Abstract
Background
Transient receptor potential vanilloid 4 (TRPV4) is activated by stretch (mechanical), warm temperature, some epoxyeicosatrienoic acids, and lipopolysaccharide. TRPV4 is expressed throughout the gastrointestinal epithelia and its activation induces adenosine triphosphate (ATP) exocytosis that is involved in visceral hypersensitivity. As an ATP transporter, vesicular nucleotide transporter (VNUT) mediates ATP storage in secretory vesicles and ATP release via exocytosis upon stimulation.
Summary
TRPV4 is sensitized under inflammatory conditions by a variety of factors, including proteases and serotonin, whereas methylation-dependent silencing of TRPV4 expression is associated with various pathophysiological conditions. Gastrointestinal epithelia also release ATP in response to hypo-osmolality or acid through molecular mechanisms that remain unclear. These synergistically released ATP could be involved in visceral hypersensitivity. Low concentrations of the first generation bisphosphate, clodronate, were recently reported to inhibit VNUT activity and thus clodronate may be a safe and potent therapeutic option to treat visceral pain.
Key Messages
This review focuses on: (1) ATP and TRPV4 activities in gastrointestinal epithelia; (2) factors that could modulate TRPV4 activity in gastrointestinal epithelia; and (3) the inhibition of VNUT as a potential novel therapeutic strategy for functional gastrointestinal disorders.
Keywords: Gastrointestinal epithelium, Adenosine triphosphate, Transient receptor potential vanilloid 4, Clodronate
Introduction
Visceral hypersensitivity to stretch (mechanical) [1], temperature [2], and acid [3] plays a role in functional gastrointestinal disorders, through molecular mechanisms that remain unclear. For example, in irritable bowel syndrome (IBS), repetitive rectal painful distention induces rectal hypersensitivity [1]. Thermal hypersensitivity was reported in IBS patients and is positively correlated with increased intestinal membrane permeability [2].
As a stretch-activated nonselective cation ion channel, transient receptor potential vanilloid 4 (TRPV4) can induce visceral hypersensitivity [4]. We found that TRPV4 and an adenosine triphosphate (ATP) transporter, vesicular nucleotide transporter (VNUT), are both expressed throughout gastrointestinal epithelia and that a VNUT inhibitor, the first-generation bisphosphonate clodronate, inhibits TRPV4-induced ATP release [5].
In this review, we consider: (1) ATP and TRPV4 activities in gastrointestinal epithelia; (2) possible factors that modulate TRPV4 activity in gastrointestinal epithelia; and (3) inhibition of VNUT as a novel therapeutic strategy for functional gastrointestinal disorders.
ATP and TRPV4 in Gastrointestinal Epithelia
ATP signaling plays an important role in a variety of gut activities. Purinergic receptors can be divided into the P2X family of ionotropic receptors and the P2Y family of G protein-coupled receptors, both of which are involved in visceral hypersensitivity [6]. In P2X3 knockout mice, ATP release in response to mechanical distension of the esophagus and stomach is similar to that seen for wild-type (WT) mice, whereas activation of afferent nerve fibers is attenuated relative to WT mice [7]. Meanwhile, a P2X3 agonist was shown to stimulate mechanosensitive vagal afferent nerves in mouse stomach and esophagus [8], suggesting that ATP release induced by mechanical stimuli and the P2X3 receptor play important roles in baroreception in the gut. Based on the possibility that epithelial ATP release in response to luminal distension could act on purinergic receptors in submucosal nerves to transduce mechanical signals to the CNS or induce enteric reflex, especially under inflammatory conditions [6], we focused on ATP release upon epithelial TRPV4 activation.
TRPV4 was originally identified as a hypoosmolarity-sensitive ion channel that is activated by mechanical stimuli, warm temperature, and epoxyeicosatrienoic acid, the levels of which are increased in inflammatory conditions [4]. In the upper gastrointestinal tract, TRPV4 is expressed in the extrinsic nerves as well as esophageal and gastric epithelia [9, 10]. TRPV4 activation promotes VNUT-mediated ATP release in the esophagus. Heat stimulus (>38.5°C) significantly increases ATP release from WT cultured mouse esophageal keratinocytes in a manner that is dependent on TRPV4 expression [9].
