Abstract
Introduction
Evidence has demonstrated greater benefit of intra-articular hyaluronic acid (IA-HA) within earlier stages of knee osteoarthritis (OA) rather than waiting for patients to have progressed to later stages of disease progression. High molecular weight (HMW) HA has also been shown to be more effective than low molecular weight (LMW) HA products in mild to moderate knee OA, providing an important distinction to make within the class of IA-HA therapies. The purpose of this study is to evaluate the cost-effectiveness of treating patients with knee OA with HMW HA compared to LMW and conservative treatment, while taking into account disease stage.
Methods
Decision analytic models were created for early/moderate, as well as late stage knee OA. Models for late stage knee OA were created by assuming a range of response rates to IA-HA treatments from 10% to 50%. These models included conservative treatment using physical therapy/exercise, braces/orthosis, and medications such as non-steroidal anti-inflammatory drugs (NSAIDs) and analgesics. The models compared the cost per quality adjusted life year (QALY) gained for these treatments to the use of either LMW or HMW HA. Incremental cost-effectiveness ratios (ICERs) were calculated for each treatment in relation to HMW HA.
Results
When evaluating treatment in early to moderate knee OA, HMW HA was dominant over LMW HA and physical therapy/exercise, as it was less expensive and provided greater benefit. HMW HA was cost-effective versus braces/orthosis and NSAID/analgesic medications based on a willingness to pay threshold of $50,000. In the model of 50% response rate to IA-HA for late stage OA, HMW HA remained cost-effective in comparison to physical therapy/exercise and braces/orthosis at a willingness to pay threshold of $50,000; but not NSAID/analgesic medications. In the worst-case scenario of a 10% responder rate to IA-HA, HMW HA was no longer cost-effective in any circumstance.
Conclusion
IA-HA, particularly HMW formulations, demonstrate cost-effectiveness when compared to conservative treatment options and LMW HA in patients with early/mid stage knee OA. The cost-effectiveness of HMW HA in patients with later stage knee OA was not as apparent, particularly because of the uncertainty in the proportion of patients with late stage OA who have a meaningful improvement after receiving IA-HA. This cost-effectiveness finding supports the use of IA-HA in patients with early and moderate knee OA, as the benefits of IA-HA are apparent within the patient population with mild to moderate knee OA. The findings of this study suggest that there is a potential cost savings benefit as a result of utilizing HMW HA in earlier stages of knee OA as opposed to later stages.
Funding
Ferring Pharmaceuticals Inc.
Electronic Supplementary Material
The online version of this article (10.1007/s12325-019-01142-x) contains supplementary material, which is available to authorized users.
Keywords: Cost analysis, Cost-effectiveness, Hyaluronic acid, Knee, Osteoarthritis, Rheumatology
Key Summary Points
| The purpose of this study is to evaluate the cost-effectiveness of treating patients with knee osteoarthritis (OA) with high molecular weight hyaluronic acid (HMW HA) compared to low molecular weight (LMW) and conservative treatment. |
| Decision analytic models were created to conduct a cost-effectiveness analysis for early/moderate, as well as late stage knee OA. |
| High molecular weight intra-articular injections with hyaluronic acid (HMW IA-HA) formulations demonstrate cost-effectiveness when compared to conservative treatment options and low molecular weight hyaluronic acid (LMW HA) in patients with early/mid stage knee OA. |
| The cost-effectiveness of HMW HA in patients with later stage knee OA was not as apparent, particularly because of the uncertainty in the proportion of patients with late stage OA who have a meaningful improvement after receiving intra-articular injections (IA-HA). |
| This cost-effectiveness finding supports the use of IA-HA in patients with early and moderate knee OA, as the benefits of IA-HA are apparent within the patient population with mild to moderate knee OA. |
Introduction
Osteoarthritis (OA) is a progressive degenerative chronic disease characterized by the degeneration of cartilage in joints, resulting in friction between bones that may cause stiffness, pain, and reduced range of motion [1]. Osteoarthritis is the most common cause of disability in older adults worldwide [2]. While it frequently affects the knees, hands, and hips, knee OA accounts for more than 80% of the total disease burden in developed nations [3]. In the USA, it is estimated that 27 million adults currently have diagnosed OA, and 9 million to 14 million adults have mild, moderate, or severe knee OA [4]. Knee OA has doubled in prevalence in the USA since the mid-twentieth century, now reaching prevalence estimates ranging from 13.8% in younger age groups to 37.4% in persons 60 years of age and older [5].
