Abstract
Background/Aims
The current treatment for anemia associated with chronic kidney disease (CKD) includes the administration of erythropoiesis stimulating agents (ESAs) combined with iron supplementation. Molidustat, a hypoxia-inducible factor prolyl hydroxylase inhibitor, has potential to treat anemia associated with CKD through increased erythropoietin production and improved iron availability. Here, we report the effect of molidustat on iron metabolism.
Method
Parameters of iron metabolism were monitored in three 16-week, randomized, controlled, phase 2 studies assessing the safety and efficacy of molidustat in the treatment of anemia associated with CKD in different populations: treatment-naïve and previously ESA-treated patients not on dialysis, and previously ESA-treated patients on hemodialysis. Iron supplementation was left at the discretion of the investigator.
Results
In treatment-naïve patients not on dialysis, transferrin saturation (TSAT), hepcidin, ferritin, and iron concentrations decreased with molidustat, whereas total iron binding capacity (TIBC) increased. Similar results were observed in previously ESA-treated patients not on dialysis, although changes in those parameters were larger in treatment-naïve than in previously ESA-treated patients. In previously ESA-treated patients receiving hemodialysis, hepcidin concentration and TIBC remained stable with molidustat, whereas TSAT and ferritin and iron concentrations increased. Generally, similar trends were observed in secondary analyses of subgroups of patients not receiving iron supplementation.
Conclusions
Molidustat is a potential alternative to standard treatment of anemia associated with CKD, with a different mechanism of action. In patients not receiving dialysis, molidustat increases iron availability. In patients receiving hemodialysis, further investigation is required to understand fully the mechanisms underlying iron mobilization associated with molidustat.
Keywords: Molidustat, Anemia, Iron metabolism, Hypoxia-inducible factor prolyl hydroxylase inhibitor, Chronic kidney disease
Introduction
Anemia, a common complication of chronic kidney disease (CKD), increases in frequency with CKD severity [1], and is associated with poor quality of life and increased risk of cardiovascular events, hospitalizations, and mortality [2, 3]. The etiology of anemia was originally attributed to impairment in erythropoietin (EPO) production in the kidneys, resulting in decreased levels of hemoglobin (Hb) [1]. However, the use of recombinant human EPO has revealed that in addition to reduced EPO production, iron deficiency is also an important factor in the pathogenesis of anemia associated with CKD [4].
Because approximately 70–80% of the body's iron is contained in Hb, iron metabolism and erythropoiesis are closely interconnected [5]. Iron homeostasis is tightly regulated via a network of proteins, such as hepcidin, transferrin, and ferritin, which are involved in absorption, transport, and storage of iron respectively [6, 7]. Dysregulation of iron homeostasis in patients with anemia associated with CKD is multifactorial. Impaired renal clearance and inflammation are 2 mechanisms thought to be involved in hepcidin excess that result in reduced iron absorption [8, 9]. In addition, some patients have decreased total body iron stores, whereas others have adequate iron stores but low circulating iron levels, leading to insufficient delivery of iron to the site of erythroblast production [7, 10].
The administration of erythropoiesis stimulating agents (ESAs) combined with iron supplementation represents the current treatment for anemia associated with CKD. Generally, the use of ESAs has improved patients' quality of life; however, growing concerns regarding their safety, including the risk of cardiovascular events and stroke, have led to a reduction in target Hb levels and doses of ESAs worldwide [11, 12, 13]. Furthermore, the use of intravenous (i.v.) iron supplementation has increased to counterbalance the decrease in ESA dose used in treating anemia associated with CKD [11]. However, in some studies i.v. iron has been associated with rare hypersensitivity reactions [14, 15]. It has also been hypothesized that excessive iron supplementation may exacerbate infections and cardiovascular events; however, the recent Proactive IV Iron Therapy in Haemodialysis Patients trial did not demonstrate any significant increase in cardiovascular events or infections in the group receiving a high dose of iron compared with the low-dose group [16]. Therefore, novel approaches to treat anemia and improve iron utilization in patients with CKD are needed.
The use of hypoxia-inducible factor prolyl hydroxylase (HIF-PH) inhibitors has the potential to increase endogenous EPO production and enhance iron availability to the bone marrow in patients with anemia associated with CKD [4, 17, 18]. Of the 3 different isoforms of HIFs, HIF-2α seems to have the most prominent role in regulation of genes involved in iron metabolism, with HIF-1α playing a smaller role [19]. Indeed, HIF-2α directly upregulates the only known iron exporter, ferroportin, resulting in increased iron availability through iron export from stores in macrophages [20]. Furthermore, HIF-2α indirectly suppresses hepcidin expression via erythropoietic activity leading to an increase in iron availability [21]. In addition, HIF-1α is involved in the regulation of critical proteins in iron mobilization, including transferrin and the transferrin receptor [22, 23].
Molidustat is an orally bioavailable HIF-PH inhibitor and, as such, stabilizes HIF-1α and HIF-2α [24]. Therefore, molidustat has the potential to improve anemia in patients with CKD through increased endogenous EPO production and improved iron utilization. In preclinical studies, it was found that molidustat was effective in raising hematocrit levels through increasing endogenous EPO levels to a normal physiological range [25, 26]. Additionally, molidustat was associated with a reduction in hepcidin mRNA expression in rats [25]. Here, we report the effect of molidustat on iron metabolism as evaluated in three phase 2b, short-term studies as part of the DaIly orAL treatment increasing endOGenoUs EPO (DIALOGUE) program. The efficacy, safety, and tolerability of molidustat in these studies were reported previously [27, 28, 29]. Molidustat was generally well tolerated and was able to elevate and maintain Hb levels within a pre-specified range in patients previously treated with ESAs and in treatment-naïve patients [27].
Materials and Methods
DIALOGUEs 1 (D1), 2 (D2), and 4 (D4) were 16-week, randomized, controlled, phase 2b studies designed to evaluate the safety and efficacy of molidustat in the treatment of anemia associated with CKD in 3 different populations. Parameters of iron metabolism were monitored as exploratory variables and represent the focus of this publication.
Study Design
D1 was a placebo-controlled, double-blind, fixed-dose trial of molidustat in ESA-treatment-naïve patients with anemia associated with CKD who were not receiving dialysis treatment [27]. D2 was an open-label, dose-optimized trial comparing molidustat and the ESA darbepoetin alfa (subsequently referred to as darbepoetin) in patients with anemia associated with CKD who were previously treated with stable doses of darbepoetin and who were not receiving dialysis treatment [27, 28]. Finally, D4 was an open-label, dose-optimized trial comparing molidustat and the ESA epoetin alfa or beta (subsequently referred to as epoetin) in patients with anemia associated with CKD who were previously treated with stable doses of epoetin and who were receiving long-term, regular hemodialysis treatment [27]. At the time of screening, participants were required to have ferritin levels ≥100 and <1,000 μg/L or transferrin saturation (TSAT) ≥20%. Additional key inclusion and exclusion criteria are summarized in online supplemental Table S1 (for all online suppl. material, see www.karger.com/doi/10.1159/000502012).
Study Treatment
In D1, patients were randomized (1:1:1:1:1:1) to oral, fixed doses of molidustat (25, 50, or 75 mg once daily, or 25 or 50 mg twice daily) or placebo. Patients discontinued the study if they experienced one of the following “stopping events”: blood Hb concentration <8.0 or >13.0 g/dL, or an increase in blood Hb concentration of >1.0 g/dL in 2 weeks.
In D2, patients were randomized (1:1:1:1) to receive 1 of 3 starting doses of oral molidustat once daily (25, 50, or 75 mg) or to remain on their current dose of darbepoetin. Dose regimens were adapted every 4 (±1) weeks for each patient based on changes in blood Hb concentrations at the previous dose to achieve or maintain Hb levels of 10.0–12.0 g/dL. Darbepoetin was dosed according to prescribing information and the site's standard practice.
In D4, patients were randomized (1:1:1:0.6:1) to receive 1 of 4 starting doses of molidustat once daily (25, 50, 75, or 150 mg) or to remain on their current dose of epoetin. Dose regimens were adapted every 4 (±1) weeks for each patient based on changes in blood Hb concentrations at the previous dose to achieve or maintain Hb levels of 10.0–11.0 g/dL. Epoetin was dosed according to prescribing information and the site's standard practice.