TRPV4 can be activated by acidity, and enhanced writhing behavior was observed within 10 min of injection of 0.7% acetate into the abdomen of mice. These effects are suppressed in TRPV4 knockout (Trpv4−/–) mice relative to WT mice [11]. In human, stimulation of esophageal epithelial cells by acid promotes ATP release [12], whereas TRPV4-mediated increases in [Ca2+]i are suppressed by extracellular protons (pH 5.0) [13]. This discrepancy could be attributed to the ability of acidic conditions to induce acid-sensing ion channel-like currents in WT or TRPV4−/– esophageal keratinocytes independent of TRPV4. Gastrointestinal epithelia cells release ATP in response to not only chemical but also acid, temperature, and hypo-osmolality (Fig. 1) [14].
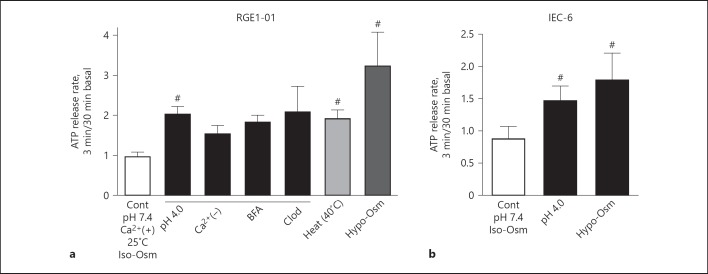
Fig. 1.
Physical stimuli-induced ATP release from gastrointestinal epithelia. a Release of ATP from the rat gastric epithelial cell line RGE1-01 in response to acid (pH 4.0), heat (40°C), and hypo-osmolality (half-normal saline). Acid-induced ATP release was not inhibited in the absence of extracellular Ca2+, the vesicle-trafficking inhibitor BFA, or the VNUT inhibitor Clod. b Rat IEC-6 also release ATP in response to acid or hypo-osmolality (# p < 0.05 vs. Cont). ATP, adenosine triphosphate; BFA, brefeldin A; Clod, clodronate; IEC, intestinal epithelia cells; Cont, control.
The VNUT modulates the storage of ATP in secretory vesicles and ATP release from these vesicles via exocytosis. TRPV4 can induce VNUT-mediated ATP exocytosis in the human gastric epithelial cell line GES-1 and activate enteric neurons [5]. Overall, the high concentrations of arachidonic acid metabolites in tissues, high temperatures, hypo-osmolality, and acidity of GI fluid may elicit ATP release from GI epithelia that in turn overstimulates GI nerves (Fig. 2).
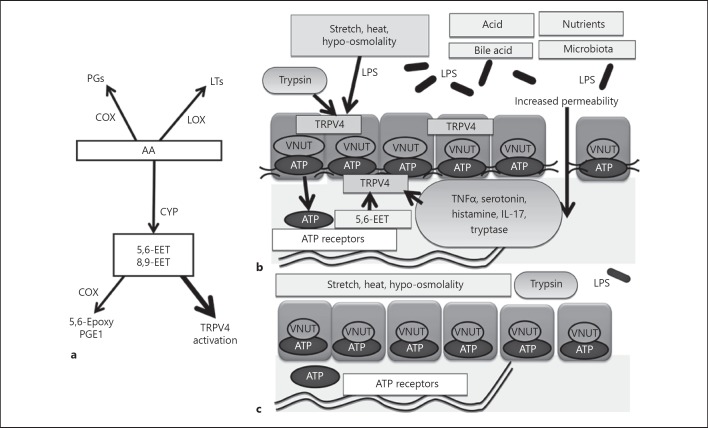
Fig. 2.