There have been many studies that illustrate the extent of socioeconomic burden in USA due to knee OA [4, 6]. There is an increasing awareness of the importance of identifying early phases of the degenerative processes in knee OA, as treatment options may provide greater benefit when employed earlier in the disease progression [7, 8]. Guideline-concordant care for knee OA varies on the basis of disease severity, patient preferences, and clinician experience [8–10]. Non-operative and non-pharmacological management of knee OA often includes education and self-management, weight loss and strengthening, and biomechanical interventions such as knee braces [11–13]. Anti-inflammatories are recommended for patients with symptomatic OA of the knee to address pain; however, prolonged use of NSAIDS increases risk of gastrointestinal (GI), cardiovascular, and renal complications [14, 15].
Studies have shown that intra-articular injections with hyaluronic acid (IA-HA) are effective in decreasing pain associated with knee OA [16]. A growing body of literature suggests that high molecular weight (HMW) HA (3000 kDa or higher) has a greater clinical benefit than its low molecular weight (LMW) (< 3000 kDa) counterpart, providing evidence of differential benefit between IA-HA treatments [17]. Evidence has also highlighted that HMW HA is of greater benefit to those with early stage OA compared to patients with later stage OA, suggesting that burden of illness and cost-effectiveness analyses of IA-HA treatments should take into account knee OA staging [18, 19]. It is important to distinguish the difference between molecular weight of IA-HA, and cross-linking. While molecular weight is a measure of the chain length of HA, cross-linking is a process in which HA chains are altered. The purpose of this study is to evaluate the cost-effectiveness of treating patients with knee OA with HMW HA compared to LMW and conservative treatment, while taking into account disease stage.
Methods
Data Sources
This study will be conducted from the payer’s perspective. Cost data, complication rates, and utility scores of patients were extracted from previously published literature. If information could not be retrieved for a specific parameter, plausible assumptions based on expert opinion or non-peer-reviewed literature are provided. Data was collected from previously conducted cost analyses and systematic reviews, with an attempt to identify results that differentiated between early/moderate or late stages of knee OA for IA-HA treatment. Particularly, the percentage of responders to IA-HA was different between early and late stage knee OA models to demonstrate the potential differences in cost-effectiveness of IA-HA based on the patients’ disease severity. A plausible range of possible responder rates from 10% to 50% was used for patients with late stage OA receiving IA-HA, as relevant literature has suggested that clinicians see reasonable success of IA-HA in 39% of patients with late stage knee OA [8]. A complete summary of the data sources utilized for analysis is provided in Supplement 1. This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Decision Tree Model
Decision analysis models were created using TreeAge Pro 2011® software to compare patients with knee OA treated with IA-HA with conservative treatment options. This comprehensive model simulates possible disease states of patients with knee OA throughout a course of treatment of 6 months with IA-HA and conservative treatment options. The standard of conservative treatments included were physical therapy and exercise, braces and orthoses, and NSAIDs. HMW HA was modeled using data pertaining to the product Euflexxa (Ferring Pharmaceuticals, Parsippany, NJ), which utilizes three injections per treatment course. LMW HA was modeled to include the requirement of five injections per treatment course. A search of previous knee OA cost analysis literature was conducted to identify references for model parameters.
Model Parameters
The cost-effectiveness of IA-HA treatment was assessed by comparing total costs (in US dollars) and utility outcomes associated with each treatment option with complication rates taken into account. All results are presented by disease severity to provide information regarding the cost-effectiveness of treatment with IA-HA in cases of early/moderate knee OA compared to IA-HA treatment of later stage knee OA. IA-HA treatments were considered to have the possibility of acute local skin reactions and serious local adverse event (AE) events such as synovitis, and sepsis. Braces/orthosis and physical therapy/exercise were both considered to have potential minor AEs, while NSAID risks associated with gastrointestinal and cardiovascular adverse events were also included in the model.
Outcomes
The outcomes examined include total costs and changes in quality adjusted life years (QALY) associated with each of the treatment options. QALY was determined by the amount of health-state utility gained multiplied by the life year time frame of 6 months (0.5 years). Cost per QALY gained for each treatment option was then used to calculate the incremental cost-effectiveness ratio (ICER) value between each of the evaluated treatment options. An ICER value threshold of $50,000 was utilized to consider cost-effectiveness of the treatments [20]. One-way sensitivity analysis on costs of physical therapy and exercise, braces and orthoses, NSAID/analgesics, and LMW HA was also conducted by adjusting by ± 10% to determine the robustness of the results of the comparisons to HMW HA. The low and high cost estimates for these treatments were used to calculate the low and high range of ICER values based on the range in cost.
