Abstract
Introduction
There is an unmet need for well-tolerated antiepileptic drugs (AEDs) that effectively control focal onset seizures. This study aimed to evaluate the economic value of new AEDs in the treatment of focal onset seizure, with or without secondary generalization, in Finnish adults and adolescents with epilepsy, comparing brivaracetam with perampanel as adjunctive AEDs.
Methods
Economic value was assessed using cost-utility analysis. Periods of AED initiation, titration, response assessment (seizure freedom, ≥ 50% reduction, no response), switching in no response or treatment-emergent adverse events (TEAEs), and death were simulated using a discrete-event simulation model. Responses and switching were simulated based on a comprehensive Bayesian network meta-analysis. The primary modeled outcome was the 3%/year discounted incremental cost-effectiveness ratio (ICER). Discounted quality-adjusted life-years (QALYs), payer costs (year 2017 Euro) per patient, and net monetary benefit (NMB) were secondary outcomes. Probabilistic and comprehensive deterministic sensitivity analyses were conducted.
Results
Brivaracetam was more efficacious and had fewer TEAEs than perampanel and other AEDs. Modeled average 5-year QALYs and costs were 3.671 and €28,297 for brivaracetam and 3.611 and €27,979 for perampanel, respectively. The resulting ICER for brivaracetam versus perampanel was only €5345/QALY gained in a deterministic base case scenario. Brivaracetam had a positive NMB and high probability of cost-effectiveness of €1190 and 71% or €1944 and 80% with the assumed willingness to pay of €25,358 or €38,036/QALY gained, respectively. The primary result was robust, with a positive NMB persistent in all sensitivity analysis scenarios. When switching from brivaracetam to perampanel was excluded from the modeling or switching from perampanel to brivaracetam was included, brivaracetam was cost-saving and more effective than perampanel (dominant).
Conclusion
These simulated comparisons demonstrated that brivaracetam was more effective and potentially also more affordable than perampanel. Thus, brivaracetam is likely a cost-effective and net beneficial alternative to perampanel for treatment of focal onset seizures.
Plain Language Summary
Plain language summary available for this article.
Electronic supplementary material
The online version of this article (10.1007/s12325-019-01155-6) contains supplementary material, which is available to authorized users.
Keywords: Brivaracetam, Economic evaluation, Epilepsy, Focal onset seizure, PICOSTEPS, Perampanel
Key Summary Points
| Why carry out this study? |
| While published evidence on benefits and costs of different treatment strategies is lacking, there is a significant unmet need for well-tolerated, effective, and affordable antiepileptic drugs for focal seizure epilepsy |
| Authors examined whether adjunctive treatment brivaracetam would provide acceptable additional effectiveness for potential additional costs compared with treatment with adjunctive perampanel |
| What was learned from the study? |
| Brivaracetam had a high probability of being cost-effective and providing acceptable additional benefit for additional costs compared with perampanel |
| With earlier brivaracetam initiation resulting in more health benefits at lower costs than achieved with later brivaracetam initiation, treatment with brivaracetam also has potential to be more affordable than treatment with perampanel |
| Indirect costs, such as work absenteeism and early retirement, associated with poorly managed epilepsy have an enormous burden for epilepsy patients and society alike and should be examined and addressed in future studies |
Plain Language Summary
Published evidence on benefits and costs of different treatment strategies for focal seizure epilepsy in Finland is lacking. We examined whether using brivaracetam as an add-on antiepileptic drug (AED) would provide acceptable additional health benefits for acceptable additional costs versus treatment with perampanel, i.e., if brivaracetam was cost-effective compared with perampanel.
We simulated the progression of epilepsy over a 5-year period, including treatment pathways, subsequent treatments, and other health care utilization. In the base case analysis, we assumed that brivaracetam or perampanel was added to treatment of two AEDs at the beginning of the simulation. We conducted extensive deterministic (based on mean values) and probabilistic (based on specified distributions) sensitivity analyses to evaluate the impact of different model inputs and treatment patterns. This included adding brivaracetam or perampanel to one AED. The treatment effects were estimated as quality-adjusted life-years, denoting survival multiplied by the expected quality of life.
Our simulations indicated that brivaracetam has a high probability of being cost-effective and likely provides sufficient additional benefit for additional costs compared with perampanel. Results also indicated that brivaracetam is likely to be cheaper and more effective than perampanel if: (1) brivaracetam is used in addition to only one AED, (2) perampanel is not used after brivaracetam, or (3) brivaracetam is also used after perampanel treatment.
Introduction
Epilepsy is a symptomatic brain disorder characterized by epileptic seizures and neurobiologic, cognitive, psychologic, and social consequences [1]. The seizures are caused by abnormal excessive or synchronous neuronal activity in the brain and are classified as generalized or focal onset seizures. Focal onset seizures were previously also known as partial-onset seizures [2, 3]. In a generalized seizure, neuronal activity begins in both hemispheres, in contrast to a focal onset seizure, which originates within specific neuronal networks within one cerebral hemisphere. Secondary generalization, or focal to bilateral tonic-clonic seizure, is initially localized to one area of the brain but then disseminates to both hemispheres [4].
Prevalence of epilepsy was estimated to be approximately 5.3–6.3 cases per 1000 individuals in Europe [5] and 6.3 per 1000 individuals for active epilepsy (one or more seizures during the previous 5 years) in Finland [6]. In Finland, the incidence of treated epilepsy was estimated in 2002 to be 0.444 per 1000 males and 0.406 per 1000 females in the 16–64 year age range [7]. The incidence of epilepsy increases with age [5, 7], and epilepsy with focal onset seizures is the most prevalent form among adults [5]. In 2017, a total of 59,972 patients in Finland who had relevant reimbursement number codes received a special reimbursement for their antiepileptic drugs (AEDs) [8], resulting in an approximate prevalence of 10.9 pharmaceutically treated epilepsy patients per 1000 individuals.
Epilepsy reduces health-related quality of life (HRQoL) [9] and increases mortality [9–11], psychiatric comorbidity [12, 13], and economic burden [14–16]. The primary aim of epilepsy treatment is to minimize the number of seizures experienced by patients and to ensure that there are as few treatment-emergent adverse events (TEAEs) as possible. Sillanpää et al. [15] estimated that epilepsy costs (in Euros) were €176 million in Finland in 2004, 54.5% of which included costs from registries that were indirect costs resulting from sick leave, early retirement, and premature deaths. In Sweden, indirect costs declined between 2005 and 2011 on increasing the use of AEDs [16].
At the same time, a higher proportion (30%) of patients with polytherapy achieved seizure freedom in 2014 than in 2004 (22%), indicating that some patients with focal onset seizures benefited from newer AEDs as an adjunctive therapy in real life [17]. Lower seizure frequency is, in turn, associated with a higher HRQoL [18–20] and a decreased incidence of accidents [9, 21].
Whereas use of older AEDs (e.g., carbamazepine, phenobarbital, phenytoin, sodium valproate) is associated with poorer tolerability and leads to more drug-drug interactions with other AEDs, use of newer AEDs (e.g., brivaracetam, perampanel) can result in improved tolerability with fewer drug-drug interactions. Brivaracetam is a well-tolerated [22–28] and efficacious [25–29] “next-generation racetam” that requires no uptitration to achieve the therapeutic dose range. Furthermore, previous treatment failures with other AEDs or racetams (e.g., levetiracetam) do not preclude the use of brivaracetam [30].
The cost-effectiveness and budget impact of, for example, lacosamide in the treatment of epilepsy patients with focal onset seizures have been previously assessed in Finland [31]. However, based on a literature search of the PubMed database, no assessments have been published on the cost-effectiveness of the most recent AEDs (brivaracetam and perampanel) in Finland. Therefore, an analysis was needed to assess the economic value (i.e., modeled cost, effectiveness, and cost-effectiveness) of brivaracetam and perampanel in the treatment of patients with focal onset seizures.
Methods
The economic value of brivaracetam was assessed with cost-utility analysis using a discrete event simulation model (DESM) developed for this purpose (see Charokopou et al. [32] and Väätäinen et al. [33]). The present analysis is based on the health economic analysis submitted as part of an application of the reasonable wholesale price and reimbursement for brivaracetam in Finland (previously reported as poster presentation; Väätäinen et al. [33]). Thus, it is in line with the official cost-effectiveness analysis guideline by the Finnish Pharmaceuticals Pricing Board [34], a health technology assessment guideline by the Finnish Medicines Agency [35], recent work by the national Current Care treatment guideline working group [36], and evidence-based medicine. The present analysis applies the Patients-Intervention-Comparator-Outcome-Setting-Time-Effects-Perspective-Sensitivity analysis (PICOSTEPS [37, 38]) principle, which describes the essential components of health economic evaluation in order of importance and has been successfully applied in multiple health economic evaluation tasks [36–41].
Patients
The relevant modeled patient cohort included adult and adolescent (≥ 16 years of age) patients with epilepsy with focal onset seizures, with or without secondary generalization (focal to bilateral tonic–clonic seizures). Based on clinical practice, the modeled patients had typically used several AEDs before inclusion and used two concurrent AEDs at the model beginning; brivaracetam or perampanel was used as the third concurrent AED. Generally, the brivaracetam and perampanel target population is drug-resistant and difficult to treat.
At model baseline, patients were on average 38.5 [standard deviation (SD) 13.0] years of age, based on the average age in placebo-controlled brivaracetam trials [42], with a potential age range of 16–99 years. Patients were modeled to have on average 10.0 seizures per month based on brivaracetam trials [42] (median 9.175 seizures/28 days, i.e., 9.175/28 × 365.25/12 seizures per month). Because of lack of data, modeled SD was set to 2.0 seizures per month, based on a 20% assumption, and the minimum rate was set to 0.08 seizures per month, based on the rationale that patients had at least one seizure per year. By sex, 49.4% of the patients were male [42], and approximately 0.2% were of Han Chinese ethnicity [43]. The effects of different baseline-relevant patient characteristics were explored in the sensitivity analyses.
Regarding compliance with ethics guidelines, this article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors. In addition, because this article reports the results of a simulated cost utility analysis and not a randomized controlled or other trial directly involving human subjects, this study is not registered with any clinical trial database.
Intervention and Comparator
Although the pharmaceutical treatment of epilepsy is always individualized, preferences for the first choice AEDs generally follow the national recommendation in Finland [44]. The modeled cost-effectiveness of brivaracetam was compared with the most relevant, recent, and similarly positioned adjunctive AED that had correspondent patient population expectations and adjunctive position in care criteria in the treatment of focal onset seizures in adult patients as a third concurrent AED. This AED was perampanel, which was considered the most relevant comparator for brivaracetam because it was used in the same patient subgroup and treatment line. In addition, perampanel was the most recently reimbursed AED before brivaracetam and was accepted as a comparator to brivaracetam for the Finnish reimbursement application.