In all 3 studies, oral and i.v. iron supplementation was dosed according to site standards at the discretion of the physician to maintain adequate levels during the study.
End Points
The primary efficacy end point in each study was the change in blood Hb concentration between baseline and the evaluation phase, defined as the average of all measurements taken during the last 4 weeks of the treatment phase. Here, we present results from the analysis of blood Hb concentrations in the molidustat combined-dose group, defined as the pooled molidustat dose groups. In addition, the exploratory variables of iron metabolism parameters (hepcidin, serum ferritin, iron concentrations, serum TSAT, and total iron binding capacity [TIBC]) at baseline, week 5, 9, 13, and 17 are also reported for the molidustat combined-dose group. With the exception of hepcidin concentration (measured by liquid chromatography-mass spectrometry/mass spectrometry), which is reported to be highly variable [30], the parameters are widely used and generally recognized as reliable, accurate, and relevant, and were evaluated using standard methods. TSAT was calculated using serum iron concentration and TIBC based on the following formula:
TSAT = (Fe/TIBC) × 100. Secondary analyses of iron metabolism parameters were performed in a subpopulation of patients not receiving oral or i.v. iron supplementation.
Statistical Analysis
The full analysis set (FAS; all patients who were randomized and received at least one dose of study agent) was used to analyze iron metabolism parameters. The change in blood Hb concentration between baseline and the evaluation phase was assessed using observed case data from the modified intention-to-treat set (mITT; all patients who were randomized, received at least one dose of study agent, and had at least one post-baseline efficacy value recorded). Data were analyzed using descriptive statistical methods, and the statistical evaluation was performed using the hosted SAS version release 9.3 (SAS Institute Inc., Cary, NC, USA).
Results
Baseline Characteristics and Patient Disposition
Baseline demographic and clinical characteristics for the FAS of all 3 of the DIALOGUE studies have been reported in detail previously [27] and are summarized in Table 1, together with the baseline characteristics of the subpopulation of patients not receiving oral or i.v. iron supplementation. Iron parameters at baseline for each study are presented in Tables 2, 3, 4.
Table 1.
Patient demographics and baseline characteristics in DIALOGUES 1, 2, and 4
| FAS |
Non-iron users |
||||||
|---|---|---|---|---|---|---|---|
| molidustat | control group | total | molidustat | control group | total | ||
| Dialogue 1 | n = 101 | n = 20 | n = 121 | n = 53 | n = 8 | n = 61 | |
| Age, years, mean (SD) | 68.7 (12.0) | 67.1 (15.9) | 68.4 (12.6) | 70.1 (11.4) | 78.6 (6.9) | 71.2 (11.3) | |
| Women, n (%) | 45 (44.6) | 11 (55.0) | 56 (46.3) | 25 (47.2) | 4 (50.0) | 29 (47.5) | |
| Race, n (%) | |||||||
| White | 63 (62.4) | 15 (75.0) | 78 (64.5) | 34 (64.2) | 6 (75.0) | 40 (65.6) | |
| Asian | 38 (37.6) | 5 (25.0) | 43 (35.5) | 19 (35.8) | 2 (25.0) | 21 (34.4) | |
| Black | 0 | 0 | 0 | 0 | 0 | 0 | |
| Other | 0 | 0 | 0 | 0 | 0 | 0 | |
| CKD duration, years, mean (SD) | 4.5 (4.5) | 3.5 (2.7) | 4.3 (4.3) | 4.5 (5.0) | 2.7 (2.3) | 4.3 (4.8) | |
| CKD etiologya, n (%) | |||||||
| Diabetes | 45 (44.6) | 9 (45.0) | 54 (44.6) | 23 (43.4) | 4 (50.0) | 27 (44.3) | |
| Hypertension | 45 (44.6) | 6 (30.0) | 51 (42.1) | 25 (47.2) | 3 (37.5) | 28 (45.9) | |
| Others | 28 (27.7) | 6 (30.0) | 34 (28.1) | 16 (30.2) | 1 (12.5) | 17 (16.4) | |
| eGFR, mL/min/1.73 m2, mean (SD) | 23.4 (12.3) | 23.0 (11.6) | 23.3 (12.1) | 25.2 (12.5) | 28.8 (13.0) | 25.7 (12.5) | |
| Hb level, g/dL, mean (SD) | 9.5 (0.7) | 9.5 (0.6) | 9.5 (0.7) | 9.4 (0.8) | 9.4 (0.7) | 9.4 (0.8) | |
| CRP, mg/L, mean (SD) | 7.2 (16.4) | 4.3 (5.1) | 6.7 (15.2) | 7.4 (20.7) | 3.3 (4.0) | 6.9 (19.4) | |
| Dialogue 2 | n = 92 | n = 32 | n = 124 | n = 42 | n = 16 | n = 58 | |
| Age, years, mean (SD) | 67.6 (10.5) | 68.8 (8.7) | 67.9 (10.0) | 67.4 (11.1) | 68.3 (8.3) | 67.6 (10.3) | |
| Women, n (%) | 47 (51.1) | 14 (43.8) | 61 (49.2) | 19 (45.2) | 6 (37.5) | 25 (43.1) | |
| Race, n (%) | |||||||
| White | 69 (75.0) | 25 (78.1) | 94 (75.8) | 33 (78.6) | 12 (75.0) | 45 (77.6) | |
| Asian | 22 (23.9) | 6 (18.8) | 28 (22.6) | 9 (21.4) | 3 (18.8) | 12 (20.7) | |
| Black | 1 (1.1) | 1 (3.1) | 2 (1.6) | 0 | 1 (6.3) | 1 (1.7) | |
| Other | 0 | 0 | 0 | 0 | 0 | 0 | |
| CKD duration, years, mean (SD) | 6.7 (6.4) | 5.8 (5.0) | 6.5 (6.1) | 7.2 (6.7) | 6.3 (5.8) | 6.9 (6.4) | |
| CKD etiologya, n (%) | |||||||
| Diabetes | 31 (33.7) | 10 (31.3) | 41 (33.1) | 14 (33.3) | 4 (25.0) | 18 (31.0) | |
| Hypertension | 31 (33.7) | 13 (40.6) | 44 (35.5) | 15 (35.7) | 8 (50.0) | 23 (39.7) | |
| Others | 43 (46.7) | 15 (46.9) | 58 (46.8) | 20 (47.6) | 8 (50.0) | 28 (48.3) | |
| eGFR, mL/min/1.73 m2, mean (SD) | 20.4 (11.4) | 21.9 (12.1) | 20.8 (11.5) | 19.0 (9.6) | 20.6 (12.3) | 19.5 (10.3 | |
| Hb level, g/dL, mean (SD) | 10.8 (0.7) | 10.9 (0.7) | 10.8 (0.7) | 10.8 (0.7) | 10.8 (0.9) | 10.8 (0.7) | |
| CRP, mg/L, mean (SD) | 7.4 (14.9) | 6.5 (11.2) | 7.2 (14.0) | 6.3 (12.8) | 8.8 (15.2) | 7.0 (13.5) | |
| Dialogue 4 | n = 157 | n = 42 | n = 199 | n = 59 | n = 15 | n = 74 | |
| Age, years, mean (SD) | 59.4 (12.5) | 58.9 (9.1) | 59.3 (11.8) | 60.8 (12.3) | 56.7 (10.1) | 60.0 (12.0) | |
| Women, n (%) | 66 (42.0) | 13 (31.0) | 79 (39.7) | 27 (45.8) | 5 (33.3) | 32 (43.2) | |
| Race, n (%) | |||||||
| White | 84 (53.5) | 18 (42.9) | 102 (51.3) | 29 (49.2) | 5 (33.3) | 34 (45.9) | |
| Asian | 29 (18.5) | 7 (16.7) | 36 (18.1) | 18 (30.5) | 3 (20.0) | 21 (28.4) | |
| Black | 40 (25.5) | 17 (40.5) | 57 (28.6) | 12 (20.3) | 7 (46.7) | 19 (25.7) | |
| Other | 4 (2.5) | 0 | 4 (2.0) | 0 | 0 | 0 | |
| CKD duration, years, mean (SD) | 6.4 (6.2) | 5.5 (4.3) | 6.2 (5.8) | 7.6 (6.8) | 5.6 (4.6) | 7.2 (6.4) | |
| Dialysis therapy duration, years, mean (SD) | 5.0 (5.2) | 5.3 (4.0) | 5.1 (5.0) | 5.6 (5.2) | 5.1 (3.9) | 5.5 (4.9) | |
| CKD etiologya, n (%) | |||||||
| Diabetes | 86 (54.8) | 24 (57.1) | 110 (55.3) | 26 (44.1) | 5 (33.3) | 31 (41.9) | |
| Hypertension | 48 (30.6) | 18 (42.9) | 66 (33.2) | 22 (37.3) | 7 (46.7) | 29 (39.2) | |
| Others | 31 (19.7) | 5 (11.9) | 36 (18.1) | 13 (22.0) | 3 (20.0) | 16 (21.6) | |
| Hb level, g/dL, mean (SD) | 10.5 (0.6) | 10.6 (0.5) | 10.5 (0.6) | 10.5 (0.6) | 10.6 (0.6) | 10.5 (0.6) | |
| CRP, mg/L, mean (SD) | 7.9 (13.5) | 7.1 (11.1) | 7.7 (13.0) | 5.4 (10.4) | 7.8 (10.8) | 5.9 (10.4) | |
One patient can have >1 etiology of CKD.
