Abstract
Background
Using a toxin-induced nonhuman primate model of acute liver failure (ALF), we previously reported that peripheral infusion of human umbilical cord mesenchymal stem cells (hUC-MSCs) strongly suppresses the activation of circulating monocytes and interleukin-6 (IL-6) production, thereby disrupting the development of a cytokine storm and improving the prognosis of monkeys. MSCs are considered to play a therapeutic role under different stresses by adaptively producing specific factors, prompting us to investigate the factors that hUC-MSCs produce in response to high serum levels of IL-6, which plays a critical role in initiating and accelerating ALF.
Methods
We stimulated hUC-MSCs with IL-6, and the hUC-MSC-derived exosomes were deeply sequenced. The miRNAs in the exosomes that have potential to suppress IL-6-associated signaling pathway were screened, and the role of one of the most possible miRNAs was tested in the mouse model of inflammatory liver injury.
Result
We determined that miR-455-3p, which is secreted through exosomes and potentially targets PI3K signaling, was highly produced by hUC-MSCs with IL-6 stimulation. The miR-455-3p-enriched exosomes could inhibit the activation and cytokine production of macrophages challenged with lipopolysaccharide (LPS) both in vivo and in vitro. In a chemical liver injury mouse model, enforced expression of miR-455-3p could attenuate macrophage infiltration and local liver damage and reduce the serum levels of inflammatory factors, thereby improving liver histology and systemic disorder.
Conclusions
miR-455-3p-enriched exosomes derived from hUC-MSCs are a promising therapy for acute inflammatory liver injury.
Keywords: Exosome, microRNA-455-3p, hUC-MSCs, Inflammation, Acute liver injury
Background
Liver damage caused by a variety of etiologies can lead to acute liver injury and even acute liver failure (ALF). Patients often die of hepatic encephalopathy, coagulopathy, multiple organ failure (MOF), etc. [1]. Previously, we established an amanitin-induced acute liver failure model in monkeys, which comprehensively revealed the pathophysiological process of ALF [2]. After the induction of toxins, the initial injury of the liver is not serious, but a secondary uncontrolled systemic inflammatory response syndrome (SIRS) is the leading cause of multiple organ failure and death in animals [2]. Most strikingly, bone marrow-derived circulating monocytes are activated, characterized by overexpression of IL-6, before the occurrence of a cytokine storm and significant liver damage. Subsequently, IL-6 becomes an important initiator of an uncontrolled cytokine storm [3]. At the same time, many circulating monocytes migrate to the liver and differentiate into mature Kupffer cells that aggravate the local liver damage, and as a result, ALF occurs. In monkey and mouse toxic liver injury models, suppression of monocyte activation, especially inhibition of IL-6 secretion, can effectively control the occurrence of a cytokine storm, ameliorate liver damage, and improve animal survival.
Mesenchymal stem cells (MSCs) are tissue stem cells that are widely distributed in various tissues, such as the bone marrow, liver, fat, placenta, and umbilical cord. MSCs have multidirectional differentiation potential. They also secrete various growth factors and cytokines that promote cell regeneration, tissue repair, and immune regulation. MSCs have been used in mouse models for the treatment of a variety of diseases, including acute lung injury, myocardial infarction, diabetes, sepsis, liver dysfunction, and acute renal failure [4, 5]. Since MSCs have shown promising clinical application prospects in animal models of human diseases, MSC-based therapies have been carried out in multiple clinical trials for the treatment of various disorders [6–8]. As shown on www.ClinicalTrials.gov, dozens of MSC-based clinical therapies are underway for different types of liver injury, including autoimmune hepatitis, alcoholic liver cirrhosis, and liver fibrosis. However, MSC-based therapies have been strongly limited by their unclear mechanism of action [5, 9].
We previously established a toxin-induced nonhuman primate model of ALF [2]. In this model, we found that early peripheral infusion of human umbilical cord mesenchymal stem cells (hUC-MSCs) significantly rescued the monkeys from lethal ALF. Mechanistically, hUC-MSCs were not found to directly differentiate into hepatocytes, promote liver regeneration, or regulate T cells and B cells. However, early treatment of hUC-MSCs strongly inhibited the overproduction of IL-6 by reducing the activation of circulating monocytes, thus significantly inhibiting cytokine storms and improving liver damage and animal survival [10]. Furthermore, in vitro experiments revealed that coculture of hUC-MSCs inhibited LPS-induced macrophage activation and inflammatory factor secretion [10]. However, the mechanism by which hUC-MSCs inhibit macrophage activation remains unclear.
Exosomes are monolayer membrane vesicles actively secreted by cells into the extracellular space. They have a lipid bilayer membrane structure with a diameter of approximately 50–150 nm and contain diverse bioactive substances, such as transcription factors, oncogenes, miRNAs, lncRNAs, and mRNAs [11].. The exosome membrane protects these molecules from degradation before they reach the target cells (receptor cells) [12]. Exosomes can be engulfed by different cells, such as macrophages, endothelial cells, and tumor cells [13, 14]. Donor cell-derived exosomes directly activate cell surface receptors via proteins and biologically active lipid ligands and deliver their effectors to recipient cells. These foreign effectors also perform their own distinct functions in the recipient cells; for example, transcription factors can directly initiate gene transcription, mRNA can be translated into proteins, and miRNAs and lncRNAs can regulate gene transcription and translation. Therefore, exosomes are an important means of intercellular communication and play important roles in immune responses, immune regulation, inflammatory responses, and stem cell phenotypic transformation [15–18].
miRNAs are small noncoding RNAs that serve as regulators of mRNA expression and translation efficiency in most cell types. miRNAs contain a 6- to 8-nucleotide seed sequence corresponding to the complementary sequence in the 3′-UTR of the target mRNAs [19]. Binding of miRNA to mRNA results in the targeted recruitment of mRNA to the RNA-induced silencing complex (RISC), leading to translational arrest and mRNA degradation. Through these mechanisms, miRNAs reduce the protein expression of target mRNAs [19]. Interestingly, miRNAs are mainly delivered to the extracellular body in the form of exosomes, which are ubiquitous in the peripheral blood and cell culture medium. The exosome membrane effectively protects miRNAs from degradation by RNase [19, 20]. In turn, miRNAs are also one of the most important effectors of exosomes. After receiving miRNA-enriched exosomes, gene expression of the receptor cell has the potential to be regulated by the foreign miRNAs [20]. Therefore, miRNA-enriched exosomes play an important role in cell-to-cell communication [21, 22].
Recent studies suggest that the protective effects of MSCs on tissue repair and immune regulation may be mainly achieved by paracrine effects [23]. MSC-derived exosomes have shown therapeutic effects in models of myocardial ischemia, acute lung injury/ischemia, and skin trauma [24, 25]. In addition, cultures containing MSC-derived exosomes have therapeutic effects in a number of preclinical models [8, 26]. Similar to MSC-based therapy, MSC-derived exosomes are also beneficial for liver diseases such as liver fibrosis, drug-induced liver injury, and liver ischemia/reperfusion injury [27]. Due to their wide plasticity, MSCs play different roles in different pathophysiological environments, which is a prerequisite for the successful treatment of various diseases [28]. We speculate that in the early stage of toxic liver injury, the rapidly increased IL-6 is one of the first internal stresses that infused hUC-MSC encounter. Therefore, in this study, to mimic the situation in vivo, we stimulated hUC-MSCs with IL-6 in vitro and determined that miR-455-3p-enriched exosomes derived from hUC-MSCs have a promising therapeutic effect on acute liver injury by inhibiting the overactivation of monocytes and macrophages.