Proposed molecular mechanism of visceral hypersensitivity or blunting with TRPV4 enhancement or suppression. a Formation of major metabolites generated from the AA cascade. CYP enzymes convert arachidonic acid into EETs. Increased amounts of 5,6-EET or 8,9-EET then activate TRPV4. b TRPV4 expressed in gastrointestinal epithelia is activated by stretch, heat, hypo-osmolality, LPS or the endogenous activators (5,6-EET and 8,9-EET). Several factors (e.g., proteases such as trypsin and tryptase, TNFα, serotonin, histamine, IL-17) enhance TRPV4 function. TRPV4 activation induces VNUT-mediated ATP exocytosis and increases cellular permeability. Acid also induces ATP release via another mechanism to induce visceral hypersensitivity. c Methylation-silencing of TRPV4 expression decreases epithelial sensitivity to physiological stimuli resulting in diminished visceral responses. AA, arachidonic acid; CYP, Cytochrome P450; EET, epoxyeicosatrienoic acids; TRPV4, transient receptor potential vanilloid 4; LPS, lipopolysaccharides; VNUT, vesicular nucleotide transporter; ATP, adenosine triphosphate; TNFα, tumor necrosis factor-α; IL-17, interleukin-17; PG, prostaglandin; LT, leukotriene; COX, cyclooxygenase; LOX, lipoxygenase.
Duodenal and intestinal microinflammation and increased permeability are pathophysiological conditions that are associated with functional dyspepsia (FD) and IBS, respectively [15, 16]. Thermal hypersensitivity in IBS is linked to increased intestinal permeability [2]. TRPV4 activation increases epithelial permeability due to endocytosis of tight junction proteins, especially claudin4, as was shown in the mammary cell line HC11 [17], and also increases the permeability of the intestinal epithelial cell line IEC6 [18]. Acid infusion in the duodenum induces symptoms in a subset of FD patients, but not in control patients [19], whereas endogenous TRPV4 agonists such as 5,6-EET and 8,9-EET increase TRPV4-mediated epithelial increased permeability and thus might be involved in visceral hypersensitivity under microinflammation conditions. Although there are no reports of TRPV4 inhibitors being administered to humans, in mice and rats such inhibitors produced no serious adverse events [20], and thus might be valuable for controlling the gut hypersensitivity.
In mouse and human colon, TRPV4 localizes to epithelial cells and as yet unidentified cells of the submucosal and muscular layers. TRPV4 agonists can increase intracellular calcium concentrations and promote chemokine release in human colon cancer cell lines and induce colitis in mice [21]. Although TRPV4 is expressed in both the epithelium and enteric neurons in the colon and TRPV4−/– mice are less sensitive to colonic distension, the tissues in which the effects of TRPV4 activity predominate are unclear. In terms of visceral sensations, TRPV4-mediated ATP exocytosis via VNUT is likely involved in response to stretch or elevated temperatures. Levels of the endogenous TRPV4 agonist 5,6-EET are increased in colon tissues from IBS patients and the increase correlates with their symptoms' severity [22]. The TRPV4 inhibitor HC067047 attenuates distension-induced neural responses in isolated human colon tissue [23]. On the other hand, the VNUT inhibitors clodronate does not inhibit acid-induced ATP release in the gastric cell line RGE1-01 (Fig. 1), suggesting that acid-sensitive receptors other than TRPV4 may contribute to the majority of acid sensitivity in the gut epithelium. Additional studies are needed to elucidate the precise mechanism mediating acid-induced ATP release in the gut.
Possible TRPV4 Modulators in Gastrointestinal Epithelia
As mentioned earlier, TRPV4 is activated by hypoosmolarity, mechanical stimuli, warm temperature, and epoxyeicosatrienoic acid [4]. Interestingly, lipopolysaccharides produced by commensal bacteria also directly activate TRPV4 [24]. Moreover, several factors are known to enhance TRPV4 function, including proteases, serotonin, histamine, tumor necrosis factor-α, interleukin-17 [25], and protease-activated receptor 2. We showed that protease-activated receptor 2 expressed in the esophageal epithelium is activated by proteases such as trypsin and tryptase and that treatment of mouse esophageal keratinocytes with trypsin enhances ATP release via TRPV4 activation [26]. Serotonin and histamine increase TRPV4 expression in mouse colon neurons [27], whereas tumor necrosis factor-α and interleukin-17 can enhance TRPV4 expression in some neuronal cell types such as DRG [20, 25].