DIALOGUE, Dally orAL treatment increasing endOGenoUs Erythropoietin; FAS, full analysis set; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; Hb, hemoglobin; CRP, C-reactive protein.
Table 2.
Iron parameters and blood Hb concentration at baseline and at week 17 in DIALOGUE 1
| Parameter | Molidustat |
Placebo |
||||||
|---|---|---|---|---|---|---|---|---|
| overall |
non-iron users |
overall |
non-iron users |
|||||
| baseline | week 17 | baseline | week 17 | baseline | week 17 | baseline | week 17 | |
| Hb, g/dL | 9.5±0.7 (101) | 11.0±1.2 (43) | 9.4±0.8 (53) | 10.8±1.3 (22) | 9.5±0.6 (20) | 9.8±14.1 (18) | 9.4±0.7 (8) | 10.05±1.0 (6) |
| Hepcidin, ng/mL | 36.0±29.9 (101) | 18.5±19.8 (40) | 40.3±35.4 (53) | 11.5±10.9 (22) | 38.0±30.8 (20) | 41.0±33.7 (18) | 35.0±15.3 (8) | 31.8±27.8 (6) |
| Ferritin, µg/L | 201.4±149.6 (101) | 98.3±89.5 (40) | 220.8±176.3 (53) | 71.7±69.4 (22) | 221.0±163.9 (20) | 215.9±149.1 (18) | 269.1±173.8 (8) | 241.2±196.0 (6) |
| Iron, µg/dL | 81.3±34.2 (101) | 67.6±29.3 (40) | 86.5±39.8 (53) | 63.5±26.6 (22) | 82.5±21.8 (20) | 68.3±28.7 (18) | 87.0±28.9 (8) | 51.6±10.0 (6) |
| TSAT, % | 34.2±13.3 (100) | 27.9±12.5 (40) | 36.3±13.6 (53) | 26.2±12.4 (22) | 35.2±9.5 (18) | 29.6±11.2 (18) | 35.1±9.8 (7) | 23.0±6.8 (6) |
| TIBC, pmol/L | 43.4±9.6 (100) | 45.6±10.3 (40) | 43.1±9.8 (53) | 46.6±10.7 (22) | 41.5±6.9 (18) | 41.3±7.0 (18) | 41.1±8.5 (7) | 41.5±6.5 (6) |
Data are mean ± SD (n).
Hb, hemoglobin; DIALOGUE, DaIly orAL treatment increasing endOGenoUs Erythropoietin; TSAT, transferrin saturation; TIBC, total iron binding capacity.
Table 3.
Iron parameters and blood Hb concentration at baseline and at week 17 in DIALOGUE 2
| Parameter | Molidustat |
Darbepoetin |
||||||
|---|---|---|---|---|---|---|---|---|
| overall |
non-iron users |
overall |
on-iron users |
|||||
| baseline | week 17 | baseline | week 17 | baseline | week 17 | baseline | week 17 | |
| Hb, g/dL | 10.8±0.7 (92) 11.8±0.8 (75) | 10.8±0.7 (42) | 11.1±0.8 (32) | 10.9±0.7 (32) | 11.0±0.8 (27) | 10.8±0.9 (16) | 10.9±0.8 (12) | |
| Hepcidin, ng/mL | 37.6±28.9 (90) 29.4±23.2 (72) | 39.1±29.0 (40) | 24.9±21.8 (30) | 34.9±29.4 (31) | 48.8±40.6 (27) | 39.2±36.9 (15) | 51.1±41.2 (12) | |
| Ferritin, µg/L | 202.9±174.5 (92) 192.9±222.4 (70) | 201.8±145.7 (42) | 139.3±118.7 (30) | 249.3±270.0 (32) | 224.2±224.0 (27) | 301.6±241.1 (16) | 254.6±193.8 (12) | |
| Iron, µg/dL | 82.3±52.6 (91) 73.7±25.2 (70) | 88.7±68.7 (42) | 75.2±25.7 (30) | 77.8±27.2 (32) | 81.5±35.2 (27) | 83.2±26.7 (16) | 89.7±45.1 (12) | |
| TSAT, % | 31.8±13.0 (89) 29.8±9.3 (70) | 32.0±13.9 (40) | 30.1±12.2 (30) | 33.8±12.2 (31) | 34.0±10.9 (27) | 36.1±12.1 (15) | 35.8±11.1 (12) | |
| TIBC, µmol/L | 44.1±9.8 (91) 44.9±8.7 (70) | 44.2±8.4 (42) | 45.7±9.0 (30) | 42.7±8.4 (31) | 42.4±9.1 (27) | 44.0±9.4 (15) | 43.1±11.8 (12) | |
Data are mean ± SD (n).
Hb, hemoglobin; DIALOGUE, DaIly orAL treatment increasing endOGenoUs Erythropoietin; TSAT, transferrin saturation; TIBC, total iron binding capacity.
Table 4.
Iron parameters and blood Hb concentration at baseline and at week 17 in DIALOGUE 4
| Parameter | Molidustat |
Darbepoetin |
||||||
|---|---|---|---|---|---|---|---|---|
| overall |
non-iron users |
overall |
non-iron users |
|||||
| baseline | week 17 | baseline | week 17 | baseline | week 17 | baseline | week 17 | |
| Hb, g/dL | 10.5±0.6 (157) | 10.2±1.1 (105) | 10.5±0.6 (59) | 10.5±1.0 (39) | 10.6±0.5 (42) | 10.4±0.7 (39) | 10.6±0.6 (15) | 10.4±0.6 (13) |
| Hepcidin, ng/mL | 72.4±40.4 (156) | 73.0±49.6 (102) | 76.1±46.8 (59) | 62.0±43.1 (39) | 70.3±35.7 (42) | 75.6±48.8 (38) | 76.2±42.1 (15) | 80.6±49.0 (13) |
| Ferritin, µg/L | 556.5±315.0 (157) | 583.0±355.1 (102) | 559.9±357.9 (59) | 484.3±346.6 (38) | 542.1±331.4 (42) | 590.1±450.0 (39) | 528.1±319.7 (15) | 489.7±341.9 (13) |
| Iron, µg/dL | 67.8±25.0 (157) | 72.7±23.4 (102) | 69.4±29.5 (59) | 72.3±27.7 (38) | 63.6±21.4 (42) | 71.1 + 30.4 (39) | 63.3±20.3 (15) | 79.5±32.4 (13) |
| TSAT, % | 33.6±10.7 (157) | 34.8±11.8 (102) | 34.0±12.4 (59) | 33.1±12.5 (38) | 33.4±12.2 (42) | 34.0±11.4 (38) | 32.4±12.0 (15) | 36.2±12.5 (13) |
| TIBC, µmol/L | 36.1±5.6 (157) | 39.7±16.4 (102) | 36.3±5.5 (59) | 43.4±25.0 (38) | 35.0±6.6 (42) | 36.8±10.5 (39) | 36.1±7.1 (15) | 39.1±5.7 (13) |
Data are mean ± SD (n).