Materials and methods
Animals and treatment
All mouse experimental procedures were approved by the Animal Care and Use Committee of Sichuan University. Eight-week-old male mice (C57BL/6) were purchased from Chengdu Dossy Experimental Animals Co., Ltd. To establish an acute liver injury model or endotoxemia model, the mice were intraperitoneally injected with 10% carbon tetrachloride (CCl4) at 10 mL/kg or LPS at 3 mg/kg. Mice were sacrificed at the indicated time points for tissue and blood harvest.
Cell lines and treatment
Human umbilical cords were donated by women who underwent cesarean sections. Informed consent was obtained from the subjects’ families. hUC-MSCs were collected at the Sichuan Stem Cell Bank, Chengdu, China, and cultured with serum-free medium (Gibco, USA). hUC-MSCs were identified by flow cytometry as described in previous studies [15]. The THP-1 cell line was obtained from Cellcook Biotech Guangzhou Ltd., and the cell line was authenticated by short tandem repeat (STR) profiling. The cells were identified as macrophages by immunohistochemical staining of CD68 (Abcam, USA) after induction with 100 μg/mL phorbol myristate acetate (PMA, Sigma-Aldrich, St. Louis, MO, USA) [20].
For the coculture system, hUC-MSCs (105/well) were cultured in the upper chamber of a Transwell plate (0.4-μm polycarbonate filter, Corning), and macrophages were grown in the lower chamber and stimulated with LPS (100 ng/mL, Sigma-Aldrich). The medium and cells were collected after 48 h. To inhibit exosome secretion, hUC-MSCs were pretreated with 10 μM GW4869 (MCE, Monmouth Junction, NJ, USA) for 24 h.
Exosome purification and characterization
hUC-MSCs were cultured with 1 ng/mL IL-6 (Sigma-Aldrich) in the medium for 48 h, and the supernatant was collected for exosome purification using an ExoQuick ULTRA EV isolation kit (SBI, Palo Alto, CA, USA). The characterization of exosomes was confirmed by electron microscopy and particle size by NanoSight analysis (Shanghai Oe Biotech Co., Ltd.) as described [19]. The expression of the exosome-specific marker TSG101 (SBI) and the EV-related protein markers CD63 and CD81 (SBI) were analyzed by western blotting. miRNAs were extracted from exosomes using TRIzol reagent (Invitrogen, USA) for qPCR analysis or sequencing at Shanghai Oe Biotech. To identify the transport of exosomes, exosomes were labeled with PKH26 fluorescent dye (Sigma-Aldrich) [19].
Cytokine analysis
A MILLIPLEX MAP Human Cytokine/Chemokine kit (Millipore, Billerica, MA, USA; cat # HCYTOMAG-60 K) was used to quantify the levels of cytokines, chemokines, and growth factors in human cell supernatants on a Luminex 200 System (Millipore) according to the manufacturer’s instructions. A MILLIPLEX MAP Mouse Cytokine/Chemokine kit (Millipore; cat # MCYTMAG-70 K-PX32) was used to quantify the levels of serum cytokines, chemokines, and growth factors in mice.
Flow cytometry analysis
Antibodies were purchased from BioLegend (San Diego, CA, USA). The proportion of monocytes/macrophages in peripheral blood was identified using fluorescently labeled antibodies. Monocytes/macrophages were detected by staining for CD45, CD14, CD16, or CCR2 [20] and analyzed using CytExpert software.
Statistical analysis
GraphPad Prism 7.0 was used for statistical analyses. All data are represented as the mean ± SEM. The statistical significance of the differences between various treatments was measured by either the two-tailed Student’s t test or one-way ANOVA with Bonferroni post-test. Differences were considered statistically significant when p < 0.05.
Results
hUC-MSC-derived exosomes inhibit macrophage activation
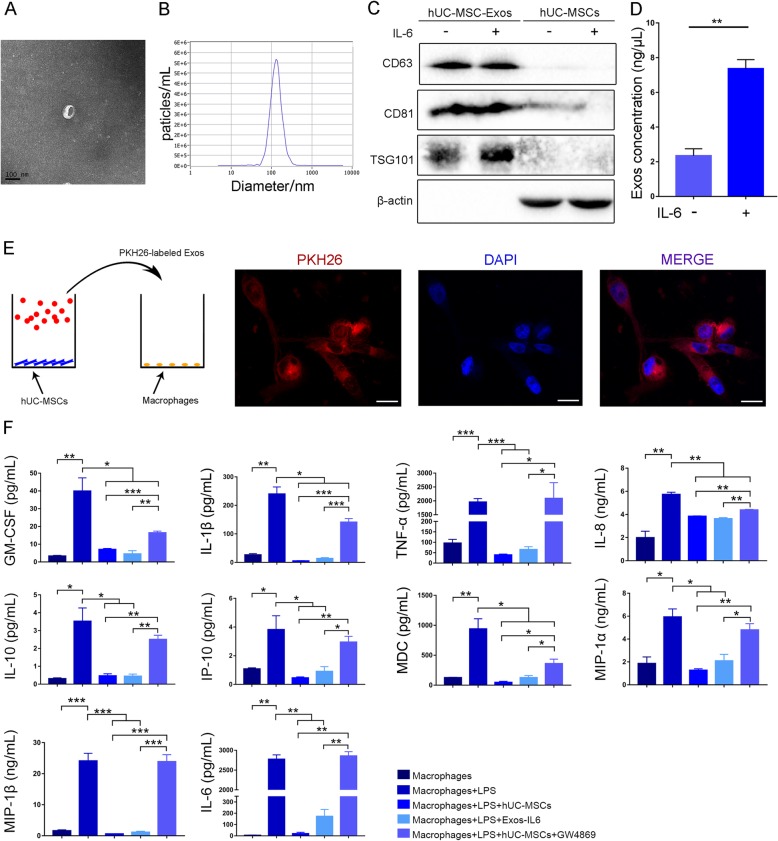
We first tested whether hUC-MSCs can secrete exosomes under IL-6 stimulation. hUC-MSCs at passage 3 were cultured with 1 ng/mL IL-6 for 48 h. After removing the dead cells and debris, an ExoQuick ULTRA EV isolation kit was used to purify exosomes from the serum-free medium. Electron microscopy and NanoSight analysis showed that the particles contained a large number of hUC-MSC-derived vesicles with a diameter of 50–150 nm (Fig. 1a, b). Western blot analysis confirmed that the vesicles did not express β-actin but expressed the exosome-specific protein markers CD63 (a four-transmembrane protein accumulated in the multivesicular body), CD81, and TSG101 (mainly present in the cytoplasm) (Fig. 1c). These observations indicated that the vesicles were MSC-derived exosomes (MSC-Exos). Notably, after IL-6 stimulation, hUC-MSCs could secrete approximately three times more exosomes (Exos-IL6) than hUC-MSCs without IL-6 stimulation (Exos-NC, negative control) (Fig. 1d).
Fig. 1.
Exos-IL6 inhibit macrophage activation. a Electron microscopy analysis of vesicles secreted by hUC-MSCs (scale bar = 100 nm). b The particle size of the vesicles secreted by hUC-MSCs was measured by NanoSight analysis. c Exosome-specific markers TSG101 and CD63 and CD81 were measured by western blot analysis. d Exosome abundance in hUC-MSC medium with (Exos-IL6) or without IL-6 (Exos-NC). e PKH26-labeled exosomes were coincubated with macrophages and examined by confocal microscopy (scale bar = 20 μm). f Exos-IL6 were added to macrophages to detect inflammatory cytokines, chemokines, and growth factors in cell culture media. GM-CSF, granulocyte-macrophage colony-stimulating factor; IL, interleukin; MIP-1, macrophage inflammatory protein-1; IP-10, interferon-inducible protein-10; MDC, macrophage-derived chemokine; MCP-1, monocyte chemoattractant protein-1; TNF-ɑ, tumor necrosis factor. Data are presented as the mean ± SEM (error bar) of at least three independent experiments. *p < 0.05, **p < 0.01, and ***p < 0.001
To confirm that MSC-Exos could be taken up by macrophages (Additional file 1: Figure S1), we labeled MSC-Exos with PKH26, which is a fluorescent dye that binds to the phospholipid bilayer membrane [19]. After a 48-h incubation, the PKH26-labeled MSC-Exos were taken up by macrophages, and the cells displayed apparent red fluorescence under a fluorescence microscope (Fig. 1e).