Results
Early/Moderate Knee OA
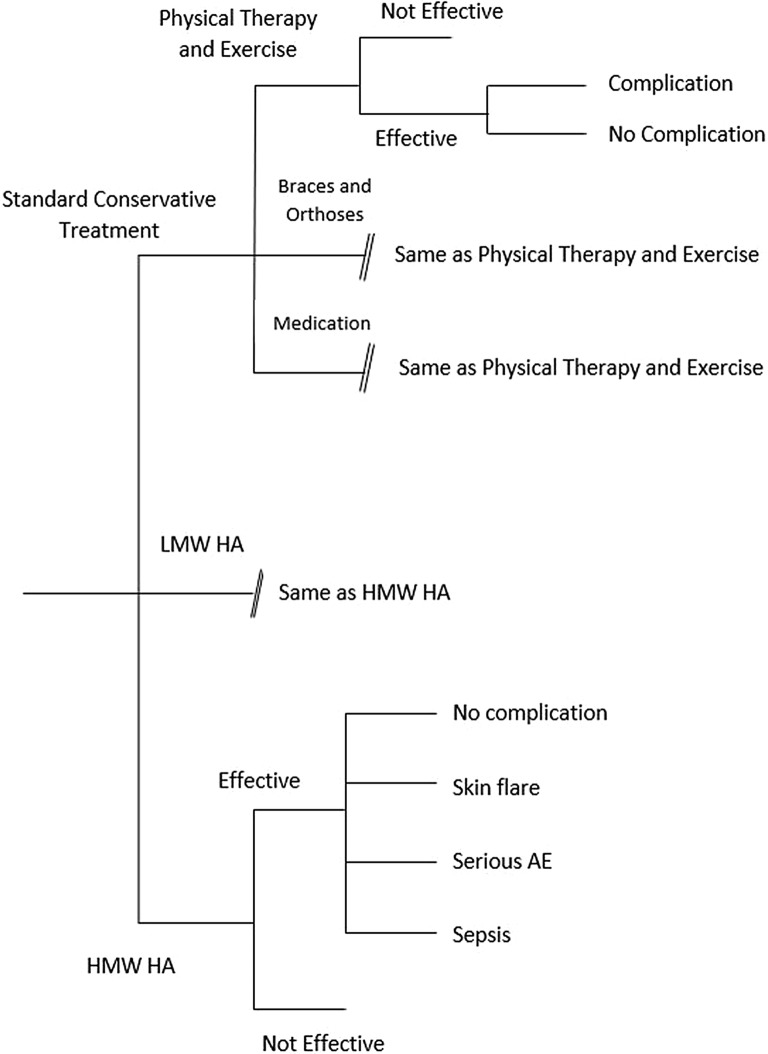
The detailed tree diagram of the model for early/moderate knee OA is provided in Fig. 1. The average cost associated with conservative treatment options over 6 months was $423. Average costs associated with physical therapy and exercise, braces and orthoses, and medication including NSAIDS over 6 months were $901, $200, and $338 respectively. The average cost associated with IA-HA treatment for patients with early and moderate knee OA over 6 months was $608 for HMW HA and $693 for LMW HA. The change in QALY among patients with knee OA using conservative treatment was 0.028 life years. The changes in QALY for patients using physical therapy and exercise, braces and orthoses, and medication were 0.044, 0.001 and 0.032 QALY, respectively. In patients with early/moderate knee OA using HMW IA-HA treatment, the change in QALY over 6 months was 0.058 life years. The change in QALY for LMW HA was 0.029 QALY.
Fig. 1.
Tree diagram for early/mid knee OA
The cost per QALY gained and ICER for each treatment relative to HMW HA are provided in Table 1. These results demonstrate that HMW HA is favorable from a cost-effectiveness perspective over each of the other treatment options for patients with early/moderate knee OA. HMW HA was dominant over LMW HA and physical therapy/exercise, as it was less expensive and provided greater benefit. HMW HA was cost-effective versus braces/orthosis and NSAID/analgesic medications based on a willingness to pay threshold of $50,000. In the sensitivity analysis, it was shown that the high and low estimates of costs for each of the treatment options provided similar conclusions as the base case analysis (Table 1).