In the modeled base case comparison, patients had either brivaracetam or perampanel added as a third concomitant AED to their existing treatment of two concurrent AEDs (“base AEDs”). This was founded on observed and expected real-world use in Finnish clinical practice. Base AEDs remained identical in both comparison arms, but they could affect the use of subsequent AED alternatives. The base AED combinations by proportions were: 79% oxcarbazepine plus lacosamide, pregabalin, or zonisamide; 7% eslicarbazepine plus lacosamide, pregabalin, or zonisamide; 7% lacosamide plus pregabalin, zonisamide, or eslicarbazepine; or 7% lamotrigine plus lacosamide, pregabalin, zonisamide, or eslicarbazepine.
Brivaracetam or perampanel was added to one of these combinations. If subsequent treatment was required after failing on brivaracetam then treatment alternatives could include zonisamide, pregabalin, lamotrigine, or perampanel, depending on which AEDs were used previously. In the perampanel arm of the model, the alternatives for subsequent treatments, after failing on perampanel, included zonisamide, pregabalin, or lamotrigine.
In the base case scenario, the subsequent AEDs after failing on brivaracetam were assumed to include perampanel as a subsequent treatment alternative. This was a conservative assumption (i.e., not favoring brivaracetam), because it assumed that brivaracetam did not replace perampanel but rather delayed its use. The costs of perampanel thus influenced both intervention and comparator. The effects of different treatment sequencing (e.g., excluding perampanel use after brivaracetam, including brivaracetam after perampanel, adding brivaracetam or perampanel only in addition to one base AED, as well as different combinations of base AEDs and subsequent AEDs) were examined in the sensitivity analyses.
In the base case analyses, both brivaracetam and perampanel doses were based on average doses examined in their respective clinical trials. These corresponded closely to brivaracetam 50 mg twice daily, which was the most common brivaracetam dose used in Finnish practice, and a perampanel dose of 8 mg once daily. Brivaracetam response is dose-independent and, moreover, in Finland has the same price for all therapeutic dose formulations. In contrast, perampanel has effects that are dose-dependent and pricing that varies according to tablet strength. Thus, different perampanel doses together with different concomitant and subsequent AEDs were explored in the sensitivity analyses. Sensitivity analyses also included a scenario in which brivaracetam was compared with placebo.
Outcomes
The primary outcome of this economic evaluation was the incremental cost-effectiveness ratio (ICER), measured as the difference (Δ) in simulated costs (in Euro) divided by the difference in simulated effectiveness [measured as quality-adjusted life-years (QALYs)]. Costs were estimated based on resources used and their respective unit costs. QALY is integral and is denoted as modeled survival multiplied by expected modeled average HRQoL.
Secondary outcomes included mean total costs, mean total QALYs gained, and net monetary benefit (NMB). Because the main aim of antiepileptic treatment is to improve the HRQoL of patients [44], use of QALYs is the most appropriate measure of effectiveness.
Setting
Individual patient-based DESM with Microsoft Excel user interface and R (v. 3.2.1) statistical software engine was used to simulate the comparison and to capture all relevant data and clinically meaningful events based on clinical consultation with neurologists specializing in epilepsy (Fig. 1; see also Charokopou et al. [32], Väätäinen et al. [33]). The DESM generated a virtual cohort of 20,000 epilepsy patients with focal onset seizures, each of whom followed an individualized clinical pathway according to their time-dependent characteristics, response to each treatment (treatment history), and risk of other events.
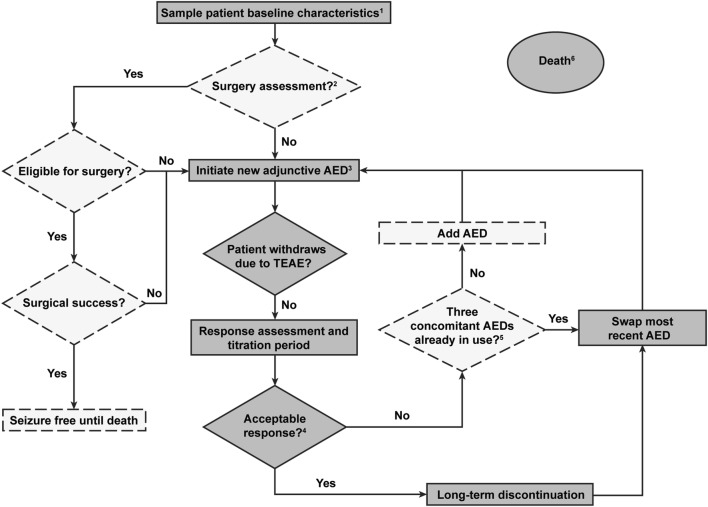
Fig. 1.
Simplified description of the discrete-event simulation model (DESM). AED antiepileptic drug, TEAE early or late onset treatment-emergent adverse event. Dashed lines denote the decisions and states excluded from the base case analysis. 1. Patient characteristics included, e.g., age, sex, seizure frequency, and ethnicity. 2. Included only in a sensitivity analysis scenario; in the base case modeling patients were assumed to have been assessed for surgery earlier based on the Finnish practice. Patients will only be assessed for the surgery once. 3. The base case modeling was initiated here, when brivaracetam or perampanel was added as a third AED on top of two base AEDs. 4. Acceptable response was seizure free or having at least a 50% reduction in seizure frequency. 5. Only relevant for a sensitivity analysis scenario. In the base case scenario, patients always had at least three concomitant AEDs. 6. Transition to death could happen at any time (absorbing, i.e., patients exit the model)
The DESM included three defined lines of monotherapy and a maximum of five defined lines of adjunctive therapy and was in line with Finnish care guidelines [44]. Modeled events included AED initiations, titration period (i.e., the AED dose was gradually increased until the patient showed optimal response), response assessment period, AED switching when there was no response, early and late TEAEs, time on AED, epilepsy surgery (only included in a sensitivity analysis scenario), and death.
In the DESM, patient’s seizure frequency influenced their monitoring, period of response assessment, and response to therapy. Ineffective treatments were not repeated in the pathway, and inappropriate/contraindicated treatment sequences and/or combinations were not used. Reasons for avoiding some combinations included contraindications (e.g., topiramate and zonisamide), no additional benefit from the combination (e.g., carbamazepine and eslicarbazepine or oxcarbazepine and eslicarbazepine and oxcarbazepine or gabapentin and pregabalin), and risk of psychologic TEAEs (e.g., levetiracetam and topiramate).
In the case of TEAEs or no acceptable response (seizure freedom or ≥ 50% reduction in seizure frequency) in the DESM, the most recently added AED was swapped for a newly selected drug from AEDs available in the next treatment line. An AED could be added when the patient had no acceptable response and had fewer than three concurrent AEDs. Discontinuation of long-term treatment because of late breakthrough seizures or loss of response was also considered. Tapering down or stopping AED treatment completely was not modeled because of rarity in this patient group and disease severity in Finland.
In the DESM, different AEDs were associated with varied titration and drug acquisition costs, which were cumulated based on modeled times on-titration and on-treatment. Monitoring costs were differentiated between time with and without seizures. Separate HRQoL was assigned depending on the patients’ treatment response and survival. Cumulative QALYs were estimated based on time spent on each of these responses and were stopped at death or at the end of the modeled time horizon. Both costs and QALYs were aggregated at the end of the modeled time horizon.
Time
A 5-year time horizon was considered sufficient to capture the relevant clinical pathway. In addition, knowledge of long-term events, effects, and discontinuations beyond 5 years is limited and more uncertain. A discount rate of 3% per year was used because the time horizon exceeded 1 year [34, 35]. The effects of time horizons and discount rates on the results were examined in the sensitivity analyses.
Effects
Although the ultimate goal of epilepsy treatment is seizure freedom [44], only 30% and 20% of all Finnish epilepsy patients with focal onset seizures and users of three concurrent AEDs achieve seizure freedom, respectively [17]. Thus, aiming for a 50% reduction in seizure frequency is clinically relevant in the patient population considered in the present analyses.
To inform the DESM, a systematic literature review (SLR) and comprehensive Bayesian network meta-analysis (BNMA) were conducted (see Borghs et al. [45], Charokopou et al. [46]) to parameterize probabilities of seizure freedom, ≥ 50% reduction in seizure frequency, and discontinuations resulting from TEAEs. The present analyses utilized a version of the BNMA used in price and reimbursed applications for Finland and the UK (Charokopou et al. [32], Väätäinen et al. [33]). Compared with the published BNMA (Borghs et al. [45], Charokopou et al. [46]), the version utilized here includes one lacosamide and one levetiracetam study less, comprising in total 63 studies instead of 65 studies in the published BNMA (see Electronic Appendix 1 for further details).
The median and mean values with corresponding 2.5–97.5 posterior distribution percentiles are reported in Table 1. The mean values and distributions were applied in the base case and in the probabilistic sensitivity analysis (PSA). Median values were used in the sensitivity analysis scenario. No interaction effects between concomitant AEDs were included because of a lack of data.
Table 1.