Our previous in vitro study demonstrated that when cocultured with hUC-MSCs, LPS-stimulated macrophages tremendously decreased the production of inflammatory factors, especially IL-6 [2]. To validate that hUC-MSCs regulate macrophage activation by exosomes, Exos-IL6 were added into the culture medium. Similar to the results of coculture with hUC-MSCs (Additional file 1: Figure S2), Exos-IL6 also significantly inhibited the secretion of various inflammatory factors in LPS-stimulated macrophages (Fig. 1f). Interestingly, when we blocked the production of MSC-Exos with GW4869, the coculture of hUC-MSCs failed to significantly decrease the release of inflammatory factors by macrophages exposed to LPS (Fig. 1f). It seems that hUC-MSCs inhibit macrophage activation mainly in an exosome-dependent manner.
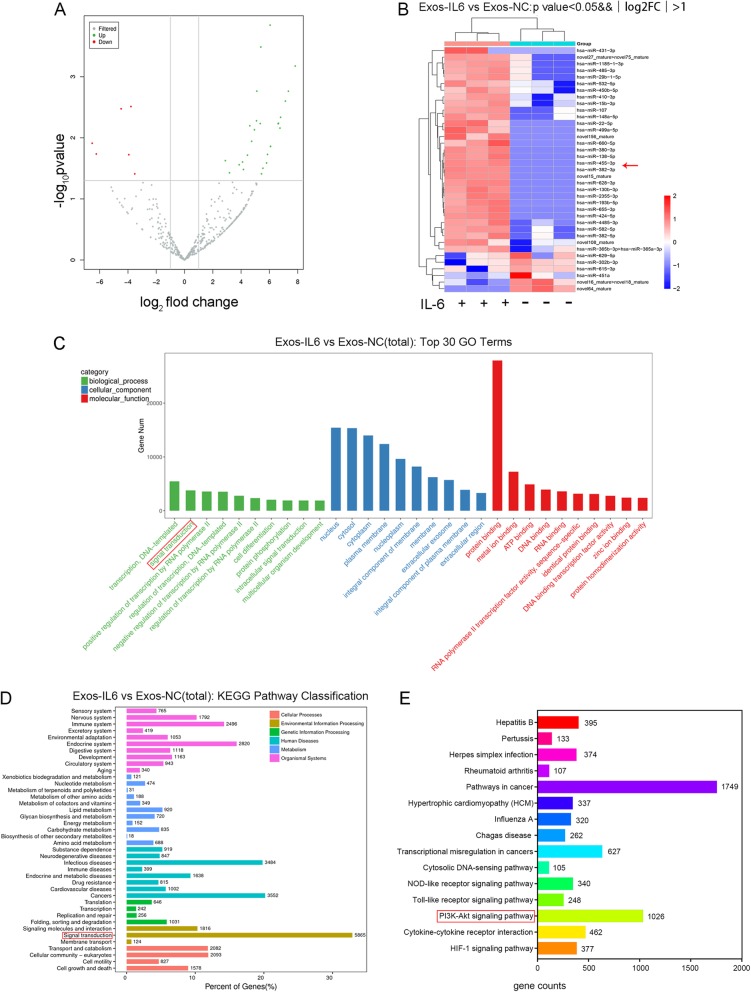
IL-6 induces changes in miRNAs in MSCs-Exos
To assess IL-6-induced miRNA changes in MSC-Exos, we conducted deep sequencing of small RNAs extracted from Exos-IL6 and Exos-NC. We identified a set of miRNAs that were significantly differentially abundant between the two groups. In comparison with those in Exos-NC, 31 miRNAs were upregulated and 6 miRNAs were downregulated in Exos-IL6 (fold change > 2, p < 0.05) (Fig. 2a, b and Additional file 1: Table S1). The miRanda algorithm was used to match miRNA and mRNA sequences to predict the target relationship between miRNAs and mRNAs. We then conducted Gene Ontology (GO) analysis and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis on the 17,589 predicted target mRNAs of the differentially expressed miRNAs. These target genes were found to be enriched in multiple signaling pathways (Fig. 2c, d). Among these signaling pathways, we focused on the signaling pathways involved in IL-6 and found that these miRNAs were widely involved in the regulation of IL-6-related signaling pathways, including pathways in cancers, the PI3K-Akt signaling pathway, cytokine-cytokine receptor interaction, and the Toll-like receptor signaling pathway (Fig. 2e). Among these upregulated miRNAs, five miRNAs, miR-455-3p, miR-424-5p, miR-485-3p, miR-431-3p, and miR-134-5p, were predicted to regulate the identified pathways.
Fig. 2.
Analysis of differentially expressed miRNA between Exos-IL6 and Exos-NC. aThirty-seven significantly differentially expressed miRNA volcanoes in Exos-IL6 (fold change > 2-fold; p < 0.05). b Heat map of differentially expressed miRNAs between Exos-IL6 and Exos-NC. c TOP 30 enriched GO terms of miRNA target genes. d The target genes of differentially expressed miRNAs were analyzed for KEGG pathway enrichment. e The number of target genes of differentially expressed miRNA target genes annotated under the IL-6-related signaling pathway
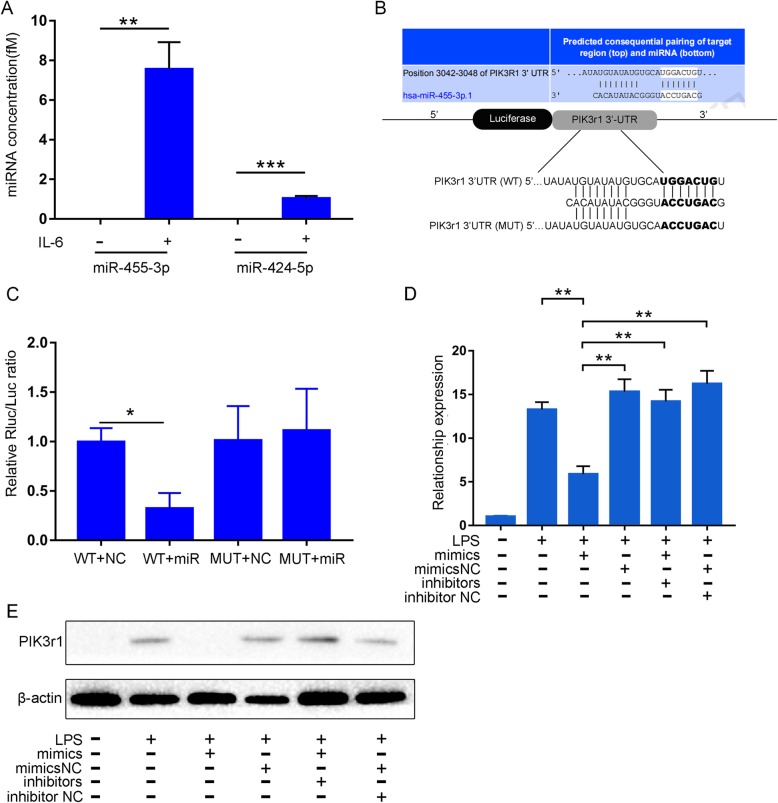
According to a fold change > 50, log2FoldChange > 5.5 and p < 0.01, we selected the upregulated miRNAs miR-455-3p and miR-424-5p to further validate the RNA sequencing results. Furthermore, absolute quantitative qPCR showed that the expression of miR-455-3p was more abundant (7.5 fM) than that of miR-424-5p (1 fM) (Fig. 3a). Therefore, we focused only on miR-455-3p in further studies.