On the other hand, gastric epithelial cells infected with Helicobacter pylori and gastric cancer cells were shown to have suppressed TRPV4 expression due to enhanced methylation of the TRPV4 gene [10]. Emerging evidence shows that dysbiosis is involved in various diseases such as IBS that involve modulations of epithelial permeability, metabolites, and immune system function [28]. Such enhanced or suppressed function of TRPV4 can be a molecular mechanism by which visceral hypersensitivity or blunting occurs (Fig. 2).
VNUT Inhibition as a Novel Therapeutic Strategy for Functional Gastrointestinal Disorders
Blockage of nerve pathways that connect TRPV4 to ATP and ATP to a purinergic receptor should ameliorate visceral pain. Although TRPV4 inhibition had no serious systemic adverse events in mice and rats [20], in humans such inhibition could promote physiological dysfunction. P2X7 receptor antagonists such as AZD-9056 were not effective in mitigating Crohn's disease symptoms. P2X3 receptor antagonists have been recommended as a possible treatment for IBS [6].
The SLC17A9 gene encodes the VNUT. VNUT knockout mice appear to be healthy but have reduced amounts of vesicular storage and release of ATP and are resistant to acute inflammatory pain [29]. Recently, the first-generation bisphosphate clodronate was shown to specifically inhibit VNUT activity at low concentrations, suggesting that this compound could be an effective agent to treat chronic pain [30]. Indeed, intravenous injection of clodronate into mice attenuated inflammatory pain by about 40% relative to untreated animals without any observed adverse effects and had an analgesic effect that was stronger than diclofenac and comparable to tramadol [29]. The clodronate Bonefos has been used worldwide for over 30 years to treat osteoporosis, bone metastasis pain, and inflammatory bowel disease [30]. Intravenous or oral administration of clodronate has been shown to have analgesic effects and does not produce severe adverse events. Generic versions of clodronate are now available. However, there are currently no clinical trials to evaluate the efficacy of clodronates for treating functional gastrointestinal disorders, in part due to the widespread use of these compounds for non-gastrointestinal purposes and the lack of approval in Japan for their use as analgesic agents.
Conclusion
In recent years, epithelial cells were shown to function as sensory tissues via the activity of ion channel receptors such as TRPV4. Future studies will likely reveal additional pressure-, temperature-, and acid-sensing mechanisms that function in digestive tract epithelia and determine whether inhibition of VNUT via clodronate could serve as an effective treatment for visceral pain.
Disclosure Statement
The authors have no conflicts of interest to disclose.
Acknowledgments
We thank T. Kozawa for her technical assistance. This review is based on the core symposium “New development of gastrointestinal functional disorder-Pathophysiology and target molecules of FD” held in 2018 and “New development of gastrointestinal functional disorder-Molecular pathogenesis and new development of IBS” presented at the 2019 meeting of The Japan Gastroenterological Association.
References
- 1.Nozu T, Kudaira M, Kitamori S, Uehara A. Repetitive rectal painful distention induces rectal hypersensitivity in patients with irritable bowel syndrome. J Gastroenterol. 2006 Mar;41((3)):217–22. doi: 10.1007/s00535-005-1748-z. [DOI] [PubMed] [Google Scholar]