Efficacy and safety probabilities of AEDs based on the comprehensive BNMA of 63 trials and the different dosing schemes of perampanel based on the FEMA of five brivaracetam and five perampanel trials
| BNMA outcome | Seizure freedom (%) | ≥ 50% seizure reduction (%) | Discontinuation due to adverse event (%) | |||
|---|---|---|---|---|---|---|
| AED | Median | Mean (2.5–97.5 percentile)a | Median | Mean (2.5–97.5 percentile)a | Median | Mean (2.5–97.5 percentile)a |
| Brivaracetam | 9.38 | 12.55 (1.8–41.44) | 32.18 | 32.99 (15.58–55.25) | 7.92 | 8.59 (3.55–17.51) |
| Carbamazepine | 6.08b | 7.56 (1.4–22.05)b | 35.03b | 35.74 (17.78–57.59)b | 17.10b | 17.78 (8.57–30.73)b |
| Eslicarbazepine | 3.71 | 4.88 (0.76–15.94) | 33.14 | 33.92 (16.24–56.02) | 12.55 | 13.31 (5.97–24.99) |
| Lacosamide | 4.20 | 6.38 (0.74–24.37) | 30.86 | 31.79 (14.36–54.65) | 14.43 | 15.50 (6.59–30.38) |
| Lamotrigine | 5.00 | 6.22 (1.23–18.51) | 28.18 | 29.15 (12.68–51.11) | 10.32 | 10.95 (4.90–20.75) |
| Levetiracetam | 6.64 | 8.10 (1.65–22.99) | 44.38 | 44.58 (24.11–66.56) | 9.74 | 10.34 (4.67–19.56) |
| Oxcarbazepine | 7.89 | 10.23 (1.59–32.11) | 36.75 | 37.56 (17.54–61.53) | 21.33 | 13.31 (5.97–24.99) |
| Perampanel | 4.50 | 6.20 (0.85–21.93) | 29.45 | 30.45 (13.93–52.19) | 10.77 | 11.56 (4.71–23.08) |
| Pregabalin | 3.50 | 4.39 (0.83–13.08) | 40.96 | 41.35 (21.57–63.75) | 12.97 | 13.68 (6.54–24.58) |
| Sodium valproate | 6.08b | 7.56 (1.4–22.05)b | 35.03b | 35.74 (17.78–57.59)b | 17.10b | 17.78 (8.57–30.73)b |
| Topiramate | 7.23 | 8.99 (1.61–27.09) | 35.30 | 35.98 (16.74–59.06) | 12.72 | 13.49 (5.72–26.18) |
| Zonisamide | 1.90 | 2.56 (0.38–8.68) | 33.73 | 34.39 (16.73–56.99) | 11.61 | 12.38 (5.19–24.14) |
| FEMA outcomec | Seizure freedom (%) | > 50% seizure reduction (%) | Discontinuation because of adverse event (%) |
|---|---|---|---|
| AED | Mean | Mean | Mean |
| Brivaracetam | 10.66 | 33.36 | 8.31 |
| Perampanel 4 mg | 5.11 | 28.59 | 8.77 |
| Perampanel 6 mgd | 5.11d | 31.13d | 8.77d |
| Perampanel 8 mg | 5.11 | 33.67 | 8.77 |
| Perampanel 10 mgd | 5.76d | 33.67d | 15.29d |
| Perampanel 12 mg | 6.40 | 33.67 | 21.81 |
See Electronic Appendix 1 for more details regarding BNMA and FEMA
AED antiepileptic drug, BNMA Bayesian network meta-analysis, FEMA fixed-effect meta-analysis
aPercentiles of posterior distribution produced by BNMA
bAssumed to be equal to the average of eslicarbazepine and oxcarbazepine (lack or limitations of evidence)
cRelative effects estimated from FEMA and anchored to BNMA mean placebo rates of 1.48%, 18.56%, and 5.33% for seizure freedom, ≥ 50% reduction in seizures and discontinuation due to adverse events, respectively
dBased on the linear interpolation of effects from the neighbor doses (no trial data available)
To put the BNMA results into a practical perspective, probabilities (and associated posterior odds; see Soini et al. [47]) that brivaracetam is better than perampanel were estimated, reflecting the greater effectiveness of brivaracetam. The probabilities of brivaracetam treatment being better and its posterior odds were 82% and 4.6, respectively, for the seizure freedom, 69% and 2.2 for the > 50% reduction in seizures, and 80% and 4.0 for no TEAE status.
In contrast to brivaracetam, perampanel had different dosing schemes resulting in varying costs and effects. Thus, the dosing scheme for perampanel was varied and was tested based on a separate fixed-effects meta-analysis (FEMA) of five placebo-controlled brivaracetam and five placebo-controlled perampanel trials identified in the SLR for the comprehensive BNMA (see Electronic Appendix 1). Table 1 also reports the dose-dependent perampanel effects based on the FEMA.
Late TEAEs were not included in the simulation because of a lack of evidence, and early TEAEs not resulting in treatment discontinuation were assumed to be similar between AEDs. The time on an AED was sampled for those patients who achieved an acceptable response to the AED and continued beyond the response assessment period.
As a result of lack of drug-specific evidence, treatment persistence after the initial response evaluation was simulated using probabilities from the National Institute for Health and Care Excellence (NICE) reference case (see NICE 2011, table 8 [48]) from the UK. Consequently, long-term discontinuation because of treatment failure was modeled to decline progressively over time, from an initial 12.6% (6-monthly risk) during the 6–12-month period after treatment initiation to 2.5% during the period 54–60 months after treatment initiation.
Health-Related Quality of Life
HRQoL in the DESM was modeled to be dependent upon the achieved level of treatment response by each patient, which was based on the significant difference in HRQoL depending on treatment responses [19, 20]. HRQoL effects associated with AEDs were assumed to take place only after completion of the titration period.
The HRQoL values used were 0.869 for seizure freedom, 0.805 for ≥ 50% and 0.623 for < 50% reduction in seizures (unpublished EQ-5D-3L data from SANAD study; see Mulhern et al. [20]). Given the Finnish population-level survey data [49], these utility values were reasonable and potentially conservative: the average EQ-5D-3L scores were 0.911 and 0.868, respectively, among the Finnish general population who was 30–44 years of age and the population with neurologic disorders who was 30–44 years of age irrespective of their diagnosis, health state, or treatment [49]. The effect of using generic versus disease-specific HRQoL values was explored in sensitivity analyses by using NEWQOL-6D values (unpublished data from SANAD study; see Mulhern et al. [20]). Additionally, the effect of using an extremely high HRQoL value based on Selai et al. [19], who reported EQ-5D values higher than those observed in the general Finnish population [49], was also explored.
Mortality
Age- and disease-specific mortalities were applied based on age-specific mortality risks of the general population [50], and adjustment to the relative all-cause mortality of patients experiencing seizures was done with a standardized mortality ratio of 2.55 (95% confidence interval 2.24, 2.91) [11]. Seizure-free patients had increased mortality risk compared with the general population [10], which was implemented using an odds ratio of 1.399, based on results from Fazel et al. [10]: (focal epilepsy odds of 112/12,841)/(general population odds of 4129/660,869). The effect of assuming that there was no increased mortality for seizure-free patients was examined in a sensitivity analysis scenario.
Changes in patient’s response status or age resulted in the re-estimation of mortality risk. The risk of death at 100 years of age was fixed at 100%.
Perspective
Based on the Finnish health economic evaluation guidelines [34, 35], only direct health care costs and travel were included, and a third-party payer perspective was applied. Thus, titration and maintenance AEDs, TEAEs, treatment, visit, hospitalization, and patient co-payment costs were included. Indirect costs, such as sickness allowances, pensions, absenteeism, presenteeism, education, unemployment, household chores, taxes and other income transfers, and time costs as a consequence of epilepsy, were excluded in the base case analysis to ensure a conservative perspective for brivaracetam.
The impact of the perspective was simulated in the sensitivity analyses because of its significance in epilepsy [15] and the significant differences observed among indirect costs for other long-term diseases in Finland [51, 52]. The modeled sensitivity analysis scenarios ranged from a narrow direct health care costs (excluding travel costs) perspective to a wide societal perspective, where, for example, short-term absenteeism, presenteeism, education, unemployment, and household chores were included [51, 52]. This was in addition to traditional societal indirect costs of sick leave, early retirement, and premature death (absenteeism; [15]).
Costs
Base case analysis considered costs related to medication, treatment initiation, and switching as well as monitoring. Drug use and costs, as well as health care resource use and associated costs, are summarized in Table 2.
Table 2.
Resource use and costs
| Phase | Titration perioda | Maintenanceb | ||
|---|---|---|---|---|
| AED | Duration, scheme, and dosing | Drug costc | Daily dosage | Daily drug costc |
| Drug use patterns and associated costs | ||||
| Brivaracetam | No titration required; titration not modeled | N/A | 2 × 50 mg | €5.53 |
| Carbamazepined | Only as base-AED in the model; titration not modeled | N/A | 2 × 400 mg | €0.39 |
| Eslicarbazepinee | Total 30 days: 2 × 200 mg 15 days → 1 × 800 mg 15 days | €76.47 | 1000 mg | €7.42 |
| Lacosamidee | Total 21 days: 2 × 50 mg 7 days → 2 × 100 mg 14 days → 2 × 150 mg 7 days | €78.04 | 2 × 200 mg | €6.40 |
| Lamotrigine | Total 70 days: 25 mg 14 days → 2 × 25 mg 14 days → 2 × 50 mg 14 days → 100 + 50 mg 14 days → 2 × 100 mg 7 days → 100 + 150 mg 7 days | €48.04 | 200 + 100 mg | €1.32 |
| Levetiracetamd,e | Total 28 days: 2 × 500 mg 28 days | €37.53 | 2 × 1000 mg | €2.52 |
| Oxcarbazepine | Only as base-AED in the model; titration not modeled | N/A | 2 × 600 mg | €1.09 |
| Perampanel | Total 28 days: 2 mg 7 days → 4 mg 7 days → 2 + 4 mg 7 days → 2 × 4 mg 7 days | €222.74 | 8 mg | €5.61 |
| Pregabalin | Total 14 days: 2 × 75 mg 7 days → 2 × 150 mg 7 days | €14.22 | 2 × 225 mg | €1.06 |
| Sodium valproated,e | Total 28 days: 2 × 300 mg for 14 days → increased by 300 mg every 7 days up to 5 × 300 mg | €17.43 | 3 × 500 mg | €0.75 |
| Topiramated,e | Total 56 days: 25 mg 7 days → 50 mg 7 days → 75 mg 7 days → 100 mg 7 days → 150 mg 7 days → 200 mg 7 days → 250 mg 7 days → 300 mg 7 days | €61.34 | 200 + 150 mg | €2.62 |
| Zonisamide | Total 28 days: 2 × 25 mg 7 days → 2 × 50 mg 7 days → 2 × 100 mg 7 days → 2 × 125 mg 7 days | €101.74 | 200 + 150 mg | €4.52 |
| Resource (special care) | Annual use, seizure free | Annual use, not seizure free | Unit cost | Daily cost, seizure free | Daily cost, not seizure free |
|---|---|---|---|---|---|
| Resources and costs associated with routine monitoring by seizure freedom status (free vs. not free)f | |||||
| Inpatient | 0.01 | 0.16 | €3132 | €0.09 | €1.37 |
| A&E visit | 0.02 | 0.27 | €471 | €0.03 | €0.35 |
| Outpatient visit | 0.50 | 3.00 | €351 | €0.48 | €2.88 |
| Nurse visit | 0.50 | 2.00 | €153 | €0.21 | €0.84 |
| Nurse call | 0.00 | 4.00 | €38 | €0.00 | €0.42 |
| Traveling | 1.03 | 2.00 | €37 | €0.11 | €0.56 |
| Total | €0.91 | €6.41 | |||
| Resource (special care) | Use | Unit cost | Total cost |
|---|---|---|---|
| Resources and cost associated with start or switch of an AEDf | |||
| Outpatient | 1.00 | €471 | €471 |
| Nurse visit | 1.00 | €153 | €153 |
| Doctor phone call | 0.83 | €83 | €69 |
| Nurse phone call | 1.67 | €38 | €64 |
| Traveling | 2.00 | €37 | €75 |
| Total | €832.23 | ||
A&E Accident and Emergency, AED antiepileptic drug, d days, DDD defined daily dose, GP general practitioner (primary care), SPC summary of product characteristics, → followed by
aTitration adapted based on SPC and Fishman et al. [54]
bMaintenance dosing based on published Finnish data by Mäkinen et al. [17] as well as SPC and DDD where feasible and needed
cCalculated using cheapest doses and pack sizes. For titration, wastage was avoided by using full packages. Drug costs represent those valid as of January 2019
dAED is only included in sensitivity analysis
eAED titration is only included in the sensitivity analysis, otherwise a base AED
fAll costs other than drug purchase prices represented at 2017 level [55]
Epilepsy treatments were excluded from generics substitution in Finland. That is, patients were not offered cheaper alternatives to the prescribed epilepsy medication brand and formulation in the pharmacy, and the full reimbursement was paid for the prescribed medication, even if cheaper alternatives were available. However, a conservative approach was assumed in the simulation, and the January 2019 cost [53] of the most affordable retail drugs (excluding value-added tax) and package sizes formulated as capsules or tablets were used. Titration periods of perampanel and the subsequent AEDs (rounded to the closest full pack) used after brivaracetam or perampanel were modeled based on the summaries of product characteristics (SPC) and a study by Fishman et al. [54]. AED dosing at the maintenance phase was based on Finnish data published by Mäkinen et al. [17]. Defined daily doses (DDDs) and recommended doses as described in the SPC were used to supplement the maintenance dosing inputs, where needed.