Table 1.
Cost-effectiveness of included treatments for early/moderate knee OA
| Treatment | Cost/QALY gained | Base case ICER (versus HMW HA) | Sensitivity | |
|---|---|---|---|---|
| Low cost ICER (versus HMW HA) | High cost ICER (versus HMW HA) | |||
| HMW HA | $10,482.76 | – | – | – |
| LMW HA | $23,896.55 | Dominated | Dominated | Dominated |
| Physical therapy and exercise | $20,477.27 | Dominated | Dominated | Dominated |
| Braces and orthosis | $200,000.00 | $7157.89 | $7508.77 | $6807.02 |
| NSAID/analgesic medication | $10,562.50 | $10,384.62 | $11,684.62 | $9084.62 |
Dominated: HMW HA was both cheaper and more effective
Late Knee OA
The model for late stage knee OA assumed a significant reduction in patient responder rates for IA-HA. The two models ranged from a 50% to 10% responder rate of the IA-HA treatments within late stage knee OA (Supplement 2). Table 2 provides a summary of cost per QALY for late stage knee OA for models of both a low and high estimate of responder rates to IA-HA. When the modeled cost-effectiveness in late stage patients was assessed, HMW HA remained dominant over LMW HA; however, the cost-effectiveness of HMW HA changes in comparison to the results of early/moderate stage knee OA. In the model of 50% response rate to IA-HA, it remains cost-effective in comparison to physical therapy/exercise and braces/orthosis at a willingness to pay threshold of $50,000. The ICER of NSAID/analgesic medications versus HMW HA is $67,000 when response to HMW HA is considered to be 50%, slightly surpassing the willingness to pay threshold of $50,000. In the worst-case scenario of a 10% responder rate to IA-HA, HMW HA was no longer cost-effective in any circumstances.
Table 2.
Cost-effectiveness in late stage knee OA
| Treatment | ICER: 50% responder rate (versus HMW HA) | ICER: 10% responder rate (versus HMW HA) |
|---|---|---|
| HMW HA | – | – |
| LMW HA | Dominated | Dominated |
| Physical therapy and exercise | $36,875 | $8027.03a |
| Braces and orthosis | $11,600 | $67,333.33 |
| NSAID/analgesic medication | $67,000 | Dominating |
Dominated: HMW HA was both cheaper and more effective
Dominating: Treatment was both cheaper and more effective than HMW HA
aIncremental cost of HMW HA and incremental effect were both negative, signifying that HMW HA is not cost-effective unless this ICER is greater than the willingness to pay threshold
Discussion
This analysis demonstrated that HMW HA is cost-effective in early/moderate knee OA when compared to LMW HA and conservative treatment options. The cost-effectiveness of HMW HA becomes less apparent in late stage knee OA, as the results were affected by a reduction in response to IA-HA treatment in these particular patients. Current literature has demonstrated that IA-HA is more effective in the earlier stages of knee OA, as opposed to being employed as a later stage treatment [18, 19]. The findings of this study suggest that this benefit is extended to the cost savings that would be a result of utilizing HMW HA in earlier stages of knee OA as opposed to later stages. Additionally, this study demonstrates the benefits that HMW HA has over LMW HA counterparts, a finding that is consistent with previous OA literature [17]. The models developed provide similar conclusions regarding the cost-effectiveness of IA-HA treatment, specifically HMW HA, while providing greater insight into the potential implications of delaying HMW HA use in patients with knee OA [20, 21]. The clinical implications for this research suggest that use of HMW HA products specifically, in earlier stages of knee OA, provide cost-effective benefits to the healthcare system that would not be apparent if the use of HMW HA is delayed until patients have progressed to a later stage in the disease.