Fig. 3.
miR-455-3p in Exos-IL6 inhibits the expression of PIK3r1 in macrophages. a qPCR analysis of miR-455-3p and miR-424-5p expression levels in Exos-IL6 and Exos-NC. b, c Schematic diagram showing the putative miR-455-3p binding sites in PIK3r1. The sequences of wild-type PIK3r1 and mutant PIK3r1 are listed as well. Luciferase reporter gene assays were performed to measure the luciferase activity in macrophages. d, e Macrophages were transfected with miR-455-3p mimics, inhibitors, and their corresponding controls (mimics NC, inhibitors NC). qPCR assay and western blot were used to analyze the mRNA and protein levels of PIK3r1, respectively. Data are presented as the mean ± SEM (error bar) of at least three independent experiments. *p < 0.05, **p < 0.01, and ***p < 0.001
miR-455-3p inhibits macrophage activation by regulating PIK3r1
According to the results of TargetScan and miRanda analyses, we found that miR-455-3p may have a binding site in the 3′-UTR of the PIK3r1 gene (phosphoinositide-3-kinase regulatory subunit 1), which encodes p85α, a subunit of PI3K. PI3K is a well-known key factor in the activation of the IL-6-related signaling pathway [29, 30], and we speculated that miR-455-3p might negatively regulate IL-6 signaling by suppressing PI3K expression. To further demonstrate this, we performed a luciferase reporter assay. PIK3r1 cDNA was cloned into the Renilla luciferase gene (hRluc, pmiR-RB-REPORT h-PIK3R1-WT) and cotransfected with miR-455-3p or negative control miRNA (miR-NC) into macrophages (Fig. 3b). The results showed that the luciferase activity in the miR-455-3p-transfected macrophages was significantly reduced compared to the miR-NC group. The luciferase activity of the PIK3r1 cDNA-mutant vector was not significantly affected by miR-455-3p (Fig. 3c). In addition, qPCR and western blot analysis showed that miR-455-3p significantly decreased PIK3r1 expression both at the mRNA and protein levels in macrophages (Fig. 3d, e). These results indicated that miR-455-3p could inhibit macrophage activation by downregulating the target gene PIK3r1.
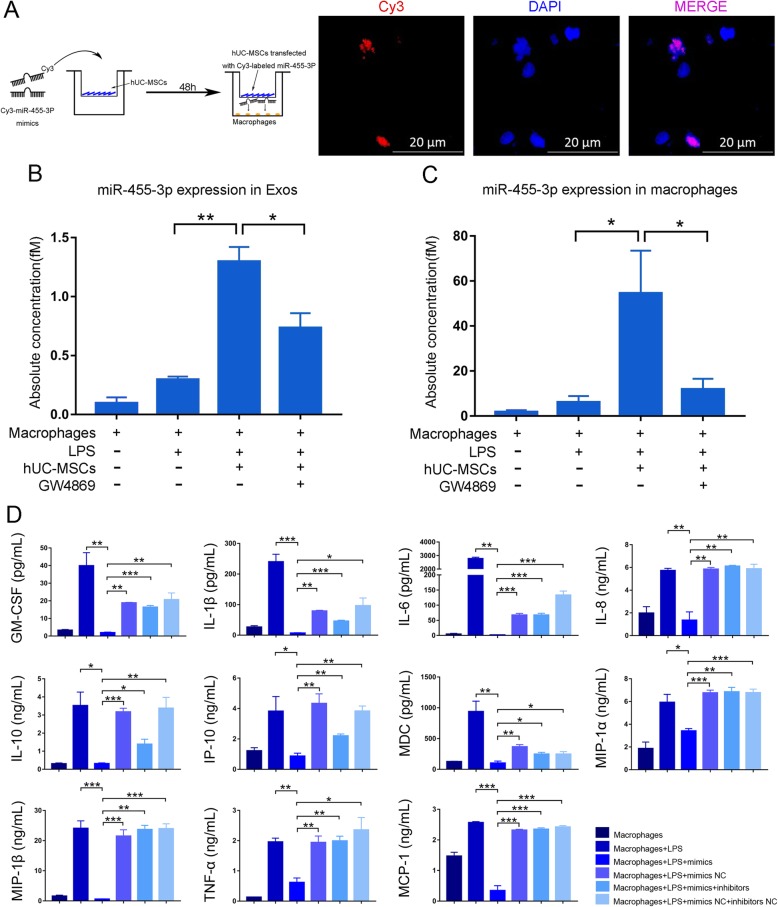
To determine whether hUC-MSCs transfer miR-455-3p to macrophages via exosomes, we performed miRNA tracing and coculture experiments. Cy3-labeled miR-455-3p mimics (purchased from Guangzhou RiboBio Co., Ltd., China) were transfected into hUC-MSCs. After removing the mimics that had not been transfected into cells, hUC-MSCs were cocultured with macrophages in a Transwell plate. The appearance of red fluorescent Cy3 dye in macrophages suggested that the Cy3-miR-455-3p mimics were delivered from the hUC-MSCs in the upper well to the macrophages in the lower well (Fig. 4a). Moreover, prior treatment with GW4869 blocked MSC-Exos production and delivery of miR-455-3p, and as a result, red fluorescence in the macrophages was significantly reduced (Additional file 1: Figure S3A). These results again indicated that miRNAs secreted by hUC-MSCs were taken up by macrophages (Figs. 1e and 4a).
Fig. 4.
hUC-MSCs secrete miR-455-3p and transport it to macrophages to inhibit macrophage secretion of inflammatory factors. a hUC-MSCs transfected with Cy3-labeled miR-455-3p mimics were cocultured with macrophages in Transwell plates (membrane well = 0.4 μm). b, c Abundance of miR-455-3p in exosomes from cell culture medium and macrophages after coculture with hUC-MSCs. d After overexpression of miR-455-3p, the macrophage inflammatory factors were tested. Data are presented as the mean ± SEM (error bar) of at least three independent experiments. *p < 0.05, **p < 0.01, and ***p < 0.001
We treated macrophages with LPS and examined the expression of miR-455-3p in the exosomes in the medium. In the presence of hUC-MSCs, miR-455-3p in the extracted exosomes was fourfold higher compared with that in the absence of hUC-MSCs (Fig. 4b). Moreover, miR-455-3p was significantly increased in the macrophages after coculture (Fig. 4c), suggesting the uptake of MSC-Exos by macrophages. Importantly, when hUC-MSCs were pretreated with GW4869 for 24 h, miR-455-3p was significantly decreased both in the medium and macrophages (Fig. 4b, c).
We next tested whether miR-455-3p alone had an inhibitory effect on macrophages. We transfected miR-455-3p mimics, inhibitors, and related controls into macrophages and cultured them for 48 h. As shown in Fig. 4d, miR-455-3p mimic transfection decreased all of the tested inflammatory factors produced by macrophages in response to LPS stimulation. When the miR-455-3p inhibitor was simultaneously added to the medium, the inhibitory effect of miR-455-3p was partially counteracted. Collectively, our data demonstrated that hUC-MSCs inhibited the activation of macrophages by secreting miR-455-3p-enriched exosomes.
miR-455-3p inhibits macrophage activation in LPS-induced endotoxemia mice
We next investigated whether miR-455-3p inhibits macrophage activation in vivo. Before the injection of human cell-derived miR-455-3p in mice, we used TargetScan software to confirm that miR-455-3p had binding sites and an identical seed sequence in both the human and mouse PIK3r1 genes (Additional file 1: Figure S3B). These results indicated that miR-455-3p is highly conserved in humans and mice and might have similar functions in mice.