The health care section of latest official full year Finnish Communal Expenses Index [55] was used to inflate the national Finnish health care unit costs [56] to 2017 values, which were applied to other health care costs, excluding drug costs. The transportation section of the Finnish Consumer Price Index [57] was applied to inflate the travel costs [58] to 2017 values. Resource use for monitoring and TEAE management was based on published results and Finnish practice. Because of lack of data, all AED initiations and switches that were modeled incur identical resource utilization. The effects of lower and higher cost inputs were examined in the sensitivity analyses.
In Finnish practice and from a proposal achieved by international consensus [59], patients were typically assessed for eligibility for epilepsy surgery after two AEDs had been tried. Thus, epileptic surgery was modeled only in a sensitivity analysis scenario. When costs [60] were inflated to the 2017 level [55], the cost for surgery assessment was €2111 and that for actual surgery was €18,204. It was estimated that approximately 10% of Finnish patients were assessed for surgery annually, with approximately 13% of those assessed found to be eligible and approximately 50% of the operated patients seizure free until death (cured, without AED, no drug costs assumed).
Willingness to Pay
The interpretation of primary outcome was complicated by the lack of official willingness-to-pay thresholds [61, 62], which could be used as the limits for additional cost to an additional QALY gained in Finland.
In Finland, the UK thresholds (converted to Euros) have previously been successfully applied in a cost-effectiveness analysis [38]. This approach was extended by adjusting the UK thresholds [63] for 2017 purchase power parity [64]. The most plausible willingness-to-pay threshold in non–end-of-life situations in the UK is £20,000 (€25,358 in 2017 purchasing power adjusted value), which may be plausible in some cases up to £30,000 (€38,036) per QALY gained. These thresholds could be potentially valid for focal onset seizures in Finland and were applied in this modeling study.
To transform the primary outcome to NMB, these two different willingness-to-pay thresholds (€25,358 and €38,036/QALY) were applied to:
where Δ denotes the difference between brivaracetam and perampanel. The NMB can be interpreted as cost savings that also cover health benefits with the given willingness-to-pay thresholds and enable straightforward cost-benefit analysis-type interpretation of the cost-effectiveness results (i.e., a positive result indicates cost savings).
Sensitivity Analyses
Robustness of the modeled primary outcome was evaluated using multiple simulated one- and multi-way sensitivity analysis scenarios as well as PSA. The sensitivity analysis scenarios varied model inputs regarding patient, intervention, comparator, time, effects, and perspective components using either (1) specific inputs based on alternative sources or (2) extreme changes assumed at ± 20% of the inputs used in the base case scenario.
PSA was implemented based on known or assumed (20% SD) distributions. Because of complex DESM computation, PSA was not conducted conventionally by iterating the DESM for thousands of times with a stable cohort size. Instead, PSA results were generated using the Sheffield Accelerated Value of Information (SAVI) tool [65], which assessed parameter uncertainty in individual patient models. SAVI used the output from smaller sampled cohorts by applying nonparametric regression to separate the variation attributed to parameter values from individual patient variation [66].
The cost-effectiveness plane depicted the joint distributions of modeled costs and QALYs. Acceptability frontier [67] described the PSA-based probability of cost-effectiveness for the optimal strategy as the function of willingness to pay [38].
Results
During the modeled 5-year time horizon, treatment with brivaracetam resulted in an average additional QALY gain of 0.059 (+ 1.6%) compared with perampanel, with an average additional cost of €318 (total + 1.1%, on average €64 per year) per patient (Table 3). Consequently, the resulting ICER, or the average cost of one additional QALY gained with brivaracetam in comparison with perampanel, was only €5345 per QALY gained in the base case simulation.
Table 3.
Base case results (5-year time horizon, 3% discount per year) per patient
| Treatment | Brivaracetam pathway | Perampanel pathway | Increment in |
|---|---|---|---|
| Investment | Average costs (€) | Average costs (€) | Costs (€) |
| AEDs | €17,148 | €16,151 | €997 |
| Monitoring, seizures | €10,166 | €10,788 | – €622 |
| Traveling | €983 | €1041 | – €58 |
| Sum | €28,297 | €27,979 | €318 |
| Outcome | QALYs | QALYs | QALYs |
| Brivaracetam/perampanel | 1.619 | 1.283 | 0.336 |
| First subsequent AED | 0.748 | 0.876 | – 0.128 |
| Reserve AED | 1.304 | 1.452 | – 0.148 |
| Sum | 3.671 | 3.611 | 0.059 |
| Outcome | ICER: brivaracetam vs. perampanel, €/QALY gained | €5345 | |
AED antiepileptic drug, QALY quality-adjusted life-year, ICER incremental cost-effectiveness ratio
NMB estimates for brivaracetam versus perampanel were €1190 and €1944 per patient with the assumed willingness to pay of €25,358 and €38,036 per QALY gained, respectively. These NMBs translate to 4.3% and 6.9% savings versus the total direct costs of perampanel. Consequently, from the perspective of NMB, each 25th or 16th relevant patient with focal onset seizures could be treated cost-free with brivaracetam versus perampanel.
The biggest differences in effectiveness were acquired during the first modeled AED. Drug costs accounted for approximately 60.6% and 57.7% of total modeled costs in the brivaracetam and perampanel arms, respectively (Table 3). Whereas the brivaracetam treatment pathway was associated with total higher average AED costs (mainly because of assumed potential subsequent perampanel treatment), the monitoring and travel costs were lower on average. In addition, the cumulative QALYs with brivaracetam alone were substantially higher (1.619) than with perampanel alone (1.283), but the differences were leveled because of modeled subsequent AEDs.
Deterministic Sensitivity Analyses
In the extensive sensitivity analyses the results were most sensitive to changes in the setting, modeled time horizon, and large-scale changes in HRQoL values (Table 4). However, brivaracetam remained the cost-effective option, and base case simulation results were found to be conservative (i.e., did not benefit brivaracetam). The modeled sensitivity analyses also demonstrated that brivaracetam should be used early rather than late.
Table 4.