Hb, hemoglobin; DIALOGUE, DaIly orAL treatment increasing endOGenoUs Erythropoietin; TSAT, transferrin saturation; TIBC, total iron binding capacity.
Of 121 patients randomized in D1, all (101 molidustat, 20 placebo) were included in the FAS and mITT population, and 60 patients (59.5%) receiving molidustat and 2 patients (10.0%) receiving placebo discontinued the study. Of those who discontinued molidustat, the majority discontinued by the last 4 weeks and had a blood Hb concentration above the upper limit of 13 g/dL or an increase in blood Hb concentration of >1.0 g/dL in 2 weeks. In D2, all of the 124 randomized patients (92 molidustat, 32 darbepoetin) were included in the FAS and mITT population, and 20 patients (21.7%) receiving molidustat and 5 patients (15.6%) receiving darbepoetin discontinued the study. All of the 199 patients randomized in D4 (157 molidustat, 42 epoetin) were included in the FAS and mITT population, and 53 patients (33.8%) receiving molidustat and 3 patients (7.1%) receiving epoetin discontinued the study.
In patients who did not receive iron supplementation, there were no differences in baseline demographic and clinical characteristics between the molidustat and control groups of all 3 DIALOGUE studies, with the exception of age in D1 in which the mean (SD) age in the placebo group was 78.6 (6.9) years compared with 70.1 (11.4) years in the molidustat group.
In D1, the number of patients not receiving iron supplementation during the study was 53 (52.4%) in the molidustat group and 8 (40.0%) in the placebo group. Of the patients randomized in D2, 42 (45.7%) in the molidustat group and 16 (50.0%) in the darbepoetin group did not receive iron supplementation. In D4, the number of patients not receiving iron supplementation during the study was 59 (37.6%) in the molidustat group and 15 (35.7%) in the placebo group.
Treatment Exposure
In D1, the mean (SD) treatment duration was 74.7 (39.7) days for molidustat with a mean (SD) total cumulative dose of 4,327.0 (2,917.9) mg. In D2, the mean (SD) treatment durations were 105.0 (25.0) days for molidustat and 103.2 (24.6) days for darbepoetin. The mean (SD) daily dose per patient was 45.4 (24.6) mg in the molidustat group and 0.03 (0.02) µg/kg in the darbepoetin group. In D4, the mean (SD) treatment durations were 94.8 (31.6) days for molidustat and 111.7 (10.6) days for epoetin. The mean (SD) daily dose per patient was 66.2 (37.6) mg in the molidustat group and 12.86 (9.9) IU/kg in the epoetin group.
Iron Metabolism
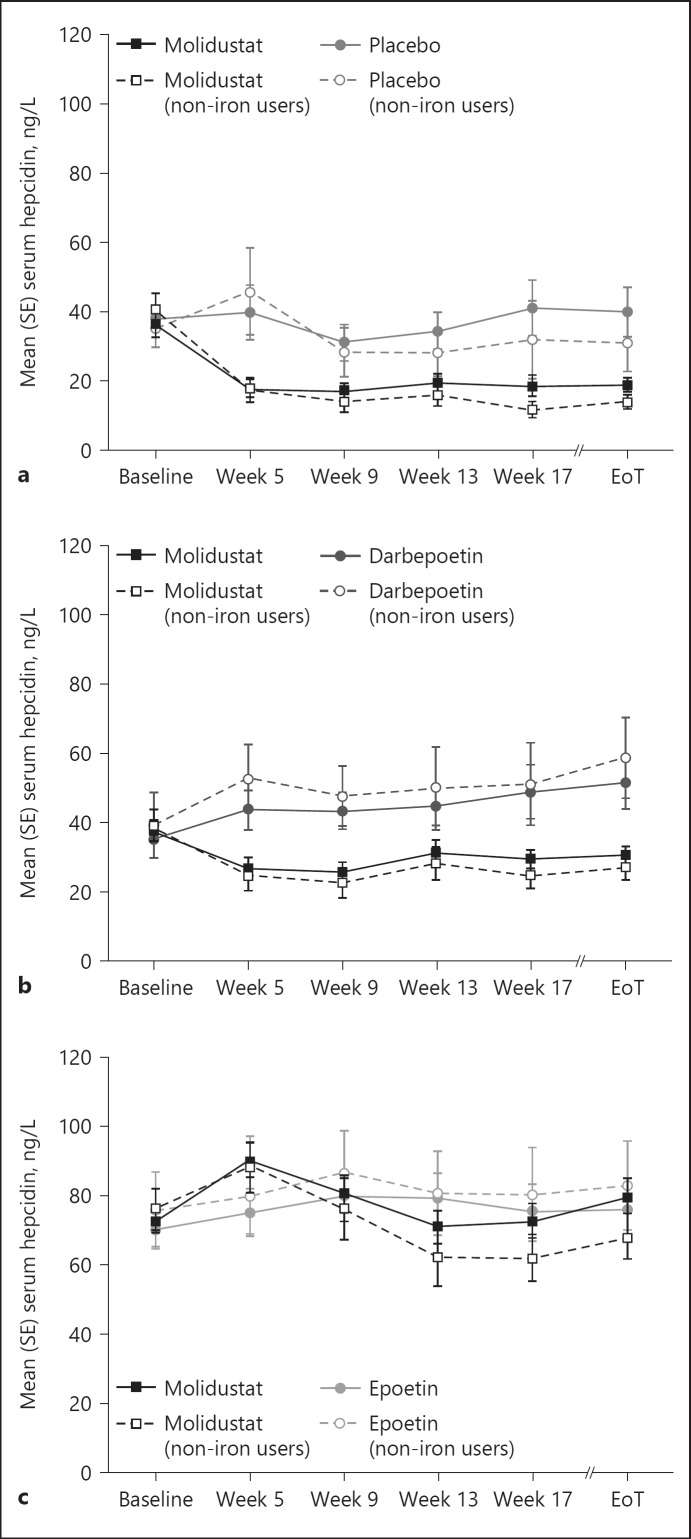
In D1, mean serum hepcidin concentrations decreased over the 16 weeks of treatment (from baseline to week 17) in patients treated with molidustat. A similar decrease was associated with molidustat in the subgroup analysis of patients who did not receive iron supplementation (online suppl. Table S2). In the placebo group, mean serum hepcidin concentrations increased over the 16 weeks of treatment in the FAS but decreased in patients not treated with iron supplementation (Table 2, Fig. 1a).
Fig. 1.
Serum hepcidin concentrations during treatment with molidustat or placebo/active comparator: (a) DIALOGUE 1, (b) DIALOGUE 2, and (c) DIALOGUE 4. Non-iron users are patients who did not receive iron supplementation during the study. EoT, end of treatment.