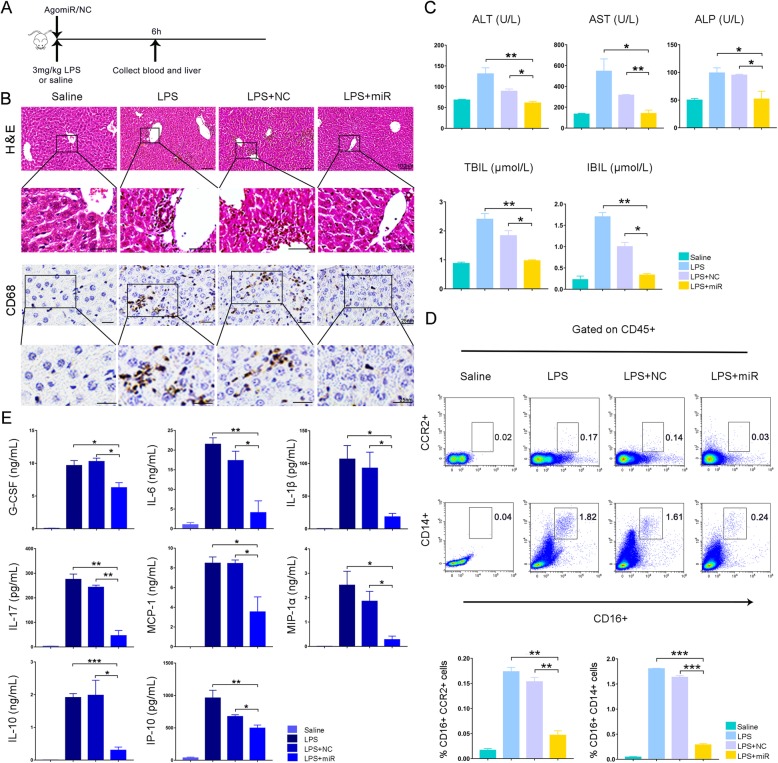
By selectively activating monocytes/macrophages, particularly resident Kupffer cells in the liver, LPS has been widely used to generate an endotoxemia mouse model [19]. Mice were intraperitoneally injected with LPS (3 mg/kg). Then, miR-455-3p agomir or agomir negative control (agomir NC) was immediately injected via the tail vein. The mice were sacrificed for tissue and blood harvest 6 h later (Fig. 5a). After LPS injection, a large number of CD68-positive cells were observed in the liver, which indicated the activation of Kupffer cells (Fig. 5b). After treatment with miR-455-3p agomir, the number of CD68+ cells was significantly reduced (Fig. 5b). Biochemical assays of hepatic indexes showed a significant improvement in liver damage after miR-455-3p treatment (Fig. 5c). Flow cytometry assays demonstrated that after miR-455-3p treatment, the ratio of activated monocytes marked by CD14+CD16+ or CCR2+CD16+ was significantly lower than that in the agomir NC group (Fig. 5d). After LPS stimulation, proinflammatory macrophages can upregulate the levels of various inflammatory factors. We assessed the serum levels of inflammatory factors, and the results showed that most inflammatory factors were significantly increased after LPS stimulation, including G-CSF, IL-1β, IL-10, IL-17, IL-6, MCP-1, MIP-1α, and IP-10. miR-455-3p reduced the levels of inflammatory factors to varying degrees, especially IL-6 (Fig. 5e). Thus, in LPS-treated mice, miR-455-3p inhibited the activation of liver Kupffer cells and the secretion of inflammatory cytokines, leading to improved liver and systemic inflammatory responses.
Fig. 5.
miR-455-3p treatment of LPS-induced endotoxemia mice. a Experimental procedure in mice. b The inflammatory infiltration in mouse tissues was visualized by H&E staining and CD68 immunohistochemistry. c Biochemical assays of hepatic indexes: alanine aminotransferase (ALT), glutamine-oxaloacetate transaminase (AST), indirect bilirubin (IBIL), alkaline phosphatase (ALP), and total bilirubin (TBIL). d Flow cytometry was used to analyze the activation rate of CD14 + CD16+ or CCR2 + CD16+ monocytes in CD45+ T cells. e The levels of inflammatory mediators in the blood were measured by Luminex. G-CSF, granulocyte colony-stimulating factor; IL, interleukin; MCP-1, monocyte chemoattractant protein-1; MIP-1ɑ, macrophage inflammatory protein-1ɑ; IP-10, interferon induction protein-10. Data are presented as the mean ± SEM (error bars). N = 5 mice per group; *p < 0.05, **p < 0.01, and ***p < 0.001. NC, miR-455-3p agomir negative control; miR, miR-455-3p agomir
miR-455-3p improves CCl4-induced acute liver injury in mice
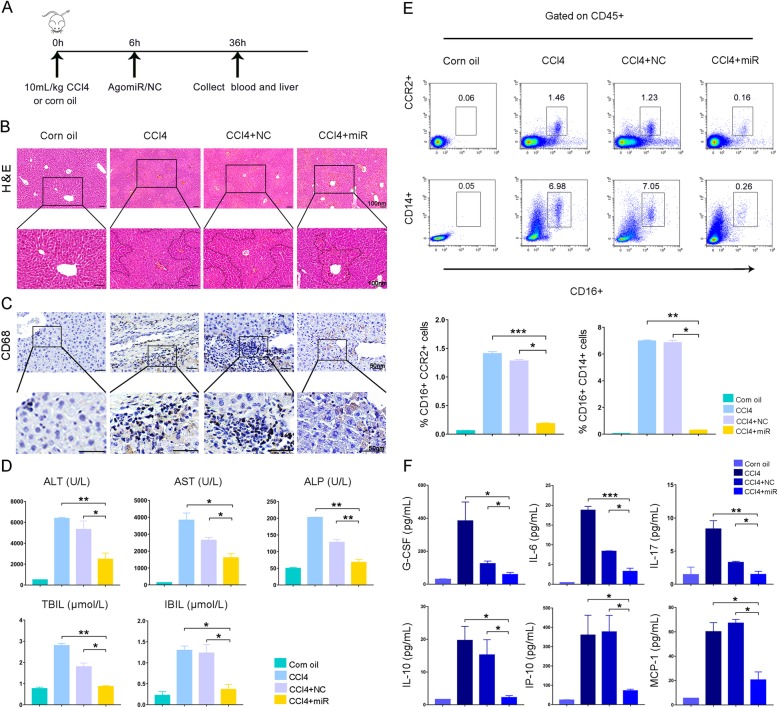
We next established an acute chemical liver injury mouse model and investigated whether miR-455-3p improved liver damage by inhibiting monocytes/macrophages activation. Six hours after intraperitoneal injection of CCl4, miR-455-3p agomir or agomir NC was injected via the tail vein. The mice were sacrificed 36 h later (Fig. 6a). Histological examination showed that after CCl4 treatment, the hepatocytes exhibited extensive swollen and eosinophilic changes, and patchy necrosis of hepatocytes was prominent in the portal area (Fig. 6b). Immunohistochemistry staining showed marked infiltration of CD68+ Kupffer cells in the liver, indicating the activation of liver macrophages (Fig. 6c). Treatment with miR-455-3p apparently reduced the edema and necrosis of hepatocytes and the infiltration of CD68+ cells (Fig. 6b, c). Biochemical assays showed that miR-455-3p agomir treatment significantly decreased the serum levels of hepatic indexes, including ALT, AST, and bilirubin (Fig. 6d). Flow cytometry analysis also showed that miR-455-3p agomir treatment decreased the proportion of CD14 + CD16+ or CCR2 + CD16+ monocytes in the peripheral blood (Fig. 6e). As expected, the serum levels of inflammatory factors, such as IL-6, G-CSF, IL-17, IL-10, IP-10, and MCP-1, were significantly decreased in response to miR-455-3p agomir infusion (Fig. 6f).