One- and multi-way sensitivity analysis results
| Scenario | Cost (€) | QALYs | ICER (€ per QALY) | NMB (€) | ||||
|---|---|---|---|---|---|---|---|---|
| BRV | PER | Δ | BRV | PER | Δ | |||
| Base case | €28,297 | €27,979 | €318 | 3.671 | 3.611 | 0.059 | €5345 | €1190 |
| Patient | ||||||||
| Mean age 20% lower: 30.8 years | €27,850 | €27,526 | €324 | 3.614 | 3.549 | 0.066 | €4947 | €1339 |
| Mean age 20% higher: 46.2 years | €28,387 | €27,981 | €406 | 3.684 | 3.615 | 0.069 | €5850 | €1353 |
| Male proportion 20% lower: 39.5% | €28,298 | €27,955 | €343 | 3.670 | 3.611 | 0.059 | €5848 | €1145 |
| Male proportion 20% higher: 59.3% | €28,297 | €27,976 | €321 | 3.670 | 3.611 | 0.060 | €5373 | €1193 |
| Seizure frequency 20% lower: 8.0/month | €28,297 | €27,979 | €318 | 3.671 | 3.611 | 0.059 | €5347 | €1189 |
| Seizure frequency 20% higher: 12.0/month | €28,523 | €28,201 | €322 | 3.702 | 3.643 | 0.059 | €5486 | €1167 |
| Comparatora | ||||||||
| Specific perampanel dose: 4 mg daily | €28,530 | €28,027 | €504 | 3.668 | 3.621 | 0.047 | €10,632 | €697 |
| Specific perampanel dose: 6 mg daily | €28,525 | €28,110 | €415 | 3.668 | 3.618 | 0.050 | €8266 | €858 |
| Specific perampanel dose: 8 mg daily | €28,520 | €28,117 | €404 | 3.660 | 3.609 | 0.051 | €7930 | €887 |
| Specific perampanel dose: 10 mg daily | €28,481 | €28,023 | €459 | 3.660 | 3.607 | 0.053 | €8701 | €878 |
| Specific perampanel dose: 12 mg daily | €28,455 | €27,920 | €535 | 3.661 | 3.597 | 0.064 | €8344 | €1091 |
| Placebo comparison: no drug costs; efficacy and safety based on BNMA placebo rates. Means: SF: 1.48%, ≥ 50% reduction: 18.56%, discontinuation due to adverse events: 5.33%; no perampanel as subsequent AED in the brivaracetam arm | €27,550 | €27,851 | –€301 | 3.658 | 3.592 | 0.067 | BRV dominant | €1991 |
| Setting | ||||||||
| Perampanel omitted from the brivaracetam arm | €27,550 | €27,979 | − €429 | 3.658 | 3.611 | 0.047 | BRV dominant | €1627 |
| Brivaracetam added to perampanel arm subsequent treatment alternatives | €28,297 | €28,599 | − €302 | 3.671 | 3.650 | 0.020 | BRV dominant | €822 |
| Brivaracetam and perampanel added on top of only one base AED, both are used for model duration, with subsequent AEDs added. Brivaracetam and perampanel not used together | €25,157 | €26,223 | − €1067 | 3.686 | 3.640 | 0.046 | BRV dominant | €2230 |
| Brivaracetam and perampanel added on top of only one base AED, both are used for model duration, with subsequent AEDs added. Brivaracetam and perampanel may be used together | €25,464 | €26,448 | − €984 | 3.687 | 3.656 | 0.031 | BRV dominant | €1764 |
| Sodium valproate, topiramate, lacosamide, and eslicarbazepine included as additional reserve AEDs to second (last) subsequent treatment line | €28,319 | €28,063 | €256 | 3.683 | 3.634 | 0.049 | €5223 | €987 |
| Sodium valproate, topiramate, lacosamide, and eslicarbazepine included as additional AEDs to both subsequent treatment lines | €28,188 | €28,110 | €78 | 3.682 | 3.636 | 0.045 | €1724 | €1075 |
| Base AEDs and subsequent therapies based on wider variety and including concomitant use of brivaracetam with levetiracetam | €25,420 | €25,320 | €99 | 3.691 | 3.645 | 0.047 | €2130 | €1082 |
| Time | ||||||||
| Discounting not applied | €30,193 | €29,838 | €354 | 3.938 | 3.875 | 0.062 | €5675 | €1229 |
| Discounting applied with higher rate: 5% p.a. | €27,162 | €26,866 | €296 | 3.511 | 3.453 | 0.058 | €5134 | €1166 |
| Time horizon shorter: 3 years | €18,445 | €18,307 | €139 | 2.268 | 2.221 | 0.047 | €2960 | €1050 |
| Time horizon longer: 10 years | €50,677 | €49,452 | €1225 | 6.741 | 6.657 | 0.084 | €14,616 | €900 |
| Effects | ||||||||
| Epilepsy-specific NEWQOL-6D scores: 0.849 for SF, 0.805 for ≥ 50%, 0.692 for < 50% reduction | €28,297 | €27,979 | €318 | 3.679 | 3.642 | 0.037 | €8584 | €621 |
| EQ-5D scores based on Selai et al. [19]: 0.942 for SF, 0.900 for ≥ 50%, 0.829 for < 50% reduction | €28,297 | €27,979 | €318 | 4.128 | 4.103 | 0.026 | €12,317 | €336 |
| AEDs HRQoL effect starts at the AED initiation | €28,297 | €27,979 | €318 | 3.683 | 3.631 | 0.052 | €6092 | €1005 |
| Medians for the efficacy and safety parameters | €28,743 | €28,205 | €539 | 3.655 | 3.593 | 0.061 | €8783 | €1016 |
| Epileptic surgery included in the DESM | €28,730 | €28,405 | €325 | 3.682 | 3.620 | 0.062 | €5260 | €1242 |
| Seizure free patient mortality assumed to be same as in the Finnish general population: SMR = 1 | €28,326 | €27,963 | €364 | 3.676 | 3.612 | 0.064 | €5685 | €1258 |
| NSF monitoring costs 20% lower: €5.13/day | €26,546 | €26,103 | €443 | 3.671 | 3.611 | 0.059 | €7456 | €1064 |
| NSF monitoring costs 20% higher: €7.70/day | €30,048 | €29,856 | €192 | 3.671 | 3.611 | 0.059 | €3234 | €1315 |
| SF monitoring costs 20% lower: €0.73/day | €28,241 | €27,941 | €300 | 3.671 | 3.611 | 0.059 | €5050 | €1207 |
| SF monitoring costs 20% higher: €1.09/day | €28,353 | €28,018 | €335 | 3.671 | 3.611 | 0.059 | €5640 | €1172 |
| Treatment switching costs 20% lower: €665.79 | €27,874 | €27,528 | €346 | 3.671 | 3.611 | 0.059 | €5814 | €1162 |
| Treatment switching costs 20% higher: €998.68 | €28,720 | €28,431 | €290 | 3.671 | 3.611 | 0.059 | €4876 | €1218 |
| Perspective | ||||||||
| Only direct medical: Travel expenses excluded | €27,314 | €26,939 | €375 | 3.671 | 3.611 | 0.059 | €6301 | €1133 |
| Direct non-medical costs included based on Sillanpää et al. [15]: NSF and SF monitoring costs excluding traveling multiplied by 2.86b | €42,594 | €42,511 | €84 | 3.688 | 3.636 | 0.052 | €1614 | €1231 |
| Traditional societal perspective: Based on Sillanpää et al. [15]: NSF and SF monitoring costs excluding traveling multiplied by 6.29b | €71,059 | €73,316 | − €2257 | 3.671 | 3.611 | 0.059 | BRV dominant | €3764 |
| Wider societal perspective: Traditional societal cost NSF and SF monitoring costs multiplied by 4.13 [51]b | €233,258 | €245,268 | − €12,010 | 3.671 | 3.611 | 0.059 | BRV dominant | €13,517 |
AED antiepileptic drug, BNMA Bayesian network meta-analysis, BRV brivaracetam, Dominant more effective and also cost saving, HRQoL health-related quality of life, ICER incremental cost-effectiveness ratio, Δ difference, NMB net monetary benefit with willingness to pay of €25,358 per QALY (purchasing parity adjusted 2017 value corresponding to £20,000 per QALY), NSF non-seizure-free, p.a. per annum, PER perampanel, QALY quality-adjusted life-year, SF seizure free
aIn comparator sensitivity analyses varying specific perampanel doses, both brivaracetam's and perampanel's relative efficacy and safety are based on fixed-effect meta-analysis anchored to BNMA placebo rates
bApplied as relative difference, assuming the same ratio for NSF and SF monitoring costs. Ratios between total direct costs: 2.86 = 28 + 52/€28 million annually in Finland, total costs 6.29 = 28 + 52 + 96/€28 million annually in Finland [15], and between wider societal and traditional perspectives 4.13 = 1570/380 € per patient annually [51]
The modeled primary results were not sensitive to changes in baseline patient characteristics. In terms of comparator, brivaracetam was more cost-effective versus the higher perampanel dosages than versus the lower perampanel dosages. Compared with BNMA pooled average placebo, brivaracetam was more effective and less expensive (i.e., dominant).
When the setting was changed, brivaracetam was dominant if: (1) perampanel was not modeled to be used as a subsequent AED in the patients receiving brivaracetam, (2) brivaracetam was added as a subsequent AED in the patients receiving perampanel, or (3) the two were assumed to be added to one base AED and both were assumed to be used for the full duration of the DESM. While changes in other subsequent AED alternatives had only minor effects on the results, the results were generally more favorable for brivaracetam than the base case.
Brivaracetam demonstrated better cost-effectiveness with shorter simulation time horizons and worse cost-effectiveness with longer time horizons. This can result from the constant drug survival rates used for all AEDs, and from the subsequent treatments used after perampanel, which are significantly more affordable than perampanel itself. Based on the evidence and development of the AED market, the 3- and 5-year scenarios were most relevant. In addition, changing discounting rates affected the results meaningfully.
Although alternative modeled effects with epilepsy-specific NEWQOL-6D HRQoL values heavily favored the less effective perampanel, the resulting ICER was only modestly higher than in the base case. In addition, applying the utility effects from the AED initiation had only minimal effect on the base case result. However, when the unrealistic (higher than average Finnish general population [49]) HRQoL values reported by Selai et al. [19] were used in an extreme sensitivity analysis, the ICER increased. Using medians instead of means for efficacy parameters resulted in a slightly higher ICER. Inclusion of surgery resulted in slightly higher costs and health benefits but did not alter the cost-effectiveness of brivaracetam. Overall, the modeled results were not sensitive to even large changes (± 20%) in monitoring and treatment-switching costs.
Limiting the perspective of simulation by excluding travel expenses resulted in a slightly higher ICER and worse cost-effectiveness for brivaracetam. On the other hand, inclusion of expenses other than direct medical costs improved the cost-effectiveness of brivaracetam. From traditional and wider societal perspectives, including indirect costs, brivaracetam was dominant. Traditional and wider societal perspectives also resulted in significantly higher total costs for the comparators (2.5–2.6-fold and 8.2–8.8-fold compared with the base case, respectively).
Probabilistic Sensitivity Analysis
The base case PSA simulation results were well in line with the deterministic base case results and the scenario sensitivity analyses. In PSA, the brivaracetam and perampanel arms resulted in mean outcomes of €28,088 and €27,353 and of 3.682 and 3.642 QALYs, respectively. Brivaracetam was associated with an average of 0.040 (95% credible interval − 0.015 to 0.100) additional QALYs at the average additional cost of €555 (− 443 to 1470). The resulting average ICER for brivaracetam versus perampanel was €14,042/QALY gained.
Brivaracetam had 71% and 80% probability of cost-effectiveness compared with perampanel at the willingness to pay of €25,358 and €38,036 per QALY gained, respectively (Fig. 2). Brivaracetam was more effective and less costly (i.e., dominant) in 12% of base case PSA simulations.
Fig. 2.