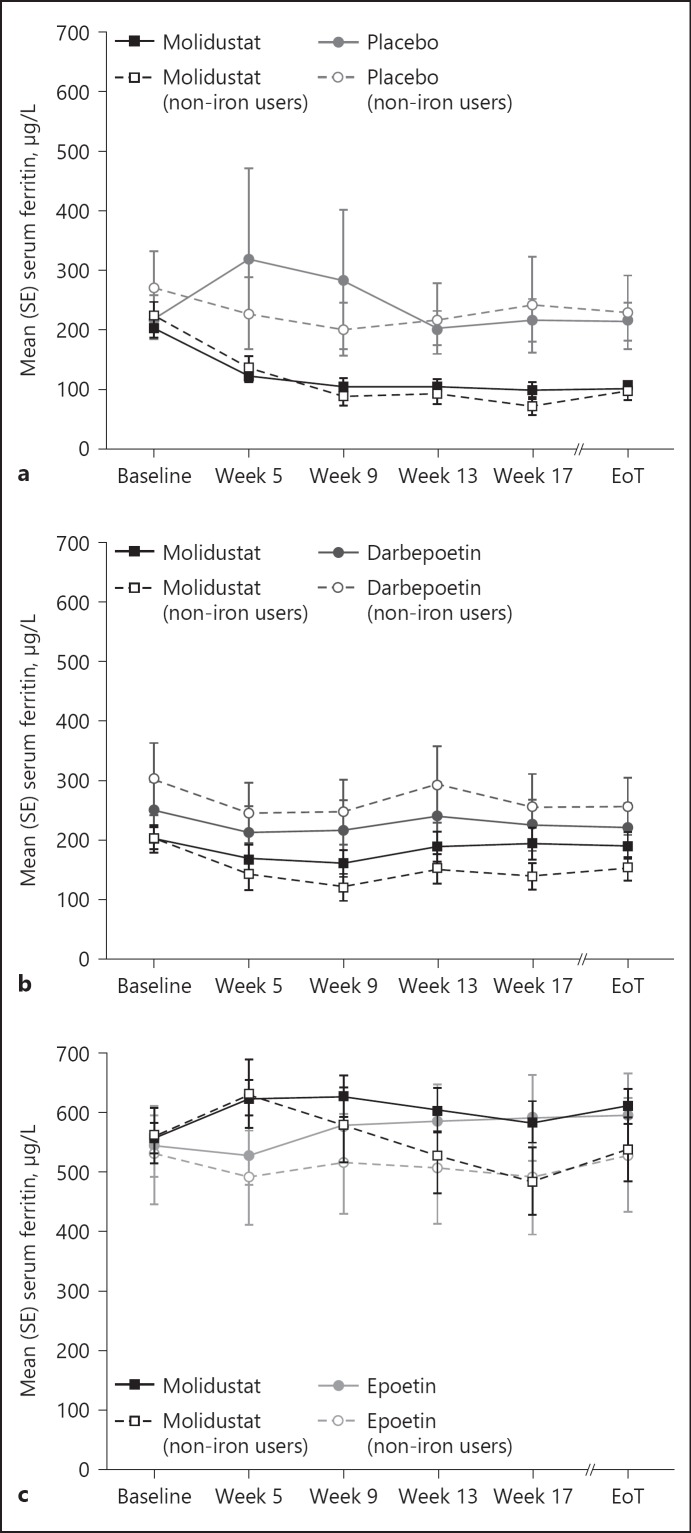
In the FAS, mean serum ferritin concentrations in patients receiving molidustat decreased over the 16 weeks of treatment (from baseline to week 17), whereas in the placebo group, mean serum ferritin concentrations initially increased before returning to baseline levels from week 13 to 17. Similarly, in patients not receiving iron supplementation, mean serum ferritin concentrations decreased in the molidustat group and remained stable in the placebo group during the 16-week treatment period (Table 2, Fig. 2a).
Fig. 2.
Serum ferritin concentrations during treatment with molidustat or placebo/active comparator: (a) DIALOGUE 1, (b) DIALOGUE 2, and (c) DIALOGUE 4. Non-iron users are patients who did not receive iron supplementation during the study. EoT, end of treatment.
In the FAS, mean serum iron concentrations and mean serum TSAT decreased over the 16 weeks of treatment (from baseline to week 17) in both molidustat and placebo groups. In patients not receiving iron supplementation, similar trends to those in the FAS were observed for mean serum iron concentrations and mean serum TSAT in both treatment groups (Table 2, online suppl. Fig. S1a, S2a).
TIBC increased in the molidustat group between baseline and week 17 but remained stable in the placebo group throughout the treatment period; for both treatment groups, similar profiles were observed in the subgroup of patients not treated with an iron supplement (Table 2, online suppl. Fig. S3a).
In D2, mean serum hepcidin concentrations decreased during the 16-week treatment period (from baseline to week 17) in patients receiving molidustat, and increased in the darbepoetin group. Similarly, in patients who did not receive iron supplementation, molidustat treatment was associated with a decrease in mean serum hepcidin concentrations, while darbepoetin treatment was associated with an increase from baseline to week 17 (Table 3, Fig. 1b). Furthermore, the decrease in hepcidin concentrations associated with molidustat treatment was numerically lower in the FAS than in patients not receiving iron supplementation.
In the FAS and in patients who did not receive iron supplementation, both molidustat and darbepoetin treatments were associated with a decrease in mean serum ferritin concentrations over the 16 weeks of treatment (Table 3, Fig. 2b, online suppl. Table S3).
Mean serum iron concentrations decreased during the 16-week treatment period (from baseline to week 17) in patients receiving molidustat and increased in the darbepoetin group in both the FAS and the subgroup of patients not receiving iron supplementation (Table 3, online suppl. Fig. S1b).
Serum TSAT and TIBC remained stable during the study and were similar in the molidustat and darbepoetin groups; for both treatment groups, similar profiles were observed in the subgroup of patients not receiving iron supplementation (Table 3, online suppl. Fig. S2b, S3b).
In D4, mean serum hepcidin concentration remained stable during the study period in patients treated with molidustat but increased in the epoetin group. In patients treated with molidustat but not receiving iron supplementation, mean serum hepcidin concentrations decreased from baseline to week 17 and increased in the epoetin group (Table 4, Fig. 1c, online suppl. Table S4).
In the FAS, mean serum ferritin concentrations increased over the 16 weeks of treatment (from baseline to week 17) in patients receiving molidustat and in those receiving epoetin. In contrast, in patients who did not receive iron supplementation, mean serum ferritin concentrations decreased from baseline to week 17 in both treatment groups (Table 4, Fig. 2c).
Both molidustat and epoetin were associated with an increase in mean serum iron concentration over the course of the study in the FAS and in the subgroup of patients not treated with iron supplementation (Table 4, online suppl. Fig. S1c).
Mean serum TSAT remained stable during the study in both treatment groups and was similar in the FAS and in patients not receiving an iron supplement (Table 4, online suppl. Fig. S2c).
In the FAS, TIBC remained stable in both treatment groups during the 16-week period. In the subgroup analysis, TIBC increased from baseline to week 17 in the molidustat group and remained stable in the epoetin group (Table 4, online suppl. Fig. S3c).
Change in Blood Hb Concentration
The changes in mean blood Hb concentration from baseline to the evaluation phase for each molidustat starting dose and for each study have been reported in detail previously [27]. Here, we present a summary of the change in mean Hb concentration in the pooled molidustat dose groups for the FAS and for the subpopulation of patients who did not receive iron supplementation.
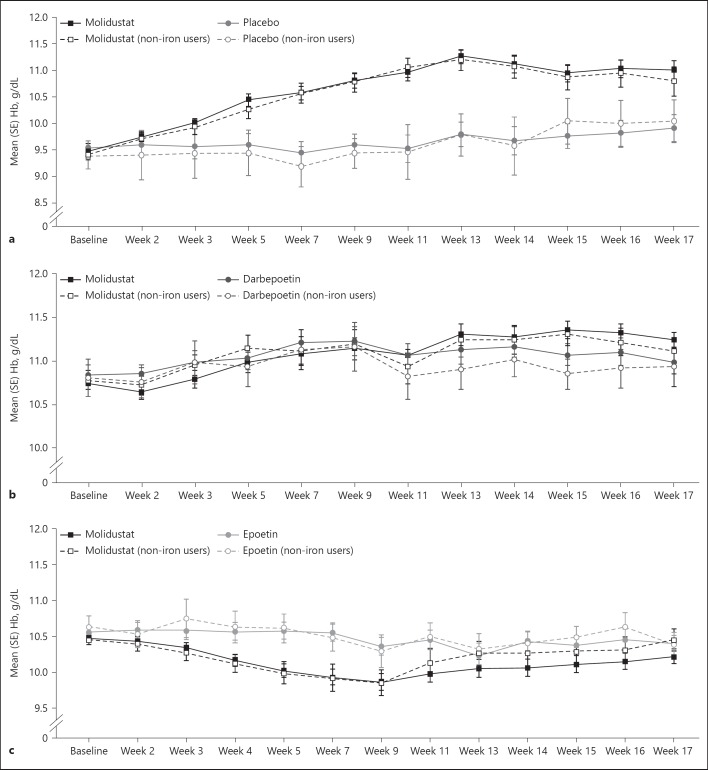
In D1, the mean blood Hb concentration in the molidustat group increased from baseline during the first 12 weeks and then plateaued during the last 4 weeks of treatment, which is largely due to discontinuations resulting from high blood Hb concentrations (Table 2, Fig. 3a). In patients who did not receive iron supplementation, molidustat was associated with an increase in mean blood Hb concentration from baseline to week 17 (Table 2, Fig. 3a). In D2, mean blood Hb concentrations in both the molidustat and darbepoetin groups were maintained within the target range (10.0–12.0 g/dL) throughout the study. Similarly, in patients not receiving iron supplementation, mean blood Hb concentrations were maintained in both treatment groups during the study (Table 3, Fig. 3b). In D4, mean blood Hb concentrations in the molidustat group fell just below the lower limit of the target range (10.0–11.0 g/dL) from week 7 to 11 and otherwise remained within the target range (Table 4, Fig. 3c). For patients who did not receive iron supplementation in the molidustat group, mean blood Hb concentrations fell just below the lower limit of the target range from week 5 to 9 and were maintained within the target range otherwise (Table 4, Fig. 3c).