Fig. 6.
miR-455-3p is used to treat CCl4-induced acute liver injury in mice. a Experimental procedure in mice. b H&E staining of liver specimens. c CD68 immunohistochemistry. d Biochemical assays of hepatic indexes. e Flow cytometry analysis of the ratio of CD14 + CD16+ or CCR2 + CD16+ monocytes in peripheral blood CD45+ T cells. f Levels of inflammatory cytokines, chemokines, and growth factors in serum. Data are presented as the mean ± SEM (error bars). N = 5 mice per group; *p < 0.05, **p < 0.01, and ***p < 0.001. NC, miR-455-3p agomir negative control; miR, miR-455-3p agomir
Discussion
Our previous studies showed that hUC-MSC therapy significantly improved ALF progression and prognosis in monkeys by suppressing circulating monocyte activation and IL-6 secretion; however, the crosstalk between hUC-MSCs and monocytes was still unclear. In this study, we revealed that hUC-MSCs produced a large amount of miR-455-3p-enriched exosomes in response to IL-6 stimulation. These exosomes inhibited the release of IL-6 as well as dozens of other inflammatory factors by LPS-challenged macrophages by targeting PIK3r1. In mouse models of endotoxemia and chemical liver injury, overexpression of miR-455-3p attenuated liver damage, macrophage infiltration, and serum levels of inflammatory factors. Therefore, hUC-MSCs might suppress the overactivation of monocytes/macrophages and improve liver damage and systemic homeostasis by secreting miR-455-3p-enriched exosomes.
Although MSC-based therapy has been widely regarded as a promising solution for various human diseases, it has been largely hindered by the unclear therapeutic mechanism, partly due to the lack of a strategy to trace the infused cells in vivo. One of the prominent features of MSCs is their strong plasticity to the varieties and quantities of local microenvironmental stimulatory factors, which helps MSCs play diverse roles in different circumstances [28]. In monkeys treated with amatoxin, exogenous hUC-MSCs encounter sharply increased IL-6 in the serum, which is one of the major changes in the internal environment. In this study, we initially simulated such stress environment with high IL-6 in vitro and found that the number of exosomes secreted by hUC-MSCs increased. Similar to hUC-MSCs, these exosomes inhibited LPS-challenged macrophages from secreting inflammatory factors, which, together with the finding that blocking exosome secretion failed to inhibit macrophage activation, suggested that hUC-MSCs suppress macrophages in an exosome-dependent manner.
Exosomes are important mediators of intercellular communication via their enclosed bioactive substances. After deep sequencing, we determined that miR-455-3p from hUC-MSCs might be one of the critical miRNAs that inhibits the activation of macrophages. Although NCBI and other publicly available databases indicate that miR-455-3p can be used as a marker for certain diseases, such as hepatocellular carcinoma, anaplastic large cell lymphoma, Alzheimer’s disease, and cerebrospinal fluid [29–32], there are no related reports showing that miR-455-3p is an anti-inflammatory factor. We found that miR-455-3p was able to suppress monocyte/macrophage activation, reduce the secretion of inflammatory factors, and significantly improve chemical liver injury. Bioinformatics analysis and luciferase reporter assay revealed that miR-455-3p directly targets the 3′-UTR of PIK3r1, which was further confirmed by the finding that overexpression of miR-455-3p significantly downregulated PIK3r1 expression at both the mRNA and protein levels. PIK3r1 is the regulatory subunit of the well-known factor phosphoinositide 3 kinase (PI3K) [33]. PI3K, as a positive regulator of IL-6, plays a key role in LPS-induced cytokine production [34, 35]. Therefore, miR-455-3p is likely to block the activation of the IL-6 signaling pathway by targeting the PIK3r1 gene.
Other reasons that limit the clinical application of stem cells include allogeneic rejection, tumorigenicity, and the tedious process of preparation and quality control. In contrast, MSC-derived exosomes can overcome the key limitations of treatment with cells. Compared with cells, exosomes have a tough lipid bilayer that enables them to be more stable [36]. Exosomes have the ability to penetrate deep tissues and avoid immune attack, which facilitates their delivery of therapeutic substances directly into target cells [37]. In this experiment, MSC-Exos could be absorbed by macrophages in vitro and act in a similar therapeutic effect as hUC-MSCs. Moreover, many in vivo studies have shown that MSC-derived exosomes can enter the liver [38], which indicates that MSC-Exos-based treatment is a promising alternative to MSC treatment, particularly in liver diseases.
There are still some limitations in the treatment with MSC-derived exosomes, including batch differences, purity, drug delivery, and off-target effects [39]. Therefore, it is particularly important to elucidate the effective components in exosomes. miRNAs are one of the most abundant components and the major bioactive substance in the exosome. Compared with exosomes that contain a great number of bioactive substances and have many unclear functions, miRNAs are more specific in function and easier to handle, making them more likely to be developed as industrialized pharmaceuticals. We demonstrated that miR-455-3p, which was markedly increased in exosomes derived from IL-6-challenged hUC-MSCs, was able to inhibit monocyte/macrophage activation both in vitro and in vivo; therefore, miR-455-3p is a promising candidate for the treatment of acute liver injury as well as other disorders characterized by the overactivation of macrophages.
There are several limitations in this study. First, IL-6 was used to simulate the inflammatory environment, and hUC-MSCs were expected to make adaptive changes and produce specific miRNA-enriched exosomes in this microenvironment. Although IL-6 is a critical factor that triggers and accelerates the inflammatory cascade, IL-6 is not the only factor evolved in this process. Stimulating hUC-MSCs with inflammatory serum might more realistically simulate the microenvironment. However, there are numerous exosomes in the serum, which hinders the identification of the hUC-MSC-derived exosomes. Second, miR-455-3p is just one of the significantly increased miRNAs in exosomes. Although miR-455-3p strongly inhibits monocyte/macrophage activation and improves acute liver injury, the roles of other miRNAs deserve further investigation. Studies have reported that one miRNA can target several genes and that one gene can be regulated by different miRNAs. The side effects of miR-455-3p are still unclear. Whether there are more effective miRNAs that inhibit IL-6 signaling still requires further investigation.
Conclusions
hUC-MSC-derived miR-455-3p-enriched exosomes are able to suppress monocyte/macrophage activation and alleviate acute liver injury by inhibiting IL-6-related signaling pathways. Our study provides a basis for miR-455-3p as a treatment for acute inflammatory liver injury.
Supplementary information
Additional file 1: Figure S1. Identification of the cell morphology of hUC-MSCs cells and macrophages. Figure S2. hUC-MSCs inhibit macrophage secretion of inflammatory factors. Figure S3. (A) The fluorescence results of hUC-MSCs pretreated with GW4869 and cocultured with macrophages. (B) The key sequences of human and murine miR-455-3p were basically identical. Table S1. After deep sequencing of Exos-IL6 and Exos-NC, 31 miRNAs were found to be upregulated and 6 miRNAs were downregulated in the Exos-IL6 group (fold change > 2, p < 0.05).
Acknowledgements
We thank Li Li and Fei Chen for the assistance provided with the pathology techniques. We would also like to thank Chengdu Dossy experimental animals Co., Ltd. for providing wild-type mice. We are grateful to Shanghai Oe Biotech Co., Ltd. for providing sequencing services.