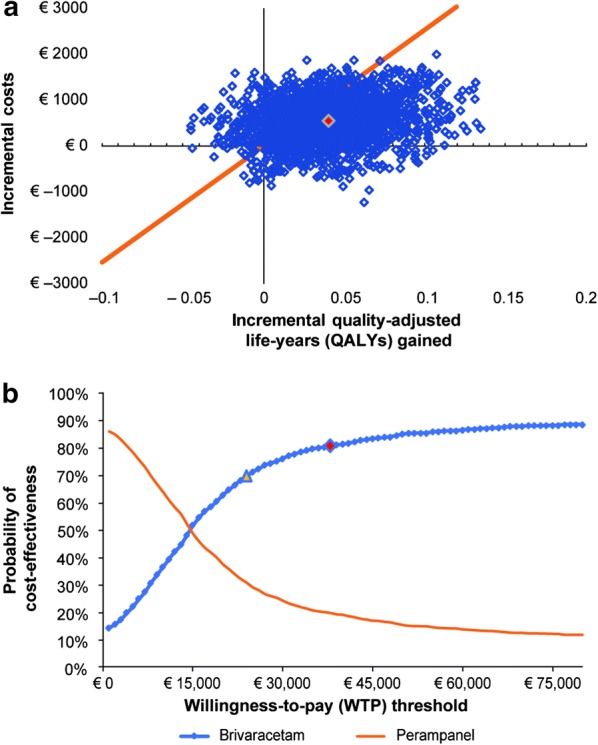
Results of the probabilistic sensitivity analysis. a Cost-effectiveness plane (CEP) and b cost-effectiveness acceptability curves (CEACs). Probabilistic sensitivity analysis results were generated using 2000 DESM iterations with cohorts of 500 patients. Marked point in CEP denotes the average results, and the line denotes the plausible willingness-to-pay threshold of €25,358 per quality-adjusted life-years (QALYs) gained. Marked points in CEAC denote the willingness to pay of €25,358 and €38,036 per QALY gained
In addition to the base case PSA, scenarios with different relevant sequences were modeled using the PSA. When the potential switch from brivaracetam to perampanel was omitted, brivaracetam had a 65% probability of dominating perampanel and 91% and 92% probability of being cost-effective with the willingness-to-pay thresholds of €25,358 and €38,046 per QALY, respectively. Mean NMB with €25,358 per QALY, cost savings, and QALYs gained were €1964, €403, and 0.062 in the modeled 5-year time horizon, respectively.
When the PSA was conducted in the setting where brivaracetam and perampanel were added on top of only one base AED, and both were used for the model duration of 5 years, brivaracetam had a 76% probability of dominating perampanel and a 90% probability of being cost-effective at both willingness-to-pay thresholds. Mean NMB, cost savings, and QALYs gained were €2192, €1114, and 0.043, respectively.
Discussion
This study simulated the cost-effectiveness of using adjunctive brivaracetam compared with perampanel in the treatment of focal onset seizures in Finland. Recently, cost savings on use of brivaracetam were demonstrated in Spain [68], in contrast to the neutral budget impact for the use of perampanel in the USA [69]; in both instances brivaracetam was compared with the current treatment practice. Here, we extended the setting to analysis of the economic value, i.e., evaluation of the modeled costs, effectiveness, and full cost-utility analysis also including NMB—comparing these two recently approved AEDs for treatment of focal onset seizures and including quality-adjusted survival measured as both QALYs and payers’ direct costs.
The expected average ICER for adjunctive brivaracetam versus adjunctive perampanel was only €5345/QALY gained in our conservative base case simulation. In the scenario analyses, where switching from brivaracetam to perampanel was excluded, or switching from perampanel to brivaracetam was included, brivaracetam demonstrated cost saving and was more effective (dominant) compared with perampanel. In a probabilistic base case scenario, NMB per patient and probability of cost-effectiveness for brivaracetam were high: €1190 and 71% or €1944 and 80% with the willingness to pay of €25,358 or €38,036/QALY gained, respectively. From the perspective of NMB, each 25th or 16th relevant epilepsy patient could be treated without any loss (“for free”) with brivaracetam versus perampanel or 4.3% or 6.9% of monetarized benefit (“savings”) could be gained. Findings are in line with the previous findings from the UK setting [32].
Based on the extensive sensitivity analyses, brivaracetam was robustly cost-effective compared with its most relevant single adjunctive AED competitor, perampanel, in the Finnish setting. When the potential switch from brivaracetam to perampanel was omitted, brivaracetam dominated perampanel in 65% of simulations and had 91% and 92% probability of being cost-effective at the willingness-to-pay thresholds. Moreover, if brivaracetam and perampanel were added on top of only one base AED, brivaracetam had 76% dominance over perampanel and was cost-effective with 90% probability.
The results of our simulation analyses were supported overwhelmingly by the clinical evidence. Brivaracetam proved its efficacy and rapid onset of therapeutic dose in the treatment of focal onset seizures [25–28] while also preserving good tolerance [22–28]. Even previous treatment failure with levetiracetam does not preclude the use of brivaracetam [30].
However, as always, modeled comparisons have assumptions or simplifications, and our study has the following five key limitations.
First, perampanel was included in the brivaracetam arm as a subsequent treatment alternative, i.e., brivaracetam did not replace perampanel but delayed its use in the base case scenario. Thus, the drug costs were largely driven by subsequent treatment with perampanel and not by brivaracetam itself. When perampanel was omitted from the brivaracetam arm, the cost-effectiveness of brivaracetam improved significantly.
Second, a maximum of three concurrent AEDs were modeled, and all other subsequent treatment alternatives were more affordable in terms of drug costs than the brivaracetam or perampanel, thus favoring perampanel in the present analyses. In more recent clinical practice, use of three or more concurrent AEDs is discouraged as much as possible, depending on the patient’s disease severity. In the sensitivity analysis scenario where brivaracetam and perampanel were added to only one base AED, brivaracetam dominated perampanel compared with ICER €5345 per QALY gained in the base case scenario where brivaracetam and perampanel were added to two base AEDs.
Third, the simulated AED costs were also based on the lowest prices and most economic package sizes; AED doses were based on published Finnish data wherever available; potential AED titration costs were incurred as a one-off cost at the start of treatment with the AED; AEDs were not tapered down, and withdrawal to monotherapy was not allowed. No interaction was modeled between the treatment effects of AEDs at adjunctive therapy, i.e., treatment effect was no different between patients receiving one or two base AEDs at baseline. AED efficacy was also unaffected by response or discontinuation of previously received AEDs. In addition, base AEDs remained unchanged during the modeled time horizon. These simplifications favored perampanel in the present comparison.
Fourth, early TEAEs that cause discontinuation during the titration and response assessment periods were simulated, and the effects of early or late TEAEs not leading to discontinuation were assumed to be negligible. When early TEAEs do not cause treatment discontinuation, the discontinuation in the long term was modeled separately using similar time-varying data for all AEDs based on the NICE (2011) guidance model [48]. In the published studies, treatment retention rates were 69.8% and 63.3% at 52 weeks after the initiation of active treatment with brivaracetam and perampanel, respectively [70]. In the current simulation study TEAEs had no impact on HRQoL. This was because TEAEs were assumed to be short-lived as the AED causing TEAEs was withdrawn. These simplifications favored perampanel in the comparison.
Fifth, drug-resistant epilepsy has a significant impact on the individuals’ everyday functioning, activities, and working capability. However, this analysis used payer perspective based on the official Finnish guidance [34, 35]. In real life, the register-based traditional indirect costs overwhelm the direct costs of epilepsy [15], which were not considered in the base case analyses. The amount and proportion of indirect costs can be even more profound than that traditionally estimated, because the indirect costs based on registers alone can significantly underestimate the total societal cost or economic burden. In a recent Finnish study that also included a wider perspective, total indirect costs of long-term diseases were four-fold those of indirect costs observed directly based on conventional national registers [51, 52]. Thus, the applied base case perspective also favored perampanel as was demonstrated by the modeled traditional and wider societal perspective sensitivity analyses. In both instances, brivaracetam dominated perampanel, and considerable changes in the expected total 5-year costs were observed (2.5–2.6-fold and 8.2–8.8-fold, respectively). The applied perspective and its potential implicit effects or biases should be considered in the interpretation of the present findings. More research is required in terms of perspectives.
Finally, more treatment options for focal onset seizures are needed. Brivaracetam has been shown to be cost-effective in the Finnish setting. In the real-world setting, brivaracetam is relatively easy to use, titration is not needed, the therapeutic dose is achieved quickly, and tolerability is good. Thus, brivaracetam is expected to be well suited to: encompassing agile and digitalized social and health care services [38, 39, 71, 72], risk-sharing [73] if needed in some settings, and the requirements of PICOSTEPS-based review [36]. In all of these, the patient is at the center; furthermore, easy applicability and follow-up of treatment are valued. Overall, the analyses with traditional register-based and considerably wider societal perspective indicate that the direct health care costs alone have limited effects and that society should be more willing to invest in larger scale studies of epilepsy-related indirect costs and losses and on how to avoid them.
Conclusions
There is a significant unmet need for new, safe, and effective epilepsy treatments. This simulated cost-utility analysis, based on clinical trial findings and payer perspective, indicated that brivaracetam is likely to be both cost-effective and net beneficial in the treatment of focal onset seizures compared with perampanel. The simulations also show that earlier treatment with brivaracetam resulted in better cost-effectiveness for brivaracetam. Brivaracetam may also provide a cost-effective alternative to treating focal onset seizures with perampanel in other countries, but studies in such settings are needed for confirmation.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank the participants of the study.
Funding
UCB Pharma Oy Finland (Espoo, Finland) funded the study and participated in the identification, design, conduct, and reporting of the study. The study sponsor also funded the journal’s Rapid Service and Open Access Fees.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authorship Contributions
The authors have contributed to the study according to the following: study: management (ES, MT), conceptualization (ES, SV, MT), design (all authors); data: acquisition (SV, JP, RK, ES, MC), interpretation (all authors); analysis: design (SV, ES), implementation (SV, ES), interpretation (all authors); manuscript: initial drafting (ES, SV), critical revision (all authors), final approval (all authors). The authors also acknowledge Michaela Fuchs, PhD, CMPP (Evidence Scientific Solutions, Horsham, UK), and Richard Fay, PhD, CMPP (Evidence Scientific Solutions, Philadelphia, PA, USA), who provided limited editorial assistance in preparing the manuscript for submission, which was funded by UCB Pharma, and Fabien Debailleul, PhD (UCB Pharma, Brussels, Belgium), for publication coordination.
Disclosures
Saku Väätäinen is an employee of ESiOR Oy, Kuopio, Finland. Erkki Soini is an employee of ESiOR Oy, Kuopio, Finland. Erkki Soini is also the founding partner and director of ESiOR. ESiOR carries out studies, statistical analysis, consultancy, education, reporting, and health economic evaluations for several pharmaceutical, food industry, diagnostics and device companies, hospitals, consultancies, academic institutions, and projects, including the producers and marketers of antiepileptic drugs. ESiOR received financial support for the study from UCB Pharma Oy Finland. Maarit Taiha is an employee of UCB Pharma. Mata Charokopou is an employee of UCB Pharma. UCB Pharma is the manufacturer and marketer of brivaracetam in Finland and other countries. Jukka Peltola has participated in clinical trials for Bial, Eisai, and UCB Pharma; received research grants from Cyberonics, Eisai, Medtronic, and UCB Pharma; received speaker honoraria from Cyberonics, Eisai, Medtronic, Orion Pharma, and UCB Pharma; received support for travel congresses from Cyberonics, Eisai, Medtronic, and UCB Pharma; and participated in advisory boards for Cyberonics, Eisai, Fenno Medical, Medtronic, Pfizer, and UCB Pharma. Reetta Kälviäinen received grants from the Academy of Finland and the Saastamoinen Foundation; speaker’s honoraria from Eisai, Orion, Sandoz, and UCB Pharma; and honoraria for the membership of advisory board from Eisai, Fenno Medical, GW Pharmaceuticals, Marinus Pharmaceuticals, Takeda, and UCB Pharma.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Data Availability
This was a simulated analysis, and no patient level data were used. Therefore, no data will be deposited in publicly available repositories or published alongside the paper as supplementary material. Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Footnotes
Enhanced Digital Features
To view enhanced digital features for this article go to 10.6084/m9.figshare.10272437.