This study demonstrated strength in its comprehensive modeling of multiple conservative treatment options compared to LMW and HMW HA. This model has utilized data from many previous cost analyses to ensure that a robust model was used to assess the cost-effectiveness of these treatment options. This study also has limitations that must be considered when interpreting the results of this study. It is important to note that the range of potential responder rates of patients with late stage knee OA receiving IA-HA was an assumed range based on the evidence of reduced effectiveness in this population. While an exact responder rate in later stages of knee OA is uncertain, this range of plausible values aided in illustrating the impact that reduced response to IA-HA would have on the cost-effectiveness of IA-HA treatments. The use of these responder rates is strengthened by the range used, as a 10% response rate to IA-HA may be considered as a very low estimate in order to provide a very conservative worst-case scenario. This wide range of possible benefit was made because of the indirect nature of the 39% responder estimate derived from clinicians’ perceptions literature, as opposed to empirical evaluation of treatment effect [8]. The model was also limited by the assumptions made within the costs associated with complication rates, and utility scores of the patient at different stages. Assumptions are a common and important component of health technology assessment that are utilized to provide plausible information to fill gaps in a comprehensive model that does not have direct data from the literature [22, 23]. The final important limitation for this study is the data used to model conservative treatment options for late stage OA. This data assumes that these conservative treatments would provide their effect in later stage patients. While this may not be an exact representation of these treatment’s effects in later stages, this assumption provides the most conservative estimates with regard to cost-effectiveness of the IA-HA treatments. For this reason, the results of this study remain as conservative evaluations of the cost-effectiveness of IA-HA in late stage patients.
It is important for future research, whether it be prospective investigations or meta-analytic efforts, to aim to provide data regarding the differential effects of IA-HA therapies on patient characteristics such as disease severity. Currently there is a large body of evidence that has evaluated IA-HA as a treatment for knee OA [16], yet there are fewer assessments that provide comprehensive insights into the implications and effects of IA-HA treatment within different patient subpopulations. This information would be important to understand IA-HA efficacy, as well as the specific cost-effectiveness of IA-HA within these subpopulations.
Conclusion
In this study, HMW IA-HA formulations demonstrated cost-effectiveness when compared to conservative treatment options and LMW counterparts in patients with early/mid stage knee OA. The cost-effectiveness of HMW HA in patients with later stage knee OA was not as apparent, particularly because of the uncertainty in responder rates of patients receiving IA-HA who would see a clinical improvement as a result of injection. This cost-effectiveness finding supports the use of IA-HA in patients with early and moderate knee OA, as the benefits of IA-HA are more apparent within patients in earlier stages of disease. The findings of this study suggest that there is a potential cost savings benefit as a result of utilizing HMW HA in earlier stages of knee OA as opposed to later stages.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Funding
This study was funded by Ferring Pharmaceuticals Inc., including the journal’s Rapid Service and Open Access Fees.
Medical Writing Assistance
Medical writing support was provided by Mark Phillips of Global Research Solutions Inc. and was funded by Ferring Pharmaceuticals Inc.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Jeffrey Rosen has nothing to disclose. Faizan Niazi is a paid employee of Ferring Pharmaceuticals. Stan Dysart has received honoraria for being a member of Ferring’s speaker’s bureau.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Footnotes
Enhanced Digital Features
To view enhanced digital features for this article go to 10.6084/m9.figshare.10048502.
References
- 1.Haq I, Murphy E, Dacre J. Osteoarthritis. Postgrad Med J. 2003;79(933):377–383. doi: 10.1136/pmj.79.933.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Laupattarakasem W, Laopaiboon M, Laupattarakasem P, Sumananont C. Arthroscopic debridement for knee osteoarthritis. Cochrane Database Syst Rev. 2008(1):CD005118. [DOI] [PMC free article] [PubMed]