Abbreviations
- ALF
Acute liver failure
- ALP
Alkaline phosphatase
- ALT
Alanine aminotransferase
- AST
Glutamine-oxaloacetate transaminase
- CCl4
Carbon tetrachloride
- Exos-IL6
hUC-MSCs with IL-6 stimulation
- Exos-NC
hUC-MSCs without IL-6 stimulation
- GM-CSF
Granulocyte-macrophage colony-stimulating factor
- GO
Gene Ontology
- hUC-MSCs
Human umbilical cord mesenchymal stem cells
- IBIL
Indirect bilirubin
- IL
Interleukin
- IL-6
Interleukin-6
- IP-10
Interferon-inducible protein-10
- KEGG
Kyoto Encyclopedia of Genes and Genomes
- LPS
Lipopolysaccharide
- MCP-1
Monocyte chemoattractant protein-1
- MDC
Macrophage-derived chemokine
- MIP-1
Macrophage inflammatory protein-1
- MOF
Multiple organ failure
- MSC-Exos
MSC-derived exosomes
- MSCs
Mesenchymal stem cells
- PI3K
Phosphoinositide 3-kinase
- RISC
RNA-induced silencing complex
- SIRS
Systemic inflammatory response syndrome
- STR
Short tandem repeat
- TBIL
Total bilirubin
- TNF-ɑ
Tumor necrosis factor
Authors’ contributions
MS, QX, and ZW performed the experiments. BZ and YZ provided the study materials. YC, YS, and XC were involved in data analysis. RY, YS, and HB designed the experiment, interpreted the data, and wrote the manuscript. All authors approved the final version of the manuscript.
Funding
The work was supported by grants from the Natural Science Foundation of China (NO. 81570564), Technology Project of Science & Technology Department of Sichuan Province (2018JY0006), and Key projects of Sichuan Health Department (18ZD002).
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Ethics approval and consent to participate
The animal protocols used in this study were approved by the Institutional Animal Care and Use Committee of the Traditional Chinese Medicine National Center (Chengdu, China) (Protocol: IACUC-2012001C). All procedures were performed in accordance with West China Hospital’s guidelines. The hUC-MSC collection and the related experiments were approved by the Ethics Committee of Sichuan Neo-Life Stem Cell Technology Co., Ltd. and Sichuan Stem Cell Bank (SCSCB).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Rong Yao, Email: yaorong@wchscu.cn.
Yujun Shi, Email: shiyujun@scu.edu.cn.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s13287-020-1550-0.
References
- 1.Robles-Diaz M, Lucena MI, Kaplowitz N, Stephens C, Medina-Cáliz I, González-Jimenez A, Ulzurrun E, Gonzalez AF, Fernandez MC, Romero-Gómez M, et al. Use of Hy’s law and a new composite algorithm to predict acute liver failure in patients with drug-induced liver injury. Gastroenterology. 2014;147:109–118. doi: 10.1053/j.gastro.2014.03.050. [DOI] [PubMed] [Google Scholar]
- 2.Guo G, Zhu Y, Wu Z, Ji H, Lu X, Zhou Y, Li Y, Cao X, Lu Y, Talbot P, et al. Circulating monocytes accelerate acute liver failure by IL-6 secretion in monkey. J Cell Mol Med. 2018;22:4056–4067. doi: 10.1111/jcmm.13673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kong LX, Zhou YJ, Bu H, Lv T, Shi Y, Yang J. Deletion of interleukin-6 in monocytes/macrophages suppresses the initiation of hepatocellular carcinoma in mice. J Exp Clin Cancer Res. 2016;35:131. doi: 10.1186/s13046-016-0412-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Campbell A, Brieva T, Raviv L, Rowley J, Niss K, Brandwein H, Oh S, Karnieli O. Concise review: process development considerations for cell therapy. Stem Cells Transl Med. 2015;4:1155–1163. doi: 10.5966/sctm.2014-0294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee CW, Chen YF, Wu HH, Lee OK. Historical perspectives and advances in mesenchymal stem cell research for the treatment of liver diseases. Gastroenterology. 2018;154:46–56. doi: 10.1053/j.gastro.2017.09.049. [DOI] [PubMed] [Google Scholar]
- 6.Kourembanas S. Exosomes: vehicles of intercellular signaling, biomarkers, and vectors of cell therapy. Annu Rev Physiol. 2015;77:13–27. doi: 10.1146/annurev-physiol-021014-071641. [DOI] [PubMed] [Google Scholar]
- 7.Jang MJ, Kim HS, Lee HG, Kim GJ, Jeon HG, Shin HS, Chang SK, Hur GH, Chong SY, Oh D, et al. Placenta-derived mesenchymal stem cells have an immunomodulatory effect that can control acute graft-versus-host disease in mice. Acta Haematol. 2013;129:197–206. doi: 10.1159/000345267. [DOI] [PubMed] [Google Scholar]
- 8.Chhabra P, Brayman KL. Stem cell therapy to cure type 1 diabetes: from hype to hope. Stem Cells Transl Med. 2013;2:328–336. doi: 10.5966/sctm.2012-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yan Y, Jiang W, Tan Y, Zou S, Zhang H, Mao F, Gong A, Qian H, Xu W. hucMSC exosome-derived GPX1 is required for the recovery of hepatic oxidant injury. Mol Ther. 2017;25:465–479. doi: 10.1016/j.ymthe.2016.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guo G, Zhuang X, Xu Q, Wu Z, Zhu Y, Zhou Y, Li Y, Lu Y, Zhang B, Talbot P, et al. Peripheral infusion of human umbilical cord mesenchymal stem cells rescues acute liver failure lethality in monkeys. Stem Cell Res Ther. 2019;10:84. doi: 10.1186/s13287-019-1184-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flaherty SE, Grijalva A, Xu X, Ables E, Nomani A, Ferrante AW, Jr, et al. A lipase-independent pathway of lipid release and immune modulation by adipocytes. Science. 2019;363:989–993. doi: 10.1126/science.aaw2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mathieu M, Martin-Jaular L, Lavieu G, Théry C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol. 2019;21:9–17. doi: 10.1038/s41556-018-0250-9. [DOI] [PubMed] [Google Scholar]
- 13.Barres C, Blanc L, Bette-Bobillo P, André S, Mamoun R, Gabius HJ, Vidal M. Galectin-5 is bound onto the surface of rat reticulocyte exosomes and modulates vesicle uptake by macrophages. Blood. 2010;115:696–705. doi: 10.1182/blood-2009-07-231449. [DOI] [PubMed] [Google Scholar]
- 14.Tian T, Wang Y, Wang H, Zhu Z, Xiao Z. Visualizing of the cellular uptake and intracellular trafficking of exosomes by live-cell microscopy. J Cell Biochem. 2010;111:488–496. doi: 10.1002/jcb.22733. [DOI] [PubMed] [Google Scholar]
- 15.Kowal J, Arras G, Colombo M, Jouve M, Morath JP, Primdal-Bengtson B, Dingli F, Loew D, Tkach M, Théry C. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc Natl Acad Sci U S A. 2016;113:E968–E977. doi: 10.1073/pnas.1521230113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Konstantin A, Krychtiuk, Lenz M, Koller L, Honeder MC, Wutzlhofer L, Zhang C, Chi L, Maurer G, Niessner A, Huber K, et al. Monocyte subset distribution is associated with mortality in critically ill patients. Thromb Haemost. 2016;116:949–957. doi: 10.1160/TH16-05-0405. [DOI] [PubMed] [Google Scholar]