References
- 1.Fisher RS, van Emde Boas W, Blume W, et al. Epileptic seizures and epilepsy: definitions proposed by the International League Against Epilepsy (ILAE) and the International Bureau for Epilepsy (IBE) Epilepsia. 2005;46:470–472. doi: 10.1111/j.0013-9580.2005.66104.x. [DOI] [PubMed] [Google Scholar]
- 2.Scheffer IE, Berkovic S, Capovilla G, et al. ILAE classification of the epilepsies: position paper of the ILAE Commission for Classification and Terminology. Epilepsia. 2017;58:512–521. doi: 10.1111/epi.13709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berg AT, Berkovic SF, Brodie MJ, et al. Revised terminology and concepts for organization of seizures and epilepsies: report of the ILAE Commission on Classification and Terminology, 2005–2009. Epilepsia. 2010;51:676–685. doi: 10.1111/j.1528-1167.2010.02522.x. [DOI] [PubMed] [Google Scholar]
- 4.Fisher RS, Cross JH, French JA, et al. Operational classification of seizure types by the International League Against Epilepsy: position paper of the ILAE Commission for Classification and Terminology. Epilepsia. 2017;58:522–530. doi: 10.1111/epi.13670. [DOI] [PubMed] [Google Scholar]
- 5.Forsgren L, Beghi E, Oun A, Sillanpää M. The epidemiology of epilepsy in Europe—a systematic review. Eur J Neurol. 2005;12:245–253. doi: 10.1111/j.1468-1331.2004.00992.x. [DOI] [PubMed] [Google Scholar]
- 6.Keränen T, Riekkinen PJ, Sillanpää M. Incidence and prevalence of epilepsy in adults in eastern Finland. Epilepsia. 1989;30:413–421. doi: 10.1111/j.1528-1157.1989.tb05320.x. [DOI] [PubMed] [Google Scholar]
- 7.Sillanpää M, Kälviäinen R, Klaukka T, Helenius H, Shinnar S. Temporal changes in the incidence of epilepsy in Finland: nationwide study. Epilepsy Res. 2006;71:206–215. doi: 10.1016/j.eplepsyres.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 8.Fimea and SII (Finnish Medicines Agency Fimea and Social Insurance Institution). Finnish statistics on medicine 2017. 2018.
- 9.Nevalainen O, Simola M, Ansakorpi H, et al. Epilepsy, excess deaths and years of life lost from external causes. Eur J Epidemiol. 2016;31:445–453. doi: 10.1007/s10654-015-0095-5. [DOI] [PubMed] [Google Scholar]
- 10.Fazel S, Wolf A, Langstrom N, Newton CR, Lichtenstein P. Premature mortality in epilepsy and the role of psychiatric comorbidity: a total population study. Lancet. 2013;382:1646–1654. doi: 10.1016/S0140-6736(13)60899-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neligan A, Bell GS, Johnson AL, et al. The long-term risk of premature mortality in people with epilepsy. Brain. 2011;134:388–395. doi: 10.1093/brain/awq378. [DOI] [PubMed] [Google Scholar]
- 12.Karouni M, Arulthas S, Larsson PG, et al. Psychiatric comorbidity in patients with epilepsy: a population-based study. Eur J Clin Pharmacol. 2010;66:1151–1160. doi: 10.1007/s00228-010-0861-y. [DOI] [PubMed] [Google Scholar]
- 13.Fiest KM, Dykeman J, Patten SB, et al. Depression in epilepsy: a systematic review and meta-analysis. Neurology. 2013;80:590–599. doi: 10.1212/WNL.0b013e31827b1ae0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Hout B, Gagnon D, Souetre E, et al. Relationship between seizure frequency and costs and quality of life of outpatients with partial epilepsy in France, Germany, and the United Kingdom. Epilepsia. 1997;38:1221–1226. doi: 10.1111/j.1528-1157.1997.tb01220.x. [DOI] [PubMed] [Google Scholar]
- 15.Sillanpää M, Andlin-Sobocki P, Lönnqvist J. Costs of brain disorders in Finland. Acta Neurol Scand. 2008;117:167–172. doi: 10.1111/j.1600-0404.2007.00973.x. [DOI] [PubMed] [Google Scholar]
- 16.Bolin K, Berggren F, Landtblom AM. Prevalence and cost of epilepsy in Sweden—a register-based approach. Acta Neurol Scand. 2015;131:37–44. doi: 10.1111/ane.12297. [DOI] [PubMed] [Google Scholar]
- 17.Mäkinen J, Rainesalo S, Raitanen J, Peltola J. The effect of newer antiepileptic drugs in combination therapy. Epilepsy Res. 2017;132:15–20. doi: 10.1016/j.eplepsyres.2017.02.020. [DOI] [PubMed] [Google Scholar]
- 18.Birbeck GL, Hays RD, Cui X, Vickrey BG. Seizure reduction and quality of life improvements in people with epilepsy. Epilepsia. 2002;43:535–538. doi: 10.1046/j.1528-1157.2002.32201.x. [DOI] [PubMed] [Google Scholar]
- 19.Selai CE, Trimble MR, Price MJ, Remak E. Evaluation of health status in epilepsy using the EQ-5D questionnaire: a prospective, observational, 6-month study of adjunctive therapy with anti-epileptic drugs. Curr Med Res Opin. 2005;21:733–739. doi: 10.1185/030079905X43695. [DOI] [PubMed] [Google Scholar]
- 20.Mulhern B, Pink J, Rowen D, et al. Comparing generic and condition-specific preference-based measures in epilepsy: EQ-5D-3L and NEWQOL-6D. Value Health. 2017;20:687–693. doi: 10.1016/j.jval.2016.03.1860. [DOI] [PubMed] [Google Scholar]
- 21.Selassie AW, Wilson DA, Martz GU, et al. Epilepsy beyond seizure: a population-based study of comorbidities. Epilepsy Res. 2014;108:305–315. doi: 10.1016/j.eplepsyres.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 22.Li-Na Z, Deng C, Hai-Jiao W, et al. Indirect comparison of third-generation antiepileptic drugs as adjunctive treatment for uncontrolled focal epilepsy. Epilepsy Res. 2018;139:60–72. doi: 10.1016/j.eplepsyres.2017.11.008. [DOI] [PubMed] [Google Scholar]
- 23.Zaccara G, Giovannelli F, Giorgi FS, et al. Tolerability of new antiepileptic drugs: a network meta-analysis. Eur J Clin Pharmacol. 2017;73:811–817. doi: 10.1007/s00228-017-2245-z. [DOI] [PubMed] [Google Scholar]
- 24.Zhu LN, Chen D, Chen T, et al. The adverse event profile of brivaracetam: a meta-analysis of randomized controlled trials. Seizure. 2017;45:7–16. doi: 10.1016/j.seizure.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 25.Toledo M, Whitesides J, Schiemann J, et al. Safety, tolerability, and seizure control during long-term treatment with adjunctive brivaracetam for partial-onset seizures. Epilepsia. 2016;57:1139–1151. doi: 10.1111/epi.13416. [DOI] [PubMed] [Google Scholar]
- 26.Klein P, Schiemann J, Sperling MR, et al. A randomized, double-blind, placebo-controlled, multicenter, parallel-group study to evaluate the efficacy and safety of adjunctive brivaracetam in adult patients with uncontrolled partial-onset seizures. Epilepsia. 2015;56:1890–1898. doi: 10.1111/epi.13212. [DOI] [PubMed] [Google Scholar]
- 27.Ryvlin P, Werhahn KJ, Blaszczyk B, Johnson ME, Lu S. Adjunctive brivaracetam in adults with uncontrolled focal epilepsy: results from a double-blind, randomized, placebo-controlled trial. Epilepsia. 2014;55:47–56. doi: 10.1111/epi.12432. [DOI] [PubMed] [Google Scholar]
- 28.Biton V, Berkovic SF, Abou-Khalil B, et al. Brivaracetam as adjunctive treatment for uncontrolled partial epilepsy in adults: a phase III randomized, double-blind, placebo-controlled trial. Epilepsia. 2014;55:57–66. doi: 10.1111/epi.12433. [DOI] [PubMed] [Google Scholar]
- 29.Karlov VA, Vlasov PN, Zhidkova IA, et al. Brivaracetam in the treatment of patients with epilepsy. Zh Nevrol Psikhiatr Im S S Korsakova. 2017;117:55–62. doi: 10.17116/jnevro20171179255-62. [DOI] [PubMed] [Google Scholar]
- 30.Asadi-Pooya AA, Sperling MR, Chung S, et al. Efficacy and tolerability of adjunctive brivaracetam in patients with prior antiepileptic drug exposure: a post hoc study. Epilepsy Res. 2017;131:70–75. doi: 10.1016/j.eplepsyres.2017.02.007. [DOI] [PubMed] [Google Scholar]
- 31.Soini E, Martikainen J, Vanoli A. Cost-effectiveness and budget impact modelling of lacosamide in the treatment of partial-onset seizures in Finland. Value Health. 2009;12:A367. [Google Scholar]
- 32.Charokopou M, Sander J, Saha R, et al. Cost-effectiveness of brivaracetam versus other antiepileptic drugs used as adjunctive treatment for focal seizures in the UK: a discrete event simulation model. Value Health. 2016;19:A365. [Google Scholar]
- 33.Väätäinen S, Soini E, Peltola J, et al. Cost-effectiveness of brivaracetam as adjunctive therapy for partial-onset epilepsy in the Finnish setting. Value Health. 2017;20:A722. [Google Scholar]
- 34.Finnish Pharmaceutical Pricing Board. Preparing a health economic evaluation to be attached to the application for reimbursement status and wholesale price for a medicinal product. Application instructions TTS 1.11.2018.