- 2.Zhou Q, Zhang B, Verne GN. Intestinal membrane permeability and hypersensitivity in the irritable bowel syndrome. Pain. 2009 Nov;146((1-2)):41–6. doi: 10.1016/j.pain.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Modlin IM, Hunt RH, Malfertheiner P, Moayyedi P, Quigley EM, Tytgat GN, et al. Vevey NERD Consensus Group Diagnosis and management of non-erosive reflux disease-the Vevey NERD Consensus Group. Digestion. 2009;80((2)):74–88. doi: 10.1159/000219365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balemans D, Boeckxstaens GE, Talavera K, Wouters MM. Transient receptor potential ion channel function in sensory transduction and cellular signaling cascades underlying visceral hypersensitivity. Am J Physiol Gastrointest Liver Physiol. 2017 Jun;312((6)):G635–G648. doi: 10.1152/ajpgi.00401.2016. [DOI] [PubMed] [Google Scholar]
- 5.Mihara H, Uchida K, Koizumi S, Moriyama Y. Involvement of VNUT-exocytosis in transient receptor potential vanilloid 4-dependent ATP release from gastrointestinal epithelium. PLoS One. 2018 Oct;13((10)):e0206276. doi: 10.1371/journal.pone.0206276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burnstock G. Purinergic Signalling therapeutic Developments. Front Pharmacol. 2017 Sep;8:661. doi: 10.3389/fphar.2017.00661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McIlwrath SL, Davis BM, Bielefeldt K. Deletion of P2X3 receptors blunts gastro-oesophageal sensation in mice. Neurogastroenterol Motil. 2009 Aug;21((8)):890-e66. doi: 10.1111/j.1365-2982.2009.01292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Page AJ, Martin CM, Blackshaw LA. Vagal mechanoreceptors and chemoreceptors in mouse stomach and esophagus. J Neurophysiol. 2002 Apr;87((4)):2095–103. doi: 10.1152/jn.00785.2001. [DOI] [PubMed] [Google Scholar]
- 9.Mihara H, Boudaka A, Sugiyama T, Moriyama Y, Tominaga M. Transient receptor potential vanilloid 4 (TRPV4)-dependent calcium influx and ATP release in mouse oesophageal keratinocytes. J Physiol. 2011 Jul;589((Pt 14)):3471–82. doi: 10.1113/jphysiol.2011.207829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mihara H, Suzuki N, Muhammad JS, Nanjo S, Ando T, Fujinami H, et al. Transient receptor potential vanilloid 4 (TRPV4) silencing in Helicobacter pylori-infected human gastric epithelium. Helicobacter. 2017 Apr;22((2)):22. doi: 10.1111/hel.12361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suzuki M, Mizuno A, Kodaira K, Imai M. Impaired pressure sensation in mice lacking TRPV4. J Biol Chem. 2003 Jun;278((25)):22664–8. doi: 10.1074/jbc.M302561200. [DOI] [PubMed] [Google Scholar]
- 12.Ma J, Altomare A, Rieder F, Behar J, Biancani P, Harnett KM. ATP a mediator for HCl-induced TRPV1 activation in esophageal mucosa. Am J Physiol Gastrointest Liver Physiol. 2011 Dec;301((6)):G1075–G1082. doi: 10.1152/ajpgi.00336.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shikano M, Ueda T, Kamiya T, Ishida Y, Yamada T, Mizushima T, et al. Acid inhibits TRPV4-mediated Ca2+ influx in mouse esophageal epithelial cells. Neurogastroenterol Motil. 2011 Nov;23((11)):1020–1028. doi: 10.1111/j.1365-2982.2011.01767.x. e497. [DOI] [PubMed] [Google Scholar]
- 14.Mihara H, Muhammad JS, Suzuki N, Tabuchi Y, Sugiyama T. Gastrointestinal Epithelia Could Be Luminal Sensors for Chemical Hypo Osmolality Temperature and Stretch via ATP Release. Gastroenterology. 2015;148((4)):S-91. [Google Scholar]
- 15.Miwa H, Oshima T, Tomita T, Fukui H, Kondo T, Yamasaki T, et al. Recent understanding of the pathophysiology of functional dyspepsia role of the duodenum as the pathogenic center. J Gastroenterol. 2019 Apr;54((4)):305–11. doi: 10.1007/s00535-019-01550-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang L, Song J, Hou X. Mast Cells and Irritable Bowel Syndrome From the Bench to the Bedside. J Neurogastroenterol Motil. 2016 Apr;22((2)):181–92. doi: 10.5056/jnm15137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reiter B, Kraft R, Günzel D, Zeissig S, Schulzke JD, Fromm M, et al. TRPV4-mediated regulation of epithelial permeability. FASEB J. 2006 Sep;20((11)):1802–12. doi: 10.1096/fj.06-5772com. [DOI] [PubMed] [Google Scholar]