Fig. 3.
Blood Hb concentrations during treatment with molidustat or placebo/active comparator: (a) DIALOGUE 1, (b) DIALOGUE 2, and (c) DIALOGUE 4. Non-iron users are patients who did not receive iron supplementation during the study. Hb, hemoglobin.
Discussion
We investigated the effect of the HIF-PH inhibitor molidustat on iron metabolism in 3 different populations of patients with CKD. In treatment-naïve patients not on dialysis, molidustat was associated with greater iron mobilization than placebo (D1). In patients not on dialysis but who had previously received treatment with the ESA darbepoetin (D2), molidustat was associated with greater changes in markers of iron metabolism than darbepoetin. Finally, in patients on hemodialysis and who had previously received ESA therapy with epoetin (D4), molidustat and epoetin had similar effects on iron parameters. In each study population, similar trends were observed in both the subgroups of patients who did not receive iron supplementation and in the FAS, with the exception of hepcidin and ferritin in the placebo group in D1 and ferritin in both treatment groups in D4. In addition, in each study population, molidustat corrected or maintained Hb concentrations within the target ranges in the FAS and in patients not receiving iron supplementation, except in D4 in which a temporary drop in Hb level below the target range was observed. This was driven by the Hb level decrease associated with lower starting doses of molidustat in the first weeks of treatment.
The reason why we observed such clinical differences between molidustat and standard of care might be due to the difference of mode of action. ESAs stimulate erythropoiesis through direct interaction with the EPO receptor on erythroid cells in the bone marrow [31, 32] and therefore are unlikely to modulate iron metabolism other than through their erythropoietic activity, whereas HIF-PH inhibitors act upstream by stimulating EPO production in the kidney via stabilization of the HIF transcription factors, which upregulate a large number of genes including those encoding key proteins involved in iron metabolism [33].
The biochemical parameters examined in this study play important roles in the mobilization of iron. Hepcidin is known to induce internalization and degradation of the iron exporter ferroportin; therefore, a decrease in hepcidin levels results in better iron absorption and an increased access to iron stores. Consequently, as iron is being exported and utilized for erythropoiesis, levels of ferritin, the protein responsible for the intracellular storage of iron, decrease. However, because hepcidin and ferritin are also acute-phase proteins, their concentrations must be interpreted cautiously in the setting of inflammation [7, 34]. Transferrin is essential in transporting iron into cells. TIBC is the sum of all iron-binding sites on transferrin, and TSAT is the ratio of the total number of occupied sites to TIBC; therefore, TIBC and TSAT are indicators of circulating iron levels.
In patients with CKD who were not on dialysis and who had not previously received ESA therapy (D1), molidustat was associated with greater decreases in levels of hepcidin and ferritin and greater increases in TIBC than placebo, consistent with enhanced iron mobilization, increased erythropoiesis, and the mechanism of action of HIF-PH inhibitors [35, 36]. Similar changes in iron metabolism were observed in studies of the HIF-PH inhibitors vadadustat and roxadustat in patients with CKD who were not on dialysis [37, 38, 39]. Not surprisingly, the decreases in mean hepcidin, ferritin, and iron levels were greater in patients not receiving iron supplementation compared with the overall study population.
When molidustat was compared with darbepoetin in patients with CKD who were not on dialysis and who had previously received ESAs (D2), both treatments were associated with a decrease in mean ferritin levels and stable TIBC and TSAT. In contrast, only molidustat was associated with decreased mean hepcidin and iron levels, suggesting that iron mobilization was greater in patients receiving molidustat than in those receiving darbepoetin. These trends were confirmed by the results of the secondary analysis in patients not receiving iron supplementation. The similar effects of molidustat and darbepoetin on ferritin, TIBC, and TSAT may be due to the common erythropoietic activity of both treatments, whereas the differences observed in hepcidin and iron levels may result from the distinct mechanism of action of molidustat, an HIF-PH inhibitor, and darbepoetin, an ESA [35, 40].
In patients not receiving dialysis, changes in iron mobilization associated with molidustat treatment, indicated by decreased hepcidin, ferritin, and iron levels, were qualitatively larger in ESA treatment-naïve patients (D1) than in previously ESA-treated patients (D2). These differences in iron parameters were also observed in the subpopulation of patients not receiving iron supplementation, and may reflect a ceiling effect for iron parameters in ESA-treated individuals.
In patients receiving hemodialysis treatment (D4), changes in iron parameters were similar in both the molidustat and epoetin groups, with ferritin levels and TIBC slightly increased, and hepcidin levels and TSAT remaining stable. However, the proportion of patients receiving i.v. iron supplementation was considerably higher in D4 than in DIALOGUEs 1 and 2, and i.v. iron supplementation has been shown to have a greater effect on ferritin levels and TSAT than oral iron supplementation [41]. In addition, hemodialysis is associated with high levels of inflammatory activity [42]; the higher baseline levels of hepcidin and ferritin in D4 than in DIALOGUEs 1 and 2 are consistent with previously reported data [8, 11] and may reflect differences in inflammatory activity between the studies [43]. Thus, i.v. iron supplementation combined with high levels of background inflammatory activity may have masked the effect of molidustat on iron availability in patients with CKD receiving hemodialysis treatment. This was further confirmed by the larger decreases in ferritin and hepcidin levels associated with molidustat compared with epoetin in patients who did not receive iron supplementation. These results are in line with those from Provenzano et al. [44] who reported a decrease in hepcidin and ferritin levels following treatment with roxadustat in patients with CKD receiving hemodialysis and not receiving i.v. iron supplementation.
Because molidustat modulates Hb and iron levels via a different mechanism of action from ESAs, it offers an alternative treatment option for patients with anemia associated with CKD. In addition, the lower hepcidin levels and enhanced iron mobilization associated with molidustat compared with darbepoetin in patients not receiving dialysis may reduce the need for iron supplementation and the risk of infection and allergic reactions associated with the use of i.v. iron.
The strength of the present data is that they were derived from 3 randomized clinical trials in 3 different populations of patients with anemia associated with CKD (dialysis, nondialysis, treatment-naïve, and previously treated). The 3 studies included either a placebo control or an ESA-control arm. However, the results presented here should be interpreted in the context of several limitations. Importantly, there was no predefined strategy for controlling iron supplementation, which was left to the discretion of the physician. The secondary analysis of iron parameters in patients not receiving iron supplementation was also not predefined. Finally, these studies were of relatively short duration, and the numbers of patients in each treatment group were low.
Conclusions
The results presented here suggest that molidustat, a novel HIF-PH inhibitor, is a potential alternative therapy with a novel mechanism of action for the treatment of anemia associated with CKD. Indeed, in patients not receiving dialysis, molidustat modulates Hb and iron metabolism in both treatment-naïve and previously treated patients. In patients receiving hemodialysis, further investigation is required to understand fully the mechanisms underlying iron mobilization associated with molidustat.
Statement of Ethics
All studies were conducted in compliance with the principles of the Declaration of Helsinki and in accordance with Good Clinical Practice guidelines. The protocols were reviewed and approved by the institutional review board or Ethics Committee of each participating center. All patients provided written, informed consent before study entry.