- 35.Fimea (Finnish Medicines Agency). Fimea kehittää, arvioi ja informoi-julkaisusarja 2/2012. Fimean suositus lääkkeiden hoidollisen ja taloudellisen arvon arvioinnista (pdf). 2., korjattu painos. Lääkealan turvallisuus-ja kehittämiskeskus Fimea 2014. 2014.
- 36.Soini E. Biologisten lääkkeiden kustannusvaikuttavuus nivelpsoriaasin hoidossa. Cost-effectiveness of biologic treatments in joint psoriasis. 2017. http://www.kaypahoito.fi/web/kh/suositukset/suositus?id=nix02465&suositusid=hoi50062. Accessed Jan 17, 2019.
- 37.Soini E, Joutseno J, Sumelahti ML. Cost-utility of first-line disease-modifying treatments for relapsing-remitting multiple sclerosis. Clin Ther. 2017;39(537–57):e10. doi: 10.1016/j.clinthera.2017.01.028. [DOI] [PubMed] [Google Scholar]
- 38.Soini E, Riekkinen O, Kröger H, et al. Cost-effectiveness of pulse-echo ultrasonometry in osteoporosis management. Clinicoecon Outcomes Res. 2018;10:279–292. doi: 10.2147/CEOR.S163237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Väätäinen S, Soini E, Arvonen S. Digitalization and customer-responsive secondary care services potentially free health care capacity: predicted monetary benefits of Virtual Hospital 2.0. Value Health. 2018;21:S151. [Google Scholar]
- 40.Soini E, Hallinen T, Laine J. Health impact modelling (HIM): concept, approach and real-world data needs for the estimation of potential effectiveness provided by a pharma company portfolio. Value Health. 2018;21:S88. [Google Scholar]
- 41.Mankinen P, Vihervaara V, Torvinen S, Martikainen J, Soini E. Costs of administration, travelling, and productivity losses associated with hospital administration of multiple myeloma drugs in Finland. J Med Econ. 2019;22:328–335. doi: 10.1080/13696998.2019.1569457. [DOI] [PubMed] [Google Scholar]
- 42.Ben-Menachem E, Mameniskiene R, Quarato PP, et al. Efficacy and safety of brivaracetam for partial-onset seizures in 3 pooled clinical studies. Neurology. 2016;87:314–323. doi: 10.1212/WNL.0000000000002864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Official Statistics of Finland. Population structure. 2019. http://www.stat.fi/til/vaerak/index_en.html. Accessed Jan 17, 2019.
- 44.The Finnish Medical Society Duodecim. Working group set up by the Finnish Medical Society Duodecim and the Finnish Neurological Association. 2014. http://www.kaypahoito.fi. Accessed Jan 17, 2019.
- 45.Borghs S, Harvey RC, Srivastava K, Townsend R, Charokopou M. Do accepted techniques for indirect comparative effectiveness reflect the need for individualized patient care in heterogeneous populations? The case of brivaracetam versus adjunctive antiepileptic medications for the treatment of adult patients with focal seizures. Value Health. 2016;19:A71. [Google Scholar]
- 46.Charokopou M, Harvey R, Srivastava K, Brandt C, Borghs S. Relative performance of brivaracetam as adjunctive treatment of focal seizures in adults: a network meta-analysis. Curr Med Res Opin. 2019;35:1345–1354. doi: 10.1080/03007995.2019.1584501. [DOI] [PubMed] [Google Scholar]
- 47.Soini E, Rissanen T, Tiihonen J, et al. Predicting forensic admission among the mentally ill in a multinational setting: a Bayesian modelling approach. Data Knowl Eng. 2009;68:1427–1440. [Google Scholar]
- 48.National Institute for Health and Care Excellence. Appendix P: economic evaluation of AEDs used as monotherapy in the treatment of adults with newly diagnosed focal seizures. 2011. http://www.nice.org.uk/guidance/CG137/documents/appendices-p-s-health-economics2. Accessed July 26, 2018.
- 49.Saarni SI, Suvisaari J, Sintonen H, et al. The health-related quality-of-life impact of chronic conditions varied with age in general population. J Clin Epidemiol. 2007;60:1288–1297. doi: 10.1016/j.jclinepi.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 50.Official Statistics of Finland. Deaths. 2019. http://www.stat.fi/til/kuol/index_en.html. Accessed Jan 17, 2019.
- 51.ESiOR. Tekemätöntä työtä, näkymättömiä kustannuksia Undone work, invisible costs. Raportti Heinäkuu. 2017. https://www.researchgate.net/publication/318420433_TekematontEa_tyota_nakymattomia_kustannuksia_-_Selvitys_tulehduksellisia_suolistosairauksia_ja_reumasairauksia_sairastavien_tyo-_ja_toimintakyvysta_seka_niiden_menetyksesta_aiheutuvista_kustannuksista. Accessed Jan 17, 2019.
- 52.Soini E. Productivity losses. In: 2nd Nordic conference on Real World Data, Collaboration between Pharma industry and Academia, September 27, 2017.
- 53.Anon. Finnish Medicines Tariff 01/2019. 2019.
- 54.Fishman J, Kalilani L, Song Y, Swallow E, Wild I. Antiepileptic drug titration and related health care resource use and costs. J Manag Care Spec Pharm. 2018;24:929–938. doi: 10.18553/jmcp.2018.17337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Official Statistics of Finland. Price index of public expenditure. 2019. http://www.stat.fi/til/jmhi/index_en.html. Accessed Jan 17, 2019.
- 56.Kapiainen S, Väisänen A, Haula T. Terveyden-ja sosiaalihuollon yksikkökustannukset Suomessa vuonna 2011 [Health and Social Care Costs in Year 2011 in Finland] Helsinki: Terveyden ja hyvinvoinnin laitos; 2014. [Google Scholar]
- 57.Official Statistics of Finland. Consumer price index. 2019. http://www.stat.fi/til/khi/index_en.html. Accessed Jan 17, 2019.
- 58.Hujanen T, Kapiainen S, Tuominen U, Pekurinen M. Terveydenhuollon yksikkökustannukset Suomessa vuonna 2006 [Health Care Unit Costs in Year 2006 in Finland] Helsinki: Stakesin Työpapereita; 2008. [Google Scholar]
- 59.Kwan P, Arzimanoglou A, Berg AT, et al. Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia. 2010;51:1069–1077. doi: 10.1111/j.1528-1167.2009.02397.x. [DOI] [PubMed] [Google Scholar]
- 60.Snellman E, Pekurinen M. Erityisen kalliit ja vaativat hoidot. Erityisen kalliin hoidon,vaativan erityistason keskitettävän hoidon ja TEO:n kriminaalipotilaiksi määrittämien hoidon kustannukset vuonna 2004. Sosiaali-ja terveysministeriön selvityksiä. 2005. https://www.julkari.fi/handle/10024/104142. Accessed Jan 17, 2019.
- 61.Soini E, Kukkonen J, Myllykangas M, Ryynänen OP. Contingent valuation of eight new treatments: what is the clinician’s and politician’s willingness to pay? Open Complement Med J. 2012;4:1–11. [Google Scholar]
- 62.Soini EJ, Hallinen T, Sokka AL, Saarinen K. Cost-utility of first-line actinic keratosis treatments in Finland. Adv Ther. 2015;32:455–476. doi: 10.1007/s12325-015-0211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.National Institute for Health and Care Excellence. Guide to the methods of technology appraisal 2013. 2013. [PubMed]
- 64.OECD. Purchasing power parities (PPP) (indicator). 2019. 10.1787/1290ee5a-en. Accessed Jan 17, 2019.
- 65.The University of Sheffield. SAVI—Sheffield Accelerated Value of Information. Release version 2.1.2. 2018. http://savi.shef.ac.uk/SAVI/. Accessed Jan 31, 2019.
- 66.Strong M, Oakley JE, Brennan A. Estimating multiparameter partial expected value of perfect information from a probabilistic sensitivity analysis sample: a nonparametric regression approach. Med Decis Mak. 2014;34:311–326. doi: 10.1177/0272989X13505910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Barton GR, Briggs AH, Fenwick EA. Optimal cost-effectiveness decisions: the role of the cost-effectiveness acceptability curve (CEAC), the cost-effectiveness acceptability frontier (CEAF), and the expected value of perfection information (EVPI) Value Health. 2008;11:886–897. doi: 10.1111/j.1524-4733.2008.00358.x. [DOI] [PubMed] [Google Scholar]
- 68.Barrachina-Martínez I, Vivas-Consuelo D, Piera-Balbastre A. Budget impact analysis of brivaracetam adjunctive therapy for partial-onset epileptic seizures in Valencia community, Spain. Clin Drug Investig. 2018;38:353–363. doi: 10.1007/s40261-017-0615-z. [DOI] [PubMed] [Google Scholar]
- 69.Tremblay G, Barghout V, Patel V, Tsong W, Wang Z. Budget impact of perampanel as adjunctive treatment of uncontrolled partial-onset and primary generalized tonic-clonic seizures in the United States. Epilepsy Behav. 2017;68:196–202. doi: 10.1016/j.yebeh.2016.12.029. [DOI] [PubMed] [Google Scholar]
- 70.Toledo M, Beale R, Evans JS, et al. Long-term retention rates for antiepileptic drugs: a review of long-term extension studies and comparison with brivaracetam. Epilepsy Res. 2017;138:53–61. doi: 10.1016/j.eplepsyres.2017.10.008. [DOI] [PubMed] [Google Scholar]
- 71.Prime Minister’s Office. Finland, a land of solutions. Mid-term review. Government Action Plan 2017–2019. 2017.
- 72.Väätäinen S, Soini E, Arvonen S. Virtual hospital 2.0—modelled cost-benefit assessment: towards potential economic efficiency with digitalization and customer responsive services. The 23rd Finnish National Conference on Telemedicine and eHealth; 2018; Helsinki, Finland and Stockholm, Sweden: Finnish Society of Telemedicine and eHealth. 2018. https://www.telemedicine.fi/images/pdf/julkaisut/978-952-68112-8-4.pdf. Accessed Jan 31, 2019.
- 73.Soini E, Asseburg C, Taiha M, et al. Modeled health economic impact of a hypothetical certolizumab pegol risk-sharing scheme for patients with moderate-to-severe rheumatoid arthritis in Finland. Adv Ther. 2017;34:2316–2332. doi: 10.1007/s12325-017-0614-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This was a simulated analysis, and no patient level data were used. Therefore, no data will be deposited in publicly available repositories or published alongside the paper as supplementary material. Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.