- 18.Yamawaki H, Mihara H, Suzuki N, Nishizono H, Uchida K, Watanabe S, et al. Role of transient receptor potential vanilloid 4 activation in indomethacin-induced intestinal damage. Am J Physiol Gastrointest Liver Physiol. 2014 Jul;307((1)):G33–G40. doi: 10.1152/ajpgi.00105.2013. [DOI] [PubMed] [Google Scholar]
- 19.Ishii M, Manabe N, Kusunoki H, Kamada T, Sato M, Imamura H, et al. Real-time evaluation of dyspeptic symptoms and gastric motility induced by duodenal acidification using noninvasive transnasal endoscopy. J Gastroenterol. 2008;43((12)):935–41. doi: 10.1007/s00535-008-2303-5. [DOI] [PubMed] [Google Scholar]
- 20.Wu Z, Wang S, Wu I, Mata M, Fink DJ. Activation of TLR-4 to produce tumour necrosis factor-α in neuropathic pain caused by paclitaxel. Eur J Pain. 2015 Aug;19((7)):889–98. doi: 10.1002/ejp.613. [DOI] [PubMed] [Google Scholar]
- 21.D'Aldebert E, Cenac N, Rousset P, Martin L, Rolland C, Chapman K, et al. Transient receptor potential vanilloid 4 activated inflammatory signals by intestinal epithelial cells and colitis in mice. Gastroenterology. 2011 Jan;140((1)):275–85. doi: 10.1053/j.gastro.2010.09.045. [DOI] [PubMed] [Google Scholar]
- 22.Cenac N, Bautzova T, Le Faouder P, Veldhuis NA, Poole DP, Rolland C, et al. Quantification and Potential Functions of Endogenous Agonists of Transient Receptor Potential Channels in Patients With Irritable Bowel Syndrome. Gastroenterology. 2015;149:433–444. doi: 10.1053/j.gastro.2015.04.011. e7. [DOI] [PubMed] [Google Scholar]
- 23.McGuire C, Boundouki G, Hockley JR, Reed D, Cibert-Goton V, Peiris M, et al. Ex vivo study of human visceral nociceptors. Gut. 2018 Jan;67((1)):86–96. doi: 10.1136/gutjnl-2016-311629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alpizar YA, Boonen B, Sanchez A, Jung C, López-Requena A, Naert R, et al. TRPV4 activation triggers protective responses to bacterial lipopolysaccharides in airway epithelial cells. Nat Commun. 2017 Oct;8((1)):1059. doi: 10.1038/s41467-017-01201-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Segond von Banchet G, Boettger MK, König C, Iwakura Y, Bräuer R, Schaible HG. Neuronal IL-17 receptor upregulates TRPV4 but not TRPV1 receptors in DRG neurons and mediates mechanical but not thermal hyperalgesia. Mol Cell Neurosci. 2013 Jan;52:152–160. doi: 10.1016/j.mcn.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 26.Suzuki N, Mihara H, Nishizono H, Tominaga M, Sugiyama T. Protease-Activated Receptor-2 Up-Regulates Transient Receptor Potential Vanilloid 4 Function in Mouse Esophageal Keratinocyte. Dig Dis Sci. 2015 Dec;60((12)):3570–8. doi: 10.1007/s10620-015-3822-6. [DOI] [PubMed] [Google Scholar]
- 27.Cenac N, Altier C, Motta JP, d'Aldebert E, Galeano S, Zamponi GW, et al. Potentiation of TRPV4 signalling by histamine and serotonin an important mechanism for visceral hypersensitivity. Gut. 2010 Apr;59((4)):481–8. doi: 10.1136/gut.2009.192567. [DOI] [PubMed] [Google Scholar]
- 28.Yang H, Duan Z. The Local Defender and Functional Mediator gut Microbiome. Digestion. 2018;97((2)):137–145. doi: 10.1159/000484687. [DOI] [PubMed] [Google Scholar]
- 29.Kato Y, Hiasa M, Ichikawa R, Hasuzawa N, Kadowaki A, Iwatsuki K, et al. Identification of a vesicular ATP release inhibitor for the treatment of neuropathic and inflammatory pain. Proc Natl Acad Sci USA. 2017 Jul;114((31)):E6297–E305. doi: 10.1073/pnas.1704847114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moriyama Y, Nomura M. Clodronate A Vesicular ATP Release Blocker. Trends Pharmacol Sci. 2018 Jan;39((1)):13–23. doi: 10.1016/j.tips.2017.10.007. [DOI] [PubMed] [Google Scholar]