7. [DOI] [PubMed] [Google Scholar]
- 21. Rodrigo-Troyano A, Suarez-Cuartin G, Peiró M, et al. Pseudomonas aeruginosa resistance patterns and clinical outcomes in hospitalized exacerbations of COPD. Respirology 2016; 21:1235–42. [DOI] [PubMed] [Google Scholar]
- 22. Mannino DM, Buist AS. Global burden of COPD: risk factors, prevalence, and future trends. Lancet 2007; 370:765–73. [DOI] [PubMed] [Google Scholar]
- 23. Rabe KF, Watz H. Chronic obstructive pulmonary disease. Lancet 2017; 389:1931–40. [DOI] [PubMed] [Google Scholar]
- 24. Gallego M, Pomares X, Espasa M, et al. Pseudomonas aeruginosa isolates in severe chronic obstructive pulmonary disease: characterization and risk factors. BMC Pulm Med 2014; 14:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sethi S, Maloney J, Grove L, et al. Airway inflammation and bronchial bacterial colonization in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2006; 173:991–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Burr LD, Rogers GB, Chen AC, et al. Macrolide treatment inhibits Pseudomonas aeruginosa quorum sensing in non-cystic fibrosis bronchiectasis. an analysis from the bronchiectasis and low-dose erythromycin study trial. Ann Am Thorac Soc 2016; 13:1697–703. [DOI] [PubMed] [Google Scholar]
- 27. Tateda K, Comte R, Pechere JC, et al. Azithromycin inhibits quorum sensing in Pseudomonas aeruginosa. Antimicrob Agents Chemother 2001; 45:1930–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nalca Y, Jänsch L, Bredenbruch F, et al. Quorum-sensing antagonistic activities of azithromycin in Pseudomonas aeruginosa PAO1: a global approach. Antimicrob Agents Chemother 2006; 50:1680–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. West SE, Zeng L, Lee BL, et al. Respiratory infections with Pseudomonas aeruginosa in children with cystic fibrosis: early detection by serology and assessment of risk factors. JAMA 2002; 287:2958–67. [DOI] [PubMed] [Google Scholar]
- 30. Garcia-Vidal C, Almagro P, Romaní V, et al. Pseudomonas aeruginosa in patients hospitalised for COPD exacerbation: a prospective study. Eur Respir J 2009; 34:1072–8. [DOI] [PubMed] [Google Scholar]
- 31. Patel IS, Vlahos I, Wilkinson TM, et al. Bronchiectasis, exacerbation indices, and inflammation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2004; 170:400–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.