- 17.Zamora R, Korff S, Mi Q, Barclay D, Schimunek L, Zucca R, Arsiwalla XD, Simmons RL, Verschure P, Billiar TR, et al. A computational analysis of dynamic, multi-organ inflammatory crosstalk induced by endotoxin in mice. PLoS Comput Biol. 2018;14:e1006582. doi: 10.1371/journal.pcbi.1006582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sendler M, Weiss FU, Golchert J, Homuth G, van den Brandt C, Mahajan UM, Partecke LI, Döring P, Gukovsky I, Gukovskaya AS, et al. Cathepsin B-mediated activation of trypsinogen in endocytosing macrophages increases severity of pancreatitis in mice. Gastroenterology. 2018;154:704–718. doi: 10.1053/j.gastro.2017.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ying W, Riopel M, Bandyopadhyay G, Dong Y, Birmingham A, Seo JB, Ofrecio JM, Wollam J, Hernandez-Carretero A, Fu W, et al. Adipose tissue macrophage-derived exosomal miRNAs can modulate in vivo and in vitro insulin sensitivity. Cell. 2017;171:372–384. doi: 10.1016/j.cell.2017.08.035. [DOI] [PubMed] [Google Scholar]
- 20.Tapp LD, Shantsila E, Wrigley BJ, Montoro-Garcia S, Lip GY. TLR4 expression on monocyte subsets in myocardial infarction. J Intern Med. 2013;273:294–305. doi: 10.1111/joim.12011. [DOI] [PubMed] [Google Scholar]
- 21.Phinney DG, Pittenger MF. Concise review: Msc-derived exosomes for cell-free therapy. Stem Cells. 2017;35:851–858. doi: 10.1002/stem.2575. [DOI] [PubMed] [Google Scholar]
- 22.Boulanger CM, Loyer X, Rautou PE, Amabile N. Extracellular vesicles in coronary artery disease. Nat Rev Cardiol. 2017;14:259–272. doi: 10.1038/nrcardio.2017.7. [DOI] [PubMed] [Google Scholar]
- 23.Zheng G, Huang R, Qiu G, Ge M, Wang J, Shu Q, Xu J, et al. Mesenchymal stromal cell-derived extracellular vesicles: regenerative and immunomodulatory effects and potential applications in sepsis. Cell Tissue Res. 2018;374:1–15. doi: 10.1007/s00441-018-2871-5. [DOI] [PubMed] [Google Scholar]
- 24.Lai RC, Chen TS, Lim SK. Mesenchymal stem cell exosome: a novel stem cell-based therapy for cardiovascular disease. Regen Med. 2011;6:481–492. doi: 10.2217/rme.11.35. [DOI] [PubMed] [Google Scholar]
- 25.Lai RC, Arslan F, Lee MM, Sze NS, Choo A, Chen TS, Salto-Tellez M, Timmers L, Lee CN, El Oakley RM, et al. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010;4:214–222. doi: 10.1016/j.scr.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 26.Mac Sweeney R, McAuley DF. Mesenchymal stem cell therapy in acute lung injury: is it time for a clinical trial? Thorax. 2012;67:475–476. doi: 10.1136/thoraxjnl-2011-201309. [DOI] [PubMed] [Google Scholar]
- 27.Zhu X, He B, Zhou X, Ren J. Effects of transplanted bone-marrow-derived mesenchymal stem cells in animal models of acute hepatitis. Cell Tissue Res. 2013;351:477–486. doi: 10.1007/s00441-012-1524-3. [DOI] [PubMed] [Google Scholar]
- 28.Murphy MB, Moncivais K, Caplan AI. Mesenchymal stem cells: environmentally responsive therapeutics for regenerative medicine. Exp Mol Med. 2013;45:54. doi: 10.1038/emm.2013.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin YH, Wu MH, Huang YH, Yeh CT, Cheng ML, Chi HC, Tsai CY, Chung IH, Chen CY, Lin KH, et al. Taurine upregulated gene 1 functions as a master regulator to coordinate glycolysis and metastasis in hepatocellular carcinoma. Hepatology. 2018;67:188–203. doi: 10.1002/hep.29462. [DOI] [PubMed] [Google Scholar]
- 30.Liu C, Iqbal J, Teruya-Feldstein J, Shen Y, Dabrowska MJ, Dybkaer K, Lim MS, Piva R, Barreca A, Pellegrino E, et al. MicroRNA expression profiling identifies molecular signatures associated with anaplastic large cell lymphoma. Blood. 2013;122:2083–2092. doi: 10.1182/blood-2012-08-447375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singh AK, Rooge SB, Varshney A, Vasudevan M, Bhardwaj A, Venugopal SK, Trehanpati N, Kumar M, Geffers R, Kumar V, et al. Global micro RNA expression profiling in the liver biopsies of hepatitis B virus infected patients suggests specific miRNA signatures for viral persistence and hepatocellular injury. Hepatology. 2018;67:1695–1709. doi: 10.1002/hep.29690. [DOI] [PubMed] [Google Scholar]
- 32.Kumar S, Vijayan M, Reddy PH. MicroRNA-455-3p as a potential peripheral biomarker for Alzheimer’s disease. Hum Mol Genet. 2017;26:3808–3822. doi: 10.1093/hmg/ddx267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leavenworth JW, Verbinnen B, Yin J, Huang H, Cantor H. A p85α-osteopontin axis couples the receptor ICOS to sustained Bcl-6 expression by follicular helper and regulatory T cells. Nat Immunol. 2015;16:96–106. doi: 10.1038/ni.3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fajgenbaum DC, Langan RA, Sada Japp A, Partridge HL, Pierson SK, Singh A, Arenas DJ, Ruth JR, Nabel CS, Stone K, et al. Identifying and targeting pathogenic PI3K/AKT/mTOR signaling in IL-6-blockade-refractory idiopathic multicentric Castleman disease. J Clin Invest. 2019;13:1558–8238. doi: 10.1172/JCI126091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hochdörfer T, Kuhny M, Zorn CN, Hendriks RW, Vanhaesebroeck B, Bohnacker T, Krystal G, Huber M. Activation of the PI3K pathway increases TLR-induced TNF-α and IL-6 but reduces IL-1β production in mast cells. Cell Signal. 2011;23:866–875. doi: 10.1016/j.cellsig.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 36.Akers JC, Ramakrishnan V, Yang I, Hua W, Mao Y, Carter BS, Chen CC. Optimizing preservation of extracellular vesicular miRNAs derived from clinical cerebrospinal fluid. Cancer Biomark. 2016;17:125–132. doi: 10.3233/CBM-160609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Elahi FM, Farwell DG, Nolta JA, Anderson JD. Preclinical Translation of Exosomes Derived from Mesenchymal Stem/Stromal Cells. Stem cells. 2019;30:1066–5099. [DOI] [PMC free article] [PubMed]
- 38.Hoshino A, Costa-Silva B, Shen TL, Rodrigues G, Hashimoto A, Tesic Mark M, Molina H, Kohsaka S, Di Giannatale A, Ceder S, et al. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527:329–335. doi: 10.1038/nature15756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marbán E. The secret life of exosomes: what bees can teach us about next-generation therapeutics. J Am Coll Cardiol. 2018;71:193–200. doi: 10.1016/j.jacc.2017.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure S1. Identification of the cell morphology of hUC-MSCs cells and macrophages. Figure S2. hUC-MSCs inhibit macrophage secretion of inflammatory factors. Figure S3. (A) The fluorescence results of hUC-MSCs pretreated with GW4869 and cocultured with macrophages. (B) The key sequences of human and murine miR-455-3p were basically identical. Table S1. After deep sequencing of Exos-IL6 and Exos-NC, 31 miRNAs were found to be upregulated and 6 miRNAs were downregulated in the Exos-IL6 group (fold change > 2, p < 0.05).
Data Availability Statement
All data generated or analyzed during this study are included in this published article.