Disclosure Statement
T.A.: has received consulting fees from Astellas, Bayer Yakuhin Ltd., GlaxoSmithKline, J.T. Pharmaceuticals, Kissei Pharmaceutical Co. Ltd., Kyowa Hakko Kirin, Nipro Corporation, Fuso Pharmaceutical Industries Ltd., and Ono Pharmaceutical Co. Ltd., and lecture fees from Bayer Yakuhin Ltd., Chugai Pharmaceutical Co. Ltd., Kyowa Hakko Kirin, and Torii Pharmaceutical Co. Ltd. I.C.M. has received research funding for the DIALOGUE studies, honoraria for steering committee activities, and speaker fees from Bayer Pharma AG; and has received research support and speakers' honoraria from Akebia, Astellas, FibroGen, and GlaxoSmithKline. J.S.B. has served on the executive committees for the DIALOGUE studies and for an Amgen-sponsored darbepoetin clinical trial. M.T. and K.I. are employees of Bayer Yakuhin Ltd. T.B. is an employee of Bayer AG.
Funding Sources
These studies were funded by Bayer AG.
Author Contributions
T.A., I.C.M., J.S.B., and T.B.: participated in the study concept and design. All the authors were involved in the acquisition, analysis, and interpretation of data. All the authors participated in preparing the manuscript.
Acknowledgments
Eriko Ogura of Bayer Yakuhin reviewed the manuscript for statistical and/or scientific accuracy. Medical writing support was provided by Dr Nicolas Bertheleme of Oxford PharmaGenesis, Oxford, UK, with funding from Bayer AG.
Availability of the data underlying this publication will be determined according to Bayer's commitment to the EFPIA/PhRMA “Principles for responsible clinical trial data sharing.” This pertains to scope, timepoint, and process of data access. As such, Bayer commits to sharing upon request from qualified scientific and medical researchers patient-level clinical trial data, study-level clinical trial data, and protocols from clinical trials in patients for medicines and indications approved in the United States and European Union as necessary for conducting legitimate research. This applies to data on new medicines and indications that have been approved by the European Union and United States regulatory agencies on or after January 1, 2014.
Interested researchers can use www.clinicalstudydatarequest.com to request access to anonymized patient-level data and supporting documents from clinical studies to conduct further research that can help advance medical science or improve patient care. Information on the Bayer criteria for listing studies and other relevant information is provided in the Study sponsors section of the portal. Data access will be granted to anonymized patient-level data, protocols, and clinical study reports after approval by an independent scientific review panel. Bayer is not involved in the decisions made by the independent review panel. Bayer will take all necessary measures to ensure that patient privacy is safeguarded.
References
- 1.Babitt JL, Lin HY. Mechanisms of anemia in CKD. J Am Soc Nephrol. 2012 Oct;23((10)):1631–4. doi: 10.1681/ASN.2011111078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Portolés J, Gorriz JL, Rubio E, de Alvaro F, García F, Alvarez-Chivas V, et al. NADIR-3 Study Group The development of anemia is associated to poor prognosis in NKF/KDOQI stage 3 chronic kidney disease. BMC Nephrol. 2013 Jan;14((1)):2. doi: 10.1186/1471-2369-14-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Nooten FE, Green J, Brown R, Finkelstein FO, Wish J. Burden of illness for patients with non-dialysis chronic kidney disease and anemia in the United States review of the literature. J Med Econ. 2010;13((2)):241–256. doi: 10.3111/13696998.2010.484307. [DOI] [PubMed] [Google Scholar]
- 4.Koury MJ, Haase VH. Anaemia in kidney disease harnessing hypoxia responses for therapy. Nat Rev Nephrol. 2015 Jul;11((7)):394–410. doi: 10.1038/nrneph.2015.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim A, Nemeth E. New insights into iron regulation and erythropoiesis. Curr Opin Hematol. 2015 May;22((3)):199–205. doi: 10.1097/MOH.0000000000000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu MM, Wang J, Xie JX. Regulation of iron metabolism by hypoxia-inducible factors. Sheng Li Xue Bao. 2017 Oct;69((5)):598–610. [PubMed] [Google Scholar]
- 7.Zumbrennen-Bullough K, Babitt JL. The iron cycle in chronic kidney disease (CKD) from genetics and experimental models to CKD patients. Nephrol Dial Transplant. 2014 Feb;29((2)):263–73. doi: 10.1093/ndt/gft443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ashby DR, Gale DP, Busbridge M, Murphy KG, Duncan ND, Cairns TD, et al. Plasma hepcidin levels are elevated but responsive to erythropoietin therapy in renal disease. Kidney Int. 2009 May;75((9)):976–81. doi: 10.1038/ki.2009.21. [DOI] [PubMed] [Google Scholar]
- 9.Antunes SA, Canziani ME. Hepcidin an important iron metabolism regulator in chronic kidney disease. J Bras Nefrol. 2016 Jul-Sep;38((3)):351–5. doi: 10.5935/0101-2800.20160053. [DOI] [PubMed] [Google Scholar]
- 10.Gaweda AE. Markers of iron status in chronic kidney disease. Hemodial Int. 2017 Jun;21((suppl 1)):S21–S27. doi: 10.1111/hdi.12556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karaboyas A, Zee J, Morgenstern H, Nolen JG, Hakim R, Kalantar-Zadeh K, et al. Understanding the recent increase in ferritin levels in United States dialysis patients potential impact of changes in intravenous iron and erythropoiesis-stimulating agent dosing. Clin J Am Soc Nephrol. 2015 Oct;10((10)):1814–21. doi: 10.2215/CJN.02600315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Evans M, Suttorp MM, Bellocco R, Hoekstra T, Qureshi AR, Dekker FW, et al. Trends in haemoglobin erythropoietin-stimulating agents and iron use in Swedish chronic kidney disease patients between 2008 and 2013. Nephrol Dial Transplant. 2016 Apr;31((4)):628–35. doi: 10.1093/ndt/gfv298. [DOI] [PubMed] [Google Scholar]
- 13.Del Vecchio L, Locatelli F. An overview on safety issues related to erythropoiesis-stimulating agents for the treatment of anaemia in patients with chronic kidney disease. Expert Opin Drug Saf. 2016 Aug;15((8)):1021–30. doi: 10.1080/14740338.2016.1182494. [DOI] [PubMed] [Google Scholar]
- 14.Macdougall IC, Bircher AJ, Eckardt KU, Obrador GT, Pollock CA, Stenvinkel P, et al. Conference Participants Iron management in chronic kidney disease conclusions from a “Kidney Disease: Improving Global Outcomes” (KDIGO) Controversies Conference. Kidney Int. 2016 Jan;89((1)):28–39. doi: 10.1016/j.kint.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 15.Hung SC, Tarng DC. ESA and iron therapy in chronic kidney disease a balance between patient safety and hemoglobin target. Kidney Int. 2014 Oct;86((4)):676–8. doi: 10.1038/ki.2014.179. [DOI] [PubMed] [Google Scholar]
- 16.Macdougall IC, White C, Anker SD, Bhandari S, Farrington K, Kalra PA, et al. Intravenous iron in patients undergoing maintenance hemodialysis. N Engl J Med. 2019 Jan;380((5)):447–58. doi: 10.1056/NEJMoa1810742. [DOI] [PubMed] [Google Scholar]
- 17.Gupta N, Wish JB. Hypoxia-inducible factor prolyl hydroxylase inhibitors A potential new treatment for anemia in patients with CKD. Am J Kidney Dis. 2017 Jun;69((6)):815–26. doi: 10.1053/j.ajkd.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 18.Yousaf F, Spinowitz B. Hypoxia-inducible factor stabilizers A new avenue for reducing BP while helping hemoglobin? Curr Hypertens Rep. 2016 Mar;18((3)):23. doi: 10.1007/s11906-016-0629-6. [DOI] [PubMed] [Google Scholar]