- 3.Vos T, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the global burden of disease study 2010. Lancet. 2012;380(9859):2163–2196. doi: 10.1016/S0140-6736(12)61729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holt HL, et al. Forecasting the burden of advanced knee osteoarthritis over a 10-year period in a cohort of 60–64 year-old US adults. Osteoarthr Cartil. 2011;19(1):44–50. doi: 10.1016/j.joca.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wallace IJ, et al. Knee osteoarthritis has doubled in prevalence since the mid-20th century. Proc Natl Acad Sci USA. 2017;114(35):9332–9336. doi: 10.1073/pnas.1703856114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Losina E, et al. Lifetime medical costs of knee osteoarthritis management in the United States: impact of extending indications for total knee arthroplasty. Arthritis Care Res (Hoboken) 2015;67(2):203–215. doi: 10.1002/acr.22412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Madry H, et al. Early osteoarthritis of the knee. Knee Surg Sports Traumatol Arthrosc. 2016;24(6):1753–1762. doi: 10.1007/s00167-016-4068-3. [DOI] [PubMed] [Google Scholar]
- 8.Rosen J, et al. Clinicians’ perspectives on the use of intra-articular hyaluronic acid as a treatment for knee osteoarthritis: a North American, multidisciplinary survey. Clin Med Insights Arthritis Musculoskelet Disord. 2016;9:21–27. doi: 10.4137/CMAMD.S34496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Altman Roy D., Schemitsch Emil, Bedi Asheesh. Assessment of clinical practice guideline methodology for the treatment of knee osteoarthritis with intra-articular hyaluronic acid. Seminars in Arthritis and Rheumatism. 2015;45(2):132–139. doi: 10.1016/j.semarthrit.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Y, et al. Using patient values and preferences to inform the importance of health outcomes in practice guideline development following the GRADE approach. Health Qual Life Outcomes. 2017;15(1):52. doi: 10.1186/s12955-017-0621-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grazina R, et al. Clinical management in early OA. Adv Exp Med Biol. 2018;1059:111–135. doi: 10.1007/978-3-319-76735-2_5. [DOI] [PubMed] [Google Scholar]
- 12.Filardo G, et al. Non-surgical treatments for the management of early osteoarthritis. Knee Surg Sports Traumatol Arthrosc. 2016;24(6):1775–1785. doi: 10.1007/s00167-016-4089-y. [DOI] [PubMed] [Google Scholar]
- 13.Jevsevar DS. Treatment of osteoarthritis of the knee: evidence-based guideline, 2nd edition. J Am Acad Orthop Surg. 2013;21(9):571–6. [DOI] [PubMed]
- 14.Ong C.K.S., Lirk P., Tan C.H., Seymour R.A. An Evidence-Based Update on Nonsteroidal Anti-Inflammatory Drugs. Clinical Medicine & Research. 2007;5(1):19–34. doi: 10.3121/cmr.2007.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jevsevar DS, et al. Mixed treatment comparisons for nonsurgical treatment of knee osteoarthritis: a network meta-analysis. J Am Acad Orthop Surg. 2018;26(9):325–336. doi: 10.5435/JAAOS-D-17-00318. [DOI] [PubMed] [Google Scholar]
- 16.Bannuru RR, et al. Comparative effectiveness of pharmacologic interventions for knee osteoarthritis: a systematic review and network meta-analysis. Ann Intern Med. 2015;162(1):46–54. doi: 10.7326/M14-1231. [DOI] [PubMed] [Google Scholar]
- 17.Altman RD, et al. Product differences in intra-articular hyaluronic acids for osteoarthritis of the knee. Am J Sports Med. 2016;44(8):2158–2165. doi: 10.1177/0363546515609599. [DOI] [PubMed] [Google Scholar]
- 18.Altman Roy D., Farrokhyar Forough, Fierlinger Anke, Niazi Faizan, Rosen Jeffrey. Analysis for Prognostic Factors from a Database for the Intra-Articular Hyaluronic Acid (Euflexxa) Treatment for Osteoarthritis of the Knee. CARTILAGE. 2015;7(3):229–237. doi: 10.1177/1947603515620890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nicholls Mathew, Shaw Peter, Niazi Faizan, Bhandari Mohit, Bedi Asheesh. The Impact of Excluding Patients with End-Stage Knee Disease in Intra-Articular Hyaluronic Acid Trials: A Systematic Review and Meta-Analysis. Advances in Therapy. 2018;36(1):147–161. doi: 10.1007/s12325-018-0847-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hatoum HT, et al. Cost-effectiveness analysis of intra-articular injections of a high molecular weight bioengineered hyaluronic acid for the treatment of osteoarthritis knee pain. J Med Econ. 2014;17(5):326–337. doi: 10.3111/13696998.2014.902843. [DOI] [PubMed] [Google Scholar]
- 21.Rosen J, et al. Cost-effectiveness of different forms of intra-articular injections for the treatment of osteoarthritis of the knee. Adv Ther. 2016;33(6):998–1011. doi: 10.1007/s12325-016-0331-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Philips Z, et al. Review of guidelines for good practice in decision-analytic modelling in health technology assessment. Health Technol Assess. 2004;8(36):iii–iv, ix–xi, 1–158. [DOI] [PubMed]
- 23.Simoens S. Health economic assessment: a methodological primer. Int J Environ Res Public Health. 2009;6(12):2950–2966. doi: 10.3390/ijerph6122950. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.