- 19.Kapitsinou PP, Liu Q, Unger TL, Rha J, Davidoff O, Keith B, et al. Hepatic HIF-2 regulates erythropoietic responses to hypoxia in renal anemia. Blood. 2010 Oct;116((16)):3039–48. doi: 10.1182/blood-2010-02-270322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taylor M, Qu A, Anderson ER, Matsubara T, Martin A, Gonzalez FJ, et al. Hypoxia-inducible factor-2α mediates the adaptive increase of intestinal ferroportin during iron deficiency in mice. Gastroenterology. 2011 Jun;140((7)):2044–55. doi: 10.1053/j.gastro.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu Q, Davidoff O, Niss K, Haase VH. Hypoxia-inducible factor regulates hepcidin via erythropoietin-induced erythropoiesis. J Clin Invest. 2012 Dec;122((12)):4635–44. doi: 10.1172/JCI63924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rolfs A, Kvietikova I, Gassmann M, Wenger RH. Oxygen-regulated transferrin expression is mediated by hypoxia-inducible factor-1. J Biol Chem. 1997 Aug;272((32)):20055–62. doi: 10.1074/jbc.272.32.20055. [DOI] [PubMed] [Google Scholar]
- 23.Tacchini L, Bianchi L, Bernelli-Zazzera A, Cairo G, Transferrin receptor induction by hypoxia HIF-1-mediated transcriptional activation and cell-specific post-transcriptional regulation. J Biol Chem. 1999 Aug;274((34)):24142–6. doi: 10.1074/jbc.274.34.24142. [DOI] [PubMed] [Google Scholar]
- 24.Beck H, Jeske M, Thede K, Stoll F, Flamme I, Akbaba M, et al. Discovery of molidustat (BAY 85-3934) a small-molecule oral HIF-prolyl hydroxylase (HIF-PH) inhibitor for the treatment of renal anemia. ChemMedChem. 2018 May;13((10)):988–1003. doi: 10.1002/cmdc.201700783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Flamme I, Oehme F, Ellinghaus P, Jeske M, Keldenich J, Thuss U. Mimicking hypoxia to treat anemia HIF-stabilizer BAY 85-3934 (Molidustat) stimulates erythropoietin production without hypertensive effects. PLoS One. 2014 Nov;9((11)):e111838. doi: 10.1371/journal.pone.0111838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boettcher M, Lentini S, Kaiser A, Flamme I, Kubitza D, Wensing G. First-in-man study with BAY 85-3934 − a new oral selective HIF-PH inhibitor for the treatment of renal anemia. J Am Soc Nephrol. 2013;24:347A. [Google Scholar]
- 27.Macdougall IC, Akizawa T, Berns JS, Bernhardt T, Krueger T. Effects of molidustat in the treatment of anemia in CKD. Clin J Am Soc Nephrol. 2019 Jan;14((1)):28–39. doi: 10.2215/CJN.02510218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Macdougall IC, Akizawa T, Berns J, Lentini S, Bernhardt T, Krüger T. Safety and efficay of molidustat in erythropoiesis stimulating agents (ESA) pre-treated anaemic patients with chronic kidney disease not on dialysis (CKD-ND) 53rd European Renal Association − European Dialysis and Transplant Association Congress. Vienna, Austria. 2016 [Google Scholar]
- 29.Macdougall IC, Lentini S, Schmidt A, Boettcher M, van der Mey D, Kaiser A, et al. Safety pharmacokinetics and pharmacodynamics of the oral HIF stabilizer moildustat in pre-dialysis patients with renal anemia ISN World Congress of Nephrology. Cape Town, South Africa. 2015 [Google Scholar]
- 30.Girelli D, Nemeth E, Swinkels DW. Hepcidin in the diagnosis of iron disorders. Blood. 2016 Jun;127((23)):2809–13. doi: 10.1182/blood-2015-12-639112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kimáková P, Solár P, Solárová Z, Komel R, Debeljak N. Erythropoietin and its angiogenic activity. Int J Mol Sci. 2017 Jul;18((7)):18. doi: 10.3390/ijms18071519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Elliott S, Pham E, Macdougall IC. Erythropoietins a common mechanism of action. Exp Hematol. 2008 Dec;36((12)):1573–84. doi: 10.1016/j.exphem.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 33.Li H, Ginzburg YZ. Crosstalk between iron metabolism and erythropoiesis. Adv Hematol. 2010;2010:605435. doi: 10.1155/2010/605435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eleftheriadis T, Liakopoulos V, Antoniadi G, Kartsios C, Stefanidis I. The role of hepcidin in iron homeostasis and anemia in hemodialysis patients. Semin Dial. 2009 Jan-Feb;22((1)):70–7. doi: 10.1111/j.1525-139X.2008.00532.x. [DOI] [PubMed] [Google Scholar]
- 35.Mastrogiannaki M, Matak P, Keith B, Simon MC, Vaulont S, Peyssonnaux C. HIF-2alpha but not HIF-1alpha promotes iron absorption in mice. J Clin Invest. 2009 May;119((5)):1159–66. doi: 10.1172/JCI38499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anderson ER, Xue X, Shah YM. Intestinal hypoxia-inducible factor-2alpha (HIF-2alpha) is critical for efficient erythropoiesis. J Biol Chem. 2011 Jun;286((22)):19533–40. doi: 10.1074/jbc.M111.238667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pergola PE, Spinowitz BS, Hartman CS, Maroni BJ, Haase VH. Vadadustat a novel oral HIF stabilizer provides effective anemia treatment in nondialysis-dependent chronic kidney disease. Kidney Int. 2016 Nov;90((5)):1115–22. doi: 10.1016/j.kint.2016.07.019. [DOI] [PubMed] [Google Scholar]
- 38.Provenzano R, Besarab A, Sun CH, Diamond SA, Durham JH, Cangiano JL, et al. Oral hypoxia-inducible factor prolyl hydroxylase inhibitor roxadustat (FG-4592) for the treatment of anemia in patients with CKD. Clin J Am Soc Nephrol. 2016 Jun;11((6)):982–91. doi: 10.2215/CJN.06890615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Besarab A, Provenzano R, Hertel J, Zabaneh R, Klaus SJ, Lee T, et al. Randomized placebo-controlled dose-ranging and pharmacodynamics study of roxadustat (FG-4592) to treat anemia in nondialysis-dependent chronic kidney disease (NDD-CKD) patients. Nephrol Dial Transplant. 2015 Oct;30((10)):1665–73. doi: 10.1093/ndt/gfv302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shah YM, Matsubara T, Ito S, Yim SH, Gonzalez FJ. Intestinal hypoxia-inducible transcription factors are essential for iron absorption following iron deficiency. Cell Metab. 2009 Feb;9((2)):152–64. doi: 10.1016/j.cmet.2008.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shepshelovich D, Rozen-Zvi B, Avni T, Gafter U, Gafter-Gvili A. Intravenous versus oral iron supplementation for the treatment of anemia in CKD an updated systematic review and meta-analysis. Am J Kidney Dis. 2016 Nov;68((5)):677–90. doi: 10.1053/j.ajkd.2016.04.018. [DOI] [PubMed] [Google Scholar]
- 42.Goldstein SL, Ikizler TA, Zappitelli M, Silverstein DM, Ayus JC. Non-infected hemodialysis catheters are associated with increased inflammation compared to arteriovenous fistulas. Kidney Int. 2009 Nov;76((10)):1063–9. doi: 10.1038/ki.2009.303. [DOI] [PubMed] [Google Scholar]
- 43.Kalantar-Zadeh K, Ikizler TA, Block G, Avram MM, Kopple JD. Malnutrition-inflammation complex syndrome in dialysis patients causes and consequences. Am J Kidney Dis. 2003 Nov;42((5)):864–81. doi: 10.1016/j.ajkd.2003.07.016. [DOI] [PubMed] [Google Scholar]
- 44.Provenzano R, Besarab A, Wright S, Dua S, Zeig S, Nguyen P, et al. Roxadustat (FG-4592) versus epoetin alfa for anemia in patients receiving maintenance hemodialysis a phase 2, randomized, 6- to 19-week, open-label, active-comparator, dose-ranging, safety and exploratory efficacy study. Am J Kidney Dis. 2016 Jun;67((6)):912–24. doi: 10.1053/j.ajkd.2015.12.020. [DOI] [PubMed] [Google Scholar]